Abstract
Context
Comprehensive assessment of metabolism in maternal obesity and pregnancy disorders can provide information about the shared maternal-fetal milieu and give insight into both maternal long-term health and intergenerational transmission of disease burden.
Objective
To assess levels, profiles, and change in the levels of metabolic measures during pregnancies complicated by obesity, gestational diabetes (GDM), or hypertensive disorders.
Design, Setting and Participants
A secondary analysis of 2 study cohorts, PREDO and RADIEL, including 741 pregnant women.
Main Outcome Measures
We assessed 225 metabolic measures by nuclear magnetic resonance in blood samples collected at median 13 [interquartile range (IQR) 12.4-13.7], 20 (IQR 19.3-23.0), and 28 (27.0-35.0) weeks of gestation.
Results
Across all 3 time points women with obesity [body mass index (BMI) ≥ 30kg/m2] in comparison to normal weight (BMI 18.5-24.99 kg/m2) had significantly higher levels of most very-low-density lipoprotein-related measures, many fatty and most amino acids, and more adverse metabolic profiles. The change in the levels of most metabolic measures during pregnancy was smaller in obese than in normal weight women. GDM, preeclampsia, and chronic hypertension were associated with metabolic alterations similar to obesity. The associations of obesity held after adjustment for GDM and hypertensive disorders, but many of the associations with GDM and hypertensive disorders were rendered nonsignificant after adjustment for BMI and the other pregnancy disorders.
Conclusions
This study shows that the pregnancy-related metabolic change is smaller in women with obesity, who display metabolic perturbations already in early pregnancy. Metabolic alterations of obesity and pregnancy disorders resembled each other suggesting a shared metabolic origin.
Keywords: diabetes, gestational, hypertension, gestational, metabolomics, nuclear magnetic resonance, biomolecular, pre-eclampsia, pregnancy, pregnant women
Maternal obesity complicates an increasing number of pregnancies. In 2016, globally 40% of women were overweight [body mass index (BMI) 25-29.99 kg/m2) and 15% obese (BMI ≥ 30 kg/m2) (1). In less than 5 years, the number of women with obesity is estimated to rise by one third to over 21% (2). Maternal overweight and obesity during pregnancy not only increase the mother’s risk for gestational diabetes (GDM), hypertensive disorders, and delivery complications (3), but also the offspring’s risk for preterm birth, intrauterine growth restriction, macrosomia, and other perinatal complications, as well as obesity, metabolic disorders, and neurodevelopmental impairment in childhood and later life (4).
While the underlying mechanisms mediating the adverse effects of maternal obesity on the offspring still remain unknown, recent studies have implicated that perturbations in the maternal metabolome during pregnancy may play a role (5,6). A series of studies have shown that higher prepregnancy BMI, GDM, and preeclampsia (PE) are associated with alterations in blood or urinary metabolome, including several lipoprotein-related variables, triglycerides, specific amino acids (AA), fatty acids (FA), and inflammatory markers (7-10). These studies are, however, limited by having measured maternal metabolic profile at only 1 time point during pregnancy or having pooled metabolome data across trimesters. Normal pregnancy is associated with profound changes in the maternal metabolism to meet the physiological demands imposed by the pregnancy and to ensure adequate growth and development of the fetus (11). Yet, it remains unknown if maternal overweight and obesity, GDM, and hypertensive disorders induce changes in the maternal metabolic signatures above and beyond to that induced by the pregnancy in itself. Studying changes in the maternal metabolome profiles during pregnancy may help to identify novel biomarkers for therapeutic targets and critical time windows for preventive measures, and potential pathways that underpin the intergenerational transmission of metabolic adversities.
Against this background, the aim of this study was to assess if maternal prepregnancy overweight and obesity, GDM, and hypertensive disorders were associated with alterations in the levels and profiles of metabolic measures and in change in the levels across 3 serial time points during pregnancy in 2 Finnish studies comprising 741 pregnant women. We used targeted high-throughput proton nuclear magnetic resonance (NMR)-based metabolomics interrogating 225 metabolic measures.
Subjects
The study population came from 2 Finnish studies: the Prediction and Prevention of Pre-eclampsia and Intrauterine Growth Restriction (PREDO) study (12) and the Finnish Gestational Diabetes Prevention (RADIEL) study (13). The flowchart is presented as Figure 1.
Figure 1.
Flowchart of the participants.
The PREDO study enrolled 1079 pregnant women between 12 and 14 weeks of gestation from 10 hospitals. Details of the enrollment are presented in Figure 1. Of the 404 women giving blood samples, a subgroup with second-degree diastolic notch in the uterine blood flow were randomized to receive low-dose aspirin (n = 61) or placebo (n = 60) for preventing PE. Women providing blood samples in the PREDO cohort were younger (32.5 vs 33.6 years; P = 0.007) and less likely to be obese (29.1% vs 39.3%; P = 0.003) than women who did not.
The RADIEL study enrolled 720 women in a randomized, controlled trial to prevent GDM by lifestyle intervention among high-risk women (prior GDM and/or prepregnancy obesity) planning a pregnancy or in the first half of pregnancy (before 20 weeks of gestation). Of the 337 women giving blood samples, 177 were randomized in the intervention group receiving advice on diet and physical activity and 160 in the control group (standard care). In the RADIEL cohort the women providing blood samples were less likely to be obese (14.0% vs 20.5%; P = 0.04) and have GDM (27.9% vs 73.2%; P < 0.0001) or PE (7.0% vs 3.3%; P = 0.04) than women who did not.
All study participants signed informed consent and the study protocols were approved by ethics committees of the Helsinki and Uusimaa Hospital District.
