Abstract
Exercise intolerance is a hallmark symptom of cardiovascular disease and likely occurs via enhanced activation of muscle metaboreflex-induced vasoconstriction of the heart and active skeletal muscle which, thereby limits cardiac output and peripheral blood flow. Muscle metaboreflex vasoconstrictor responses occur via activation of metabolite-sensitive afferent fibers located in ischemic active skeletal muscle, some of which express transient receptor potential vanilloid 1 (TRPV1) cation channels. Local cardiac and intrathecal administration of an ultrapotent noncompetitive, dominant negative agonist resiniferatoxin (RTX) can ablate these TRPV1-sensitive afferents. This technique has been used to attenuate cardiac sympathetic afferents and nociceptive pain. We investigated whether intrathecal administration (L4–L6) of RTX (2 µg/kg) could chronically attenuate subsequent muscle metaboreflex responses elicited by reductions in hindlimb blood flow during mild exercise (3.2 km/h) in chronically instrumented conscious canines. RTX significantly attenuated metaboreflex-induced increases in mean arterial pressure (27 ± 5.0 mmHg vs. 6 ± 8.2 mmHg), cardiac output (1.40 ± 0.2 L/min vs. 0.28 ± 0.1 L/min), and stroke work (2.27 ± 0.2 L·mmHg vs. 1.01 ± 0.2 L·mmHg). Effects were maintained until 78 ± 14 days post-RTX at which point the efficacy of RTX injection was tested by intra-arterial administration of capsaicin (20 µg/kg). A significant reduction in the mean arterial pressure response (+45.7 ± 6.5 mmHg pre-RTX vs. +19.7 ± 3.1 mmHg post-RTX) was observed. We conclude that intrathecal administration of RTX can chronically attenuate the muscle metaboreflex and could potentially alleviate enhanced sympatho-activation observed in cardiovascular disease states.
Keywords: cardiovascular performance, exercise pressor reflex, resiniferatoxin, TRPV1, ventricular function
INTRODUCTION
Whole body dynamic exercise presents one of the greatest challenges to cardiovascular control as heart rate and cardiac output rise from resting values to maximal levels during peak workloads (1–13). Blood flow to inactive vascular beds is reduced in many species and vasodilation in the active skeletal muscle and even the coronary circulation is restrained by the substantial increases in sympathetic activity (14–17). These marked changes in autonomic activity occur through the action and likely interaction between activation of central command, resetting of the arterial baroreflex, and activation of skeletal muscle afferents. In cardiovascular dysfunction, these challenges often become exacerbated as systemic perfusion and cardiac function may be compromised resulting in massive sympathetic activation. One potential mechanism mediating the enhanced sympathetic activity during exercise in cardiovascular disease is overactivation of skeletal muscle afferents, in particular those activated via accumulation of metabolites due to suboptimal oxygen delivery—termed as the muscle metaboreflex (18–33). Even in normal subjects this reflex is thought to become tonically active at relatively modest workloads and activation of this reflex can cause substantial increases in sympathetic activity, which elicits increases in cardiac output via tachycardia, increased ventricular contractility, and central blood volume mobilization (5–7, 9–13, 34–41). Arterial elastance rises in parallel with ventricular maximal elastance, thereby optimizing ventricular-vascular coupling and energy transfer from the left ventricle to the systemic circulation (11, 42, 43). Peripheral vasoconstriction can also occur which is countered by adrenal epinephrine release and β2-mediated vasodilation (16, 34, 38, 40). Normally, via the large increases in total blood flow (e.g., cardiac output) coupled with increased O2-carrying capacity of the blood (via splanchnic vasoconstriction causing red blood cell mobilization), the muscle metaboreflex acts to correct deficits in blood flow and O2 delivery to the active muscle (7, 29, 44). In pathophysiological states, even during moderate workloads this reflex likely becomes markedly overactivated and often profound vasoconstriction occurs in inactive vascular beds and even the ischemic active muscle and heart are vasoconstricted (15, 17, 41, 45–49). This exacerbates an already precarious situation leading to a positive feedback amplification of sympathetic activity (16, 17). The potentially extreme levels of sympathetic activity may pose increased risks for adverse cardiovascular events such as stroke, myocardial infarction, ventricular arrhythmia, and sudden cardiac death. Thus, mechanisms to ameliorate overactivation of skeletal muscle afferents during exercise in patients with cardiovascular disease may be beneficial.
Skeletal muscle metabo-sensitive afferents respond to several substances via stimulation of a variety of receptors. These receptors include TRPV1 channels which are nonselective cation channels known to be stimulated by a variety of stimuli such as temperature, and H+ ion concentration (18, 50–53). Recent studies have investigated the impact of stimulating or antagonizing TRPV1-expressing afferents using capsaicin, capsazepine, their analogs, and other various pharmaceutical compounds (33, 54–61). These studies found that activation of TRPV1 receptors has been linked to not only initiation of blood pressure responses but also the sensation of nociceptive pain (56, 57, 62–68). Short-term attenuation of groups III and IV afferents including those expressing TRPV1, during exercise, decreases the sensation of muscle fatigue and the pressor responses during exercise via activation of μ opioid channels. (69–71). To what extent TRPV1 receptors are stimulated during exercise and mediate the muscle metaboreflex responses is controversial (23, 33, 57, 58, 72–75). However, group IV afferents contain TRPV1 receptors (76), and thus this can be used as a tool to alter their activity. Our goal was to assess if metaboreflex responses can be chronically attenuated in a conscious model using the availability of TRPV1 receptors on metabo-sensitive afferents as a tool for ablation. We hypothesized that chronic ablation of TRPV1-expressing afferent neurons in the hindlimbs could be used to chronically reduce the strength or gain of the muscle metaboreflex. In a longitudinally designed study, we first established the strength of the muscle metaboreflex in chronically instrumented canines during mild exercise. We then used an ultrapotent-dominant negative agonist of capsaicin, resiniferatoxin (RTX) injected into the intrathecal space to partially destroy afferents expressing TRPV1 receptors. We repeated the experiments from 7 to 78 ± 14 days after injection and found that the strength of the muscle metaboreflex was chronically attenuated by over 50%. We conclude that this approach could be useful to attenuate exaggerated sympathetic activation during exercise in patients with cardiovascular disease.
METHODS
Experimental Subjects
Six adult mongrel canines (1 male, 5 females) of ∼19–25 kg were selected based on their willingness to walk on a motor-driven treadmill. We have previously shown that sex does not affect the strength or mechanisms of muscle metaboreflex activation (77). Canines were acclimatized to exercise at a workload of 3.2 km/h with a 0% grade. All experimental and surgical procedures used for this study comply with the National Institutes of Health Guide to the Care and Use of Laboratory Animals and were approved by the Wayne State University Institutional Animal Care and Use Committee (IACUC). Animals used in this study underwent a 14-day acclimation period with the laboratory surroundings and personnel and exercised on their own volition during all experiments.
Surgical Instrumentation
All animals underwent a series of surgical procedures over a period of 4 wk using standard aseptic surgical techniques. Before each surgery, animals were sedated with an intramuscular injection of acepromazine (0.4–0.5 mg/kg) and administered a subcutaneous injection of slow-release analgesic buprenorphine SR (0.03 mg/kg) 30 min before induction of anesthesia. Induction was achieved by intravenous administration of ketamine (5 mg/kg) and diazepam (0.2–0.3 mg/kg). Anesthesia was maintained pre and intra-operatively with (1%–3%) isoflurane gas. Additional preoperative analgesic included intravenous administration of carprofen (4.4 mg/kg). Postoperatively acepromazine (0.2–0.3 mg/kg iv) and buprenorphine (0.01–0.03 mg/kg im) were administered as needed. Acute proactive antibiotic (cephalexin 30 mg/kg iv) was administered pre- and postoperatively. Prophylactic antibiotic (cephalexin 30 mg/kg orally twice a day) were administered to prevent microbial infection.
