Abstract
Background and aims
Coronavirus disease (COVID-19) still becomes a global burden that affected people in different groups. The aim of this study was to evaluate the association between thyroid disease and the outcome of COVID-19 patients.
Method
This was a meta-analysis study from articles obtained through a systematic literature search to investigate the relationship between thyroid disease and COVID-19 outcomes. Composite poor outcomes comprised of severity, mortality, intensive care unit (ICU) admission, and hospitalization.
Results
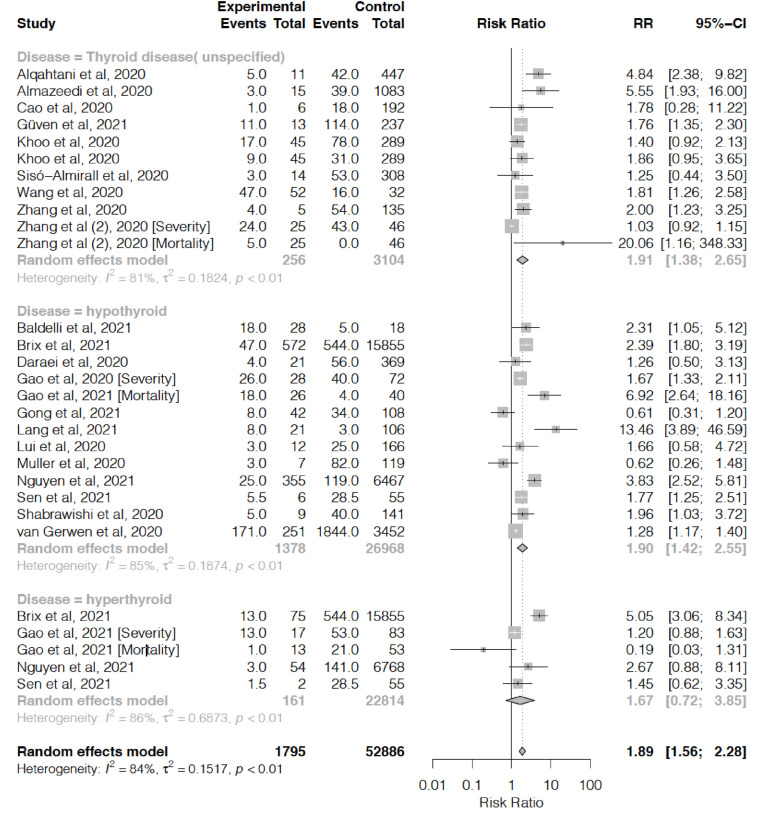
A total of 31339 patients from 21 studies included in this study. Thyroid disorder was associated with increased composite poor outcome (risk ratio (RR) 1.87 [95% confidence interval (CI) 1.53, 2.27], p < 0.001; I2 = 84%, p < 0.01), this included higher disease severity (RR 1.92 [1.40, 2.63], p < 0.05; I2 = 86%, p < 0.01), ICU admission (RR 1.61 [1.12, 2.32], p > 0.05; I2 = 32%, p < 0.05), mortality (RR 2.43 [1.44, 4.13], p < 0.05; I2 = 83%, p < 0.01), and hospitalization (RR 1.28 [1.17, 1.39], p < 0.05; I2 = 0%, p < 0.96). Meta-regression analysis indicated that age (p = 0.002) was a significant influence that affects the association. Also, the presence of unspecified thyroid disease (RR 1.91 [1.38, 2.65], p < 0.05; I2 = 81%, p < 0.01) and hypothyroidism (RR 1.90 [1.45, 2.55], p < 0.05; I2 = 85%, p < 0.01) during admission were associated with poor outcomes.
Conclusion
Thyroid abnormalities increased the risk of COVID-19 composite poor outcomes and were influenced by the patient's age. Abnormal thyroid and hypothyroidism, but not hyperthyroidism, were associated with poor COVID-19 outcomes.
Keywords: COVID-19, Hyperthyroidism, Hypothyroidism, Outcome, Thyroid disease
1. Introduction
The growing number of cases and the rapidly evolving SARS-CoV-2 has caused coronavirus disease 2019 (COVID-19) to become a global burden that affected people from different groups of age [1,2]. As the spectrum of signs and symptoms among COVID-19-infected individuals is broad–ranging from mild to severe illness requiring intensive care, identifying risk factors that may predict the patients’ outcome is imperative for a better resource allocation in mitigating this current COVID-19 outbreak [3,4].
