Abstract
Background
Fatty liver disease is a common metabolic abnormality in adolescents with obesity but remains understudied in early childhood.
Objectives
To describe hepatic fat deposition in prepubertal children and examine cross-sectional associations with metabolic markers and body composition.
Methods
Data were from 286 children ages 4 to 8 years old in the Healthy Start Study, a longitudinal prebirth cohort in Colorado (USA). Assessments included magnetic resonance imaging to quantify hepatic and abdominal fats, fasting blood draws to measure metabolic markers, and air displacement plethysmography to measure body composition (fat mass and fat-free mass).
Results
The median (interquartile range) for hepatic fat was 1.65% (1.24%, 2.11%). Log-transformed hepatic fat was higher in Hispanic [mean (95% CI): 0.63 (0.52, 0.74)] vs non-Hispanic white children [0.46 (0.38, 0.53), P = 0.01] and children with overweight/obesity [0.64 (0.49, 0.79)] vs normal-weight [0.47 (0.40, 0.53), P = 0.02]. Higher log-hepatic fat was associated with higher insulin [β (95% CI): 1.47 (0.61, 2.33) uIU/mL, P = 0.001] and estimated insulin resistance (homeostatic model assessment) [0.40 (0.20, 0.60), P < 0.001] in the full sample and glucose [5.53 (2.84, 8.21) mg/dL, P < 0.001] and triglycerides [10.92 (2.92,18.91) mg/dL, P = 0.008] in boys, in linear regression models adjusted for sociodemographics, maternal/perinatal confounders, and percentage body fat. Log-hepatic fat was also associated with abdominal subcutaneous adipose tissue [SAT; 7.37 (1.12,13.60) mm2, P = 0.02] in unadjusted models, but this was attenuated and insignificant after adjusting for confounders.
Conclusions
While hepatic fat was low in children 4 to 8 years old, it was independently associated with estimated insulin resistance and exhibited sex-specific associations with glucose and triglycerides, suggesting hepatic fat may be an early indicator of metabolic dysfunction in youth.
Keywords: hepatic steatosis, insulin resistance, pediatrics, prepubertal, dyslipidemia, magnetic resonance imaging, NAFLD
In parallel with the rise of childhood and adolescent obesity over recent decades (1), there has been an increase in historically adult-onset chronic diseases among pediatric populations (2,3). One such obesity-related condition is pediatric nonalcoholic fatty liver disease (NAFLD), defined by hepatic fat accumulation in the absence of excess alcohol intake or other steatosis-inducing liver diseases (4). The typical presentation for NAFLD is in children around or after the onset of puberty (>10 years) (5-7). Children, however, can vary substantially in disease severity at the time of diagnosis, with some having nonalcoholic steatohepatitis, characterized by steatosis with inflammation and cell injury, and/or advanced fibrosis (8). This observation suggests that liver-related changes due to hepatic fat accumulation likely begin much earlier than the time of clinical diagnosis in some children, but they are asymptomatic.
To date, very few studies have examined NAFLD at younger ages (<9 years), prior to the typical age of clinical diagnosis (9). Also, most prevalence studies thus far have relied on hepatic ultrasound or elevations in alanine aminotransferase to detect NAFLD, which are limited in their sensitivity and specificity in detecting early steatosis (9,10). Notably, in a 2006 study from the greater San Diego, California area, researchers examined autopsies from 742 children from 1999 to 2003 to estimate the prevalence of NAFLD by liver histology (defined as hepatic steatosis >5%) (11). In this study, NAFLD prevalence was low in children 2 to 4 year olds (0.7%) and 5 to 9 year olds (3.3%), but jumped to 11.3% in 10 to 14 year olds (11). While these findings support that clinically defined NAFLD is rare in younger children, this study did not define whether subclinical hepatic fat (ie, below the clinical-histological threshold of 5%) was present in these younger children. It is also unclear whether the spectrum of hepatic fat that occurs at this young age is associated with metabolic impairments. In addition, nearly 20 years have passed since this autopsy study, warranting more contemporary studies based on multiethnic populations.
As such, the current study leveraged data/samples from the Healthy Start Study, a longitudinal prebirth cohort in Colorado (USA), which began in 2014 and initially enrolled >1400 pregnant mothers in early pregnancy (<25 weeks’ gestation). Mothers and their offspring children then returned for several follow-up visits, including in late pregnancy, at delivery, and when offspring were in infancy and childhood. The most recent study visit was completed when offspring were in early childhood, between 4 and 8 years old, and included the assessment of hepatic steatosis by magnetic resonance imaging (MRI). The objectives of this study were to (1) quantify hepatic fat by MRI in a general risk cohort of young children (4-8 years old), both overall and according to key child characteristics and (2) determine associations between hepatic fat content at 4 to 8 years of age with metabolic markers and body composition.
Materials and Methods
Study Population
The Healthy Start Study is an ongoing, longitudinal prebirth cohort in Colorado (USA) that recruited pregnant women from the obstetric clinic at the University of Colorado from 2009 to 2014. Mothers were eligible if they met the following criteria: >15 yrs old, no history of chronic disease or obstetric complications, <25 weeks’ gestation, and singleton pregnancy. Participants initially completed in-person study visits in early pregnancy (median 17 weeks gestation, mid pregnancy (median 27 weeks gestation), delivery (median 1 day post-delivery). For Healthy Start Phase 2, offspring participants completed an additional follow-up visit at ages 4 to 8 years old, at which time a sample of children underwent abdominal MRI to assess hepatic fat and abdominal fat deposition. Thus, the eligible sample was 286 children who underwent MRI at the Healthy Start Phase 2 visit when they were 4 to 8 years old. When we compared the characteristics of this subsample of 286 children to all other children who completed the Healthy Start Phase 2 visit (n = 645), as shown in Supplementary Table 1 (12), we found no significant differences in terms of sociodemographics (age, sex, race/ethnicity, and household income) or body mass index (BMI) categories; however, mean total cholesterol and low-density lipoprotein (LDL)-cholesterol were lower in the excluded sample vs included sample (total cholesterol: 142.0 ± 22.8 vs 146.2 ± 23.4 mg/dL, P = 0.039; LDL-cholesterol: 80.9 ± 19.2 vs 85.0 ± 19.7 mg/dL, P = 0.016), and fat-free mass (FFM) was higher in the excluded sample vs included sample (14.8 ± 2.8 vs 14.3 ± 2.7 kg, P = 0.001) [Supplementary Table 1 (12)]. All protocols and data collection for the Healthy Start Study were approved by the Colorado Multiple Institutional Review Board. All mothers provided written informed consent prior to the first study visit and at subsequent visits. The Healthy Start Study is registered as an observational study at ClinicalTrials.gov (NCT02273297).
