Abstract
Context
Studies in rodents and humans suggest that high-fructose corn syrup (HFCS)–sweetened diets promote greater metabolic dysfunction than sucrose-sweetened diets.
Objective
To compare the effects of consuming sucrose-sweetened beverage (SB), HFCS-SB, or a control beverage sweetened with aspartame on metabolic outcomes in humans.
Methods
A parallel, double-blinded, NIH-funded study. Experimental procedures were conducted during 3.5 days of inpatient residence with controlled feeding at a research clinic before (baseline) and after a 12-day outpatient intervention period. Seventy-five adults (18-40 years) were assigned to beverage groups matched for sex, body mass index (18-35 kg/m2), and fasting triglyceride, lipoprotein and insulin concentrations. The intervention was 3 servings/day of sucrose- or HFCS-SB providing 25% of energy requirement or aspartame-SB, consumed for 16 days. Main outcome measures were %hepatic lipid, Matsuda insulin sensitivity index (ISI), and Predicted M ISI.
Results
Sucrose-SB increased %hepatic lipid (absolute change: 0.6 ± 0.2%) compared with aspartame-SB (-0.2 ± 0.2%, P < 0.05) and compared with baseline (P < 0.001). HFCS-SB increased %hepatic lipid compared with baseline (0.4 ± 0.2%, P < 0.05). Compared with aspartame-SB, Matsuda ISI decreased after consumption of HFCS- (P < 0.01) and sucrose-SB (P < 0.01), and Predicted M ISI decreased after consumption of HFCS-SB (P < 0.05). Sucrose- and HFCS-SB increased plasma concentrations of lipids, lipoproteins, and uric acid compared with aspartame-SB. No outcomes were differentially affected by sucrose- compared with HFCS-SB. Beverage group effects remained significant when analyses were adjusted for changes in body weight.
Conclusion
Consumption of both sucrose- and HFCS-SB induced detrimental changes in hepatic lipid, insulin sensitivity, and circulating lipids, lipoproteins and uric acid in 2 weeks.
Keywords: Sugar-sweetened beverages, high-fructose corn syrup, sucrose, liver fat, insulin sensitivity, lipids
Over the past decade the prevalence of both nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2D) have dramatically increased worldwide, establishing both as global health concerns (1, 2). More than 65% to 70% of T2D patients have NAFLD and their coexistence leads to a greater risk of disease-related complications and development of cardiovascular disease (CVD) (3, 4). Among T2D patients, cardiovascular complications are the leading cause of morbidity and mortality worldwide (2). While the prevalence of NAFLD and T2D among older adults is well recognized, the increasing number of cases among young people is of particular concern as earlier onset increases susceptibility to long-term complications (5-7).
NAFLD may precede and/or promote the development of T2D as increased hepatic lipid synthesis and deposition can contribute to altered glucose homeostasis and insulin resistance and/or beta-cell dysfunction (8). Diet and other lifestyle habits are among some of the leading factors that modulate the development and progression of hepatic steatosis, T2D, and CVD (2, 9). Epidemiological research has linked sugar-sweetened beverage (SB) consumption to these adverse health outcomes (10-12). A recent meta-analysis of twelve observational studies found that a higher intake of sugar-SBs was associated with an increased risk of NAFLD (1.39-fold increase, 95% CI 1.29-1.50, P < 0.00001) (13). Experimental evidence shows fructose consumption directly alters hepatic lipid metabolism and insulin sensitivity in both animals and humans (14-22). Plausible mechanisms related to the increased de novo lipogenesis (DNL) and uric acid production caused by the unregulated uptake of fructose by the liver support this evidence (18, 23-25). High-fructose corn syrup (HFCS) and sucrose are the leading added sugars consumed in the United States and the main sources of added fructose in the diet. HFCS is the main sweetener in sugar-SBs (26). With a self-reported intake of about 144 kcal per day, equivalent to 1 12oz can of soda, sugar-SBs are the primary source of added sugar in the diet among youth and adults (27, 28).
Evidence from several studies conducted in rodents indicates that compared with diets sweetened with sucrose, HFCS increases hepatocellular lipid content and decreases hepatic insulin sensitivity (29-31). Epidemiological evidence suggests that countries utilizing HFCS as a sweetener in their food supply have a ~20% higher T2D prevalence than countries not utilizing HFCS (32). An acute crossover study found that compared with sucrose, a 24-oz serving of HFCS led to greater systemic exposure of fructose and more deleterious metabolic effects in men and women (33). However, there is limited clinical evidence from sustained dietary intervention studies comparing the metabolic effects of HFCS and sucrose consumption. The main evidence comes from a large industry-funded study that reported that there were no differences between the metabolic effects of HFCS and sucrose when consumed for 10 weeks at low, medium or high doses (~8, 18, and 33% of energy requirement (Ereq) with the sugars dissolved in milk) (34, 35). However, the results of these studies also led the authors to conclude that there were no significant differences between the effects of high vs low doses of sugar on low-density lipoprotein cholesterol (LDL-C) (34), 24-hour triglyceride (TG), or uric acid (35). In contrast, we have reported that subjects consuming 0, 10, 17.5, or 25% of Ereq as HFCS for 2 weeks exhibited dose-dependent increases in all 3 outcomes; LDL-C: P < 0.0001, 24-hour TG area under the curve (AUC): P < 0.05, uric acid AUC: P < 0.0001 (36).
Therefore, the objective of this study was to compare the metabolic effects of consuming sucrose- or HFCS-SB at 25% Ereq with aspartame-SB as a control in young, healthy male and female adults. We hypothesized that hepatic lipid content, lipid/lipoproteins and uric acid levels would be increased, and insulin sensitivity decreased in subjects consuming either HFCS- or sucrose-SB compared with those consuming aspartame-SB.
Materials and Methods
Participants
This paper reports the results of a subgroup of 75 participants from an NIH-funded investigation in which a total of 187 participants assigned to 8 experimental groups were studied. The 75 adults were assigned to consume 3 sweetened beverages/day containing either aspartame (n = 23) or 25% of their Ereq as HFCS (n = 28) or sucrose (n = 24) for 2 weeks.
This study protocol was approved by the University of California (UC), Davis Institutional Review Board and is registered with Clinical Trials.gov: NCT01103921. Participants provided written informed consent. Recruitment was through an Internet listing (craigslist.com) and local flyer postings. To assess eligibility potential participants underwent telephone and in-person interviews for medical history, a complete blood count and serum biochemistry panel. Inclusion criteria included age 18-40 years and body mass index (BMI) 18 to 35 kg/m2 with a self-report of stable body weight during the prior 6 months. Exclusion criteria included diabetes (fasting glucose >125 mg/dL), evidence of renal or hepatic disease according to aspartate aminotransferase (AST) and alanine aminotransferase (ALT) 1.5 normal limits ratio, fasting plasma TG >400 mg/dL, hypertension (>140/90 mmHg), hemoglobin <8.5 g/dL, and surgery for weight loss. Individuals who reported that they smoked, regularly ingested >2 alcoholic beverages or sugar-SB/day, exercised >3.5 hours/week at a level more vigorous than walking, or used thyroid, lipid-lowering, glucose-lowering, antihypertensive, antidepressant, or weight loss medications were also excluded. By design, assignment to the experimental groups was not randomized; the experimental groups were matched for sex, BMI, and concentrations of fasting TG, LDL-C, high-density lipoprotein cholesterol (HDL-C), and insulin measured in serum collected during the in-person interviews. Subjects who were scheduled for participation were asked to limit daily consumption of sweet beverages to no more than 1 8-oz serving of 100% fruit juice and to discontinue consumption of any vitamin, mineral, dietary, or herbal supplements 5 weeks before the start of study.
Study Protocol
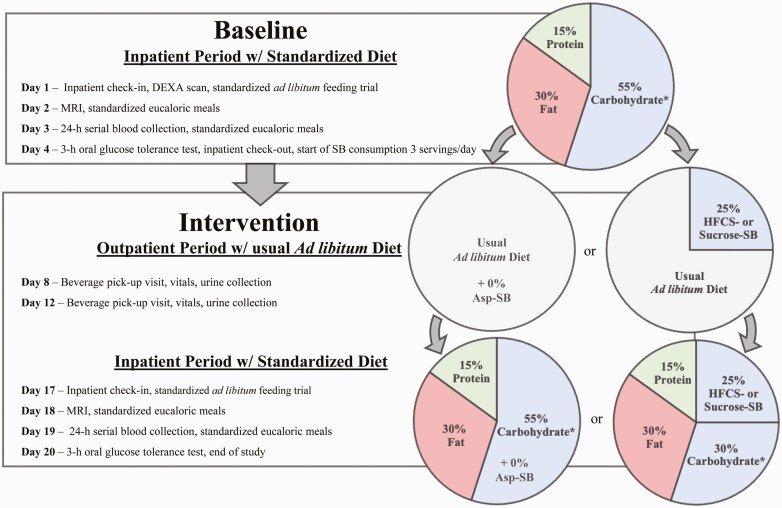
This was a parallel-arm, double-blinded diet intervention study with 3 phases (Fig. 1): (1) a 3.5-day inpatient baseline period during which subjects resided at the UC Davis Clinical and Translational Science Center Clinical Research Center (CCRC), consumed a standardized baseline diet, and participated in experimental procedures; (2) a 12-day outpatient intervention period during which subjects consumed their assigned sweetened beverages containing aspartame or 25% Ereq as sucrose or HFCS along with their usual ad libitum diets; and (3) a 3.5-day inpatient intervention period during which subjects resided at the CCRC and consumed standardized diets that included the sweetened beverages and all experimental procedures were repeated (Fig. 1). All participants were asked to maintain their usual exercise habits throughout the study.
Figure 1.
