Abstract
Purpose
Early detection of SARS-CoV-2 patients is essential to contain the pandemic and keep the hospital secure. The rapid antigen test seems to be a quick and easy diagnostic test to identify patients infected with SARS-CoV-2. To assess the possible role of the antigen test in the Emergency Department (ED) assessment of potential SARS-CoV-2 infection in both symptomatic and asymptomatic patients.
Methods
Between 1 July 2020 and 10 December 2020, all patients consecutively assessed in the ED for suspected COVID-19 symptoms or who required hospitalisation for a condition not associated with COVID-19 were subjected to a rapid antigen test and RT-PCR swab. The diagnostic accuracy of the antigen test was determined in comparison to the SARS-CoV-2 PCR test using contingency tables. The possible clinical benefit of the antigen test was globally evaluated through decision curve analysis (DCA).
Results
A total of 3899 patients were subjected to antigen tests and PCR swabs. The sensitivity, specificity and accuracy of the antigen test were 82.9%, 99.1% and 97.4% (Cohen's K = 0.854, 95% CI 0.826–0.882, p < 0.001), respectively. In symptomatic patients, sensitivity was found to be 89.8%, while in asymptomatic patients, sensitivity was 63.1%. DCA appears to confirm a net clinical benefit for the preliminary use of antigen tests.
Conclusions
The antigen test performed in the ED, though not ideal, can improve the overall identification of infected patients. While it appears to perform well in symptomatic patients, in asymptomatic patients, although it improves their management, it seems not to be definitive.
Keywords: Rapid antigen test, COVID-19, SARS-CoV-2, Emergency medicine, Emergency Department, RT-PCR
1. Introduction
Since the beginning of the SARS-CoV-2 pandemic, the rapid recognition and isolation of infected patients have proven to be crucial factors in limiting the spread of the virus and containing the pandemic [1,2]. The early and accurate identification of SARS-CoV-2 patients in the Emergency Department (ED) remains a major challenge [2,3]. In addition to the confirmation or exclusion of SARS-CoV-2 infection in symptomatic patients, the ED must simultaneously manage a large number of patients with pathological conditions other than COVID-19 [4]. These patients may be asymptomatic carriers of SARS-CoV-2 infection or, if not infected, they should be separated from patients infected with SARS-CoV-2 [5]. The consequences of failing to correctly identify a SARS-CoV-2-infected patient, whether symptomatic or asymptomatic, can be catastrophic [1,5].
Currently, the diagnostic reference standard for the detection of SARS-CoV-2 is the real-time polymerase chain reaction (RT-PCR) test for viral RNA, primarily from orotracheal secretions [6]. Although the molecular techniques have progressed in recent months and the overall performance of the RT-PCR test has been improving, there are still some difficulties in its application in the daily routine of EDs [7]. For instance, the test is not immediately available for every patient (few procedural sessions per day). The delay in test results and the lack of dedicated analysers in every ED are just some of the conditions that limit the effectiveness of the RT-PCR test for COVID-19 in the complicated clinical context of the ED [7,8].
The US Food and Drug Administration approved the first COVID-19 antigen test, which is rapid, direct and economical, at the end of August 2020. This test detects specific proteins attached to the surface of SARS-CoV-2 in samples obtained from the upper airways using an immuno-chromatographic procedure [9]. Early laboratory evidence suggested a good correlation between antigen testing and RT-PCR performed on samples with a high viral load [9,10].
A recent systemic review synthesized the evidence on the performance of the antigen test, evaluating 943 samples from five different studies. Although the sensitivity is not optimal (average sensitivity 56.2%), the specificity above 99% may suggest a preliminary role for rapid antigen tests in a more complete clinical evaluation for the determination of SARS-CoV-2 infection [11]. The results of the studies included in the review, which were largely based on remnant laboratory samples, currently have limited clinical applicability due to the absence of evidence in real clinical contexts (e.g. the ED) and the lack of information about the symptomatic status of the patients, the timing of symptom onset and the time elapsed since possible exposure [11].
This study reports the performance of the rapid antigen test in the identification of SARS-CoV-2-infected patients in the clinical context of the ED. Both symptomatic patients suspected of having COVID-19 and asymptomatic patients who needed hospital evaluation for conditions other than COVID-19 were evaluated.
2. Methods
2.1. Setting and sample
This retrospective observational study was conducted in the ED of the General Hospital of Merano (70,000 visits per year). The study period was from 1 July 2020 to 10 December 2020. This study considers patients with data previously published in the form of a preliminary report [12]. The data reported in this study are the conclusion of the previous report [12].
In July a new clinical protocol for the management of patients requiring an ED evaluation was introduced considering the use of rapid antigen tests for the timely identification patients with a SARS-CoV-2 infection.