Hepatic and Abdominal Fat Assessments at 4 to 8 Years
A series of T1-weighted coronal images were taken by abdominal MRIs performed by a trained technician using a 3T HDx imager (General Electric, Waukesha, WI, USA) with participants in the supine position. Hepatic imaging was performed using a breath-hold, multi-inference, 6-point MRI-proton density fat fraction technique, which has been validated against magnetic resonance spectroscopy (MRS) and shown to have a diagnostic sensitivity of 98% and specificity of 88%. Hepatic fat, measured by MRI-proton density fat fraction, was calculated from the mean pixel signal intensity for each flip angle acquisition [(SIin-phase–SIout-of-phase)/2 SIin-phase)] using the Osirix, Lipoquant plug-in (13). We determined the prevalence of clinical NAFLD based on a cutoff of >5.5% (14) and an alternate cutoff of >3% (15). In addition, 1 axial T1-weighted image at the L4/L5 disc space was analyzed to determine abdominal SAT and visceral adipose tissue (VAT) area using a modification of the Engelson method, where adipose tissue regions were differentiated by their signal intensity and location, as previously reported (16,17). The VAT-to-SAT ratio (VAT/SAT) was also calculated to assess patterns of abdominal fat partitioning.
Anthropometric and Body Composition Assessments at 4 to 8 Years
Body composition [fat-mass (FM) and FFM] were measured using whole-body air displacement plethysmography in children at 4 to 8 years old during the Healthy Start Phase 2 visit. The protocol was performed twice and a third measurement was taken if the first 2% body fat values differed by >2%, as described previously (18-20). Reported values are the average of the 2 most similar measurements. Percentage body fat was calculated as FM (kg)/body weight (kg). Anthropometrics including standing height (cm) and weight (kg) were also performed by trained staff when children were 4 to 8 years old, and age and sex-adjusted BMI z-scores and percentiles were calculated using 2000 Centers for Disease Control and Prevention growth charts (21). Participant BMIs were categorized as follows: underweight (<5th percentile) normal (5th to <85th percentile), overweight (85th to <95th percentile), and obesity (≥95th percentile). Due to the small sample size of children with obesity (n = 15; 5%), we grouped these children in the overweight category.
Metabolic Assessments at 4 to 8 Years
Fasting blood draws were performed on children at 4 to 8 years by certified bilingual phlebotomists after an overnight fast. Glucose was analyzed by a Beckman Coulter Instrument and insulin was analyzed by radioimmunoassay (Millipore). Based on fasting glucose and insulin, we assessed insulin resistance by the homeostatic model of assessment-insulin resistance (HOMA-IR) (22,23). Total cholesterol, high-density lipoprotein cholesterol, and triglyceride levels were measured by a Beckman Coulter Instrument and LDL cholesterol was calculated by the Friedewald equation (24). Adipokines (adiponectin and leptin) were assessed by a Millipore Multiplex assay kit with the following within-day precision, between-day precision, and sensitivity: adiponectin = 3.90%, 8.50%, 1.0 ug/mL, respectively; leptin = 5.90%, 5.80%, 0.5 ng/mL, respectively. All assays were performed by the Colorado Clinical Translational Science Institute Core Laboratory using standard methods. Blood pressure was also assessed at 4 to 8 years old during physical exams. Specifically, systolic and diastolic blood pressure were measured in triplicate after the participant rested for 5 minutes using a Dinamap manometer (GE Carescape, Dinamap V100), and these values were averaged before statistical analyses.
Covariate Assessments
Maternal age was determined at the first study visit in pregnancy. Prepregnancy BMI (kg/m2) was calculated using height measured at the first study visit and last measured weight before pregnancy in clinical records. Gestational weight gain was calculated as the difference between the last weight measurement during pregnancy and prepregnancy weight (20). Maternal diet was measured by monthly 24-h dietary recalls during pregnancy using the Automated Self-Administered 24-Hour Dietary Recall tool, and diet quality was assessed by Healthy Eating Index-2010 total scores (25), aligning with dietary guidelines at that time. Physical activity during pregnancy was assessed by the Pregnancy Physical Activity Questionnaire (26) and estimated as average metabolic equivalents in hours/week. All women were screened for gestational diabetes mellitus (GDM) during pregnancy by routine clinical screening at 24 to 28 weeks with a 2-step procedure (50 g glucose challenge + 100 g oral glucose tolerance test), and results were obtained from medical records, as previously described (27). History of type 1 or type 2 diabetes prior to pregnancy was also ascertained by surveys at the first study visit; one mother reported a history of type 2 diabetes and was grouped into the maternal GDM category for analyses. Offspring sex and race/ethnicity were ascertained at delivery based on self-report. Gestational age was estimated by calculating an average conception date, which was determined using both medical record data and ultrasound reports, and birth length and weight were measured at delivery by trained nurses, as previously described (18,19,28). Children were grouped as low birth weight (<2500 g), normal, or high birth weight (>4000 g). We also calculated weight for gestational age z-scores (29) and categorized children as small for gestational age (SGA) if birth weight was below the 10th percentile based on reference data (29). Breastfeeding exclusivity and duration were determined by maternal interviews at 4 to 6 months and 18 to 24 months, and children were categorized based on whether they were exclusively breastfed for 6 months.