The interaction between thyroid hormone and immune regulation has been explored extensively in both physiological and pathological settings. As the regulation of the immune system plays an integral part in determining COVID-19 patients’ disease progressivity, evidence is required to accentuate the correlation between thyroid hormone and COVID-19 outcomes [5,6]. In light of this, several studies have been carried out to emphasize the relationship between thyroid status or thyroid abnormalities and the outcome of COVID-19. However, the correlation between the two remains elusive as indicated by the variability of reported study results. Thus, in this present systematic review and meta-analysis, we determined to summarize recent findings on the relationship between thyroid disease and COVID-19 outcome.
2. Material and methods
This systematic review was presented consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRIMSA) [7]. A detailed protocol has been previously registered in PROSPERO (CRD42021267278).
2.1. Eligibility criteria
Inclusion and exclusion criteria were defined according to the PECO (Population, Exposure, Comparison, Outcome) structure. The population of this study was COVID-19 patients confirmed with either polymerase chain reaction (PCR), serum antibody or antigen testing, or highly suspected cases based on clinical presentation. Exposure and comparator were defined as patients with known thyroid disorders, hypothyroidism defined as measured thyroid levels below normal range during admission, and hyperthyroidism defined as measured thyroid levels above normal reference during admission.
The inclusion criteria were research articles and letters that report COVID-19 patients with information on thyroid disorders that were presented as categorical data along with measurable composite poor outcomes–mortality/severity/critically ill/intensive care unit (ICU) admission/patients required hospitalization.
The following types of articles were excluded: case report, non-research letter, editorial, invited commentary, review, and abstract-only article. Also, studies that reported only continuous variables of thyroid hormone level were excluded. There was no language restriction applied in this study.
2.2. Search strategy and study selection
A systematic search of the literature was carried out on multiple databases–PubMed and Cochran Central Database pooling related articles from the beginning to 20th of August 2021 by two independent investigators (FAD AND MHB). The following search terms were used (("Thyroid Gland"[Mesh] OR "Thyroid (USP)"[Mesh] OR "Hyperthyroidism"[Mesh] OR "Hypothyroidism"[Mesh] OR "Thyroid Hormones"[Mesh] OR “thyroid disease”[tiab] OR “hyperthyroid”[tiab] OR “hypothyroid”[tiab] OR “thyroid”[tiab] OR thyroid OR “TSH”[tiab] OR “T3”[tiab] OR “T4”[tiab]) AND ("COVID-19"[Mesh] OR "SARS-CoV-2"[Mesh] OR COVID-19)). We used the ‘related articles’ feature and hand-searched the reference lists of the included articles to expand the search and obtain additional studies. Duplicate results were removed using a reference tool and screening was done manually after the initial search.
2.3. Data extraction
Data extraction was conducted independently by two authors (FAD and MHB). Standardized forms contained author, year, study design, sample size, cut-off value, gender, age, related comorbidities–hypertension (HT), diabetes mellitus (DM), obesity, and chronic obstructive pulmonary disease (COPD)–and measured outcomes. Data extraction was performed independently, and different perceptions were resolved through discussion and consensus between authors.
The primary exposure was thyroid abnormalities preexisting thyroid disease from baseline characteristics or a group of patients with lower- or higher-than-normal measured thyroid hormone levels during admission. The type of thyroid measurement during admission to categorize the patient's type of thyroid level abnormality was thyroid-stimulating hormone (TSH), free triiodothyronine (fT3), or free thyroxine (fT4). The patient was considered hypothyroidism if the level of thyroid hormone was lower than normal reference according to the specified cut-off defined by each study. Conversely, the patient was considered hyperthyroidism if the level of measured thyroid hormone was higher than the normal range. Furthermore, we also performed subgroup analyses on each type of thyroid abnormalities–undefined, hypothyroidism, and hyperthyroidism.
The measured outcome of interest was composite poor outcomes that comprised of mortality, COVID-19 severity, intensive care unit (ICU) admission, and hospitalization requirement. Severe COVID-19 generally was characterized as patients who met the criteria of respiratory distress (respiratory rate ≥30 breaths/min); pulse oxygen saturation ≤93% on room air; and low arterial oxygenation ratio (PaO2/fraction of inspired oxygen ≤300). Subsequently, critically ill patients were indicated if they had respiratory failure requiring a form of mechanical ventilation; shock; or had complications with other organ failures that require monitoring and treatment in the ICU. Further, when the outcome was split into multiple groups (mild, moderate, severe, and critically ill COVID-19), mild and moderate subjects were combined into a single group, and severe to critically ill patients were also considered into one group.
