Abstract
Introduction
The COVID-19 pandemic has resulted in severe ongoing blood shortages across the US, despite employment of numerous blood-conservation measures. Massive transfusion protocols (MTP) are one resource-intensive practice that utilize significant amounts of blood products. Alterations to the composition of MTP parameters to conserve scarce biologic resources have hitherto not been examined during the pandemic.
Methods
An anonymous 18-question survey was administered to 115 hospitals with valid email contact information. Survey questions addressed whether institutions have altered their MTPs due to the COVID-19 pandemic and blood shortages, and if so, what adjustments they have made. Additional details concerning potential differences in the number and cycles of MTPs and blood product wastage during the COVID-19 pandemic compared to the year prior were assessed.
Results
50 responses were received (43 % response rate). 10 % (5/50) of institutions altered their MTPs utilizing a variety of approaches in attempt to conserve blood during the COVID-19 pandemic. Four additional institutions intend to alter them if it becomes necessary. Following onset of the COVID-19 pandemic, 24 % of institutions (12/50) reported an increase in monthly MTP activations, while 16 % (8/50) reported decreased activations compared to prior to the pandemic. 22 % (11/50) of institutions experienced increased blood wastage, whereas 16 % (8/50) reported decreased waste compared to pre-pandemic.
Discussion
The results of this survey highlight a variety of mechanisms by which institutions have attempted to conserve blood via altering MTPs. Whether an institution adjusted their MTP does not correlate with changes in blood product wastage compared to pre-pandemic.
Keywords: Massive transfusion protocols, Patient blood management, COVID-19, Pandemic, Blood shortage
1. Introduction
The COVID-19 pandemic has become an unprecedented event in modern human history, disrupting all aspects of life over the previous 18 months. One consequence of the numerous infections, fear of the virus, and measures implemented to mitigate escalation of the pandemic is the ongoing disturbance in the blood supply. Soon after the first cases were confirmed in the United States (US), blood donations began to decrease secondary to canceled blood drives, lockdowns, social distancing measures, and a general reluctance of donors to gather in public [[1], [2], [3]]. To avert the impending blood shortage, hospitals began altering their practices to minimize blood usage, primarily focusing on postponement of elective surgeries and procedures [2,3]. Initially, these temporizing measures were successful in preventing shortages; however, as hospitals have resumed normal operations, in conjunction with the addition of postponed procedures, the demand for blood products has approached pre-pandemic levels, while the blood supply has not recovered [[4], [5], [6]].
To ameliorate the ongoing blood shortage, additional strategies have been implemented to reduce blood usage, including prospective auditing of transfusion requests [7], altering red blood cell (RBC) exchange programs for sickle cell patients [8], enforcing and strictly adhering to guidelines for anemia management [9], considering the lowering of transfusion thresholds [10], and various other patient blood management techniques [11].
One resource-intensive transfusion practice that has not been examined during the pandemic is the massive transfusion protocol (MTP). MTPs represent an important evidence-based strategy to prevent exsanguination and coagulopathy in patients with large-volume hemorrhage via constant provision of a pre-determined volume and composition of blood products [12,13]. As a result, large numbers of blood components are often dispensed during MTPs, which can lead to a significant amount of blood wastage during these procedures [[14], [15], [16], [17]]. MTPs therefore represent a crucial area to address during blood supply constraints, as a single MTP is capable of depleting the blood inventory for an entire hospital or region [18]. An AABB survey administered over a 4-week period in the early stages of the pandemic (March 23, 2020 – April 13, 2020) found that a minority of institutions had limited the number of blood components available during MTPs, but did not explore these changes further [10].
We sought to expand upon the AABB survey findings regarding MTP practices during the COVID-19 pandemic. We analyzed how institutions have utilized their MTPs throughout the pandemic, whether changes have been made to conserve blood, and if so, whether these changes are permanent. Additionally, we sought to understand if blood product wastage has changed in the context of MTPs since the beginning of the pandemic.
2. Methods
An anonymous 18-question survey (Table 1 ) was constructed utilizing Research Electronic Data Capture (REDCap) [19]. The questions were developed by the authors to assess whether institutions have altered their MTPs due to the COVID-19 pandemic and blood shortages, as well as whether institutions have observed differences in the number and cycles of MTPs and blood product wastage during the COVID-19 pandemic compared to the year before.
Table 1.