The first surgical procedure was a left thoracotomy through the left 3rd/4th intercostal space to expose the heart. The pericardium was incised and parted for placement of a telemetric pressure transducer tip (TA11 PA-D70, DSI) into the apex of the left ventricle that was tunneled from the telemeter body tethered subcutaneously caudal to the thoracotomy incision site. The upper section of pericardium was then retracted dorsally to expose the aorta and pulmonary vein. A section of the aortic trunk was used for placement of a blood flow transducer (20 PAU, Transonic Systems) for measure of cardiac output. Unrelated to the investigations of this study, four stainless steel pacing leads (0-FLexon, Ethicon) were attached to the right ventricle free wall. All leads and cables were tunneled subcutaneously and exteriorized between the scapulae. The pericardium and ribs were reapproximated and the chest was closed in layers.
The final surgical procedure occurred at a minimum of 14 days postthoracotomy. A left retroperitoneal approach was used to access the terminal aorta. All arterial blood vessels caudal to the renal artery and cranial to the edge of the incision above the iliac crest were ligated. The most accessible cranial arterial branch caudal to the renal artery was catheterized with a 19-gauge polyvinyl catheter (Tygon, S54-HL, Norton) for measure of arterial pressure. In order, cranial to caudal, placed caudal to the arterial catheter, a blood flow transducer (10 PAU, Transonic Systems) and two 8–10 mm hydraulic occluders (DocXS Biomedical Products) were placed to measure and manipulate hindlimb blood flow. All cables and occluder lines were tunneled subcutaneously and exteriorized between the scapulae.
Intrathecal RTX Administration and Terminal Capsaicin Response
Administration of RTX was performed after completion of all control experiments. Before this procedure animals were sedated, induced, anesthetically maintained, and treated with the same analgesics and aseptic techniques as described in Surgical Instrumentation. Flow probes and pressure transducers were connected. Heart rate and mean arterial pressure responses were taken during a 1-min steady state before administration of each pharmaceutical agent, and peak responses postadministration for analysis. Before placement of the spinal needle, intra-arterial capsaicin (20 µg/kg) was administered through the catheter placed in the terminal aorta. After recovery from capsaicin administration a 20- to 22-gauge spinal needle was placed into the intrathecal space between the L4–L5 and L5–L6 vertebrae. Placement of the spinal needle was done based on animal positioning, alignment, and successful observation of cerebrospinal fluid through the needle. Spinal fluid equal to the volume amount of intrathecal RTX administered to reach the desired dose volume (2 µg/kg) plus 0.5 mL to use as a flush was removed. RTX was then administered through the spinal needle at a concentration of 2 µg/kg followed by flush of 0.5 mL spinal fluid and removal of the spinal needle. After ∼30–60 min and recovery from responses to RTX, a second intra-arterial injection of capsaicin (20 µg/kg) was performed. Animals were monitored postoperatively and administered acepromazine (0.2–0.3 mg/kg iv) and buprenorphine (0.01–0.03 mg/kg im) as needed. Metaboreflex experiments were repeated after a 7- to 10-day recovery period. After completion of all experiments a final terminal anesthetic procedure was performed to assess the long-term efficacy of the RTX injection. Animals were sedated, induced, treated with analgesics, and anesthetically maintained as described above in the surgical procedures. All equipment was connected and steady-state values of in vivo hemodynamics were taken during a 1-min steady-state before intra-arterial administration of capsaicin (20 µg/kg).
Data Acquisition
Animals were acclimated 10–20 min in the laboratory setting and then led to the treadmill and equipment. Arterial pressure was measured by connection of the 19-gauge polyvinyl catheter (Tygon, S54-HL, Norton) to a pressure transducer (Transpac iv, ICU Medical). Left ventricular pressure telemeter (DSI) and blood flow transducers were connected to their appropriate instrumentation. All flows and pressures were monitored and recorded in real time through an A/D converter (IWorx). A/D output was run through Labscribe4 acquisition and analysis software (IWorx).
Experimental Procedures
All hemodynamic parameters were measured under steady-state conditions during rest, mild free flow exercise (3.2 km/h 0% grade), and sustained mild free flow exercise with successive reductions in hindlimb blood flow used to activate the muscle metaboreflex. Hydraulic vascular occluders (DocXS) were used to achieve reductions in hindlimb blood flow to ∼30% of free flow conditions during exercise for both control and RTX-treated animals. Experiments were repeated after RTX, thus each animal served as its own control in this longitudinally designed study.
Analysis
All in vivo hemodynamic parameters were continuously recorded during each experiment. One-minute averaged steady-state values were taken after a 3-min acclimation period at rest, free flow exercise, and during steady-state at each reduction of hindlimb blood flow (HLBF). Measures of mean arterial pressure, cardiac output, and hindlimb blood flow were measured in vivo. Heart rate was derived from the cardiac output waveform. All other hemodynamic parameters were mathematically derived from in vivo measurements.
Statistical Analysis
Systat Software (Systat 13.0) was used for statistical analysis. Data are reported as means ± SE and statistical significance was determined by an α level of P < 0.05. Data were analyzed using a two-way ANOVA for repeated measures, and when a significant interaction was observed, individual means were compared using C-matrix test for simple effects. Direct observations on changes in muscle metaboreflex gain, and comparison in changes observed during surgical procedures were assessed by a Student’s paired t test.
RESULTS
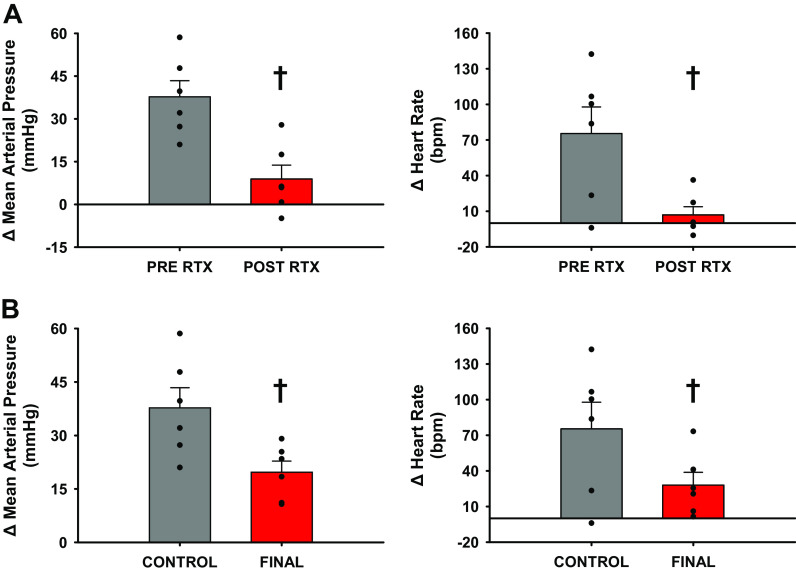
Figure 1A shows the pressor responses to intra-arterial capsaicin (20 µg/kg) before and ∼30 min post-RTX injection. RTX markedly attenuated the pressor and tachycardic responses to capsaicin. Figure 1B shows the responses to intra-arterial capsaicin performed in a terminal procedure 78 ± 14 days after intrathecal administration of RTX. Compared to the responses observed before RTX, the pressor and tachycardic responses to capsaicin were significantly attenuated by ∼50%.
Figure 1.
A: intraoperative responses to intra-arterial administration of capsaicin (20 µg/kg) while under isoflurane anesthesia. Pre-RTX (gray) and post-RTX (red) are the Δ responses comparing a 1-min steady-state before capsaicin administration to the maximal response for each variable after administration. B: intraoperative responses to intra-arterial capsaicin (20 µg/kg) before administration of RTX and during a terminal experiment performed 78 ± 14 days postinjection of RTX. Bar graphs show standard error of the mean with individual data points overlain. Standard error shown on bar graphs with actual data points overlain. †Statistical significance is shown compared with previous condition (P < 0.05, Student’s paired t test), n = 6 animals, RTX, resiniferatoxin.