We used Newcastle-Ottawa Scale (NOS) to assess the quality of included observational cohort studies. Assessment of the included studies was done independently by four authors (GRM, RI, AHS, and H). The following aspects were taken into consideration in the assessment: cohort selection, the comparability of cohort-based design or analysis, the way exposure is determined, and the way of outcomes of interest is evaluated. Discrepancies of perception in determining study quality were resolved by discussion.
2.4. Statistical analysis
All statistical analysis was performed using R (version 4.0.4, The R Foundation, Vienna, Austria) [8]. We used the Mantel-Haenszel method formula to generate pooled effect estimates in a form of risk ratio (RR) along with their 95% confidence interval (CI). The inconsistency index (I2) and subgroup analysis using the Chi-square test was used to investigate potential sources of heterogeneity. An I2 of more than 50% and a p-value of less than 0.05 were considered significant for heterogeneity [9]. To consider interstudy variability, a random-effects model was used regardless of study heterogeneity [10]. We used a two-tailed p-value with statistical significance was accounted with p ⩽ 0.05.
Subgroup analyses were performed only when at least two studies on each at least two subsets were available. Subgroup analysis was carried out on each component of composite poor outcome and the type of thyroid abnormalities specified in the studies. Further, we also conducted a restricted-maximum likelihood random-effects meta-regression analysis to study the influence of the following covariates–gender, age, hypertension, coronary artery disease/cardiovascular disease (CAD/CVD), diabetes mellitus (DM), obesity, and chronic obstructive pulmonary disease (COPD).
Analyses of publication bias were presented qualitatively using a contour-enhanced funnel plot and quantitatively assessed using Egger's linear regression test to indicate small-study effects [11,12]. Sensitivity analysis was performed under the leave-one-out method to single out the cause of study heterogeneity and statistical significance. When publication bias was detected, trim-and-fill analysis was carried out to evaluate the potential sources of publication bias.
3. Results
3.1. Study selection
The selection flow to obtain included studies in this meta-analysis is shown in (Fig. 1 ). The initial search pooled 459 articles from PubMed and Cochran Collaboration Central Register of Controlled Clinical Trials. Among 459 studies, two articles were removed automatically due to duplication, yielding 457 articles that were eligible to be screened and sought for retrieval. From all 457 screened articles, we assessed 30 studies for each individual's eligibility. Among 30 studies, a total of nine full-text articles were excluded from further analysis due to; a) five articles did not present the outcome of interest and did not categorize patients into different thyroid statuses and b) four articles present thyroid hormones as continuous numerical data. Thereby, 21 studies were included for further qualitative and quantitative analyses [[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33]].
Fig. 1.
PRISMA literature search flowchart.
3.2. Characteristics of included studies
The characteristics of all included studies are available in Table 1 . There were 31339 patients from 21 studies included in this present systematic review and meta-analysis. All patients were adults with confirmed COVID-19 cases. The setting of the study varied from China, Denmark, India, Iran, Italy, Kuwait, Saudi Arabia, Spain, Turkey, United Kingdom, and the United States. Among all included studies, 15 studies were retrospective cohort studies, five studies were prospective cohort studies, and one studies were cross-sectional. Different cut-offs were used between studies that grouped the patients by their thyroid hormones level during admission (Supplementary Table 2). In addition, we extracted the cumulative incidence of comorbidities reported in each study.
Table 1.
Characteristics of the included studies.