Methods
Metabolic Profiling Using the NMR Platform
In both cohorts, venous blood samples were drawn from the antecubital vein between 7 and 10 am after at least a 10-h overnight fast. In the PREDO study plasma and in the RADIEL study serum was separated immediately and stored at −80°C until analysis, in which 225 metabolic markers were quantified by using a high-throughput proton NMR metabolomics platform (Nightingale Health Ltd, Helsinki, Finland). These metabolic measures cover multiple metabolic pathways, including 186 lipoprotein lipids and their subclasses, 9 FA and 7 ratios of FA, 5 other lipids, 8 AA, 3 ketone bodies, and 2 metabolites related to fluid balance and three to gluconeogenesis and 1 to inflammation. Following the lead of earlier studies using this metabolomics platform, we used 68 of these metabolic measures as our primary outcomes (9,14). However, we show the results also for the entire metabolomics platform. Details of the experimentation and applications of the NMR metabolomics platform have been described previously (15). In brief, the thawed samples (260 μL) were carefully mixed with sodium phosphate buffer (260 μL) and moved to NMR tubes. The setup is a combination of Bruker AVANCE III 500 MHz (a selective inverse room temperature probe head) and Bruker AVANCE III HD 600 MHz spectrometers (a cryogenically cooled triple resonance probe head; CryoProbe Prodigy TCI), both with the SampleJet robotic sample changer. The lipid extraction procedure was done manually (Integra Biosciences VIAFLO 96 channel electronic pipette) based on multiple extraction steps containing saturated sodium chloride solution, methanol, dichloromethane, and deuterochloroform and data were collected in full automation with the 600 MHz instrument. Computers that controlled the spectrometers do the Fourier transformations to NMR spectra and automated phasing. A centralized server performs various automated spectral processing steps, including overall signal check for missing/extra peaks, background control, baseline removal, and spectral area-specific signal alignments, and the spectral information was compared to 2 quality control samples. This NMR platform has been used in studies of pregnant and nonpregnant populations (9,14,16). Of all the metabolites 37 have been validated against the standard clinical chemistry methods.
Prepregnancy Overweight/Obesity, Gestational Diabetes, and Hypertensive Disorders
Prepregnancy BMI was calculated from prepregnancy weight and height recorded in antenatal clinic records and the Medical Birth Register and, when available, from prepregnancy weight and height measurements (the participants recruited before pregnancy) in the RADIEL study. In both cohorts, diagnoses of GDM and hypertensive disorders were extracted from medical records and verified by a jury comprising of a research nurse and 2 or more medical doctors.
Normal weight (BMI 18.5-24.99 kg/m2), overweight (BMI 25-30 kg/m2), and obesity (BMI ≥ 30 kg/m2) were defined according to World Health Organization guidelines (17). The diagnostic thresholds for GDM were, according to the Finnish guidelines, 5.3, 10.0, and 8.6 mmol/L in a 2-h 75-g oral glucose tolerance test (18). Hypertensive disorders were assessed according to the criteria of the American College of Obstetricians and Gynecologists recommendations (19). Definition for chronic hypertension (HT) was systolic/diastolic blood pressure ≥ 140/90 mmHg present prepregnancy or diagnosed before 20 weeks of gestation or medication for HT before 20 weeks of gestation. Definition for gestational HT was systolic/diastolic blood pressure ≥ 140/90 mmHg occurring after 20 weeks of gestation in a previously normotensive woman, and definition for PE was systolic/diastolic blood pressure ≥ 140/90 mmHg with proteinuria ≥ 300 mg/24 h or equivalent with dipstick in two consecutive measurements.
Covariates
We chose the covariates included in the models based on previous literature. In all models we first adjusted for maternal age (9), cohort, and gestational week at the time of blood sampling (Model 1). Next, we adjusted for level of maternal education (basic/secondary vs tertiary) (9), parity (9,14), and substance (tobacco and alcohol no vs yes) use during pregnancy (14) (Model 2). In additional models (Model 3), overweight and obesity were further adjusted for GDM and hypertensive disorders, and analyses of GDM and hypertensive disorders were additionally adjusted for BMI (9), and GDM further for hypertensive disorders, and hypertensive disorders for GDM. We also assessed the potential confounding of the intervention trials in the PREDO and RADIEL studies. Supplementary Figures 1 and 2 (20) show that interventions were not associated with the metabolic markers during pregnancy, thus, intervention was not accounted for in the analyses. The effect of different samples, serum, and plasma was accounted by the adjustment for cohort.
Statistical Analysis
To study associations of maternal overweight/obesity, GDM, and hypertensive disorders with the levels of and with change in the levels of metabolic measures during pregnancy, we applied individual-participant data meta-analytic approach by using mixed model regression analyses. In these analyses, the repeated metabolic measures represented the within-person outcome variables, and gestational week at the blood sampling the time-varying within-person predictor variable. Normal weight vs overweight/ obesity, normoglycemia vs GDM, normoglycemia vs insulin/ diet treated GDM, and normotension vs HT/gestational HT/PE were included into these models as between-person fixed effects to test if the levels of maternal metabolic measures differed according to these pregnancy conditions. Interaction between normal weight vs overweight/obesity, normoglycemia vs GDM, and normotension vs HT/gestational HT/PE × gestational week at blood sampling tested if the within-person change in the levels of the metabolic measures during pregnancy differed between these pregnancy conditions. We defined unstructured covariance and first-order autoregressive error covariance matrices, used the cohort as a fixed effect, and allowed random effects to account for individual differences in the intercept and in the time-varying gestational week-related slopes.
To identify women with different metabolic profiles during pregnancy we applied latent class analysis (LCA). For these analyses we pooled data for each metabolic measure from the 3 sampling points into a grand average. We compared solutions with 2 to 6 latent classes. Based on criteria for the optimal number of classes described by Kongsted and Nielsen (21), the optimal solution was based on (1) goodness-of-fit criteria (Akaike information criterion, Bayesian information criterion), (2) reasonable distribution of participants across subgroups (at least 10% of the sample), (3) high certainty of classification identified by posterior probabilities, and (4) clear clinical characteristics of the participants within each of the identified groups. We applied logistic regression analysis to examine if the odds to belong to latent classes, identified by the LCA as the optimal, varied according to the pregnancy conditions.
The associations were adjusted for all covariates. Data were missing for substance use and education level (Table 1) and missing values in these variables were coded into a separate category.
Table 1.