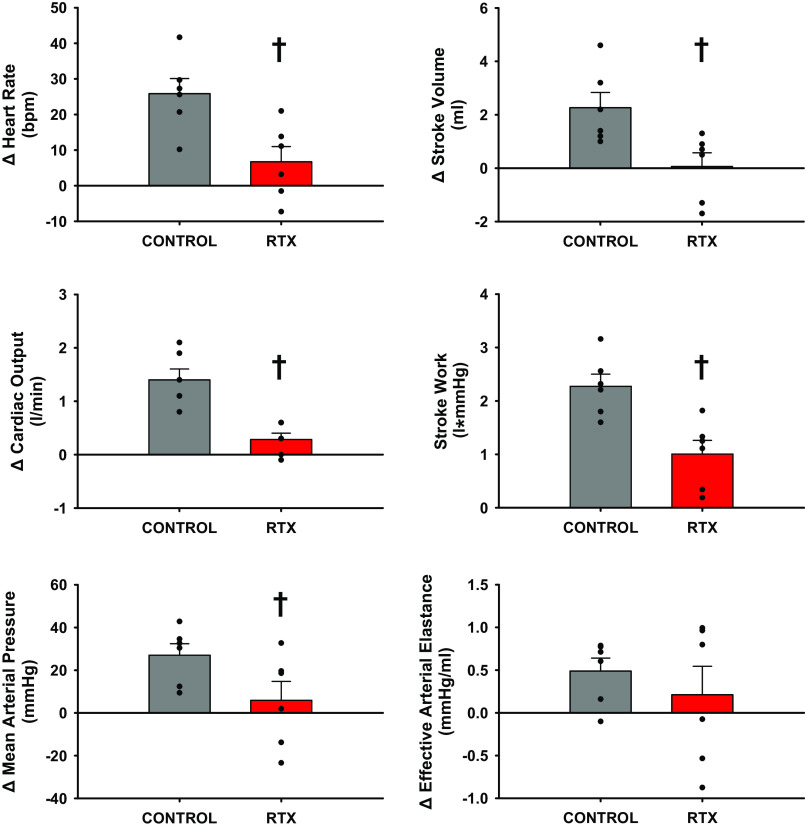
Figure 2 shows that in control experiments, heart rate (HR), stroke volume (SV), cardiac output (CO), and stroke work (SW) increased significantly from rest to exercise, whereas there were no significant changes in mean arterial pressure (MAP) or effective arterial elastance [EaZ, an index of vascular load (42, 78)] likely as a result of the significant increases in nonischemic vascular conductance (NIVC, vascular conductance to all vascular beds except the hindlimbs calculated as (CO-HLBF)/MAP). During muscle metaboreflex activation HR, SV, CO, SW, EaZ, and MAP, all increased significantly with no significant increases in NIVC when compared to the free flow exercise condition. Intrathecal RTX injection had no effect on MAP, HR, SV, CO, NIVC, or EaZ at rest and during steady-state exercise. The significant increase in stroke work from rest to steady state exercise was lost. However, after RTX muscle metaboreflex responses were virtually abolished: only SW showed a small but statistically significant increases in response to similar reductions in HLBF.
Figure 2.
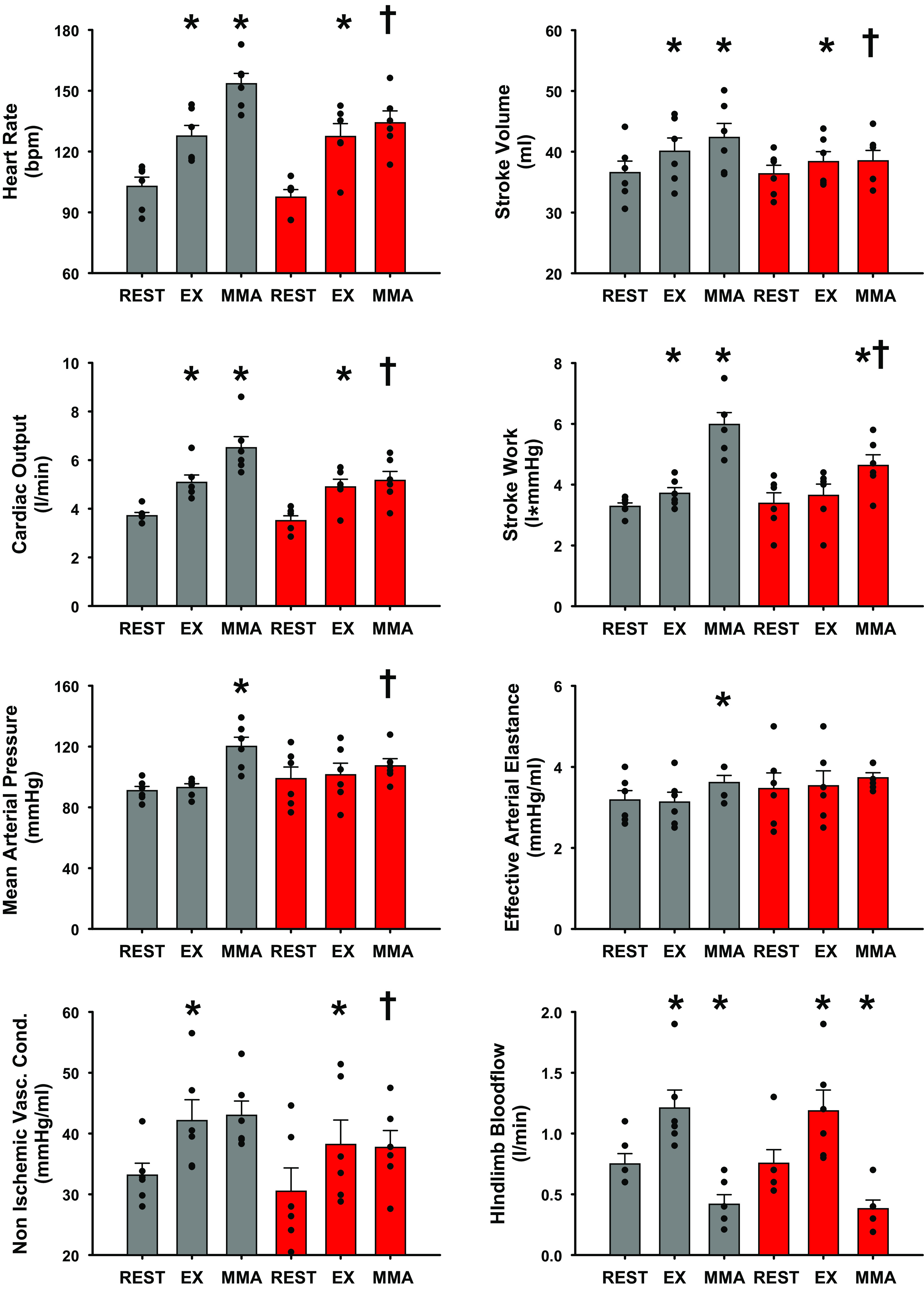
One-minute averaged hemodynamic responses taken during steady states at rest, exercise (3.2 km/h, 0% grade), and exercise with muscle metaboreflex activation before (gray) and after administration of intrathecal RTX (2 µg/kg) (L4–L6) (red). Bar graphs show means ± SE with individual data points overlain. Standard error shown on bar graphs with actual data points overlain. *Statistical significance (P < 0.05) compared with previous workload. †Significance to previous condition (P < 0.05, Two way ANOVA), n = 6 animals, Ex, exercise; MMA, muscle metaboreflex activation; RTX, resiniferatoxin.
Figure 3 shows a comparison of changes observed in the hemodynamic values between steady-state mild exercise (3.2 km/h 0% grade) and steady-state maximal muscle metaboreflex activation. Increases in HR and SV were significantly attenuated which thereby lowered CO after intrathecal administration of RTX. The significant reduction in the increase of MAP after intrathecal RTX is primarily a result of the lack of CO response and a reduction in stroke work. The rise in EaZ was not significantly different before or after administration of RTX.
Figure 3.
Average change between 1-min steady-state values taken during exercise (3.2 km/h, 0% grade) and during exercise with muscle metaboreflex activation before (gray) and after administration of intrathecal RTX (2 µg/kg) (L4–L6) (red). Standard error shown on bar graphs with actual data points overlain. †Statistical significance (P < 0.05, Student’s paired t test) compared with previous condition (n = 6 animals). RTX, resiniferatoxin.