| Authors | Study Design | Samples | Male (%) | Age (years) | Hypertension (%) | CAD/CVD (%) | DM (%) | Obesity (%) | COPD (%) | Outcome | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alqahtani et al., 2020 [51] | Retrospective cohort | 458 | 86.9 | N/A | 10.94 | 1.97 | 13.6 | 57.1 | N/A | Severity | 8 |
| Almazeedi et al., 2020 [52] | Retrospective cohort | 1096 | 81 | 41 | 16.1 | 4.3 | 14.1 | 21.8 | 0.5 | Severity | 6 |
| Baldelli et al., 2021 [53] | Retrospective cohort | 46 | 69.5 | 59.6 | N/A | N/A | N/A | N/A | N/A | ICU | 7 |
| Brix et al., 2021 [54] | Retrospective cohort | 16502 | 45.6 | 57.3 | 26.73 | 17.35 | 11.96 | 10.9 | 5.8 | Mortality | 7 |
| Cao et al., 2020 [55] | Retrospective cohort | 198 | 51 | 50.1 | 21.2 | 6 | 7.6 | N/A | N/A | ICU | 7 |
| Daraei et al., 2020 [56] | Retrospective cohort | 390 | 67.7 | 58.1 | N/A | N/A | N/A | N/A | N/A | Mortality | 6 |
| Gao et al., 2020 [57] | Retrospective cohort | 100 | 52 | 62.3 | N/A | N/A | N/A | N/A | N/A | Severity and Mortality | 9 |
| Gong et al., 2021 [58] | Retrospective cohort | 150 | 54 | 69.8 | N/A | 11.33 | N/A | N/A | N/A | Mortality | 9 |
| Güven et al., 2021 [59] | Prospective cohort | 250 | 63 | 68 | N/A | N/A | N/A | N/A | N/A | ICU | 8 |
| Khoo et al., 2020 [60] | Prospective cohort | 334 | 60.8 | 66.1 | 48.5 | 23.7 | 39.5 | N/A | 17.4 | Mortality and ICU | 9 |
| Lang et al., 2021 [61] | Retrospective cohort | 127 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Mortality | 7 |
| Lui et al., 2020 [62] | Prospective cohort | 191 | 51.8 | 53.5 | 27.2 | 6.3 | 13.1 | N/A | 3.1 | Severity | 8 |
| Muller et al., 2020 [63] | Prospective cohort | 145 | 61.3 | 66.9 | N/A | N/A | N/A | N/A | N/A | ICU | 6 |
| Nguyen et al., 2021 [64] | Cross sectional | 6822 | 49.6 | 48 | N/A | N/A | N/A | N/A | N/A | Mortality | N/A |
| Sen et al., 2021 [65] | Prospective cohort | 60 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Severity | 6 |
| Shabrawishi et al., 2020 [66] | Retrospective cohort | 150 | 60 | 46.1 | 28.8 | 8 | 26 | N/A | 0.7 | Severity | 6 |
| Sisó-Almirall et al., 2020 [67] | Retrospective cohort | 322 | 50 | 56.7 | 33.9 | 7.8 | 14.3 | 14.3 | 5.9 | Hospitalization | 7 |
| van Gerwen et al., 2020 [68] | Retrospective cohort | 3703 | 55.3 | 56,9 | N/A | N/A | N/A | 57.4 | N/A | Hospitalization | 8 |
| Wang et al., 2020 [69] | Retrospective cohort | 84 | 63.1 | 57.3 | N/A | N/A | N/A | N/A | N/A | Severity | 9 |
| Zhang et al., 2020 [70] | Retrospective cohort | 140 | 50.7 | 56.3 | 30 | 7.1 | 12.1 | N/A | 1.4 | Severity | 7 |
| Zhang et al., 2020 [71] | Retrospective cohort | 71 | 56.3 | 62.7 | 28.2 | 21.1 | 18.3 | N/A | N/A | Severity and Mortality | 7 |
CAD/CVD: Coronary artery disease/cardiovascular disease; COPD: Chronic Obstructive Pulmonary Disease; DM: Diabetes Mellitus; HTN: Hypertension; N/A: Not applicable; NOS: Newcastle-Ottawa Scale.
3.3. Thyroid abnormalities and poor outcomes
Meta-analysis showed that abnormal thyroid was associated with higher risk of COVID-19 composite poor outcome (RR 1.87 [1.53, 2.27], p < 0.001; I2 = 84%, p < 0.01) (Fig. 2 ). Subgroup analysis in patients with thyroid abnormalities also showed a significant increased risk of higher disease severity (RR 1.92 [1.40, 2.63], p < 0.05; I2 = 86%, p < 0.01), ICU admission (RR 1.61 [1.12, 2.32], p > 0.05; I2 = 32%, p < 0.05), mortality (RR 2.43 [1.44, 4.13], p < 0.05; I2 = 83%, p < 0.01), and hospitalization (RR 1.28 [1.17, 1.39], p < 0.05; I2 = 0%, p < 0.96) (see Fig. 3).
Fig. 2.
Thyroid abnormalities and COVID-19 composite poor outcome with subgroup analysis based on each outcome.
Fig. 3.
Thyroid abnormalities and COVID-19 composite poor outcome with subgroup analysis based on each disease type.
