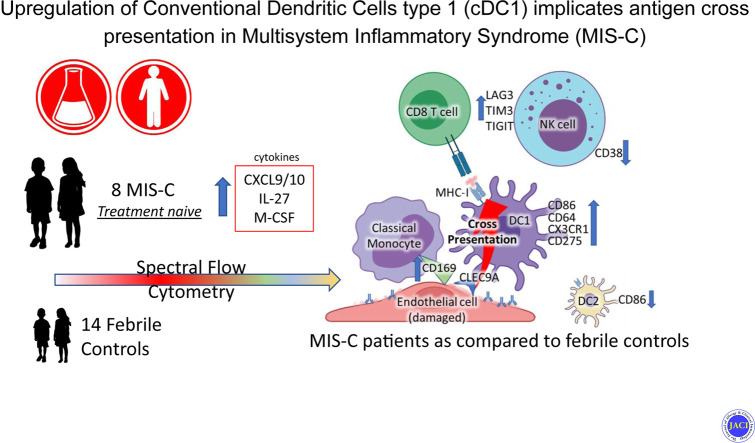
Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) is an acute, febrile, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-associated syndrome, often with cardiohemodynamic dysfunction. Insight into mechanism of disease is still incomplete.
Objective
Our objective was to analyze immunologic features of MIS-C patients compared to febrile controls (FC).
Methods
MIS-C patients were defined by narrow criteria, including having evidence of cardiohemodynamic involvement and no macrophage activation syndrome. Samples were collected from 8 completely treatment-naive patients with MIS-C (SARS-CoV-2 serology positive), 3 patients with unclassified MIS-C–like disease (serology negative), 14 FC, and 5 MIS-C recovery (RCV). Three healthy controls (HCs) were used for comparisons of normal range. Using spectral flow cytometry, we assessed 36 parameters in antigen-presenting cells (APCs) and 29 in T cells. We used biaxial analysis and uniform manifold approximation and projection (UMAP).
Results
Significant elevations in cytokines including CXCL9, M-CSF, and IL-27 were found in MIS-C compared to FC. Classic monocytes and type 2 dendritic cells (DCs) were downregulated (decreased CD86, HLA-DR) versus HCs; however, type 1 DCs (CD11c+CD141+CLEC9A+) were highly activated in MIS-C patients versus FC, expressing higher levels of CD86, CD275, and atypical conventional DC markers such as CD64, CD115, and CX3CR1. CD169 and CD38 were upregulated in multiple monocyte subtypes. CD56dim/CD57−/KLRGhi/CD161+/CD38− natural killer (NK) cells were a unique subset in MIS-C versus FC without macrophage activation syndrome.
Conclusion
Orchestrated by complex cytokine signaling, type 1 DC activation and NK dysregulation are key features in the pathophysiology of MIS-C. NK cell findings may suggest a relationship with macrophage activation syndrome, while type 1 DC upregulation implies a role for antigen cross-presentation.
Key words: Multisystem inflammatory syndrome in children (MIS-C), Kawasaki disease (KD), dendritic cells, antigen cross-presentation, CLEC9A, NK cell cytotoxicity
Graphical abstract
Multisystem inflammatory syndrome in children (MIS-C) is an acute febrile illness temporally associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 The manifestations include distributive shock, myocardial mechanical dysfunction, or both, as well as several cutaneous features reminiscent of both Kawasaki disease (KD) and toxic shock syndrome.2 Therapy includes immunosuppressive agents, especially corticosteroids, as well as intravenous immunoglobulin and biologic therapies.1 , 3 , 4 Corticosteroid therapy is effective in terms of improving outcomes.5 , 6 These findings, along with the observation of a delay between initial infection and the subsequent febrile inflammatory episode, has suggested that an anomalous and excessive immune response to infection may underlie the pathophysiology of MIS-C.4 , 7, 8, 9
Several clinical and translational studies investigating the immunologic underpinnings of MIS-C have been published in the last 12 months. A broad set of findings and hypotheses for the etiology of the syndrome have emerged; however, the mechanisms of disease remain opaque. Broadly, consistent findings thus far are an upregulation of CD64 on monocytes and neutrophils, upregulation of the IFN-γ axis, and findings suggesting endothelial injury, including, notably, upregulation of CX3CR1-expressing T cells and HLA class I–associated expansion of T cells.10, 11, 12, 13, 14, 15, 16
We sought to comprehensively phenotype cellular markers in the antigen-presenting and T-cell compartments, with the aim of gaining further insight into the mechanisms of the disease. Some previous studies have compared patients with MIS-C to either patients with acute SARS-CoV-2 infection or HCs.7 , 8 , 11, 12, 13, 14, 15 However, our goal was to identify features that differentiate treatment-naive MIS-C patients from age-similar febrile controls (FCs) who were seen in the acute setting during the same time period. We hypothesized that this approach would allow us to analyze the individual features and mechanisms that would prove to be highly specific for MIS-C.
Methods
Patients and exclusion criteria
Samples from MIS-C patients were obtained from September 2020 to June 2021. The definition we used of MIS-C met the criteria of both the US Centers for Disease Control and Prevention and the World Health Organization:17 subjects were serology positive for SARS-CoV-2 and PCR negative, without a remote exposure history, along with having evidence of cardiovascular involvement (shock, mechanical cardiac dysfunction, or serologic evidence of cardiovascular stress such as rapidly increasing N-terminal pro–B-type natriuretic peptide [ntBNP] or elevated troponin), and having lymphopenias without macrophage activation syndrome or hemophagocytic lymphohistiocytosis (HLH). FCs were defined as any patient seen in the emergency department who had febrile symptoms that had no initial obvious source, but were then later found to have a known diagnosis that was not infection with coronavirus disease 2019 (COVID-19) (and a negative SARS-CoV-2 antibodies). Febrile patients with a history of a serious comorbid condition (ie, malignancy, congenital) were excluded. Initially, 18 patients were included as FCs. Four were excluded. Two of these patients were excluded because they had uncertain diagnoses and had either recent or unknown COVID-19 exposure and had tested positive for SARS-CoV-2 antibodies. Two additional patients were excluded because their ages (<1 year) were younger than any of the MIS-C patients. One patient with positive SARS-CoV-2 antibodies remained included in the FC group because of the development of fulminant macrophage activation syndrome and no recent exposure history. Details regarding all included patients are presented in Table E1 in this article’s Online Repository at www.jacionline.org.
MIS-C and FC samples were collected at the time of first presentation, and in all cases, samples were collected before any therapy with corticosteroids or intravenous immunoglobulin. Peripheral blood was collected in lavender-topped EDTA tubes, then processed to obtain plasma and cell fractions. Peripheral blood mononuclear cells were obtained by standard Ficoll gradient methods. Peripheral blood mononuclear cells were then cryopreserved in a dimethyl sulfoxide/fetal bovine serum (1:9) mixture, frozen, and stored in a liquid nitrogen freezer until samples were run for cytometry. Median time from collection to processing was 7 hours. Plasma was stored at −80°C until used for cytokine analysis.