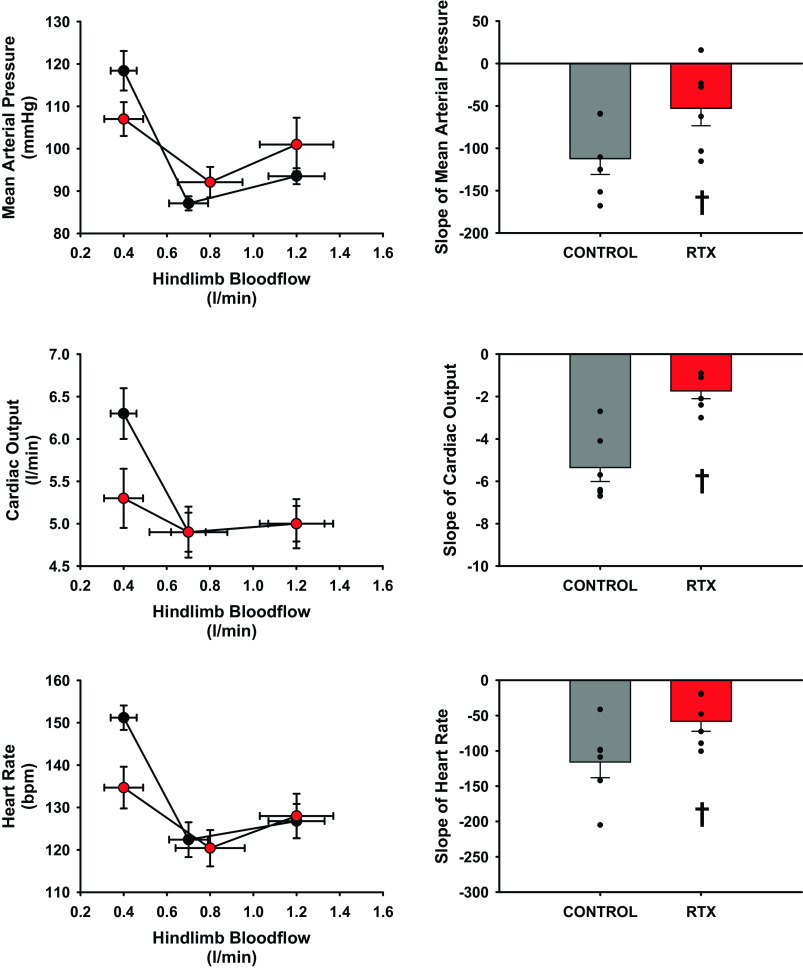
Figure 4 is a comparison of the reflex gain defined as the slope of the regression line from the threshold of reflex to peak muscle metaboreflex activation of between MAP, CO, and HR versus hindlimb blood flow before and after RTX. The slopes of MAP, CO, and HR were significantly attenuated after treatment with RTX. The slope of CO relationship showed the largest reduction by ∼70% compared with reductions of MAP and HR of ∼50%.
Figure 4.
Assessment of changes in the gain of the muscle metaboreflex determined by the slope of the linear regression taken from 1-min averaged steady states at each successive reduction in hindlimb blood flow from threshold to peak reflex activation before (black circles, gray bars) and after administration of RTX (red circles, red bars). Line graphs should be observed from the right point (exercise) to the middle point (reflex threshold) to the final point left (peak muscle metaboreflex activation) as hindlimb blood flow is reduced. Standard error shown on bar graphs with actual data points overlain. †Statistical significance between control (gray) and after administration of intrathecal RTX (2 µg/kg) (L4–L6) (red) (P < 0.05, Student’s paired t test), n = 6 animals. RTX, resiniferatoxin.
DISCUSSION
This is the first study to demonstrate that the cardiovascular responses to muscle metaboreflex activation evoked during volitional treadmill exercise in conscious animals, can be chronically attenuated by ablation of TRPV1-sensitive afferents via intrathecal administration of RTX. We determined that intrathecal RTX attenuates the muscle metaboreflex primarily through significant reductions in heart rate and stroke volume and attenuation of ventricular performance as indexed by stroke work. These reduced responses lower the reflex increase in cardiac output. Inasmuch as in normal subjects during submaximal dynamic exercise, the increase in cardiac output is the major mechanism mediating the rise in arterial pressure, with the reduced rise in cardiac output the metaboreflex pressor response was significantly attenuated.
Traditionally, the muscle metaboreflex increases cardiac output via the combined effects of tachycardia, increased ventricular contractility, and enhanced central blood volume mobilization (10, 11, 34, 39). This reflex arises due to the activation of metabo-sensitive skeletal muscle afferents (19, 23, 24, 26, 27, 32, 79). These afferents are primarily group IV, but some group III afferents, which primarily respond to mechanical stimulation, are also metabo-sensitive (23, 24, 33, 58, 76, 80). These metabo-sensitive afferents respond to TRPV1 agonists (33, 50, 56, 76, 81). Although, these afferents respond to TRPV1 receptor activation, there is controversy regarding whether these receptors mediate the muscle metaboreflex under physiological conditions. Studies in unanesthetized, acutely decerebrated rats (58, 72, 74, 75) and cats (73) concluded that TRPV1 receptors are not responsible for activation of the muscle metaboreflex. Most of the evidence in these studies is centered around the nonphysiological conditions required to activate TRPV1, limited activators, and the inability of TRPV1 inhibition to prevent reflex responses to static muscle contraction and skeletal muscle circulatory occlusion (58, 72, 73, 75). Alternatively, others have reported that muscle metaboreflex responses can be attenuated by antagonism of TRPV1 receptors (33, 57). TRPV1 receptor sensitivity can vary across species (52) and substances related to tissue injury (82–84) can interact with TRPV1 receptors and possibly reduce activation thresholds (85). Therefore, there is potential for TRPV1 activation to elicit or enhance pressor responses via an altered inflammatory state such as heart failure and peripheral arterial disease. In chronic heart failure, TRPV1 expression is downregulated in the skeletal muscle (86), and this may be a result of overactivation due to tissue injury as a result of hypoperfusion, although no study to date has addressed this aspect of TRPV1 receptors. Our study neither supports nor refutes the possible implications of TRPV1 activation eliciting the muscle metaboreflex. We used the ability to selectively ablate capsaicin-sensitive skeletal muscle afferents that express ASIC, P2X, and EP receptors that are known modulators of the muscle metaboreflex (21, 81, 87–91). These receptors are coexpressed with the TRPV1 receptor and thus are also eliminated with the use of resiniferatoxin. Thus, the existence of TRPV1 receptors on group IV afferents served as a convenient tool to ablate these neurons which contain a variety of other receptors likely involved in triggering metaboreflex responses.
In the present study, we used a noncompetitive agonist to ablate TRPV1-sensitive afferent fibers in the hindlimb skeletal muscle. Intrathecal RTX itself usually caused a pressor response likely due to irreversible activation of the TRPV1 receptors. Thirty minutes to one hour later, the reflex responses to intra-arterial capsaicin were significantly attenuated. Metaboreflex experiments were performed 7 to 78 ± 14 days after RTX and the responses were consistently reduced. In the terminal experiment, the responses to intra-arterial capsaicin was reduced by ∼50%, indicating sustained significant ablation of afferents containing TRPV1 receptors after 78 ± 14 days. The lack of complete ablation is likely a result of multiple factors such as toxin volume delivered, intrathecal flow, and the levels of TRPV1 receptors available per neuron to accept RTX and achieve the level of cation flow required for cytotoxicity. Furthermore, expression patterns of TRPV1 neurons likely vary per neuron and their upregulation and downregulation from various stimuli may play a role in injection efficacy. Wang et al. (86) showed that TRPV1 protein levels can vary based on pathological state possibly acting as a compensatory mechanism in response to enhanced levels of metabolites in muscle with reduced blood supply.