Massive Transfusion Protocol (MTP) survey.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A Web-based browser search was performed to identify health systems with valid email contact information for a representative cross-section of transfusion medicine specialists. We utilized publicly available databases, including those by the AABB and the Society for the Advancement of Blood Management, and cross-referenced institutions with Level 1 trauma accreditation. We also employed a free language search using key search words including “blood bank,” “transfusion medicine,” “massive transfusion,” and “bloodless medicine” to identify healthcare facilities likely to have an MTP. Personalized links for accessing the survey in the REDCap system were sent from July – August 2021 via email to individuals within the blood bank, transfusion medicine, or clinical/laboratory medicine department at 115 institutions. These personalized links prevented duplicative responses. Survey responses were automatically uploaded and de-identified within the database.
3. Results
50 unique institutions responded to the survey (43 % response rate), and all have an MTP in place. The characteristics of the institutions that responded are represented in Table 2 .
Table 2.
Characteristics of survey respondents.
| Hospital category | Beds | Trauma center | Patient population | MTP | |||||
|---|---|---|---|---|---|---|---|---|---|
| Academic medical center/university hospital | 45 (90 %) | >900 | 13 (26 %) | Level 1 | 40 (80 %) | Adults | 11 (22 %) | Yes | 50 (100 %) |
| State, community, or city hospital | 2 (4 %) | 751−900 | 10 (20 %) | Level 2 | 1 (2 %) | Pediatrics | 4 (8 %) | No | 0 (0 %) |
| Federal government/military facility | 0 (0 %) | 601−750 | 10 (20 %) | Level 3 | 1 (2 %) | Both | 35 (70 %) | ||
| Private, nonprofit (1) | 3 (6 %) | 451−600 | 7 (14 %) | No | 8 (16 %) | ||||
| Private, profit | 0 (0 %) | 301−450 | 9 (18 %) | ||||||
| 150−300 | 1 (2 %) | ||||||||
| <150 | 0 (0 %) | ||||||||
MTP, massive transfusion protocol.
3.1. MTP composition
Among the institutions that serve all patient populations, 12 have developed MTPs that are unique to adult, pediatric, and obstetric patients, while 11 others have specific adult and pediatric MTPs.
All responding institutions utilize a fixed number and ratio of blood products in their MTP, while 8 of these institutions also use point-of-care coagulation testing to determine the composition of their MTP. One institution that incorporates a fixed number and ratio of products also employs threshold-based approaches to transfusion with concomitant point-of-care coagulation testing to guide their MTPs. 24 % (12/50) use low-titer group O whole blood in their MTP. Significant variation in MTP component ratios exists among the responding institutions. The most common ratios among respondents are 6 RBCs : 6 FFP : 1 apheresis platelet (17/50) and 4 RBCs : 4 FFP : 1 apheresis platelet (11/50), but 17 unique protocols were identified.
3.2. COVID-19 MTP alterations
10 % (5/50) of institutions altered their MTPs during the COVID-19 pandemic due to blood product inventory availability, and 4 additional institutions intend to alter them if it becomes necessary. Although all institutions that changed their MTP expect to revert to their old ratio following stabilization of the blood supply, 1 institution does plan to permanently implement the new ratio for MTPs that persist beyond a certain cycle number.
To conserve blood, the institutions that altered their MTP components did so in a variety of ways including:
-
•
Altering the MTP ratio from 6:6:1 to 4:4:1 (RBCs : FFP : apheresis platelet)
-
•
Decreasing the frequency of platelets from 1 unit of apheresis platelets in every cycle to 1 unit every other cycle
-
•
Issuing one-half of the MTP cycle at a time (e.g., providing 3 RBCs, 2 FFP, and 1 apheresis platelet every other cycle instead of 6 RBCs, 4 FFP, and 1 apheresis platelet every cycle), effectively splitting the number of RBC and FFP units in half, and providing platelets in every other cycle
-
•
Reducing the number of RBC units by half for patients using type O units following the 3rd MTP cycle (originally 6 RBCs : 6 FFP: 1 apheresis platelet)
No institution altered their transfusion thresholds or changed the amount or frequency of low-titer group O whole blood in their MTP.
