Abstract
Context
Histone deacetylases (HDACs) and histone acetyltransferases (HAT) have an important role in the regulation of gene transcription as well as in the development and function of CD4+Foxp3+ T regulatory (Treg) cells. Our group and others have reported that patients with autoimmune thyroid disease (AITD) show abnormalities in the levels and function of different Treg cell subsets.
Objective
We aimed to analyze the levels of expression of several HDACs and the Tip60 HAT in the thyroid gland and immune cells from patients with AITD.
Methods
The expression of HDAC1-11 and the Tip60 HAT, at RNA and protein levels, were determined in thyroid tissue from 20 patients with AITD and 10 healthy controls and these findings were correlated with clinical data. HDAC9 and Tip60 levels were also analyzed in thyroid cell cultures, stimulated or not with proinflammatory cytokines, as well as in different cell subsets from peripheral blood mononuclear cells.
Results
Altered expression of different HDACs was observed in thyroid tissue from AITD patients, including a significant increase in HDAC9, at RNA and protein levels. Likewise, HDAC9 expression was increased in peripheral blood mononuclear cells particularly in Treg cells in patients with AITD. In contrast, Tip60 expression was reduced in thyroid gland samples from patients with Hashimoto thyroiditis.
Conclusion
Our results indicate that HDAC expression is dysregulated in thyroid gland and immune cells from patients with AITD, suggesting involvement in the pathogenesis of this condition.
Keywords: histone deacetylases (HDACs), autoimmune thyroid diseases (AITD), Graves’ disease (GD), Hashimoto’s thyroiditis (HT), Graves’ ophthalmopathy (GO)
Autoimmune thyroid diseases (AITD) result from a dysregulation of the immune system, which causes a loss of immune tolerance toward thyroid self-antigens, with a variable degree of inflammation and gland dysfunction. The main phenotypes of AITD are Hashimoto’s thyroiditis (HT) and Graves´ disease (GD). In HT the autoimmune response is accompanied by a heavy inflammatory cell infiltrate, followed by thyroid gland damage and hypothyroidism. In contrast, GD is characterized by gland hyperplasia and hyperthyroidism, induced by the presence of autoantibodies directed against the receptor for thyrotropin (thyroid-stimulating hormone, TSH). In both conditions, other autoantibodies can be detected, mainly those directed against thyroid peroxidase (TPO) and thyroglobulin (1-3).
Despite the high prevalence of AITD (around 5% of the population in industrialized countries), the pathogenesis of this condition has not yet been fully elucidated (4). However, it is evident that genetic and environmental factors as well as epigenetic mechanisms and alterations in immune regulation have an important role in this condition (5). In this regard, we and others have previously described abnormalities in the levels and function of different T regulatory (Treg) cells, including CD4+Foxp3+ Treg lymphocytes (6-10). Moreover, single nucleotide polymorphisms (SNPs) of different genes as well as modifications in DNA methylation and histone acetylation also appear to participate in the pathogenesis of the immune dysregulation observed in AITD. Accordingly, it has been described that the levels of histone acetylation have an important influence in the development and maintenance of immune tolerance to self-antigens as well as in the expression of the transcription factor Foxp3 and the differentiation of a subset of Treg lymphocytes (5, 11).
Histone deacetylases (HDACs) are involved in the removal of acetyl groups from histone lysine residues, inducing chromatin compaction. This effect leads to an epigenetic repression, decreasing the expression of their target genes (12, 13). HDACs are classified into 4 groups: type I (HDACs 1, 2, 3 and 8); type II (HDACs 4, 5, 6, 7, 9 and 10); type III or sirtuins (SIRT1-7), and type IV (HDAC11). They all share a similar sequence and conserved catalytic and noncatalytic domains (14). As expected, the activity of these enzymes has an important effect on different relevant physiological and pathological phenomena, including cell growth and differentiation, intracellular signal transduction, and malignant transformation (15, 16). In this regard, it has been described that the deregulation of HDAC activity can promote the expression of proinflammatory genes and other mediators of immunity, contributing thus to the pathogenesis of chronic inflammatory and autoimmune diseases (17).
Accordingly, HDAC inhibitors may exert anti-inflammatory and immunosuppressive effects that may be helpful in the treatment of autoimmune diseases, such as inflammatory bowel diseases, systemic lupus erythematosus, multiple sclerosis (MS) and rheumatoid arthritis (RA) (17-22).
Histone acetyltransferases (HATs) are enzymes that promote the transfer of acetyl groups to histone lysine residues, resulting in chromatin relaxation and increasing gene transcription. The Tip60 HAT belongs the MYST family of these enzymes, which mainly induce the acetylation of the H2A, H3, and H4 histones (23). As expected, these enzymes counteract the activity of HDACs, also exerting important effects under physiological and pathological conditions. In this regard, it has been described that HATs are able to regulate the stability and transcriptional activity of the FOXP3 gene, regulating thus the differentiation of CD4+Foxp3+ Treg cells and Th17 lymphocytes (24, 25). Accordingly, patients with rheumatoid arthritis show a diminished activity of Tip60, which contributes to a defective expression of Foxp3 and a deficient function of Treg cells (26).
In this study we assessed the level of expression of different HDACs and the HAT Tip60 in thyroid gland samples and immune cells from patients with AITD and controls. Our data indicate that there is an altered regulation of the expression of several HDACs and Tip60 in AITD, suggesting their involvement in the pathogenesis of this autoimmune condition.
Methods
Patients
Fresh-frozen thyroid tissue samples from 20 patients with AITD (10 with HT, and 10 with GD) and 10 control individuals were collected. Peripheral blood mononuclear cells (PBMCs) were obtained from 27 patients with AITD (12 with HT, 15 with GD) and healthy controls (n = 13). Clinical diagnoses were all reviewed by a single experienced endocrinologist; these were based on standard clinical, laboratory, and histological criteria. In all patients, serum free thyroxine (T4), thyroid stimulating hormone (TSH), and levels of antibodies against thyroglobulin (Tg), thyroperoxidase (TPO), and TSH receptor (TSHR) were determined, at the time of thyroid surgery (7). Clinical data are summarized in Tables 1 and 2.
Table 1.
Clinical features of AITD patients from PBMC samples
| PBMCs | HT | GD | Controls |
|---|---|---|---|
| N | 12 | 15 | 13 |
| Gender (F/M) | 11/1 | 12/3 | 9/4 |
| Age, yrears | 39 (28-50) | 50 (35-59) | 31 (27-34) |
| Ophthalmopathy | 0 | 3 | 0 |
| TSH, mU/mL | 9.12 (6.93-16.85) | 0 | 1.95 (1.76-3.11) |
| T4, ng/dL | 1.02 (0.73-1.17) | 2.81 (2.47-3.73) | 1.12 (1.09-1.25) |
| Tg-Ab, UI/mL | 403 (20-1475.25) | 20 (20-505.25) | 20 (20-20) |
| TPO-Ab, UI/mL | 697.5 (338.75-1264.25) | 144.5 (20-417.5) | 20 (20-20) |
| TSHR-Ab, U/L | 1 (0.56-1.5) | 4.44 (2.48-11.85) | - |
Values show number for categorical values and median (interquartile intervals 25%-75%) for continuous variables.
Abbreviations: F/M = female/male; T4, thyroxine (normal range = 0.93-1.7); Tg-Ab, anti-thyroglobulin antibody (negative < 344); TPO-Ab, anti-thyroid peroxidase antibody (negative < 100); TSH, thyrotropin (normal range = 0.27-4.20); TSHR-Ab, anti-thyrotropin receptor antibody (negative < 0.7).
Table 2.
