Abstract
Objective
The coronavirus 2019 (COVID-19) pandemic caused suspension of directly observed therapy (DOT) for patients with active tuberculosis (TB). This study aimed to estimate the outcomes of pandemic-related DOT suspension and the cost-effectiveness of video-observed therapy (VOT) during the pandemic.
Methods
A decision-analytic model was constructed to project outcomes of adult patients with active TB from the perspective of a US healthcare provider. Two model-based analyses were conducted: (1) before (with DOT) and during [with self-administered therapy (SAT)] the pandemic; and (2) VOT vs SAT during the pandemic. The primary outcome measures were direct medical costs and disability-adjusted life years (DALYs).
Results
In the base-case analysis, care during the pandemic (with SAT) increased the cost (by US$285 per patient) and DALYs (by 0.2155 per patient) in comparison with DOT. Care with VOT reduced DALYs (by 0.4870) and costs (by US$1797) in comparison with SAT. On probabilistic sensitivity analysis, care during the pandemic (with SAT) increased DALYs in 100% of 10,000 simulations, and increased costs in 55.52% of instances. Care with VOT reduced DALYs and costs in 99.7% and 68.79% of instances, respectively. The probability of VOT being cost-effective was 99.4% at the willingness-to-pay threshold of 50,000 US$/DALY.
Conclusion
Suspension of DOT during the COVID-19 pandemic worsened treatment outcomes. VOT was found to be a cost-effective option for active TB care in an outpatient setting.
Keywords: Video-observed therapy, COVID-19 pandemic, Tuberculosis, Cost-effectiveness, High income
Introduction
There were approximately 10 million cases of tuberculosis (TB) worldwide in 2019, and 2.9% occurred in the Americas (World Health Organization, 2020). Data and statistics from the US Centers for Disease Control and Prevention indicated that there were 8916 reported cases of TB in 2019 (2.7 per 100,000 persons) (Centres for Disease Control and Prevention, 2020). Despite the low incidence of TB in the USA, the cost of TB management is still substantial. The estimated direct medical cost per case in the USA, a high-income country, was US$19,000 in 2018 (Marks et al., 2014; Aslam et al., 2018).
An inadequate level of patient adherence to TB treatment is a well-documented risk of treatment failure and drug resistance (Weis et al., 1994). A systematic review and meta-analysis on the association between adherence interventions and TB treatment outcomes found that the use of directly observed therapy (DOT) was significantly associated with improved treatment outcomes (Alipanah et al., 2018).
The coronavirus disease 2019 (COVID-19) pandemic has drawn resources away from the usual programmatic TB services (Migliori et al., 2020). Patient–staff interactions for usual TB management were re-engineered, and DOT was suspended to comply with social distancing (Burzynski et al., 2020). In 2017, the World Health Organization endorsed the use of video-observed therapy (VOT) as a suitable alternative to DOT for monitoring treatment, and published guidance on its implementation (World Health Organization, 2017). Clinical findings have shown that VOT was preferred by most patients, with high adherence in developed countries (Garfein et al., 2018; Story et al., 2019). During the COVID-19 pandemic, some healthcare systems swiftly implemented telehealth (delivery of healthcare services at a distance using digital technology) services to reduce non-urgent clinic visits (Burzynski et al., 2020; Migliori et al., 2020; Visca et al., 2020). This study aimed to estimate the impact of pandemic-related DOT suspension on TB treatment outcomes, and evaluate the cost-effectiveness of applying VOT for patients with active TB in the ambulatory care setting of a high-income country during the pandemic.
Methods
Model design
A decision-analytic model was constructed to evaluate the clinical and economic outcomes of a hypothetical cohort of adult patients with active drug-susceptible TB managed in an ambulatory setting. A two-part model-based analysis was performed to simulate the health outcomes of TB management, including direct medical costs and disability-adjusted life years (DALYs), over a 1-year timeframe.
Part 1 (outcome) analysis examined the TB treatment outcomes before and during the pandemic. In both scenarios, patients with TB were treated with the recommended 6-month drug regimen for drug-susceptible TB: a 2-month intensive phase (7 days/week) of isoniazid, rifampicin, pyrazinamide and ethambutol; and a 4-month continuation phase (7 days/week) with isoniazid and rifampicin (Nahid et al., 2016). Usual in-person clinic visits were provided for patients with TB in both scenarios (Burzynski et al., 2020). In the scenario prior to the pandemic, DOT was one of the standard activities for TB case management. The case management applied DOT on 5 days/week (weekdays) at a healthcare facility, and self-administered therapy (SAT) for the weekend doses (loaded in a pillbox) (Nahid et al., 2016). In the scenario during the pandemic (i.e. DOT suspended), SAT was applied for TB case management. Outcomes for the patients who received DOT or SAT were treatment success (cured or treatment completed), treatment failure (not cured or treatment incomplete), death, or lost to follow-up (if treatment failure or death was not documented) (World Health Organization, 2013) (Figure 1 ).
