Abstract
Adrenal tumors are commonly discovered incidentally on cross-sectional abdominal imaging performed for reasons other than adrenal mass. Incidence of adrenal tumors increased 10-fold in the past 2 decades, with most diagnosed in older adults. In any patient with a newly discovered adrenal mass, determining whether the adrenal mass is malignant and whether it is hormonally active is equally important to guide the best management. Malignancy is diagnosed in 5% to 8% of patients with adrenal tumors, with a higher risk in young patients, if history of extra-adrenal malignancy, in those with large adrenal tumors with indeterminate imaging characteristics, and in bilateral adrenal tumors. Although overt hormone excess is uncommon in adrenal incidentalomas, mild autonomous cortisol secretion can be diagnosed in up to 30% to 50% of patients. Because autonomous cortisol secretion is associated with increased cardiovascular morbidity and metabolic abnormalities, all patients with adrenal incidentalomas require work up with dexamethasone suppression test. Management of adrenal tumors varies based on etiology, associated comorbidities, and patient’s preference. This article reviews the current evidence on the diagnosis and evaluation of patients with adrenal mass and focuses on management of the most common etiologies of adrenal incidentalomas.
Keywords: adrenal mass, diagnosis, malignancy, Hounsfield units, hormonal work up, dexamethasone suppression test, imaging
Case 1
The patient was a 61-year-old woman who presented for evaluation of continuous weight gain of 50 pounds over 5 to 7 years. She was recently diagnosed with diabetes mellitus type 2 (hemoglobin A1C [HbA1C] = 7.1%, not on medications). Other comorbidities included hypertension treated with lisinopril 10 mg daily and amlodipine 5 mg daily. Two years prior, she was diagnosed with osteoporosis (treated only with calcium and vitamin D supplements daily). Several months prior, she was incidentally discovered with a 2.2 cm left adrenal mass. Physical examination was positive for body mass index (BMI) of 41.3 kg/m2 and blood pressure of 138/86 mm Hg, but no features of Cushing syndrome.
On unenhanced computed tomography (CT) of abdomen, the left adrenal mass was lipid rich (-12 Hounsfield units [HU]), measuring 2.2 cm in the largest diameter (Fig. 1A). The right adrenal gland appeared normal. The baseline laboratory test results are shown in Table 1.
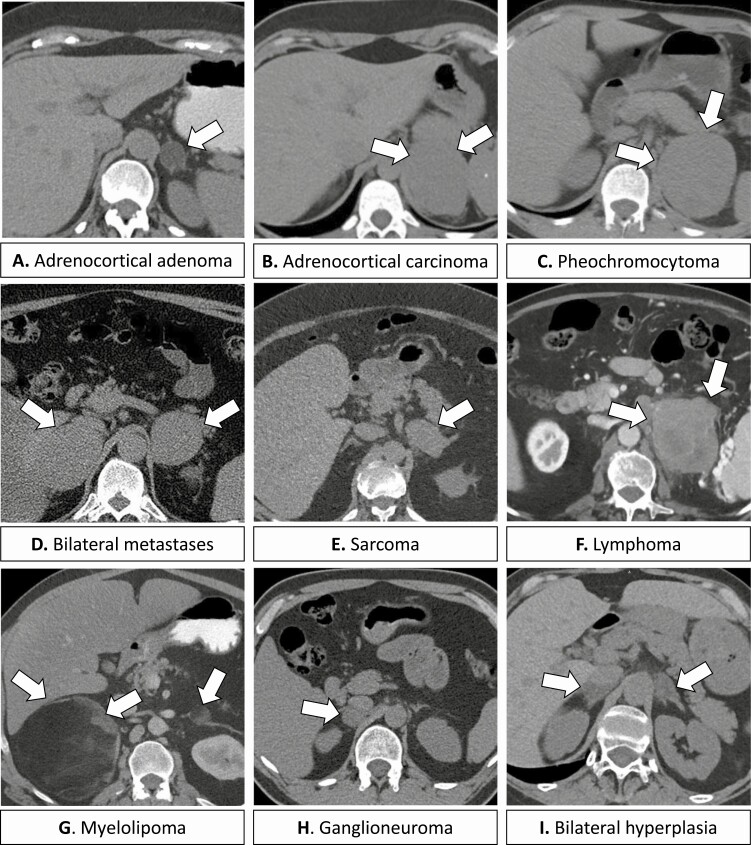
Figure 1.
Computed tomography (CT) imaging of adrenal incidentalomas. (A) Adrenocortical adenoma, left, 2.2 cm, Hounsfield units (HU) of -12 on unenhanced CT. (B) Adrenocortical carcinoma, left, 6.8 cm, HU of 35 on unenhanced CT. (C) Pheochromocytoma, left, 7.7 cm, HU 42 on unenhanced CT. (D) Bilateral adrenal metastases, melanoma, 4.5 right and 2.8 cm left, HU of 25 to 27 on unenhanced CT. (E) Sarcoma, left, 5.1 cm, HU of 36 on unenhanced CT. (F). Lymphoma, left, 9.7 cm, heterogeneous on contrast enhanced CT. (G) Bilateral myelolipomas, 12.7 cm right and 2.6 left, with areas of macroscopic fat on contrast enhanced CT. (H) Ganglioneuroma, right, 2.6 cm, HU of 18 on unenhanced CT. (I) Bilateral adrenal hyperplasia, 2.4 cm right and 2.6 cm left, HU of 7 on unenhanced CT.
Table 1.
Laboratory work up for case 1, case 2, and case 3
| Biochemical testing | Case 1 | Case 2 | Case 3 | Reference range |
|---|---|---|---|---|
| Morning cortisol following the 1-mg overnight DST, mcg/dL | 2.9 | 3.3 | 22 | <1.8 |
| ACTH, pg/mL | <5 | 14 | <5 | 7.2-63 |
| DHEA-S, mcg/dL | 26 | 485 | <15 | 15-200 (case 1); 44-332 (case 2); 18-284 (case 3) |
| Aldosterone, ng/dL | 8 | 5 | 4 | <21 |
| Plasma renin activity, ng/mL/h | 4.1 | 1.7 | <0.6 | ≤0.6-3 |
| Urine metanephrines, mcg/24 h | - | 227 | - | <400 |
| Urine normetanephrine, mcg/24 h | - | 356 | - | <900 |
| Urine free cortisol, mcg/24 h | 27 | 38 | 389 | 3.5-45 |
Abbreviations: DHEA-S, dehydroepiandrosterone sulfate; DST, dexamethasone suppression test.
Case 2
The patient was a 24-year-old woman who presented for evaluation of an incidentally discovered adrenal mass on a CT scan performed in the emergency room several months earlier. She was then diagnosed and treated for appendicitis. Since then, she fully recovered and was asymptomatic. She was not taking any medications or supplements. Her physical examination was normal, without any signs of cortisol or androgen excess. Her BMI was 23.6 kg/m2 and blood pressure 115/64 mm Hg.
Her CT scan was reviewed and demonstrated a left adrenal mass at 6.8 cm with HU of 36 (Fig. 1B). When compared with previous imaging 4 months earlier, an enlargement by 1 cm was noted. Laboratory tests were obtained (Table 1).
Case 3
A 47-year-old woman presented for evaluation of bilateral adrenal tumors. Over the past 3 years, she progressively gained weight (27 pounds, mostly over abdominal area), developed supraclavicular and dorsocervical fat pads, striae over upper thighs and abdomen, anxiety, and insomnia. In addition, she reported a new onset hypertension and a new-onset diabetes mellitus type 2 (HbA1C = 7.8%) diagnosed 8 months previously. At the time of evaluation, medications included lisinopril, amlodipine, hydrochlorothiazide, and metformin. On physical examination, her blood pressure was 163/97 mm Hg and BMI was 31.7 kg/m2. She had abdominal and upper thigh striae, facial rounding and erythema, dorsocervical pad, and supraclavicular pads. Cushing syndrome was suspected, and laboratory tests were obtained (Table 1). Imaging was reviewed and revealed bilateral adrenal nodules, 2.2 cm on the right and 2.4 cm on the left, consistent with adrenal adenomas (HU of 5 bilaterally).
Clinical Relevance of Adrenal Incidentaloma
Adrenal tumors are commonly discovered on cross-sectional abdominal imaging in up 5% to 7% of patients (1). Incidence of adrenal tumors increased 10-fold in the past 2 decades, in parallel to the increase in the number of abdominal CT and magnetic resonance imaging (MRI) studies (2). Most of the increase in incidence was due to a more frequent discovery of smaller adrenal adenomas in older adults >65 years old, whereas the number of large and malignant tumors remained the same (2). Adrenal tumors are seen almost equally in men (45%) and women (55%), with a median age of diagnosis of 62 years. Adrenal tumors are very uncommon in children, with only 1% of all adrenal tumors diagnosed in patients <18 years old (2).
Adrenal tumors can be broadly divided in 5 categories: (1) adrenal adenomas and nodular hyperplasia; (2) other benign lesions (myelolipomas, cysts, hematomas, other); (3) adrenocortical carcinomas (ACC); (4) other malignant tumors (metastases, sarcomas, lymphoma); and (5) pheochromocytomas.
Population-based data demonstrated that malignant adrenal tumors are diagnosed in 8.6% of all cases, with a majority representing adrenal metastases, and only 0.3% representing ACC (2). The distribution of pathologies is different in patients referred to endocrinology, where most patients with malignant tumors are diagnosed with ACC, and only a minority with other malignant tumors (3, 4). This is likely because patients with suspected metastasis or lymphoma are evaluated by other specialties, such as medical oncology, pulmonology, and urology. In a recent study of patients with adrenal metastases, only 30% of patients with adrenal metastases were evaluated by endocrinology (5). Similarly, only 10% of patients referred for adrenal biopsy are evaluated by endocrinologists (6).
Most benign tumors are adrenal cortical adenomas, representing 85% of all patients with adrenal tumors (2). Adrenal cortical adenomas demonstrate biochemical evidence of adrenal hormone excess in around 50% to 60% of cases, most commonly mild autonomous cortisol secretion (MACS) (1). As demonstrated by a recent population-based study, only a minority of patients with adrenal tumors undergo optimal work up for hormone excess, missing in particular patients with MACS, but also likely patients with primary aldosteronism and milder forms of Cushing syndrome (CS) (2). If untreated, adrenal hormone excess is associated with increased cardiometabolic risk, multiple comorbidities, and increased mortality (7, 8). Thus, determining whether the adrenal mass is malignant and whether the adrenal mass is hormonally active is equally important to determine the best management.
Etiology of Adrenal Incidentaloma
Adrenal Adenoma
Adrenal adenomas represent the majority of incidentally discovered adrenal tumors in both the population setting (88%) (2) as well as in the endocrine clinic (81%-88%) (3, 9, 10). At the time of diagnosis, median tumor size of adrenal adenomas is 1.5 to 2.5 cm, with 95% to 98% being <4 cm in size (2, 7, 9). Bilateral adrenal adenomas are demonstrated in approximately 15% of patients. On imaging, around 60% of adenomas are lipid rich, with unenhanced HU <10, 25% with HU between 10 and 19, and 15% with HU >20. Autonomous cortisol secretion is diagnosed in up to 35% to 50% of patients (Table 2) (1, 2). Notably, adenomas with overt hormone excess are often diagnosed only after incidental discovery with adrenal mass, rather than upon presentation with features of CS or primary aldosteronism (3, 10, 11).
Table 2.