Study design, experimental testing days, and dietary protocol. Asp (Aspartame). SB (sweetened beverage). Dual energy x-ray absorptiometry (DEXA). *<2% added sugar. % = % of energy requirement.
Inpatient Meals
Identical low sugar ad libitum meals were served to all subjects on Day 1 and Day 17 (Fig. 1). On Days 2 and 3 all subjects consumed standardized eucaloric meals containing 55% Ereq as complex carbohydrate, 30% fat, 15% protein. Ereq was calculated using the Mifflin equation (37) with a 1.5 adjustment for activity level during Day 2 and a 1.3 adjustment on Day 3 when activity was minimized by the 24-hour serial blood collection. The meals consumed during the intervention testing period included the assigned study beverages. The Day 18 and 19 eucaloric meals were as identical as possible to baseline meals, except for the isocaloric substitution of the sugar-SB for complex carbohydrate. The timing and energy distribution of the meals were as follows: breakfast 9:00 hours, 25% Ereq; lunch 13:00 hours, 35% Ereq; dinner 18:00 hours, 40% Ereq.
Beverages
Beverages were prepared by a designated staff member at the UC Davis Department of Nutrition Ragle Clinical Research Center. HFCS-containing beverages were sweetened with HFCS-55 (ISOSWEET 5500, 55% fructose, 45% glucose; Tate & Lyle). Sucrose-containing beverages were sweetened with C&H® cane sugar (Domino Foods Inc). The sugar-SBs were flavored with unsweetened Kool-Aid® drink mix and formulated as 15% sugar in water (w/w). Aspartame-containing beverages were prepared using fruit flavored Market Pantry™ drink mix. Beverages were provided as 3 servings/day with amounts standardized among the 3 groups and based on Ereq (Mifflin equation with a 1.5 adjustment for activity level (37)). During the 12-day outpatient phase, participants were instructed to consume their usual diet, to drink 1 serving of the assigned study beverage with each meal, and to not consume any other sugar-containing beverages including 100% fruit juice. Participants were blinded to their beverage assignments, as were all CCRC staff and study personnel who interacted with participants or analyzed samples. Beverages contained a biomarker (riboflavin) that was measured fluorometrically in urine samples collected twice weekly to index compliance of beverage consumption. Subjects were informed that they were being monitored for beverage consumption but were not briefed about the method. Riboflavin was assessed in fasting urine samples collected during baseline, on the 8th and 12th days of outpatient intervention, and during inpatient intervention. Urinary riboflavin concentrations did not differ among groups and concentrations during outpatient beverage consumption were not different from those during the inpatient period when beverage consumption was monitored (36, 38).
Body Composition and Body Weight
Percent body fat at baseline was determined by dual energy x-ray absorptiometry (DEXA) on Day 1. Subjects were weighed in the fasting state with standardized attire on Day 1 and Day 17.
Hepatic Lipid Imaging
Magnetic resonance imaging (MRI) for hepatic lipid content was conducted on Day 2 (baseline) and Day 17 (intervention). While the baseline and intervention scans were always conducted at the same time for each subject, scheduling logistics required that some scans were scheduled in the morning and some in the afternoon. Subjects were transported from the CCRC to the UC Davis Medical Center, Ambulatory Care Center and were scanned using a confounder-corrected chemical shift-encoded liver fat quantification MRI technique on a 1.5-T system (General Electric HDxt, with an 8-channel body coil). To estimate liver proton density fat fraction (PDFF), a quantitative image biomarker of liver fat content, axial 2D, T1-independent, T2*-corrected, 6-echo gradient-recalled-echo images were acquired (repetition time [TR] = 125ms, echo time [TE] = 2.3, 4.6, 6.9, 9.2, 11.5, 13.8 ms, 8-mm slice thickness, 256 × 192 matrix size) (39).
Hepatic Lipid Content Quantification
Sixty-six of the 75 subjects had quantifiable baseline and intervention scans that were included in the analyses of hepatic lipid content. Of the 9 sets of missing scans, 7 were due to scanner unavailability (HFCS-SB: 5; Sucrose-SB: 1; Aspartame-SB: 1), 1 was due to subject discomfort with the procedure (aspartame-SB), and 1 was due to corrupt file format (aspartame-SB). Thus, the sample sizes of the groups analyzed for hepatic lipid content were 20, 23, and 23 for the aspartame, sucrose, and HFCS groups, respectively. MRI-PDFF was measured with Osirix software (OsiriX MD versions 10 and 11; Pixmeo, Geneva, Switzerland) and the LIPO-Quant (Liver Imaging of Phase-interference related signal Oscillation and Quantification) plugin (40), which computed liver PDFF parametric maps pixel-by-pixel from source images. PDFF values were obtained by placing regions of interest (ROIs) in representative portions of the liver on those maps to ensure adequate sampling of the liver. PDFF values of all ROIs were averaged to provide whole-liver estimates of PDFF. The MR technologists performing the scans and the image analyst placing the ROIs on the liver PDFF parametric maps were blinded to any subject data including assigned beverage group. To ensure consistency of PDFF methodology, 31 scans were randomly selected and analyzed by 2 additional blinded image analysts. Intraclass correlation coefficient for the 3 analyses of PDFF was 0.99.
Insulin Sensitivity
Three-hour oral glucose tolerance tests (OGTTs) were performed on Day 4 (baseline) and Day 20 (intervention) following a 14-hour overnight fast at the CCRC. A fasting blood sample was collected at 8:00 hours through intravenous catheter. Following a 75-g oral glucose load, blood samples were collected 30, 60, 90, 120, and 180 minutes later. Plasma samples were analyzed for glucose concentrations using an YSI glucose analyzer and for insulin concentrations using radioimmunoassay (Millipore Inc., St. Charles, MO). Insulin sensitivity was calculated using the 2-hour OGTT data with both the Matsuda insulin sensitivity index (ISI) (41) and the Predicted M ISI (42). The more recently developed Predicted M ISI utilizes the oral glucose insulin sensitivity index (43) and includes an adjustment for BMI (42). The baseline and intervention Predicted M ISI for this report were calculated utilizing the BMIs specific to each timepoint, therefore the ΔPredicted M ISI includes adjustment for Δbody weight.
Twenty-four Hour Serial Blood Collections
Twenty-four hour serial blood samples were collected on the Day 3 (baseline) and Day 19 (intervention) via intravenous catheter. Starting at 8:00 hours, 3 fasting samples followed by 29 postprandial blood samples were collected at 30- to 60-minute intervals until 8:00 hours the following morning. An additional 6mL of blood were collected at fasting time points (8:00, 8:30, 9:00 hours) and at late evening time points (22:00, 23:00, 24:00 hours). The additional plasma from the 3 fasting draws was pooled as was that from the late-evening postprandial draws, and each pool was aliquoted and frozen as 24 identical samples. The timing of the late evening postprandial draws was based on the late-evening peak of postprandial TG concentrations observed in our previous study (20). The plasma concentrations of TG and uric acid were measured at all time points and were calculated for total 24-hour AUC by the trapezoidal method. The concentrations of cholesterol, LDL-C, HDL-C, apolipoprotein B (apoB), and apolipoprotein CIII (apoCIII) were measured during the fasting and late evening postprandial periods. Lipid and lipoprotein and uric acid concentrations were measured with a Polychem Chemistry Analyzer (PolyMedCo Inc.) with reagents from MedTest DX. The intra- and interassay CVs for all assays were as follows: glucose: 3.6%, 4.5%; insulin: 6.5%, 7.6%; TG: 2.2%, 7.2%; total cholesterol: 1.4%, 4.2%; LDL-C: 2.7%, 5.7%; HDL-C: 2.7%, 5.7%; uric acid: 1.9%, 14.5%; apoB: 2.4%, 5.7%; apoCIII: 0.9%, 5.5%.
Statistical Analyses
The primary sample size calculation included in the funded NIH grant application was based on the effect sizes for fasting apoB and small dense LDL-C (1.23 and 0.96 respectively) obtained from our previous study comparing the effects of consuming fructose- or glucose-sweetened beverages at 25% Ereq (20). The results indicated that 25 subjects per group would allow detection of a difference in apoB or small dense LDL-C between subjects consuming aspartame or 25% Ereq as fructose-SB at P < 0.05 and 80% power in the 7-group analysis proposed in the application. Due to funding limitations this sample size was not achieved in all groups. In order to accomplish the aims of an NIH-funded ancillary project (1R01HL107256) the sample size was exceeded in the 25% Ereq fructose-, glucose-, and HFCS-SB groups.
Baseline and intervention outcomes were log transformed when the baseline or absolute change values were not normally distributed. The absolute change (∆) at intervention compared to baseline for each outcome was analyzed using a general linear 2-factor (sweetened beverage [SB] group, sex) analysis of covariance (ANCOVA) adjusted for SB group x sex, outcome at baseline, and BMI at baseline (SAS 9.4, SAS, Cary, NC). The interaction term and/or covariates that did not improve the sensitivity of the models were removed. Significant differences between groups were identified using Tukey’s multiple-comparisons test. Significant within-group changes (intervention value compared with baseline value) were identified as least squares mean (LS mean) of the change significantly different from 0. Secondary ANCOVAs were conducted that included adjustment for Δbody weight (except Predicted M ISI) or substituted %body fat at baseline for BMI at baseline. Statistical significance was considered at P < 0.05. Data are reported as mean ± standard error of the mean unless otherwise specified.
Results
There were no significant differences among the 3 SB groups in the baseline characteristics shown in Table 1. The absolute values of body weight, hepatic lipid content and indices of insulin sensitivity at baseline and intervention are presented in Table 2, along with the P values for the effect of SB group in the primary ANCOVA and the effect of SB group and Δbody weight in the secondary ANCOVA. Each outcome is designated by a numerical superscript in order to detail the covariates included in the ANCOVA model in the legend for Table 2.