Figure 1.
Simplified decision-analytical model for tuberculosis (TB) management (a) before and during the coronavirus disease 2019 pandemic; and (b) care with video-observed therapy (VOT) vs self-administered therapy (SAT) during the pandemic. DOT, directly observed therapy.
Part 2 (cost-effectiveness) analysis examined the costs and DALYs of using VOT compared with SAT for TB case management during the pandemic. In both VOT and SAT arms, patients were treated with the 6-month drug regimen (as described above) and followed-up at usual clinic visits. In the VOT group, patients communicated daily with a healthcare provider using a video-conferencing platform (Holzman et al., 2018; Browne et al., 2019). The healthcare provider observed the administration of medication by patients via videoconferencing. Outcomes for patients in both the VOT and SAT groups were treatment success, treatment failure, lost to follow-up or death.
Clinical inputs
All model inputs are shown in Table 1 . The clinical model inputs were retrieved from published literature. A MEDLINE search was conducted for 2000–2021 using keywords such as ‘tuberculosis’, ‘self-administered therapy’, ‘directly observed therapy’, ‘video-observed therapy’, ‘telehealth’, ‘telemedicine’ and ‘tuberculosis treatment outcomes’. The inclusion criteria for published articles were: (1) written in English; (2) patients aged ≥18 years with active TB; (3) use of an adherence intervention (SAT, DOT or VOT); and (4) treatment outcomes were reported. A study was included if data relevant to the model inputs were available. Preferred study types were meta-analyses and randomized controlled trials. If multiple sources were found for a model input, the weighted average was used as the base-case value, and the high and low values formed the range for sensitivity analysis.
Table 1.
Model input parameters
| Parameters | Base case value | Range for sensitivity analysis | Distribution | Reference |
|---|---|---|---|---|
| Clinical inputs | ||||
| SAT | (Alipanah et al., 2018) | |||
| Proportion of treatment success | 0.66 | 0.53–0.80 | Beta | |
| Treatment success not achieved | ||||
| Treatment failure | 0.06 | 0.05–0.07 | Beta | |
| Death | 0.23 | 0.18–0.27 | Beta | |
| Risk ratio of event with DOT vs SAT | (Alipanah et al., 2018) | |||
| Treatment success | 1.14 | 1.07–1.24 | Triangular | |
| Treatment failure | 1.0 | 0.8–1.2 | Triangular | |
| Death | 0.74 | 0.59–0.89 | Triangular | |
| Risk ratio of event with VOT vs DOT | (Alipanah et al., 2018) | |||
| Treatment success | 1.0 | 0.8–1.2 | Triangular | |
| Treatment failure | 1.0 | 0.8–1.2 | Triangular | |
| Death | 1.0 | 0.8–1.2 | Triangular | |
| Proportion of patients achieved ≥80% compliance on DOT | 0.31 | 0.25–0.37 | Beta | (Story et al., 2019) |
| Relative increment in proportion of patients achieved ≥80% compliance on VOT vs DOT | 2.26 | 1.81–2.72 | Triangular | (Story et al., 2019) |
| Proportion of hospitalization among patients with treatment failure and lost to follow-up | 0.31 | 0.25–0.37 | Beta | (Wada et al., 2020) |
| Utility inputs | ||||
| Mean age at active TB diagnosis (years) | 52 | 25–85 | Triangular | (Wada et al., 2020) |
| Age-specific utility | (Gold et al., 1998) | |||
| <18 years | 1 | — | ||
| 18–65 years | 0.92 | — | ||
| >65 years | 0.84 | — | ||
| TB treatment success | 0.88 | 0.70–1 | Uniform | (Kittikraisak et al., 2012) |
| Treatment failure or lost to follow-up | 0.68 | 0.54–0.86 | Uniform | (Wirth et al., 2017) |
| Hospitalization | 0.59 | 0.47–0.71 | Uniform | (Guo et al., 2008) |
| Cost inputs | ||||
| Cost (US$) | ||||
| VOT (per session) | 6.89 | 5.51–8.27 | Gamma | (Lam et al., 2019) |
| DOT (per session) | 9.81 | 7.85–11.77 | Gamma | (Lam et al., 2019) |
| TB outpatient clinic visit (per case) | 478 | 239–716 | Gamma | (Oh et al., 2017) |
| TB-related hospitalization (per day) | 7980 | 6384–9576 | Gamma | (Center for Medicare and Meidcaid Services, 2016) |
| Drug treatment in treatment success (per case) | 1921 | 1537–2305 | Gamma | (Drugs.com, 2021) |
| Drug treatment in treatment failure (per case) | 4001 | 3201–4802 | Gamma | (Drugs.com, 2021) |
| Length of TB-related hospitalization (days) | 9.5 | 7–11 | Triangular | (Wada et al., 2020) |
| Number of DOT sessions | 120 | 72–168 | Triangular | (Nahid et al., 2016) |
| Number of VOT sessions | 168 | 120–168 | Triangular | (Holzman et al., 2018; Browne et al., 2019) |
DOT, directly observed treatment; SAT, self-administered treatment; TB, tuberculosis; VOT, video-observed treatment.