Characteristics of the study participants by cohort
| PREDO (N = 404) | RADIEL (N = 337) | |
|---|---|---|
| Gestational age, mean (range) | ||
| At the first blood sampling point | 13.0 (11.1-16.7) | 13.0 (6.0-17.7) |
| At the second blood sapling point | 19.4 (17.1-22.9) | 23.1 (20.1-27.6) |
| At the third blood sampling point | 27.0 (24.1-31.1) | 35.1 (30.6-38.9) |
| Maternal age, years, mean (SD) | 32.6 (5.2) | 33.4 (4.5) |
| Data not available | 0 | 0 |
| Education level, n (%) | ||
| Secondary or lower | 196 (49.5) | 232 (69.0) |
| Tertiary | 200 (51.5) | 104 (31.0) |
| Data not available | 8 (1.1) | 1 (0.3) |
| Parity, n (%) | ||
| Primiparous | 128 (31.7) | 114 (33.8) |
| Multiparous | 276 (68.3) | 223 (66.2) |
| Data not available | 0 | 0 |
| Smoking during pregnancy, n (%) | ||
| No | 374 (93.3) | 323 (96.1) |
| Smoked at any time during pregnancy | 27 (6.7) | 13 (3.9) |
| Data not available | 3 (0.7) | 1 (0.3) |
| Alcohol use during pregnancy, n (%) | ||
| No | 308 (86.5) | 315 (95.2) |
| Yes | 48 (13.5) | 16 (4.8) |
| Data not available | 48 (11.9) | 6 (1.8) |
| Body mass index category, n (%) | ||
| Normal weight (18.5-24.99 kg/m2) | 195 (48.3) | 69 (20.7) |
| Overweight (25-29.99 kg/m2) | 85 (21.0) | 45 (13.4) |
| Obese (≥30 kg/m2) | 124 (30.7) | 223 (66.2) |
| Data not available | 0 | 0 |
| Hypertensive disorders, n (%) | ||
| Normotension | 254 (62.9) | 292 (86.7) |
| Gestational hypertension | 36 (8.9) | 16 (4.8) |
| Preeclampsia | 43 (10.6) | 11 (3.3) |
| Chronic hypertension | 71 (17.6) | 18 (5.4) |
| Data not available | 0 | 0 |
| Gestational diabetes mellitus, n (%) | ||
| Normoglycemia | 314 (77.7) | 243 (71.22) |
| Gestational diabetes mellitus | 90 (22.3) | 94 (27.9) |
| Data not available | 0 | 0 |
The metabolic measures were log-transformed to normalize their distributions. We analyzed the values in standardized units with the SDs summarized in the combined sample so that they had the same value in both cohorts. Due to significant amount of collinearity in the metabolomics data, standard Bonferroni correction for multiple testing may be overly conservative and increase the risk of type II error (22). To overcome this risk, we applied principal components analysis approach, which is one of the most commonly used methods to reduce multidimensionality in metabolomics data and determine the number of independent tests (14,16,23-25) and is suggested as the first step in approaching metabolomics data analysis (22). This approach is analogous to multiple comparison correction routinely applied in genome-wide association studies, where the significance level is set up based on the assumption of the number of independent loci in the genome (26-29). Hence, by using the principal components analysis approach, we identified 25 principal components, which explained over 99% of the variation in the 68 metabolic measures that we used as the primary outcomes. Therefore, 2-sided P < 0.002 (0.05/25) was used to infer statistical significance.
As effect size indicators we present estimates and their 99.8% CIs (mixed model) and odds ratios and their 95% CIs (logistic regression models). Estimates represent mean differences (pooling data from the 3 sampling points into a grand average) and differences in the change (estimate of slope) of the metabolic measures across the three sampling points between women with and without the pregnancy condition. If the estimate reflecting differences in the level of change is negative, the metabolic measure increases less or decreases more, and if the estimate is positive, the metabolic measure increases more or decreases less during pregnancy in women with the disorder compared to women without the disorder.
Statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA). The circular diagrams were created using R (R Core Team 2020) EpiViz package (30-32).
Results
Women in the PREDO study were younger, had higher education, were less often obese, and had more often chronic or gestational HT or PE than women in the RADIEL study (Table 1). The second and third sampling points in the PREDO study were at an earlier gestational stage than in the RADIEL study. Of the study population, 524 (70.7%) women provided all 3 blood samples, 169 (22.8%) 2 samples, and 48 (6.5%) 1 sample (Table 1) and the number of samples at first time point was 625; at second, 666; and at third, 667.
The results for all the 225 metabolic measures are presented as circular diagrams in the supplementary material [see Supplemental Figures 3-5 in (20)], and results of the 68 metabolic measures used as the primary outcomes are presented in Figures 2 to 5.
Figure 2.
Mean differences [pooled mean across the 3 consecutive measurement points (A)] and differences in the change [slopes (A)] of metabolic measures during pregnancy between women with prepregnancy overweight or obesity in comparison to women with normal weight. Dots refer to mean differences and change per 1 pregnancy week in the metabolic measures in SD units and error bars, to their 99.8% CIs between overweight (gray) and normal weight women and between obese (black) and normal weight women. In the analyses of mean differences (main effect models), the associations were adjusted for gestational week at the time of blood sampling, cohort, and maternal age and the analyses of change (interaction models) additionally for the main effects of prepregnancy overweight/obesity (Model 1; dots and bars); further adjustments included parity, education, and substance use during pregnancy (significance is indicated with OW2 for overweight and OB2 for women with obesity), and gestational diabetes and hypertensive disorders (significance is indicated with OW3 for overweight and OB3 for women with obesity).
Figure 5.
Mean differences [pooled mean across the 3 consecutive measurement points (A)] and differences in the change [slopes (B)] of metabolic measures during pregnancy between women with chronic hypertension in comparison to normotensive women. Dots refer to mean differences and change per 1 pregnancy week in the metabolic measures in SD units and error bars, to their 99.8% CIs. In the analyses of mean differences (main effect models) the associations were adjusted for gestational week at the time of blood sampling, cohort, and maternal age, and the analyses of change (interaction models) additionally for the main effects of chronic hypertension (Model 1; dots and bars); further adjustments included parity, education, and substance use during pregnancy (significance is indicated with HT2), and body mass index and gestational diabetes (significance is indicated with HT3).