According to the clinical protocol, the rapid antigen test for SARS-CoV-2 was performed at the initial triage and at the same time as the RT-PCR swab for SARS-CoV-2 (with two different swabs) in all the patients reporting: 1) symptoms suspicious for COVID-19 infection (fever, dyspnoea, cough, sore throat, diarrhoea, vomiting, asthenia, myalgias, conjunctivitis and deficits in smell and taste); 2) all patients without COVID-19-like symptoms but with an increased temperature (>37.3 °C); and 3) one positive epidemiological criterion, such as i) provenance from areas with a high incidence of SARS-CoV-2 cases, ii) coming from other European countries (independently if as a tourist or worker), or iii) reporting contacts with a person who tested positive for SARS-CoV-2. In addition, in order to prevent the possible intra-hospital spread of the infection, we included 4) all patients evaluated in the ED for other problems not related to COVID-19 infection and who needed hospitalisation underwent both a rapid antigen test and the RT-PCR swab for SARS-CoV-2. Data from all patients who received a rapid antigen test and at least one RT-PCR swab consecutively in the ED were retrieved from the electronic database and retrospectively evaluated.
The study was conducted in accordance with the local ethical committee (Comitato etico per la sperimentazione clinica, Azienda Sanitaria dell'Alto Adige, Bolzano, Italia, approval number 57-2020) and was conducted according to the Declaration of Helsinki regarding the Ethical Principles for Medical Research Involving Human Subjects.
2.2. Identification methods and patients
All of the patients who received a rapid antigen test and at least one RT-PCR swab consecutively in the ED were retrospectively evaluated. The patient extraction procedure was performed as follows: all the electronic ED folders of patients who had performed the rapid antigenic test in the ED (computer registered request) were extracted from the ED computer database using the dedicated management software QlickView (QlikTech, Pennsylvania, PA, US). The files of the patients thus identified were manually re-evaluated by a group of ED physicians and nurses (GTu, AZ, SS, GTe, NP, DA) and only those files in which a rapid antigenic test and an RT-PCR test were registered were considered. If other RT-PCR swabs were performed after the one performed in the ED (up to a maximum of three swabs within 15 days) the results were recorded and considered. No other rapid antigen tests were performed in addition to the one performed in the ED as the test was only available in the ED for initial screening.
The rapid antigen test and the RT-PCR swab for SARS-CoV-2 were performed in the ED by appropriately trained nurses. For each patient, one nurse collected both of the swabs.
Based on the symptomatology presented at ED admission, the patients were divided into two groups: symptomatic for COVID-19 and asymptomatic for COVID-19.
The rapid antigen test for COVID-19 was performed with the STANDARD Q COVID-19 Ag (R-Ag) (SD BIOSENSOR, KR), which is a ready-to-use test, according to the manufacturer's instructions. A control line is included in the test to assess the migration of the sample. Visual interpretation of the result was performed between 15 and 30 min. The test result was reported for each patient in the personal triage record. In the case of an invalid test, a second test was performed. RT-PCR was performed with the laboratory machine GeneXpert DX System (Cepheid, CA, US), the laboratory-processed swabs were developed with XPRSARS-COV2-10 (Cepheid, CA, US). The system has been approved by the World Health Organization (WHO), and the development of RT-PCR has been conducted in accordance with the WHO guidelines. The nasopharyngeal swab is collected and placed into a transport tube containing 3 mL of saline. The specimen is briefly mixed by rapidly inverting the collection tube 5 times. Using the supplied transfer pipette, the sample is transferred to the sample chamber of the Xpert Xpress SARS-CoV-2 cartridge. The GeneXpert cartridge is loaded onto the GeneXpert DX System platform, which performs hands-off, automated sample processing, and real-time RT-PCR for detection of viral RNA.
2.3. Statistical analysis
The continuous variables are expressed as median and interquartile range (25th–75th percentile), while the categorical variables are reported as percentage and total number of events. Comparisons were made using the Mann–Whitney test, Fisher's exact test or Chi-square test, as appropriate. The performance of the antigen test was determined by analysing sensitivity, specificity and accuracy using a 2 × 2 table with the result of the RT-PCR test, with 95% confidence intervals (95% CI) reported.
The concordance between the antigen test and the RT-PCR test was evaluated with Cohen's kappa coefficient. The clinical benefit that may be provided by testing ED patients with antigen tests was evaluated through decision curve analysis (DCA) [13]. DCA is a new simple statistical method that allows calculating the clinical practicality of predictive models and can lead to clinical considerations for decision-making. DCA is a plot of net clinical benefit (y-axis) against threshold probability (x-axis) [13]. The possible net clinical benefit of performing rapid antigen tests on ED arrival is compared with the two default strategies of “all patients are infected with SARS-CoV-2 “ (assuming all patients are positive) and “no patient is infected with SARS-CoV-2” (assuming all patients are negative). A higher net benefit within a wide threshold range than the two standard strategies indicate the model has more potential in clinical application.