A meta-analysis (n=129 clinical trials) evaluated the association between treatment adherence interventions and TB outcomes (Alipanah et al., 2018). The weighted average event rates associated with the SAT arm were first pooled from studies included in the meta-analysis, and were adopted as the event rates of the SAT group in the present model. The risk ratios of event in the patients who used DOT (vs SAT) and VOT (vs DOT) were estimated from the pooled event rates in studies with the DOT and VOT groups (Alipanah et al., 2018).
The proportions of patients who achieved ≥80% compliance with DOT and VOT were adopted from the findings of a multi-centre, randomized controlled trial of VOT vs DOT in patients with active TB (n=226) (Story et al., 2019). The TB-related hospitalization rate was retrieved from the findings of a 10-year disease burden study of patients with active TB (n=1957) in an US health system (Wada et al., 2020).
Utility inputs
Expected DALYs was estimated using the time spent in a health state and the corresponding utility reduction of the health state (when compared with age-specific health utility). The base-case value of age (52 years) of patients with TB was retrieved from the disease burden study of TB in the USA (Wada et al., 2020), and the age-specific health utilities derived from the US national health measures and surveys were adopted (Gold et al., 1998). The utilities of TB-related health states (treatment success, treatment failure and lost to follow-up) were estimated from the findings of health-related quality-of-life studies in patients with TB (Guo et al., 2008; Kittikraisak et al., 2012), and adopted from the utility input of model-based health economic analysis on treatment of active TB (Wirth et al., 2017). DALYs resulting from TB-related mortality was approximated by the age-specific remaining life expectancy [from US life tables (Arias and Xu, 2020)] and age-specific health utilities. Mortality-related DALYs was discounted to 2021 by an annual rate of 3%.
Cost inputs
The cost analysis was performed from the perspective of a US healthcare provider. Cost items included direct medical costs of DOT, VOT, drug treatment, outpatient clinic visits and TB-related hospitalization. The costs per session of DOT and VOT were adopted from the findings of a cost-minimization analysis of various types of observed therapy for TB management in the USA (Lam et al., 2019). The cost per case of TB outpatient clinic care was retrieved from the results of a direct cost analysis of TB in the USA (Oh et al., 2017). Drug treatment costs were estimated using the drug costs listed in an online pharmacy (Drugs.com, 2021). The inpatient costs were retrieved from diagnosis-related group data reported by the Centers for Medicare and Medicaid Services (Center for Medicare and Medicaid Services, 2016). The length of hospital stay for active TB in the USA was reported to be 9.5 days in the TB disease burden study in the USA (Wada et al., 2020). All costs were adjusted to 2021.
Base-case analysis
All analyses were performed using TreeAge Pro 2021 (TreeAge Software Inc., Williamstown, MA, USA) and Excel 2016 (Microsoft Corp., Redmond, WA, USA). Expected direct medical costs and DALYs were calculated for Part 1 (outcome) and Part 2 (cost-effectiveness) analyses. In the Part 2 (cost-effectiveness) analysis, a strategy was classed as dominant when it had higher DALYs at higher cost than another option, and the dominant option was eliminated from further cost-effectiveness analyses. If a strategy resulted in lower DALYs at higher cost than another alternative, the incremental cost per DALY averted (ICER) of the more effective strategy was calculated: ICER=∆Cost/∆DALYs
A willingness-to-pay (WPT) threshold of 50,000 US$/DALY was adopted in the cost-effectiveness analysis. A strategy was preferred if it: (1) resulted in lower DALYs at lower cost; or (2) resulted in lower DALYs at higher cost and ICER was less than the WTP threshold.
Sensitivity analysis
In the one-way sensitivity analysis, each model input was varied over the range of sensitivity analysis (specified in Table 1) to examine the most influential parameters on the base-case results. The probabilistic sensitivity analysis was performed using Monte Carlo simulation to examine the impact of uncertainty in all variables simultaneously. Direct costs and DALYs were recalculated 10,000 times by randomly drawing each of the model inputs from the parameter-specific distribution (Table 1). The probability of each alternative being accepted as the preferred option was determined over a wide range of WTP from 0 to 100,000 US$/DALY by the acceptability curves.
Results
Part 1 (outcome) analysis: before and during the pandemic
Compared with DOT (before the pandemic), care with SAT (during the pandemic) increased both costs (by US$285 per patient) and DALYs (by 0.2155 per patient) (Table 2 ).
Table 2.