Eight MIS-C samples were used for flow cytometry and 7 for cytokine analysis. Data from 1 additional MIS-C patient were obtained from a collaborator at University of Buffalo and were analyzed as a separate patient. Five patients with MIS-C who had been treated 2 weeks previously were used as recovery (RCV) samples. (Two of these patients were also part of the 8 patients in the MIS-C set.) Fourteen samples from FCs were used for flow cytometry and 7 for cytokine analysis. FC patients had sought care at the emergency department while aged 0 to 21 with long-standing (>3 days) fevers, or acute high fever and symptoms concerning for MIS-C and no significant medical comorbidities. Data from an additional patient were obtained from a collaborator in Buffalo so that we could include an age-similar FC.
Three additional samples were obtained as healthy controls (HCs) from patients in the emergency room without febrile illnesses (eg, minor injuries or headaches). Finally, an additional 3 samples were obtained from patients with a phenotype that appeared similar to either MIS-C or KD, but who were serologically negative for SARS-CoV-2 (or who had tested positive several months earlier) or were atypical for KD. For the purposes of this study, we characterized these patents as MIS-C–like.
The patients chosen for cytokine analysis were randomly chosen from all patients and from patients who had more sample volume. Sample volumes of whole blood ranged from 10 mL to as little as 1 to 2 mL, and in cases where sample volumes were minimal, only flow cytometry was performed to preserve as many cells as possible. The choice of 7 patients for cytokine analysis was based on a power analysis indicating that we would require approximately this number of patients to detect a difference of 50% cytokine concentration with a large (33%) variability with a power of 80% and an alpha of 0.05. Additionally, recovery samples were run for cytokine analysis; however, these samples were unable to be accurately measured as a result of the samples’ high viscosity, a result of the intravenous immunoglobulins in the samples. A substantial number of these samples were reported as “no result.” All cytokine results are included in Table E2 in this article’s Online Repository at www.jacionline.org.
The study was reviewed and approved by the institutional review board at Columbia University school of medicine (IRB AAAS5915) and the University of Buffalo school of medicine (IRB Study00004340).
Cytokine analysis
Cytokine assay was performed using a 48-plex cytokine panel (Eve Technologies, Calgary, Alberta, Canada). Samples were sent by dry ice and were validated as being in a frozen condition upon arrival. The following cytokines were assayed: sCD40L, EGF, eotaxin, FGF-2, Flt-3 ligand, fractalkine, G-CSF, GM-CSF, GRO-α, IFN-α2, IFN-γ, IL-1α, IL-1β, IL-1ra, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17A, IL-17E/IL-25, IL-17F, IL-18, IL-22, IL-27, IP-10, MCP-1, MCP-3, M-CSF, MDC (CCL22), MIG, MIP-1α, MIP-1β, PDGF-AA, PDGF-AB/BB, RANTES, TGF-α, TNF-α, TNF-β, and VEGF-A.
High-dimensional flow cytometry assays
All samples were studied in an APC panel and a T-cell panel. The T-cell panel was composed of 29 fluorochrome-tagged antibodies, and the APC panel was composed of 36 fluorochrome-tagged antibodies. Details of antibodies are provided in Table E3 in this article’s Online Repository at www.jacionline.org. The cryopreserved peripheral blood mononuclear cell preparations were rapidly thawed, then washed 3 times with 50 mL volumes of cold saline. Viability was assessed by staining with Ghost Dye according to the manufacturer’s protocol (Tonbo Biosciences, San Diego, Calif); after washing, the preparation was suspended at room temperature in PBS-BSA containing 0.1% NaN3. Approximately 105 cells were then pelleted and resuspended in a freshly prepared antibody cocktail mixture to a final total volume of 200 μL. After a 30-minute incubation at room temperature in the dark, the cells were washed twice with PBS-BSA containing 0.1% NaN3 and immediately analyzed in an Aurora flow cytometer equipped with 5 lasers (Cytek Biosciences, Fremont, Calif). Essentially the entire volume was analyzed. Reference single-stained controls for spectral unmixing were either beads or cells, according to the frequency of cells expressing the candidate antigen. The unmixed FSC computer files were imported into FCS Express software (De Novo Software, Pasadena, Calif) and analyzed. Samples also included the same reference control healthy blood donor for each run, and a mix of MIS-C, FCs, and other patients was assayed in each run to minimize any batch effect. For some fluorochrome-tagged antibodies, as a result of the large number of fluorochromes, compensation in spectral cytometry results in compensated “negative” mean fluorescence intensity. To ensure validity for these, fluorescence minus 1 staining was used to confirm negative and positive values for continuously distributed molecules including HLA-DR, CD38, CD57, CD64, CD169, CXCR3, and CD62L.
Flow cytometry analysis and uniform manifold approximation and projection
Acquired data were analyzed using manual gating (biaxial analysis, uniform manifold approximation and projection, UMAP) using FCS Express 7 (De Novo Software) and FlowJo 10.7.1 (Treestar, Ashland, Ore) (manual gating, biaxial analysis). A subset of 1000 cells was selected with weighted density sampling for each of 32 samples (13 FC, 8 MIS-C, 3 MIS-C–like, 5 RCV, 3 HCs). UMAP analysis of APC panel was conducted on a concatenated file containing 32,000 APCs (HLA-DR+CD3/CD5/CD19−). UMAP analysis of the T-cell panel was conducted on a concatenated file containing 32,000 viable lymphocytes. Manual gating results were projected on the UMAP dot plot. Intensities for parameters of interest were overlaid to show the expression on different cell islands. The gating strategy is described in Fig E1 in this article’s Online Repository at www.jacionline.org.
Statistical analysis
Statistical comparisons and graphical presentation were made by GraphPad Prism 9.0.0 software (GraphPad Software, La Jolla, Calif). Comparisons between MIS-C, FCs, and RCV were evaluated with the Kruskal-Wallis test, followed by post hoc multiple comparison Dunn test (indicated as significant on figures by ∗P < .05, ∗∗P < .01). The Wilcoxon rank sum test was used (indicated as significant on the figures by #P < .05) when direct comparisons were made between HC index patients and MIS-C patients.
Results
Patient characteristics
Demographic and clinical data from the MIS-C and FC patients, as well as serology-negative patients with MIS-C/KD–like illness, is presented in Table I . MIS-C and FC patients were about the same median age (9 vs 9.5 years). All FC patients had no preexisting diagnoses at the time of contact with the research team or the blood draw procedure. A detailed list of the ultimate diagnoses, laboratory findings by patient, and course of hospitalization for all patients in this study is provided in Table E1.
Table I.