Statistical analyses were performed with STATA 13.0 software (StataCorp, College Station, TX, USA).
3. Results
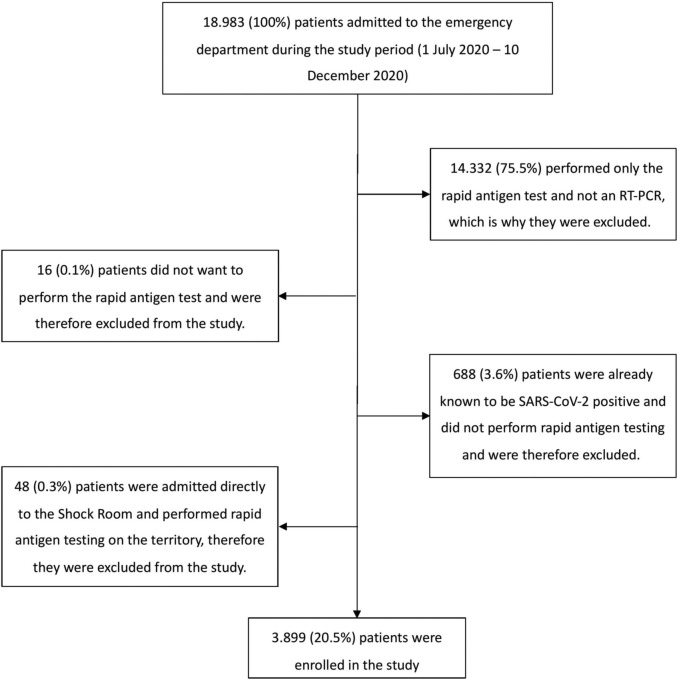
During the study period, 3899 patients required an ED evaluation and were tested with both rapid antigen tests and RT-PCR tests (Fig. 1 ). Of these, 30.5% (1191/3899) complained of at least one symptom possibly associated with SARS-CoV-2 infection. According to the RT-PCR results, a positive result for SARS-CoV-2 infection was found in 10.2% (397/3899) of the patients. Among the patients evaluated in the ED for a symptom that could be associated with a SARS-CoV-2 infection (n = 1191), 24.7% (294/1191) tested positive for SARS-CoV-2 (86.2% in the ED, 13.8% at 48/72 h, 0% at 14 days), and among asymptomatic patients (n = 2708), 3.8% (103/2708) tested positive with the RT-PCR test (95% in the ED, 3.1% at 48/72 h, 1.9% at 14 days). 0.2% (8/3899) reported an invalid rapid antigen test; all these patients underwent a second rapid antigen test with a valid result. The patients' demographic and clinical characteristics recorded upon ED access are reported in Table 1 .
Fig. 1.
Flow chart of patients enrolled in the study.
Table 1.
Demographic and baseline characteristics of all enrolled patients, divided according to RT-PCR result for SARS-CoV-2. * = less than 14 years; $ = over than 65 years.
| Variable | Global | COVID-19 negative | COVID-19 positive | p |
|---|---|---|---|---|
| Patients, n (%) | 3899 (100) | 3502 (89.8) | 397 (10.2) | |
| Sex, n (%) | 0.501 | |||
| Male | 1992 (51.1) | 1789 (51.1) | 212 (53.4) | |
| Female | 1907 (48.9) | 1713 (48.9) | 185 (46.6) | |
| Age, years, median (IQR) | 69 (49–82) | 69 (49–82) | 68 (47–81) | 0.830 |
| Paediatrics population*, n (%) | 91 (2.3) | 85 (2.4) | 6 (1.5) | 0.296 |
| Elderly population$, n (%) | 1866 (47.9) | 1672 (47.7) | 194 (48.9) | 0.672 |
| Arrival mode, n (%) | 0.005 | |||
| Walk-in/Private vehicle | 1740 (44.6) | 1612 (46.0) | 128 (32.2) | |
| Ambulance | 1798 (46.1) | 1551 (44.3) | 247 (62.2) | |
| Emergency medical service | 361 (9.3) | 339 (9.7) | 22 (5.5) | |
| Days of the week, n (%) | 0.132 | |||
| During the week | 2998 (76.9) | 2705 (77.2) | 293 (73.8) | |
| Weekend | 901 (23.1) | 797 (22.8) | 104 (26.2) | |
| Access during night (20.00–08.00), n (%) | 789 (20.2) | 718 (20.5) | 71 (17.9) | 0.236 |
| Tourist, n (%) | 235 (6.0) | 227 (6.0) | 8 (2.0) | <0.001 |
| Triage code, n (%) | 0.016 | |||
| Blue and Green | 1666 (42.7) | 1469 (41.9) | 197 (49.6) | |
| Yellow | 1501 (38.5) | 1369 (39.1) | 132 (33.2) | |
| Orange and Red | 732 (18.8) | 664 (19.0) | 68 (17.1) | |
| COVID-19 symptoms | 1191 (30.5) | 897 (25.6) | 294 (74.1) | <0.001 |
| Area of treatment, n (%) | <0.001 | |||