Clinical, imaging, and biochemical presentation of adrenal tumors
| Adrenocortical adenoma | Other benign mass | Adrenocortical carcinoma | Other malignant mass | Pheochromocytoma | |
|---|---|---|---|---|---|
| Prevalence | |||||
| Population | 84% | 7% | 0.3% | 8% | 1% |
| Endocrine clinic | 85%-90% | 3%-7% | 1%-5% | 1%-3% | 1%-8% |
| Mode of discovery | |||||
| Incidental | 85% | 90% | 40% | 35% | 60% |
| Cancer staging imaging | 7% | 4% | <1% | 50% | <1% |
| Symptoms of hormone excess | 7% | <1% | 40% | <1% (adrenal insufficiency) | 30% |
| Abdominal mass effect | <1% | 5% | 15% | 5% | <1% |
| Other | <1% | 1% | 5% | 10% | 10% (genetic screening) |
| Imaging | |||||
| Tumor size | |||||
| Median | 1.5-2.5 cm | 2-3 cm | 10 cm | 3 cm | 4-5 cm |
| <4 cm (%) | 95% | 60%-70% | 1-2% | 60% | 45% |
| Tumor laterality | 15%-20% bilateral | 5%-10% bilateral | <0.1% bilateral | 24%-43% bilateral | 5%-10% bilateral |
| Tumor growth | <1 cm/12 mo | <1 cm/12 mo | >1 cm/3-6 mo | >1 cm/3-6 mo | <1 cm/12 mo |
| Unenhanced CT (HU) | HU < 10: 50%-60% | HU: variable | HU < 10: 0% | HU < 10: 0% | HU < 10: 0% |
| HU 10-20: 20%-30% | HU < 0: myelolipomas | HU 10-20: 1%-2% | HU 10-20: 1%-4% | HU 10-20: 1%-3% | |
| HU > 20: 10%-20% | HU > 100: calcifications | HU > 20: 98%-99% | HU > 20: 96%-98% | HU > 20: 97%-99% | |
| MRI | Variable (depending on etiology) | ||||
| Chemical shift present | 60%-80%a | 0%a | 0%b | 0%b | |
| Chemical shift absent | 20%-40%a | 100%a | 100%b | 100%b | |
| Adrenal hormone excess | |||||
| Hormonally inactive | 50%-60% | 100%b | 20%-50% show no hormone excess during routine biochemical testing (only 5% with urine steroid metabolomics).Functioning tumors most commonly cause glucocorticoid and androgen excess (alone or in combination). Isolated aldosterone excess is extremely rare. | 100% | 5%-10% |
| Glucocorticoid excess | 40-50% MACS; 1%-3% CS | - | - | - | |
| Aldosterone excess | 5%-10% | - | - | - | |
| Androgen excess | <0.1% | - | - | - | |
| Catecholamine excess | - | - | - | 90%-95% | |
| Other | Consider CAH in case of bilateral masses | Consider CAH in large bilateral myelolipomas | Consider primary adrenal insufficiency if bilateral adrenal involvement | - |
Abbreviations: CAH, congenital adrenal hyperplasia; CS, Cushing syndrome; CT, computed tomography; HU, Hounsfield unit; MACS, mild autonomous cortisol secretion; MRI, magnetic resonance imaging.
aData on the accuracy of MRI in diagnosing malignancy and pheochromocytoma is limited; definitions of chemical shift may vary between users. Considering these limitations, a clearly lipid-rich lesion (chemical shift present) excludes malignancy and pheochromocytoma.
bRarely, adrenal hormone excess can be detected in a patient with myelolipoma (autonomous cortisol secretion, primary hyperaldosteronism). In these situations, a concomitant adenoma or hyperplasia is usually diagnosed.
Myelolipomas and Other Benign Adrenal Masses
Adrenal myelolipomas are diagnosed in 3.3% to 6.2% of patients with adrenal incidentalomas (Table 2). Patients usually present with a unilateral adrenal myelolipoma, at a median size of around 2 to 2.5 cm; however, size may range considerably between 0.5 and >15 cm (12). Bilateral myelolipomas occur in 5% of all patients, and in 20% of patients with large tumors >6 cm (12).
Benign noncortical adrenal tumors other than myelolipomas are rare, representing altogether 1% to 2%, and include ganglioneuromas, cysts, hemangiomas, lymphangiomas, and schwannomas (2, 3, 9, 10, 13, 14). Imaging characteristics of these tumors vary, and diagnosis is frequently made on histology after adrenalectomy for a suspicious mass.
Pheochromocytoma
Pheochromocytomas represent 1.1% of patients with adrenal tumors in a population, and 4% to 8.5% of patients evaluated in the endocrine setting (Table 2) (2-4). Only 27% of patients are diagnosed with pheochromocytomas based on evaluation for symptoms of catecholamine excess, with the majority discovered incidentally (61%) or based on genetic case detection testing (12%) (15). When diagnosed incidentally or based on symptoms, pheochromocytomas are usually large tumors with a median size of 4 to 5 cm, and bilateral tumors in in 4% to 10% of cases (15). However, when diagnosed based on case detection imaging in those with known genetic predisposition, pheochromocytomas are usually smaller and more likely to be bilateral (15). Pheochromocytomas usually demonstrate HU > 20 on unenhanced CT scan (92%), with only 7.5% presenting HU between 10 and 20, and 0.5% HU of exactly 10. No pheochromocytomas have been reported to have HU <10 (11, 15-17). Around 4% of pheochromocytomas may be biochemically silent with normal testing for catecholamine excess (15), either from lack of catecholamine secretion, or when detected at a smaller size with catecholamine secretion under the standard of care cutoff for measurements.
Adrenocortical Carcinoma
ACCs are rare malignant adrenal tumors representing 0.3% of all adrenal tumors and 3.6% of malignant adrenal tumors in a population setting (2). However, ACC is the most common adrenal malignancy evaluated in the endocrine clinic, representing around 5% of patients seen by endocrinologist (Table 2) (9). ACCs are discovered incidentally in 42% to 44% of cases (9, 11), though the subsequent work up for hormonal excess is frequently abnormal (9). ACC presents as a unilateral mass, with most ACCs being large at the time of discovery, with a median size of 10 cm (9, 11). On imaging, ACCs are either heterogeneous tumors or demonstrate HU > 20 on unenhanced CT, with a median of 35 HU (9, 11). Only 2% of ACCs are discovered at tumor size <4 cm and only 1% have HU between 10 and 20 on unenhanced CT scan (9).
Adrenal Metastasis or Other Malignant Mass
Adrenal metastasis is the most common etiology of a malignant adrenal mass, representing 7.5% of all adrenal tumors and 86% of all malignant adrenal tumors in a population-based study (2). However, adrenal metastases are uncommon in the endocrine clinic, with only 1 in 4 patients with adrenal metastasis undergoing endocrine work up (2, 5, 6). Although the majority of adrenal metastases are discovered during imaging for cancer staging, 36% are detected incidentally (5). Patients usually present with median mass size of 3 cm, ranging between 0.5 and 20 cm (Table 2) (5). Bilateral adrenal metastases are common, with 24% of patients demonstrating bilateral disease at the time of initial diagnosis and 43% of patients developing bilateral disease during follow-up (5). Preclinical or symptomatic primary adrenal insufficiency can be diagnosed in 12% of patients with bilateral adrenal metastases (5). Other very rare adrenal malignancies include lymphomas, sarcomas, and neuroblastomas (2, 9, 11).
Diagnosing Adrenal Malignancy
Diagnosis of adrenal malignancy is based on pertinent history, imaging with secondary testing including urine and serum steroid profiling, rarely, adrenal biopsy, or diagnostic adrenalectomy (Table 2, Fig. 2).
Figure 2.
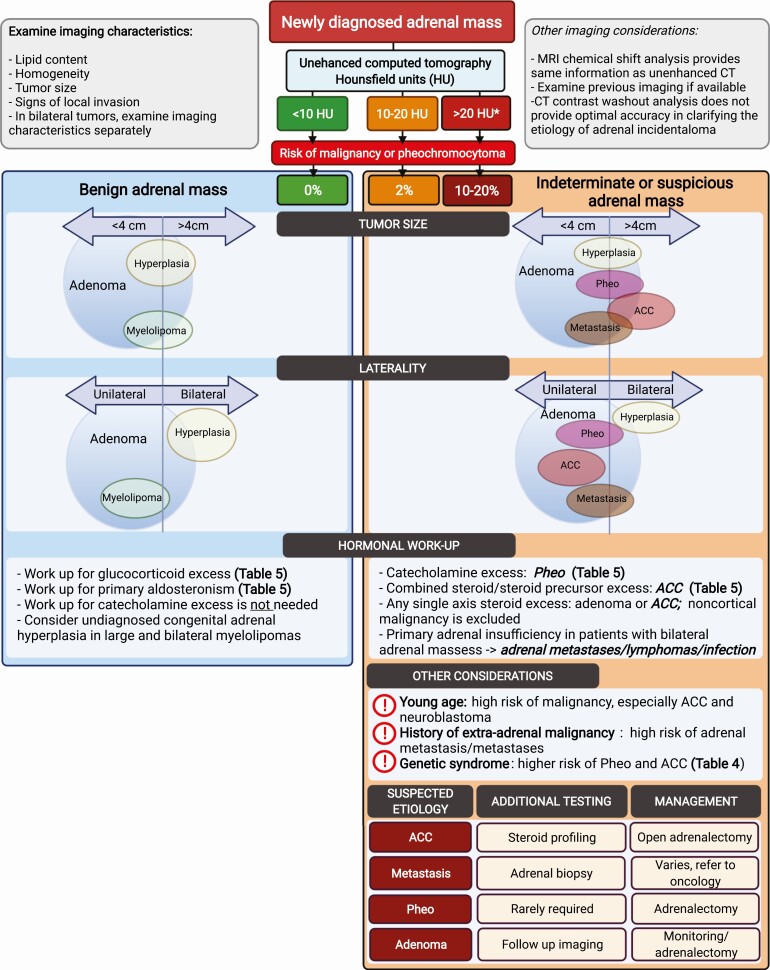
Adrenal incidentaloma work up: evaluation for etiology. In a patient with newly diagnosed incidental adrenal mass, imaging characteristics should be examined. Risk of malignancy and/or pheochromocytoma is stratified based on the unenhanced computed tomography attenuation of the adrenal mass (measured in HU). If HU of a homogeneous adrenal mass is <10, the benign adrenal mass pathway should be followed. If the HU of a homogeneous adrenal mass is >10, or the mass is heterogeneous, the pathway for the indeterminate or suspicious adrenal mass should be followed. Tumor size and laterality of the adrenal mass should be examined next. The size of the circle is proportional to the prevalence of each entity. The line that divides the tumor size (<4 cm and >4 cm) and laterality (unilateral vs bilateral) further indicates the proportion of each tumor entity falling within each cutoff. Further considerations in the indeterminate/suspicious adrenal mass pathway include the (1) findings of the hormonal work up, (2) demographics, (3) history of extra-adrenal malignancy, and (4) presence of the genetic syndrome. Additional testing and management are suggested based on suspected etiology. Abbreviations: ACC, adrenocortical carcinoma; CT, computed tomography; HU, Hounsfield units; MRI, magnetic resonance imaging; Pheo, pheochromocytoma. *If the tumor is heterogeneous on imaging, proceed with the pathway for “>20 HU.” Created with BioRender.com.
Mode of Discovery
The circumstances around the discovery of the adrenal mass could help estimate the risk of malignancy. Only 3.3% of all adrenal incidentalomas are malignant, compared with 43% of adrenal tumors discovered on cancer staging imaging in those with history of extra-adrenal malignancy (2). Of all patients discovered with an adrenal mass, only 3% are diagnosed based on symptoms of overt hormone excess (2). Circumstances around the discovery of the adrenal mass should be interpreted along with imaging characteristics of the adrenal mass (Table 2, Fig. 2).
Imaging Characteristics
Unenhanced CT, contrast-enhanced CT with washout characteristics, and MRI with chemical shift analysis are the most commonly used imaging techniques in evaluating adrenal tumors (18-20). 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) scan is usually reserved for patients with history of extra-adrenal malignancy (18-20). In addition to characterization of tumor size, tumor borders and heterogeneity of the adrenal mass, imaging characteristics of the adrenal mass provide valuable information in diagnosis of malignant adrenal mass and pheochromocytoma (16, 17, 20) (Fig. 2).
Tumor size and laterality
The risk of malignancy is proportional to tumor size. In a population study of 1287 patients, 6% of adrenal tumors <2 cm and 9% of adrenal tumors between 2 and 4 cm were malignant, as opposed to 34% of adrenal tumors >4 cm being malignant (2). In a study of 705 patients with large adrenal tumors >4 cm evaluated in a tertiary center, prevalence of malignancy was similar to the population setting (31%) (11). Because the median size of ACCs at the time of presentation is 10 cm, larger tumor size makes the likelihood of this diagnosis higher; however, tumor size does not perform as well in adrenal metastases (median size at diagnosis, 3 cm), possibly because of the earlier discovery through cancer staging performed in patients with extra-adrenal malignancy (5, 9, 11). ACC is almost always a unilateral adrenal mass, whereas 24% to 43% of adrenal metastases are bilateral (5, 11). As such, in a population setting in which most malignant adrenal tumors were adrenal metastases, bilaterality was associated with a 2.3 times higher risk for malignancy when compared with unilateral adrenal tumors (2).