Base-case analysis results
| Strategy | Cost (US$) | Incremental costs (US$) | DALYs | Additional DALYs |
|---|---|---|---|---|
| Part 1 (outcome) analysis: before and during the pandemic | ||||
| With DOT (before pandemic) | 14,049 | - | 1.3192 | - |
| With SAT (during pandemic) | 14,334 | 285 | 1.5346 | 0.2155 |
| Part 2 (cost-effectiveness) analysis: TB care with VOT vs SAT during the pandemic | ||||
| With VOT | 12,537 | - | 1.0477 | - |
| With SAT | 14,334 | 1797 | 1.5346 | 0.4870 |
DALYs, disability-adjusted life-years; DOT, directly observed treatment; SAT, self-administered treatment; TB, tuberculosis;VOT, video-observed treatment.
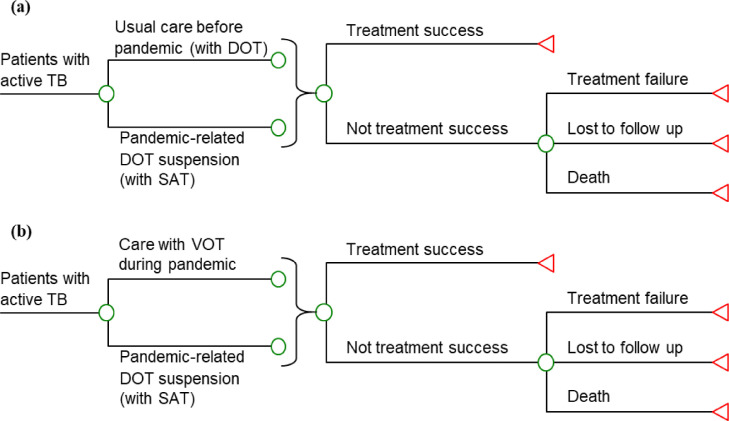
One-way deterministic sensitivity analyses were conducted for all model inputs. The base-case DALYs were robust to the variation of all model inputs, and the base-case costs were sensitive to the risk ratio of treatment success with DOT vs SAT. TB outpatient care during the pandemic (with SAT) would become less costly than care with DOT (before the pandemic) if the risk ratio of treatment success with DOT vs SAT was <1.10 (base-case value: 1.14) (Figure 2 ).
Figure 2.
(a) Costs and (b) disability-adjusted life-years (DALYs) of tuberculosis (TB) outpatient care with directly observed therapy (DOT) (before the coronavirus disease 2019 pandemic) and with self-administered therapy (SAT) (during the pandemic) against the risk ratio (RR) of treatment success with DOT vs SAT.
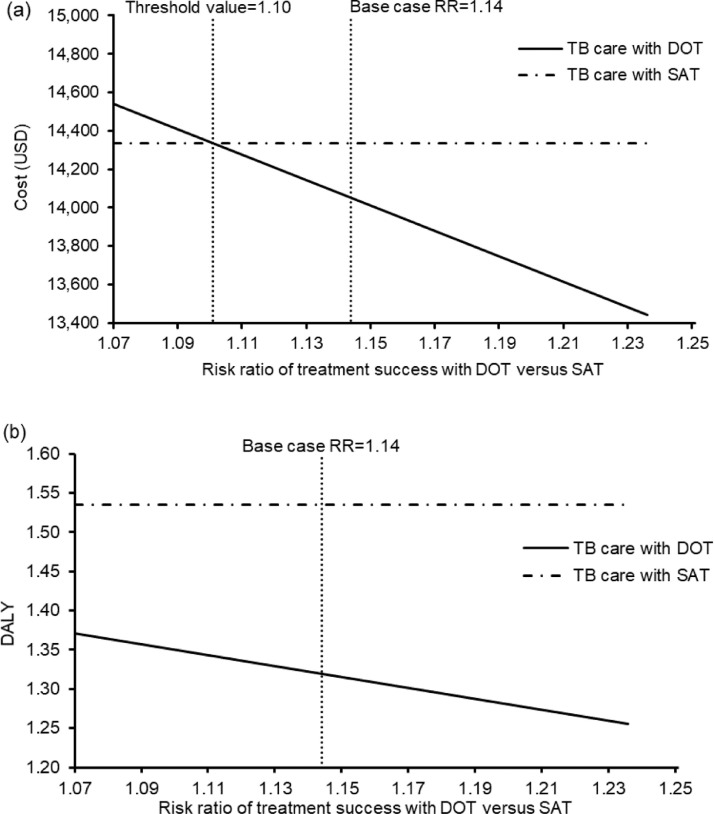
The change in direct medical costs and DALYs for TB outpatient care with SAT during the pandemic (vs care with DOT before the pandemic) in the 10,000 Monte Carlo simulations is shown in a scatterplot (Figure 3 ). TB care during the pandemic (with SAT) increased both DALYs [by 0.1954; 95% confidence interval (CI) 0.1941–0.1966; P<0.01] and cost (by US$277; 95% CI US$245–310; P<0.01). Compared with TB care with DOT before the pandemic, care with SAT during the pandemic had higher DALYs in 100% of simulations and increased costs in 55.52% of instances.