Table 1.
Participant characteristics at baseline
| Parameter | Aspartame | HFCS | Sucrose |
|---|---|---|---|
| Age (year) | 25.4 ± 6.2a | 26.8 ± 6.6 | 25.9 ± 6.3 |
| Sex (M/F) | 11/12 | 15/13 | 12/12 |
| Weight (kg) | 71.8 ± 10.6 | 72.9 ± 14.5 | 71.9 ± 12.1 |
| BMI (kg/ m2) | 24.8 ± 3.3 | 24.9 ± 4.0 | 25.3 ± 3.4 |
| Waist circumference (cm) | 75.2 ± 6.4 | 77.0 ± 10.1 | 75.4 ± 7.2 |
| Body fat (%) | 27.1 ± 9.6 | 26.0 ± 9.7 | 29.1 ± 11.5 |
| Energy requirement (kcal/d) | 2354 ± 322 | 2390 ± 350 | 2351 ± 335 |
| Systolic blood pressure (mmHg) | 112.3 ± 11.5 | 117.1 ± 10.0 | 114.3 ± 8.4 |
| Diastolic blood pressure (mmHg) | 69.2 ± 8.6 | 72.7 ± 7.2 | 72.2 ± 5.5 |
| Total cholesterol (mg/dL) | 148.9 ± 25.5 | 157.6 ± 34.3 | 159.1 ± 23.1 |
| Fasting HDL cholesterol (mg/dL) | 39.4 ± 7.4 | 45.6 ± 13.7 | 42.9 ± 6.6 |
| Fasting AST (mg/dL) | 23.1 ± 8.4 | 22.1 ± 5.6 | 24.1 ± 7.6 |
| Fasting ALT (mg/dL) | 23.4 ± 20.1 | 21.5 ± 8.9 | 24.1 ± 15.8 |
a Values are mean ± standard deviation.
Table 2.
Body weight, hepatic lipid content and indices of insulin sensitivity (mean ± SEM) at baseline and intervention
| Outcome | Aspartame (n = 23) | HFCS (n = 28) | Sucrose (n = 24) | Testing effect of: | P value |
|---|---|---|---|---|---|
| Body weight (kg) 1 | SB group* | 0.080 | |||
| Baseline | 71.8 ± 2.2 | 72.9 ± 2.7 | 71.9 ± 2.5 | — | — |
| Intervention | 71.7 ± 2.2 | 73.7 ± 2.8 | 72.4 ± 2.6 | — | — |
| Hepatic lipid (MRI-PDFF, %) 2 | SB group | 0.020 | |||
| Baseline | 1.6 ± 0.8 | 2.3 ± 0.8 | 1.9 ± 0.4 | SB group w/ΔBWa | 0.027 |
| Intervention | 1.4 ± 0.7 | 2.8 ± 0.9 | 2.6 ± 0.5 | ΔBWb | 0.041 |
| Matsuda ISI (arbitrary units) 3 | SB group | 0.0022 | |||
| Baseline | 3.6 ± 0.3 | 3.3 ± 0.2 | 3.6 ± 0.3 | SB group w/ΔBW | 0.0011 |
| Intervention | 3.9 ± 0.3 | 3.0 ± 0.3 | 3.1 ± 0.3 | ΔBW | 0.19 |
| Predicted M ISI (arbitrary units) 4 | SB group | 0.045 | |||
| Baseline | 1.5 ± 0.14 | 1.5 ± 0.16 | 1.5 ± 0.18 | — | — |
| Intervention | 1.6 ± 0.055 | 1.5 ± 0.065 | 1.4 ± 0.058 | — | — |
| OGTT glucose AUC (mg/dL × 3 hours) 5 | SB group | 0.0063 | |||
| Baseline | 27893 ± 926 | 27719 ± 755 | 27572 ± 834 | SB group w/ΔBW | 0.0048 |
| Intervention | 26788 ± 810 | 29246 ± 1137 | 28605 ± 661 | ΔBW | 0.42 |
| OGTT insulin AUC (µU/mL × 3 hours) 6 | SB group | 0.0003 | |||
| Baseline | 15943 ± 2205 | 14631 ± 1156 | 15541 ± 2359 | SB group w/ΔBW | 0.0003 |
| Intervention | 13882 ± 1666 | 17916 ± 1761 | 17311 ± 1757 | ΔBW | 0.53 |
| FST glucose (mg/dL)7 | SB group | 0.38 | |||
| Baseline | 91.1 ± 1.4 | 90.5 ± 1.3 | 93.4 ± 1.2 | SB group w/ΔBW | 0.32 |
| Intervention | 88.6 ± 1.4 | 89.3 ± 1.2 | 92.0 ± 1.1 | ΔBW | 0.36 |
| FST insulin (µU/mL) 8 | SB group | 0.40 | |||
| baseline | 13.2 ± 1.0 | 13.2 ± 0.9 | 13.3 ± 1.0 | SB group w/ΔBW | 0.33 |
| Intervention | 13.1 ± 1.1 | 14.5 ± 1.3 | 14.4 ± 1.1 | ΔBW | 0.45 |
| HOMA-IR (arbitrary units)9 | SB group | 0.48 | |||
| Baseline | 3.0 ± 0.2 | 3.0 ± 0.2 | 3.1 ± 0.2 | SB group w/ΔBW | 0.41 |
| Intervention | 2.9 ± 0.2 | 3.2 ± 0.3 | 3.3 ± 0.3 | ΔBW | 0.46 |
*Effect of SB group on the absolute change of outcome in the primary 2-factor (SB group, sex) ANCOVA model that included adjustment for BMI1,2,4,7,8,9, outcome at baseline3,5,6,7,9, and/or SB group × sex6,8,9; log-transformed values used 2,6,9; aeffect of SB group in the ANCOVA that included adjustment for the ΔBW; beffect of ΔBW in the secondary ANCOVA; Δ, absolute change; ISI, insulin sensitivity index; FST, fasting.
Body Weight
While subjects consuming HFCS-SB gained body weight during the 2-week intervention (0.8 ± 0.3 kg; P = 0.0015 vs baseline, LS mean of Δ different from 0), the changes in body weight (sucrose-SB: 0.5 ± 0.3 kg, P = 0.079; aspartame-SB: –0.05 ± 0.2 kg, P = 0.90 vs baseline) were not significantly different (P = 0.080, effect of SB group). Males, however, gained more body weight (0.8 ± 0.2 kg, P = 0.0004 vs baseline) than females (0.1 ± 0.2 kg, P = 0.017, effect of sex).
Hepatic Lipid Content (MRI-PDFF)
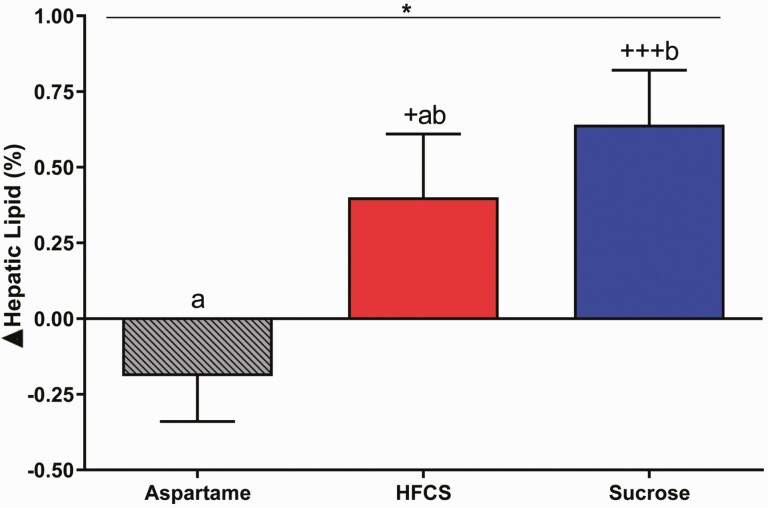
As shown in Table 2, the absolute change in %hepatic lipid (MRI-PDFF) was significantly affected by SB group (P = 0.020). Subjects consuming sucrose-SB had increased hepatic lipid compared with subjects consuming aspartame-SB (P = 0.016, Tukey’s multiple-comparisons test), and consumption of both sucrose- (P = 0.0008) and HFCS-SB (P = 0.041) increased hepatic lipid content compared with levels at baseline (Fig. 2).
Figure 2.
Changes of hepatic lipid content: the mean ± SEM of the absolute change (intervention—baseline) of hepatic lipid content in subjects consuming aspartame- (n = 20), HFCS- (n = 23), or sucrose-sweetened beverages (n = 23) for 2 weeks. *P < 0.05, effect of SB group, 2-factor (SB group, sex) ANCOVA with adjustment for BMI. +P < 0.05, +++P < 0.001, LS mean different from 0; a different from b, Tukey’s.