Demographic and laboratory findings in patients at acute presentation
| Characteristic | MIS-C (n = 8) | FC (n = 14) | MIS-C–like (n = 3) |
|---|---|---|---|
| Demographics | |||
| Age (years), median (25th-75th percentile) | 9.5 (4.7-15.25) | 9.0 (2-14.5) | 4 (4-11) |
| Positive COVID-19 serology | 8/8 (100%) | 3/14 (21%) | 1/3 (33%) |
| Known recent COVID-19 exposure | 6/8 (75%) | 0 | 0 |
| Meets criteria for KD | 2/8 (25%) | 0 | 2/3 (66%) |
| Days of fever, median (25th/75th percentile) | 5.5 (4-7.25) | 5 (3-7) | 4 (3.5-9) |
| Laboratory findings | |||
| Platelet count (× 109 cells/L), median | 219 | 324 | 482 |
| Lymphocyte count (× 109 cells/L), median | 0.78 | 3.47 | 1.6 |
| Serum sodium (mEq/L), median | 134.5 | 138 | 135 |
| C-reactive protein (mg/L), median | 111.72 | 13.55 | 72.55 |
| N-terminal pro–B-type natriuretic peptide (ntBNP) peak (pg/mL), median (range) | 3318 (1075-34,875) | 75 (10.9-891.2) | 184.6 (12.4-8907) |
| Troponin peak (ng/L), median (range) | 27.5 (0-235) | 0 (0-22) | Not sampled |
Cytokine assay demonstrates a strong IFN-γ signal and IL-27 upregulation, along with a subset of APC and cytotoxic T-cell–stimulating cytokines and chemokines
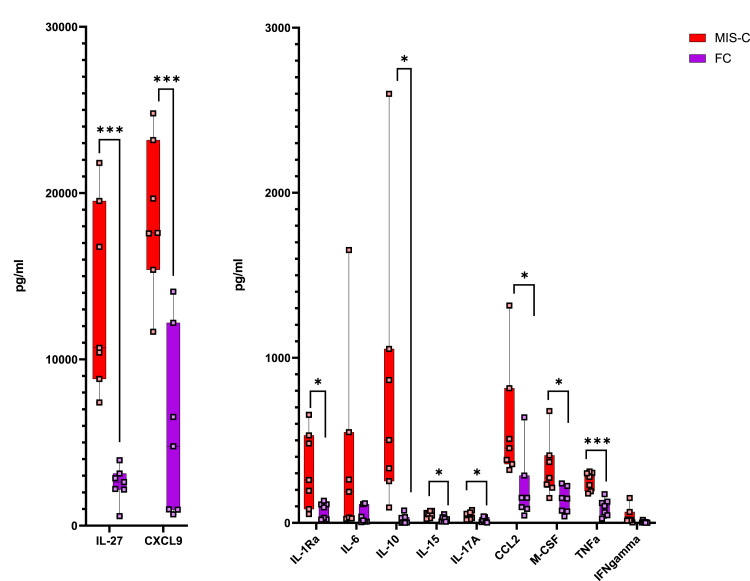
Forty-eight individual chemokines and cytokines were assayed from 7 FC and 7 MIS-C patients. Several of these markers demonstrated strong statistically significant differences in patients with MIS-C versus patients with FC, as shown in Fig 1 . Those upregulated in MIS-C included IL-1ra, IL-3, IL-10, IL-15, IL-17A, IL-27, MCP-1/CCL2, M-CSF (CD115 ligand), CXCL9, TNF-α, and eotaxin. Of those, CXCL9, IL-27, and TNF-α were among the most highly elevated, with the greatest degree of statistical significance. Epidermal growth factor was downregulated in patients with MIS-C. IL-6, notably, was elevated in both groups, but not significantly more so in patients with MIS-C. These findings demonstrate a strong IFN-γ signal, as evidenced by CXCL9 elevation,18 along with IL-15, a cytokine important for CD8 and natural killer (NK) cytotoxic activation.19 , 20 CXCL10 levels were also extremely elevated in all MIS-C patients (median 74,337 pg/mL vs 378 pg/mL FCs), but for 2 MIS-C patients, they were above the range of detection for the assay; data could thus not be compared for this chemokine. Together, CXCL9 and CXCL10 upregulation suggest a strong IFN-γ signal. IFN-γ itself also had an upregulated trend (P = .11).
Fig 1.
Cytokine concentrations in 7 patients with MIS-C versus 7 FC patients. IL-27 and CXCL9 are the 2 major cytokines most markedly and significantly elevated; a slight elevation but high significance is also seen in TNF-α.
CD64 is upregulated in monocytes in MIS-C versus RCV and on type 1 dendritic cells in MIS-C compared to FCs
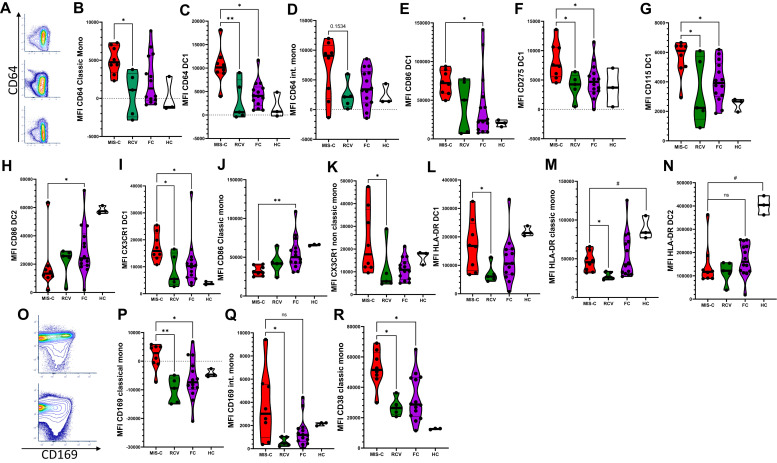
In our analysis, we focused first on APC phenotypes. In the first biaxial analyses of our samples, we saw that MIS-C patients demonstrated upregulation of CD64 expression compared to RCV and FC in classic monocytes and type 1 dendritic cells (DC1). Slight elevations were seen in CD64 expression in intermediate monocytes (Fig 2 , A-D), and no significant expression changes were observed in nonclassic monocytes (data not shown). CD64 is typically a marker of monocyte lineage and is not highly expressed on conventional DCs; however, subsets of conventional DCs have been described that do express CD64.21 This finding of elevated CD64 expression of monocytes has been previously described both in MIS-C versus HCs15 and in a comparison of patients with KD, FCs, and patients with an autoinflammatory syndrome.22 Our findings discriminating CD64 expression between MIS-C and FC raises the possibility of using it as an actionable biomarker. Subcategorization of CD64 expression by DC subtype has not been performed previously in KD.
Fig 2.
Expression of markers on the APC compartment. (A) Biaxial cytometry demonstrating CD64 upregulation on CD11c+ cells in concatenated MIS-C (top), FCs (middle), and HCs (bottom). (B-D) Mean fluorescence index (MFI) of CD64 on APCs. (E-H) MFI CD86, CD275, CD115, and CX3CR1 in MIS-C versus FC and RCV. (I, J) MFI CD86 in MIS-C versus classic monocytes and DC2. (K) MFI CX3CR1 in nonclassic monocytes in MIS-C versus FC and RCV. (L-N) MFI HLA-DR expression in DC1 cells, classic monocytes, and DC2 cells. (O) Biaxial cytometry demonstrating concatenated CD169/CD38 in FC (bottom) and MIS-C (top). (P-R) MFI CD169 in classic and intermediate monocytes, and MFI CD38 in classic monocytes. ∗∗P < .01, ∗P < .05 (with multiple comparisons, Kruskal Wallis test with Dunn correction), #P < .05 (single comparisons, Mann-Whitney U test without correction); when no comparison bar is shown with HCs, a statistical test was not performed, and HC information is shown to represent an index of normal range.