Peripheral vascular responses were altered post-RTX. We observed a significant reduction in nonischemic vascular resistance during muscle metaboreflex activation likely as a result of reduced β2-mediated vasodilation observed in previous studies (16,17, 38). We have shown that muscle metaboreflex activation enhances ventricular maximal elastance and effective arterial elastance such that ventricular-vascular coupling is maintained, and stroke work is optimal (11, 42). In this study, intrathecal RTX significantly attenuated increases in stroke work with no significant changes in effective arterial elastance. Reductions in stroke work is probable evidence of some degree of ventricular-vascular uncoupling, likely a result of reductions in ventricular maximal elastance during muscle metaboreflex activation post-RTX. In addition to overall reductions in hemodynamic responses to graded reductions in hindlimb blood flow, we also observed significant reductions in the gain of the muscle metaboreflex. Calculation of muscle metaboreflex gain (strength) is quantified via the slope of the regression line between a given hemodynamic parameter versus the reduction in hindlimb blood flow once beyond metaboreflex threshold. We observed that not only are maximum hemodynamic values attenuated but also the slope of mean arterial pressure, heart rate, and cardiac output relationships versus hindlimb blood flow are significantly reduced with no change in threshold, indicating that reflex gain is reduced for all observed hemodynamic parameters. These experiments were performed only during mild exercise, and to what extent metaboreflex responses after RTX are different at higher workloads remains to be investigated.
Limitations
This study used resiniferatoxin and the availability of TRPV1 receptors on skeletal muscle afferents to achieve a chronic ablation. We did not address the role of TRPV1 receptors in mediating the muscle metaboreflex. Furthermore, no specific compounds traditionally used to assess metaboreflex activation in cats and rats such as lactic acid, adenosine, or potassium were used to assess any residual metaboreflex capabilities with or without intact TRPV1-expressing afferents.
We did not quantify exercise capacity or tolerance before or after administration of RTX. The animals exercised voluntarily and received no negative stimuli to reinforce exercise. Anecdotally, we did observe what appeared to be improved exercise performance during occlusions after administration of RTX. Amann et al. (69, 92–94) concluded that anesthetization of skeletal muscle afferents via intrathecal fentanyl infusion in humans lessened the sensation of fatigue during leg exercise, which could improve exercise tolerance.
In this study, we were unable to directly address if ablation of group IV afferents causes enhanced group III mechanosensitive afferent activation as was concluded by Smith et al. (58). However, RTX did not affect the normal cardiovascular responses to steady-state mild exercise before metaboreflex activation indicating that there was no enhanced activation of skeletal muscle mechano-receptors at this workload. We observed that the muscle metaboreflex was significantly reduced after application of RTX; however, there was not a complete loss of the response. This could be a result of group III afferent compensation, or more likely a result of still intact group IV afferents due to inefficient toxin delivery, lack of TRPV1 quantity and availability to interact with RTX and induce neuronal toxicity, or lastly compensation from alternative cardiovascular reflexes such as the baroreflex.
No morphological analyses of dorsal root ganglia were conducted during this study to show any potential off-target sensory neuron ablation. Sensory afferent function was conducted after ablation by veterinary staff to address any loss of proprioception in the hindlimb and none was noted. Morphological analyses have been conducted using this technique and found that RTX ablates dorsal root ganglia in a canine model of bone cancer (95, 96) and no off-target effects were noted in those studies. We are confident that our functional assessments (responses to capsaicin infusion and metaboreflex activation) strongly support the conclusions that RTX administration partially ablated group IV afferents which mediate the muscle metaboreflex.
Perspectives and Significance
Several cardiovascular diseases (e.g., heart failure, hypertension, and peripheral vascular disease) alter the strength and mechanisms of the muscle metaboreflex (15, 34, 43, 46–49). Impaired ventricular function can occur not only from the direct effect of the disease, but also from enhanced reflex coronary vasoconstriction (15, 48), which thereby contributes to the attenuated ability to raise cardiac output and thus correct metabolic mismatches in ischemic active skeletal muscle. Not only the heart, but also the ischemic muscle becomes a target for metaboreflex-induced vasoconstriction (17). Inability to improve perfusion of ischemic active skeletal muscle in heart failure causes enhanced sympathetic activation due to overactivation of the muscle metaboreflex (15, 17). In heart failure, ventricular-vascular interaction is already uncoupled and enhanced sympathetic outflow further decouples this relationship, which thereby lessens the ability to sustain or improve skeletal muscle blood flow (42). Impaired ventricular function and ventricular-vascular interactions leads to an enhanced vasoconstriction even within the ischemic muscle (17). Limitations in raising skeletal muscle blood flow are thus both the cause and effect of an enhanced metabolic mismatch that causes the enhanced sympathetic activation in heart failure and likely other cardiovascular pathologies such as hypertension (48, 49, 70, 97, 98). To date, no study has assessed the effect of chronic chemical ablation of skeletal muscle afferents in cardiovascular disease during exercise. A common symptom of these pathologies is exercise intolerance and fatigue, such that quality of life and the will to exercise are reduced. Studies have shown that exercise training regimens are capable of improving, and in some cases, correcting abnormal cardiovascular responses during exercise (62, 99–106). Furthermore, multiple studies have shown that attenuation of skeletal muscle afferents by way of activation of μ opioid receptors located on lower body afferents improves cardiovascular responses during exercise and reduces fatigue (69, 70, 79, 92, 107). In the present study, we observed that a reduction of skeletal muscle afferents via intrathecal RTX was capable of significantly attenuating muscle metaboreflex responses during exercise. In addition, anecdotally we observed animals treated with RTX could maintain workload performance with greater reduction in hindlimb blood flow. Direct experimental assessments of fatigue and performance were not measured and need to be addressed in future studies involving RTX to address if treatment may also lessen the sensation of fatigue during exercise as were observed in studies utilizing intrathecal opioids (57, 68–70, 79, 90–94). Thus, chronic ablation of skeletal muscle afferents may allow greater exercise-induced increases in cardiovascular fitness, less exercise intolerance, and improved overall quality of life for patients with various cardiovascular pathologies.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants HL-55473, HL-126706, and HL-120822, and in part supported by NIGMS/NIH R25-GM058905.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M. and D.S.O'L. conceived and designed research; J.M., M.-H.A., B.L., A.A., D.S., and D.S.O'L. performed experiments; J.M., M.-H.A., B.L., A.A., D.S., and D.S.O'L. analyzed data; J.M. and D.S.O'L. interpreted results of experiments; J.M. and D.S.O'L. prepared figures; J.M. and D.S.O'L. drafted manuscript; J.M. and D.S.O'L. edited and revised manuscript; J.M. and D.S.O'L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Audrey Nelson for expert technical assistance and animal care.