Prepregnancy Overweight and Obesity
Compared to normal-weight women, women with obesity had higher mean levels (pooled across the 3 measurement points) of many lipoprotein lipids including all very-low-density lipoprotein (VLDL) subclasses and mean diameter of VLDL particles, small high-density (HDL) particles, cholesterol and triglycerides in VLDL, and total triglycerides; monounsaturated FAs (MUFA), saturated fatty acids (SFA), and MUFA to total FA ratio; branched-chain AAs (BCAA) and aromatic AAs; and inflammation marker glycoprotein acetyls (GlycA) in the fully adjusted model, including adjustment for GDM and hypertensive disorders (Fig. 2A). Women with obesity had lower mean levels of very large and large HDL lipoprotein subclasses and mean diameter for HDL particles, and some FA ratios, including polyunsaturated fatty acids (PUFA) to total FA ratio. Out of the 68 metabolic measures, the change in the levels of 43 measures across the 3 sampling points was significantly different (smaller increase in 41 measures, greater decrease in valine and smaller decrease in albumin) between obese and normal weight women in the fully adjusted model [Fig. 2B; also see Supplementary Figure 6 in (20)]. The results were similar when comparing overweight women with normal-weight women, although the levels of metabolic measures and their change were less pronounced and not always statistically significant.
Gestational Diabetes
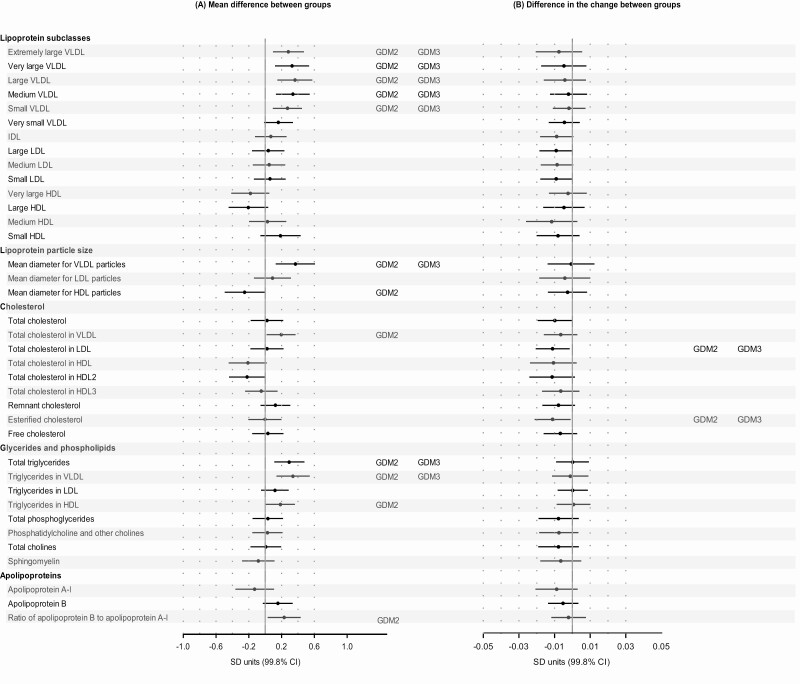
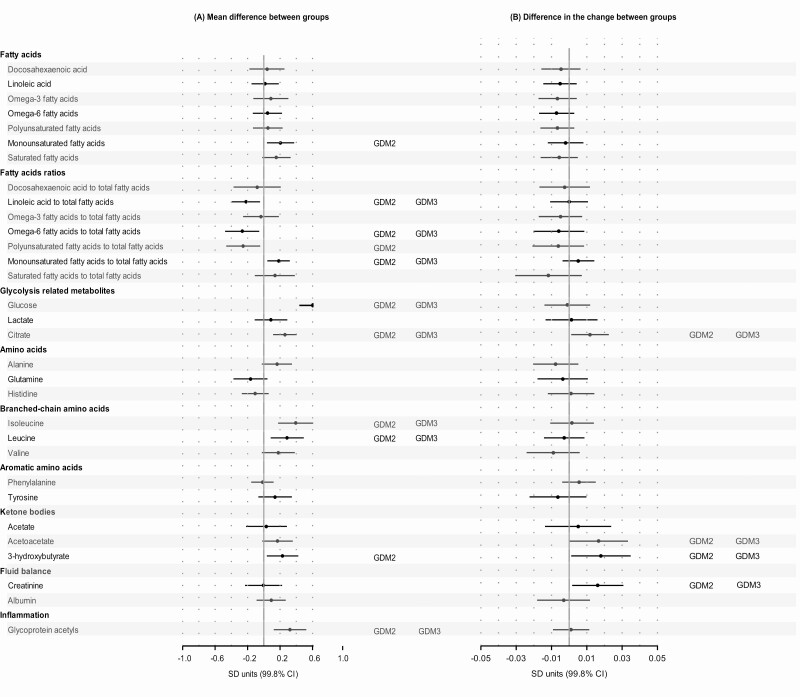
Compared to normoglycemic women, women with GDM had higher/lower mean levels of many of the same metabolites as obesity (Fig. 3A). Of the 68 metabolic measures, 23 associations were significant in the Model 1, but when fully adjusted, including adjustment for BMI and hypertensive disorders, 9 of the associations were rendered nonsignificant (Fig. 3A). The associations that remained significant after full adjustment included all VLDL subclasses (except for very small size), mean diameter for VLDL, VLDL and total triglycerides, BCAA isoleucine and leucine, linoleic to total FA ratio, and the inflammation marker GlycA. Out of the 68 metabolic measures, the change in the levels of 6 measures across the 3 sampling points differed between GDM and normoglycemic women in the fully adjusted model [Fig. 3B; also see Supplemental Figure 7 in (20)]. The differences between normoglycemic and GDM women were more pronounced in insulin-treated than in diet-treated group [see Supplementary Figure 8 in (20)].
Figure 3.