Dendritic cell subtype DC1 cells are activated in MIS-C versus FCs
Further analysis of our data demonstrated that in the MIS-C patient population, DC1 (CLECL9A+ CD141+) cells were selectively activated compared to FCs. DC1s show upregulation of CD86, CD64, CD275, CD115, and CX3CR1 (Fig 2, E-H), whereas classic monocytes and DC2 cells do not show these findings. In fact, classic monocytes and DC2 cells display downregulation of CD86 versus FCs (Fig 2, I and J). CD115 is the receptor for M-CSF,23 , 24 which itself is elevated significantly in the cytokines for our study’s MIS-C patients. CD115 is more typically a monocyte marker, but it can be expressed in DCs.24 CD115 is also, along with CX3CR1 (also upregulated), a marker along the development pathway for inflammatory DCs. Increased expression of CX3CR1 may thus indicate the activation of this inflammatory development pathway,23 as well as the potential for homing to vasculature, as with T cells’ expressing CX3CR1, previously described in MIS-C.14 Additionally, we also see elevated CX3CR1 in nonclassic monocytes in patients with MIS-C versus RCV (Fig 2, K) and a trend versus FCs.
HLA-DR is downregulated in MIS-C on classic monocytes and DC2 cells versus HCs, but not versus FCs, and HLA-DR is relatively upregulated in DC1 cells versus RCV
It was previously reported that HLA-DR is downregulated in monocytes and DCs in patients with MIS-C versus HCs;15 these findings are observed in our study, with HLA-DR downregulated in classic monocytes and DC2 cells in our study’s MIS-C cohort versus HCs (with our data showing statistical significance with direct comparison between MIS-C and HCs, but not significant when correcting for multiple comparisons and including FCs) (Fig 2, L and M; direct comparisons indicated for significance #P < .05). However, by dissecting DC/monocyte subtypes, we find that in DC1 cells, HLA-DR is not significantly up- or downregulated versus FCs, but it is still downregulated (without statistical significance in this small set) versus HCs. We also find that a subset of our FC patients demonstrate monocyte HLA-DR downregulation. Critically, downregulation of HLA-DR and CD86 in all APCs with the exception of DC1 cells is also described in adult patients with severe SARS-CoV-2 syndrome.25
CD169 and CD38 are upregulated in the monocyte subtypes of our cohort
Increased expression of CD169 in monocyte subsets in MIS-C versus FCs and/or RCV was clearly evident in our cohort of patients (Fig 2, O-Q). CD169 was upregulated in classic monocytes in MIS-C compared to FCs and RCV, and was upregulated in intermediate monocytes compared to RCV. CD38 is also upregulated in classic monocytes (Fig 2, R).
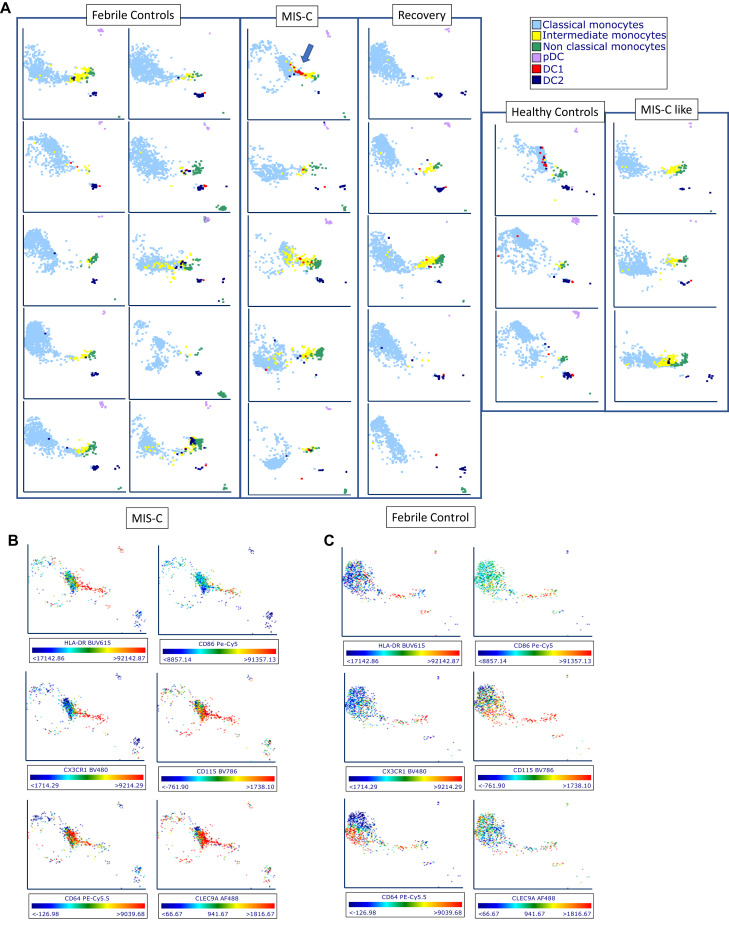
MIS-C patients demonstrate change in phenotype of DC1 cells toward that of intermediate and nonclassical monocytes
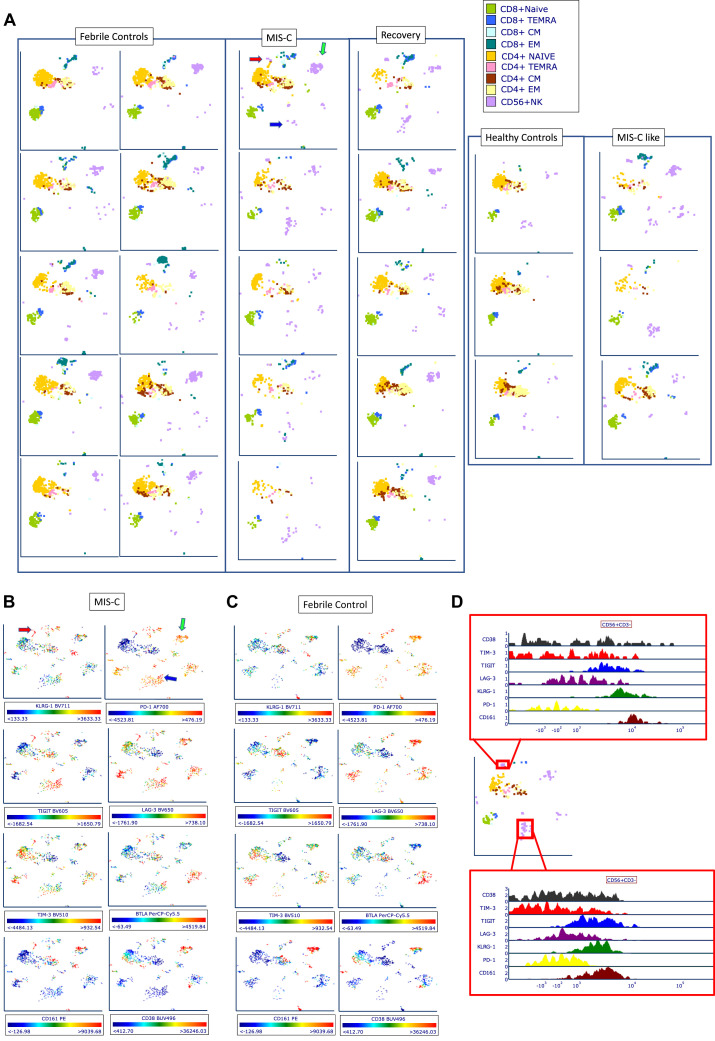
Using UMAP analysis (Fig 3 , A), DC1 cells appeared, uniquely, to change in character (and location within UMAP); they were found in the nonclassic and intermediate monocyte vicinity rather than alongside DC2 cells, as they are in most FCs and HCs. This is consistent with the appropriation by the DC1 cells of various more monocyte typical receptors, such as CD64, CD115, and CX3CR1, indicating the inflammatory character of this DC1 phenotype. In RCV, these cells were found adjacent to DC2 cells in a UMAP analysis, thus suggesting that RCV induces a reversion to a more normative DC1 phenotype. Numerically, no difference in percentage