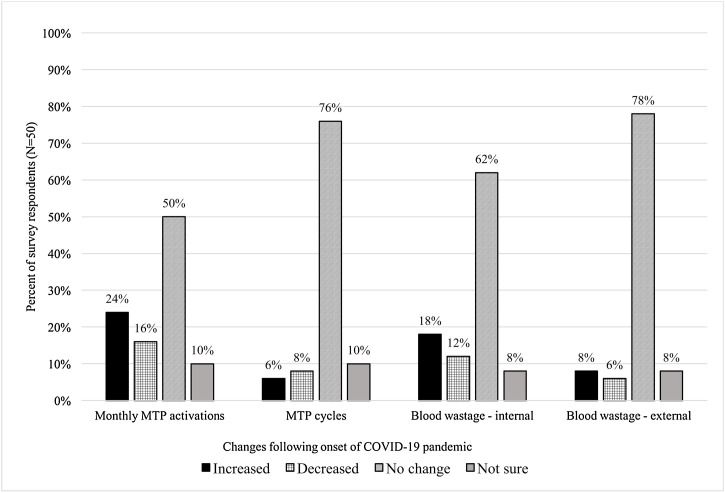
24 % of institutions (12/50) reported that monthly MTP activations increased following onset of the COVID-19 pandemic compared to the baseline pre-pandemic level (Fig. 1 ). The number of institutions reporting increases in the number of MTP cycles (3/50; 6 %) and decreased cycles (4/50; 8 %) was similar.
Fig. 1.
Changes in massive transfusion protocols and blood wastage following the onset of the COVID-19 pandemic compared to the previous year.
3.3. Blood product usage, wastage, and comments
Following onset of the COVID-19 pandemic, 22 % (11/50) of institutions reported increased blood wastage compared to pre-pandemic. 7/11 (64 %) hospitals attributed this waste to internal blood bank variables, with the primary source being internal product outdating; 3/11 (27 %) attributed it to external variables including poor product handling and products being returned out of the appropriate temperature range; and 1/11 (9 %) associated the increased waste with both internal and external variables. Institutions also attributed wastage to staff shortages both within and external to the clinical laboratory, including challenges with adequately transporting MTPs, resulting in suboptimal processes. There is no apparent correlation between changes in blood product waste and MTP alterations, as institutions that altered their MTPs reported both increased and decreased blood wastage.
To mitigate potential issues with blood wastage and MTPs during blood inventory shortages, institutions made concerted efforts to assure adherence to blood management policies. Additional changes that institutions incorporated include more readily switching to RhD-positive units in all populations; attempting to include more product derivatives such as factor concentrates during MTPs; standardizing “time out” episodes during active resuscitation at the bedside to discuss prognostic implications of high-volume blood use; ongoing MTP utilization review; and establishing triggers to modify MTPs based on the number of units in the inventory.
4. Discussion
The change in the number of MTP activations following the onset of the COVID-19 pandemic compared to the year prior is highly variable, with almost one-quarter of institutions reporting increased MTP activations, while 16 % activated fewer MTPs. The etiology for this variation is unclear, and may simply represent random fluctuations. However, it is essentially impossible to definitively exclude unique “pandemic-related” factors that could theoretically influence trauma-related and non-trauma-related MTP activations, including the patterns and length of community responses and measures taken to mitigate spread of the virus such as lockdowns and “stay-at-home” orders, variations in traffic patterns and accidental traumas, and general societal and geopolitical factors [20,21].
Interestingly, while many hospitals have struggled with blood shortages at different time-points throughout the pandemic, several respondents to our survey have not experienced blood shortages, or are just now beginning to face shortages. These findings highlight the need for enhanced communication among blood donor centers and hospitals. This inter-institutional collaboration would ensure adequate blood product inventories in regions with more significant shortages and higher numbers of MTPs.
Hospitals have employed numerous approaches to mitigate wastage during periods of blood supply constraints. Almost one-fifth of institutions in our study altered or have plans in place to adjust their MTPs, particularly focusing on limiting the number of cycles per MTP if necessary. The institutions that did modify their MTPs made changes such as decreasing the number of RBCs and plasma in every cycle and decreasing the frequency with which platelets were provided. Data have shown that a 1:1:1 ratio of RBCs, plasma, and platelets is currently considered the optimal approach for stabilization of hemorrhaging patients [22,23]; thus, the consequences of intermittently adjusting the ratio of blood products in MTPs are unknown. However, it should be noted that despite this expert opinion considering a 1:1:1 product ratio as the “gold standard,” the randomized trials from which much of this data were derived failed to show significant differences in mortality at 24 h or 30 days in patients who received a 1:1:1 product ratio compared to a 1:1:2 ratio (FFP, platelets, RBCs) [22,23]. Furthermore, the significant variability in MTP practices that exists among institutions [24] serves to highlight the knowledge gaps that exist. Therefore, the MTP alterations made by the responding institutions appear to have occurred at an institutional level with little published data to support these implementations. Instead, these changes seem to predominantly reflect decisions made to ensure conservation of blood product inventories at the expense of altering established practices.