Mean differences [pooled mean across the 3 consecutive measurement points (A)] and differences in the change [slopes (B)] of metabolic measures during pregnancy between women with gestational diabetes in comparison to normoglycemic women. Dots refer to mean differences and change per 1 pregnancy week in the metabolic measures in SD units and error bars, to their 99.8% CIs. In the analyses of mean differences (main effect models) the associations were adjusted for gestational week at the time of blood sampling, cohort, and maternal age, and the analyses of change (interaction models) additionally for the main effects of gestational diabetes (model 1; dots and bars); further adjustments included parity, education and substance use during pregnancy (significance is indicated with GDM2), and body mass index and hypertensive disorders (significance is indicated with GDM3).
Hypertensive Pregnancy Disorders
PE was associated with higher/lower mean levels of many of the same metabolites as obesity. Of the 68 metabolic measures, 19 association were significant in Model 1, but when fully adjusted, including adjustment for BMI and GDM, 9 were rendered nonsignificant (Fig. 4A). The associations that remained significant after full adjustment were 5 lipoprotein subclasses (from extremely large to small VLDL), total triglycerides and triglycerides in VLDL, MUFA, isoleucine, and leucine. Out of the 68 metabolic measures, the change in the levels of 2 measures across the 3 sampling points differed between women with PE and normotension in the fully adjusted model [Fig. 4B; also see Supplementary Figure 9 in (20)].
Figure 4.
Mean differences [pooled mean across the 3 consecutive measurement points (A)] and differences in the change [slopes (B)] of metabolic measures during pregnancy between women with preeclampsia in comparison to normotensive women. Dots refer to mean differences and change per one pregnancy week in the metabolic measures in SD units and error bars, to their 99.8% CIs. In the analyses of mean differences (main effect models) the associations were adjusted for gestational week at the time of blood sampling, cohort, and maternal age, and the analyses of change (interaction models) additionally for the main effects of preeclampsia (Model 1; dots and bars); further adjustments included parity, education, and substance use during pregnancy (significance is indicated with PE2) and body mass index and gestational diabetes (significance is indicated with PE3).
HT was also associated with higher/lower mean levels of many of the same metabolites as obesity, but many of them were rendered nonsignificant after adjustment for BMI and GDM. Out of the 68 metabolic measures, 24 of the 29 significant associations (in Model 1) became nonsignificant (Fig. 5A). The associations that remained significant were total triglycerides, MUFA, citrate, isoleucine, and GlycA. Out of the 68 metabolic measures, change in the levels of 3 measures across the 3 sampling points differed between women with HT and normotension in models adjusted for all covariates [Fig. 5B; also see Supplementary Figure 9 in (20)].
Gestational HT was not associated significantly with any of the metabolic measures during pregnancy [see Supplementary Figure 10 in (20)].
Metabolic Profiles: Latent Class Analysis
The optimal LCA solution identified 3 classes of women who differed significantly for 52 out of 68 metabolic measures; in addition, 9 metabolic measures differed significantly between 2 classes [see Supplementary Tables 1 and 2 in (20)]. Supplemental Table 3 (20) shows the number of women in the 3 latent classes according to different pregnancy conditions. Metabolic profile of women in the class 3 was characterized by higher levels of lipoproteins, cholesterol, triglycerides, AA, and GlycA and a lower ratio of PUFA to total FA. With the exception of acetate and some FA ratios, the levels of most metabolites gradually increased from classes 1 to classes 2 and 3 [see Supplementary Table 2 in (20)]. Across all adjustment models women with obesity compared to women with normal weight had significantly higher odds to belong to class 3 than class 1, and women with PE compared with those with normotension had a significantly higher odds to belong to class 2 than class 1 (Table 2).
Table 2.
Odds ratio with 95% CI for women with overweight, obesity, gestational diabetes, and hypertensive disorders to belong to latent classes with different metabolic profiles during pregnancy
| Latent class 2 vs latent class 1 | Latent class 3 vs latent class 1 | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Overweight versus normal weight | ||||||
| Model 1 | 1.32 | 0.77, 2.26 | 0.31 | 1.75 | 0.90, 3.43 | 0.10 |
| Model 2 | 1.46 | 0.83, 2.56 | 0.19 | 1.90 | 0.96, 3.78 | 0.07 |
| Model 3 | 1.29 | 0.73, 2.30 | 0.38 | 1.74 | 0.87, 3.51 | 0.12 |
| Obesity versus normal weight | ||||||
| Model 1 | 1.74 | 1.10, 3.43 | 0.02 | 2.02 | 1.16, 3.35 | 0.01 |
| Model 2 | 1.64 | 1.01, 2.64 | 0.04 | 2.12 | 1.19, 3.80 | 0.01 |
| Model 3 | 1.46 | 0.89, 2.40 | 0.13 | 1.95 | 1.08, 3.52 | 0.03 |
| Gestational diabetes versus no diabetes | ||||||
| Model 1 | 1.51 | 0.92, 2.47 | 0.11 | 1.39 | 0.78, 2.47 | 0.26 |
| Model 2 | 1.53 | 0.92, 2.55 | 0.10 | 1.34 | 0.75, 2.41 | 0.33 |
| Model 3 | 1.41 | 0.84, 2.36 | 0.19 | 1.23 | 0.68, 2.22 | 0.49 |
| Gestational hypertension versus normotension | ||||||
| Model 1 | 1.11 | 0.53, 2.29 | 0.79 | 1.20 | 0.48, 3.00 | 0.69 |
| Model 2 | 1.07 | 0.51, 2.26 | 0.86 | 1.18 | 0.47, 2.98 | 0.72 |
| Model 3 | 1.03 | 0.49, 2.19 | 0.94 | 1.10 | 0.43, 2.79 | 0.84 |
| Preeclampsia versus normotension | ||||||
| Model 1 | 2.34 | 1.04, 5.27 | 0.04 | 2.32 | 0.85, 6.32 | 0.10 |
| Model 2 | 2.80 | 1.17, 6.72 | 0.02 | 2.73 | 0.95, 7.82 | 0.06 |
| Model 3 | 2.58 | 1.06, 6.23 | 0.04 | 2.36 | 0.81, 6.84 | 0.11 |
| Chronic hypertension versus normotension | ||||||
| Model 1 | 2.63 | 1.31, 5.29 | 0.007 | 3.06 | 1.33, 7.01 | 0.008 |
| Model 2 | 2.25 | 1.11, 4,59 | 0.03 | 2.81 | 1.21, 6.49 | 0.02 |
| Model 3 | 2.04 | 0.99, 4.21 | 0.054 | 2.37 | 1.01, 5.58 | 0.05 |
Model 1 is adjusted for maternal age and cohort, Model 2 is additionally adjusted for maternal education, parity, and substance use during pregnancy, and Model 3 is additionally adjusted for gestational diabetes and hypertensive disorders (in analyses of overweight and obesity), body mass index and hypertensive disorders (in analyses of gestational diabetes), or body mass index and gestational diabetes (in analyses of hypertensive disorders).