of frequency of DC subsets was seen versus FCs; however, plasmacytoid DCs were sharply decreased in frequency versus HCs in MIS-C (0.5% of all DRhi cells vs 3.5% in HCs, P < .0001), as previously described.12 , 14 Evaluation of expression of individual cellular markers again demonstrated upregulation of CD86, CD68, CD275, CD115, and CX3CR1 in this DC1 cell (CLEC9A+) population on UMAP (Fig 3, B and C). Expression of CLEC9A on UMAP parameter analysis for 6 MIS-C patients and 10 FCs is shown in Fig E2 in this article’s Online Repository at www.jacionline.org.
Fig 3.
UMAP analysis of APC populations. (A) Representative plots were selected from 10 FC, 5 MIS-C, 5 RCV, 5 HC, and 3 MIS-C–like subjects. MIS-C patients demonstrate small but evident population of DC1 cells concentrated at the intermediate monocyte/nonclassic monocyte location (blue arrow). This characteristic finding in our DC patients suggests that DC1 cells have altered their phenotype to take on characteristics of these monocytes, but that they are playing a unique role via the function of CLEC9A, which is typically for antigen cross-presentation. (B, C) Two selected patients, 1 with MIS-C (B) and 1 FC (C), demonstrating expression of HLA-DR, CD86, CD115, CX3CR1, CD64, and CLEC9A. Cells in the same region demonstrate upregulation of these markers, with the exception of CLEC9A, which is only seen robustly expressed in the MIS-C patient.
MIS-C–like and patients from another institution share features with our study’s MIS-C patients
Upregulation of inflammatory makers of DC1 cells in MIS-C–like patients resembles the characteristics of true MIS-C patients in flow cytometry findings. Overall, MIS-C–like patients show findings similar to MIS-C, but in some cases intermediate between MIS-C and FCs (see Fig E3, A, in this article’s Online Repository at www.jacionline.org) in terms of expression of markers including DC1, CD64, CD86, HLA-DR, CX3CR1, CD115, and classic monocyte CD169 versus FCs and MIS-C patients. This may indicate that these patients share some, but not all, immunologic features of MIS-C, much as clinically they share some, but not all, features. We additionally analyzed one patient from another institution classified there as definitely MIS-C (University of Buffalo) with a sample obtained before any treatment. This patient shared features such as upregulated CD86 and CD68 on DC1 cells, downregulated CD86, and HLA-DR on DC2 (Fig E3, A).
T-cell lymphopenia, and T-cell activation and exhaustion in our study’s MIS-C cohort
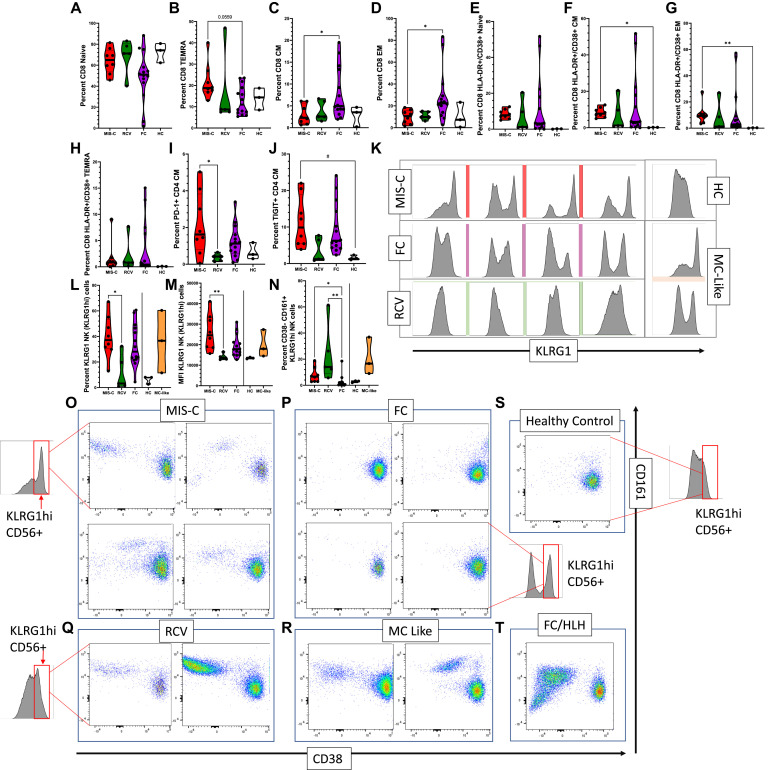
Consistent with all other studies on MIS-C, our cohort demonstrates profound lymphopenia, including the T-cell compartment.7 , 10, 11, 12, 13, 14 Median lymphocyte count (Table I) in our study’s MIS-C patients was 780 × 109 cells/L, which are 25% that of FCs. In our cohort within the CD8 compartment, a trend toward higher terminally differentiated effector memory (TEMRA) T cells, as well as lower central memory and effector memory subsets, is evident (Fig 4 , A-D). CD4 subsets did not differ from each other in this regard (data not shown). CD8 T cells were activated versus HCs, as described previously14 , 26 (Fig 4, E-H). Markers of exhaustion such as PD-1 and TIGIT were upregulated as a percentage of positive cells versus RCV or HCs, consistent with previous descriptions of T-cell exhaustion14 , 27 (Fig 4, I and J), especially noted in the CD4 central memory cells in our cohort; however, generally, these markers were also upregulated in FCs.