Insulin Sensitivity
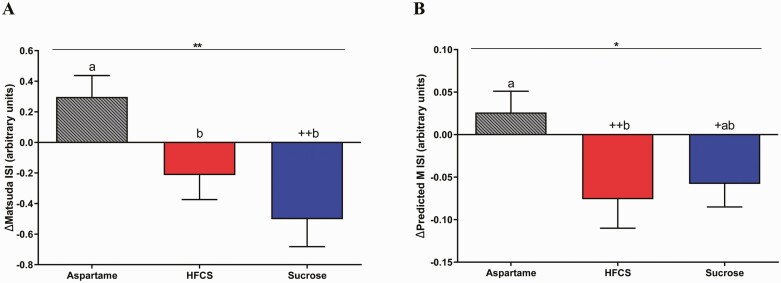
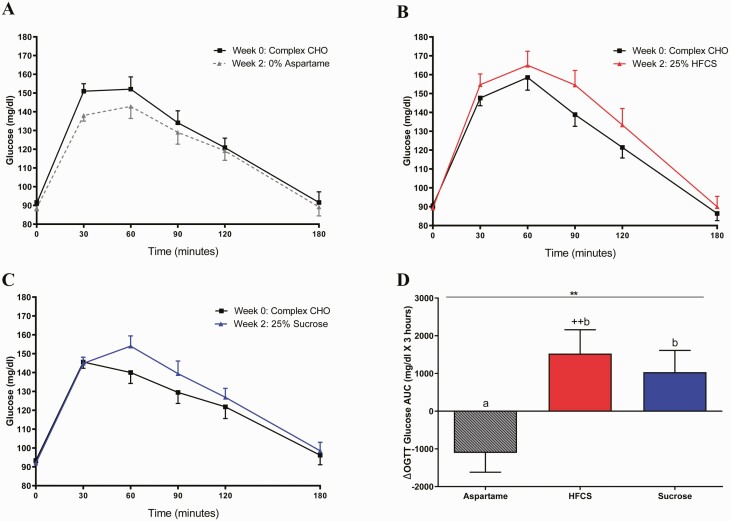
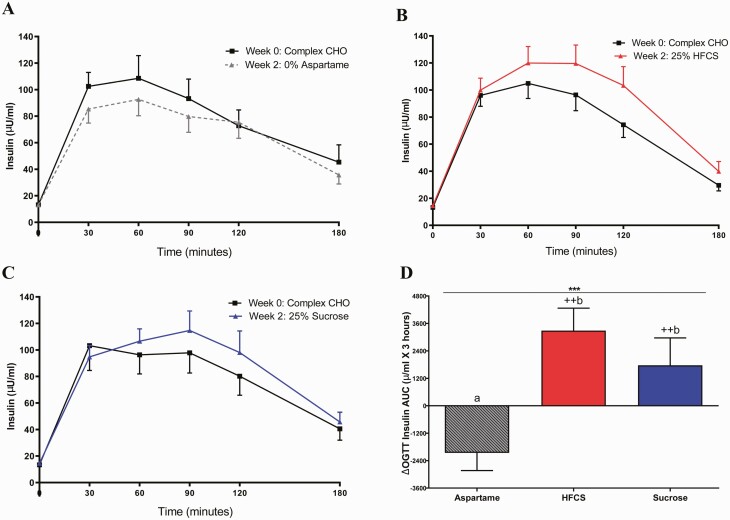
The SB group significantly affected insulin sensitivity, as assessed by the Matsuda ISI (P = 0.0022) and the Predicted M ISI (P = 0.045) (Table 2). Consumption of both HFCS- and sucrose-SB decreased Matsuda ISI compared with aspartame-SB (HFCS: P = 0.029; sucrose: P = 0.0020), but only subjects consuming sucrose-SB exhibited decreases compared with baseline (HFCS: P = 0.091; sucrose: P = 0.0031) (Fig. 3A). Predicted M ISI was decreased by HFCS-SB compared with aspartame-SB (HFCS: P = 0.049; sucrose: P = 0.12) and decreased by both sugars compared with baseline (HFCS: P = 0.009; sucrose: P = 0.047) (Fig. 3B). Compared with aspartame-SB, both HFCS- and sucrose-SB increased the 3-hour glucose (Fig. 4A-D HFCS: P = 0.0063; sucrose: P = 0.043) and insulin AUC (Fig. 5A-5D HFCS: P = 0.0014; sucrose: P = 0.0011). In contrast, the consumption of HFCS- and sucrose-SB for 2 weeks did not significantly affect fasting glucose and insulin concentrations, nor HOMA-IR (Table 2).
Figure 3.
Changes of Matsuda ISI and Predicted M ISI: the mean ± SEM of the absolute changes (intervention—baseline) of Matsuda (A) and Predicted M (B) ISI in subjects consuming HFCS-, sucrose, or aspartame-sweetened beverages for 2 weeks. *P < 0.05, **P < 0.01, effect of SB group, 2-factor (SB group, sex) ANCOVA with adjustment for outcome at baseline or BMI. +P < 0.05, ++P < 0.01, LS mean different from 0; a different from b, Tukey’s. Predicted M ISI: aspartame- (n = 22), HFCS- (n = 27), or sucrose-sweetened beverages (n = 23).
Figure 4.
Plasma glucose excursions during OGTT: glucose concentrations during OGTT at baseline and after consuming aspartame- (n = 23) (A), HFCS- (n = 28) (B), or sucrose-sweetened beverages (n = 24) (C) for 2 weeks (intervention). Δ 3-hour Glucose AUC (intervention—baseline) during OGTT in subjects consuming either aspartame, HFCS-, or sucrose- sweetened beverages for 2 weeks (D). **P < 0.01, effect of SB group, 2-factor (SB group, sex) ANCOVA with adjustment for outcome at baseline. ++P < 0.01, LS mean different from 0; a different from b, Tukey’s.
Figure 5.
Plasma insulin excursions during OGTT: insulin concentrations during OGTT at baseline and after consuming aspartame- (n = 23) (A), HFCS- (n = 28) (B), or sucrose-sweetened beverages (n = 24) (C) for 2 weeks. Δ3-hour insulin AUC (intervention—baseline) during OGTT in subjects consuming aspartame, HFCS-, or sucrose-SB for 2 weeks (D). ***P < 0.001, effect of SB group, 2-factor (SB group, sex) ANCOVA with adjustment for sugar*sex. ++P < 0.01, LS mean different from 0; a different from b, Tukey’s.
Circulating Lipid and Lipoprotein
Table 3 presents the absolute values of plasma lipid, lipoprotein, and uric acid concentrations at baseline and intervention using the same format as Table 2. The data from subjects consuming sucrose-SB have not been previously reported, however the data from the HFCS- and aspartame-SB groups were included in a publication reporting the dose response effects of consuming 0, 10, 17.5, and 25% Ereq as HFCS-SB (36), and in a more recent publication comparing consumption of HFCS-SB with glucose- and fructose-SB (38).
Table 3.
Circulating lipid, lipoproteins and uric acid concentrations (Mean ± SEM) at baseline and intervention
| Outcome | Aspartame (n = 23) | HFCS (n = 28) | Sucrose (n = 24) | Testing effect of: | P value |
|---|---|---|---|---|---|
| FST TG (mg/dL) 1 | SB group* | 0.045 | |||
| Baseline | 101 ± 111 | 108 ± 9 | 114 ± 10 | SB group w/ΔBWa | 0.11 |
| Intervention | 98 ± 10 | 119 ± 10 | 132 ± 13 | ΔBWb | 0.13 |
| 24-hour TG AUC (mg/dL × 24 hours)2 | SB group | 0.0020 | |||
| Baseline | 2605 ± 331 | 2847 ± 246 | 3125 ± 314 | SB group w/ΔBW | 0.0039 |
| Intervention | 2486 ± 287 | 3170 ± 284 | 3599 ± 384 | ΔBW | 0.53 |
| PP TG (mg/dL) 3 | SB group | <0.0001 | |||
| Baseline | 94 ± 14 | 108 ± 11 | 115 ± 13 | SB group w/ΔBW | <0.0001 |
| Intervention | 94 ± 13 | 145 ± 14 | 170 ± 19 | ΔBW | 0.88 |
| FST apoCIII (mg/dL) 4 | SB group | 0.0006 | |||
| Baseline | 7.3 ± 0.5 | 8.2 ± 0.5 | 7.1 ± 0.4 | SB group w/ΔBW | 0.0012 |
| Intervention | 7.3 ± 0.5 | 8.8 ± 0.5 | 8.2 ± 0.5 | ΔBW | 0.0042 |
| PP apoCIII (mg/dL) 5 | SB group | <0.0001 | |||
| Baseline | 6.7 ± 0.6 | 7.4 ± 0.5 | 6.7 ± 0.4 | SB group w/ΔBW | <0.0001 |
| Intervention | 6.5 ± 0.5 | 8.5 0.5 | 8.4 ± 0.6 | ΔBW | 0.14 |
| FST LDL-C (mg/dL) 6 | SB group | <0.0001 | |||
| Baseline | 84 ± 5 | 91 ± 5 | 95 ± 5 | SB group w/ΔBW | 0.0003 |
| Intervention | 83 ± 5 | 107 ± 6 | 106 ± 5 | ΔBW | 0.17 |
| PP LDL-C (mg/dL) 7 | SB group | <0.0001 | |||
| Baseline | 80 ± 5 | 86 ± 5 | 93 ± 4 | SB group w/ΔBW | 0.001 |
| Intervention | 81 ± 4 | 105 ± 6 | 106 ± 4 | ΔBW | 0.51 |
| FST non-HDL cholesterol (mg/dL) 8 | SB group | 0.0002 | |||
| Baseline | 110 ± 5 | 112 ± 6 | 116 ± 5 | SB group w/ΔBW | 0.0007 |
| Intervention | 107 ± 5 | 128 ± 6 | 128 ± 6 | ΔBW | 0.18 |
| PP non-HDL-C (mg/dL) 9 | SB group | <0.0001 | |||
| Baseline | 101 ± 5 | 103 ± 5 | 111 ± 5 | SB group w/ΔBW | <0.0001 |
| Intervention | 99 ± 5 | 124 ± 6 | 128 ± 6 | ΔBW | 0.27 |
| FST apoB (mg/dL) 10 | SB group | 0.0010 | |||
| Baseline | 64.8 ± 3.6 | 69.6 ± 3.5 | 73.6 ± 3.3 | SB group w/ΔBW | 0.0048 |
| Intervention | 65.1 ± 3.0 | 80.0 ± 4.1 | 79.3 ± 3.6 | ΔBW | 0.10 |
| PP apoB (mg/dL) 11 | SB group | <0.0001 | |||
| Baseline | 62.0 ± 3.6 | 65.3 ± 3.4 | 71.4 ± 3.0 | SB group w/ΔBW | 0.0002 |
| Intervention | 61.3 ± 3.0 | 77.4 ± 4.2 | 78.3 ± 3.2 | ΔBW | 0.27 |
| FST uric acid (mg/dL) 12 | SB group | <0.0001 | |||
| Baseline | 4.6 ± 0.2 | 4.5 ± 0.2 | 4.6 ± 0.2 | SB group w/ΔBW | 0.0004 |
| Intervention | 4.5 ± 0.2 | 5.0 ± 0.2 | 4.9 ± 0.2 | ΔBW | 0.19 |
| 24-hour uric acid AUC (mg/dL × 24 hours)13 | SB group | <0.0001 | |||
| Baseline | 104.2 ± 5.0 | 102.3 ± 5.1 | 106.6 ± 4.1 | SB group w/ΔBW | <0.0001 |
| Intervention | 101.0 ± 4.6 | 116.4 ± 5.8 | 115.4 ± 4.6 | ΔBW | 0.16 |
*Effect of SB group on the absolute change of outcome in the primary 2-factor (SB group, sex) ANCOVA that included adjustment for BMI3,5-11, outcome at baseline2,3,5-13, and/or SB group × sex4,6-13; log-transformed values used 1-3,5,10,11; aeffect of SB group in the secondary ANCOVA that also included adjustment for the ΔBW; beffect of ΔBW in the secondary ANCOVA; Δ, absolute change; AUC, area under the curve; FST, fasting; PP, postprandial.