Abbreviation: OR, odds ratio.
Discussion
Our study shows that women with prepregnancy obesity have adverse levels of metabolic measures throughout 3 time points during pregnancy and smaller pregnancy-induced changes in the levels compared to normal weight women. Women with obesity displayed higher lipoprotein levels during pregnancy, their fatty acid levels were characterized by higher MUFA and SFA and lower relative levels of PUFA to total FA, their amino acid levels were characterized by higher BCAA and aromatic AA, and they displayed higher levels of GlycA when compared to normal-weight women. The metabolic profile of women with prepregnancy obesity was characterized by a pattern that recapitulated the bivariate associations and pointed to profound and broad metabolic perturbations. Metabolic alterations related with GDM, PE, and HT resembled the alterations related with obesity.
Our study clearly highlights the broad attenuated metabolic response to pregnancy among women with obesity. Most metabolic markers demonstrated smaller changes across pregnancy in obese than in normal-weight women. Metabolic response to pregnancy, evaluated by insulin resistance, converges by the end of pregnancy between women with severe obesity and normal weight according to a study by Forbes et al (33). We have now shown the same kind of convergence in a broader set of metabolic markers. In another study the ability of pregnant women with obesity to adapt to changes in energy fuel demands (eg, from fasting to a postabsorptive state) was less flexible, and they displayed higher inflammation marker levels after test meal (34). Obesity, metabolic inflexibility, and inflammation may enhance each other resulting in adverse long-term effects, such as increased triglycerides and impaired glucose metabolism and insulin resistance (35). Interestingly, in our study, adaptability to pregnancy in women with GDM, PE, or HT seemed, in turn, to be quite similar to women without these complications.
We showed that prepregnancy obesity was associated with atherogenic alterations in lipoproteins consisting of higher levels and larger VLDL particles, smaller HDL particles, and higher levels of triglycerides as well as with high levels of MUFA and SFA and low relative levels of PUFA across pregnancy. Similar adverse lipoprotein levels have been previously presented in cross-sectional studies (9,36). Women with obesity demonstrate net lipolysis (eg, release of free FA mainly from adipose tissue) throughout pregnancy, in contrast with normal-weight women who demonstrate anabolic lipogenesis in early gestation and lipolysis in late gestation (11). Accordingly, the levels of FA in women with obesity in our study were unfavorable already in early pregnancy and stayed at a perturbed level across pregnancy. Obesity-enhanced lipolysis, insulin resistance, and increased inflammation induce hypertriglyceridemia and VLDL secretion from liver (37). Also, reduced activity of lipoprotein lipase results in higher levels of circulating VLDL lipoproteins and triglycerides (37). Excess VLDL may provoke endothelial and placental dysfunction, which have been suggested to explain the associations between maternal hyperlipidemia, obesity, PE, and GDM (38). The high MUFA levels in obesity and pregnancy disorders are probably a consequence of increased lipolysis, lack of fatty acid oxidation, and increased de novo lipogenesis (39). In our study, obesity was associated with a lower ratio of PUFA to total FA that is mainly a consequence of higher total levels of MUFA and SFA. The impact of low relative levels of PUFA on the fetal development should be studied further.
Our longitudinal study strengthens the findings of cross-sectional studies showing prepregnancy obesity to be associated with high levels of BCAA and aromatic AA (9,36). Reduced utilization of BCAAs in liver and adipose tissue, and de novo synthesis of BCAAs by gut microbiota contribute to accumulation of BCAAs in plasma, and obesity is tightly related to reduced activity of BCAA catabolism enzymes and to the changes in the microbiota (40). BCAAs have also been causally linked with insulin resistance (40). In contrast to leucine and isoleucine, we found valine levels decreasing during pregnancy, as seen before (14). Additionally, we demonstrated a greater decrease in obese compared to normal-weight women. It has been hypothesized that valine might have different metabolic effects depending on the adiposity status (40).
Underlying pathophysiologic processes, insulin resistance, low-grade inflammation, oxidative stress, and endothelial dysfunction (41), along with coexistence of obesity and pregnancy disorders, may explain the similarities in metabolic profiles of obesity, GDM, PE, and HT. The origins of GDM, PE, and HT are, however, complex and multifactorial, related to genetic predisposition or lifestyle factors (42). In our study, metabolic measures, which remained significantly associated with GDM and PE in fully adjusted models, were many VLDL measures, triglycerides, some FAs and BCAAs, isoleucine, and leucine, as seen also in the previous cross-sectional studies (9,43-45). In nonpregnant populations HT has also been associated with increased concentrations of many lipids like VLDL and triglycerides (46) which was also seen in our study but rendered nonsignificant after adjustment for BMI and GDM.
We demonstrated persistently higher levels of inflammation marker GlycA across pregnancy complicated by obesity, GDM, and HT. GlycA is a marker of inflammation associated with multiple metabolic aberrations including type 2 diabetes and cardiovascular disease (47). GlycA levels elevate during normal pregnancy (14) and are higher in obese than in overweight pregnant women (36). In our study PE was not independently associated with GlycA levels, but inflammation of PE could have been demonstrated by using a broader panel of inflammation markers.