In addition, subgroup analyses on different type of thyroid abnormalities were also conducted. The presence of unspecified thyroid disease showed a significant increased risk of composite poor outcome (RR 1.91 [1.38, 2.65], p < 0.05; I2 = 81%, p < 0.01). Similarly, patients with hypothyroidism during admission also showed to have a higher risk of composite COVID-19 poor outcome (RR 1.90 [1.45, 2.55], p < 0.05; I2 = 85%, p < 0.01). In contrast, hyperthyroidism patients did not pose a significant higher risk of poor outcomes (RR 1.67 [0.72, 3.85], p > 0.05; I2 = 85%, p < 0.01). Sensitivity analysis by removing each study did not indicate any alteration in statistical robustness and study heterogeneity (Supplementary Fig. 2).
3.4. Meta-regression
Meta-regression showed that the association between thyroid abnormalities and composite poor outcome was significantly influenced by age (p = 0.002), but not affected by gender (p = 0.22), hypertension (p = 0.13), cardiovascular disease (p = 0.23), diabetes mellitus (p = 0.43), obesity (p = 0.94), and COPD (p = 0.15) (Fig. 4 ).
Fig. 4.
Meta-regression analysis showed that the association between abnormal thyroid and increased risk of poor outcome was influenced by age, but not comorbidities–CAD/CVD and hypertension.
3.5. Publication bias
Publication bias assessment demonstrated an asymmetrical funnel plot for the association between thyroid abnormalities and composite poor outcome (Supplementary Fig. 1a). Regression-based Egger's test indicated small-study effects in the association between thyroid abnormalities and composite poor outcomes (p = 0.007) (Supplementary Fig. 3). Trim-and-fill analysis showed a reduced pooled effect estimate after imputing 9 studies without altering its significance (RR 1.33 [1.08, 1.63], p = 0.007; I2 = 88%, p < 0.01) (Supplementary Fig. 1b).
4. Discussion
The outcomes of COVID-19 patients worldwide are diverse and can be caused by any comorbidities, one of which is thyroid abnormalities. This systematic review presented a summary of hospital-based data, describing the comparison of primary outcomes, including mortality rate, ICU admission, severity, and hospitalization among patients with euthyroid and thyroid abnormalities.
In this present study, the presence of thyroid abnomalities–hypothyroidism, hyperthyroidism, or unspecified thyroid disorder–increased the risk of COVID-19 composite poor outcomes that consist of disease severity, ICU admission, mortality, and hospitalization. Contrary to the cumulative result, Zhang et al. and Lui et al found that the presence of thyroid disease was not associated with more severe COVID-19 [24,33]. In addition, Lui et al. also showed a similar result of the incidence of thyroid abnormalities between mild, moderate, and severe COVID-19 patients [24]. The authors suggested that the result may be affected by the level of SARS-CoV-2 viral load that ought to be confirmed through qRT-PCR [24]. Muller et al. and Gong et al. in their study did not demonstrate a positive and significant association between abnormal thyroid and ICU admission and mortality, respectively [20,25].
Furthermore, meta-regression analyses showed that the correlation between thyroid abnormalities and poor outcomes was affected by the increasing age, not due to other comorbidities. This association because the levels of TSH and thyroxine will decrease gradually and deteriorated physiological response that occurs with increasing age [1,[34], [35], [36]]. In addition, the iodine nutritional status of the populations may result in different thyroid function patterns during aging [35,36].
Although the interplay between thyroid hormones and immune response has been largely investigated, the exact mechanism involved in the setting of COVID-19 infection remains elusive. Like other viral infections, the immune response has an important role in the outcome of SARS-CoV-2 infection [5,6]. Previous hypotheses have postulated that thyroid hormones play a role in modulating the cellular level of both innate and adaptive immune responses [37,38]. The thyroid hormone demonstrates its effects on the immune system at both nuclear levels by activating transcription factors that are responsible for intracellular signaling and at cellular levels by modulating cytokine release on multiple cells when the innate immune response is taking place [39,40]. Several studies have shown that in the acute phase of critically ill patients, the concentration of T3 was markedly decreased [13,14,19]. This abnormal release of thyroid hormone affects the robustness of the immune response that is integral in maintaining COVID-19 outcomes. Moreover, during the process of cytokine storm, the body overproduces cytokines and chemokines triggered by SARS-CoV-2 infection [26]. If this period is prolonged, suppression of the thyroid axis can occur in critically ill patients. It can lead to a worsening of outcomes in patients with thyroid abnormalities [19]. In addition, thyroid hormones also influence other metabolic diseases such as hypertension, diabetes, and cardiovascular disease–those comorbidities are strongly related to an increased risk of poor COVID-19 composite poor outcomes [37,41].