Clinical features of AITD patients from thyroid tissue samples
| Thyroid tissue | HT | GD |
|---|---|---|
| 10 | 10 | |
| Gender (F/M) | 10 | 9/1 |
| Age (yr) | 60 (57-69) | 47 (40-57) |
| Ophthalmopathy | 0 | 7 |
| TSH mU/mL | 2.36 (1, 27-3, 12) | 0.44 (0.01-0.8) |
| T4 ng/dL | N/A | 1.29 (1.02-1.54) |
| Tg-Ab UI/mL | 948(449.5-9330) | 20 (20-2279) |
| TPO-Ab UI/mL | 640.5 (102.75-834.5) | 169(20-578.5) |
| TSHR-Ab U/L | N/A | 5.63 (0.91-7.67) |
Values show number for categorical values and median (interquartile intervals 25-75) for continuous variables.
Abbreviations: F/M = female/male; T4, thyroxine (normal range = 0.93-1.7); Tg-Ab, anti-thyroglobulin antibody (negative < 344); TPO-Ab, anti-thyroid peroxidase antibody (negative < 100); TSH, thyrotropin (normal range = 0.27-4.20); TSHR-Ab, anti-thyrotropin receptor antibody (negative < 0.7).
This study was approved by the Internal Ethical Review Committee of the Hospital de la Princesa (Committee Register Number: 2796), and a written informed consent was obtained from all patients, in accordance with the Declaration of Helsinki.
Tissue and PBMC Samples
All thyroid tissues were obtained from surgical thyroidectomies performed in our hospital and evaluated by an experienced pathologist. Control thyroid samples were collected from nonthyroid pathology laryngectomy samples or healthy organ donors from Institut d’Investigació en Ciències de la Salut Germans Trias i Pujol (IGTP-HUGTIP) Biobank. As the samples were provided from healthy donors, we do not have clinical data of hormones and antibodies from these samples. Samples were immediately snap-frozen in liquid nitrogen–cooled isopentane and transferred to a −80 °C freezer for long-term preservation. A total of 5 sections of 30 µm thickness were used for RNA extraction. PBMCs were isolated by Ficoll-Paque (Lonza Ibérica) density-gradient centrifugation.
RNA Isolation and Quantitative Reverse Transcription–Polymerase Chain Reaction
RNA from the 30 fresh-frozen thyroid tissues was isolated by using the miRNeasy Mini Kit (Qiagen), according to the manufacturer’s instructions. RNA from PBMCs was isolated using TRIzol, and the quality and quantity of RNA were evaluated by NanoDrop ND-1000 analysis. First-strand cDNA was generated with a cDNA synthesis kit (Applied Biosystems), and quantitative reverse transcription–polymerase chain reaction (RT-qPCR) was performed by triplicate, by using high-capacity cDNA reverse transcription kit with a ribonuclease inhibitor (Thermo Fisher), and with the CFX384 Touch Real-Time PCR Detection System (Bio-Rad). Primer sequences employed for the RT-qPCR analyses (27) are shown in Table 3.
Table 3.
HDACs 1-11 and TIP60 primer sequences
| Primer | Orientation | Sequence |
|---|---|---|
| HDAC1 | FW | CTATCAAAGGACACGCCAAGTG |
| RV | ACCGGGCAACGTTACGAAT | |
| HDAC2 | FW | CATGGTGATGGTGTTGAAGAAG |
| RV | TCATTGGAAAATTGACAGCATAGT | |
| HDAC3 | FW | TTGAGTTCTGCTCGCGTTACA |
| RV | CCCAGTTAATGGCAATATCACAGAT | |
| HDAC4 | FW | AATCTGAACCACTGCATTTCCA |
| RV | GGTGGTTATAGGAGGTCGACACT | |
| HDAC5 | FW | TTGGAGACGTGGAGTACCTTACAG |
| RV | GACTAGGACCACATCAGGTGAGAAC | |
| HDAC6 | FW | TGGCTATTGCATGTTCAACCA |
| RV | GTCGAAGGTGAACTGTGTTCCT | |
| HDAC7 | FW | CTGGCACAGCGGATGTTTG |
| RV | CTGCATTGGAGGAATGAAGCT | |
| HDAC8 | FW | TCCCGAGTATGTCAGTATGTGTGA |
| RV | GCTTCAATCAAAGAATGCACCAT | |
| HDAC9 | FW | AGTAGAGAGGCATCGCAGAGA |
| RV | GGAGTGTCTTTCGTTGCTGAT | |
| HDAC10 | FW | CAGTTCGACGCCATCTACTTC |
| RV | CAAGCCCATTTTGCACAGCTC | |
| HDAC11 | FW | ACCCAGACAGGAGGAACCATA |
| RV | TGATGTCCGCATAGGCACAG | |
| TIP60 | FW | CGTAAGAACAAGAGTTATTCCCA |
| RV | GTCTTCCGTTGATTCTTTCTCC |
Thyroid Cell Cultures
The NThy-ORi 3-1 cell line (kindly provided by Dr. Pilar Santisteban, Instituto de Investigaciones Biomédicas “Alberto Sols,” Spain) was cultured in RPMI 1640 medium supplemented with Gluta-MAX, 10% fetal bovine serum (Hyclone), 1% of penicillin/streptomycin (Gibco), and 10 mU/mL TSH (Sigma-Aldrich), as described (28).
Thyroid follicular cells were prepared from 6 thyroid gland surgical fragments, as described (29). In brief, thyroid tissue was minced and digested with collagenase (1.0 mg/mL Sigma-Aldrich) in HBSS (Lonza Ibérica) for 1 hour at 37 °C, and red blood cells were lysed with an erythrocytes lysis buffer for 5 minutes (Qiagen). The resulting cell suspensions was cultured overnight in DMEM medium supplemented with 10% fetal bovine serum (Hyclone), and penicillin-streptomycin (Gibco), at 37 °C and 5% CO2. These cells were incubated with or without the addition of 1000 U/mL interferon-γ (IFN-γ) (R&D systems) and 800 mU/mL TNF-α (R&D systems). All experimental conditions were run by triplicate, and 3 independent experiments were carried out.
Immunofluorescence Microscopy Analyses
Cells were cultured on round coverslips in 6-well plates. After incubation under the indicated conditions, cells were washed with PBS and fixed with 4% paraformaldehyde for 15 minutes, and then permeabilized with 0.1% Triton X-100 for 10 minutes at room temperature. Permeabilized cells were blocked with 5% bovine serum albumin and 10% normal goat serum in PBS for 15 minutes. Then 5-µm thyroid-sections from 9 frozen thyroid tissue samples (3 HT, 3 GD, and 3 controls) were fixed with 4% paraformaldehyde and permeabilized with PBS 0.1% Triton buffer. Nonspecific binding was blocked using 5% bovine serum albumin and 10% normal goat serum for 15 minutes.
Thereafter, tissue sections or cultured cells were incubated with an anti-HDAC9 antibody (Abcam Cat# ab109446, RRID:AB_10861804), anti-Foxp3 (Abcam Cat# ab20034, RRID:AB_445284), anti-CD19 (Agilent Cat# IR656, RRID:AB_10861804), anti-Foxp3 (Abcam Cat# ab20034, RRID:AB_445284), anti-CD19 (Agilent Cat# IR656, RRID:AB_2892575) and anti-CD68 (Agilent Cat# M0876, RRID:AB_2074844) overnight at 4 ºC. Then, slides were washed 3 times with PBS and incubated 1 hour with an Alexa Fluor 488 labeled goat anti-rabbit IgG antibody (Thermo Fisher Scientific Cat# A32731, RRID:AB_2633280) and Alexa Fluor 568 labeled goat anti-mouse IgG antibody (Thermo Fisher Scientific Cat# A-11031, RRID:AB_144696). Finally, cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) and sections were analyzed in a Leica Sp5 confocal microscopy (Leica).
Western Blot Analysis
Thyroid tissue samples were disaggregated and resuspended in RIPA buffer (Sigma-Aldrich) containing a protease inhibitor (Halt, Thermo Fisher Scientific). After 30 minutes on ice, samples were sonicated and cell lysates were centrifuged at 12700 rpm for 10 minutes and 4 ºC and the supernatant was recovered.