The strength of our study lies in its longitudinal study design, which allowed us not only to study mean levels of the metabolic markers but change in their levels across 3 serial time points during pregnancy. The targeted panel of metabolic measures we used has been widely studied previously in pregnant and nonpregnant populations, and some of the metabolites have been proved to give quantitative results comparable to conventional laboratory techniques (15). Furthermore, our sample included women at risk for GDM and PE. This resulted in higher number of women with overweight/obesity, GDM and hypertensive disorders in our sample than seen in a general population of pregnant women, which provided higher statistical power to detect associations. Despite the large sample size, in latent class analyses using categorical rather than continuous outcome, the power was still limited as our predictor variables were dichotomous. The targeted metabolomics panel precludes discovery of novel molecules and high-risk sample limits generalizations to all pregnant women. Generalizability may also be limited by the fact that both study populations came from a Nordic high-income country. The studies collected different samples, plasma and serum, but to our knowledge, the plausible bias due to different samples is minimal (48), and we have addressed the issue by applying the statistical methods with SD scaling and adjustment for cohort. Combining 2 cohorts generates a challenge of a wide time range in blood sampling points, which might diminish some of the findings.
In conclusion, our findings indicate that, when compared to normal weight, women with prepregnancy obesity have profoundly perturbed metabolic levels and profiles during pregnancy and display smaller pregnancy-induced change in the levels of the metabolic measures. The metabolic perturbations in pregnancies complicated by GDM, PE, and HT resembled the perturbations seen in obesity, but some of these associations were explained by BMI. Future studies are warranted to explore the influence of disturbed maternal metabolome on long-term maternal health as well as newborn metabolic health and growth.
Acknowledgments
Author Contributions : The authors’ responsibilities were as follows: K.R., H.M.L., E.K., P.M.V., E.H., S.B.K., J.G.E., E.H., and B.A.S.L. designed the research; K.R., H.M.L., E.K., P.M.V., E.H., S.B.K., J.G.E., E.H., and B.A.S.L. conducted research; P.V.G., J.K., and H.S-H. analyzed the data and performed statistical analysis; J.K., H.S-H., P.V.G., K.R., and S.B.K. wrote the manuscript; and J.K., H.S-H., K.R. and S.B.K. had primary responsibility for final content. All authors read and approved the final manuscript.
Financial Support: The PREDO project has been supported by EVO research funding (a special Finnish state subsidy for health science research), Academy of Finland, Signe and Ane Gyllenberg Foundation, Sigrid Juselius Foundation, University of Helsinki Research Funds, Finnish Medical Foundation, Juho Vainio Foundation, Novo Nordisk Foundation, Jane and Aatos Erkko Foundation, and Päivikki and Sakari Sohlberg Foundation. The RADIEL project has been supported by the Alfred Kordelin Foundation, Juho Vainio Foundation, Ahokas Foundation, the Finnish Foundation for Cardiovascular Disease, special state subsidy for health science research of Helsinki University Hospital (HUH), Samfundet Folkhälsan, Finska Läkaresällskapet, Viipuri Tuberculosis Foundation, The Finnish Diabetes Research Foundation. R.R. acknowledges the support of the British Heart Foundation (RE/18/5/34216).
Additional Information
Disclosure Summary: The authors report no conflicts of interest.
Data Availability
Data sets generated during the current study are not publicly available but will be made available upon reasonable request. Requests are subject to further review by the national register authority and by the ethical committees.
References
- 1. World Health Organization. Obesity and overweight. Accessed July 7, 2020. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santos S, Voerman E, Amiano P, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts. Bjog. 2019;126(8):984-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Godfrey KM, Reynolds RM, Prescott SL, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5(1):53-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hellmuth C, Lindsay KL, Uhl O, et al. Maternal metabolomic profile and fetal programming of offspring adiposity: identification of potentially protective lipid metabolites. Mol Nutr Food Res. 2019;63(1):e1700889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kadakia R, Nodzenski M, Talbot O, et al. ; HAPO Study Cooperative Research Group . Maternal metabolites during pregnancy are associated with newborn outcomes and hyperinsulinaemia across ancestries. Diabetologia. 2019;62(3):473-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mills HL, Patel N, White SL, et al. ; UPBEAT Consortium . The effect of a lifestyle intervention in obese pregnant women on gestational metabolic profiles: findings from the UK Pregnancies Better Eating and Activity Trial (UPBEAT) randomised controlled trial. BMC Med. 2019;17(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacob S, Nodzenski M, Reisetter AC, et al. ; HAPO Study Cooperative Research Group . Targeted metabolomics demonstrates distinct and overlapping maternal metabolites associated with BMI, glucose, and insulin sensitivity during pregnancy across four ancestry groups. Diabetes Care. 2017;40(7):911-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor K, Ferreira DLS, West J, Yang T, Caputo M, Lawlor DA. Differences in pregnancy metabolic profiles and their determinants between White European and South Asian women: findings from the Born in Bradford cohort. Metabolites. 2019;9:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelly RS, Croteau-Chonka DC, Dahlin A, et al. Integration of metabolomic and transcriptomic networks in pregnant women reveals biological pathways and predictive signatures associated with preeclampsia. Metabolomics. 2017;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938-948. [DOI] [PubMed] [Google Scholar]
- 12. Girchenko P, Lahti M, Tuovinen S, et al. Cohort profile: prediction and prevention of preeclampsia and intrauterine growth restriction (PREDO) study. Int J Epidemiol. 2017;46(5): 1380-1381g. [DOI] [PubMed] [Google Scholar]