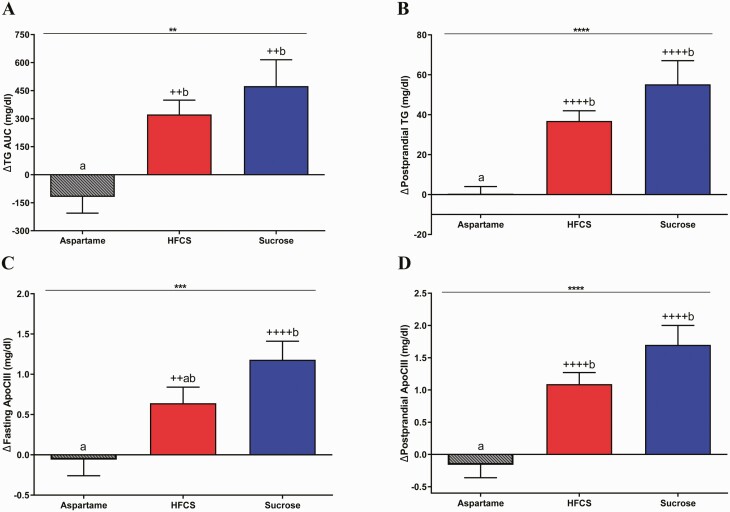
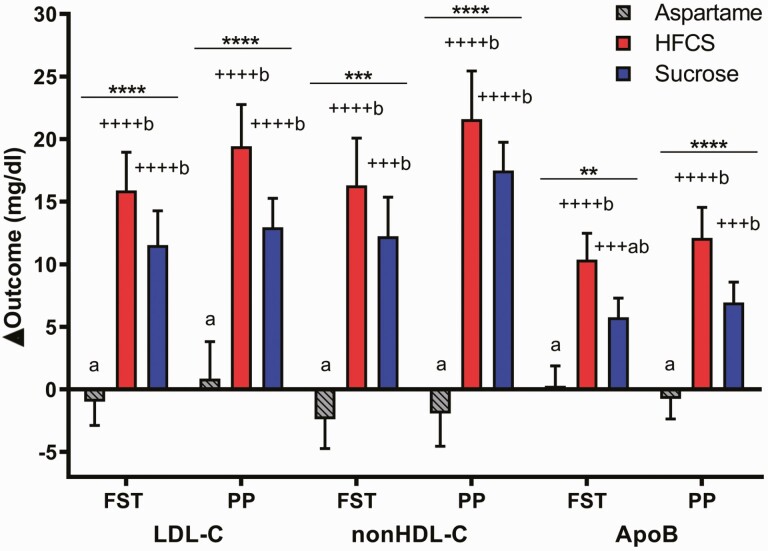
Sucrose- and HFCS-SB significantly increased fasting TG compared with baseline (sucrose: 18.6 ± 5.9 mg/dL, P = 0.011; HFCS: 12.7 ± 4.4 mg/dL, P = 0.009), but not compared with aspartame-SB (0.5 ± 3.7 mg/dL, P = 0.07 vs both sucrose and HFCS-SB). In contrast, both sucrose- and HFCS-SB increased 24-hour TG AUC (sucrose: P = 0.0028; HFCS: P = 0.011) and postprandial TG concentrations (sucrose and HFCS: P < 0.0001) compared with aspartame-SB (Fig. 6A and 6B). Sucrose-SB significantly increased fasting apoCIII (Sucrose: P = 0.0003; HFCS: P = 0.065 vs aspartame-SB), and both sucrose- (P < 0.0001) and HFCS-SB (P = 0.0006) increased postprandial apoCIII compared with aspartame (Fig. 6C and 6D). As presented in Fig. 7, fasting and postprandial LDL-C, non-HDL-C, and postprandial apoB concentrations were all significantly increased in subjects consuming HFCS- and sucrose-SB compared with aspartame (all P < 0.01) and compared with baseline concentrations (all P < 0.001). Fasting apoB was significantly increased by HFCS-SB compared with aspartame-SB (HFCS: P = 0.0006; Sucrose: P = 0.056) and increased by both HFCS- (P < 0.0001) and sucrose-SB compared with baseline (P = 0.0008).
Figure 6.
Changes of plasma TG and apoCIII concentrations: the mean ± SEM of the absolute change (intervention – baseline) of 24-hour TG AUC (5A), postprandial TG (5B), fasting (5C) and postprandial (5D) apoCIII concentrations in subjects consuming aspartame- (n = 23) (A), HFCS- (n = 28) (B), or sucrose-sweetened beverages (n = 24) (C) for 2 weeks, **P < 0.01, ***P < 0.001, ****P < 0.0001, effect of SB group, 2-factor (SB group, sex) ANCOVA with adjustment for outcome at baseline, BMI and/or SB group × sex. ++P < 0.01, ++++P < 0.0001, LS mean different from 0; a different from b, Tukey’s.
Figure 7.
Change of plasma LDL-C, non-HDL-C, and apoB concentrations: the mean ± SEM of the absolute change (intervention – baseline) in fasting (FST) and postprandial (PP) LDL-C, non-HDL-C, and apoB concentrations in subjects consuming aspartame- (n = 23), HFCS- (n = 28), or sucrose-sweetened beverages (n = 24) for 2 weeks. **P < 0.01, ***P < 0.001, ****P < 0.0001, effect of SB group, 2-factor (SB group, sex) ANCOVA with adjustment for outcome at baseline, BMI and SB group × sex. +++P < 0.001, ++++P < 0.0001, LS mean different from 0; a different from b, Tukey’s; FST, fasting; PP, postprandial.
Uric Acid
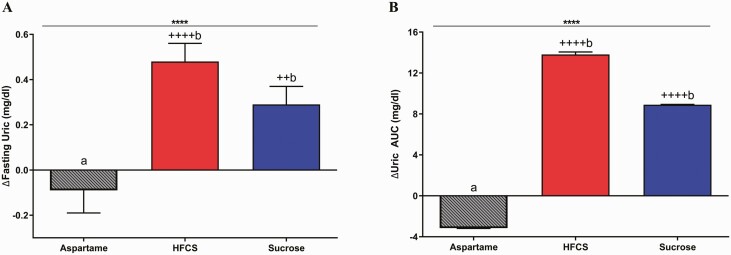
As shown in Fig. 8, both fasting and total 24-hour uric acid AUC significantly increased in subjects consuming HFCS- (fasting and AUC: P < 0.0001) and sucrose-SB (fasting: P = 0.0083; AUC: P < 0.0001) compared with subjects consuming aspartame-SB.
Figure 8.
Change in circulating uric acid concentrations: the mean ± SEM of the absolute change (intervention—baseline) of fasting uric acid (7A) and 24-hour uric acid AUC (7B) in subjects consuming aspartame- (n = 23), HFCS- (n = 28), or sucrose-sweetened beverages (n = 24) for 2 weeks. ****P < 0.0001, effect of SB group, 2-factor (SB group, sex) ANCOVA with adjustment for outcome at baseline, BMI and SB group × sex. ++P < 0.01, ++++P < 0.0001, LS mean different from 0; a different from b, Tukey’s.
Sucrose- vs HFCS-SB
There were no significant effects of sucrose- compared with HFCS-SB on any of the outcomes. The most differential effects were observed for postprandial apoCIII, which tended to be more increased in subjects consuming sucrose-SB (P = 0.13, sucrose- vs HFCS-SB, Tukey’s); and for 24-hour uric acid AUC, which tended to be more increased in subjects consuming HFCS-SB (P = 0.17, sucrose- vs HFCS-SB, Tukey’s). P values for the effects of sucrose- vs HFCS-SB on the changes of indices of insulin sensitivity (Matsuda ISI, Predicted M ISI, glucose OGTT AUC, insulin OGTT AUC) were all >0.9 (Tukey’s).
Secondary ANCOVAs With Adjustment for Body Weight
All outcomes that showed significant effects of SB group retained significance when the statistical models were adjusted for Δbody weight (Table 2). The Δbody weight was a significant contributor to the change of hepatic lipid content (P = 0.041) and fasting apoCIII (P = 0.0042). In simple regression analyses, Δbody weight was positively associated with both outcomes (hepatic lipid R2 = 0.10, P = 0.009; fasting apoCIII: R2 = 0.13, P = 0.002.) All other outcomes were not affected by Δbody weight.