Tumor growth
Examining prior available imaging may be valuable in assessing the risk of malignancy. As reported in a large systematic review and meta-analysis of adrenal adenomas followed for a mean of 50 months, mean tumor growth was 2 mm, and the overall tumor growth of at least 10 mm was noted in 2.5% of patients (7). Imaging evidence of tumor growth in adenomas also depends on when in its natural history adenoma is initially detected. Smaller adenomas (<2.5 cm) at baseline were reported to grow more than larger adenomas (>2.5 cm) (7). Adenoma growth was also more likely to occur with longer duration of follow-up (>24 months) (7). No malignant transformation was reported in any patient initially classified to have a benign adrenal tumor (7, 12).
Pheochromocytomas grow slowly, around 3 to 5 mm/y (21, 22), though the data are scarce because adrenalectomy is the standard of care for any patient with pheochromocytoma.
The tumor growth of malignant adrenal tumors is variable. Studies reporting on tumor growth of malignant tumors are difficult to interpret because of concomitant therapies that may affect tumor growth, short duration of follow-up because of adrenalectomy, or possible bias toward cases with unusual natural history. A mean of 60 mm/y tumor growth was reported in 1 small study (23). Another study reported that an absolute growth of 8 mm in 2 to 12 months had sensitivity of 72% and specificity of 81% in diagnosing malignancy (24).
Computed tomography and magnetic resonance imaging
Both unenhanced CT and MRI with chemical shift analysis assess lipid content within the adrenal mass. HU measurement <10 on unenhanced CT indicates a lipid-rich lesion. HU cutoff >10 demonstrates a sensitivity of 100% and specificity of 33% to 72% to detect malignancy in patients with adrenal mass (20, 25). In other words, a homogeneous adrenal mass with HU <10 can be definitely diagnosed as benign. This cutoff can be accurately applied to large adrenal tumors >4 cm and to patients at high risk for malignancy, such as those with extra-adrenal malignancy. For example, in a study of 705 patients with adrenal tumors >4 cm, the minimum HU on unenhanced CT in pheochromocytomas, ACC, and other malignancy was 18, 18, and 14, respectively (11). In another study of 353 patients referred for adrenal biopsy because of concern of malignancy, the median unenhanced HU was 36 with the minimum HU of 11 in patients ultimately diagnosed with malignant lesions (25).
Chemical shift analysis on MRI demonstrated a sensitivity of 86% to 90% and specificity of 85% in diagnosing malignant adrenal mass, with most studies being small, and with some concern of user variability (20). Because both unenhanced CT and MRI assess lipid content, in general it is not useful to obtain both studies to characterize the adrenal mass.
CT washout analysis was reported to have a suboptimal performance in a systematic review and meta-analysis of accuracy of imaging in diagnosing malignancy, with limited data available, and sensitivity of only 16% (95% CI, 3-40) and specificity of 86% (95% CI, 64-97) in diagnosing malignancy (20). Taking together the scarce data, reported suboptimal accuracy, and associated cost, CT washout analysis was not recommended as a second-line testing of adrenal mass by the most recent guidelines (18).
18F-fluorodeoxyglucose positron emission tomography imaging
In a systematic review and meta-analysis of diagnostic accuracy of imaging, FDG-PET scan was reported to have sensitivity of 82% to 100% and specificity of 96% when adrenal-to-liver standardized uptake value (SUV) ratio is used (20). A subsequent study of 89 patients with 44 malignant and 36 benign adrenal tumors, FDG-PET adrenal-to-liver ratio >1.8 demonstrated a sensitivity equal to specificity of 86% (25). Another study of 70 benign adrenal tumors, 35 adrenocortical carcinomas, and 12 adrenal metastases demonstrated similar results: FDG-PET adrenal-to-liver ratio of 2.5 yielded 85% sensitivity and 90% specificity, whereas a cutoff of 3.4 had 83% sensitivity and 90% specificity (26). False positives may occur in functioning adrenal adenomas and false-negative results may occur in small metastases and when significant necrosis is present (25, 26). Pheochromocytomas are FDG-avid; however, FDG-PET in patients with pheochromocytomas is not usually indicated unless metastatic or multifocal disease is suspected.
Steroid Profiling
Both serum and urine steroid profiling can be valuable in the diagnostic work up of an adrenal mass. Steroid profiling has been investigated in the diagnosis of cortisol and aldosterone excess as well as in the diagnosis of ACC (9, 27-32). Urine steroid profiling has been recently prospectively validated in a large multicenter study of 2017 patients demonstrating a high accuracy in diagnosing ACC, especially when combined with imaging characteristics (tumor size > 4 cm and HU > 20 on unenhanced CT) (9). The basis of steroid profiling is that ACCs secrete steroid precursors in increased amounts, sometimes as high as 10 to 40 times higher than in other adrenal tumors (33). Thus, even “nonfunctioning” ACC on standard-of-care testing will be detected through urine steroid profiling, unless the tumor has lost its capacity for steroidogenesis, which is a very rare occurrence (Table 2) (9).
Adrenal Biopsy
Adrenal biopsy is rarely needed in the diagnostic work up of adrenal mass. It may be useful in indeterminate nonfunctioning adrenal masses where the results of biopsy will change management, such as patients suspected to have malignant adrenal tumors other than ACC (adrenal metastasis, sarcoma, lymphoma) or an infectious etiology of the adrenal mass (fungal, tuberculosis) (18, 34). Sensitivity and specificity of adrenal biopsy in making the diagnosis of adrenal malignancy were reported to be 89% to 100% and 91% to 99%, respectively (6, 35-38).
Adrenal biopsy has poor accuracy in diagnosing ACC and may present with a higher risk of needle track seeding and a worse prognosis; thus, noninvasive diagnosis of ACC is key. Notably, adrenal biopsy has a nondiagnostic rate of 3% to 8.7%, and a complication rate of up to 4% (6, 35, 36). Reported complications are usually mild, and include periadrenal hemorrhage, asymptomatic pneumothorax, and pain. Complications associated with the endoscopic ultrasound-guided fine needle aspiration are lower than in the CT-guided biopsy (38). Notably, case series describe inadvertent biopsy of pheochromocytoma in 0.7% to 1.7% of cases (6, 39), which can precipitate hypertension, tremors, and tachycardia (6, 35). It is not surprising because only one-quarter of patients are tested for possible pheochromocytoma before biopsy (6).
Diagnosing Adrenal Hormone Excess
Clinical Evaluation
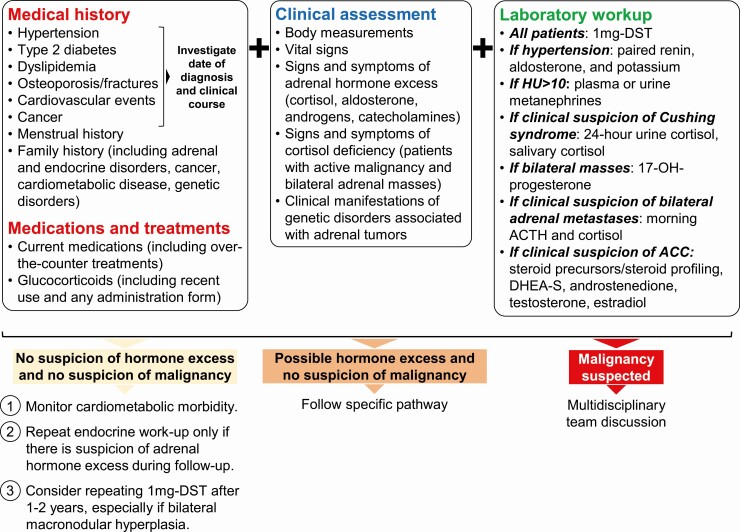
The first step in assessing possible hormone excess in patients with adrenal incidentaloma is obtaining a detailed medical history, including family history and current medications that can affect the hormonal tests (Fig. 3). The clinical course and combination of comorbidities should be investigated as well because this may be a clue to hormone excess (Table 3). For example, a recent diagnosis of dyslipidemia and hypertension in a previously healthy 40-year-old individual, even if well-controlled with medical treatment, may be more relevant than a diagnosis of type 2 diabetes 10 years before the incidental finding of the adrenal mass. Questions regarding current and recent use of glucocorticoids, which can both affect laboratory investigations and be responsible for iatrogenic CS, are a key part of the initial assessment of adrenal incidentaloma.
Figure 3.
Adrenal incidentaloma evaluation: clinical and hormonal assessment. Abbreviations: 1-mg DST, 1-mg overnight dexamethasone suppression test; ACC, adrenocortical cancer; DHEA-S, dehydroepiandrosterone sulfate.
Table 3.
Clinical manifestations of adrenal hormone excess
| Hormone excess | General considerations | Clinical manifestations | |
|---|---|---|---|
| Cortisol excess | MACS | • Diagnosed in up to 50% of patients with incidentalomas. • Absence of stigmata of overt cortisol excess (Cushing syndrome). • Associated with frailty and an increased cardiometabolic morbidities. |
• Metabolic syndrome: hyperglycemia; hypertension; dyslipidemia; obesity. • Cardiovascular events. • Atrial fibrillation and other arrhythmias. • Osteoporosis and fragility fractures (high prevalence of asymptomatic vertebral fractures). • Anxiety/depression. • Chronic kidney disease. • Frailty |
| Cushing syndrome | • Rare (3%-5% of patients with adrenal adenomas). • Up to 50% are diagnosed during adrenal incidentaloma work up. • Requires prompt recognition and work up to confirm the diagnosis and offer treatment. • If untreated, it is associated with high morbidity and mortality. • Concomitant adrenal androgen excess indicates adrenocortical carcinoma. |
• Stigmata of cortisol excess: facial plethora; dorsocervical fat pad; supraclavicular fat pads; muscle loss, proximal myopathy, and muscle weakness; easy bruising; red stretchmarks. • Increased mortality risk, mainly driven by cardiovascular and thromboembolic events. • Metabolic syndrome: hyperglycemia; hypertension; dyslipidemia; obesity. • Osteoporosis and fragility fractures. • Immunosuppression and susceptibility to infections. • Depression and other psychiatric disorders, insomnia, memory loss, irritability, panic attacks. • Amenorrhea and reduced fertility. • Heart failure. |
|
| Aldosterone excess | • Incidentalomas causing primary aldosteronism are almost invariably benign. • Hypokalemia is not required to make the diagnosis. • Associated with increased cardiometabolic risk. • Concomitant cortisol excess (usually MACS) is common. |
• Hypertension. • Hypokalemia (10%-40% of cases). • Symptoms and complications related to hypokalemia: polyuria and nocturia; fatigue and weakness; muscle cramps; constipation. • Cardiovascular and cerebrovascular events. • Chronic kidney disease. • Heart failure and atrial fibrillation. • Left ventricular hypertrophy. • Metabolic syndrome: hyperglycemia; obesity. • Osteoporosis. • Depression. • The spectrum of biochemically proven primary aldosteronism includes also subjects with normal blood pressure. Primary aldosteronism should be suspected in young normotensive subjects with unexplained hypokalemia. |
|
| Androgen excess | • Adrenal androgen excess typically indicates adrenocortical carcinoma. Androgen-producing adenomas are extremely rare. • Androgen and cortisol co-secretion (both MACS and Cushing syndrome) are common in adrenocortical carcinoma. |
Women can present with: • Hirsutism. • Oily skin and acne. • Hair loss. • Changes in libido. • Menstrual irregularities. • Metabolic syndrome (chronic androgen excess). |
|
| Catecholamine excess | • Pheochromocytomas are increasingly diagnosed incidentally (60%). • Most patients do not have the classic “spell” symptoms. • Germline pathogenic variants are common (up to 40%-50%). Syndromic associations can be a clue to the diagnosis (Table 4). |
• Hypertension in up to 90% cases (paroxysmal in 50%). • Classic triad (“spell”): headaches, sweating, palpitations. • Pallor, nausea, tremor, anxiety. • Postural hypotension • Supraventricular tachycardia. • Myocardial ischemia/ infarction. • Cardiomyopathy and heart failure (takotsubo syndrome). • Hypertensive crisis triggered by stressors (e.g., surgery, colonoscopy, some medications). |
|
Abbreviation: MACS, mild autonomous cortisol secretion.