Several respondents remarked that they have encountered significant difficulty in altering the composition of their MTPs or instituting other blood-conservation measures in response to blood shortages. These decisions, including whether to alter MTPs, thereby undoing institutional experience and expert opinion, or alternatively risk depleting the blood product inventory, have been extremely challenging. Institutions report that oftentimes, disagreements have occurred among members of the blood bank/transfusion medicine service and clinical providers in various departments regarding resource allocation and transfusion priority. Furthermore, staffing issues secondary to COVID-19 infection and quarantine measures, both inside and external to the blood bank, have decreased compliance with standardized protocols and hampered blood conservation efforts at many institutions. Therefore, system-wide enhancements to collaborative measures and intra-hospital committees are critical to ensure that patient blood management and blood conservation strategies are implemented and adhered to during periods of supply constraints. Furthermore, hospital ethics committees are a valuable resource during these challenging decisions regarding the allocation of scarce resources.
The COVID-19 pandemic is an historical event that modern medicine has never confronted. Thus, it represents a challenging, but unique scenario in which transfusion medicine and blood banks can analyze blood usage during significant inventory fluctuations and devise methods to mitigate waste in real time. The results of this survey are unable to definitively conclude that altering MTP ratios, and the actual ratios themselves, affect blood wastage or mitigate blood shortages; however, the marked variability in MTP practice among institutions highlighted by this survey despite the generally accepted notion that the 1:1:1 approach is “optimal” begs the question as to why there are so many variations in practice. Given this significant variation and the 43 % response rate, the results obtained in this study cannot be considered representative of the entire population enrolled. Nevertheless, this survey provides a foundation to analyze what effect potential changes to MTPs may have on blood product waste, optimal practice, and patient outcomes during times of blood shortages.
CRediT authorship contribution statement
Jeremy W. Jacobs: formulated the research aims, performed data investigation and analysis, wrote the original draft, and approved the final draft. Garrett S. Booth: formulated the research aims, reviewed and revised the original draft, provided supervision, and approved the final draft.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.World Health Organization; 2021. Listings of WHO’s response to COVID-19.https://www.who.int/news/item/29-06-2020-covidtimeline Accessed August 12. [Google Scholar]
- 2.Stanworth S.J., New H.V., Apelseth T.O., Brunskill S., Cardigan R., Doree C., et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020;7(10):e756–e764. doi: 10.1016/S2352-3026(20)30186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S., Azim D., Nasim S., Hashmi S.H. Dwindling blood reserves: an ominous downside of COVID-19 pandemic. Transfus Apher Sci. 2020;59(5) doi: 10.1016/j.transci.2020.102818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeSimone R.A., Costa V.A., Kane K., Sepulveda J.L., Ellsworth G.B., Gulick R.M., et al. Blood component utilization in COVID-19 patients in New York City: transfusions do not follow the curve. Transfusion. 2021;61(3):692–698. doi: 10.1111/trf.16202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy C., Jackson B., Fontaine M. Tools for rapid analysis of blood usage and inventory during the COVID-19 pandemic. Transfusion. 2020;60(10):2199–2202. doi: 10.1111/trf.15996. [DOI] [PubMed] [Google Scholar]
- 6.Murphy C., Fontaine M., Luethy P., McGann H., Jackson B. Blood usage at a large academic center in Maryland in relation to the COVID-19 pandemic in 2020 [published online ahead of print, 2021 Apr 20] Transfusion. 2021 doi: 10.1111/trf.16415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey H.C., Coshic P., C S C, Arcot P.J., Kumar K. Blood supply management in times of SARS-CoV-2 pandemic - challenges, strategies adopted, and the lessons learned from the experience of a hospital-based blood centre. Vox Sang. 2021;116(5):497–503. doi: 10.1111/vox.13019. [DOI] [PubMed] [Google Scholar]