Effect of Sex
In response to sucrose- or HFCS-SB consumption, males tended to have larger increases in the lipid and lipoprotein outcomes than females, and the sex differences were significant for TG (fasting, AUC, and late-night postprandial), non-HDL-C (fasting and postprandial), and postprandial LDL-C. The sex differences remained significant for TG (fasting, AUC, and late-night postprandial) and postprandial nonHDL-C in the secondary ANCOVA that included adjustment for Δbody weight.
Sex did not significantly affect the changes in hepatic lipid content in the primary ANCOVA model that included adjustment for BMI at baseline (Table 4). However, in a secondary ANCOVA, in which adjustment for BMI was replaced with adjustment for %body fat at baseline, both %body fat and male sex were significant positive contributors to the changes of hepatic lipid content. ANCOVA 3 confirms that adjustment with %body fat is required to show a significant effect of sex and ANCOVA 4 demonstrates that the reverse is true; adjustment of sex is required to show a significant effect of %body fat (Table 4). Substituting BMI with %body fat in secondary ANCOVAs of the changes of postprandial apoCIII, TG, and 24-hour TG AUC also revealed significant or more significant effects of sex (Table 5).
Table 4.
The effect of sex on hepatic lipid content with and without adjustment for BMI or % body fat (%BF)
| Primary ANCOVA | P value | Secondary ANCOVA | P value | ANCOVA 3 | P-value | ANCOVA 4 | P-value |
|---|---|---|---|---|---|---|---|
| BMI | 0.10 | %BF | 0.0497 | — | — | %BF | 0.58 |
| Sex | 0.32 | Sex | 0.044 | Sex | 0.47 | — | — |
| SB group | 0.02 | SB group | 0.027 | SB group | 0.013 | SB group | 0.014 |
Table 5.
The effect of sex on postprandial apolipoprotein CIII (apoCIII) and triglyceride (TG) with adjustment for BMI or % body fat (%BF)
| Outcome | Postprandial apoCIII | Postprandial TG | 24-hour TG AUC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Primary ANCOVA | BMI | Sex | SB group | BMI | Sex | SB group | BMI | Sex | SB group |
| P value | 0.19 | 0.27 | <0.0001 | 0.22 | 0.030 | <0.0001 | 0.66 | 0.015 | 0.002 |
| Secondary ANCOVA | %BF | Sex | SB group | %BF | Sex | SB group | %BF | Sex | SB group |
| P value | 0.038 | 0.028 | <0.0001 | 0.021 | 0.003 | <0.0001 | 0.085 | 0.005 | 0.002 |
Discussion
These findings demonstrate that compared with aspartame-SB, the consumption of HFCS- or sucrose-SB at 25% Ereq for 2 weeks increased hepatic lipid content and decreased insulin sensitivity in healthy young adults, but there were no differences between effects of the 2 different sugars.
During the outpatient intervention period, the current study utilized an ad libitum feeding protocol in which subjects were required to consume the experimental beverages, but were free to consume as much or as little of their usual diet as they wished. This is in contrast to hypercaloric feeding protocols in which subjects are required to consume experimental beverages or sugar supplements in addition to diets containing their usual caloric requirement. The majority of the studies reporting fructose, sucrose, or candy and soda consumption increased liver fat utilized these types of hypercaloric feeding protocols (44-47). Therefore, a 2014 meta-analysis reporting on the effects of sugar consumption on liver fat concluded that results were confounded by excess energy intake, and there was insufficient evidence to draw a conclusion for the effects of HFCS or sucrose on NAFLD (48).
However, during a 6-month intervention trial, overweight adults consuming 1 liter/day of sucrose-SB had a greater increase in liver fat compared with control groups consuming aspartame-SB, semi-skim milk or water even though the increases of body weight were comparable in the groups consuming sucrose-SB (+1.25 kg) and milk (+1.29 kg) (49). For the current study, while the effects of SB group on body weight were not significant, subjects consuming HFCS-SB gained weight compared with baseline. Secondary analyses that included adjustment for change of body weight showed that this increase of body weight was a significant contributor to the changes in liver fat, while not eliminating the significant effect of sugar and improving the R2 of the statistical model from 0.19 to 0.27. This suggests that both body weight gain and consumption of sucrose- and/or HFCS-SB are independent risk factors for NAFLD. The independent role of sucrose-SB and HFCS-SB will need to be confirmed in additional dietary intervention studies in which sucrose- and/or HFCS-SB are consumed with eucaloric diets that prevent body weight gain. Importantly, in an 8-day eucaloric crossover study, healthy young adult males exhibited increased DNL, reduced fat oxidation, and increased liver fat while consuming 25% Ereq as fructose-SB compared with isocaloric complex carbohydrate despite the absence of body weight change (15). Also, 4 dietary intervention studies revealed marked decreases in liver fat following restriction of sugar-SB (50), fructose (51, 52), or added sugar (53) consumption for periods ranging from 9 days to 12 weeks. In 2 of these studies, the decreases in liver fat were accompanied by significant decreases in body weight (52, 53), and in the other 2 studies, they were not (50, 51).
Our findings that the consumption of HFCS- and sucrose-SB decreased the Matsuda ISI and Predicted M ISI are novel. An extensive meta-analysis revealed no effects of fructose consumption on fasting glucose, fasting insulin, or HOMA-IR and our results agree with this (54). Indeed, our discordant findings for HOMA-IR vs the Matsuda ISI and Predicted M ISI support the conclusion of Shaibi and colleagues (55), and our own recent results (56), that HOMA-IR lacks the sensitivity to detect changes of whole-body insulin sensitivity that are detected by more sophisticated methods employing OGTTs and frequently sampled intravenous glucose tolerance tests. The meta-analysis (54) also concluded that fructose consumption does not affect peripheral or muscle insulin sensitivity index based on 3 studies (14, 15, 57) that assessed whole body glucose disposal under euglycemic hyperinsulinemic clamp conditions. The reductions of insulin sensitivity that we observed, based on both the Matsuda ISI and Predicted M ISI, are not in agreement with this conclusion. Both Matsuda ISI (41) and Predicted M ISI (42) are indices derived by modeling OGTT results on clamp results generated in the same subjects, and both methods have been shown to be well correlated with glucose disposal during clamps in follow-up studies (58-60). Additionally, the recently developed Predicted M ISI was modeled to predict the clamp-derived M value and thus allow comparisons between studies conducting clamps and those conducting OGTT (42).
Older (61-63) and more recent studies (56) have also shown that high sucrose diets increased glucose and insulin response during OGTT. The discrepancy between the results generated during OGTT and those generated during clamp (54) could be due to the type of sugar consumed (fructose vs sucrose) or may be due to the sensitivity, repeatability and reproducibility of euglycemic hyperinsulinemic clamps compared to OGTTs. The euglycemic hyperinsulinemic clamp has long been considered the gold standard for assessing insulin sensitivity, however it has also been described as labor-intensive, operator dependent, and nonphysiological (64). With regard to the latter, the oral route of glucose delivery during OGTT is more physiological than the intravenous glucose infusion utilized during euglycemic hyperinsulinemic clamps. Sustained sugar-SB consumption could mediate changes in incretin responses or gastric emptying that affect insulin sensitivity and secretion, and these effects would not be apparent during the intravenous glucose infusion. More studies testing the effects of sugar consumption utilizing both OGTT and clamps within the same subjects would be of value.
While we have previously published the results showing that consumption of HFCS-SB at 25% Ereq increases fasting and postprandial plasma concentrations of TG, lipoproteins, and uric acid compared with aspartame-SB, these results are included in this report to allow direct comparisons with sucrose-SB (36). The effects of sucrose-SB on these outcomes, and on all of the other reported outcomes, did not differ from the effects of HFCS-SB. However, compared with aspartame-SB, sucrose-SB significantly increased postprandial TG, fasting and postprandial apoCIII, LDL-C, nonHDL-C, uric acid, and postprandial apoB. These results support previous dietary intervention studies that showed increases in TG, lipoproteins and uric acid in subjects consuming sucrose (14, 49, 56, 65).
Our observation that males had or tended to have higher increases in lipid and lipoprotein risk factors than females in response to sugar-SB consumption is in agreement with the findings of previous studies (20, 36, 65). Both male sex and %body fat at baseline contributed to the increases in hepatic lipid content and postprandial apoCIII. Importantly, adjusting the statistical model for BMI rather than %body fat attenuated the significant contributions of sex on these outcomes. Since females have higher %body fat than males of comparable BMI, adjustment for BMI did not separate the larger increases of hepatic fat that occurred in females due to higher body fat from the larger increases that occurred in males due to sex. The common practice of relying on BMI to adjust for body adiposity status may obscure significant effects of male sex and %body fat on outcomes that are sensitive to these variables.
The changes we report in hepatic lipid content and insulin sensitivity, as well as the changes of fasting and postprandial lipids, lipoproteins and uric acid, all support the conclusion that consumption of both HFCS- and sucrose-SB increase risk factors for cardiometabolic disease compared with aspartame-SB, and that the effects of the 2 sugars are comparable. A study funded by the Corn Refiners Association also detected no differences between the effects of HFCS- or sucrose-SB on all of the reported outcomes (34, 35). However, contrary to our findings, this study also detected no within-group changes in hepatic fat content, 24-hour TG and uric acid AUC, or fasting LDL-C concentrations in subjects who consumed 30% Ereq as either HFCS- and sucrose-sweetened milk for 10 weeks (34, 35). Explanations for these discordant results have been previously reviewed (23). Also, the industry-funded study, which utilized computed tomography imaging rather than MRI (39, 66), may have lacked the sensitivity and the power to detect changes of hepatic lipid content. The effect size for the significant changes in hepatic lipid content between the subjects consuming sucrose-SB (n = 23) and those consuming aspartame-SB (n = 20) in the current study was 0.94. To detect a 0.94 effect size in a 6-group comparison requires n ≥ 31 participants per group. The number of subjects in the 6 groups assessed for hepatic lipid content in the industry-funded study ranged from 8 to 13 per group (34).