A minority of adrenal tumors can be manifestations of numerous genetic syndromes: up to 5% to 10% of ACC, 25% of bilateral macronodular hyperplasia, and 40% of catecholamine-secreting tumor cases harbor a germline mutation (40-42). It is important, therefore, to evaluate for possible syndromic associations that can be clues to an underlying genetic disorder during the physical examination and when collecting the medical history (Table 4) (40-53). Other elements that can point toward a germline pathogenic variant are young age at the time of diagnosis, familial occurrence, and bilateral adrenal masses.
Table 4.
Genetic disorders associated with adrenal tumors
| Genetic disorder | Affected gene (mode of inheritance) | Adrenal involvement | Extra-adrenal manifestations |
|---|---|---|---|
| Genetic disorders associated with pheochromocytomas | |||
| Von-Hippel-Lindau disease type 2 | VHL (autosomal dominant) | Pheochromocytomas are diagnosed in 10%-20% of patients and are often bilateral (~40%). Malignant cases have been reported. | • Hemangioblastomas of the brain and spine. • Retinal angiomas. • Renal cell cancer. • Pancreatic neuroendocrine neoplasms. • Pancreatic serous cystadenomas. • Endolymphatic sac tumors of the middle ear. • Papillary cystadenomas of the epididymis and broad ligament. |
| Multiple endocrine neoplasia syndrome type 2 | RET (autosomal dominant) | Pheochromocytomas are common (~50% of cases), often bilateral (~60%), and rarely malignant. |
MEN2A: • Medullary thyroid carcinoma. • Hyperparathyroidism. • Cutaneous lichen amyloidosis. • Hirschsprung disease. MEN2B: • Medullary thyroid carcinoma. • Mucocutaneous neuromas of the tongue, lips, and eyelids. • Marfanoid habitus. • Decreased upper/lower body ratio. • Kyphoscoliosis/lordosis. • Joint laxity. • Myelinated corneal nerves. • Intestinal ganglioneuromas, associated with constipation and megacolon. |
| Paraganglioma syndrome type 1 | SDHD (autosomal dominant; maternal imprinting) | Pheochromocytomas are found in 10%-25% of cases and can be bilateral. Malignancy is uncommon. | • Paragangliomas. • Renal cell cancer. • Pituitary adenomas. • GISTs. |
| Paraganglioma syndrome type 4 | SDHB (autosomal dominant) | Pheochromocytomas are found in ~25% of cases and can be bilateral. Malignancy is common (>40%). | • Paragangliomas. • Renal cell cancer. • Pituitary adenomas. • GISTs. • Pulmonary chondromas. |
| Paraganglioma syndrome type 5 | SDHA (autosomal dominant) | Pheochromocytomas are rare (very low penetrance). Bilateral disease and malignancy have been reported. | • Paragangliomas. • Pituitary adenomas. • GISTs. • Pulmonary chondromas. |
| MAX mutation | MAX (autosomal dominant) | Pheochromocytomas are often bilateral (60%-70%) and can be malignant (10%-25%). | Paragangliomas. |
| TMEM127 mutation | TMEM127 (autosomal dominant) | Pheochromocytomas are often bilateral (~40%). Moderate risk of malignancy (~10%). | Renal cell cancer. |
| Pheochromocytoma/paraganglioma-somatostatinoma-polycythemia syndrome | EPAS1 (somatic mosaicism) | Pheochromocytomas have been described. | • Paragangliomas. • Polycythemia. • Somatostatinoma. • Retinal abnormalities. • Organ cysts. |
| Genetic disorders associated with both pheochromocytomas and adrenocortical tumors | |||
| Neurofibromatosis type 1 | NF1 (autosomal dominant) | • Pheochromocytomas are diagnosed in 0.1%-6% of cases. They can be bilateral (~15%) and malignant (3%-12%). • poradic cases of adrenocortical carcinoma have been reported. |
• Central nervous system gliomas (especially of the optic pathway). • Café-au-lait spots. • Freckling. • Neurofibromas (can have malignant transformation). • Lisch nodules. • Short stature. • Scoliosis. • Cognitive deficits. • Seizures. • Macrocephaly. • Paragangliomas (rare). • Rhabdomyosarcomas. • GISTs. • Juvenile myelomonocytic leukemia. • Breast cancer. • Congenital heart disease. |
| Hereditary leiomyomatosis and renal cell cancer | FH (autosomal dominant) | • Adrenal masses have been observed in ~8% of cases. • Patients can present with bilateral adrenal tumors (~15%). Cases of ACTH-independent Cushing syndrome have been described. • Pheochromocytomas are rare. They can be bilateral and carry a moderate/high risk of malignancy. |
• Renal cell cancer. • Cutaneous leiomyomas. • Uterine leiomyomas. • Paragangliomas. |
| Beckwith-Wiedemann syndrome | Chromosome 11p15.5 region. Mostly sporadic disease. Familial transmission occurs in ~15% of cases. |
Adrenal involvement has been described only in children: • Adrenocortical adenomas and hyperplasia (including association with androgen and cortisol excess) are common. • Numerous cases of adrenal cysts. • Some cases of adrenocortical carcinomas. • Sporadic cases of pheochromocytoma (including bilateral disease). |
• Several childhood cancers, including Wilms tumor, hepatoblastoma, rhabdomyosarcoma, neuroblastoma. • Macrosomia. • Omphalocele. • Macroglossia. |
| Genetic disorders associated with adrenocortical carcinomas only | |||
| Li-Fraumeni syndrome | TP53 (autosomal dominant) | Accounts for 3%-7% of adrenocortical carcinoma cases in adults (50%-80% in children). Adrenocortical carcinoma is often the presenting malignancy of the syndrome. | Numerous malignancies, including brain, breast, and lung cancer, sarcomas, leukemias, choroid plexus tumors. |
| Lynch syndrome | MLH1, MSH2, MSH6, PMS2, EPCAM (autosomal dominant) | Accounts for 3% of adrenocortical carcinoma cases in adults. | • Numerous malignancies, including colorectal, endometrial, small bowel, genitourinary system, pancreatobiliary system, ovarian, stomach, brain, prostate. • Sebaceous skin neoplasms. • Keratoacanthomas. |
| Carney complex | PRKAR1A (autosomal dominant) | Cases of adrenocortical carcinomas reported. ACTH-independent Cushing syndrome secondary to primary pigmented nodular adrenocortical disease is much more common. | • Lentiginous skin pigmentation. • Cutaneous myxomas. • Thyroid nodules (including cancer). • Growth hormone hypersecretion, with or without evidence of pituitary adenoma/hyperplasia. • Cardiac myxomas. • Schwannomas. • Large cell calcifying Sertoli cell tumors (males). • Ovarian cysts and tumors. • Benign breast tumors. |
| Birt-Hogg-Dubé syndrome | FLCN (autosomal dominant) | Cases of adrenocortical tumors with uncertain malignant potential (oncocytomas) have been reported. | • Skin fibrofolliculomas. • Pulmonary cysts and spontaneous pneumothorax. • Renal oncocytomas. • Renal cell cancer. |
| Genetic disorders associated with both adrenocortical carcinomas and adenomas | |||
| Multiple endocrine neoplasia syndrome type 1 | MEN1 (autosomal dominant) | Adrenocortical tumors have been reported in ~10% of cases and can be bilateral (~15%). The risk of malignancy is high (~15%). Hormone hypersecretion is found in ~15% of cases (mainly primary aldosteronism and ACTH-independent Cushing syndrome). Cases of pheochromocytoma have been described, but this is an extremely rare occurrence. |
• Primary hyperparathyroidism. • Pituitary adenomas. • Neuroendocrine neoplasms. • Lipomas. • Facial angiofibromas. • Collagenomas. |
| Familial adenomatous polyposis | APC (autosomal dominant) | Adrenocortical adenomas have been described in 7%-13% of patients (including bilateral tumors in ~20% of cases). Rare cases of adrenocortical carcinomas have been reported. | • Colorectal polyps and cancer. • Upper gastrointestinal polyps. • Desmoid tumors. • Brain tumors. • Thyroid nodules and cancer. • Hepatoblastomas. |
| Genetic disorders associated with adrenocortical adenomas only | |||
| PBMAH from ARMC5 mutations | ARMC5 (autosomal dominant) | Most cases of PBMAH are sporadic. Germline mutations of ARMC5 are found in ~25% of all case of PBMAH. However, ~50% of those with PBMAH and severe Cushing syndrome and ~80% of those with a clear family history of PBMAH have a germline mutation. | Meningiomas have been described in patients harboring ARMC5 mutations. |
| Carney triad | Mostly unknown. Germline variants of SDHA, SDHB, or SDHC have been found in ~10% of cases. | It is sporadic and affects mostly young women. Adrenocortical adenomas have been described in 20% of cases (mostly nonfunctioning tumors). |
Triad: paragangliomas + GISTs + pulmonary chondromas. Other manifestations: esophageal leiomyomas. |
| Partial glucocorticoid resistance associated with bilateral adrenal hyperplasia | Heterozygous mutations of NR3C1, encoding for the glucocorticoid receptor | Mutations have been identified in 5% of patients with bilateral adrenal incidentalomas combined with hypertension and/or MACS. Patients with mutations: • Were younger and had a lower prevalence of hypertension than nonmutated patients. • All but one failed the 1-mg DST. • All but 1 had higher urinary free cortisol excretion than nonmutated patients. • All but 1 had normal or high ACTH levels. • Had lower potassium (<4 mEq/L), renin, and aldosterone (<2.7 ng/dL) levels than patients without mutations. |
Patients typically lack clinical hypercortisolism or hyperandrogenism and present incidentally with bilateral adrenal enlargement. The combination of low potassium, low aldosterone, elevated urinary free cortisol, and abnormal 1-mg DST are clues to the diagnosis. |
Abbreviations: 1-mg DST, 1-mg overnight dexamethasone suppression test; ARMC5, Armadillo Repeat Containing 5 gene; GIST, gastrointestinal stromal tumor; MACS, mild autonomous cortisol secretion; PBMAH, primary bilateral macronodular adrenal hyperplasia.
Laboratory Assessment
MACS (adrenal incidentaloma + abnormal 1-mg dexamethasone suppression test [DST] + absent stigmata of CS) is the most common hormonal abnormality diagnosed in 30% to 50% of patients with adrenal mass (1, 18, 54); thus, all patients should undergo a 1-mg overnight DST to exclude cortisol excess (Table 5, Fig. 4) (18, 19). The rest of the initial laboratory assessment should be personalized based on clinical and imaging characteristics: (1) if there is a history of hypertension or unexplained hypokalemia, primary aldosteronism should be ruled out by paired morning plasma renin and aldosterone measurement (55, 56); (2) if unenhanced CT HU are >10, work up for catecholamine excess with plasma or urine metanephrines should be performed (57); and (3) if ACC is suspected, elevated sex hormones and steroid precursors can noninvasively confirm this diagnosis before adrenalectomy (18) (Table 5). Additional testing may be required in patients with abnormal 1-mg DST and signs suggestive of CS (Table 5, Fig. 4). In patients with bilateral adrenal tumors (adenomas or myelolipomas), congenital adrenal hyperplasia should be considered. Finally, in patients with high suspicion of primary adrenal insufficiency from bilateral infiltrative disease (bilateral adrenal metastases), morning ACTH and cortisol should be measured (Table 5) (18).
Table 5.