| Surgical area | 1032 (26.5) | 985 (28.1) | 47 (11.8) | |
| Internal medicine area | 2279 (58.5) | 1945 (55.5) | 334 (84.1) | |
| Gynaecological area | 189 (4.8) | 182 (5.2) | 7 (1.8) | |
| Trauma area | 399 (10.2) | 390 (11.1) | 9 (2.3) | |
| Rapid antigen test for COVID-19, n (%) | <0.001 | |||
| Negative antigen test | 3538 (90.7) | 3470 (99.1) | 68 (17.1) | |
| Positive antigen test | 361 (9.3) | 32 (0.9) | 329 (82.9) |
The antigen test for SARS-CoV-2 was positive in 9.3% (361/3899) of all patients. Among patients with SARS-CoV-2 (n = 397), rapid antigen tests were positive in 82.9% (329/397) of cases. Among patients not infected by SARS-CoV-2 (n = 3502), a false-positive antigen test occurred in 0.9% (32/3502) of patients.
Overall, the sensitivity and specificity of the antigen test for detection of SARS-CoV-2 infection were 82.9% (95% CI, 81.0–84.8) and 99.1% (95% CI, 98.8–99.3), respectively (Table 2 ). The accuracy of the antigen test is 97.4% (95% CI, 97.1–97.6, Cohen's K = 0.854, 95% CI 0.826–0.882, p < 0.001).
Table 2.
One 2 × 2 contingency table on rapid antigen test performance in the assessment of patients with COVID-19 among all patients.
| Global population (n = 3899) | Patients COVID-19 non-infected | Patients COVID-19 infected |
|---|---|---|
| Negative antigen test for COVID-19 | 3470 | 68 |
| Positive antigen test for COVID-19 | 32 | 329 |
| Sensitivity | 82.9% (81.0–84.8) | |
| Specificity | 99.1% (98.8–99.3) | |
| Positive predictive value | 91.1% (89.7–92.5) | |
| Negative predictive value | 98.1% (97.8–98.3) | |
| Accuracy (correctly classified) | 97.4% (97.1–97.6) | |
3.1. Performance of the SARS-CoV-2 antigen test in symptomatic and asymptomatic patients
The clinical characteristics of the patients evaluated in the ED for COVID-19-like symptoms are reported in Table 3 . (See Table 4.)
Table 3.
Demographic and baseline characteristics of the patients evaluated in the ED for symptoms suspicious for SARS-CoV-2.
| Variable | Negative antigen test for COVID-19 | Positive antigen test for COVID-19 | p |
|---|---|---|---|
| Patients, n (%) | 906 (76.1) | 285 (23.9) | |
| SARS-CoV-2 positive | 30 (3.3) | 264 (92.6) | <0.001 |
| Age, years, median (IQR) | 75 (58–84) | 68 (50–8) | 0.002 |
| Sex, n (%) | 0.556 | ||
| Male | 486 (53.7) | 161 (56.4) | |
| Female | 420 (46.3) | 124 (43.6) | |
| COVID-19 symptoms, n (%) | |||
| Fever or history of fever | 451 (52.7) | 148 (53.2) | 0.890 |
| Cough | 112 (13.2) | 84 (30.2) | <0.001 |
| Dyspnoea | 327 (38.2) | 114 (41.2) | 0.395 |
| Gastroenterological | 204 (23.8) | 61 (21.9) | 0.568 |
| Other symptoms | 369 (43.2) | 137 (49.5) | 0.071 |
| Time of onset of symptoms, days, median (IQR) | 2 (1–3) | 2 (2–4) | <0.001 |
| Comorbidity, n (%) | 415 (48.5) | 91 (33.0) | <0.001 |
Table 4.