Fig 4.
Expression of markers on the T and NK cell compartment. (A-D) Percentage of T-cell subtypes in patients with MIS-C versus FC and RCV. (E-H) Percentage of CD38+HLA-DR+ T-cell subsets in patients with MIS-C versus FC and RCV. (I, J) Percentage TIGIT+ and PD-1+ in CD4 T-cell CM. (K) Histogram showing upregulated KLRG1 in MIS-C patients versus RCV, HC, and FC subjects. (L) Percentage of KLRG1hi NK cells seen in MIS-C versus RCV and FC patients. (M) MFI in MIS-C patients versus RCV and FC patients; MIS-C–like patients are shown for comparison but statistical analysis not performed (line indicating where no statistical analysis performed); (N) Percentage of KLRG1hi, CD38−CD161+ NK cells in MIS-C versus RCV and FC patients, MIS-C–like patients again included. (O-T) Biaxial analysis of CD38 and CD161 expression in KLRG1hi NK cells from MIS-C, FC, RCV, HC, and MIS-C–like subjects and in a FC with HLH. KLRG1hi NK cells were gated in all subgroups from the same KLRG1hi region (red box, demonstrated visually on the graph in some of the subgroups for explanation; the same region was sampled in the FC/HLH and the MIS-C–like patients). ∗∗P < .01, ∗P < .05 (with multiple comparisons, Kruskal Wallis test with Dunn correction), #P < .05 (single comparisons, Mann-Whitney U test without correction); when no comparison bar is shown with HCs, a statistical test was not performed, and HC information is shown to represent an index of normal range. HCs and MIS-C–like patients included for (M) and (N). CM, Central memory; EM, effector memory; MFI, mean fluorescence index.
Dysregulated NK cell phenotype is a hallmark of MIS-C and MIS-C–like syndromes, and persists in early RCV
Further interrogation of NK cell phenotypes demonstrated surprising patterns of marked differences in specific subpopulations of NK cells in patients with MIS-C as well as MIS-C–like features. In MIS-C patients, NK cells demonstrated a subpopulation of CD56dim CD57lo KLRG1+ NK cells with very high mean fluorescence intensity for KLRG1 compared to RCV or HCs, with a trend of increase versus FCs (Fig 4, K-M). Within this group of KLRG1 cells, a prominent group of CD161+/CD38− NK cells was seen (Fig 4, N). This was highly restricted to patients in the MIS-C, MIS-C–like, and RCV groups, but not FCs or HCs. Notably, this pattern of expression, strongly enhanced, was also seen in 1 FC patient—a patient presenting with HLH (Fig 4, O-T). This finding was also seen in the MIS-C patient sample provided to us by our collaborator (M. Hicar) at the University of Buffalo (Fig E3, B). Further review of the NK cell compartment via UMAP analysis demonstrated marked and obvious changes in the phenotype of NK cells in MIS-C and MIS-C–like patients. MIS-C patients demonstrated an increase in 2 particular NK cell phenotypes (Fig 5 , A, blue arrow and red arrow) consisting of cells that appear to be expressing exhaustion/senescence markers KLRG1, TIGIT, LAG3, BTLA, and PD-1 (Fig 5, B-D). This NK phenotype, however, was seen in 2 of 3 HCs and occasionally in FC. MIS-C patients also demonstrated another, smaller group (Fig 5, A, red arrow) showing expression of CD161, KLRG1, and TIGIT. A third group of NK cells (Fig 5, A, green arrow) tended to be seen more typically in both MIS-C and FC patients but not in HCs. This NK phenotype appears to have sparse CD38 expression (see Fig E4 in this article’s Online Repository at www.jacionline.org). We speculate that these findings, taken together, may suggest an exhausted phenotype of NK cell after extended inflammation in MIS-C patients.
Fig 5.
UMAP analysis of T-cell and NK cell populations. (A) Ten selected FC, 5 MIS-C, 5 recovery, 5 HC, and 3 MIS-C subjects. MIS-C patients demonstrate increased number of NK cells of 2 phenotypes, indicated by a red arrow and blue arrow in the UMAP of the first MIS-C patients. These cell populations are seen also in MIS-C–like patients. MIS-C patients and FCs showed decreased expression of a third phenotype (green arrow). (B, C) Representative expression of cellular markers from 1 patient with MIS-C (B) and 1 FC (C) demonstrating KLRG1, PD-1, TIGIT, LAG3, TIM3, BTLA, CD161, and CD38. (D) Histogram of clusters indicated on previous diagrams with red arrow and blue arrow, with relative expression levels of KLRG1, TIGIT, CD161, and CD38.
Discussion
Here we describe several novel findings in the immune phenotypes of DCs, monocytes, and NK cells in MIS-C. The strengths of our study are the completely treatment-naive status of the acute MIS-C samples and the use of FCs as comparators. To our knowledge, few studies11 , 28 have succeeded in having mostly or all treatment-naive patients.
A central finding is that DC1 cells are preferentially activated, while DC2 and classic monocytes are downregulated. This unique pattern may suggest that antigen cross-presentation is part of the cellular and molecular mechanism of MIS-C. Antigen cross-presentation is mediated in large part by actions of CLEC9A, 1 of 2 major markers, along with BDCA3/CD141 of DC1 cells.29 , 30 This activated DC1 finding has been described in SARS-CoV-2 syndrome.25 CLEC9A also mediates the uptake of cellular fragments from damaged or necrosed cells by DC1 cells.29 Because endothelial damage7 mediated by antibody interaction with endothelial cells13 is believed to be a central feature of MIS-C, antigen cross-presentation would be entirely consistent as a next step in disease. Endothelial damage is present in MIS-C to such a degree that denuded endothelial cells are seen in microscopic sections from autopsy patients.7 , 31 Because antigen cross-presentation occurs via MHC-1, restricted HLA-1–associated expansion of T cells, as previously described,10 would also be consistent with our findings. CX3CR1 in these DC1 cells complements the T-cell findings, showing similar upregulation.14
Monocyte CD169 plays an important role in cross-priming T cells during DC1 antigen cross-presentation,32 and it thus is another key piece of evidence for this process in MIS-C. Downregulation of CD169 in our 2-week posttreatment samples (RCV) suggests that CD169 responds quickly to treatment—and thus the necessity of obtaining pretreatment samples. Upregulation of CD169 has been described in SARS-CoV-2 syndrome, with speculation that it may be related to disease severity and mechanism.33 Upregulated CD38, a marker of monocyte/macrophage activation,34 has also been observed in adults with severe SARS-CoV-2 syndrome.25
We also find that exhausted NK cell phenotypes are a major feature within our cohort, as evidenced by a population of KLRG1hi, LAG3, TIGIT, PD-1, and TIM-3 expressing NK cells, and that downregulated CD38 in a subpopulation of KLRG1hi cells may be a marker for this. LAG3, TIGIT, and TIM3 are known markers of NK cell dysfunction/exhaustion.35, 36, 37, 38, 39 In T cells, these markers are all upregulated by IL-27,38 , 39 which is highly upregulated in our cohort. The function of IL-27 toward NK cells is less clear;40 however, this cytokine may also be promoting suppression in NK cells. CD161 and CD38 are markers that remain under exploration in NK cell function, but they may have a relationship to NK activation. CD161 is highly expressed on NK cells, and it is believed to be a marker of proinflammatory status.41 CD38 was originally described in NK cells as playing a role in cytotoxicity, and NK CD38 expression appears to decrease in fatal COVID-19 cases, along with perforin expression.42 The finding that our FC patient with HLH shared the upregulated CD161+CD38− pattern with our study’s MIS-C also raises questions as to whether MIS-C shares some features with HLH/macrophage activation syndrome (MAS).43 Persistence of these abnormalities in RCV patients at 2 weeks may explain why some of these patients rebound without lengthy corticosteroid tapering therapy.