Statistical Analyses
Before analyses, continuous variables were assessed for normality and hepatic fat was log-transformed to ensure normally distributed residuals. We performed descriptive statistics to describe the characteristics of the sample and then reported least-squares means and 95% CIs for log-hepatic fat according to childhood characteristics. P-values were calculated by pairwise Student’s t test compared to a reference category. Multivariable-adjusted linear regression models were used to examine associations between log-hepatic fat at 4 to 8 years as the independent variable and each metabolic or body composition outcome as the dependent variable. Model 1 was unadjusted. Model 2 was adjusted for child sex, race/ethnicity, and age at the Healthy Start Phase 2 visit. Model 3 was adjusted for Model 2 covariates plus household income, SGA, breastfeeding exclusivity, and maternal/perinatal covariates (maternal age, parity, prepregnancy BMI, maternal GDM, gestational weight gain, and maternal smoking, physical activity, and diet quality based on Healthy Eating Index-2010 total score during pregnancy). For models where a metabolic marker was the outcome, Model 4 was adjusted for Model 3 covariates plus percentage body fat at 4 to 8 years to examine whether associations were confounded by concurrent total adiposity. Results were reported as estimates and 95% CIs for the association of a 1 unit increase in log-hepatic fat with each outcome. Analyses were performed using a complete-case approach, and the sample size for each model was reported in each table. We tested for effect modification by sex or race/ethnicity using 2-way product terms. Due to small sample sizes for non-Hispanic black (n = 33 or 12%) and non-Hispanic other (n = 24 or 8%), we grouped these participants with non-Hispanic white and assessed effect modification for Hispanic vs non-Hispanic ethnicity. Stratified estimates were reported based on interaction P < 0.05. Sensitivity analyses were performed to examine the robustness of findings. First, we assessed whether results differed after excluding participants with clinical NAFLD to assess whether associations were driven by these participants. Second, we compared results if we adjusted for birth weight category (low birth weight, normal, high birth weight) as a potential perinatal confounder instead of SGA in Model 3, given evidence that either birth outcome has been associated with hepatic fat later in life (30,31). Third, we compared results if we adjusted for abdominal VAT area at 4 to 8 years instead of percentage body fat in Model 4. SAS v9.4 (Cary, NC, USA) was used for all analyses. Statistical significance was determined based on P < 0.05.
Results
A total of 286 offspring participants were eligible for analyses based on undergoing the abdominal MRI at the Healthy Start Phase 2 visit (between the ages of 4 to 8 years old). The characteristics of this sample are shown in Table 1. The mean age was 4.8 ± 0.8 years at the time of hepatic fat assessment; 76% were 4 to 8 years old, 49% were boys, and 27% were Hispanic. The median (interquartile range) for hepatic fat was 1.65% (1.24%, 2.11%), and the range was 0.37% to 15.70%. A total of 4 participants (1.4%) had clinical NAFLD based on an MRI-hepatic fat threshold >5.5% (14), and 23 (8.0%) had NAFLD based on a more lenient MRI-hepatic fat threshold >3% (15). Average log-hepatic fat according to child characteristics are also shown in Table 2. There were no differences in hepatic fat by age, sex, or income, but hepatic fat was significantly higher in Hispanic children [mean (95% CI): 0.63 (0.52, 0.74)] vs non-Hispanic white children as the reference [0.46 (0.38, 0.53)] (Table 2). As expected, hepatic fat was also higher in children with overweight or obesity [0.64 (0.49, 0.79)] vs normal-weight children [0.47 (0.40, 0.53)], defined based on age and sex-adjusted BMI percentiles (Table 2).
Table 1.
Demographic and clinical characteristics of the sample in early childhood (4-8 years): the Healthy Start Study
| Variables | Observations, n | Result |
|---|---|---|
| Demographic | ||
| Age category | 286 | |
| 4 to <5 years | 218 (76) | |
| 5 to <6 years | 43 (15) | |
| ≥ 6 years | 25 (9) | |
| Male sex | 286 | 140 (49) |
| Race/ethnicity | 284 | |
| Hispanic | 76 (27) | |
| Non-Hispanic white | 151 (53) | |
| Non-Hispanic black | 33 (12) | |
| Non-Hispanic other | 24 (8) | |
| Household income | 286 | |
| < $50 000 | 100 (35) | |
| $50 000 - $100 000 | 85 (30) | |
| > $100 000 | 91 (32) | |
| BMI category | 285 | |
| Underweight: <5th percentile | 22 (8) | |
| Normal: 5th to <85th percentile | 226 (79) | |
| Overweight/obesity: ≥85th percentile | 37 (13) | |
| Clinical | ||
| Glucose, mg/dL | 209 | 81.7 (7.0) |
| Insulin, uIU/mL | 212 | 6.2 (3.0) |
| HOMA-IR | 195 | 1.3 (0.7) |
| Triglycerides, mg/dL | 213 | 56.8 (20.0) |
| Total cholesterol, mg/dL | 213 | 146.2 (23.4) |
| LDL-cholesterol, mg/dL | 213 | 85.0 (19.7) |
| HDL-cholesterol, mg/dL | 213 | 49.8 (9.1) |
| Adiponectin, ug/dL | 230 | 15.4 (6.1) |
| Leptin,a ng/mL | 230 | 5.5 (3.8-7.5) |
| Diastolic BP, mmHg | 284 | 56.5 (5.1) |
| Systolic BP, mmHg | 284 | 97.5 (8.3) |
| Abdominal VAT, mm2 | 275 | 11.4 (3.9) |
| Abdominal SAT, mm2 | 275 | 46.8 (25.2) |
| VAT/SAT ratio | 275 | 0.3 (0.1) |
| Fat mass, kg | 283 | 3.7 (1.6) |
| Fat-free mass, kg | 283 | 14.3 (2.7) |
| Body fat, % | 283 | 20.6 (6.7) |
| Hepatic fat,a % | 286 | 1.6 (1.2-2.1) |
Unless otherwise noted, data are given number (percentage) for demographic variables and mean (SD) for clinical variables.
Abbreviations: BMI, body mass index; HOMA-IR, homeostatic model of assessment-insulin resistance; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BP, blood pressure; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue.
a Data given as median (interquartile range).
Table 2.
Log-hepatic fat in early childhood (4-8 years) according to demographic characteristics: the Healthy Start Study
| Characteristic at 4-8 years | Mean (95% CI) | P-valuea |
|---|---|---|
| Age category | ||
| 4 to <5 years | 0.49 (0.42, 0.55) | Reference |
| 5 to <6 years | 0.57 (0.42, 0.71) | 0.33 |
| ≥ 6 years | 0.56 (0.37, 0.75) | 0.46 |
| Child sex | ||
| Female | 0.55 (0.47, 0.63) | Reference |
| Male | 0.46 (0.38, 0.54) | 0.13 |
| Race/ethnicity | ||
| Hispanic | 0.63 (0.52, 0.74) | 0.01 |
| Non-Hispanic white | 0.46 (0.38, 0.53) | Reference |
| Non-Hispanic black | 0.48 (0.32, 0.65) | 0.80 |
| Non-Hispanic other | 0.43 (0.52, 0.74) | 0.79 |
| Household income | ||
| < $50 000 | 0.56 (0.46, 0.65) | 0.33 |
| $50 000 to $100 000 | 0.44 (0.34, 0.54) | 0.50 |
| > $100 000 | 0.49 (0.39, 0.59) | Reference |
| BMI category | ||
| Underweight: <5th BMI percentile | 0.66 (0.46, 0.86) | 0.08 |
| Normal: 5th to <85th BMI percentile | 0.47 (0.40, 0.53) | Reference |
| Overweight/Obesity: ≥85th BMI percentile | 0.64 (0.49, 0.79) | 0.02 |
a P-values calculated using pairwise Student’s t-test compared to the reference category. Bolding indicates P < 0.05.