Two 2 × 2 contingency tables on antigen rapid test performance in the assessment of patients with COVID-19. The first table focuses on the rapid antigen test among symptomatic patients for SARS-CoV-2 and the table below focuses on the rapid antigen test among asymptomatic patients for SARS-CoV-2.
| Patients COVID-19 non-infected | Patients COVID-19 infected | |
|---|---|---|
| Only considering symptomatic patients for COVID-19 | ||
| Negative antigen test for COVID-19 | 876 | 30 |
| Positive antigen test for COVID-19 | 21 | 264 |
| Sensitivity | 89.8% (88.0–91.5) | |
| Specificity | 97.6% (97.1–98.1) | |
| Positive predictive value | 92.6% (91.0–94.1) | |
| Negative predictive value | 96.7% (96.0–97.2) | |
| Accuracy (correctly classified) | 95.7% (95.1–96.3) | |
| Only considering asymptomatic patients for COVID-19 | ||
| Negative antigen test for COVID-19 | 2594 | 38 |
| Positive antigen test for COVID-19 | 11 | 65 |
| Sensitivity | 63.1% (58.4–67.8) | |
| Specificity | 99.6% (99.5–99.7) | |
| Positive predictive value | 85.5% (81.5–89.5) | |
| Negative predictive value | 98.5% (98.3–98.7) | |
| Accuracy (correctly classified) | 98.2% (97.9–98.4) | |
A total of 3.3% (30/906) of patients with a negative result in the antigen test were found to be infected with SARS-CoV-2 via the RT-PCR test, while 7.4% (21/285) of patients with positive antigen test were not to be infected with SARS-CoV-2 in a RT-PCR test.
In symptomatic patients, the sensitivity of the antigen test for the detection of SARS-CoV-2 infection was 89.8% (95% CI, 88.0–91.5), specificity was 97.6% (95% CI, 97.1–98.1) and accuracy was 95.7% (95% CI, 95.1–96.3, Cohen's K = 0.884, 95% CI 0.852–0.915, p < 0.001).
Finally, among asymptomatic patients, the sensitivity, specificity and accuracy of the antigen test compared to RT-PCR are 63.1% (95% CI, 58.4–67.8), 99.6% (95% CI, 99.5–99.7) and 98.2% (95% CI, 97.9–98.4, Cohen's K = 0.717, 95% CI 0.642–0.793, p < 0.001).
3.2. SARS-CoV-2 antigen test and decision curve analysis
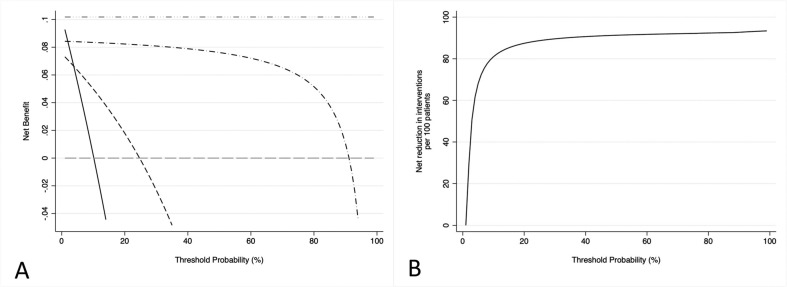
In addition to the assessment of the diagnostic accuracy of the rapid antigen test, a wider evaluation on the possible global clinical benefit from the use of rapid antigen test to identify COVID-19-infected patients in ED was performed through DCA. The DCA plot seems to suggest that the use of rapid antigen tests as an initial screening tool in EDs can provide an important clinical benefit, especially when considering a population that includes asymptomatic individuals and in which the prevalence of infection appears close to its true prevalence in the general population. The inclusion of rapid antigen tests in ED had a net clinical benefit superior to clinical evaluation alone over a wide range of threshold probabilities, demonstrating the usefulness of this strategy in ED. Between a threshold probability (disease prevalence) of 20% and 30%, the use of rapid antigen tests resulted in a net clinical benefit of between 3% and 5%, suggesting the possibility of detecting up to 5 additional true positives per 100 patients admitted in the ED who were not correctly identified by the clinical evaluation alone (Fig. 2A). In fact, compared to testing all ED patients immediately with RT-PCR, at low disease prevalence rates (<20%) of SARS-CoV-2, the preliminary use of the rapid antigen test leads to a net clinical benefit of approximately 8%. The net clinical benefit is gradually reducing as the disease prevalence increases and at prevalence over 60%, indicating severe levels of infection circulating in the general population, the use of a preliminary screening test may not be a useful strategy, becoming useless for disease prevalence above 90%. However, it must be considered that at high disease prevalence's, above 90%, it would not be useful to apply the screening test at ED admission to identify COVID-19 positive patients. Moreover, clinical suspicion of SARS-CoV-2 infection based on the patients' history and signs and symptoms (clinical evaluation) does not seem to be a useful strategy for discriminating patients infected with SARS-CoV-2 admitted in ED, especially due to asymptomatic patients (Fig. 2A). For disease prevalence values around 10%, the implementation of the antigen test strategy leads to the detection of 8 true positives per 100 RT-PCR tests performed, with a stable performance from a threshold probability above 22%. In addition, a hypothetical strategy involving adding the rapid antigen test to the clinical study of the symptomatology presented prior to subjecting all ED patients to the RT-PCR swab could improve by 65 out of 100 RT-PCR tests when the prevalence of SARS-CoV-2 is lower than 10% (Fig. 2B).