Protein samples from PBMCs were obtained from the phenol/chloroform interphase after RNA extraction, following TRIzol standard protocol (Life Technologies). At the end of this procedure, the protein pellet was shaken for 1 hour, sonicated, and centrifuged for 10 minutes at 12700 rpm and 4 ºC. Finally, the supernatant containing the proteins was transferred to another tube and stored at −80 ºC until use.
Protein samples were resolved in an 8% to 15% mini-protean TGX precast gel (Bio-Rad) and transferred to nitrocellulose membranes, which were blocked with 10% skimmed milk and incubated with anti-HDAC9 (Abcam Cat# ab109446, RRID:AB_10861804), anti-Tip60 (Santa Cruz Biotechnology Cat# sc-166323, RRID:AB_2296327) and anti-β–actin (Santa Cruz Biotechnology Cat# sc-47778, RRID:AB_626632) polyclonal antibodies overnight at 4 ºC. The next day, membranes were washed 3 times, incubated with secondary antibodies conjugated to horseradish peroxidase and visualized using a chemiluminescent detection reagent kit (ThermoScientific). ImageJ software (National Institutes of Health) was used to quantify the amount of protein of each band.
Flow Cytometry Analysis
HDAC9 expression was evaluated by a multiparametric flow cytometry analysis in 5 HT, 5 GD, and 6 healthy controls in different cell subsets by using anti-CD3-PE (BD Biosciences Cat# 555333, RRID:AB_395740); anti-CD4-PerCP (BD Biosciences Cat# 345770, RRID:AB_2868798); anti-CD25-PE (Miltenyi Biotec Cat# 130-113-286, RRID:AB_2733792); anti-CD14-FITC (BD Biosciences Cat# 555397, RRID:AB_395798); anti-CD19-PE (BD Biosciences Cat# 555413, RRID:AB_395813); IL-17-APC (Thermo Fisher Scientific Cat# 17-7179-42, RRID:AB_1582221); HLA-DR-PerCP (BD Biosciences Cat# 347364, RRID:AB_400292); CD11c-Pacific Blue (BD Biosciences Cat# 560369, RRID:AB_1645557); BDCA1-PE (BD Biosciences Cat# 564900, RRID:AB_2739006) monoclonal antibodies. Intracellular stainings were performed following permeabilization with 0.3% saponin. In addition, cells were fixed and permeabilized with the FOXP3 Fix/Perm kit (eBioscience) and stained with anti-Foxp3-FITC (Thermo Fisher Scientific Cat# 11-4777-42, RRID:AB_11149498) and anti-HDAC9 (Abcam Cat# ab109446, RRID:AB_10861804) followed by an Alexa Fluor 647 (Abcam Cat# ab150075, RRID:AB_2752244). Data were acquired on a FACS Canto II flow cytometer (BD Biosciences), and data were analyzed by using the FlowJo software v7.6 (Tree Star).
Statistical Analysis
Results were expressed as the arithmetic mean and SD, and differences among groups were compared by the Mann-Whitney U test or Kruskal-Wallis analysis. Association analyses were performed by using the Spearman correlation test. A P value < 0.05 was considered as significant. All statistical analyses were performed with the GraphPad Prism 5.0 software (GraphPad Software).
Results
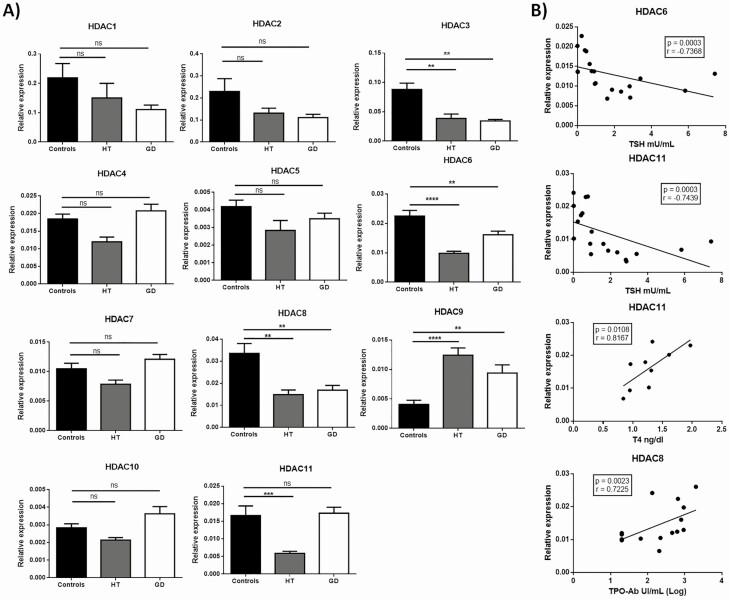
We first analyzed the expression of different HDAC genes in thyroid tissue from patients with AITD and controls. A significant downregulation of HDAC3 and HDAC6 was observed in samples from patients with either HT or GD, compared with those from healthy controls (P < 0.01 in all cases, Fig. 1A). In addition, significantly low levels of expression of HDAC8 and HDAC11 genes were observed in patients with HT (P < 0.05, compared with controls, in both cases) but not in those with GD (Fig. 1A). In contrast, the expression of HDAC9 gene was clearly increased in samples from HT and GD, compared with controls (P < 0.01 in both cases, Fig. 1A). Accordingly, similar levels of HDAC9 gene expression were observed in HT and GD (P > 0.05, Fig. 1A).
Figure 1.
HDAC gene expression in AITD thyroid gland. A) Thyroid gland samples from HT, GD, and control individuals were obtained and the expression of HDAC1-11 genes at mRNA level was analyzed by RT-qPCR. Data correspond to the arithmetic mean ± SD of 30 samples (10 controls, 10 patients with HT, and 10 patients with GD). B) Correlation analyses of the levels of gene expression of the indicated HDACs in thyroid gland samples and different clinical laboratory parameters. Abbreviations: HDAC, histone deacetylase; ns, not significant; TPO-Ab, anti-thyroid peroxidase antibody; TSH, thyrotropin. *P < 0.05; **P < 0.01; ***P < 0.005.
When the possible association of clinical laboratory parameters and HDAC gene expression was analyzed in AITD patients, a negative tight correlation was observed between TSH concentration and the levels of expression of HDAC6 (r = −0.73, P = 0.0003) or HDAC11 (r = −0.74, P = 0.0003) genes (Fig. 1B). In addition, free T4 hormone concentrations showed a positive significant association with HDAC11 gene expression levels (r = 0.82, P = 0.01). Finally, HDAC8 expression and the levels of anti-TPO antibodies also showed a significant positive association (r = 0.72, P = 0.0023).