Study Strengths
A strength of this study was the use of an advanced MRI techniques, validated against magnetic resonance spectroscopy and considered a surrogate for liver biopsy, for the quantification of hepatic lipid content (67). MRI for assessment of the hepatic fat fraction has been demonstrated to be superior to ultrasound and computed tomography (39, 66). Another strength of the study was the use of a biomarker in the beverages to assess compliance in comparison to the use of self-reported checklists or the return of empty beverage containers (34). Also, the 3.5-day inpatient baseline and intervention testing periods with standardized diets and activity monitoring minimized variation inherent in data collected under outpatient conditions.
Study Limitations
The 25% Ereq dose of sugar utilized for this study does not represent average added sugar intake for the United States population. However, the prevalence of excessive sugar-SB consumption (≥500 kcals/day or ≥25% of Ereq for a typical 2000 kcal diet) was reported to be 5% in children, 16% in adolescents, and 12% in adults in the United States (68). Furthermore, individuals from racial minorities and low-socioeconomic status, who are more susceptible to the development of metabolic disease, are more likely to consume excess sugar-SB than nonminority or higher socioeconomic groups (69). Understanding the health effects of sugar-SB at high levels of consumption is particularly important for populations experiencing health disparities. A potential study limitation is that the 2-week intervention was relatively short. However, it illustrates how quickly excess sugar consumption can lead to metabolic dysregulation in young adults who are normal weight, overweight, or obese. During the 12-day outpatient period, the subjects consumed their usual ad libitum diets, thus the exact level of sugar consumed by each subject during this period is unknown.
Finally, it is important to note that the beverages consumed by the participants in this study were prepared by study staff, and the HFCS utilized contained 55% fructose. While it is assumed that commercially available sugar-SB is made with HFCS that also contains 55% fructose, an analysis of 23 HFCS-containing sodas, purchased from the grocery store, showed that the fructose constituted an average of 59% of the sugar content with a range of 47% to 65% (70, 71). Therefore, a study comparing the metabolic effects of commercially available HFCS-SB with sucrose-SB could yield different results and would be warranted.
Conclusion
In conclusion, the results of this study demonstrate that consumption of either HFCS- or sucrose-SB provided at 25% Ereq for 2 weeks increased hepatic lipid content, decreased insulin sensitivity, and increased circulating lipids, lipoproteins and uric acid concentrations compared with aspartame-SB in young adults. While these results do not indicate that consuming 25% Ereq as HFCS- and sucrose-SB for 2 weeks causes clinically relevant increases in disease risk, they are indicative of the pattern of early phase metabolic dysfunction that underlies the epidemics of metabolic syndrome, CVD, T2D, and NAFLD (18). These results, in which both the consumption of HFCS- and sucrose-SB significantly increased risk factors for NAFLD, T2D, and CVD when compared with aspartame-SB consumption, do not support the data from some studies in rodent models and humans suggesting that consumption of HFCS results in greater metabolic dysregulation than sucrose. These results are important for shaping public health policy and consumer choices, in part because it has been reported that many consumers believe that HFCS and aspartame are more detrimental to human health than sucrose (72).
Acknowledgments
We thank James Graham (UC Davis) for excellent technical support and our undergraduate student interns and employees (UC Davis) for their help with meal and sample processing. We also thank the nursing staff at the CCRC for their committed support.
Financial Support: This study was supported by the NIH/National Heart, Lung and Blood Institute 1R01 HL091333 and 1R01 HL107256; National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research UL1 RR024146; a Building Interdisciplinary Research Careers in Women’s Health award (K12 HD051958), funded by the National Institute of Child Health and Human Development, Office of Research on Women’s Health, Office of Dietary Supplements, and the National Institute of Aging (to K.L.S); and intramural USDA Agricultural Research Service project (5306-51530-022-00-D) (to N.L.K). D.M.S., C.A.P. and Y.B. were supported by diversity supplements from the NIH/National Heart, Lung and Blood Institute: R01-HL-121324 and R01-HL-137716. B.H. was supported by a research fellowship from the German Research Foundation (Deutsche Forschungsgemeinschaft) HI 2113/1-1.
Clinical Trial Registration: NCT01103921.
Glossary
Abbreviations
- ALT
alanine aminotransferase
- ANCOVA
analysis of covariance
- apoB
apolipoprotein B
- apoCIII
apolipoprotein CIII
- AST
aspartate aminotransferase
- AUC
area under the curve
- BMI
body mass index
- CCRC
Clinical and Translational Science Center Clinical Research Center
- CVD
cardiovascular disease
- DEXA
dual energy x-ray absorptiometry
- DNL
de novo lipogenesis
- Ereq
energy requirement
- FST
fasting
- HDL-C
high-density lipoprotein cholesterol
- HFCS
high-fructose corn syrup
- ISI
insulin sensitivity index
- LDL-C
low-density lipoprotein cholesterol
- LS
Least squares
- MRI
magnetic resonance imaging
- NAFLD
nonalcoholic fatty liver disease
- OGTT
oral glucose tolerance test
- PDFF
proton density fat fraction
- Predicted M
Predicted mean glucose infusion rate
- PP
postprandial
- ROI
region of interest
- SB
sweetened beverage
- T2D
type 2 diabetes
- TE
echo time
- TG
triglyceride
- TR
repetition time
- UC
University of California
- VLDL
very low-density lipoprotein
Additional Information
Disclosure Statement: V.M. serves in the Advisory board of Alexion Pharmaceuticals. M.I.G. serves as Scientific Advisor to Yumi infant food and receives royalties for Sugarproof published by Penguin Random House. C.S. reports grants from GE, Siemens, Philips, Bayer, Foundation of NIH, Gilead, and Pfizer (grant is to UW-Madison; UCSD is a subcontract to UW-Madison); personal consultation fees from Blade, Boehringer, and Epigenomics; consultation under the auspices of the University to AMRA, BMS, Exact Sciences, GE Digital, IBM-Watson, and Pfizer; lab service agreements from Enanta, Gilead, ICON, Intercept, Nusirt, Shire, Synageva, Takeda; royalties from Wolters Kluwer for educational material outside the submitted work; honoraria to the institution from Medscape for educational material outside the submitted work; ownership of stock options in Livivos; unpaid position in advisory board to Quantix Bio. All other authors have no conflicts of interest to declare.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve subject confidentially. The corresponding authors will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84. [DOI] [PubMed] [Google Scholar]
- 2. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88-98. [DOI] [PubMed] [Google Scholar]
- 3. Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65(8):1096-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40(3):419-430. [DOI] [PubMed] [Google Scholar]
- 5. Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6(1):69-80. [DOI] [PubMed] [Google Scholar]
- 6. Doycheva I, Watt KD, Alkhouri N. Nonalcoholic fatty liver disease in adolescents and young adults: the next frontier in the epidemic. Hepatology. 2017;65(6):2100-2109. [DOI] [PubMed] [Google Scholar]
- 7. Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr. 2013;162(3):496-500 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510(7503):84-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686-690. [DOI] [PubMed] [Google Scholar]
- 10. Ma J, Fox CS, Jacques PF, et al. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J Hepatol. 2015;63(2):462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drouin-Chartier JP, Zheng Y, Li Y, et al. Changes in consumption of sugary beverages and artificially sweetened beverages and subsequent risk of type 2 diabetes: results from three large prospective U.S. cohorts of women and men. Diabetes Care. 2019;42(12):2181-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malik VS, Li Y, Pan A, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139(18):2113-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen H, Wang J, Li Z, et al. Consumption of sugar-sweetened beverages has a dose-dependent effect on the risk of non-alcoholic fatty liver disease: an updated systematic review and dose-response meta-analysis. Int J Environ Res Public Health. 2019;16(12):2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aeberli I, Hochuli M, Gerber PA, et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care. 2013;36(1):150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwarz JM, Noworolski SM, Wen MJ, et al. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J Clin Endocrinol Metab. 2015;100(6):2434-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jensen T, Abdelmalek MF, Sullivan S, et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68(5):1063-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. 2013;57(6):2525-2531. [DOI] [PubMed] [Google Scholar]
- 18. Hannou SA, Haslam DE, McKeown NM, Herman MA. Fructose metabolism and metabolic disease. J Clin Invest. 2018;128(2):545-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schaefer EJ, Gleason JA, Dansinger ML. Dietary fructose and glucose differentially affect lipid and glucose homeostasis. J Nutr. 2009;139(6):1257S-1262S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balakumar M, Raji L, Prabhu D, et al. High-fructose diet is as detrimental as high-fat diet in the induction of insulin resistance and diabetes mediated by hepatic/pancreatic endoplasmic reticulum (ER) stress. Mol Cell Biochem. 2016;423(1-2):93-104. [DOI] [PubMed] [Google Scholar]