Hormonal work up in patients with adrenal tumors
| Adrenal hormone abnormality | Indication for testing | First-line testing | Second-line or confirmatory testing | Other considerations and remarks |
|---|---|---|---|---|
| Cortisol excess | Anyone with adrenal mass, regardless of symptoms | 1-mg DST. Abnormal result: serum cortisol >1.8 mcg/dL. The ESE-ENSAT guidelines on adrenal incidentalomas define MACS as “possible” when 1-mg DST serum cortisol is 1.8-5.0 mcg/dL and “confirmed” when 1-mg DST serum cortisol is >5.0 mcg/dL. Possible causes of false-positive results: • Oral estrogens (e.g., OCP) • CYP3A4 inducers. • Exogenous glucocorticoids (assay interference). • Uncontrolled hyperglycemia. • Alcoholism. • Psychiatric disorders. • Morbid obesity. • Pregnancy. • Chronic active hepatitis. • Kidney failure • Older age • Dementia |
• ACTH. • DHEAS. • 24-h urine free cortisol (if Cushing syndrome is suspected). • Salivary cortisol (if Cushing syndrome is suspected). • In selected cases: repeat 1-mg DST; perform 8-mg DST; 2-day, low-dose DST (Liddle test); CRH test. |
• ACTH-independent cortisol excess must be confirmed before considering adrenal surgery. • Patients with adrenal hypercortisolism have abnormal DST, low ACTH, and DHEA-S. • 24h urine free cortisol is usually normal in MACS. • The accuracy of salivary cortisol in MACS is low. |
| Aldosterone excess | Anyone with hypertension, with or without spontaneous hypokalemia | Morning aldosterone + renin (DRCorPRA). Abnormal result: aldosterone >10 ng/dL and suppressed renin (DRC or PRA). Possible causes of false-positive results:a • Beta-blockers. • α-methyldopa • NSAIDs. • Oral estrogens (renin measured as DRC). • Testing during the luteal phase (women of reproductive age). • Impaired renal function with hyperkalemia. Possible causes of false-negative results:a • Mineralocorticoid receptor antagonists. • Diuretics. • Dihydropyridine calcium channel blockers. • Inhibitors. • Angiotensin II receptor blockers. • SSRIs. • SGLT2-inhibitors. |
Unnecessary if positive first-line test and spontaneous hypokalemia. Otherwise: salt loading test, saline infusion test, captopril challenge, or fludrocortisone test. |
• Patients with confirmed primary aldosteronism will need subtype evaluation with imaging and adrenal vein sampling. • Imaging finding of adrenal mass is accurate only in 60% of cases in subtype determination. • Cortisol co-secretion is highly prevalent in primary aldosteronism (abnormal 1-mg DST is found in up to 22% of patients).b |
| Catecholamine excess | Anyone with indeterminate adrenal mass (HU ≥ 10), with or without symptoms | Plasma or 24-h urine metanephrines. Abnormal result: usually >2× upper limit of normal. Possible causes of false-positive results: • Collection of plasma metanephrines under not controlled conditions. • Medications: tricyclic antidepressants; psychoactive agents; prochlorperazine; L-dopa; adrenergic receptor agonists; phenoxybenzamine. |
• Usually not needed unless false-positive results are suspected.d • Urinary or plasma dopamine or plasma methoxy tyramine is a possible add-on test to detect tumors with dopamine hypersecretion (increased risk of malignancy). |
• 24-h urine fractionated metanephrines have excellent sensitivity and specificity. • Plasma fractionated metanephrines have excellent sensitivity but suboptimal specificity if not collected appropriately. Fasting collection after 30 min in the supine position and the use of age-adjusted upper limits of normal increase specificity. |
|
• Physical stress or illness: significant illness requiring hospitalization; congestive heart failure; panic attacks; subarachnoid bleeding; obstructive sleep apnea. • Withdrawal from alcohol, clonidine, and other drugs. Possible causes of false-negative results: small pheochromocytomas (especially with maximum diameter < 2 cm).c |
• In patients with suspected pheochromocytoma, extra-adrenal disease and associated genetic predisposition need to be considered (Table 4). |
|||
| Suspected steroid precursor, androgen, or estrogen excess | Anyone suspected to have adrenocortical carcinoma, with or without symptoms | DHEA-S, progesterone, 17-OH-progesterone, 17-OH-pregnenolone, 11-deoxycortisol, androstenedione, testosterone (women), estradiol (men, postmenopausal women). If available, consider urine multisteroid profiling. |
• Avoid adrenal biopsy. • Open adrenalectomy is usually recommended. • Experienced adrenal surgeon is key! |
|
| Anyone with bilateral adenomas or myelolipomas should be evaluated for congenital adrenal hyperplasia | Early-morning 17-OH-progesterone (to be collected during the early follicular phase in menstruating females). | ACTH stimulation test for cortisol and 17-OH-progesterone. | Consider genetic testing. | |
| Adrenal insufficiency | Anyone with indeterminate bilateral masses likely to be adrenal metastases or bilateral infiltration of other causes | Morning ACTH and cortisol. If electrolyte abnormalities, test for aldosterone deficiency (paired aldosterone and renin). |
Potential need for additional dynamic testing such as ACTH stimulation. |
aWe advise against routine washout from potential interfering medications when assessing a patient with a newly diagnosed adrenal mass. The main issue with interfering medications is false-negative results for mild or optimally treated primary aldosteronism cases (mostly from a nonsuppressed renin). Case detection testing can be done even in patients treated with mineralocorticoid receptor antagonists because patients with hypertension are usually treated with doses lower than those required to fully block the mineralocorticoid receptor. A suppressed renin in this scenario (especially if hypokalemia is present) makes the diagnosis of primary aldosteronism very likely. If retesting is required, this should be carried out in the morning, 2 to 4 h after waking up. If it is safe to do so, interfering medications should be stopped for 2 to 4 weeks before testing. Alternative treatments that do not interfere with the test are verapamil, doxazosin, prazosin, terazosin, hydralazine, and moxonidine.
bCortisol co-secretion is not only relevant from a cardiometabolic risk perspective; it may also affect the performance of adrenal vein sampling, the risk of developing adrenal insufficiency after unilateral adrenalectomy, and the rate of clinical success after surgery.
cSmaller catecholamine-secreting tumors may have negative metanephrine test results. This is relevant for smaller incidentalomas with HU > 10 and especially for patients with known genetic disorders associated with pheochromocytoma who undergo routine surveillance imaging for other manifestations of their disease. In such cases, it is therefore appropriate to retest patients if the initial test was negative and the adrenal mass increases in size and/or the patient develops symptoms of catecholamine excess during follow-up.
dTricyclic antidepressants and other psychoactive agents should be tapered down and discontinued at least 2 wk before retesting. Selective serotonin reuptake inhibitors should not significantly affect the screening tests.
Abbreviations: CRH, corticotropin releasing hormone; CYP3A4, cytochrome P450 3A4; DHEA-S, dehydroepiandrosterone sulfate; DRC, direct renin concentration; DST, dexamethasone suppression test; MACS, mild autonomous cortisol secretion; NSAIDs, nonsteroidal anti-inflammatory drugs; OCP, oral contraceptive pill; PRA, plasma renin activity; SGLT2, sodium-glucose co-transporter-2.
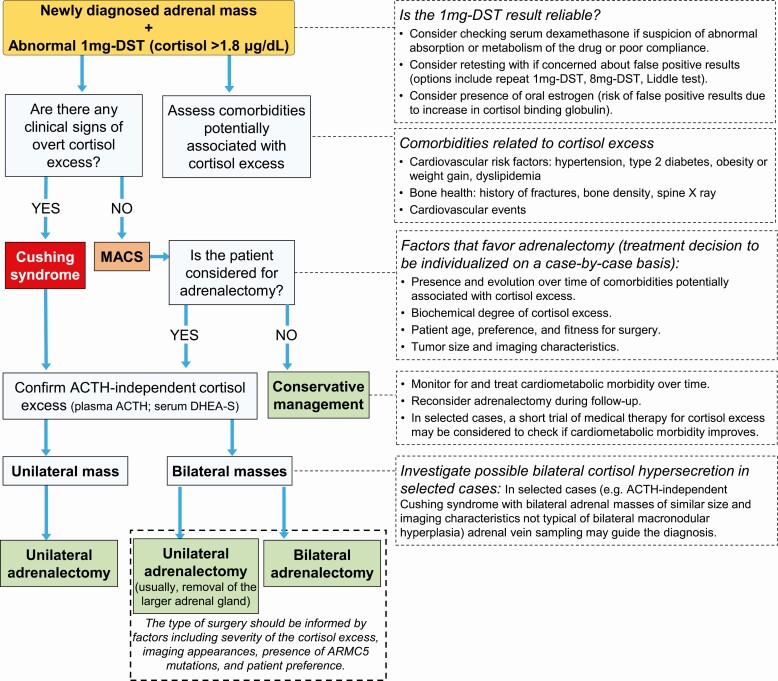
Figure 4.
Approach to adrenal adenoma with autonomous cortisol secretion. Abbreviations: 1-mg DST, 1-mg overnight dexamethasone suppression test; 8-mg DST, 8-mg overnight dexamethasone suppression test; ARMC5, Armadillo Repeat Containing 5 gene; DHEA-S, dehydroepiandrosterone sulfate; MACS, mild autonomous cortisol secretion.
Laboratory Assessment of Cortisol Excess
The 1-mg DST is the best initial test to diagnose MACS, which is suspected if serum cortisol is >1.8 mcg/dL (Table 5) (18). The concomitant measurement of serum dexamethasone can inform about the bioavailability of the drug if false-positive results from abnormal metabolism or absorption of the drug are suspected (58-60) (Table 5, Fig. 4). Additional tests, such as dehydroepiandrosterone sulfate (DHEA-S) and corticotropin (ACTH) can confirm the degree of cortisol excess and ACTH independence. When CS is suspected, 24-hour urine cortisol and late-night salivary cortisol can be used to confirm the diagnosis (61). Notably, in patients with MACS, 24-hour urine cortisol and midnight salivary cortisol are frequently normal (62-69).
Laboratory Assessment of Aldosterone Excess
Numerous antihypertensive classes and other medications can affect renin and aldosterone measurements; this should be taken into consideration when interpreting the results (Table 5) (56, 70). However, we advise against routinely discontinuing medications to screen for primary aldosteronism; this is more convenient for patients and results can often offer valuable information to guide future tests, if necessary (Table 5) (55, 56).
Laboratory Assessment of Catecholamine Excess
Urinary or plasma metanephrines are the test of choice for case detection of pheochromocytoma (71). Both tests have excellent diagnostic sensitivity (72), with plasma metanephrines having a slightly better performance if measured in controlled conditions (72, 73). Several medications, diseases, and preanalytical factors can lead to false-positive results and must be taken into consideration when testing patients (Table 5).
Management of Adrenal Incidentalomas
The initial management of adrenal incidentalomas is guided by imaging characteristics, clinical evaluation, and the hormonal assessment. Challenging cases should be discussed in a multidisciplinary expert meeting, especially if malignancy is suspected, if the laboratory evaluation suggests a functioning adrenal tumor and if adrenal surgery is considered (18). The approaches to patients with suspected adrenocortical cancer, primary aldosteronism, or catecholamine excess have recently been published (55, 74-77). In this review, we focus on the management of the most common diagnoses in patients with adrenal incidentalomas: benign nonfunctioning tumors, MACS, and bilateral adrenal disease.
Management of Nonfunctioning Benign Adrenal Masses: Is Imaging Follow-up Necessary?
The European Society of Endocrinology Consensus Guidelines advise against repeated imaging in homogeneous adrenal incidentalomas <4 cm with unenhanced CT tumor attenuation of HU ≤10 (18). This recommendation should also be extended to clearly benign adrenal diseases such as hemorrhage without an underlying solid component, simple cysts, and small myelolipomas. A prospective study in 2017 patients with newly diagnosed adrenal tumors (9) and 2 large retrospective studies in adrenal incidentalomas (78, 79) have recently confirmed that the risk of ACC in adrenal masses <4 cm (regardless of HU) is extremely low. These studies also provide strong evidence that in addition to the tumor size cutoff of 4 cm, the HU cutoff of 20 is more specific to exclude ACC (9, 78, 79). A systematic review and meta-analysis investigating the natural history of adrenal incidentalomas found no cases of malignant transformation in more than 2800 adrenal incidentalomas initially classified as benign adrenocortical adenomas (7).