REFERENCES
- 1.Augustyniak RA, Ansorge EJ, O'Leary DS. Muscle metaboreflex control of cardiac output and peripheral vasoconstriction exhibit different latencies. Am J Physiol Heart Circ Physiol 278: H530–H537, 2000. doi: 10.1152/ajpheart.2000.278.2.H530. [DOI] [PubMed] [Google Scholar]
- 2.Boushel R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol (Oxf) 199: 367–383, 2010. doi: 10.1111/j.1748-1716.2010.02133.x. [DOI] [PubMed] [Google Scholar]
- 3.Choi HM, Stebbins CL, Nho H, Kim KA, Kim C, Kim JK. Skeletal muscle metaboreflex is enhanced in postmenopausal women. Eur J Appl Physiol 112: 2671–2678, 2012. doi: 10.1007/s00421-011-2245-0. [DOI] [PubMed] [Google Scholar]
- 4.Crisafulli A, Piras F, Filippi M, Piredda C, Chiappori P, Melis F, Milia R, Tocco F, Concu A. Role of heart rate and stroke volume during muscle metaboreflex-induced cardiac output increase: differences between activation during and after exercise. J Physiol Sci 61: 385–394, 2011. doi: 10.1007/s12576-011-0163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crisafulli A, Salis E, Pittau G, Lorrai L, Tocco F, Melis F, Pagliaro P, Concu A. Modulation of cardiac contractility by muscle metaboreflex following efforts of different intensities in humans. Am J Physiol Heart Circ Physiol 291: H3035–H3042, 2006. doi: 10.1152/ajpheart.00221.2006. [DOI] [PubMed] [Google Scholar]
- 6.Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJ, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc 35: 221–228, 2003. doi: 10.1249/01.MSS.0000048639.02548.24. [DOI] [PubMed] [Google Scholar]
- 7.Donal S, O'Leary RAA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1399–H1403, 1999. doi: 10.1152/ajpheart.1999.276.4.H1399. [DOI] [PubMed] [Google Scholar]
- 8.Ichinose M, Sala-Mercado JA, Coutsos M, Li Z, Ichinose TK, Dawe E, Fano D, O'Leary DS. Dynamic cardiac output regulation at rest, during exercise, and muscle metaboreflex activation: impact of congestive heart failure. Am J Physiol Regul Integr Comp Physiol 303: R757–R768, 2012. doi: 10.1152/ajpregu.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur J, Alvarez A, Hanna HW, Krishnan AC, Senador D, Machado TM, Altamimi YH, Lovelace AT, Dombrowski MD, Spranger MD, O'Leary DS. Interaction between the muscle metaboreflex and the arterial baroreflex in control of arterial pressure and skeletal muscle blood flow. Am J Physiol Heart Circ Physiol 311: H1268–H1276, 2016. doi: 10.1152/ajpheart.00501.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol Heart Circ Physiol 275: H220–H224, 1998. doi: 10.1152/ajpheart.1998.275.1.H220. [DOI] [PubMed] [Google Scholar]
- 11.Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O'Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290: H751–H757, 2006. doi: 10.1152/ajpheart.00869.2005. [DOI] [PubMed] [Google Scholar]
- 12.Sheriff DD, Augustyniak RA, O'Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998. doi: 10.1152/ajpheart.1998.275.3.H767. [DOI] [PubMed] [Google Scholar]
- 13.Shoemaker JK, Mattar L, Kerbeci P, Trotter S, Arbeille P, Hughson RL. WISE 2005: stroke volume changes contribute to the pressor response during ischemic handgrip exercise in women. J Appl Physiol (1985) 103: 228–233, 2007. doi: 10.1152/japplphysiol.01334.2006. [DOI] [PubMed] [Google Scholar]
- 14.Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O'Leary DS. Muscle metaboreflex-induced coronary vasoconstriction functionally limits increases in ventricular contractility. J Appl Physiol (1985) 109: 271–278, 2010. doi: 10.1152/japplphysiol.01243.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O'Leary DS. Muscle metaboreflex-induced coronary vasoconstriction limits ventricular contractility during dynamic exercise in heart failure. Am J Physiol Heart Circ Physiol 304: H1029–H1037, 2013. doi: 10.1152/ajpheart.00879.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur J, Machado TM, Alvarez A, Krishnan AC, Hanna HW, Altamimi YH, Senador D, Spranger MD, O'Leary DS. Muscle metaboreflex activation during dynamic exercise vasoconstricts ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 309: H2145–H2151, 2015. doi: 10.1152/ajpheart.00679.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaur J, Senador D, Krishnan AC, Hanna HW, Alvarez A, Machado TM, O'Leary DS. Muscle metaboreflex-induced vasoconstriction in the ischemic active muscle is exaggerated in heart failure. Am J Physiol Heart Circ Physiol 314: H11–H18, 2018. doi: 10.1152/ajpheart.00375.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelhamid RE, Kovács KJ, Honda CN, Nunez MG, Larson AA. Resiniferatoxin (RTX) causes a uniquely protracted musculoskeletal hyperalgesia in mice by activation of TRPV1 receptors. J Pain 14: 1629–1641, 2013. doi: 10.1016/j.jpain.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol (1985) 82: 1811–1817, 1997. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- 20.O'Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995. doi: 10.1152/ajpheart.1995.268.3.H980. [DOI] [PubMed] [Google Scholar]
- 21.Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol (1985) 94: 1437–1445, 2003. doi: 10.1152/japplphysiol.01011.2002. [DOI] [PubMed] [Google Scholar]
- 22.Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol Heart Circ Physiol 280: H2153–H2161, 2001. doi: 10.1152/ajpheart.2001.280.5.H2153. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol Respir Environ Exerc Physiol 55: 105–112, 1983. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol Respir Environ Exerc Physiol 57: 644–650, 1984. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 26.Pickar JG, Hill JM, Kaufman MP. Dynamic exercise stimulates group III muscle afferents. J Neurophysiol 71: 753–760, 1994. doi: 10.1152/jn.1994.71.2.753. [DOI] [PubMed] [Google Scholar]
- 27.Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol (1985) 64: 2306–2313, 1988. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- 28.Sheriff DD. Latency of muscle chemoreflex to vascular occlusion of active muscle during dynamic exercise. Am J Physiol Heart Circ Physiol 272: H1981–H1985, 1997. doi: 10.1152/ajpheart.1997.272.4.H1981. [DOI] [PubMed] [Google Scholar]
- 29.Sheriff DD, Wyss CR, Rowell LB, Scher AM. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise? Am J Physiol Heart Circ Physiol 253: H1199–H1207, 1987. doi: 10.1152/ajpheart.1987.253.5.H1199. [DOI] [PubMed] [Google Scholar]
- 30.Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol (1985) 66: 429–436, 1989. doi: 10.1152/jappl.1989.66.1.429. [DOI] [PubMed] [Google Scholar]
- 31.Sinoway LI, Smith MB, Enders B, Leuenberger U, Dzwonczyk T, Gray K, Whisler S, Moore RL. Role of diprotonated phosphate in evoking muscle reflex responses in cats and humans. Am J Physiol Heart Circ Physiol 267: H770–H778, 1994. doi: 10.1152/ajpheart.1994.267.2.H770. [DOI] [PubMed] [Google Scholar]
- 32.Sinoway LI, Wroblewski KJ, Prophet SA, Ettinger SM, Gray KS, Whisler SK, Miller G, Moore RL. Glycogen depletion-induced lactate reductions attenuate reflex responses in exercising humans. Am J Physiol Heart Circ Physiol 263: H1499–H1505, 1992. doi: 10.1152/ajpheart.1992.263.5.H1499. [DOI] [PubMed] [Google Scholar]
- 33.Smith SA, Leal AK, Williams MA, Murphy MN, Mitchell JH, Garry MG. The TRPv1 receptor is a mediator of the exercise pressor reflex in rats. J Physiol 588: 1179–1189, 2010. doi: 10.1113/jphysiol.2009.184952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ansorge EJ, Augustyniak RA, Perinot ML, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, O'Leary DS. Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. Am J Physiol Heart Circ Physiol 288: H1381–H1388, 2005. doi: 10.1152/ajpheart.00985.2004. [DOI] [PubMed] [Google Scholar]
- 35.Fisher JP, Bell MP, White MJ. Cardiovascular responses to human calf muscle stretch during varying levels of muscle metaboreflex activation. Exp Physiol 90: 773–781, 2005. doi: 10.1113/expphysiol.2005.030577. [DOI] [PubMed] [Google Scholar]
- 36.Fisher JP, Seifert T, Hartwich D, Young CN, Secher NH, Fadel PJ. Autonomic control of heart rate by metabolically sensitive skeletal muscle afferents in humans. J Physiol 588: 1117–1127, 2010. doi: 10.1113/jphysiol.2009.185470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen J, Thomas GD, Jacobsen TN, Victor RG. Muscle metaboreflex triggers parallel sympathetic activation in exercising and resting human skeletal muscle. Am J Physiol Heart Circ Physiol 266: H2508–H2514, 1994. doi: 10.1152/ajpheart.1994.266.6.H2508. [DOI] [PubMed] [Google Scholar]