- 22. Bremer AA, Stanhope KL, Graham JL, et al. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci. 2011;4(4):243-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stanhope KL. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit Rev Clin Lab Sci. 2016;53(1):52-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin R, Vos MB. Fructose and liver function–is this behind nonalcoholic liver disease? Curr Opin Clin Nutr Metab Care. 2015;18(5):490-495. [DOI] [PubMed] [Google Scholar]
- 25. Softic S, Cohen DE, Kahn CR. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci. 2016;61(5):1282-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537-543. [DOI] [PubMed] [Google Scholar]
- 27. Rosinger A, Herrick K, Gahche J, Park S. Sugar-sweetened beverage consumption among U.S. adults, 2011-2014. NCHS Data Brief. 2017;( 270):1-8. [PubMed] [Google Scholar]
- 28. Rosinger A, Herrick K, Gahche J, Park S. Sugar-sweetened beverage consumption among U.S. youth, 2011-2014. NCHS Data Brief. 2017;( 271):1-8. [PubMed] [Google Scholar]
- 29. Mock K, Lateef S, Benedito VA, Tou JC. High-fructose corn syrup-55 consumption alters hepatic lipid metabolism and promotes triglyceride accumulation. J Nutr Biochem. 2017;39:32-39. [DOI] [PubMed] [Google Scholar]
- 30. Bocarsly ME, Powell ES, Avena NM, Hoebel BG. High-fructose corn syrup causes characteristics of obesity in rats: increased body weight, body fat and triglyceride levels. Pharmacol Biochem Behav. 2010;97(1):101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yuruk AA, Nergiz-Unal R. Maternal dietary free or bound fructose diversely influence developmental programming of lipogenesis. Lipids Health Dis. 2017;16(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goran MI, Ulijaszek SJ, Ventura EE. High fructose corn syrup and diabetes prevalence: a global perspective. Glob Public Health. 2013;8(1):55-64. [DOI] [PubMed] [Google Scholar]
- 33. Le MT, Frye RF, Rivard CJ, et al. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism. 2012;61(5):641-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bravo S, Lowndes J, Sinnett S, Yu Z, Rippe J. Consumption of sucrose and high-fructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Appl Physiol Nutr Metab. 2013;38(6):681-688. [DOI] [PubMed] [Google Scholar]
- 35. Yu Z, Lowndes J, Rippe J. High-fructose corn syrup and sucrose have equivalent effects on energy-regulating hormones at normal human consumption levels. Nutr Res. 2013;33(12):1043-1052. [DOI] [PubMed] [Google Scholar]
- 36. Stanhope KL, Medici V, Bremer AA, et al. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr. 2015;101(6):1144-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241-247. [DOI] [PubMed] [Google Scholar]
- 38. Hieronimus B, Medici V, Bremer AA, et al. Synergistic effects of fructose and glucose on lipoprotein risk factors for cardiovascular disease in young adults. Metabolism. 2020;112:154356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34(4):729-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yokoo T, Shiehmorteza M, Bydder M, et al. Spectrally-modeled hepatic fat quantification by multi-echo gradient-recalled-echo magnetic resonance imaging at 3.0T. Proc Intl Soc Mag Reson Med. 2009;17. [Google Scholar]
- 41. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462-1470. [DOI] [PubMed] [Google Scholar]
- 42. Tura A, Chemello G, Szendroedi J, et al. Prediction of clamp-derived insulin sensitivity from the oral glucose insulin sensitivity index. Diabetologia. 2018;61(5):1135-1141. [DOI] [PubMed] [Google Scholar]
- 43. Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24(3):539-548. [DOI] [PubMed] [Google Scholar]
- 44. Sevastianova K, Santos A, Kotronen A, et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr. 2012;96(4):727-734. [DOI] [PubMed] [Google Scholar]
- 45. Lecoultre V, Egli L, Carrel G, et al. Effects of fructose and glucose overfeeding on hepatic insulin sensitivity and intrahepatic lipids in healthy humans. Obesity (Silver Spring). 2013;21(4):782-785. [DOI] [PubMed] [Google Scholar]
- 46. Ngo Sock ET, Lê KA, Ith M, Kreis R, Boesch C, Tappy L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br J Nutr. 2010;103(7):939-943. [DOI] [PubMed] [Google Scholar]
- 47. Surowska A, Jegatheesan P, Campos V, et al. Effects of dietary protein and fat content on intrahepatocellular and intramyocellular lipids during a 6-day hypercaloric, high sucrose diet: a randomized controlled trial in normal weight healthy subjects. Nutrients. 2019;11(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chung M, Ma J, Patel K, Berger S, Lau J, Lichtenstein AH. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100(3):833-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maersk M, Belza A, Stødkilde-Jørgensen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95(2):283-289. [DOI] [PubMed] [Google Scholar]
- 50. Campos V, Despland C, Brandejsky V, et al. Sugar- and artificially sweetened beverages and intrahepatic fat: a randomized controlled trial. Obesity (Silver Spring). 2015;23(12):2335-2339. [DOI] [PubMed] [Google Scholar]
- 51. Ibarra-Reynoso LDR, López-Lemus HL, Garay-Sevilla ME, Malacara JM. Effect of restriction of foods with high fructose corn syrup content on metabolic indices and fatty liver in obese children. Obes Facts. 2017;10(4):332-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schwarz JM, Noworolski SM, Erkin-Cakmak A, et al. Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology. 2017;153(3):743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schwimmer JB, Ugalde-Nicalo P, Welsh JA, et al. Effect of a low free sugar diet vs usual diet on nonalcoholic fatty liver disease in adolescent boys: a randomized clinical trial. JAMA. 2019;321(3):256-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ter Horst KW, Schene MR, Holman R, Romijn JA, Serlie MJ. Effect of fructose consumption on insulin sensitivity in nondiabetic subjects: a systematic review and meta-analysis of diet-intervention trials. Am J Clin Nutr. 2016;104(6):1562-1576. [DOI] [PubMed] [Google Scholar]
- 55. Shaibi GQ, Davis JN, Weigensberg MJ, Goran MI. Improving insulin resistance in obese youth: choose your measures wisely. Int J Pediatr Obes. 2011;6(2-2):e290-e296. [DOI] [PubMed] [Google Scholar]
- 56. Price CA, Medici V, Nunez MV, et al. A pilot study comparing the effects of consuming 100% orange juice or sucrose-sweetened beverage on risk factors for cardiometabolic disease in women. Nutrients. 2021;13(3):760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Johnston RD, Stephenson MC, Crossland H, et al. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology. 2013;145(5):1016-1025.e2. [DOI] [PubMed] [Google Scholar]
- 58. Rebelos E, Honka MJ. PREDIM index: a useful tool for the application of the euglycemic hyperinsulinemic clamp. J Endocrinol Invest. 2021;44(3):631-634. [DOI] [PubMed] [Google Scholar]
- 59. Yang G, Li C, Gong Y, et al. Assessment of insulin resistance in subjects with normal glucose tolerance, hyperinsulinemia with normal blood glucose tolerance, impaired glucose tolerance, and newly diagnosed type 2 diabetes (prediabetes insulin resistance research). J Diabetes Res. 2016;2016:9270768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Otten J, Ahrén B, Olsson T. Surrogate measures of insulin sensitivity vs the hyperinsulinaemic-euglycaemic clamp: a meta-analysis. Diabetologia. 2015;57:1781-1788. [DOI] [PubMed] [Google Scholar]
- 61. Lewis AS, McCourt HJ, Ennis CN, et al. Comparison of 5% versus 15% sucrose intakes as part of a eucaloric diet in overweight and obese subjects: effects on insulin sensitivity, glucose metabolism, vascular compliance, body composition and lipid profile. A randomised controlled trial. Metabol. 2013;62(5):694-702. [DOI] [PubMed] [Google Scholar]
- 62. Reiser S, Bohn E, Hallfrisch J, Michaelis OE 4th, Keeney M, Prather ES. Serum insulin and glucose in hyperinsulinemic subjects fed three different levels of sucrose. Am J Clin Nutr. 1981;34(11):2348-2358. [DOI] [PubMed] [Google Scholar]
- 63. Reiser S, Handler HB, Gardner LB, Hallfrisch JG, Michaelis OE 4th, Prather ES. Isocaloric exchange of dietary starch and sucrose in humans. II. Effect on fasting blood insulin, glucose, and glucagon and on insulin and glucose response to a sucrose load. Am J Clin Nutr. 1979;32(11):2206-2216. [DOI] [PubMed] [Google Scholar]
- 64. Dube S, Errazuriz I, Cobelli C, Basu R, Basu A. Assessment of insulin action on carbohydrate metabolism: physiological and non-physiological methods. Diabet Med. 2013;30:664-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reiser S, Bickard MC, Hallfrisch J, Michaelis OE 4th, Prather ES. Blood lipids and their distribution in lipoproteins in hyperinsulinemic subjects fed three different levels of sucrose. J Nutr. 1981;111(6):1045-1057. [DOI] [PubMed] [Google Scholar]
- 66. Gu J, Liu S, Du S, et al. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol. 2019;29(7):3564-3573. [DOI] [PubMed] [Google Scholar]
- 67. Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology. 2018;68(2):763-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Han E, Powell LM. Consumption patterns of sugar-sweetened beverages in the United States. J Acad Nutr Diet. 2013;113(1):43-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ramphal L, Zhang J, Suzuki S. Ethnic disparities in the prevalence of the metabolic syndrome in American adults: data from the examination of national health and nutrition examination survey 1999-2010. Proc (Bayl Univ Med Cent). 2014;27(2):92-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ventura EE, Davis JN, Goran MI. Sugar content of popular sweetened beverages based on objective laboratory analysis: focus on fructose content. Obesity (Silver Spring). 2011;19(4):868-874. [DOI] [PubMed] [Google Scholar]
- 71. Walker RW, Dumke KA, Goran MI. Fructose content in popular beverages made with and without high-fructose corn syrup. Nutrition. 2014;30(7-8):928-935. [DOI] [PubMed] [Google Scholar]
- 72. Drewnowski A, Tappy L, Forde CG, et al. Sugars and sweeteners: science, innovations, and consumer guidance for Asia. Asia Pac J Clin Nutr. 2019;28(3):645-663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve subject confidentially. The corresponding authors will on request detail the restrictions and any conditions under which access to some data may be provided.