Figure 3.
Scatterplot of change in costs against change in disability-adjusted life-years (DALYs) by tuberculosis outpatient care with self-administered therapy during the coronavirus disease 2019 pandemic (vs care before the pandemic with directly observed therapy) in 10,000 Monte Carlo simulations.
Part 2 (cost-effectiveness) analysis: TB care with VOT vs SAT during the pandemic
The base-case expected costs and DALYs of each strategy during the pandemic are shown in Table 2. TB care with VOT reduced DALYs (by 0.4870) and costs (by US$1797) (ICER= -3690 US$/DALY), and VOT was therefore the preferred cost-effective option.
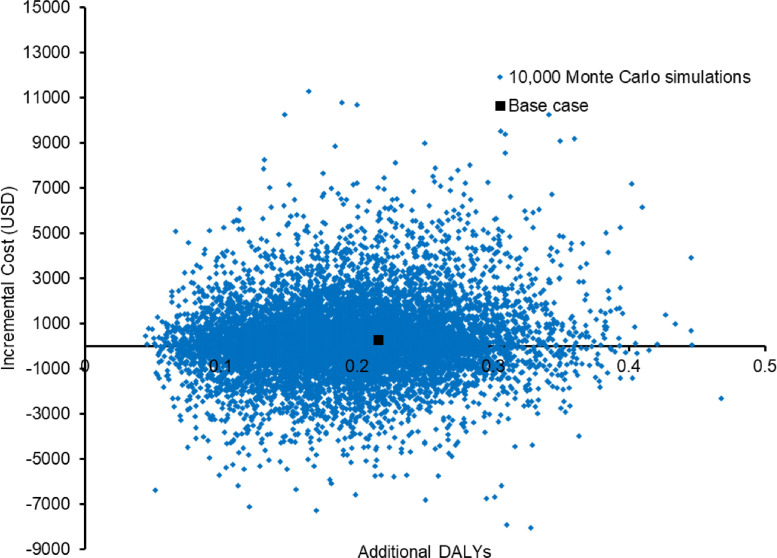
The base-case results were robust to the variation of all model inputs in the one-way sensitivity analysis, and no threshold value was identified. Six influential parameters (i.e. changed ICER by >15% from base-case ICER) are shown in the tornado diagram (Figure 4 ), and the risk ratio of treatment success with VOT vs DOT was the most influential parameter on the base-case ICER. Further, the one-way analysis was performed separately on direct medical costs and DALYs. TB care with VOT continued to avert DALYs when compared with SAT throughout the variation of all model inputs. The cost of TB care with VOT became higher compared with SAT when the risk ratio of treatment success with VOT (vs DOT) was <0.89 (base-case value: 1.0).
Figure 4.
Tornado diagram of six influential factors identified in one-way sensitivity analysis on the incremental cost‐effectiveness ratio (ICER) of video-observed therapy (VOT) vs self-administered therapy (SAT) during the coronavirus disease 2019 pandemic. DOT, directly observed therapy; WTP, willingness-to-pay; TB, tuberculosis.
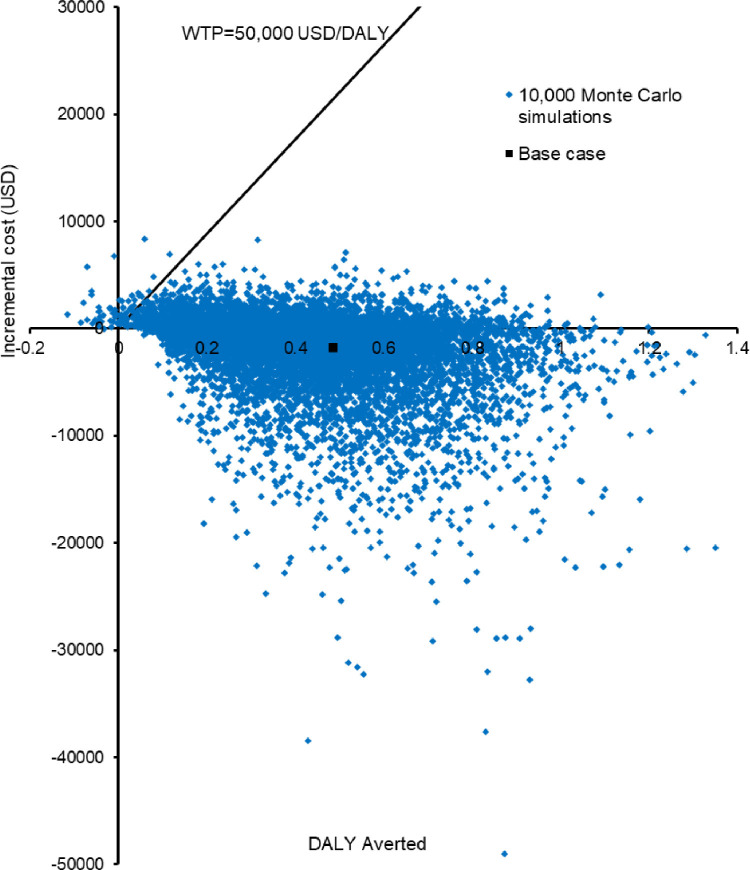
Probabilistic sensitivity analysis was performed by recalculating the costs and DALYs 10,000 times with Monte Carlo simulation. Incremental costs against DALYs averted by TB care with VOT vs SAT are shown in a scatterplot (Figure 5 ). Compared with TB care with SAT, care with VOT reduced DALYs by 0.4299 (95% CI 0.4358–0.4440; P<0.01) with a cost-saving of US$1871 (95% CI US$1797–1944; P<0.01). Care with VOT reduced DALYs and costs in 99.7% and 68.79% of instances, respectively. Care with VOT averted DALYs at a higher cost in 30.91% of instances (30.61% and 0.3% were below and above the WTP threshold, respectively).