In the case of nonfunctioning lipid-poor small adrenal incidentaloma, additional testing is warranted and may include (1) follow-up imaging in 3 to 12 months; (2) additional testing at the time of the diagnosis, such as alternative imaging or steroid profiling; or (3) adrenalectomy (Fig. 2). Follow-up should be selected based on the pretest probability of pheochromocytoma, smaller ACC, or other malignancy.
Another class of adrenal incidentalomas that may benefit from repeated imaging is larger myelolipomas. Despite being benign tumors, myelolipomas >3.5 cm tend to grow over time and carry a small risk of compressive symptoms and bleeding when the maximum diameter exceeds 6 cm (12).
Management of Nonfunctioning Benign Adrenal Masses: Is Clinical and Hormonal Follow-up Necessary?
Patients with benign nonfunctioning adrenal adenomas carry a surprisingly high prevalence of cardiometabolic disease at the time of diagnosis and were also found to have an increased risk of incident prediabetes and type 2 diabetes during follow-up (1, 7, 80), which is possibly linked to a mild cortisol excess that is not picked up by the 1-mg DST (80, 81). Therefore, it is prudent to screen these patients for comorbid conditions and optimize their treatment as appropriate.
The risk of developing overt hormone excess in patients initially diagnosed with either nonfunctioning adrenal adenoma or mild autonomous cortisol secretion during long-term follow-up is very low (<0.1%) (7); however, 4% to 12% of patients with adrenal incidentalomas initially classified as nonfunctioning can develop an abnormal 1-mg DST during follow-up (7, 54, 62, 82). Therefore, a repeated 1-mg DST can be considered to detect possible MACS; we otherwise advise against routine repeated hormonal investigations in patients with nonfunctioning adrenal incidentaloma unless there is a clinical concern (e.g., onset or worsening of a comorbid condition potentially linked to cortisol excess or new unexplained hypokalemia possibly pointing to previously unrecognized primary aldosteronism) (7, 18). Exceptions are small lipid-poor adrenal incidentalomas with a higher pretest probability of pheochromocytoma (e.g., known germline mutation associated with catecholamine-secreting tumors), where repeated biochemical testing is warranted in case of tumor growth, and repeated imaging should be considered if increase in metanephrines is noted (Table 5).
Management of Benign Adrenal Mass and Autonomous Cortisol Secretion
Adrenalectomy is the therapy of choice in patients with primary adrenal CS. In any patient with an abnormal 1-mg DST, however, it is imperative to confirm that the cortisol excess is ACTH-independent before referring the patients for adrenal surgery because more than 30% of patients with Cushing disease can have adrenal nodules that are either coincidental or secondary to chronic ACTH stimulation (83, 84).
MACS is increasingly recognized as a clinically relevant cardiometabolic condition. A systematic review and meta-analysis of more than 4000 patients with benign adrenal incidentalomas found a prevalence of hypertension (64%), obesity (41%), dyslipidemia (34%), type 2 diabetes (28%), and cardiovascular events (6%) in patients with MACS that is remarkably higher than expected for Western populations (7). An increased risk of frailty, cardiovascular events, and mortality has also been reported (7, 85-89). Patients with MACS have also been found to carry a significant risk of osteoporosis and (mostly asymptomatic) vertebral fractures (46%-82%), as compared with 13% to 23% of patients with nonfunctioning adrenal incidentalomas (90-92). A population-based study in patients with adrenal incidentalomas that included a mix of patients with nonfunctioning adrenal adenomas and patients with MACS demonstrated an increased prevalence and incidence of fractures when compared with reference subjects from the same population (93).
The detection of MACS poses the therapeutic dilemma of whether to carefully observe and pursue medical management of metabolic comorbidities or to select adrenalectomy. A meta-analysis of small, mostly retrospective studies with heterogeneous definitions of MACS has shown that patients with MACS undergoing adrenalectomy experience an improvement in blood pressure, glycemic control, and dyslipidemia, and a reduction of body weight (94). Another study found that adrenalectomy reduces the risk of incident vertebral fractures in patients with MACS (95). Three small-scale studies have tested the potential use of medical treatment with mifepristone or metyrapone to reduce the cortisol excess in MACS and the authors reported beneficial effects on several metabolic parameters (96-98). These data suggest that MACS is a remediable cause of increased cardiometabolic risk; however, the evidence is mostly based on small, heterogeneous studies; randomized controlled trials comparing adrenalectomy to conservative management in this population are needed to conclusively answer this question. Until more evidence becomes available and reliable markers for metabolic risk stratification are identified, the therapeutic decision must be individualized considering clinical, biochemical, imaging parameters, as well as patient’s preferences (Fig. 4). A patient who is younger at the time of diagnosis, presenting with a higher degree of MACS, a more recent onset, or exacerbation of potential cortisol-related comorbidities is more likely to benefit from adrenalectomy, both in the short and long term. Plasma ACTH and serum DHEA-S can help defining the severity of the cortisol excess (99-101), even though the first may not be reliable because of preanalytical challenges of sample collection and the latter is typically reduced in postmenopausal women who make up the vast majority of MACS cases (54). Higher cortisol concentrations during the 1-mg DST are also associated with a higher risk of cardiometabolic disease and mortality (18, 54, 88).
If it is decided to manage MACS conservatively, it is imperative to monitor the patient over time and optimize the treatment of the comorbidities potentially associated with cortisol excess, including hypertension, type 2 diabetes, dyslipidemia, bone loss, and asymptomatic vertebral fractures. The patient’s overall risk profile should be reevaluated every 1 to 2 years and adrenalectomy rediscussed, if appropriate.
Management of Bilateral Adrenal Masses
Bilateral disease is found in approximately 20% of patients with newly diagnosed adrenal masses (9). Bilateral adrenal masses carry a higher risk of MACS (42% vs 12%-23% in nonfunctioning tumors), malignancy (16% vs 7% in unilateral tumors), and germline mutations causing primary bilateral macronodular adrenal hyperplasia and bilateral pheochromocytomas (ARMC5, VHL, RET, SDHx, MAX, TMEM127, NF1, FH, MEN1, and APC) (Table 4) (2, 9, 40, 102, 103). Other etiologies of bilateral adrenal lesions include congenital adrenal hyperplasia, ACTH-dependent CS, myelolipomas, infections, hemorrhage, or heterozygous NR3C1 mutations (104). Patients with bilateral adrenal incidentalomas should undergo the same initial assessment as those with unilateral disease (18). In addition, 17-hydroxyprogesterone measurement should be considered in cases of bilateral adenomas or myelolipomas, and assessment for primary adrenal insufficiency should be performed if high suspicion of large adrenal metastases (Table 5).
The high prevalence of cortisol excess in patients with bilateral adrenal incidentalomas (more frequently MACS) poses diagnostic and therapeutic challenges when surgery is thought to be beneficial (Fig. 3). Several authors have suggested the removal of the largest adrenal gland if the masses are unequal in size (18). This approach prevents lifelong need for glucocorticoid and mineralocorticoid replacement and is associated with an initial remission rate of up to 97%, including temporary postoperative adrenal insufficiency and improvement of comorbidities related to cortisol excess (104). Nonetheless, recurrence is observed in 20% to 70% of cases, and up to 33% of patients undergo contralateral adrenalectomy during follow-up to control the hypercortisolism (104, 105). Bilateral adrenalectomy as the initial treatment strategy can be considered in patients with ACTH-independent CS and bilateral adrenal enlargement of similar size (104). Adrenal venous sampling (AVS) to identify the site of dominant cortisol secretion is a promising technique that has shown good performance but is not standardized (106, 107).
Back to the Patients
Case 1
Malignancy was excluded based on the imaging phenotype (<10 HU). MACS was diagnosed based on an abnormal dexamethasone suppression test (cortisol > 1.8 mcg/dL), as well as low ACTH and DHEA-S. The patient was counseled about the possible relationship of MACS and cardiovascular comorbidities (hypertension, diabetes, obesity). Conservative management vs adrenalectomy was discussed. The patient decided on adrenalectomy that was performed laparoscopically. Pathology demonstrated an adrenocortical adenoma.
Postoperative adrenal insufficiency was diagnosed based on cortisol of 2.1 mcg/dL the morning after surgery. Hydrocortisone supplementation was initiated with a plan to reassess adrenal function periodically. At the 6-month follow-up, the patient’s hypothalamic-pituitary-adrenal axis recovered and hydrocortisone was stopped. Clinically, the patient lost 15 pounds of body weight and her blood pressure measurements improved (on same antihypertensive therapy). Follow-up HbA1C decreased to 6.5% 6 months after surgery.
Case 2
The patient was advised that the most likely diagnosis of the adrenal mass was ACC (because of a significant tumor growth, large tumor size, indeterminate imaging characteristics, and a combined glucocorticoid and androgen excess). She underwent open resection of the left adrenal mass. On pathology, the mass measured 5.5 × 7.5 × 6.9 cm. ACC was diagnosed with a Weiss score of 5 and KI67 of 10%. The patient was dismissed from the hospital on glucocorticoid therapy and opted to initiate postoperative treatment with mitotane 4 weeks later. Mitotane therapy was continued for 15 months and was stopped because of gastrointestinal side effects. Five years after adrenalectomy, the patient was doing well and continued to be in remission.
Case 3
Imaging showed benign bilateral adrenal adenomas (HU < 10) of nearly equal size. AVS was performed to identify the source of autonomous cortisol secretion. In preparation for the AVS procedure, dexamethasone 0.5 mg was initiated on the morning the day prior and administered every 6 hours, with the last dose on the morning of AVS. Successful bilateral adrenal vein canulation was confirmed based on epinephrine concentrations in the adrenal veins (Table 6). Left adrenal vein to inferior vena cava cortisol ratio was 17, and the left adrenal vein to right adrenal vein cortisol ratio was 6.1, consistent with the ACTH-independent hypercortisolism from the dominant left adrenal adenoma. The patient was treated with a laparoscopic left adrenalectomy that revealed an adrenocortical adenoma. Following adrenalectomy, she developed adrenal insufficiency and initiated glucocorticoid therapy. Postoperatively, both diabetes mellitus type 2 and hypertension resolved, she lost 15 pounds, and demonstrated a significant improvement in anxiety.
Table 6.
Adrenal vein sampling in case 3
| Biochemical testing | Right adrenal vein | Inferior vena cava | Left adrenal vein | Comment |
|---|---|---|---|---|
| Epinephrine, ng/dL | 2895 | 82 | 1342 | Adrenal vein epinephrine concentrations of at least 100 pg/mL more than concentrations in the inferior vena cava were used to confirm successful adrenal vein cannulation. |
| Cortisol, mcg/dL | 53 | 19 | 323 | Adrenal vein sampling was completed following administration of dexamethasone of 0.5 mg every 6 h started 24 h prior. |
| Cortisol adrenal vein/ inferior vena cava ratio | 2.8 (53/19) | 17 (323/19) | Ratio > 6.5 confirmed autonomous cortisol secretion from the left adrenal gland. | |
| Ratio < 3.3 excludes autonomous cortisol secretion from the right adrenal gland. | ||||
| Cortisol left adrenal vein/ right adrenal vein ratio | 6.1 (17/2.8) | Lateralization of cortisol to the left was confirmed by the left to right adrenal vein ratio of >2.3. |
Acknowledgments
The views expressed are those of the author(s) and not necessarily those of the National Institutes of Health USA.
Funding: This work was supported by Diabetes UK (Sir George Alberti Research Training Fellowship 18/0005782 to A.P.). This work was partially supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award K23DK121888 (to I.B.).
Glossary
Abbreviations
- ACC
adrenocortical carcinoma
- AVS
adrenal venous sampling
- BMI
body mass index
- CS
Cushing syndrome
- CT
computed tomography
- DHEA-S
dehydroepiandrosterone sulfate
- DST
dexamethasone suppression test
- FDG-PET
18F-fluorodeoxyglucose positron emission tomography
- HbA1C
hemoglobin A1C
- HU
Hounsfield unit
- MACS
mild autonomous cortisol secretion
- MRI
magnetic resonance imaging
- SUV
standardized uptake value.