Abbreviations: BMI, body mass index.
Associations of hepatic fat with metabolic markers and body composition at 4 to 8 years based on stepwise, linear regression models are shown in Table 3. In the full sample, higher log-hepatic fat was associated with higher glucose [β (95% CI): 3.82 (1.88, 5.75) mg/dL], insulin [1.55 (0.74, 2.38) uIU/mL], HOMA-IR [0.42 (0.22, 0.61)], and triglycerides [6.19 (0.63, 11.75) mg/dL] in unadjusted analyses, and these associations remained significant when we adjusted for child demographics in Model 2, maternal/perinatal confounders in Model 3, and percentage total body fat in Model 4 (Table 3). Higher log-hepatic fat was also associated with higher abdominal SAT [β (95% CI): 7.37 (1.12, 13.60) mm2] and a lower VAT/SAT ratio [−0.03 (−0.06, −0.01)] in unadjusted analyses, but associations were attenuated to the null after adjusting for confounders (Table 3).
Table 3.
Estimates for associations of log-hepatic fat with metabolic and body composition variables in early childhood (4-8 years): the Healthy Start Study
| Model 1a | Model 2b | Model 3c | Model 4d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | n | β (95% CI)e | P | n | β (95% CI)e | P | n | β (95% CI)e | P | n | β (95% CI)e | P |
| Metabolic | ||||||||||||
| Glucose, mg/dL | 209 | 3.82 (1.88, 5.75) | <0.001 | 208 | 3.09 (1.23, 4.95) | 0.001 | 188 | 3.38 (1.36, 5.40) | 0.001 | 186 | 3.44 (1.41, 5.47) | 0.001 |
| Insulin, uIU/mL | 212 | 1.55 (0.73, 2.38) | <0.001 | 211 | 1.38 (0.55, 2.22) | 0.001 | 189 | 1.47 (0.60, 2.34) | 0.001 | 187 | 1.47 (0.61, 2.33) | 0.001 |
| HOMA-IR | 195 | 0.42 (0.22, 0.61) | <0.001 | 194 | 0.37 (0.17, 0.56) | <0.001 | 175 | 0.40 (0.20, 0.60) | <0.001 | 173 | 0.40 (0.20, 0.60) | <0.001 |
| Triglycerides, mg/dL | 213 | 6.19 (0.63, 11.75) | 0.029 | 212 | 5.36 (−0.28, 11.00) | 0.06 | 192 | 5.95 (0.12, 11.78) | 0.046 | 190 | 6.10 (0.22, 11.98) | 0.042 |
| Total C, mg/dL | 213 | 3.97 (−2.60, 10.54) | 0.23 | 212 | 4.98 (−1.68, 11.64) | 0.14 | 192 | 5.39 (−1.71, 12.49) | 0.14 | 190 | 5.26 (−1.91, 12.43) | 0.15 |
| LDL-C, mg/dL | 213 | 4.14 (−2.38, 9.65) | 0.14 | 212 | 5.01 (−0.56, 10.58) | 0.08 | 192 | 5.06 (−0.86, 10.99) | 0.09 | 190 | 5.01 (−0.97, 10.99) | 0.10 |
| HDL-C, mg/dL | 213 | −1.41 (−3.96, 1.15) | 0.28 | 212 | −1.10 (−3.62, 1.43) | 0.39 | 192 | −0.86 (−3.57, 1.84) | 0.53 | 190 | −0.96 (−3.68, 1.75) | 0.48 |
| Adiponectin, ug/mL | 230 | −0.72 (−2.41, 0.97) | 0.40 | 229 | −0.63 (−2.34, 1.09) | 0.47 | 204 | −0.89 (−2.65, 0.88) | 0.32 | 202 | −0.88 (−2.64, 0.89) | 0.33 |
| Leptin, ng/mL | 230 | 1.29 (−0.01, 2.56) | 0.05 | 229 | 0.86 (−0.40, 2.13) | 0.18 | 204 | 0.71 (−0.42, 1.83) | 0.22 | 202 | 0.57 (−0.50, 1.64) | 0.30 |
| Diastolic BP, mm Hg | 284 | −0.23 (−1.46, 1.00) | 0.71 | 283 | −0.30 (−1.54, 0.94) | 0.63 | 252 | −0.40 (−1.74, 0.93) | 0.55 | 249 | −0.36 (−1.72, 0.99) | 0.60 |
| Systolic BP, mm Hg | 284 | 0.80 (−1.22, 2.82) | 0.44 | 283 | 0.76 (−1.29, 2.81) | 0.47 | 252 | 0.35 (−1.85, 2.55) | 0.75 | 249 | 0.31 (−1.94, 2.55) | 0.79 |
| Body composition | ||||||||||||
| Abdominal VAT, mm2 | 275 | 0.41 (−0.56, 1.38) | 0.40 | 273 | 0.17 (−0.77, 1.10) | 0.73 | 242 | 0.19 (−0.75, 1.13) | 0.69 | |||
| Abdominal SAT, mm2 | 275 | 7.37 (1.12, 13.60) | 0.021 | 273 | 4.41 (−1.49, 10.31) | 0.14 | 242 | 2.37 (−2.62, 7.37) | 0.35 | |||
| VAT/SAT ratio | 275 | −0.03 (−0.06, −0.01) | 0.029 | 273 | −0.02 (−0.05, 0.01) | 0.12 | 242 | −0.01 (−0.04, 0.02) | 0.48 | |||
| Fat mass, kg | 283 | 0.28 (−0.11, 0.67) | 0.16 | 281 | 0.21 (−0.18, 0.60) | 0.28 | 250 | 0.18 (−0.20, 0.56) | 0.35 | |||
| Fat-free mass, kg | 283 | −0.03 (−0.68, 0.63) | 0.94 | 281 | −0.12 (−0.60, 0.36) | 0.63 | 250 | −0.15 (−0.63, 0.33) | 0.54 | |||
| Body fat, % | 283 | 1.22 (−0.40, 2.84) | 0.14 | 281 | 1.12 (−0.50, 2.73) | 0.18 | 250 | 0.98 (−0.72, 2.69) | 0.26 |
Bolding indicates P < 0.05.