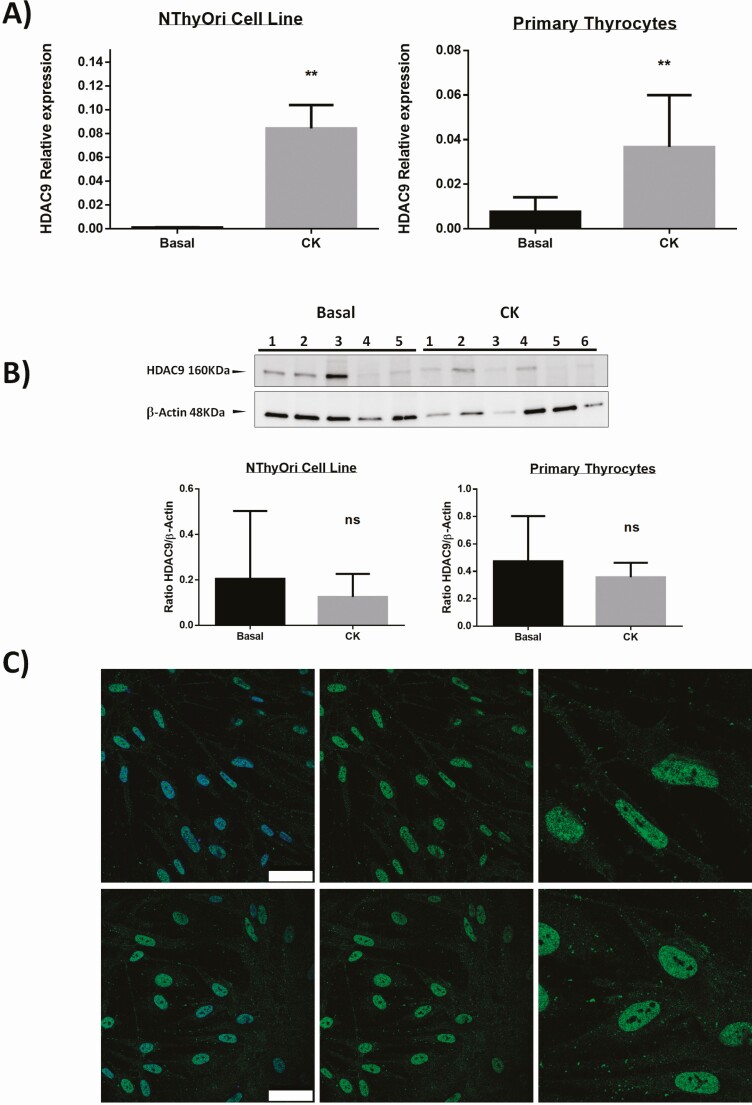
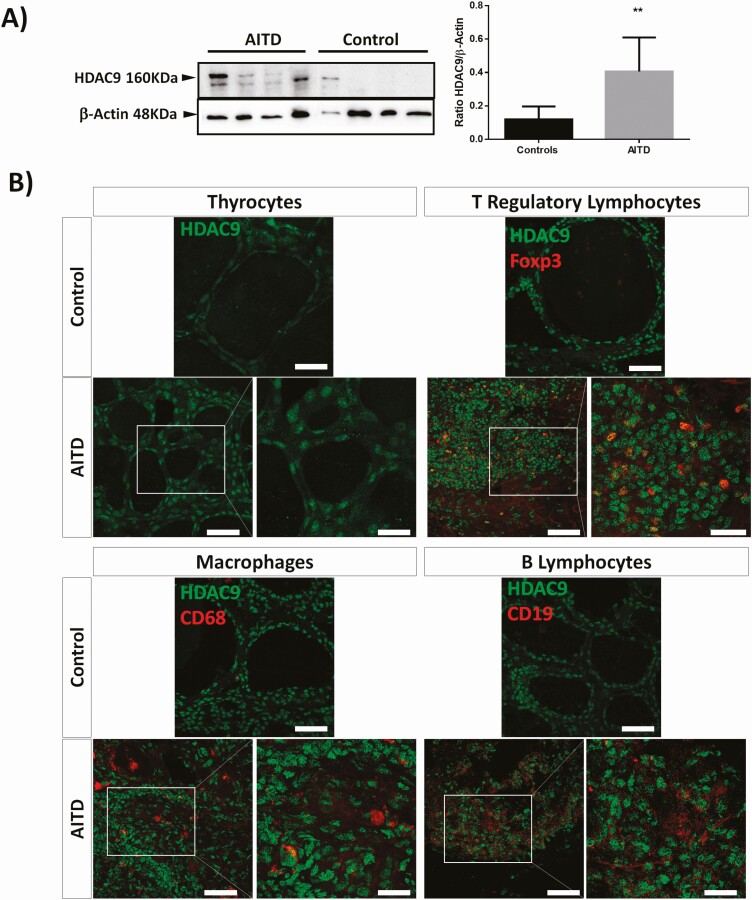
We then analyzed the possible in vitro effect of proinflammatory cytokines (IFN-γ and TNF-α) on the expression of HDAC genes, at mRNA and protein levels, in cultured thyroid follicular cells. As shown in Fig. 2A, cytokines induced a significant expression of HDAC9 gene in both primary thyrocytes and the NThyOri cell line (P < 0.01, in both cases, compared to baseline levels). In contrast, no significant induction of other HDAC genes was observed under these culture conditions (Fig. 2A and data not shown). In addition, when the expression of the HDAC9 gene was analyzed at protein level (Western blot), no significant induction of HDAC9 by the proinflammatory cytokines was observed (Fig. 2B). However, differences in the subcellular distribution of HDAC9 were observed by immunofluorescence microscopy, detecting a predominance of cytoplasmic HDAC9 staining in cultures stimulated with cytokines, whereas nonstimulated cell cultures mainly showed nuclear staining (Fig. 2C). Furthermore, the analysis of HDAC9 by Western blot of thyroid tissue homogenates revealed enhanced levels of 2 isoforms of this enzyme in samples from AITD patients compared with healthy tissue (Fig. 3A, P < 0.01 in both cases). Accordingly, immunofluorescence microscopy analysis of thyroid tissue sections showed an enhanced staining of HDAC9 in thyrocytes from AITD samples (Fig. 3B). Then, we performed double immunofluorescences to identify Tregs (FOXP3/HDAC9), macrophages (CD68/HDAC9), and B lymphocytes (CD19/HDAC9). Our results showed almost no expression of HDAC9 in macrophages from AITD patients and HDAC9 expression in both B lymphocytes and in Tregs (Fig. 3B).
Figure 2.
HDAC gene expression in thyroid cell cultures. A) Primary thyrocytes and NThyOri cell were cultured in the presence or not of IFN-γ and TNF-α (CK), and then the levels of HDAC9 mRNA were analyzed by RT-qPCR. A significant induction of HDAC9 gene is shown in both cell types. B) Primary thyrocytes and NThyOri cell were cultured in the presence or not of IFN-γ and TNF-α (CK), and then the levels of HDAC9 were analyzed by Western blot. No significant differences in the levels of HDAC9 synthesis induction was detected. C) Immunofluorescence microscopy analysis of HDAC9 expression (green fluorescence) in primary thyroid cell cultures, in the presence or not of IFN-γ and TNF-α (CK). Cell nuclei were stained with DAPI (blue fluorescence). Scale bar = 50µm. Data correspond to the arithmetic mean ± SD. Abbreviations: ns, not significant. *P < 0.05; **P < 0.01; ***P < 0.005.
Figure 3.
Analysis of HDAC9 levels in thyroid gland samples from AITD. A) Western blot analysis of tissue sample homogenates from healthy and AITD thyroid glands. A significant overexpression of HDAC9 was detected in AITD thyroid gland samples. **P < 0.01. B) Double immunofluorescence microscopy analysis of HDAC9 expression (green fluorescence), Foxp3, CD68, and CD19 (red fluorescence) in thyroid gland tissue sections from controls and patients with AITD. An increased immunostaining of HDAC9 is observed in samples from AITD. Cell nuclei were stained with DAPI (blue fluorescence). Scale bar = 50 µm and 25 µm (details in white square).