Fig. 2.
A) Decision curve analysis and its distribution. Grey dashed line: assume no patients have COVID-19. Black line: assume all patients have COVID-19. Grey dash-dotted line: a hypothetical perfect test. Black dashed line: the strategy of discovering COVID-19-infected patients only on the basis of their symptoms. Black dash-dotted line: the strategy of performing antigen tests on patients in the ED. The X-axis indicates the threshold probability and the Y-axis indicates the net benefit. The black line assumes that all the patients would be SARS-CoV-2 infected, while the grey line reflects the assumption that no patients would be SARS-CoV-2 infected. The dashed black line represents the net clinical benefit provided by the clinical evaluation and the grey dashed and dotted line represents the net clinical benefit provided by the introduction of rapid antigen tests in the ED. As demonstrated in the graph, rapid antigen tests achieved greater clinical utility in the threshold probability, indicating that rapid antigen tests may be a valuable tool in identifying SARS-CoV-2 positive patients. B) Decision curve analysis plotting the decrease in RT-PCR swabs due to the clinical evaluation plus the implementation of the antigen test based on the prevalence of SARS-CoV-2 in the population.
4. Discussion
Using a large cohort of patients consecutively managed in the ED, the study presents the diagnostic performance of the rapid antigen test compared to the RT-PCR swab when used in a clinical setting to identify SARS-CoV-2-infected patients in both symptomatic and asymptomatic patients managed daily in the ED. The antigen test achieved good specificity and accuracy values, as reported in previous laboratory studies, with a fair sensitivity. The results of the present study confirm the previously published data on a partial cohort of this study, confirming the usefulness of the strategy of performing the antigen test on all patients presenting in the ED, as demonstrated by DCA [12]. Even when considering 489 more patients than in the previously published preliminary report, the performance of the rapid antigen test did not change, demonstrating a clearly superior performance in symptomatic patients compared to asymptomatic patients [12].
To the best of our knowledge, this is the study with the largest cohort that has evaluated antigen tests in daily ED clinical practice con una that has considered patients who are symptomatic as well as patients who came to the ED for other reasons and who may be asymptomatic carriers of the virus. Since the hospitalisation of one of these patients (asymptomatic carriers) incorrectly identified as SARS-CoV-2 negative can lead to dramatic consequences, the evaluation of appropriate strategies in order to prevent this is crucial [14,15].
Some of the characteristics of the rapid antigen test described at the time of commercialisation seem to adapt well to the ED scenario [9,11]. Its simplicity, repeatability, rapidity of execution and, more importantly, immediate results could overcome the limitations presented by the RT-PCR swab, adapting better to the dynamic nature of EDs [[9], [10], [11]].
The first laboratory studies for the validation of the methodology have provided good initial indications for different types of antigen tests. The laboratory comparison with RT-PCR performed on the same microbiological sample revealed a sensitivity of 68%, a specificity of 100% and an accuracy of 72% for the antigen test [16]. Only 31 out of 239 patients were negative in the detection of the viral RNA, indicating a high prevalence of the disease in the limited study cohort [16]. In addition to the high prevalence of infection, the absence of clinical information may limit the application of these results in clinical practice. Similarly, Porte et al., who tested 127 samples and found 82 to be positive by RT-PCR, confirmed a 100% specificity of the antigen test and indicated a sensitivity and accuracy of 93.9% and 96.1%, respectively [10]. Despite the high prevalence of SARS-CoV-2 in the present study, the sensitivity of the antigen test seems to improve as the viral load presented in the samples increases. In the case of reduced prevalence and reduced viral load, the antigen test seems to have a decreased ability to identify patients infected with SARS-CoV-2 [10,17]. More recently, Cerutti et al. reported the first indications of the antigen test in a group of asymptomatic patients (travellers returning from risk areas) [18]. The sensitivity and specificity of the antigen test in this group of asymptomatic patients were 40% and 100%, respectively, with a RT-PCR swab agreement close to 98%, higher than in the symptomatic group [18].
An initial systematic review of the performance of the antigen test was conducted in an attempt to provide precise indications that can be applied in clinical practice [11]. By grouping 943 tests from five studies, the sensitivity of the antigen tests was found to be relatively low (56.2%, 95% CI 29.5%–79.8%), but a consistently high level of specificity was observed (mean 99.5%, 95% CI 98.1%–99.9%) [11]. However, the absence of information on patient symptomatology limits the evaluation of the usefulness of the antigen test in clinical practice.