Our results showed abnormal thyroid and hypothyroidism were significantly associated with a higher risk of composite COVID-19 poor outcomes. In contrast, hyperthyroidism patients did not pose different risks in COVID-19 poor outcomes. This discrepancy between these findings can be potentially affected by several factors. It has been emphasized that thyroid hormone affects the renin-angiotensinogen system (RAS) [42,43]. Thus, patients with thyroid abnormalities might have dysregulated RAS which contributes to increased angiotensin-converting enzyme 2 (ACE2) expression [44]. ACE2 plays an essential role in SARS-CoV-2 infection as the key receptor of the virus to enter the host [[45], [46], [47], [48]]. The relationship between thyroid hormones and immune response may be bidirectional. However, cautious considerations should be considered when interpreting the relationship between thyroid hormones and COVID-19 outcomes. First, the effect of excessive thyroid hormone in hyperthyroidism patients may not significantly alter the physiologic regulation of RAS [38]. Second, the dysregulated RAS caused by hyperthyroidism may also not significantly influence the expression of ACE2–yielding to a similar amount of viral entry and disease outcome [44]. Third, the association between hyperthyroidism and poor COVID-19 outcome may be affected by multiple factors. This was demonstrated in the study by Brix et al. that shows a significantly higher risk of poor COVID-19 outcome in hyperthyroidism patients with crude analysis but posed a similar risk ratio of poor outcomes in propensity score weighted analysis [16]. Lastly, a relatively smaller sample and studies involving hyperthyroidism patients may also be one of the possible causes of these findings.
This present study supports the previous meta-analyses exploring the relationship between thyroid abnormalities with COVID-19 poor outcomes. Hariyanto et al. found in their meta-analysis that the presence of thyroid abnormalities was associated with severe COVID-19 outcomes [49]. However, the study only included eight studies with unspecified thyroid abnormalities. On the one hand, this current study included more studies yielding a relatively higher sample, subgroup analyses based on disease outcomes and thyroid abnormalities, and further meta-regression which was not previously conducted. Ultimately, the evidence of the association between thyroid abnormalities and COVID-19 poor outcomes is strengthened by our present results.
Although this present analysis addresses the association between thyroid hormones and COVID-19 outcomes in a larger population, this study has several limitations. Studies that were included in our analyses were largely conducted in a retrospective fashion that may impose a relatively greater potential risk of bias. The association between COVID 19 severity and the degree of thyroid dysfunction could not be assessed due to the paucity of reported studies on each subset. In addition, one cross-sectional study with a relatively larger sample was included that potentially affects cumulative effect estimates. It is important to note that iodine intake may vary across the country. Similarly, ethnicity may also take place to the varied iodine intake levels, especially in a large country [50]. However, we did not perform subgroup analysis under the sociodemographic subset due to study paucity and underreported characteristics on each country or ethnic group of each study. Nevertheless, our study demonstrated a moderate-to-high quality of evidence illustrating those thyroid abnormalities and hypothyroidisms were associated with COVID-19 poor outcomes. Moreover, the generalizability of our findings can be ascertained as the populations included in the analyses were diverse.
5. Conclusion
Thyroid abnormalities are associated with a higher risk of disease severity, mortality, ICU admission, and hospitalization among COVID-19 patients. This association was significantly influenced by an increase in age. Also, the presence of thyroid disorder and hypothyroidism, but not hyperthyroidism, are associated with a higher risk of poor outcomes.
Financial support
None.