As with previous studies,13 , 15 we also found downregulation of HLA-DR in most APC populations, although it was less pronounced in the DC1 compartment. Elevated IL-10 is known to be a factor in downregulation of MHC-2, particularly in monocytes;44 however, this may be one factor in a complex pathway of regulation. Downregulation of HLA-DR is described in settings in which patients are septic or significant systemic inflammation, representing a sort of immunoparalysis that can place patients at risk of secondary infections45 and may therefore not be specific for MIS-C. A subset of our FC patients, those with long-standing fevers (median fever of 5 days, similar to our study’s MIS-C population), had downregulated HLA-DR as well. However, an alternative possibility is that HLA-DR downregulation may lead to cessation of regulatory signals to some DC types (especially DC1) from suppressive Treg cells, leading to severe systemic autoimmune response.46
The study is limited by relatively small numbers (8 MIS-C patients, although many studies published in the past 15 months have had similar numbers), a lack of a KD cohort from before the pandemic (which indeed would have been extremely fascinating to investigate), and, as in all studies, the lack of a validated case definition for MIS-C. This lack of case definition makes it difficult to define MIS-C in borderline cases. One patient who was placed in the FC group had positive serologies but presented with fulminant HLH/MAS. Because this patient’s pathology differed significantly from our other MIS-C patients (none of whom developed HLH/MAS), we included this patient in our FC group because clinically, in our institution, we did not categorize this case as MIS-C, and the patient did not present with the classic features or laboratory findings (lymphopenia, relative thrombocytopenia) seen in MIS-C patients.
Although we used all patients for flow cytometry analysis, the cytokine analysis was limited by studying only a subset of patients. Thus, the cytokine findings are not directly correlative of all of the flow cytometry data. Finally, the study is also limited by the lack of more extensive markers to further evaluate NK cell activity, such as perforin, NKD2D, and CD16. This was the result of our expectation that the primary pathology would be found in the T-cell compartment, and although T-cell abnormalities were found in our data, we were somewhat surprised by the evident NK cell findings. Future studies will more comprehensively analyze the changes in these cells.
Key messages.
-
•
DC1s appear upregulated, while DC2s appear downregulated, in flow cytometric analysis of treatment-naive peripheral blood. This may indicate antigen cross-presentation as a mechanism of disease in MIS-C.
-
•
NK cells show downregulation of CD38 in a subset of cells, suggesting downregulated cytotoxicity, which is also a feature of macrophage activation syndrome.
Footnotes
Supported in part by the National Institutes of Health (grant K08 HL155033 to M. Gorelik). Core flow cytometry was suported by the United States Public Health Service (grant S10RR027050) and the American Heart Association (grant 19TPA34910217).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Supplementary data
References
- 1.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395(10239):1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bautista-Rodriguez C., Sanchez-de-Toledo J., Clark B.C., Herberg J., Bajolle F., Randanne P.C., et al. Multisystem inflammatory syndrome in children: an international survey. Pediatrics. 2021;147 doi: 10.1542/peds.2020-024554. e2020024554. [DOI] [PubMed] [Google Scholar]
- 3.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouldali N., Toubiana J., Antona D., Javouhey E., Madhi F., Lorrot M., et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. 2021;325:855–864. doi: 10.1001/jama.2021.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belhadjer Z., Auriau J., Meot M., Oualha M., Renolleau S., Houyel L., et al. Addition of corticosteroids to immunoglobulins is associated with recovery of cardiac function in multi-inflammatory syndrome in children. Circulation. 2020;142:2282–2284. doi: 10.1161/CIRCULATIONAHA.120.050147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diorio C., Henrickson S.E., Vella L.A., McNerney K.O., Chase J., Burudpakdee C., et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130:5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee P.Y., Day-Lewis M., Henderson L.A., Friedman K.G., Lo J., Roberts J.E., et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porritt R.A., Paschold L., Noval Rivas M., Cheng M.H., Yonker L.M., Chandnani H., et al. HLA class I–associated expansion of TRBV11-2 T cells in multisystem inflammatory syndrome in children. J Clin Invest. 2021;131:e146614. doi: 10.1172/JCI146614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consiglio C.R., Cotugno N., Sardh F., Pou C., Amodio D., Rodriguez L., et al. the immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–981.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruber C.N., Patel R.S., Trachtman R., Lepow L., Amanat F., Krammer F., et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183:982–995.e14. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramaswamy A., Brodsky N.N., Sumida T.S., Comi M., Asashima H., Hoehn K.B., et al. Immune dysregulation and autoreactivity correlate with disease severity in SARS-CoV-2–associated multisystem inflammatory syndrome in children. Immunity. 2021;54:1083–1095.e7. doi: 10.1016/j.immuni.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vella L.A., Giles J.R., Baxter A.E., Oldridge D.A., Diorio C., Kuri-Cervantes L., et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci Immunol. 2021;6:eabf7570. doi: 10.1126/sciimmunol.abf7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter M.J., Fish M., Jennings A., Doores K.J., Wellman P., Seow J., et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26:1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Smith J.J., Verweyen E.L., Clay G.M., Esteban Y.M., de Loizaga S.R., Baker E.J., et al. Inflammatory biomarkers in COVID-19–associated multisystem inflammatory syndrome in children, Kawasaki disease, and macrophage activation syndrome: a cohort study. Lancet Rheumatol. 2021;3:e574–e584. doi: 10.1016/S2665-9913(21)00139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsaied T., Tremoulet A.H., Burns J.C., Saidi A., Dionne A., Lang S.M., et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. 2021;143:78–88. doi: 10.1161/CIRCULATIONAHA.120.049836. [DOI] [PubMed] [Google Scholar]
- 18.Metzemaekers M., Vanheule V., Janssens R., Struyf S., Proost P. Overview of the mechanisms that may contribute to the non-redundant activities of interferon-inducible CXC chemokine receptor 3 ligands. Front Immunol. 2017;8:1970. doi: 10.3389/fimmu.2017.01970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M., Wen B., Anton O.M., Yao Z., Dubois S., Ju W., et al. IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc Natl Acad Sci U S A. 2018;115:E10915–E10924. doi: 10.1073/pnas.1811615115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J., Giuntoli R.L., 2nd, Omiya R., Kobayashi H., Kennedy R., Celis E. Interleukin 15 promotes antigen-independent in vitro expansion and long-term survival of antitumor cytotoxic T lymphocytes. Clin Cancer Res. 2002;8:3877–3884. [PubMed] [Google Scholar]