The findings of the current study are the first to translate the evidence from previous laboratory studies into clinical practice. Due to the fact that the consequences of a single error in the detection of a SARS-CoV-2-infected patient accessing the hospital can have catastrophic consequences, in daily ED practice, the confirmed high levels of specificity do not seem to be sufficient to compensate for the sub-optimal levels of sensitivity and to provide perfect flow management based only on antigen tests [14,15,19]. However, at the indicated levels of prevalence, a positive antigen test in symptomatic patients could allow early and rapid identification of the infection while, in case of a negative antigen test, the maintenance of a high clinical suspicion could guarantee safe management until successive microbiological confirmations. Moreover, in asymptomatic patients, the use of the antigen test, which alone cannot guarantee that all SARS-CoV-2-positive patients are identified, could allow an immediate identification of a portion of patients and thus improve the entire ED decision-making process. The ability of asymptomatic patients to spread the infection is well known [14,20,21]. Hospitalisation of an undiagnosed asymptomatic patient in a non-COVID-19 department can lead to intra-hospital spread of the virus, affecting patients and healthcare workers [14,20,21]. The development of specific strategies for these patients is important. The use of the antigen test in the ED, as suggested by the DCA, provides a net clinical benefit in the process of identifying infected individuals. Although it is still far from the performance of an ‘ideal test’, the antigen test seems to be a good initial strategy in both symptomatic patients, in whom it is combined with clinical evaluation, and asymptomatic patients, where it can immediately identify a portion of patients who otherwise would not have been rapidly identified.
The study presents some limitations. First, there are no quantitative data available about the viral load to confirm a possible condition of reduced viral load in the false-negative antigen tests. Second, there are no analyses available about the risk of viral transmission by patients who received a false-negative antigen test. Third, asymptomatic patients who were discharged after ED evaluation and did not require hospitalisation were not included in the study because they were not subjected to a RT-PCR swab.
5. Conclusions
Rapid and accurate identification of patients with SARS-CoV-2 infection in the ED is essential to contain the progression of the pandemic and keep the hospital secure. The overall sensitivity, specificity and accuracy of the rapid antigen test were very good, yet with low sensitivity in asymptomatic patients. The assessment of patients admitted to the ED for a SARS-CoV-2 infection during the initial triage via rapid antigen test has an additional clinical benefit. In symptomatic patients arriving in the ED, where the prevalence of SARS-CoV-2 infection is higher, a positive antigen test can accelerate and optimise the management of the infected patient. A negative antigen test in these symptomatic patients should however be followed by RT-PCR testing to confirm the absence of disease. In asymptomatic patients, for whom more secure and precise strategies need to be implemented, an initial antigen test seems to identify patients with SARS-CoV-2 infection who otherwise would not have been rapidly detected. However, a negative rapid antigen test in asymptomatic patients is not sufficient to safely exclude SARS-CoV-2 infection.
Authors' contributors
GTu, AZ, NP conceived and designed the project.
GTu, AZ, GTe, SS, NP, AB acquired, analyzed, and interpreted the data.
GTu, AZ and DA wrote the original draft manuscript.
GTu and AZ wrote, revised and edited the manuscript.
The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Funding
This study was not funded.
Data availability statement
Data are available on request.
Declaration of Competing Interest
None declared.
References
- 1.Wu X., Zhou H., Wu X., Huang W., Jia B. Strategies for qualified triage stations and fever clinics during the outbreak of COVID-2019 in the county hospitals of Western Chongqing. J Hosp Infect. 2020 Jun;105(2):128–129. doi: 10.1016/j.jhin.2020.03.021. (Epub 2020 Mar 20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wee L.E., Fua T., Chua Y.Y., Ho A.F.W., Sim X.Y.J., Conceicao E.P., et al. Containing COVID-19 in the emergency department: the role of improved case detection and segregation of suspect cases. Acad Emerg Med. 2020 May;27(5):379–387. doi: 10.1111/acem.13984. (Epub 2020 May 11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshi R.P., Pejaver V., Hammarlund N.E., Sung H., Lee S.K., Furmanchuck A., et al. A predictive tool for identification of SARS-CoV-2 RT-PCR-negative emergency department patients using routine test results. J Clin Virol. 