- 38.Kaur J, Spranger MD, Hammond RL, Krishnan AC, Alvarez A, Augustyniak RA, O'Leary DS. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in β2-mediated vasodilation. Am J Physiol Heart Circ Physiol 308: H524–H529, 2015. doi: 10.1152/ajpheart.00648.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Leary DS, Senador D, Augustyniak RA. Muscle metaboreflex-induced central blood volume mobilization in heart failure. Am J Physiol Heart Circ Physiol 316: H1047–H1052, 2019. doi: 10.1152/ajpheart.00805.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O'Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001. doi: 10.1152/ajpheart.2001.280.4.H1645. [DOI] [PubMed] [Google Scholar]
- 41.Shoemaker JK, Kunselman AR, Silber DH, Sinoway LI. Maintained exercise pressor response in heart failure. J Appl Physiol (1985) 85: 1793–1799, 1998. doi: 10.1152/jappl.1998.85.5.1793. [DOI] [PubMed] [Google Scholar]
- 42.Mannozzi J, Kaur J, Spranger MD, Al-Hassan MH, Lessanework B, Alvarez A, Chung CS, O'Leary DS. Muscle metaboreflex-induced increases in effective arterial elastance: effect of heart failure. Am J Physiol Regul Integr Comp Physiol 319: R1–R10, 2020. doi: 10.1152/ajpregu.00040.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sala-Mercado JA, Hammond RL, Kim JK, McDonald PJ, Stephenson LW, O'Leary DS. Heart failure attenuates muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 292: H2159–H2166, 2007. doi: 10.1152/ajpheart.01240.2006. [DOI] [PubMed] [Google Scholar]
- 44.White S, Patrick T, Higgins CB, Vatner SF, Franklin D, Braunwald E. Effects of altering ventricular rate on blood flow distribution in conscious dogs. Am J Physiol 221: 1402–1407, 1971. doi: 10.1152/ajplegacy.1971.221.5.1402. [DOI] [PubMed] [Google Scholar]
- 45.Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- 46.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000. doi: 10.1152/ajpheart.2000.278.3.H818. [DOI] [PubMed] [Google Scholar]
- 47.O'Leary DS, Sala-Mercado JA, Augustyniak RA, Hammond RL, Rossi NF, Ansorge EJ. Impaired muscle metaboreflex-induced increases in ventricular function in heart failure. Am J Physiol Heart Circ Physiol 287: H2612–H2618, 2004. doi: 10.1152/ajpheart.00604.2004. [DOI] [PubMed] [Google Scholar]
- 48.Spranger MD, Kaur J, Sala-Mercado JA, Krishnan AC, Abu-Hamdah R, Alvarez A, Machado TM, Augustyniak RA, O'Leary DS. Exaggerated coronary vasoconstriction limits muscle metaboreflex-induced increases in ventricular performance in hypertension. Am J Physiol Heart Circ Physiol 312: H68–H79, 2017. doi: 10.1152/ajpheart.00417.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spranger MD, Kaur J, Sala-Mercado JA, Machado TM, Krishnan AC, Alvarez A, O'Leary DS. Attenuated muscle metaboreflex-induced pressor response during postexercise muscle ischemia in renovascular hypertension. Am J Physiol Regul Integr Comp Physiol 308: R650–R658, 2015. doi: 10.1152/ajpregu.00464.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhaka A, Uzzell V, Dubin AE, Mathur J, Petrus M, Bandell M, Patapoutian A. TRPV1 is activated by both acidic and basic pH. J Neurosci 29: 153–158, 2009. doi: 10.1523/JNEUROSCI.4901-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elokely K, Velisetty P, Delemotte L, Palovcak E, Klein ML, Rohacs T, Carnevale V. Understanding TRPV1 activation by ligands: Insights from the binding modes of capsaicin and resiniferatoxin. Proc Natl Acad Sci USA 113: E137–145, 2016. doi: 10.1073/pnas.1517288113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, Zhang TJ, Viswanadhan VN, Toth A, Pearce LV, Vanderah TW, Porreca F, Blumberg PM, Lile J, Sun Y, Wild K, Louis JC, Treanor JJ. Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem 279: 20283–20295, 2004. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- 53.Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Arch 451: 143–150, 2005. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- 54.Hollis M, Wang DH. Transient receptor potential vanilloid in blood pressure regulation. Curr Opin Nephrol Hypertens 22: 170–176, 2013. doi: 10.1097/MNH.0b013e32835c8d4c. [DOI] [PubMed] [Google Scholar]
- 55.Horton JS, Buckley CL, Stokes AJ. Successful TRPV1 antagonist treatment for cardiac hypertrophy and heart failure in mice. Channels (Austin) 7: 17–22, 2013. doi: 10.4161/chan.23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jankowski MP, Rau KK, Ekmann KM, Anderson CE, Koerber HR. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol 109: 2374–2381, 2013. [Erratum in J Neurophysiol 113(2): 677, 2015]. doi: 10.1152/jn.01067.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol 589: 6191–6204, 2011. doi: 10.1113/jphysiol.2011.214429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith SA, Williams MA, Mitchell JH, Mammen PP, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation 111: 2056–2065, 2005. doi: 10.1161/01.CIR.0000162473.10951.0A. [DOI] [PubMed] [Google Scholar]
- 59.Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 64: 745–755, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshie K, Rajendran PS, Massoud L, Kwon O, Tadimeti V, Salavatian S, Ardell JL, Shivkumar K, Ajijola OA. Cardiac vanilloid receptor-1 afferent depletion enhances stellate ganglion neuronal activity and efferent sympathetic response to cardiac stress. Am J Physiol Heart Circ Physiol 314: H954–H966, 2018. doi: 10.1152/ajpheart.00593.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zahner MR, Li DP, Chen SR, Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol 551: 515–523, 2003. doi: 10.1113/jphysiol.2003.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antunes-Correa LM, Nobre TS, Groehs RV, Alves MJ, Fernandes T, Couto GK, Rondon MU, Oliveira P, Lima M, Mathias W, Brum PC, Mady C, Almeida DR, Rossoni LV, Oliveira EM, Middlekauff HR, Negrao CE. Molecular basis for the improvement in muscle metaboreflex and mechanoreflex control in exercise-trained humans with chronic heart failure. Am J Physiol Heart Circ Physiol 307: H1655–H1666, 2014. doi: 10.1152/ajpheart.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown DC. Resiniferatoxin: the evolution of the “molecular scalpel” for chronic pain relief. Pharmaceuticals (Basel) 9: 47, 2016. doi: 10.3390/ph9030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown JD, Saeed M, Do L, Braz J, Basbaum AI, Iadarola MJ, Wilson DM, Dillon WP. CT-guided injection of a TRPV1 agonist around dorsal root ganglia decreases pain transmission in swine. Sci Transl Med 7: 305ra145, 2015. doi: 10.1126/scitranslmed.aac6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leffler A, Mönter B, Koltzenburg M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience 139: 699–709, 2006. doi: 10.1016/j.neuroscience.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 66.Leo M, Schulte M, Schmitt LI, Schäfers M, Kleinschnitz C, Hagenacker T. Intrathecal resiniferatoxin modulates TRPV1 in DRG neurons and reduces TNF-induced pain-related behavior. Mediators Inflamm 2017: 2786427, 2017. doi: 10.1155/2017/2786427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sapio MR, Neubert JK, LaPaglia DM, Maric D, Keller JM, Raithel SJ, Rohrs EL, Anderson EM, Butman JA, Caudle RM, Brown DC, Heiss JD, Mannes AJ, Iadarola MJ. Pain control through selective chemo-axotomy of centrally projecting TRPV1+ sensory neurons. J Clin Invest 128: 1657–1670, 2018. doi: 10.1172/JCI94331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vianna LC, Fernandes IA, Barbosa TC, Teixeira AL, Nóbrega ACL. Capsaicin-based analgesic balm attenuates the skeletal muscle metaboreflex in healthy humans. J Appl Physiol (1985) 125: 362–368, 2018. doi: 10.1152/japplphysiol.00038.2018. [DOI] [PubMed] [Google Scholar]