- 21.Schraml B.U., van Blijswijk J., Zelenay S., Whitney P.G., Filby A., Acton S.E., et al. Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell. 2013;154:843–858. doi: 10.1016/j.cell.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Hokibara S., Kobayashi N., Kobayashi K., Shigemura T., Nagumo H., Takizawa M., et al. Markedly elevated CD64 expression on neutrophils and monocytes as a biomarker for diagnosis and therapy assessment in Kawasaki disease. Inflamm Res. 2016;65:579–585. doi: 10.1007/s00011-016-0942-1. [DOI] [PubMed] [Google Scholar]
- 23.Auffray C., Fogg D.K., Narni-Mancinelli E., Senechal B., Trouillet C., Saederup N., et al. CX3CR1+CD115+CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald K.P., Rowe V., Bofinger H.M., Thomas R., Sasmono T., Hume D.A., et al. The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J Immunol. 2005;175:1399–1405. doi: 10.4049/jimmunol.175.3.1399. [DOI] [PubMed] [Google Scholar]
- 25.Kvedaraite E., Hertwig L., Sinha I., Ponzetta A., Hed Myrberg I., Lourda M., et al. Major alterations in the mononuclear phagocyte landscape associated with COVID-19 severity. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2018587118. e2018587118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreews M., Le Gouge K., Khaldi-Plassart S., Pescarmona R., Mathieu A.-L., Malcus C., et al. Polyclonal expansion of TCR Vbeta 21.3+ CD4+ and CD8+ T cells is a hallmark of multisystem inflammatory syndrome in children. Sci Immunol. 2021;6:eabh1516. doi: 10.1126/sciimmunol.abh1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckmann N.D., Comella P.H., Cheng E., Lepow L., Beckmann A.G., Mouskas K., et al. Cytotoxic lymphocytes are dysregulated in multisystem inflammatory syndrome in children. Nat Commun. 2021;12:4854. doi: 10.1038/s41467-021-24981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esteve-Sole A., Anton J., Pino-Ramirez R.M., Sanchez-Manubens J., Fumadó V., Fortuny C., et al. Similarities and differences between the immunopathogenesis of COVID-19–related pediatric multisystem inflammatory syndrome and Kawasaki disease. J Clin Invest. 2021;131:e144554. doi: 10.1172/JCI144554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreibelt G., Klinkenberg L.J., Cruz L.J., Tacken P.J., Tel J., Kreutz M., et al. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood. 2012;119:2284–2292. doi: 10.1182/blood-2011-08-373944. [DOI] [PubMed] [Google Scholar]
- 30.Cueto F.J., Del Fresno C., Sancho D. DNGR-1, a dendritic cell–specific sensor of tissue damage that dually modulates immunity and inflammation. Front Immunol. 2019;10:3146. doi: 10.3389/fimmu.2019.03146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duarte-Neto A.N., Caldini E.G., Gomes-Gouvea M.S., Kanamura C.T., de Almeida Monteiro R.A., Ferranti J.F., et al. An autopsy study of the spectrum of severe COVID-19 in children: from SARS to different phenotypes of MIS-C. EClinicalMedicine. 2021;35 doi: 10.1016/j.eclinm.2021.100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grabowska J., Lopez-Venegas M.A., Affandi A.J., den Haan J.M.M. CD169+ macrophages capture and dendritic cells instruct: the interplay of the gatekeeper and the general of the immune system. Front Immunol. 2018;9:2472. doi: 10.3389/fimmu.2018.02472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park M.D. Macrophages: a Trojan horse in COVID-19? Nat Rev Immunol. 2020;20:351. doi: 10.1038/s41577-020-0317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amici S.A., Young N.A., Narvaez-Miranda J., Jablonski K.A., Arcos J., Rosas L., et al. CD38 is robustly induced in human macrophages and monocytes in inflammatory conditions. Front Immunol. 2018;9:1593. doi: 10.3389/fimmu.2018.01593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller-Durovic B., Lanna A., Covre L.P., Mills R.S., Henson S.M., Akbar A.N. Killer cell lectin-like receptor G1 inhibits NK cell function through activation of adenosine 5′-monophosphate–activated protein kinase. J Immunol. 2016;197:2891–2899. doi: 10.4049/jimmunol.1600590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Judge S.J., Murphy W.J., Canter R.J. Characterizing the dysfunctional NK cell: assessing the clinical relevance of exhaustion, anergy, and senescence. Front Cell Infect Microbiol. 2020;10:49. doi: 10.3389/fcimb.2020.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson A.C., Joller N., Kuchroo V.K. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chihara N., Madi A., Kondo T., Zhang H., Acharya N., Singer M., et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature. 2018;558(7710):454–459. doi: 10.1038/s41586-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu C., Sakuishi K., Xiao S., Sun Z.Y., Zaghouani S., Gu G.X., et al. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nat Commun. 2015;6:6072. doi: 10.1038/ncomms7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ochayon D.E., Waggoner S.N. The effect of unconventional cytokine combinations on NK-cell responses to viral infection. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.645850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurioka A., Cosgrove C., Simoni Y., van Wilgenburg B., Geremia A., Bjorkander S., et al. CD161 defines a functionally distinct subset of pro-inflammatory natural killer cells. Front Immunol. 2018;9:486. doi: 10.3389/fimmu.2018.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilk A.J., Lee M.J., Wei B., Parks B., Pi R., Martínez-Colón G.J., et al. Multi-omic profiling reveals widespread dysregulation of innate immunity and hematopoiesis in COVID-19. J Exp Med. 2021;218:e20210582. doi: 10.1084/jem.20210582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ombrello M.J., Schulert G.S. COVID-19 and cytokine storm syndrome: are there lessons from macrophage activation syndrome? Transl Res. 2021;232:1–12. doi: 10.1016/j.trsl.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koppelman B., Neefjes J.J., de Vries J.E., de Waal Malefyt R. Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 1997;7:861–871. doi: 10.1016/s1074-7613(00)80404-5. [DOI] [PubMed] [Google Scholar]
- 45.Landelle C., Lepape A., Voirin N., Tognet E., Venet F., Bohe J., et al. Low monocyte human leukocyte antigen–DR is independently associated with nosocomial infections after septic shock. Intensive Care Med. 2010;36:1859–1866. doi: 10.1007/s00134-010-1962-x. [DOI] [PubMed] [Google Scholar]
- 46.Wohn C., Le Guen V., Voluzan O., Fiore F., Henri S., Malissen B. Absence of MHC class II on cDC1 dendritic cells triggers fatal autoimmunity to a cross-presented self-antigen. Sci Immunol. 2020;5:eaba1896. doi: 10.1126/sciimmunol.aba1896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.