Source of financial support
None.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dsx.2021.102312.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Li X., Marmar T., Xu Q., Tu J., Yin Y., Tao Q., et al. Predictive indicators of severe COVID-19 independent of comorbidities and advanced age: a nested case-control study. Epidemiol Infect. 2020;148:e255. doi: 10.1017/S0950268820002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung J.Y., Thone M.N., Kwon Y.J. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soetedjo N.N.M., Iryaningrum M.R., Damara F.A., Permadhi I., Sutanto L.B., Hartono H., et al. Clinical Nutrition ESPEN; 2021. Prognostic properties of hypoalbuminemia in COVID-19 patients: a systematic review and diagnostic meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik P., Patel U., Mehta D., Patel N., Kelkar R., Akrmah M., et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2020 doi: 10.1136/bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui N., Yan R., Qin C., Zhao J. Clinical characteristics and immune responses of 137 deceased patients with COVID-19: a retrospective study. Front Cell Infect Microbiol. 2020;10:595333. doi: 10.3389/fcimb.2020.595333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 [PMC free article] [PubMed] [Google Scholar]
- 8.Team R.C.R. 2013. A language and environment for statistical computing. [Google Scholar]
- 9.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Br Med J Int Ed. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riley R.D., Moons K.G.M., Snell K.I.E., Ensor J., Hooft L., Altman D.G., et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;364:k4597. doi: 10.1136/bmj.k4597. [DOI] [PubMed] [Google Scholar]
- 11.Peters J.L., Sutton A.J., Jones D.R., Abrams K.R., Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alqahtani A.M., AlMalki Z.S., Alalweet R.M., Almazrou S.H., Alanazi A.S., Alanazi M.A., et al. Assessing the severity of illness in patients with coronavirus disease in Saudi Arabia: a retrospective descriptive cross-sectional study. Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.593256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almazeedi S., Al-Youha S., Jamal M.H., Al-Haddad M., Al-Muhaini A., Al-Ghimlas F., et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldelli R., Nicastri E., Petrosillo N., Marchioni L., Gubbiotti A., Sperduti I., et al. Thyroid dysfunction in COVID-19 patients. J Endocrinol Invest. 2021:1–5. doi: 10.1007/s40618-021-01599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brix T.H., Hegedüs L., Hallas J., Lund L.C. Risk and course of SARS-CoV-2 infection in patients treated for hypothyroidism and hyperthyroidism. Lancet Diabetes Endocrinol. 2021;9(4):197–199. doi: 10.1016/S2213-8587(21)00028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao M., Zhang D., Wang Y., Lu Y., Zhu X., Li Y., et al. 2020. Clinical features of patients infected with the 2019 Novel coronavirus (COVID-19) in Shanghai, China. medRxiv. [Google Scholar]
- 18.Daraei M., Hasibi M., Abdollahi H., Mirabdolhagh Hazaveh M., Zebaradst J., Hajinoori M., et al. Possible role of hypothyroidism in the prognosis of COVID-19. Intern Med J. 2020;50(11):1410–1412. doi: 10.1111/imj.15000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao W., Guo W., Guo Y., Shi M., Dong G., Wang G., et al. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinol Invest. 2021;44(5):1031–1040. doi: 10.1007/s40618-020-01460-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong J., Wang D.K., Dong H., Xia Q.S., Huang Z.Y., Zhao Y., et al. Prognostic significance of low TSH concentration in patients with COVID-19 presenting with non-thyroidal illness syndrome. BMC Endocr Disord. 2021;21(1):111. doi: 10.1186/s12902-021-00766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Güven M., Gültekin H. The prognostic impact of thyroid disorders on the clinical severity of COVID-19: results of single-centre pandemic hospital. Int J Clin Pract. 2021;75(6) doi: 10.1111/ijcp.14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoo B., Tan T., Clarke S.A., Mills E.G., Patel B., Modi M., et al. Thyroid function before, during, and after COVID-19. J Clin Endocrinol Metab. 2021;106(2):e803–e811. doi: 10.1210/clinem/dgaa830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang S., Liu Y., Qu X., Lu R., Fu W., Zhang W., et al. Association between thyroid function and prognosis of COVID-19: a retrospective observational study. Endocr Res. 2021:1–8. doi: 10.1080/07435800.2021.1924770. [DOI] [PubMed] [Google Scholar]