Figure 5.
Scatterplot of incremental cost against disability-adjusted life-years (DALYs) averted by tuberculosis care with video-observed therapy (VOT) vs self-administered therapy (SAT) during the coronavirus disease 2019 pandemic in 10,000 Monte Carlo simulations. WTP, willingness-to-pay.
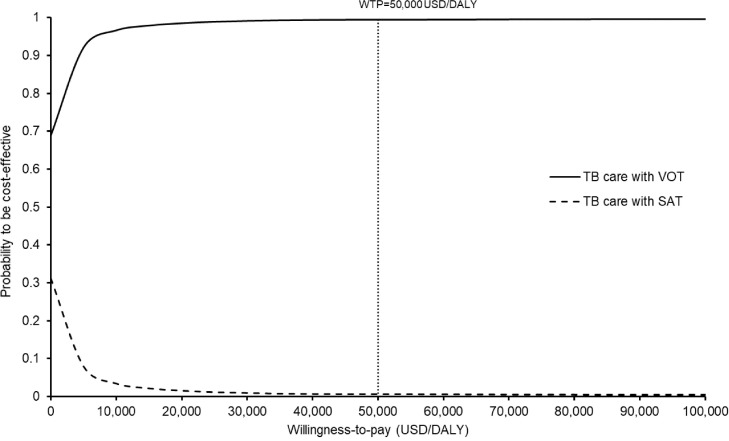
The probabilities of each strategy being accepted as cost-effective during the pandemic were presented in the acceptability curves over a range of WTP (0–100,000 US$/DALY). The VOT was accepted to be cost-effective in 99.4% of instances at a WTP threshold of 50,000 US$/DALY (Figure 6 ).
Figure 6.
Acceptability curves of care with video-observed therapy (VOT) and self-administered therapy (SAT) for treatment of active tuberculosis (TB) during the coronavirus disease 2019 pandemic to be cost-effective against willingness-to-pay (WTP).
Discussion
To the authors’ knowledge, this is the first outcome analysis to estimate the impact of the COVID-19 pandemic on TB treatment outcomes in the ambulatory care setting when DOT was suspended, and evaluate the cost-effectiveness of switching to VOT for active TB management during the pandemic in the USA. Compared with standard care (with DOT) before the pandemic, care with SAT (during the pandemic) increased the costs of TB management (by US$285 per patient) and resulted in higher DALYs (by 0.2155 per patient) over a 1-year period. At the beginning of the pandemic in the USA, routine in-person services for TB management were restricted to implement social distancing. The volume of in-person clinic visits was reduced and DOT was suspended (Burzynski et al., 2020). The findings of one-way sensitivity analysis showed that the increased DALYs associated with pandemic-related DOT suspension was robust to variation of all model inputs. The direct medical costs during the pandemic (when DOT was suspended) were sensitive to variation of the risk ratio of treatment success with DOT vs SAT. Probabilistic sensitivity analysis further supported the robustness of base-case findings that care with SAT during the pandemic increased DALYs (100% of instances) at increased cost (>55% of instances).
The use of VOT during the pandemic to manage patients with active TB reduced costs (by US$1797 per patient) and averted DALYs (by 0.4870 per patient) in a 1-year time frame. The reduction in DALYs was generated by the improved treatment success rate associated with VOT compared with SAT. The costs saved by care with VOT (vs SAT) were primarily due to a considerable decrease in hospitalization costs resulting from the higher treatment success rate. The one-way sensitivity analysis found that the cost-effectiveness of VOT was highly robust, and no influential parameter (with threshold value) was identified throughout variation of all model inputs. The results of probabilistic sensitivity analysis also supported VOT to be the cost-effective and preferred strategy over a wide range of WTP thresholds in the 10,000 Monte Carlo simulations.