- 13. Rönö K, Stach-Lempinen B, Klemetti MM, et al. ; RADIEL group . Prevention of gestational diabetes through lifestyle intervention: study design and methods of a Finnish randomized controlled multicenter trial (RADIEL). BMC Pregnancy Childbirth. 2014;14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Q, Würtz P, Auro K, et al. Metabolic profiling of pregnancy: cross-sectional and longitudinal evidence. BMC Med. 2016;14(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soininen P, Kangas AJ, Würtz P, Suna T, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet. 2015;8(1):192-206. [DOI] [PubMed] [Google Scholar]
- 16. Würtz P, Cook S, Wang Q, et al. Metabolic profiling of alcohol consumption in 9778 young adults. Int J Epidemiol. 2016;45(5):1493-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii. [PubMed] [Google Scholar]
- 18. Kaaja R, Kivelä R, Kukkonen-Harjula K, et al. Raskausdiabetes: Käypä hoito -suositus. Duodecim. 2008;124:1556-1569. [Google Scholar]
- 19. American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstetr Gynecol. 2013;122:1122-1131. [DOI] [PubMed] [Google Scholar]
- 20.Kivelä J, Sormunen-Harju H, Girchenko P, et al. Data from: Longitudinal metabolic profiling of maternal obesity, gestational diabetes and hypertensive pregnancy disorders. Supplemental material for the original research article. Deposited 28 Jun 2021. https://zenodo.org/record/5036704#.YNnIoBMzbCV. [DOI] [PMC free article] [PubMed]
- 21. Kongsted A, Nielsen AM. Latent class analysis in health research. J Physiother. 2017;63(1):55-58. [DOI] [PubMed] [Google Scholar]
- 22. Alonso A, Marsal S, Julià A. Analytical methods in untargeted metabolomics: state of the art in 2015. Front Bioeng Biotechnol. 2015;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sliz E, Kettunen J, Holmes MV, et al. Metabolomic consequences of genetic inhibition of PCSK9 compared with statin treatment. Circulation. 2018;138(22):2499-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bell JA, Carslake D, Wade KH, et al. Influence of puberty timing on adiposity and cardiometabolic traits: a Mendelian randomisation study. PloS Med. 2018;15(8):e1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beynon RA, Richmond RC, Santos Ferreira DL, et al. ; ProtecT Study Group; PRACTICAL consortium . Investigating the effects of lycopene and green tea on the metabolome of men at risk of prostate cancer: the ProDiet randomised controlled trial. Int J Cancer. 2019;144(8):1918-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gao X, Starmer J, Martin ER. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol. 2008;32(4):361-369. [DOI] [PubMed] [Google Scholar]
- 27. Galwey NW. A new measure of the effective number of tests, a practical tool for comparing families of non-independent significance tests. Genet Epidemiol. 2009;33(7):559-568. [DOI] [PubMed] [Google Scholar]
- 28. Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb). 2005;95(3):221-227. [DOI] [PubMed] [Google Scholar]
- 29. Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74(4):765-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee MA, Mahmoud P, Hughes D, et al. Epiviz: an implementation of Circos plots for epidemiologists. https://github.com/mattlee821/EpiViz. Accessed October 6, 2020.
- 31. Gu Z, Gu L, Eils R, Schlesner M, Brors B. circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30(19):2811-2812. [DOI] [PubMed] [Google Scholar]
- 32. Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32(18):2847-2849. [DOI] [PubMed] [Google Scholar]
- 33. Forbes S, Barr SM, Reynolds RM, et al. Convergence in insulin resistance between very severely obese and lean women at the end of pregnancy. Diabetologia. 2015;58(11):2615-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tinius RA, Blankenship MM, Furgal KE, et al. Metabolic flexibility is impaired in women who are pregnant and overweight/obese and related to insulin resistance and inflammation. Metabolism. 2020;104:154142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell Metab. 2017;25(5):1027-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Houttu N, Mokkala K, Laitinen K. Overweight and obesity status in pregnant women are related to intestinal microbiota and serum metabolic and inflammatory profiles. Clin Nutr. 2018;37(6 Pt A):1955-1966. [DOI] [PubMed] [Google Scholar]
- 37. Bays HE, Toth PP, Kris-Etherton PM, et al. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7(4):304-383. [DOI] [PubMed] [Google Scholar]
- 38. Contreras-Duarte S, Carvajal L, Garchitorena MJ, et al. Gestational diabetes mellitus treatment schemes modify maternal plasma cholesterol levels dependent to women´s weight: possible impact on feto-placental vascular function. Nutrients. 2020;12:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frigolet ME, Gutiérrez-Aguilar R. The role of the novel lipokine palmitoleic acid in health and disease. Adv Nutr. 2017;8(1):173S-181S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newgard CB. Metabolomics and metabolic diseases: where do we stand? Cell Metab. 2017;25(1):43-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McElwain CJ, Tuboly E, McCarthy FP, McCarthy CM. Mechanisms of endothelial dysfunction in pre-eclampsia and gestational diabetes mellitus: windows into future cardiometabolic health? Front Endocrinol (Lausanne). 2020;11:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15(3):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mokkala K, Vahlberg T, Pellonperä O, Houttu N, Koivuniemi E, Laitinen K. Distinct metabolic profile in early pregnancy of overweight and obese women developing gestational diabetes. J Nutr. 2020;150(1):31-37. [DOI] [PubMed] [Google Scholar]
- 44. Villa PM, Laivuori H, Kajantie E, Kaaja R. Free fatty acid profiles in preeclampsia. Prostaglandins Leukot Essent Fatty Acids. 2009;81(1):17-21. [DOI] [PubMed] [Google Scholar]
- 45. White SL, Lawlor DA, Briley AL, et al. ; UPBEAT Consortium . Early antenatal prediction of gestational diabetes in obese women: development of prediction tools for targeted intervention. Plos One. 2016;11(12):e0167846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Onuh JO, Aliani M. Metabolomics profiling in hypertension and blood pressure regulation: a review. Clin Hypertens. 2020;26(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ritchie SC, Würtz P, Nath AP, et al. The biomarker GlycA is associated with chronic inflammation and predicts long-term risk of severe infection. Cell Syst. 2015;1(4):293-301. [DOI] [PubMed] [Google Scholar]
- 48. Jiménez B, Holmes E, Heude C, et al. Quantitative lipoprotein subclass and low molecular weight metabolite analysis in human serum and plasma by 1H NMR spectroscopy in a multilaboratory trial. Anal Chem. 2018;90(20):11962-11971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sets generated during the current study are not publicly available but will be made available upon reasonable request. Requests are subject to further review by the national register authority and by the ethical committees.