Abbreviations BP, blood pressure; HDL-C, high-density lipoprotein-cholesterol; HOMA-IR, homeostatic model of assessment-insulin resistance; LDL-C, low-density lipoprotein cholesterol; SAT, subcutaneous adipose tissue; Total C, total cholesterol; VAT, visceral adipose tissue.
a Model 1: unadjusted.
b Model 2: adjusted for child sex, Hispanic ethnicity, and age (years) at the Healthy Start Phase 2 visit.
c Model 3: adjusted for Model 2 covariates plus household income, small for gestational age, breastfeeding, maternal age (years), parity, prepregnancy body mass index (kg/m2), gestational weight gain (kg/wk), maternal diabetes mellitus during pregnancy, maternal smoking, physical activity (average metabolic equivalent hours/week), and diet quality (Healthy Eating Index-2010 score) during pregnancy.
d Model 4: adjusted for Model 3 covariates plus percentage body fat at the Healthy Start Phase 2 visit. Results were similar when adjusted for VAT (mm2) at the Healthy Start Phase 2 visit.
e B-coefficients are calculated for the effect of a 1 unit increase in log-hepatic fat at 4 to 8 years on the dependent variable.
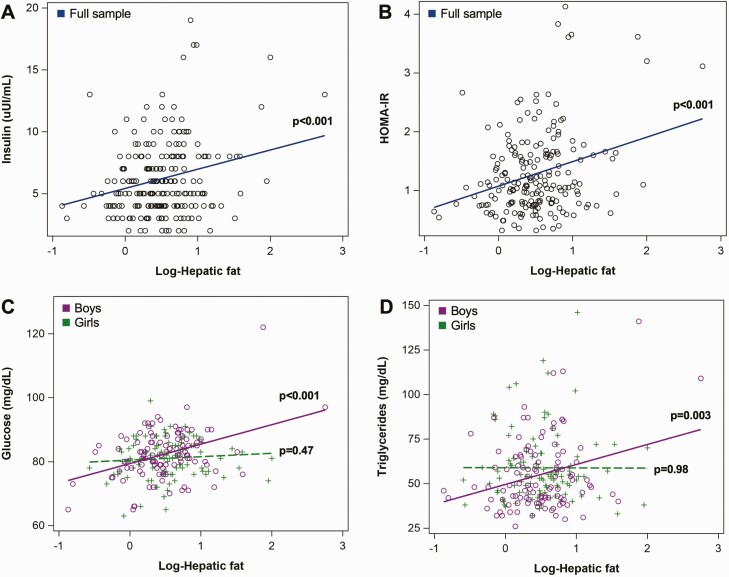
We also found evidence of effect modification by sex for associations of hepatic fat with fasting glucose (p-interaction = 0.030) and triglycerides (p-interaction = 0.045). After stratification, hepatic fat at 4 to 8 years was positively associated with fasting glucose and triglycerides in boys only (Table 4). Associations between log-hepatic fat and fasting glucose, insulin, HOMA-IR, and triglycerides, either in the sample overall or stratified by sex, if appropriate, are visualized in Figure 1.
Table 4.
Stratified estimates for associations of log-hepatic fat with select metabolic variables in early childhood (4-8 years): the Healthy Start Study
| Model 1a | Model 2b | Model 3c | Model 4d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | n | β (95% CI)e | P-value | n | β (95% CI)e | P-value | n | β (95% CI)e | P-value | n | β (95% CI)e | P-value |
| Glucose, mg/dL | ||||||||||||
| Girls | 101 | 1.06 (−1.83, 3.94) | 0.47 | 100 | 0.82 (−1.93, 3.57) | 0.56 | 92 | 0.85 (−2.1, 3.79) | 0.57 | 91 | 0.88 (−2.08, 3.85) | 0.56 |
| Boys | 108 | 6.08 (3.54, 8.63) | <0.001 | 108 | 4.91 (2.44, 7.37) | <0.001 | 96 | 5.44 (2.78, 8.11) | <0.001 | 95 | 5.53 (2.84, 8.21) | <0.001 |
| Triglycerides, mg/dL | ||||||||||||
| Girls | 103 | −0.11 (−8.29, 8.06) | 0.98 | 102 | −0.34 (−8.55, 7.87) | 0.94 | 94 | 1.77 (−6.73, 10.27) | 0.68 | 93 | 1.85 (−6.72, 10.42) | 0.67 |
| Boys | 110 | 11.20 (3.74, 18.67) | 0.003 | 110 | 10.27 (2.65, 17.90) | 0.009 | 98 | 10.73 (2.8, 18.66) | 0.008 | 97 | 10.92 (2.92, 18.91) | 0.008 |
Bolding indicates P < 0.05.
a Model 1: unadjusted.
b Model 2: adjusted for Hispanic ethnicity and age (yrs) at the Healthy Start Phase 2 visit.
c Model 3 adjusted for Model 2 covariates plus household income, small-for-gestational age, breastfeeding, maternal age (yrs), parity, pre-pregnancy body mass index (kg/m2), gestational weight gain (kg/wk), maternal DM during pregnancy, maternal smoking, physical activity (average metabolic equivalent hours/week), and diet quality (Healthy Eating Index-2010 score) during pregnancy.
d Model 4: adjusted for Model 3 covariates plus percent body fat at the Healthy Start Phase 2 visit. Results were similar when adjusted for VAT (mm2) at the Healthy Start Phase 2 visit.
e B-coefficients are calculated for the effect of a 1 unit increase in log-hepatic fat at 4 to 8 years on the dependent variable.
Figure 1.
Associations of log-hepatic fat and metabolic markers at 4 to 8 years in the Healthy Start Study. (A) Fasting insulin (uIU/mL) in the full sample; (B) homeostatic model of assessment for insulin resistance in the full sample; (C) Fasting glucose (mg/dL) stratified by sex; (D) triglycerides (mg/dL) stratified by sex. P-values were calculated from unadjusted linear regression models.
Results from sensitivity analyses are in the Supplementary Tables 2 through 5 (12). First, we tested whether results differed if we excluded the 4 participants with clinical NAFLD (hepatic fat >5.5%) and found that associations of hepatic fat with glucose and HOMA-IR were similar, but associations with triglycerides were attenuated to the null, both overall and among boys [Supplementary Tables 2 and 3 (12)]. Other associations were slightly strengthened when we excluded NAFLD participants, particularly positive associations of hepatic fat with LDL-cholesterol [β (95% CI): 7.51 (0.62, 14.39) mg/dL, P = 0.033, in fully adjusted Model 4] [Supplementary Table 2 (12)]. In other sensitivity analyses, we tested whether results differed if we adjusted for birth weight, instead of SGA, in Model 3 or abdominal VAT area (mm2), instead of percentage body fat, in Model 4. Results were relatively unchanged, both in models overall and stratified by sex (Supplementary Tables 4 and 5, respectively) (12).