To the authors’ knowledge, this is the first health economic analysis to evaluate the impact of pandemic-related suspension of DOT on the outcomes of TB management, measured as direct medical costs and DALYs, in a high-income country setting. Prior health economics analyses of VOT were limited to the impact on the cost component, either comparing the costs of VOT and DOT (cost-minimization analysis) (Lam et al., 2019; Beeler et al., 2020), or focusing on the costs of VOT (cost-analysis) (Mirsaeidi et al., 2015). The present study is a full-scale health economic analysis, comparing both the costs and effectiveness (measured as DALYs) of management of active TB with VOT compared with SAT during the COVID-19 pandemic. The model included all key treatment outcomes of active TB (treatment success, treatment failure, lost to follow-up and death) for estimation of the costs and DALYs of the two strategies (VOT and SAT) applied to TB case management during the pandemic.
This study had some limitations. Model-based analyses are, in general, subject to uncertainty of model inputs. Rigorous sensitivity analyses were therefore performed to examine the impact of model input uncertainty and assumption on the base-case results. The present study used a simplified decision model to represent treatment adherence strategies and the corresponding outcomes in patients with drug-susceptible TB. The negative impact of the patient's comorbidities on TB treatment outcomes was not incorporated in the present model. The results therefore only represent the relative difference in outcomes (as measured by costs and DALYs) associated with the treatment adherence strategies (SAT, DOT and VOT). The search of model inputs was performed in English publications, and may have missed relevant data published in non-English languages. The present model time horizon was limited to a short period of time (1 year) to resemble the timeframe with the most stringent social distancing restrictions for COVID-19 control in the USA. The cost analysis was performed from the perspective of a healthcare provider, and indirect costs (productivity loss) were not considered. The cost-saving associated with VOT during the pandemic may therefore have been underestimated.
DOT has long been adopted as a key component of standard care for active TB management in high-income countries, but it was necessary to suspended DOT in order to implement social distancing during the COVID-19 pandemic. The sudden global outbreak of COVID-19 shifted the treatment strategy from DOT to VOT in some settings with adequate resources and trained staff. The study findings demonstrate that, without VOT, the pandemic-related suspension of DOT would have resulted in higher costs and worsened treatment outcomes (as indicated by increased DALYs). In those settings where switching to VOT was feasible, care with VOT improved treatment outcome (as indicated by reduced DALYs) and lowered direct medical costs.
Despite the well-established effectiveness of VOT for patients with active TB, many clinical settings have adopted the practice of DOT and did not have any urgency to implement VOT prior to the pandemic. The COVID-19 pandemic sped up the implementation of telehealth in many medical disciplines. With global efforts to control the spread of COVID-19, the pandemic will surely end. The development and sustainability of telehealth technology such as VOT in the post-pandemic era will require both clinical evidence and health economics findings to support the informed decision-making process of resource allocation. Furthermore, health economic evaluation of VOT-based care is highly warranted in high-TB-burden and low-resource settings.
In conclusion, the suspension of DOT for ambulatory care of active TB during the COVID-19 pandemic appeared to worsen treatment outcomes (with higher DALYs) and increase costs. Switching to VOT during the pandemic was a cost-effective option to improve the treatment outcomes of active TB by reducing both DALYs and direct medical costs from the perspective of a US healthcare provider.
Conflict of interest statement
None declared.
Acknowledgments
Funding
None
Ethical approval
Not required
References
- Alipanah N, Jarlsberg L, Miller C, Linh NN, Falzon D, Jaramillo E, et al. Adherence interventions and outcomes of tuberculosis treatment: a systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias E, Xu J. United States life tables, 2018. National Vital Statistics Reports 2020;69. Available at: https://www.cdc.gov/nchs/data/nvsr/nvsr69/nvsr69-12-508.pdf (accessed 15 March 2021) [PubMed]
- Aslam MV, Owusu-Edusei K, Marks SM, Asay GRB, Miramontes R, Kolasa M, et al. Number and cost of hospitalizations with principal and secondary diagnoses of tuberculosis, United States. Int J Tuberc Lung Dis. 2018;22:1495–1504. doi: 10.5588/ijtld.18.0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler Asay GR, Lam CK, Stewart B, Mangan JM, Romo L, Marks SM, et al. Cost of tuberculosis therapy directly observed on video for health departments and patients in New York City; San Francisco, California; and Rhode Island (2017–2018) Am J Public Health. 2020;110:1696–1703. doi: 10.2105/AJPH.2020.305877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SH, Umlauf A, Tucker AJ, Low J, Moser K, Gonzalez Garcia J, et al. Wirelessly observed therapy compared to directly observed therapy to confirm and support tuberculosis treatment adherence: a randomized controlled trial. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynski J, Macaraig M, Nilsen D, Schluger NW. Transforming essential services for tuberculosis during the COVID-19 pandemic: lessons from New York City. Int J Tuberc Lung Dis. 2020;24:735–736. doi: 10.5588/ijtld.20.0283. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2020. Tuberculosis – data and statistics.https://www.cdc.gov/tb/statistics/default.htm Available at. (accessed 10 January 2021) [Google Scholar]
- Center for Medicare and Medicaid Services. 100% MEDPAR inpatient hospital data for fiscal year 2016. Available at: www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareFeeforSvcPartsAB/Downloads/DRGState16.pdf (accessed 2 March 2021).