Additional Information
Disclosure Statement: I.B. reports advisory board/consulting with Corcept, HRA Pharma, Strongbridge, Sparrow pharmaceutics, and Adrenas outside the submitted work.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Reimondo G, Castellano E, Grosso M, et al. Adrenal incidentalomas are tied to increased risk of diabetes: findings from a prospective study. J Clin Endocrinol Metab. 2020;105(4):e973-e981. [DOI] [PubMed] [Google Scholar]
- 2. Ebbehoj A, Li D, Kaur RJ, et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(11):894-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ichijo T, Ueshiba H, Nawata H, Yanase T. A nationwide survey of adrenal incidentalomas in Japan: the first report of clinical and epidemiological features. Endocr J. 2020;67(2):141-152. [DOI] [PubMed] [Google Scholar]
- 4. Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85(2):637-644. [DOI] [PubMed] [Google Scholar]
- 5. Mao JJ, Dages KN, Suresh M, Bancos I. Presentation, disease progression and outcomes of adrenal gland metastases. Clin Endocrinol (Oxf). 2020;93(5):546-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delivanis DA, Erickson D, Atwell TD, et al. Procedural and clinical outcomes of percutaneous adrenal biopsy in a high-risk population for adrenal malignancy. Clin Endocrinol (Oxf). 2016;85(5):710-716. [DOI] [PubMed] [Google Scholar]
- 7. Elhassan YS, Alahdab F, Prete A, et al. Natural history of adrenal incidentalomas with and without mild autonomous cortisol excess: a systematic review and meta-analysis. Ann Intern Med. 2019;171(2):107-116. [DOI] [PubMed] [Google Scholar]
- 8. Li D, El Kawkgi OM, Henriquez AF, Bancos I. Cardiovascular risk and mortality in patients with active and treated hypercortisolism. Gland Surg. 2020;9(1):43-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bancos I, Taylor AE, Chortis V, et al. ; ENSAT EURINE-ACT Investigators . Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol. 2020;8(9):773-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cyranska-Chyrek E, Szczepanek-Parulska E, Olejarz M, Ruchala M. Malignancy risk and hormonal activity of adrenal incidentalomas in a large cohort of patients from a single tertiary reference center. Int J Environ Res Public Health. 2019;16(10):1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2018;2(1):30-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamidi O, Raman R, Lazik N, et al. Clinical course of adrenal myelolipoma: a long-term longitudinal follow-up study. Clin Endocrinol (Oxf). 2020;93(1):11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dages KN, Kohlenberg JD, Young WF Jr, et al. Presentation and outcomes of adrenal ganglioneuromas: a cohort study and a systematic review of literature. Clin Endocrinol (Oxf). 2021;95(1):47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahn SH, Kim JH, Baek SH, et al. Characteristics of adrenal incidentalomas in a large, prospective computed tomography-based multicenter study: the COAR Study in Korea. Yonsei Med J. 2018;59(4):501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gruber LM, Hartman RP, Thompson GB, et al. Pheochromocytoma characteristics and behavior differ depending on method of discovery. J Clin Endocrinol Metab. 2019;104(5):1386-1393. [DOI] [PubMed] [Google Scholar]
- 16. Canu L, Van Hemert JAW, Kerstens MN, et al. CT characteristics of pheochromocytoma: relevance for the evaluation of adrenal incidentaloma. J Clin Endocrinol Metab. 2019;104(2):312-318. [DOI] [PubMed] [Google Scholar]
- 17. Gruber LM, Strajina V, Bancos I, et al. Not all adrenal incidentalomas require biochemical testing to exclude pheochromocytoma: Mayo Clinic experience and a meta-analysis. Gland Surg. 2020;9(2):362-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1-G34. [DOI] [PubMed] [Google Scholar]
- 19. Vaidya A, Hamrahian A, Bancos I, Fleseriu M, Ghayee HK. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25(2):178-192. [DOI] [PubMed] [Google Scholar]
- 20. Dinnes J, Bancos I, Ferrante di Ruffano L, et al. Management of endocrine disease: imaging for the diagnosis of malignancy in incidentally discovered adrenal masses: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(2):R51-R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Michałowska I, Ćwikła JB, Michalski W, et al. Growth rate of paragangliomas related to germline mutations of the Sdhx genes. Endocr Pract. 2017;23(3):342-352. [DOI] [PubMed] [Google Scholar]
- 22. Sanford T, Gomella PT, Siddiqui R, et al. Long term outcomes for patients with von Hippel-Lindau and pheochromocytoma: defining the role of active surveillance. Urol Oncol. 2021;39(2):134.e1-134.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corwin MT, Navarro SM, Malik DG, et al. Differences in growth rate on CT of adrenal adenomas and malignant adrenal nodules. AJR Am J Roentgenol. 2019;213(3):632-636. [DOI] [PubMed] [Google Scholar]
- 24. Pantalone KM, Gopan T, Remer EM, et al. Change in adrenal mass size as a predictor of a malignant tumor. Endocr Pract. 2010;16(4):577-587. [DOI] [PubMed] [Google Scholar]
- 25. Delivanis DA, Bancos I, Atwell TD, et al. Diagnostic performance of unenhanced computed tomography and 18 F-fluorodeoxyglucose positron emission tomography in indeterminate adrenal tumours. Clin Endocrinol (Oxf). 2018;88(1):30-36. [DOI] [PubMed] [Google Scholar]
- 26. He X, Caoili EM, Avram AM, Miller BS, Else T. 18F-FDG-PET/CT evaluation of indeterminate adrenal masses in noncancer patients. J Clin Endocrinol Metab. 2021;106(5):1448-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Athimulam S, Grebe S, Bancos I. Steroid profiling in the diagnosis of mild and overt Cushing’s syndrome. Best Pract Res Clin Endocrinol Metab. 2021;35(1):101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arlt W, Lang K, Sitch AJ, et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. 2017;2(8):e93136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bancos I, Arlt W. Diagnosis of a malignant adrenal mass: the role of urinary steroid metabolite profiling. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):200-207. [DOI] [PubMed] [Google Scholar]
- 30. Kerkhofs TM, Kerstens MN, Kema IP, Willems TP, Haak HR. Diagnostic value of urinary steroid profiling in the evaluation of adrenal tumors. Horm Cancer. 2015;6(4):168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schweitzer S, Kunz M, Kurlbaum M, et al. Plasma steroid metabolome profiling for the diagnosis of adrenocortical carcinoma. Eur J Endocrinol. 2019;180(2):117-125. [DOI] [PubMed] [Google Scholar]
- 32. Taylor DR, Ghataore L, Couchman L, et al. A 13-steroid serum panel based on LC-MS/MS: use in detection of adrenocortical carcinoma. Clin Chem. 2017;63(12):1836-1846. [DOI] [PubMed] [Google Scholar]
- 33. Hines JM, Bancos I, Bancos C, et al. High-resolution, accurate-mass (HRAM) mass spectrometry urine steroid profiling in the diagnosis of adrenal disorders. Clin Chem. 2017;63(12):1824-1835. [DOI] [PubMed] [Google Scholar]
- 34. Herndon J, Nadeau AM, Davidge-Pitts CJ, Young WF, Bancos I. Primary adrenal insufficiency due to bilateral infiltrative disease. Endocrine. 2018;62(3):721-728. [DOI] [PubMed] [Google Scholar]
- 35. Bancos I, Tamhane S, Shah M, et al. Diagnosis of endocrine disease: the diagnostic performance of adrenal biopsy: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(2):R65-R80. [DOI] [PubMed] [Google Scholar]
- 36. Zhang CD, Delivanis DA, Eiken PW, Atwell TD, Bancos I. Adrenal biopsy: performance and use. Minerva Endocrinol. 2019;44(3):288-300. [DOI] [PubMed] [Google Scholar]
- 37. Patel S, Jinjuvadia R, Devara A, et al. Performance characteristics of EUS-FNA biopsy for adrenal lesions: a meta-analysis. Endosc Ultrasound. 2019;8(3):180-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang CD, Erickson D, Levy MJ, et al. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of adrenal metastasis in a high-risk population. Endocr Pract. 2017;23(12):1402-1407. [DOI] [PubMed] [Google Scholar]
- 39. McDermott E, Kilcoyne A, O’Shea A, Cahalane AM, McDermott S. The role of percutaneous CT-guided biopsy of an adrenal lesion in patients with known or suspected lung cancer. Abdom Radiol (NY). 2021;46(3):1171-1178. [DOI] [PubMed] [Google Scholar]
- 40. Bouys L, Chiodini I, Arlt W, Reincke M, Bertherat J. Update on primary bilateral macronodular adrenal hyperplasia (PBMAH). Endocrine. 2021;71(3):595-603. [DOI] [PubMed] [Google Scholar]
- 41. Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14(2):108-119. [DOI] [PubMed] [Google Scholar]
- 42. Petr EJ, Else T. Adrenocortical carcinoma (ACC): when and why should we consider germline testing? Presse Med. 2018;47(7-8 Pt 2):e119-e125. [DOI] [PubMed] [Google Scholar]
- 43. Bausch B, Schiavi F, Ni Y, et al. ; European-American-Asian Pheochromocytoma-Paraganglioma Registry Study Group . Clinical characterization of the pheochromocytoma and paraganglioma susceptibility genes SDHA, TMEM127, MAX, and SDHAF2 for gene-informed prevention. JAMA Oncol. 2017;3(9):1204-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Benn DE, Robinson BG, Clifton-Bligh RJ. 15 years of paraganglioma: clinical manifestations of paraganglioma syndromes types 1-5. Endocr Relat Cancer. 2015;22(4):T91-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boikos SA, Xekouki P, Fumagalli E, et al. Carney triad can be (rarely) associated with germline succinate dehydrogenase defects. Eur J Hum Genet. 2016;24(4):569-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carney JA. Carney triad: a syndrome featuring paraganglionic, adrenocortical, and possibly other endocrine tumors. J Clin Endocrinol Metab. 2009;94(10):3656-3662. [DOI] [PubMed] [Google Scholar]
- 47. Crona J, Taïeb D, Pacak K. New perspectives on pheochromocytoma and paraganglioma: toward a molecular classification. Endocr Rev. 2017;38(6):489-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Else T, Greenberg S, Fishbein L. Hereditary paraganglioma-pheochromocytoma syndromes. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews((R)). Seattle (WA): University of Washington; 1993. [Google Scholar]
- 49. Gatta-Cherifi B, Chabre O, Murat A, et al. Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d’etude des Tumeurs Endocrines database. Eur J Endocrinol. 2012;166(2):269-279. [DOI] [PubMed] [Google Scholar]
- 50. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665-675. [DOI] [PubMed] [Google Scholar]
- 51. MacFarland SP, Mostoufi-Moab S, Zelley K, et al. Management of adrenal masses in patients with Beckwith-Wiedemann syndrome. Pediatr Blood Cancer. 2017;64(8):e26432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shuch B, Ricketts CJ, Vocke CD, et al. Adrenal nodular hyperplasia in hereditary leiomyomatosis and renal cell cancer. J Urol. 2013;189(2):430-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vitellius G, Trabado S, Hoeffel C, et al. ; investigators of the MUTA-GR Study . Significant prevalence of NR3C1 mutations in incidentally discovered bilateral adrenal hyperplasia: results of the French MUTA-GR Study. Eur J Endocrinol. 2018;178(4):411-423. [DOI] [PubMed] [Google Scholar]
- 54. Di Dalmazi G, Vicennati V, Garelli S, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2(5):396-405. [DOI] [PubMed] [Google Scholar]
- 55. Vaidya A, Carey RM. Evolution of the primary aldosteronism syndrome: updating the approach. J Clin Endocrinol Metab. 2020;105(12):3771-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916. [DOI] [PubMed] [Google Scholar]
- 57. Buitenwerf E, Berends AMA, van Asselt ADI, et al. Diagnostic accuracy of computed tomography to exclude pheochromocytoma: a systematic review, meta-analysis, and cost analysis. Mayo Clin Proc. 2019;94(10):2040-2052. [DOI] [PubMed] [Google Scholar]