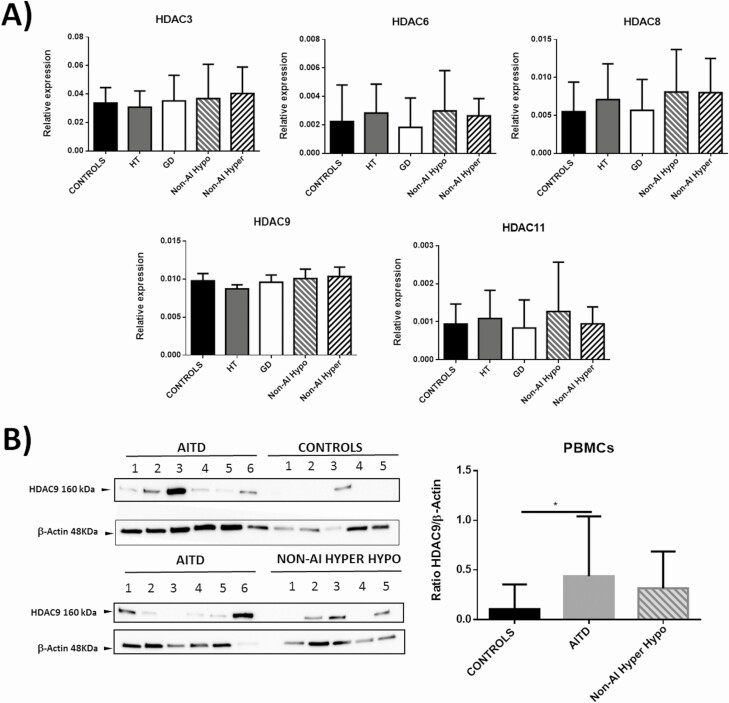
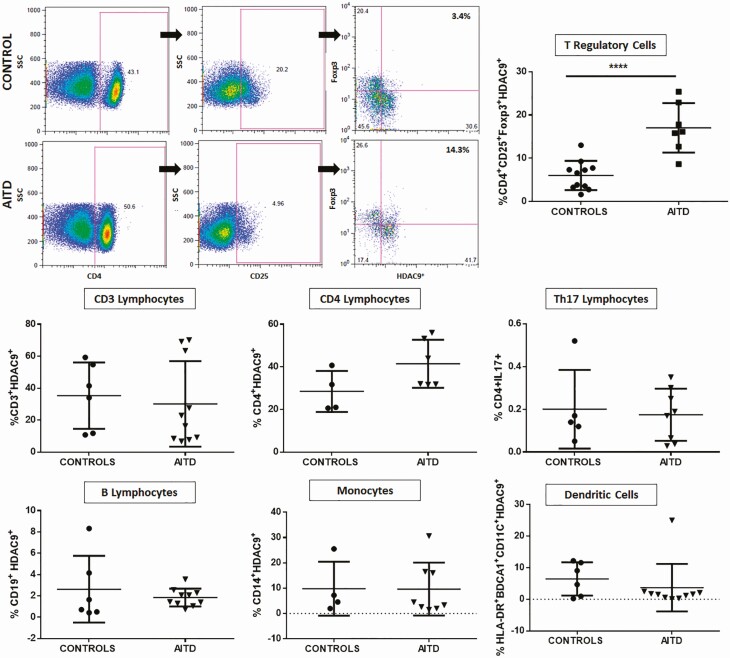
We then analyzed in PBMCs the mRNA levels of those HDACs that showed an altered expression in thyroid tissue from AITD patients. As shown in Fig. 4A, similar levels of HDAC3, 6, 8, 9, and 11 gene expression were observed in cells from healthy controls, and patients with HT, GD, and nonautoimmune thyroid disease. However, analysis of these samples at protein level (Western blot) showed a significant increase of HDAC9 concentration in cells from patients with AITD (P < 0.05, Fig. 4B). Accordingly, when HDAC9 was analyzed by flow cytometry in different subpopulations (hematopoietic-derived cells (CD3+), global lymphocytes T (CD4+), lymphocytes Th17 (CD4+ IL-17+), Treg lymphocytes (CD4+CD25+Foxp3+), lymphocytes B (CD19+), monocytes (CD14+), and dendritic cells (HLA-DR+ CD11c+ BDCA1+). We observed an increased proportion of CD4+CD25+Foxp3+HDAC9+ cells in samples from AITD patients (P < 0.01), compared with healthy controls (Fig. 5).
Figure 4.
Analysis of expression of HDAC3, 6, 8, 9, and 11 by PBMCs from patients with AITD and controls. A) Blood samples were obtained from healthy controls (n = 13) and patients with HT (n = 12), GD (n = 15), nonautoimmune hypothyroidism (n = 8), or hyperthyroidism (n = 10) and their PBMCs were analyzed by RT-qPCR for the levels of HDAC3, 6, 8, 9, and 11 mRNA. No significant differences were detected among the groups. B) Western blot analysis of HDAC9 expression in PBMCs homogenates from the indicated individuals. A significantly higher amount of HDAC9 was observed in samples from patients with AITD. *P < 0.05.
Figure 5.
Flow cytometry analysis of HDAC9 expression by different cell subsets from patients with AITD. Peripheral blood mononuclear cells from patients with AITD and healthy controls were immunostained for CD3+ (T lymphocytes), CD4+CD25+ Foxp3+ (T regulatory cells), CD19+ (B lymphocytes), CD14+ (monocytes) and HLA-DR+CD11c+ BDCA1+ (myeloid dendritic cells), and HDAC9, and the cells were analyzed by flow cytometry, as stated in “Methods.” Analysis strategy is shown in the dot plot left panel. A significant increase in the percent of HDAC9+ Treg (CD4+CD25+Foxp3+) cells was detected in patients with AITD compared with healthy controls. (right panel). *P < 0.05; ****P < 0.001.
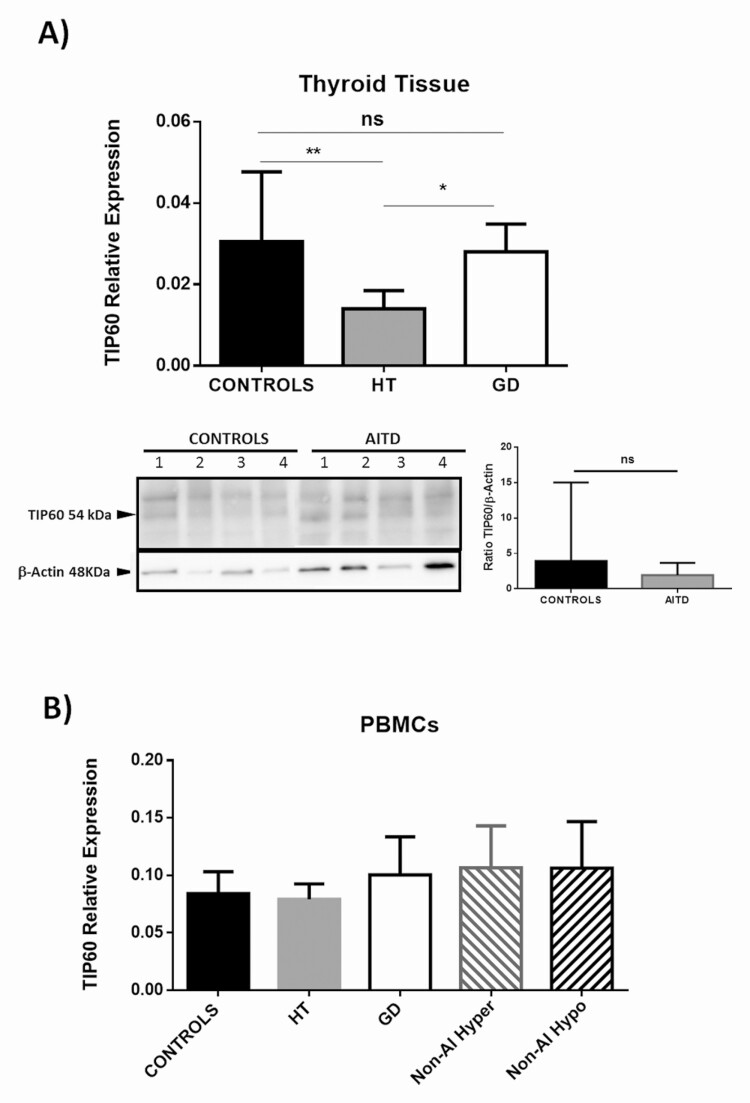
Finally, we studied the expression of the Tip60 HAT in both thyroid tissue and PBMCs from AITD patients and healthy controls. As shown in Fig. 6A, a significantly diminished expression of Tip60 gene was observed in thyroid gland samples from patients with HT (P < 0.01), but not from GD, compared with healthy tissue. Accordingly, the levels of Tip60 mRNA were higher in thyroid samples from patients with GD compared with those from HT (P < 0.05, Fig. 6A). In contrast, similar levels of Tip60 gene expression (at mRNA and protein levels) were observed in PBMC from AITD patients and healthy controls (Fig. 6B, and data not shown).