Discussion
This is the largest study to quantify hepatic fat deposition by MRI among prepubertal children from the general risk population. We demonstrated that hepatic fat was low (<2%) in our sample of 286 young children (4-8 years old), and only 4 participants (1%) were diagnosed with clinical NAFLD (MRI-hepatic fat >5.5%). Levels were similar in boys and girls, but Hispanic children and children with overweight/obesity had higher hepatic fat compared to non-Hispanic and normal-weight children, reflecting the patterns reported in older children and adolescents (11,32). This supports that hepatic steatosis begins in early childhood for some individuals but is often clinically silent until early adolescence. Importantly, despite hepatic fat being largely subclinical, we found positive associations between hepatic fat and estimated insulin sensitivity, independent of demographics, maternal perinatal covariates, and total or visceral adiposity. This association remained significant even after we excluded the 4 participants with clinical NAFLD and suggests that even during early childhood and below clinical thresholds of NAFLD, higher hepatic fat is already associated with markers of cardiometabolic risk. We also discovered subtle sex-specific findings, such that higher hepatic fat deposition was associated with higher fasting glucose and triglycerides in boys only. This supports that there may be an early sexual dimorphism in the metabolic correlates of hepatic fat in childhood.
While numerous studies have reported on associations between hepatic fat and markers of metabolic dysfunction and/or body composition in older children and adolescents (32-43), there is a paucity of data examining these associations in young children before the onset of puberty—a sensitive period for the development of future cardiometabolic disease risk. In 2008, a study by D’Adamo et al evaluated 100 prepubertal Italian children with obesity (mean age 8.6 ± 2.2 years) who were diagnosed with or without steatosis by ultrasound and found that children with obesity and steatosis had higher HOMA-IR and triglycerides and lower whole-body insulin sensitivity index (assessed by oral glucose tolerance test) and high-density lipoprotein cholesterol compared to those without hepatic fat on ultrasound (44). This study was limited, however, by the use of ultrasound, which has limited accuracy in grading the severity of steatosis and can lead to high rates of false positives, particularly in detecting mild steatosis, when compared to MRS (45,46). Another 2008 study by Maffeis et al examined 30 prepubertal Italian children with overweight or obesity (mean age 10.3 ± 1.6 years) but assessed hepatic fat by MRI, a more robust technique, and found that hepatic fat content was associated with lower insulin sensitivity (assessed by intravenous glucose tolerance test), higher FM, and higher abdominal SAT and VAT (47). In the United States, a 2012 study by Bennett et al assessed hepatic fat by MRS in a multiethnic sample of 123 prepubertal children (mean age 8.0 ± 0.8 years) in Louisiana (USA), who were primarily non-Hispanic white (63%) or African American (33%), and found that hepatic fat was higher in children with obesity and inversely correlated with insulin sensitivity (assessed by intravenous glucose tolerance test) (48). A subsequent study by Larson-Meyer et al further explored associations between hepatic fat and metabolic markers in the same Louisiana-based cohort and found that hepatic fat was associated with higher body fat, abdominal SAT and VAT, total and LDL-cholesterol, and systolic blood pressure, in addition to insulin resistance (49).
In the present study, we expanded on these prior studies by examining MRI-assessed hepatic fat in a large cohort of more than 250 young children, all under 9 years old, which was also enriched with Hispanic participants (27% of the sample). Overall, average hepatic fat in our study (1.9%) was markedly lower compared to average hepatic fat in the study by Maffeis et al (9.1%), which also assessed hepatic fat by MRI. This difference is likely because the study by Maffeis et al only examined children with overweight or obesity, whereas the majority of children in our sample (79%) were normal weight. However, similar to the previous studies, we found that hepatic fat was higher in children with overweight or obesity based on BMI percentile categories and was independently associated with markers of glucose-insulin homeostasis, including fasting insulin concentrations and estimated insulin sensitivity (HOMA-IR), which supports a link between hepatic fat and insulin resistance. In contrast, we did not find associations with other metabolic markers reported in the previous studies, such as blood pressure. Given our sample was slightly younger (mean age: 4.8 ± 0.8 years) than prior studies, with less overweight and obesity, as previously mentioned, it is possible that a longer duration or magnitude of hepatic fat deposition is required for these other associations to become evident or that they are only present in the context of obesity. We also did not find many significant associations between hepatic fat and other body composition outcomes, such as abdominal VAT or percentage body fat. The exception was a positive association with abdominal SAT in unadjusted analyses, but this was attenuated when we adjusted for potential confounders in subsequent models. It is possible that the effect sizes for these continuous, dose-response associations were too subtle to detect in our sample.
We also evaluated whether associations between hepatic fat content and metabolic outcomes differed by sex or race/ethnicity. This was novel and revealed that higher hepatic fat at 4 to 8 years was associated with higher fasting glucose and triglycerides in boys only. These sex-specific findings suggest that even early on in development and prior to pubertal onset, boys are more susceptible to hepatic fat-associated metabolic impairments than girls. This also aligns with the notion that the sexual dimorphism in metabolic profiles may be due to other factors, beyond sex hormones alone, such as underlying genetic or epigenetic regulation or gut microbiota differences (50). It should be noted that associations of hepatic fat with triglycerides were attenuated and insignificant in sensitivity analyses excluding participants with clinical NAFLD, suggesting this association is otherwise much weaker among participants with only subclinical hepatic fat. This may, therefore, reflect a compensatory mechanism already at play among children with clinically elevated hepatic fat characterized by increased secretion of triglyceride-rich, very-low-density lipoproteins to remove excess hepatic triglycerides (51,52)—a pathway that can also be stimulated by elevations in plasma glucose (53). An association between hepatic fat deposition and triglycerides in some children this age is also concerning given evidence suggesting that elevated triglycerides is an indicator of an atherogenic blood lipid profile consisting of higher apolipoprotein B particles and small, dense LDL particles (54). Future studies utilizing more sophisticated blood lipid assessments will be needed to confirm whether this atherogenic pattern is also associated with increased hepatic fat in early childhood.