- 58. Ceccato F, Artusi C, Barbot M, et al. Dexamethasone measurement during low-dose suppression test for suspected hypercortisolism: threshold development with and validation. J Endocrinol Invest. 2020;43(8):1105-1113. [DOI] [PubMed] [Google Scholar]
- 59. Ueland GÅ, Methlie P, Kellmann R, et al. Simultaneous assay of cortisol and dexamethasone improved diagnostic accuracy of the dexamethasone suppression test. Eur J Endocrinol. 2017;176(6):705-713. [DOI] [PubMed] [Google Scholar]
- 60. Vogg N, Kurlbaum M, Deutschbein T, Gräsl B, Fassnacht M, Kroiss M. Method-specific cortisol and dexamethasone thresholds increase clinical specificity of the dexamethasone suppression test for Cushing syndrome. Clin Chem. 2021;67(7):998-1007. [DOI] [PubMed] [Google Scholar]
- 61. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Giordano R, Marinazzo E, Berardelli R, et al. Long-term morphological, hormonal, and clinical follow-up in a single unit on 118 patients with adrenal incidentalomas. Eur J Endocrinol. 2010;162(4):779-785. [DOI] [PubMed] [Google Scholar]
- 63. Prete A, Paragliola RM, Bottiglieri F, et al. Factors predicting the duration of adrenal insufficiency in patients successfully treated for Cushing disease and nonmalignant primary adrenal Cushing syndrome. Endocrine. 2017;55(3):969-980. [DOI] [PubMed] [Google Scholar]
- 64. Raff H, Auchus RJ, Findling JW, Nieman LK. Urine free cortisol in the diagnosis of Cushing’s syndrome: is it worth doing and, if so, how? J Clin Endocrinol Metab. 2015;100(2):395-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sereg M, Toke J, Patócs A, et al. Diagnostic performance of salivary cortisol and serum osteocalcin measurements in patients with overt and subclinical Cushing’s syndrome. Steroids. 2011;76(1-2):38-42. [DOI] [PubMed] [Google Scholar]
- 66. Ueland GÅ, Grinde T, Methlie P, Kelp O, Løvås K, Husebye ES. Diagnostic testing of autonomous cortisol secretion in adrenal incidentalomas. Endocr Connect. 2020;9(10):963-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ceccato F, Barbot M, Albiger N, et al. Daily salivary cortisol and cortisone rhythm in patients with adrenal incidentaloma. Endocrine. 2018;59(3):510-519. [DOI] [PubMed] [Google Scholar]
- 68. Hurtado MD, Cortes T, Natt N, Young WF Jr, Bancos I. Extensive clinical experience: hypothalamic-pituitary-adrenal axis recovery after adrenalectomy for corticotropin-independent cortisol excess. Clin Endocrinol (Oxf). 2018;89(6):721-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Delivanis DA, Iñiguez-Ariza NM, Zeb MH, et al. Impact of hypercortisolism on skeletal muscle mass and adipose tissue mass in patients with adrenal adenomas. Clin Endocrinol (Oxf). 2018;88(2):209-216. [DOI] [PubMed] [Google Scholar]
- 70. O’Shea PM, Griffin TP, Denieffe S, Fitzgibbon MC. The aldosterone to renin ratio in the diagnosis of primary aldosteronism: promises and challenges. Int J Clin Pract. 2019;73(7):e13353. [DOI] [PubMed] [Google Scholar]
- 71. Lenders JW, Duh QY, Eisenhofer G, et al. ; Endocrine Society . Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. [DOI] [PubMed] [Google Scholar]
- 72. Därr R, Kuhn M, Bode C, et al. Accuracy of recommended sampling and assay methods for the determination of plasma-free and urinary fractionated metanephrines in the diagnosis of pheochromocytoma and paraganglioma: a systematic review. Endocrine. 2017;56(3):495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Eisenhofer G, Prejbisz A, Peitzsch M, et al. Biochemical diagnosis of chromaffin cell tumors in patients at high and low risk of disease: plasma versus urinary free or deconjugated O-methylated catecholamine metabolites. Clin Chem. 2018;64(11):1646-1656. [DOI] [PubMed] [Google Scholar]
- 74. Turcu AF, Auchus R. Approach to the patient with primary aldosteronism: utility and limitations of adrenal vein sampling. J Clin Endocrinol Metab. 2021;106(4):1195-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552-565. [DOI] [PubMed] [Google Scholar]
- 76. Fassnacht M, Dekkers OM, Else T, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the study of adrenal tumors. Eur J Endocrinol. 2018;179(4):G1-G46. [DOI] [PubMed] [Google Scholar]
- 77. Fassnacht M, Assie G, Baudin E, et al. ; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org . Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1476-1490. [DOI] [PubMed] [Google Scholar]
- 78. Kahramangil B, Kose E, Remer EM, et al. A modern assessment of cancer risk in adrenal incidentalomas: analysis of 2219 patients. Ann Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 79. Hong AR, Kim JH, Park KS, et al. Optimal follow-up strategies for adrenal incidentalomas: reappraisal of the 2016 ESE-ENSAT guidelines in real clinical practice. Eur J Endocrinol. 2017;177(6):475-483. [DOI] [PubMed] [Google Scholar]
- 80. Lopez D, Luque-Fernandez MA, Steele A, Adler GK, Turchin A, Vaidya A. “Nonfunctional” adrenal tumors and the risk for incident diabetes and cardiovascular outcomes: a cohort study. Ann Intern Med. 2016;165(8):533-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Arlt W, Biehl M, Taylor AE, et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab. 2011;96(12):3775-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Morelli V, Reimondo G, Giordano R, et al. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab. 2014;99(3):827-834. [DOI] [PubMed] [Google Scholar]
- 83. Albiger NM, Occhi G, Sanguin F, et al. Adrenal nodules in patients with Cushing’s disease: prevalence, clinical significance and follow-up. J Endocrinol Invest. 2011;34(8):e204-e209. [DOI] [PubMed] [Google Scholar]
- 84. Borretta G, Terzolo M, Cesario F, Meineri I, Pia A, Angeli A. Coexistence of unilateral adrenal macronodule and Cushing’s disease. Report of two cases. J Endocrinol Invest. 1996;19(2):131-135. [DOI] [PubMed] [Google Scholar]
- 85. Di Dalmazi G, Vicennati V, Pizzi C, et al. Prevalence and incidence of atrial fibrillation in a large cohort of adrenal incidentalomas: a long-term study. J Clin Endocrinol Metab. 2020;105(8):e2770-e2777. [DOI] [PubMed] [Google Scholar]
- 86. Morelli V, Palmieri S, Lania A, et al. Cardiovascular events in patients with mild autonomous cortisol secretion: analysis with artificial neural networks. Eur J Endocrinol. 2017;177(1):73-83. [DOI] [PubMed] [Google Scholar]
- 87. Singh S, Atkinson EJ, Achenbach SJ, LeBrasseur N, Bancos I. Frailty in patients with mild autonomous cortisol secretion is higher than in patients with nonfunctioning adrenal tumors. J Clin Endocrinol Metab. 2020;105(9):e3307-e3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kjellbom A, Lindgren O, Puvaneswaralingam S, Londahl M, Olsen H. Association between mortality and levels of autonomous cortisol secretion by adrenal incidentalomas: a cohort study. Ann Intern Med. 2021;42(1):164-173. [DOI] [PubMed] [Google Scholar]
- 89. Zhang CD, Li D, Kaur RJ, et al. Cardiometabolic outcomes and mortality in patients with adrenal adenomas: a population-based cohort study. J Clin Endocrinol Metab. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chiodini I, Morelli V, Masserini B, et al. Bone mineral density, prevalence of vertebral fractures, and bone quality in patients with adrenal incidentalomas with and without subclinical hypercortisolism: an Italian multicenter study. J Clin Endocrinol Metab. 2009;94(9):3207-3214. [DOI] [PubMed] [Google Scholar]
- 91. Chiodini I, Guglielmi G, Battista C, et al. Spinal volumetric bone mineral density and vertebral fractures in female patients with adrenal incidentalomas: the effects of subclinical hypercortisolism and gonadal status. J Clin Endocrinol Metab. 2004;89(5):2237-2241. [DOI] [PubMed] [Google Scholar]
- 92. Chiodini I, Viti R, Coletti F, et al. Eugonadal male patients with adrenal incidentalomas and subclinical hypercortisolism have increased rate of vertebral fractures. Clin Endocrinol (Oxf). 2009;70(2):208-213. [DOI] [PubMed] [Google Scholar]
- 93. Li D, Kaur RJ, Zhang CD, et al. Risk of bone fractures after the diagnosis of adrenal adenomas: a population-based cohort study. Eur J Endocrinol. 2021;184(4):597-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bancos I, Alahdab F, Crowley RK, et al. Therapy of endocrine disease: improvement of cardiovascular risk factors after adrenalectomy in patients with adrenal tumors and subclinical Cushing’s syndrome: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(6):R283-R295. [DOI] [PubMed] [Google Scholar]
- 95. Salcuni AS, Morelli V, Eller Vainicher C, et al. Adrenalectomy reduces the risk of vertebral fractures in patients with monolateral adrenal incidentalomas and subclinical hypercortisolism. Eur J Endocrinol. 2016;174(3):261-269. [DOI] [PubMed] [Google Scholar]
- 96. Belokovskaya R, Ravikumar A, Arumugam D, et al. Mifepristone treatment for mild autonomous cortisol secretion due to adrenal adenomas: a pilot study. Endocr Pract. 2019;25(8):846-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Debono M, Harrison RF, Chadarevian R, Gueroult C, Abitbol JL, Newell-Price J. Resetting the abnormal circadian cortisol rhythm in adrenal incidentaloma patients with mild autonomous cortisol secretion. J Clin Endocrinol Metab. 2017;102(9):3461-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Debono M, Chadarevian R, Eastell R, Ross RJ, Newell-Price J. Mifepristone reduces insulin resistance in patient volunteers with adrenal incidentalomas that secrete low levels of cortisol: a pilot study. Plos One. 2013;8(4):e60984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dennedy MC, Annamalai AK, Prankerd-Smith O, et al. Low DHEAS: a sensitive and specific test for the detection of subclinical hypercortisolism in adrenal incidentalomas. J Clin Endocrinol Metab. 2017;102(3):786-792. [DOI] [PubMed] [Google Scholar]
- 100. Yener S, Yilmaz H, Demir T, Secil M, Comlekci A. DHEAS for the prediction of subclinical Cushing’s syndrome: perplexing or advantageous? Endocrine. 2015;48(2):669-676. [DOI] [PubMed] [Google Scholar]
- 101. Carafone LE, Zhang Z, Li D, et al. Diagnostic accuracy of dehydroepiandrosterone sulfate and corticotropin in autonomous cortisol secretion. Biomedicines. 2021;9:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vassiliadi DA, Ntali G, Vicha E, Tsagarakis S. High prevalence of subclinical hypercortisolism in patients with bilateral adrenal incidentalomas: a challenge to management. Clin Endocrinol (Oxf). 2011;74(4):438-444. [DOI] [PubMed] [Google Scholar]
- 103. Olsen H, Nordenström E, Bergenfelz A, Nyman U, Valdemarsson S, Palmqvist E. Subclinical hypercortisolism and CT appearance in adrenal incidentalomas: a multicenter study from Southern Sweden. Endocrine. 2012;42(1):164-173. [DOI] [PubMed] [Google Scholar]
- 104. Bourdeau I, El Ghorayeb N, Gagnon N, Lacroix A. Management of endocrine disease: differential diagnosis, investigation and therapy of bilateral adrenal incidentalomas. Eur J Endocrinol. 2018;179(2):R57-R67. [DOI] [PubMed] [Google Scholar]
- 105. Osswald A, Reincke M. Response to the letter to the editor: “Long-Term Outcome of Primary Bilateral Macronodular Adrenocortical Hyperplasia After Unilateral Adrenalectomy.” J Clin Endocrinol Metab. 2020;105(3):e922-e923. [DOI] [PubMed] [Google Scholar]
- 106. Young WF Jr, du Plessis H, Thompson GB, et al. The clinical conundrum of corticotropin-independent autonomous cortisol secretion in patients with bilateral adrenal masses. World J Surg. 2008;32(5):856-862. [DOI] [PubMed] [Google Scholar]
- 107. Ueland GÅ, Methlie P, Jøssang DE, et al. Adrenal venous sampling for assessment of autonomous cortisol secretion. J Clin Endocrinol Metab. 2018;103(12):4553-4560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.