- 8.Uter S., An H.H., Linder G.E., Kadauke S., Sesok-Pizzini D., Kim H.C., et al. Measures to reduce red cell use in patients with sickle cell disease requiring red cell exchange during a blood shortage. Blood Adv. 2021;5(12):2586–2592. doi: 10.1182/bloodadvances.2021004395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolich D., Auron M., McCoy K., Dargis M., Quraishy N. Blood management during the COVID-19 pandemic [published online ahead of print, 2020 Aug 7] Cleve Clin J Med. 2020 doi: 10.3949/ccjm.87a.ccc053. [DOI] [PubMed] [Google Scholar]
- 10.Pagano M.B., Rajbhandary S., Nunes E., Cohn C.S. Transfusion services operations during the COVID-19 pandemic: results from AABB survey. Transfusion. 2020;60(11):2760–2762. doi: 10.1111/trf.15986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shander A., Goobie S.M., Warner M.A., Aapro M., Bisbe E., Perez-Calatayud A.A., et al. Essential role of patient blood management in a pandemic: a call for action. Anesth Analg. 2020;131(1):74–85. doi: 10.1213/ANE.0000000000004844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yazer M.H., Sperry J.L., Cap A.P., Seheult J.H. If not now, when? The value of the MTP in managing massive bleeding. Blood Transfus. 2020;18(6):415–418. doi: 10.2450/2020.0275-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young P.P., Cotton B.A., Goodnough L.T. Massive transfusion protocols for patients with substantial hemorrhage. Transfus Med Rev. 2011;25(4):293–303. doi: 10.1016/j.tmrv.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunbar N.M., Olson N.J., Szczepiorkowski Z.M., Martin E.D., Tysarcyk R.M., Triulzi D.J., et al. Blood component transfusion and wastage rates in the setting of massive transfusion in three regional trauma centers. Transfusion. 2017;57(1):45–52. doi: 10.1111/trf.13880. [DOI] [PubMed] [Google Scholar]
- 15.Paganini M., Abowali H., Bosco G., Balouch M., Enten G., Deng J., et al. Quality improvement project of a massive transfusion protocol (MTP) to reduce wastage of blood components. Int J Environ Res Public Health. 2021;18(1):274. doi: 10.3390/ijerph18010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balvers K., Coppens M., van Dieren S., van Rooyen-Schreurs I.H., Klinkspoor H.J., Zeerleder S.S., et al. Effects of a hospital-wide introduction of a massive transfusion protocol on blood product ratio and blood product waste. J Emerg Trauma Shock. 2015;8(4):199–204. doi: 10.4103/0974-2700.166597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanderson B., Coiera E., Asrianti L., Field J., Estcourt L.J., Wood E.M. How well does your massive transfusion protocol perform? A scoping review of quality indicators. Blood Transfus. 2020;18(6):423–433. doi: 10.2450/2020.0082-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohn C.S., Pagano M.B., Allen E.S., Frey K.P., Gniadek T., Lokhandwala P.M., et al. How do I manage long-term blood component shortages in a hospital transfusion service? Transfusion. 2020;60(9):1897–1904. doi: 10.1111/trf.15857. [DOI] [PubMed] [Google Scholar]
- 19.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NHTSA . 2020. Fatality data show increased traffic fatalities during pandemic.https://www.nhtsa.gov/press-releases/2020-fatality-data-show-increased-traffic-fatalities-during-pandemic [Google Scholar]
- 21.Braun A.L., Gorlin J.B., Peters J., Murphy S., Van Buren N.L. The effect of the SARS-CoV-2 pandemic and civil unrest on massive transfusion protocol activations in Minneapolis 2020. Transfusion. 2021;61(8):2250–2254. doi: 10.1111/trf.16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holcomb J.B., del Junco D.J., Fox E.E., Wade C.E., Cohen M.J., Schreiber M.A., et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–136. doi: 10.1001/2013.jamasurg.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holcomb J.B., Tilley B.C., Baraniuk S., Fox E.E., Wade C.E., Podbielski J.M., et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etchill E., Sperry J., Zuckerbraun B., Alarcon L., Brown J., Schuster K., et al. The confusion continues: results from an American Association for the Surgery of Trauma survey on massive transfusion practices among United States trauma centers. Transfusion. 2016;56(10):2478–2486. doi: 10.1111/trf.13755. [DOI] [PubMed] [Google Scholar]