We utilized data from a large cross-sectional cohort study, with a sample size larger than all previous investigations of hepatic fat deposition in young children. The prospective design enabled us to consider various potential confounding variables that were collected earlier in pregnancy and infancy. Our use of MRI to assess hepatic fat deposition, as well as abdominal VAT and SAT deposition, is also a strength given MRI is safe and provides a more accurate and reliable measurement of hepatic fat compared to ultrasound or noninvasive biomarkers. We also used air displacement plethysmography to assess FM, FFM, and percentage body fat, which provides more accurate estimates than anthropometrics. The demographic characteristics of children in the Healthy Start Study, which includes both boys and girls, a diverse racial/ethnic distribution, and a range of BMI categories, is also a strength that increases the generalizability of findings to the general public. At the same time, we included only a subsample of offspring in the Healthy Start Study, particularly those who underwent MRI at 4 to 8 years to assess hepatic fat. This reduced sample size may have limited our statistical power and ability to detect more subtle effect sizes at younger ages. Also, the observational nature of our study limits our ability to establish causality.
Overall, the findings in this study advanced our knowledge of average hepatic fat deposition (measured by MRI) in generally healthy, prepubertal children and the metabolic correlates of hepatic fat deposition as a continuous variable at this age. We showed that hepatic fat at 4 to 8 years is highest in Hispanic children and children with overweight and obesity. Despite hepatic fat being below typical clinical thresholds for NAFLD in most children, it was still an independent risk factor for estimated insulin resistance overall and higher fasting glucose in boys, even after adjusting for total or visceral adiposity, supporting that low levels of hepatic fat deposition may reflect the initiation of metabolic alterations involved in the pathogenesis of insulin resistance and hyperglycemia. Hepatic fat was also associated with triglycerides in boys, particularly those who already have clinically elevated hepatic fat. Collectively, these findings suggest that the current screening guidelines for certain high-risk subgroups (ie, boys, Hispanic children, and/or children with obesity) may need to be altered since these individuals exhibited higher levels of hepatic fat at this age compared to other children and/or stronger associations with metabolic outcomes. Study visits are currently ongoing for the next phase of follow-up for the Healthy Start Study, when children are between 8 and 10 years of age, which will enable us to explore the natural history of early-onset hepatic fat deposition and longitudinal associations with markers of metabolic dysfunction and body composition over time. Our findings may also have significant therapeutic implications. Specifically, our findings support that interventions aiming to prevent hepatic fat accumulation may also be effective in preventing the onset and/or progression of insulin resistance and other cardiometabolic disease risk factors in youth and that such interventions should start early on in life. Future directions will include examining the influence of modifiable, lifestyle behaviors on hepatic fat deposition at this age, as well as underlying pathophysiological mechanisms linking hepatic fat with cardiometabolic dysfunction.
Acknowledgments
Financial Support: This work was funded by the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) grant no. R01-DK076648 to D.D. Dr. C.C.C. is supported by NIDDK grant no. T32-DK07658. W.P. is supported by the NIH/NCATS Colorado CTSA grant no. KL2-TR002534.
Author Contributions: C.C.C. performed the research, analyzed the data, and wrote the manuscript. W.P., S.S., K.S., and D.D. assisted with writing the paper and with data interpretation. A.S. analyzed imaging data and assisted with writing the paper. D.D. acquired funding for the research. All authors critically reviewed and revised the paper.
Additional Information
Disclosure Statement: The authors have no competing financial interests to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999-2016. Pediatrics. 2018;141(3):e20173459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weiss R, Bremer AA, Lustig RH. What is metabolic syndrome, and why are children getting it? Ann N Y Acad Sci. 2013;1281:123-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. l’Allemand-Jander D. Clinical diagnosis of metabolic and cardiovascular risks in overweight children: early development of chronic diseases in the obese child. Int J Obes (Lond). 2010;34(Suppl 2):S32-S3 6. [DOI] [PubMed] [Google Scholar]
- 4. Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease in the pediatric population. Clin Liver Dis. 2004;8(3):549-58, viii. [DOI] [PubMed] [Google Scholar]
- 5. Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children. J Pediatr Gastroenterol Nutr. 2017;64(2):319-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J; Nonalcoholic Steatohepatitis Clinical Research Network. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135(6):1961-1971.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carter-Kent C, Brunt EM, Yerian LM, et al. Relations of steatosis type, grade, and zonality to histological features in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2011;52(2):190-197. [DOI] [PubMed] [Google Scholar]
- 8. Schwimmer JB, Newton KP, Awai HI, et al. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38(10):1267-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PloS One. 2015;10(10):e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldner D, Lavine JE. Nonalcoholic fatty liver disease in children: unique considerations and challenges. Gastroenterology. 2020;158(7):1967-1983.e1. [DOI] [PubMed] [Google Scholar]
- 11. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388-1393. [DOI] [PubMed] [Google Scholar]
- 12. Cohen CC. Supplementary materials for: Hepatic fat in early childhood is independently associated with estimated insulin resistance: the Healthy Start Study. 2021. 10.6084/m9.figshare.14755293.v2 [DOI] [PMC free article] [PubMed]
- 13. Smits LP, Coolen BF, Panno MD, et al. Noninvasive differentiation between hepatic steatosis and steatohepatitis with mr imaging enhanced with USPIOs in patients with nonalcoholic fatty liver disease: a proof-of-concept study. Radiology. 2016;278(3):782-791. [DOI] [PubMed] [Google Scholar]
- 14. Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462-E468. [DOI] [PubMed] [Google Scholar]
- 15. Nasr P, Forsgren MF, Ignatova S, et al. Using a 3% proton density fat fraction as a cut-off value increases sensitivity of detection of hepatic steatosis, based on results from histopathology analysis. Gastroenterology. 2017;153(1):53-55.e7. [DOI] [PubMed] [Google Scholar]