Figure 6.
Analysis of Tip60 expression in thyroid glands and PBMCs from patients with AITD and controls. A) Significant diminished levels of Tip60 were detected in gland samples from patients with HT compared with both GD and controls (upper panel). Homogenates of thyroid gland samples analyzed by Western blot from patients with HT and GD showed a tendency of decreased levels of Tip60 HAT (54 kDa band, lower panel) compared with controls. Data correspond to the arithmetic mean and SD. Abbreviation: ns, not significant; *P < 0.05; **P < 0.01. B) Analysis of Tip60 mRNA levels in PBMCs from patients with AITD (HT and GD) as well as from patients with nonautoimmune hyper- and hypothyroidism. Cells from healthy controls were also analyzed. No significant differences among all the groups studied were observed.
Discussion
Histone acetylation has an important effect on the regulation of gene expression, and the removal of acetyl groups from the lysine residues of these molecules induces chromatin compaction, and thus inhibition of gene expression. Accordingly, the expression and activity of those enzymes that regulate histone acetylation (eg, HDACs and HATs) exert an important effect in different cellular phenomena, under physiological and pathological conditions (16, 30). In autoimmune diseases it has been considered that an upregulation of HDACs could promote the expression of proinflammatory genes, since their inhibition has anti-inflammatory effects, being considered as possible targeted approaches (17, 18). In rheumatoid arthritis (RA) synovial fibroblasts, the selective inhibition of class I HDACs (HDACs 1-3) with MS-275, diminishes proinflammatory cytokines, angiogenic factors, and metalloproteinases, pointing to a key role of HDACs in promoting RA symptoms (31). In multiple sclerosis (MS), HDAC3 is upregulated in MS plaques and also in PBMCs from patients compared with controls. HDAC3 regulates genes involved in cell proliferation and their apoptosis, thus this increase may be related with the presence of autoreactive T cells and resistance to apoptosis in MS (32). Regarding class IIa HDACs, HDAC5 has been described to be downregulated in fibroblast-like-synoviocytes by inflammatory cytokines, such as IL-1β and TNF, promoting the transcription of IFN-I response genes. The authors conclude that restoring HDAC5 expression may become a novel strategy in RA treatment (33). Concerning class IIb HDACs, HDAC6 is the most described. In RA, the inhibition with tubastatin, a selective HDAC6i, promoted antirheumatic and anti-inflammatory conditions, which suggest a role of this HDAC in promoting this autoimmune disease (34). HDAC11, the unique member of class IV HDACs, has been reported to be increased in female patients with MS compared with controls linked to a marked oligodendroglial histone deacetylation at early stages of the disease (35). Therefore, we decided to explore the degree of expression, at mRNA and protein levels, of different HDACs and the Tip60 HAT in thyroid gland samples and immune cells from patients with AITD.
We have observed an abnormal and complex pattern of expression of HDACs in patients with AITD, with diminished levels of different types of these enzymes (HDAC3, 6, 8, 11) and enhanced expression of HDAC9 in thyroid gland tissue, including thyrocytes and thyroid infiltrating mononuclear cells. Likewise, an increased expression (at protein level) of HDAC9 was also observed in PBMC from these patients. In this regard, it is of interest that human Tregs have higher levels of HDAC9 than Teffector cells even upon Treg activation, which suggests a selective expression of HDAC9 and its important role in human Tregs functions. In this regard, it is of interest that this enzyme seems to have an important role in the regulation of the FOXP3 gene, since human Tregs have higher levels of HDAC9 than Teffector cells even upon Treg activation (18, 36, 37). Indeed, upon TCR stimulation, HDAC9 is phosphorylated and translocated to the cytoplasm allowing Foxp3 acetylation and promoting the survival and development of Treg suppressive functions (18). In this context, previous studies have shown that blocking HDAC9 activity in HDAC9-/- mice modeling colitis promotes resistance to the development of this disease and Tregs have a greater suppressive and protective function (38) (Fig. 7). Therefore, it is feasible that this increased expression of HDAC9 has a relevant role in the defective function of CD4+CD25+ Treg cells that we and others have previously observed in patients with AITD (8). In addition, the dysregulated expression of different HDACs and the Tip60 HAT detected in our study may have a role in the inflammatory phenomenon observed in AITD, mainly in HT. In this regard, it has been described that, in different animal models of chronic inflammatory and autoimmune diseases, the administration of synthetic inhibitors of HDACs (such as Trichostatin A, a class I and II inhibitor; SAHA/vorinostat, a class I, II, and IV inhibitor; or valproic acid, a potent class I and also class II inhibitor, among others) exerts a beneficial effect, by diminishing the inflammatory phenomenon through the induction of CD4+CD25+ Treg lymphocytes and enhancing the suppressive properties of Foxp3-dependent Treg cells (17, 20, 36, 37, 39-42). This effect has been observed in animal models of RA, inflammatory bowel disease, and systemic lupus erythematosus, among others (19, 43, 44). Accordingly, it has been proposed—and different preclinical data indicate—that the inhibition of the activity of different HDACs may have a relevant therapeutic effect in chronic inflammatory and autoimmune diseases (17, 36, 45). Interestingly, in a phase I/II clinical trial, treatment with the oral HDAC inhibitor vorinostat in patients receiving allogeneic hematopoietic cell transplant reduced inflammatory responses of PBMC and also increased suppresive capacity and levels of Tregs (46). Hence, it is important to perform in vitro and in vivo experiments using isoform-selective HDAC inhibitors to determine the specific role of each HDAC and to provide a more specific immunomodulatory effect in autoimmune diseases (17).
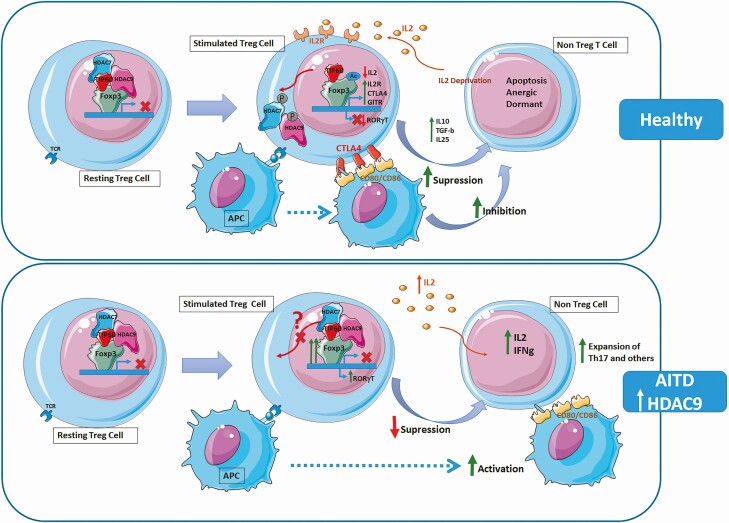
Figure 7.