- Drugs.com. 2021. Available at: https://www.drugs.com/ (accessed 2 March 2021).
- Garfein RS, Liu L, Cuevas-Mota J, Collins K, Munoz F, Catanzaro DG, et al. Tuberculosis treatment monitoring by video directly observed therapy in 5 health districts, California, USA. Emerg Infect Dis. 2018;24:1806–1815. doi: 10.3201/eid2410.180459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses – using national measures to create condition-specific values. Med Care. 1998;36:778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- Guo N, Marra CA, Marra F, Moadebi S, Elwood RK, Fitzgerald JM. Health state utilities in latent and active tuberculosis. Value Health. 2008;11:1154–1161. doi: 10.1111/j.1524-4733.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- Holzman SB, Zenilman A, Shah M. Advancing patient-centered care in tuberculosis management: a mixed-methods appraisal of video directly observed therapy. Open Forum Infect Dis. 2018;5 doi: 10.1093/ofid/ofy046. ofy046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittikraisak W, Kingkaew P, Teerawattananon Y, Yothasamu J, Natesuwan S, Manosuthi W, et al. Health related quality of life among patients with tuberculosis and HIV in Thailand. PLoS One. 2012;7:e29775. doi: 10.1371/journal.pone.0029775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CK, Fluegge K, Macaraig M, Burzynski J. Cost savings associated with video directly observed therapy for treatment of tuberculosis. Int J Tuberc Lung Dis. 2019;23:1149–1154. doi: 10.5588/ijtld.18.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks SM, Flood J, Seaworth B, Hirsch-Moverman Y, Armstrong L, Mase S, et al. TB Epidemiologic Studies Consortium. Treatment practices, outcomes, and costs of multidrug-resistant and extensively drug-resistant tuberculosis, United States, 2005–2007. Emerg Infect Dis. 2014;20:812–821. doi: 10.3201/eid2005.131037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliori GB, Thong PM, Akkerman O, Alffenaar JW, Alvarez-Navascues F, Assao-Neino MM, et al. Worldwide effects of coronavirus disease pandemic on tuberculosis services, January–April 2020. Emerg Infect Dis. 2020;26:2709–2712. doi: 10.3201/eid2611.203163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsaeidi M, Farshidpour M, Banks-Tripp D, Hashmi S, Kujoth C, Schraufnagel D. Video directly observed therapy for treatment of tuberculosis is patient-oriented and cost-effective. Eur Respir J. 2015;46:871–874. doi: 10.1183/09031936.00011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63:e147–e195. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh P, Pascopella L, Barry PM, Flood JM. A systematic synthesis of direct costs to treat and manage tuberculosis disease applied to California, 2015. BMC Res Notes. 2017;10:1–7. doi: 10.1186/s13104-017-2754-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story A, Aldridge RW, Smith CM, Garber E, Hall J, Ferenando G, et al. Smartphone-enabled video-observed versus directly observed treatment for tuberculosis: a multicentre, analyst-blinded, randomised, controlled superiority trial. Lancet. 2019;393:1216–1224. doi: 10.1016/S0140-6736(18)32993-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visca D, Tiberi S, Pontali E, Spanevello A, Migliori GB. Tuberculosis in the time of COVID-19: quality of life and digital innovation. Eur Respir J. 2020;56 doi: 10.1183/13993003.01998-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada PY, Lee-Rodriguez C, Hung YY, Skarbinski J. Burden of active tuberculosis in an integrated health care system, 1997–2016: incidence, mortality, and excess health care utilization. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa015. ofaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SE, Slocum PC, Blais FX, King B, Nunn M, Matney GB, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med. 1994;330:1179–1184. doi: 10.1056/NEJM199404283301702. [DOI] [PubMed] [Google Scholar]
- Wirth D, Dass R, Hettle R. Cost-effectiveness of adding novel or group 5 interventions to a background regimen for the treatment of multidrug-resistant tuberculosis in Germany. BMC Health Serv Res. 2017;17:182. doi: 10.1186/s12913-017-2118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2013. Definitions and reporting framework for tuberculosis –2013 revision: updated December 2014 and January 2020.https://www.who.int/tb/publications/definitions/en/ Available at. (accessed 22 February 2021) [Google Scholar]
- World Health Organization . WHO; Geneva: 2017. Guidelines for treatment of drug-susceptible tuberculosis and patient care.https://www.who.int/tb/publications/2017/dstb_guidance_2017/en/ Available at. (accessed 14 January 2021) [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. Global tuberculosis report 2020.https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf?ua=1 Available at. (accessed 14 January 2021) [Google Scholar]