- 69.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283, 2009. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barbosa TC, Vianna LC, Fernandes IA, Prodel E, Rocha HN, Garcia VP, Rocha NG, Secher NH, Nobrega AC. Intrathecal fentanyl abolishes the exaggerated blood pressure response to cycling in hypertensive men. J Physiol 594: 715–725, 2016. doi: 10.1113/JP271335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Estrada JA, Kaufman MP. Mu opioid receptors inhibit the exercise pressor reflex by closing N-type calcium channels but not by opening GIRK channels in rats. Am J Physiol Regul Integr Comp Physiol 314: R693–R699, 2018. doi: 10.1152/ajpregu.00380.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ducrocq GP, Estrada JA, Kim JS, Kaufman MP. Blocking the transient receptor potential vanilloid-1 does not reduce the exercise pressor reflex in healthy rats. Am J Physiol Regul Integr Comp Physiol 317: R576–R587, 2019. doi: 10.1152/ajpregu.00174.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kindig AE, Heller TB, Kaufman MP. VR-1 receptor blockade attenuates the pressor response to capsaicin but has no effect on the pressor response to contraction in cats. Am J Physiol Heart Circ Physiol 288: H1867–H1873, 2005. doi: 10.1152/ajpheart.00735.2004. [DOI] [PubMed] [Google Scholar]
- 74.Li J, Maile MD, Sinoway AN, Sinoway LI. Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol (1985) 97: 1709–1714, 2004. doi: 10.1152/japplphysiol.00389.2004. [DOI] [PubMed] [Google Scholar]
- 75.Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010. doi: 10.1152/ajpheart.00141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with ending in skeletal muscle. Circ Res 50: 133–139, 1982. doi: 10.1161/01.res.50.1.133. [DOI] [PubMed] [Google Scholar]
- 77.Laprad SL, Augustyniak RA, Hammond RL, O'Leary DS. Does gender influence the strength and mechanisms of the muscle metaboreflex during dynamic exercise in dogs? Am J Physiol Regul Integr Comp Physiol 276: R1203–R1208, 1999. doi: 10.1152/ajpregu.1999.276.4.R1203. [DOI] [PubMed] [Google Scholar]
- 78.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation 86: 513–521, 1992. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 79.Sidhu SK, Weavil JC, Mangum TS, Jessop JE, Richardson RS, Morgan DE, Amann M. Group III/IV locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin Neurophysiol 128: 44–55, 2017. doi: 10.1016/j.clinph.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. doi: 10.1113/jphysiol.2001.012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, Zipkin RE, Daddario N, Appendino G, Di Marzo V, Walker JM. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem 278: 13633–13639, 2003. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- 83.Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA 99: 8400–8405, 2002. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Olah Z, Karai L, Iadarola MJ. Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J Biol Chem 276: 31163–31170, 2001. doi: 10.1074/jbc.M101607200. [DOI] [PubMed] [Google Scholar]
- 85.Ji R-R, Samad TA, Jin S-X, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation Increases TRPV1 levels and maintains heat hyperalgesia. Neuron 36: 57–68, 2002. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 86.Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol 588: 5033–5047, 2010. doi: 10.1113/jphysiol.2010.199562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Campos MO, Mansur DE, Mattos JD, Paiva ACS, Videira RLR, Macefield VG, da Nóbrega ACL, Fernandes IA. Acid-sensing ion channels blockade attenuates pressor and sympathetic responses to skeletal muscle metaboreflex activation in humans. J Appl Physiol (1985) 127: 1491–1501, 2019. doi: 10.1152/japplphysiol.00401.2019. [DOI] [PubMed] [Google Scholar]
- 88.Greaney JL, Matthews EL, Boggs ME, Edwards DG, Duncan RL, Farquhar WB. Exaggerated exercise pressor reflex in adults with moderately elevated systolic blood pressure: role of purinergic receptors. Am J Physiol Heart Circ Physiol 306: H132–H141, 2014. doi: 10.1152/ajpheart.00575.2013. [DOI] [PubMed] [Google Scholar]
- 89.Notarius CF, Atchison DJ, Rongen GA, Floras JS. Effect of adenosine receptor blockade with caffeine on sympathetic response to handgrip exercise in heart failure. Am J Physiol Heart Circ Physiol 281: H1312–H1318, 2001. doi: 10.1152/ajpheart.2001.281.3.H1312. [DOI] [PubMed] [Google Scholar]
- 90.Queme LF, Ross JL, Lu P, Hudgins RC, Jankowski MP. Dual modulation of nociception and cardiovascular reflexes during peripheral ischemia through p2y1 receptor-dependent sensitization of muscle afferents. J Neurosci 36: 19–30, 2016. doi: 10.1523/JNEUROSCI.2856-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stone AJ, Copp SW, Kim JS, Kaufman MP. Combined, but not individual, blockade of ASIC3, P2X, and EP4 receptors attenuates the exercise pressor reflex in rats with freely perfused hindlimb muscles. J Appl Physiol (1985) 119: 1330–1336, 2015. doi: 10.1152/japplphysiol.00630.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol 589: 5299–5309, 2011. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amann M, Sidhu SK, Weavil JC, Mangum TS, Venturelli M. Autonomic responses to exercise: group III/IV muscle afferents and fatigue. Auton Neurosci 188: 19–23, 2015. doi: 10.1016/j.autneu.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MA, Jonathan Groot H, Walter Wray D, Stehlik J, Richardson RS. Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol 174: 368–375, 2014. doi: 10.1016/j.ijcard.2014.04.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown DC, Agnello K, Iadarola MJ. Intrathecal resiniferatoxin in a dog model: efficacy in bone cancer pain. Pain 156: 1018–1024, 2015. doi: 10.1097/j.pain.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, Olah Z, Mannes AJ. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology 103: 1052–1059, 2005. doi: 10.1097/00000542-200511000-00020. [DOI] [PubMed] [Google Scholar]
- 97.Carlson SH, Shelton J, White CR, Wyss JM. Elevated sympathetic activity contributes to hypertension and salt sensitivity in diabetic obese Zucker rats. Hypertension 35: 403–408, 2000. doi: 10.1161/01.hyp.35.1.403. [DOI] [PubMed] [Google Scholar]
- 98.Grassi G, Dell'Oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F, Gamba PL, Mancia G. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia 48: 1359–1365, 2005. doi: 10.1007/s00125-005-1798-z. [DOI] [PubMed] [Google Scholar]
- 99.Ahmet I, Lakatta EG, Talan MI. Pharmacological stimulation of beta2-adrenergic receptors (beta2AR) enhances therapeutic effectiveness of beta1AR blockade in rodent dilated ischemic cardiomyopathy. Heart Fail Rev 10: 289–296, 2005. doi: 10.1007/s10741-005-7543-3. [DOI] [PubMed] [Google Scholar]
- 100.Andrade DC, Arce-Alvarez A, Toledo C, Díaz HS, Lucero C, Schultz HD, Marcus NJ, Del Rio R. Exercise training improves cardiac autonomic control, cardiac function, and arrhythmogenesis in rats with preserved-ejection fraction heart failure. J Appl Physiol (1985) 123: 567–577, 2017. doi: 10.1152/japplphysiol.00189.2017. [DOI] [PubMed] [Google Scholar]
- 101.Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, Binder L, Topper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Loffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol 58: 1780–1791, 2011. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 102.Harthmann AD, De Angelis K, Costa LP, Senador D, Schaan BD, Krieger EM, Irigoyen MC. Exercise training improves arterial baro- and chemoreflex in control and diabetic rats. Auton Neurosci 133: 115–120, 2007. doi: 10.1016/j.autneu.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 103.Iellamo F, Legramante JM, Massaro M, Raimondi G, Galante A. Effects of a residential exercise training on baroreflex sensitivity and heart rate variability in patients with coronary artery disease: a randomized, controlled study. Circulation 102: 2588–2592, 2000. doi: 10.1161/01.cir.102.21.2588. [DOI] [PubMed] [Google Scholar]
- 104.Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrão CE, Rondon MU. Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension 49: 1298–1306, 2007. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- 105.Rame JE. Chronic heart failure: a reversible metabolic syndrome? Circulation 125: 2809–2811, 2012. doi: 10.1161/CIRCULATIONAHA.112.108316. [DOI] [PubMed] [Google Scholar]
- 106.Wang HJ, Pan YX, Wang WZ, Gao L, Zimmerman MC, Zucker IH, Wang W. Exercise training prevents the exaggerated exercise pressor reflex in rats with chronic heart failure. J Appl Physiol (1985) 108: 1365–1375, 2010. doi: 10.1152/japplphysiol.01273.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109: 966–976, 2010. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]