2020 Aug;129:104502. doi: 10.1016/j.jcv.2020.104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casalino E., Choquet C., Bouzid D., Peyrony O., Curac S., Ravue E., et al. Analysis of emergency department visits and hospital activity during influenza season, COVID-19 epidemic, and lockdown periods in view of managing a future disaster risk: a multicenter observational study. Int J Environ Res Public Health. 2020 Nov 10;17(22) doi: 10.3390/ijerph17228302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X., Yang R. COVID-19 transmission through asymptomatic carriers is a challenge to containment. Influenza Other Respi Viruses. 2020 Jul;14(4):474–475. doi: 10.1111/irv.12743. (Epub 2020 Apr 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu S.Y., Yu M.Y., Tsang H.F., Chan L.W.C., Cho W.C.S., Yu A.K.Y., et al. The diagnostic methods in the COVID-19 pandemic, today and in the future. Expert Rev Mol Diagn. 2020 Sep;20(9):985–993. doi: 10.1080/14737159.2020.1816171. (Epub 2020 Sep 16) [DOI] [PubMed] [Google Scholar]
- 7.Zitek T. The appropriate use of testing for COVID-19. West J Emerg Med. 2020 Apr 13;21(3):470–472. doi: 10.5811/westjem.2020.4.47370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter C.R., Mudd P.A., West C.P., Wilber E., Wilber S.T. Diagnosing COVID-19 in the emergency department: a scoping review of clinical examinations, laboratory tests, imaging accuracy, and biases. Acad Emerg Med. 2020 Jun 16 doi: 10.1111/acem.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissleder R., Lee H., Ko J., Pittet M.J. COVID-19 diagnostics in context. Sci Transl Med. 2020 Jun 3;12(546) doi: 10.1126/scitranslmed.abc1931. [DOI] [PubMed] [Google Scholar]
- 10.Porte L., Legarraga P., Vollrath V., Aguilera X., Munita J.M., Araos R., et al. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int J Infect Dis. 2020 Oct;99:328–333. doi: 10.1016/j.ijid.2020.05.098. (Epub 2020 Jun 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinnes J., Deeks J.J., Adriano A., Berhane S., Davenport C., Dittrich S., et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020 Aug 26;8 doi: 10.1002/14651858.CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turcato G., Zaboli A., Pfeifer N., Ciccariello L., Sibilio S., Tezza G., et al. Clinical application of a rapid antigen test for the detection of SARS-CoV-2 infection in symptomatic and asymptomatic patients evaluated in the emergency department: a preliminary report. J Infect. 2021 Mar;82(3):e14–e16. doi: 10.1016/j.jinf.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vickers A.J., van Calster B., Steyerberg E.W. A simple, step-by-step guide to interpreting decision curve analysis. Diagn Progn Res. 2019 Oct 4;3:18. doi: 10.1186/s41512-019-0064-7. (eCollection 2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavezzo E., Franchin E., Ciavarella C., Cuomo-Dannenburg G., Barzon L., Del Vecchio C., et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020 Aug;584(7821):425–429. doi: 10.1038/s41586-020-2488-1. (Epub 2020 Jun 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Sadeq D.W., Nasrallah G.K. The incidence of the novel coronavirus SARS-CoV-2 among asymptomatic patients: a systematic review. Int J Infect Dis. 2020 Sep;98:372–380. doi: 10.1016/j.ijid.2020.06.098. (Epub 2020 Jul 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diao B., Wen K., Chen J., Liu Y., Yuan Z., Han C., et al. 2021. Diagnosis of acute respiratory syndrome coronavirus 2 infection by detection of nucleocapsid protein. [DOI] [Google Scholar]
- 17.Hirotsu Y., Maejima M., Shibusawa M., Nagakubo Y., Hosaka K., Amemiya K., et al. Comparison of automated SARS-CoV-2 antigen test for COVID-19 infection with quantitative RT-PCR using 313 nasopharyngeal swabs, including from seven serially followed patients. Int J Infect Dis. 2020 Oct;99:397–402. doi: 10.1016/j.ijid.2020.08.029. Published online 2020 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerutti F., Burdino E., Milia M.G., Allice T., Gregori G., Bruzzone B., et al. Urgent need of rapid tests for SARS CoV-2 antigen detection: evaluation of the SD-Biosensor antigen test for SARS-CoV-2. J Clin Virol. 2020 Nov;132:104654. doi: 10.1016/j.jcv.2020.104654. (Epub 2020 Sep 29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scohy A., Anantharajah A., Bodéus M., Kabmba-Mukadi B., Verroken A., Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol. 2020 Aug;129:104455. doi: 10.1016/j.jcv.2020.104455. (Epub 2020 May 21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao M., Yang L., Chen X., Deng Y., Yang S., Xu H., et al. A study on infectivity of asymptomatic SARS-CoV-2 carriers. Respir Med. 2020 Aug;169:106026. doi: 10.1016/j.rmed.2020.106026. (Epub 2020 May 13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L., Zhang X., Zhang X., Wei Z., Zhang L., Xu J., et al. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16-23 years outside Wuhan and characteristics of young patients with COVID-19: a prospective contact-tracing study. J Infect. 2020 Jun;80(6):e1–e13. doi: 10.1016/j.jinf.2020.03.006. (Epub 2020 Apr 10) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request.