- 16. Maligie M, Crume T, Scherzinger A, Stamm E, Dabelea D. Adiposity, fat patterning, and the metabolic syndrome among diverse youth: the EPOCH study. J Pediatr. 2012;161(5):875-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bellatorre A, Scherzinger A, Stamm E, Martinez M, Ringham B, Dabelea D. Fetal overnutrition and adolescent hepatic fat fraction: the Exploring Perinatal Outcomes in Children Study. J Pediatr. 2018;192:165-170.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crume TL, Brinton JT, Shapiro A, et al. Maternal dietary intake during pregnancy and offspring body composition: the Healthy Start Study. Am J Obstet Gynecol. 2016;215(5):609.e1-609.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shapiro AL, Kaar JL, Crume TL, et al. Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start Study. Int J Obes (Lond). 2016;40(7):1056-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Starling AP, Brinton JT, Glueck DH, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start Study. Am J Clin Nutr. 2015;101(2):302-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1-190. [PubMed] [Google Scholar]
- 22. Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57-63. [DOI] [PubMed] [Google Scholar]
- 23. Emoto M, Nishizawa Y, Maekawa K, et al. Homeostasis model assessment as a clinical index of insulin resistance in type 2 diabetic patients treated with sulfonylureas. Diabetes Care. 1999;22(5):818-822. [DOI] [PubMed] [Google Scholar]
- 24. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502. [PubMed] [Google Scholar]
- 25. Guenther PM, Kirkpatrick SI, Reedy J, et al. The Healthy Eating Index-2010 Is a Valid and Reliable Measure of Diet Quality According to the 2010 Dietary Guidelines for Americans. J Nutr. 2014;144(3):399-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc. 2004;36(10):1750-1760. [DOI] [PubMed] [Google Scholar]
- 27. Sauder KA, Starling AP, Shapiro AL, et al. Diet, physical activity and mental health status are associated with dysglycaemia in pregnancy: the Healthy Start Study. Diabet Med. 2016;33(5):663-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crume TL, Shapiro AL, Brinton JT, et al. Maternal fuels and metabolic measures during pregnancy and neonatal body composition: the healthy start study. J Clin Endocrinol Metab. 2015;100(4):1672-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Faienza MF, Brunetti G, Ventura A, et al. Nonalcoholic fatty liver disease in prepubertal children born small for gestational age: influence of rapid weight catch-up growth. Horm Res Paediatr. 2013;79(2):103-109. [DOI] [PubMed] [Google Scholar]
- 31. Newton KP, Feldman HS, Chambers CD, et al. ; Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN). Low and high birth weights are risk factors for nonalcoholic fatty liver disease in children. J Pediatr. 2017;187:141-146.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tricò D, Caprio S, Rosaria Umano G, et al. Metabolic features of nonalcoholic fatty liver (NAFL) in obese adolescents: findings from a multiethnic cohort. Hepatology. 2018;68(4):1376-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cali AM, De Oliveira AM, Kim H, et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49(6):1896-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen M, Syme C, Deforest M, et al. Ectopic fat in youth: the contribution of hepatic and pancreatic fat to metabolic disturbances. Obesity (Silver Spring). 2014;22(5):1280-1286. [DOI] [PubMed] [Google Scholar]
- 35. D’Adamo E, Cali AM, Weiss R, et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;33(8):1817-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jin R, Le N-a, Cleeton R, et al. Amount of hepatic fat predicts cardiovascular risk independent of insulin resistance among Hispanic-American adolescents. Lipids Health Dis. 2015:14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim JS, Lê KA, Mahurkar S, Davis JN, Goran MI. Influence of elevated liver fat on circulating adipocytokines and insulin resistance in obese Hispanic adolescents. Pediatr Obes. 2012;7(2):158-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fonvig CE, Chabanova E, Andersson EA, et al. 1H-MRS measured ectopic fat in liver and muscle in Danish lean and obese children and adolescents. PloS One. 2015;10(8):e0135018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nissen A, Fonvig CE, Chabanova E, et al. 1H-MRS measured ectopic fat in liver and muscle is associated with the metabolic syndrome in Danish girls but not in boys with overweight and obesity. Obes Sci Pract. 2016;2(4):376-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee S, Rivera-Vega M, Alsayed HM, Boesch C, Libman I. Metabolic inflexibility and insulin resistance in obese adolescents with non-alcoholic fatty liver disease. Pediatr Diabetes. 2015;16(3):211-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wolfgram PM, Connor EL, Rehm JL, Eickhoff JC, Reeder SB, Allen DB. Ethnic differences in the effects of hepatic fat deposition on insulin resistance in nonobese middle school girls. Obesity (Silver Spring). 2014;22(1):243-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alderete TL, Toledo-Corral CM, Desai P, Weigensberg MJ, Goran MI. Liver fat has a stronger association with risk factors for type 2 diabetes in African-American compared with Hispanic adolescents. J Clin Endocrinol Metab. 2013;98(9):3748-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cioffi CE, Narayan KMV, Liu K, et al. Hepatic fat is a stronger correlate of key clinical and molecular abnormalities than visceral and abdominal subcutaneous fat in youth. BMJ Open Diabetes Res Care. 2020;8(1):e001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. D’Adamo E, Impicciatore M, Capanna R, et al. Liver steatosis in obese prepubertal children: a possible role of insulin resistance. Obesity (Silver Spring). 2008;16(3):677-683. [DOI] [PubMed] [Google Scholar]
- 45. Bohte AE, Koot BG, van der Baan-Slootweg OH, et al. US cannot be used to predict the presence or severity of hepatic steatosis in severely obese adolescents. Radiology. 2012;262(1):327-334. [DOI] [PubMed] [Google Scholar]
- 46. Awai HI, Newton KP, Sirlin CB, Behling C, Schwimmer JB. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin Gastroenterol Hepatol. 2014;12(5):765-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maffeis C, Manfredi R, Trombetta M, et al. Insulin sensitivity is correlated with subcutaneous but not visceral body fat in overweight and obese prepubertal children. J Clin Endocrinol Metab. 2008;93(6):2122-2128. [DOI] [PubMed] [Google Scholar]
- 48. Bennett B, Larson-Meyer DE, Ravussin E, et al. Impaired insulin sensitivity and elevated ectopic fat in healthy obese vs. nonobese prepubertal children. Obesity (Silver Spring). 2012;20(2):371-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Larson-Meyer DE, Newcomer BR, Ravussin E, et al. Intrahepatic and intramyocellular lipids are determinants of insulin resistance in prepubertal children. Diabetologia. 2011;54(4):869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chella Krishnan K, Mehrabian M, Lusis AJ. Sex differences in metabolism and cardiometabolic disorders. Curr Opin Lipidol. 2018;29(5):404-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fabbrini E, deHaseth D, Deivanayagam S, Mohammed BS, Vitola BE, Klein S. Alterations in fatty acid kinetics in obese adolescents with increased intrahepatic triglyceride content. Obesity (Silver Spring). 2009;17(1):25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106(36):15430-15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Adiels M, Taskinen MR, Packard C, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49(4):755-765. [DOI] [PubMed] [Google Scholar]
- 54. Bril F, Sninsky JJ, Baca AM, et al. Hepatic steatosis and insulin resistance, but not steatohepatitis, promote atherogenic dyslipidemia in NAFLD. J Clin Endocrinol Metab. 2016;101(2):644-652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.