HDAC-mediated regulation of Treg cells. (Upper panel: Healthy condition) In resting Treg cells, FOXP3 recruits the histone acetyl transferase (HAT) TIP60 (in red) and the class IIa histone deacetylase (HDAC) HDAC7 (in blue) and HDAC9 (in pink) inhibiting FOXP3 (in green) transcription. Under TCR stimulation, HDAC7 and HDAC9 phosphorylate and shuttle from the nucleus to the cytoplasm and dissociate from FOXP3. FOXP3 is then acetylated by HATs, such as TIP60, and binds to the promoter regions of target genes such as IL2R (CD25), CTLA4, or GITR. Expression of high IL2R by Treg cells results in IL-2 deprivation of effector cells. CTLA4, downmodulates CD80/CD86 expression by antigen-presenting cells (APCs). The secretion of anti-inflammatory cytokines, such as IL-10, IL-35, and TGF-β and the deprivation of co-stimulatory signal inhibit responder T cells and lead them to apoptosis. (Lower panel: AITD condition) The increased expression of HDAC9 in Tregs may affect its phosphorylation or translocation to the cytoplasm, resulting in a retention of the HDAC complex and supressing Treg activation. APC are activated leading to expansion of Th17 cells and other Teffector cells with an increase in the production of inflammatory cytokines.
Although the precise role of HDACs in the pathogenesis of AITD has not been fully elucidated, a significant association of 2 single nucleotide polymorphisms of the SIRT1 gene (which encodes for Sirtuin1, a Class 3 NAD-dependent histone deacetylase) with high titers of antithyroid antibodies in patients with AITD has been previously reported (47). In addition, Yin et al have detected a diminished expression of Sirtuin1 in both, thyroid gland samples and PBMC from patients with GD (48). Interestingly, the increased expression of this deacetylase was associated with an increased activation of the transcription factor NF-kB and the induction of the synthesis of proinflammatory cytokines (48). Furthermore, it has been reported that PBMCs from patients with GD show a diminished level of histone 4 acetylation, which is accompanied by an increased expression of HDAC1 and HDAC2 (49). However, in this report no significant differences in the expression of the SIRT1 gene were observed in cells from patients with GD compared with those from healthy individuals (49). All the above data, along with our results on the increased expression of HDAC9 in thyroid gland, PBMCs, and CD4+CD25+ Treg lymphocytes from AITD patients, further suggest that the levels of histone acetylation and the expression of different enzymes that promote the deacetylation of these nuclear proteins have an important role in the pathogenesis of this condition. This pathogenic effect seems to be mediated by the dysregulated expression of the transcription factor Foxp3. In this regard, it is of interest to note the significant defective expression of the Tip60 HAT, which promotes histone acetylation and the transcription of the FOXP3 gene, in the samples from AITD patients that we studied. In agreement with these data, Yang et al have reported that CD4+CD25+ Treg cells from patients with HT show a diminished expression of Foxp3 and a decreased level of acetylation of FOXP3 gene, accompanied by a low number and a defective function of these regulatory cells (50). Furthermore, it has been described that the immune cells from patients with systemic lupus erythematosus show a dysregulated balance between the levels and activity of different HDACs and HATs, which results in abnormal levels of H4 histone acetylation, a phenomenon that is favored by the interferon regulatory factor 1 (IRF1) (51). Accordingly, investigators have observed, in an animal model of inflammatory bowel disease, the therapeutic effect of the inhibition of the expression of HDAC9 or the blockade of its enzymatic activity, by Trichostatin A, SAHA, and entinostat (a selective class I inhibitor). These authors observed an induction of Treg cells using pan-HDAC inhibitors, but not class I–specific HDAC inhibitors, indicating the role of class II HDACs (including HDAC9) in controlling Treg function (38).
In summary, we consider that our data further support the importance of an improper balance between the enzymes that promote the acetylation and deacetylation of histones (HATs and HDACs, respectively, among others) in the pathogenesis of chronic inflammatory and autoimmune diseases, including HT and GD. In this regard, it is expected that increased levels of expression/function of HDAC9 and the downregulation of Tip60 significantly contributes to the altered function of the immune regulatory mechanisms observed in patients with AITD. Thus, our data further suggest that the selective inhibition of some HDACs is an alternative and a rational approach for the therapy of thyroid autoimmunity.
Acknowledgments
We thank Francisca Molina-Jimenez from the Instituto Universitario de la Princesa for technical assistance with confocal microscopy. We also warmly thank all the participants included in the study for their selfless participation. We also thank the Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER GCV14/ER/12) for their support.
Financial Support: This work was supported by the following grants: Proyectos de Investigacion en Salud (FIS) PI16-02091 and PI19-00584 (funded by Instituto de Salud Carlos III), TIRONET2-CM, B2017/BMD-3724 (funded by Comunidad de Madrid), and cofinanced by FEDER funds (to M.M.); and predoctoral fellowship funded by Comunidad de Madrid (PEJD-2019-PRE/BMD-14876) and by Instituto de Salud Carlos III (FI20/00035) (to P.S.G.). The funders had no role in study design, data collection, data analysis, interpretation, or writing of the report.
Glossary
Abbreviations
- AITD
autoimmune thyroid disease(s)
- DAPI
4′,6-diamidino-2-phenylindole
- GD
Graves’ disease
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- HT
Hashimoto’s thyroiditis
- IFN-γ
interferon-γ
- MS
multiple sclerosis
- PBMC
peripheral blood mononuclear cell
- RA
rheumatoid arthritis
- RT-qPCR
quantitative reverse transcription–polymerase chain reaction
- SIRT
sirtuin
- TNF
tumor necrosis factor
- TPO
thyroid peroxidase
- Treg
T regulatory cells
- TSH
thyrotropin (thyroid-stimulating hormone)
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartalena L, Fatourechi V. Extrathyroidal manifestations of Graves’ disease: a 2014 update. J Endocrinol Invest. 2014;37(8):691-700. [DOI] [PubMed] [Google Scholar]
- 3. Degroot LJ, Quintans J. The causes of autoimmune thyroid disease. Endocr Rev. 1989;10(4):537-562. [DOI] [PubMed] [Google Scholar]
- 4. Rapoport B, McLachlan SM. Thyroid autoimmunity. J Clin Invest. 2001;108(9):1253-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang B, Shao X, Song R, Xu D, Zhang JA. The emerging role of epigenetics in autoimmune thyroid diseases. Front Immunol. 2017;8:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. García-López MA, Marazuela M, Sánchez-Madrid F, et al. Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab. 2006;91(9):3639-3646. [DOI] [PubMed] [Google Scholar]
- 7. Vitales-Noyola M, Serrano-Somavilla A, Martínez-Hernández R, et al. Patients with autoimmune thyroiditis show diminished levels and defective suppressive function of Tr1 regulatory lymphocytes. J Clin Endocrinol Metab. 2018;103(9):3359-3367. [DOI] [PubMed] [Google Scholar]
- 8. González-Amaro R, Marazuela M. T regulatory (Treg) and T helper 17 (Th17) lymphocytes in thyroid autoimmunity. Endocrine. 2016;52(1):30-38. [DOI] [PubMed] [Google Scholar]
- 9. Mao C, Wang S, Xiao Y, et al. Impairment of regulatory capacity of CD4+CD25+ regulatory T cells mediated by dendritic cell polarization and hyperthyroidism in Graves’ disease. J Immunol. 2011;186(8):4734-4743. [DOI] [PubMed] [Google Scholar]
- 10. Verginis P, Li HS, Carayanniotis G. Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4+CD25+ T cells. J Immunol. 2005;174(11):7433-7439. [DOI] [PubMed] [Google Scholar]
- 11. Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev. 2015;14(2):174-180. [DOI] [PubMed] [Google Scholar]
- 12. Brownell JE, Allis CD. An activity gel assay detects a single, catalytically active histone acetyltransferase subunit in Tetrahymena macronuclei. Proc Natl Acad Sci U S A. 1995;92(14):6364-6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hubbert C, Guardiola A, Shao R, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417(6887):455-458. [DOI] [PubMed] [Google Scholar]
- 14. Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26(37):5310-5318. [DOI] [PubMed] [Google Scholar]
- 15. Park SY, Kim JS. A short guide to histone deacetylases including recent progress on class II enzymes. Exp Mol Med. 2020;52(2):204-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10(1):32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Regna NL, Reilly CM. Isoform-selective HDAC inhibition in autoimmune disease. J Clin Cell Immunol. 2014;5(2). doi: 10.4172/2155-9899.1000207 [DOI] [Google Scholar]
- 18. Wang L, de Zoeten EF, Greene MI, Hancock WW. Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nat Rev Drug Discov. 2009;8(12):969-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glauben R, Siegmund B. Inhibition of histone deacetylases in inflammatory bowel diseases. Mol Med. 2011;17(5-6):426-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tao R, de Zoeten EF, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13(11):1299-1307. [DOI] [PubMed] [Google Scholar]
- 21. Saouaf SJ, Li B, Zhang G, et al. Deacetylase inhibition increases regulatory T cell function and decreases incidence and severity of collagen-induced arthritis. Exp Mol Pathol. 2009;87(2):99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reilly CM, Thomas M, Gogal R Jr, et al. The histone deacetylase inhibitor trichostatin A upregulates regulatory T cells and modulates autoimmunity in NZB/W F1 mice. J Autoimmun. 2008;31(2):123-130. [DOI] [PubMed] [Google Scholar]
- 23. Sapountzi V, Logan IR, Robson CN. Cellular functions of TIP60. Int J Biochem Cell Biol. 2006;38(9):1496-1509. [DOI] [PubMed] [Google Scholar]
- 24. Xiao Y, Nagai Y, Deng G, et al. Dynamic interactions between TIP60 and p300 regulate FOXP3 function through a structural switch defined by a single lysine on TIP60. Cell Rep. 2014;7(5):1471-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geng J, Yu S, Zhao H, et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat Immunol. 2017;18(7):800-812. [DOI] [PubMed] [Google Scholar]
- 26. Su Q, Jing J, Li W, et al. Impaired Tip60-mediated Foxp3 acetylation attenuates regulatory T cell development in rheumatoid arthritis. J Autoimmun. 2019;100:27-39. [DOI] [PubMed] [Google Scholar]
- 27. Waltregny D, North B, Van Mellaert F, de Leval J, Verdin E, Castronovo V. Screening of histone deacetylases (HDAC) expression in human prostate cancer reveals distinct class I HDAC profiles between epithelial and stromal cells.Eur J Histochem. 2004;48(3):273-290 . [PubMed]
- 28. Martínez-Hernández R, Serrano-Somavilla A, Ramos-Leví A, et al. Integrated miRNA and mRNA expression profiling identifies novel targets and pathological mechanisms in autoimmune thyroid diseases. Ebiomedicine. 2019;50:329-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marazuela M, Postigo AA, Acevedo A, Díaz-González F, Sánchez-Madrid F, De Landázuri MO. Adhesion molecules from the LFA-1/ICAM-1, 3 and VLA-4/VCAM-1 pathways on T lymphocytes and vascular endothelium in Graves’ and Hashimoto’s thyroid glands. Eur J Immunol. 1994;24(10):2483-2490. [DOI] [PubMed] [Google Scholar]
- 30. Gatla H, Muniraj N, Thevkar P, Yavvari S, Sukhavasi S, Makena M. Regulation of chemokines and cytokines by histone deacetylases and an update on histone decetylase inhibitors in human diseases. Int J Mol Sci. 2019;20(5):1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Choo Q-Y, Ho PC, Tanaka Y, Lin H-S. Histone deacetylase inhibitors MS-275 and SAHA induced growth arrest and suppressed lipopolysaccharide-stimulated NF- B p65 nuclear accumulation in human rheumatoid arthritis synovial fibroblastic E11 cells. Rheumatology 2010;49(8):1447-1460. [DOI] [PubMed] [Google Scholar]
- 32. Zhang F, Shi Y, Wang L, Sriram S. Role of HDAC3 on p53 expression and apoptosis in T cells of patients with multiple sclerosis. Plos One. 2011;6(2):e16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Angiolilli C, Grabiec AM, Ferguson BS, et al. Inflammatory cytokines epigenetically regulate rheumatoid arthritis fibroblast-like synoviocyte activation by suppressing HDAC5 expression. Ann Rheum Dis. 2016;75(2):430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vishwakarma S, Iyer LR, Muley M, et al. Tubastatin, a selective histone deacetylase 6 inhibitor shows anti-inflammatory and anti-rheumatic effects. Int Immunopharmacol. 2013;16(1):72-78. [DOI] [PubMed] [Google Scholar]
- 35. Pedre X, Mastronardi F, Bruck W, López-Rodas G, Kuhlmann T, Casaccia P. Changed histone acetylation patterns in normal-appearing white matter and early multiple sclerosis lesions. J Neurosci. 2011;31(9):3435-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akimova T, Ge G, Golovina T, et al. Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs. Clin Immunol. 2010;136(3):348-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lucas JL, Mirshahpanah P, Haas-Stapleton E, Asadullah K, Zollner TM, Numerof RP. Induction of Foxp3+ regulatory T cells with histone deacetylase inhibitors. Cell Immunol. 2009;257(1-2):97-104. [DOI] [PubMed] [Google Scholar]
- 38. de Zoeten EF, Wang L, Sai H, Dillmann WH, Hancock WW. Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology. 2010;138(2):583-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Camelo S, Iglesias AH, Hwang D, et al. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;164(1-2):10-21. [DOI] [PubMed] [Google Scholar]
- 40. Glauben R, Batra A, Fedke I, et al. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol. 2006;176(8):5015-5022. [DOI] [PubMed] [Google Scholar]
- 41. Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest. 2003;111(4):539-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin HS, Hu CY, Chan HY, et al. Anti-rheumatic activities of histone deacetylase (HDAC) inhibitors in vivo in collagen-induced arthritis in rodents. Br J Pharmacol. 2007;150(7):862-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Regna NL, Chafin CB, Hammond SE, Puthiyaveetil AG, Caudell DL, Reilly CM. Class I and II histone deacetylase inhibition by ITF2357 reduces SLE pathogenesis in vivo. Clin Immunol. 2014;151(1):29-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Joosten LA, Leoni F, Meghji S, Mascagni P. Inhibition of HDAC activity by ITF2357 ameliorates joint inflammation and prevents cartilage and bone destruction in experimental arthritis. Mol Med. 2011;17(5-6):391-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Akimova T, Beier UH, Liu Y, Wang L, Hancock WW. Histone/protein deacetylases and T-cell immune responses. Blood. 2012;119(11):2443-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Choi SW, Gatza E, Hou G, et al. Histone deacetylase inhibition regulates inflammation and enhances Tregs after allogeneic hematopoietic cell transplantation in humans. Blood. 2015;125(5):815-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sarumaru M, Watanabe M, Inoue N, et al. Association between functional SIRT1 polymorphisms and the clinical characteristics of patients with autoimmune thyroid disease. Autoimmunity. 2016;49(5):329-337. [DOI] [PubMed] [Google Scholar]
- 48. Yin Q, Shen L, Qi Y, et al. Decreased SIRT1 expression in the peripheral blood of patients with Graves’ disease. J Endocrinol. 2020;246(2):161-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yan N, Zhou JZ, Zhang JA, et al. Histone hypoacetylation and increased histone deacetylases in peripheral blood mononuclear cells from patients with Graves’ disease. Mol Cell Endocrinol. 2015;414:143-147. [DOI] [PubMed] [Google Scholar]
- 50. Yang X, Lun Y, Jiang H, et al. SIRT1-regulated abnormal acetylation of FOXP3 induces regulatory T-cell function defect in Hashimoto’s thyroiditis. Thyroid. 2018;28(2):246-256. [DOI] [PubMed] [Google Scholar]
- 51. Leung YT, Shi L, Maurer K, et al. Interferon regulatory factor 1 and histone H4 acetylation in systemic lupus erythematosus. Epigenetics. 2015;10(3):191-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.