- 24.Lui D.T.W., Lee C.H., Chow W.S., Lee A.C.H., Tam A.R., Fong C.H.Y., et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab. 2021;106(2):e926–e935. doi: 10.1210/clinem/dgaa813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller I., Cannavaro D., Dazzi D., Covelli D., Mantovani G., Muscatello A., et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8(9):739–741. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen C., Yale K., Ghigi A., Zheng K., Mesinkovska N.A., Wambier C.G., et al. SARS-CoV-2 infection in patients with thyroid disease: a cross-sectional study. Ann Thyroid. 2022;6 doi: 10.21037/aot-21-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sen K., Sinha A., Sen S., Chakraborty S., Alam M.S. Thyroid function test in COVID-19 patients: a cross-sectional study in a tertiary care hospital. Indian J Endocrinol Metab. 2020;24(6):532–536. doi: 10.4103/ijem.IJEM_779_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shabrawishi M., Al-Gethamy M.M., Naser A.Y., Ghazawi M.A., Alsharif G.F., Obaid E.F., et al. Clinical, radiological and therapeutic characteristics of patients with COVID-19 in Saudi Arabia. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siso-Almirall A., Kostov B., Mas-Heredia M., Vilanova-Rotllan S., Sequeira-Aymar E., Sans-Corrales M., et al. Prognostic factors in Spanish COVID-19 patients: a case series from Barcelona. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Gerwen M., Alsen M., Little C., Barlow J., Naymagon L., Tremblay D., et al. Outcomes of patients with hypothyroidism and COVID-19: a retrospective cohort study. Front Endocrinol. 2020;11:565. doi: 10.3389/fendo.2020.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W., Su X., Ding Y., Fan W., Zhou W., Su J., et al. Thyroid function abnormalities in COVID-19 patients. Front Endocrinol. 2020;11 doi: 10.3389/fendo.2020.623792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Lin F., Tu W., Zhang J., Choudhry A.A., Ahmed O., et al. Thyroid dysfunction may be associated with poor outcomes in patients with COVID-19. Mol Cell Endocrinol. 2021;521 doi: 10.1016/j.mce.2020.111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aggarwal N., Razvi S. Thyroid and aging or the aging thyroid? An evidence-based analysis of the literature. J Thyroid Res. 2013 doi: 10.1155/2013/481287. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbesino G. Thyroid function changes in the elderly and their relationship to cardiovascular health: a mini-review. Gerontology. 2019;65(1):1–8. doi: 10.1159/000490911. [DOI] [PubMed] [Google Scholar]
- 36.Bremner A.P., Feddema P., Leedman P.J., Brown S.J., Beilby J.P., Lim E.M., et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab. 2012;97(5):1554–1562. doi: 10.1210/jc.2011-3020. [DOI] [PubMed] [Google Scholar]
- 37.Kumari K., GBN Chainy, Subudhi U. Prospective role of thyroid disorders in monitoring COVID-19 pandemic. Heliyon. 2020;6(12) doi: 10.1016/j.heliyon.2020.e05712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubingh J., van der Spek A., Fliers E., Boelen A. The role of thyroid hormone in the innate and adaptive immune response during infection. Comp Physiol. 2020;10(4):1277–1287. doi: 10.1002/cphy.c200003. [DOI] [PubMed] [Google Scholar]
- 39.Montesinos M.D.M., Pellizas C.G. Thyroid hormone action on innate immunity. Front Endocrinol. 2019;10:350. doi: 10.3389/fendo.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Luca R., Davis P.J., Lin H.Y., Gionfra F., Percario Z.A., Affabris E., et al. Thyroid hormones interaction with immune response, inflammation and non-thyroidal illness syndrome. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.614030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X., Zhong X., Wang Y., Zeng X., Luo T., Liu Q. Clinical determinants of the severity of COVID-19: a systematic review and meta-analysis. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0250602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park C.W., Shin Y.S., Ahn S.J., Kim S.Y., Choi E.J., Chang Y.S., et al. Thyroxine treatment induces upregulation of renin-angiotensin-aldosterone system due to decreasing effective plasma volume in patients with primary myxoedema. Nephrol Dial Transplant. 2001;16(9):1799–1806. doi: 10.1093/ndt/16.9.1799. [DOI] [PubMed] [Google Scholar]
- 43.Vargas F., Rodriguez-Gomez I., Vargas-Tendero P., Jimenez E., Montiel M. The renin-angiotensin system in thyroid disorders and its role in cardiovascular and renal manifestations. J Endocrinol. 2012;213(1):25–36. doi: 10.1530/JOE-11-0349. [DOI] [PubMed] [Google Scholar]
- 44.Diniz G.P., Senger N., Carneiro-Ramos M.S., Santos R.A., Barreto-Chaves M.L. Cardiac ACE2/angiotensin 1-7/Mas receptor axis is activated in thyroid hormone-induced cardiac hypertrophy. Ther Adv Cardiovasc Dis. 2016;10(4):192–202. doi: 10.1177/1753944715623228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vieira C., Nery L., Martins L., Jabour L., Dias R., Simoes E.S.A.C. Downregulation of membrane-bound angiotensin converting enzyme 2 (ACE2) receptor has a pivotal role in COVID-19 immunopathology. Curr Drug Targets. 2021;22(3):254–281. doi: 10.2174/1389450121666201020154033. [DOI] [PubMed] [Google Scholar]
- 46.Aleksova A., Ferro F., Gagno G., Cappelletto C., Santon D., Rossi M., et al. COVID-19 and renin-angiotensin system inhibition: role of angiotensin converting enzyme 2 (ACE2) - is there any scientific evidence for controversy? J Intern Med. 2020;288(4):410–421. doi: 10.1111/joim.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ni W., Yang X., Yang D., Bao J., Li R., Xiao Y., et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24(1):422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kragstrup T.W., Singh H.S., Grundberg I., Nielsen A.L., Rivellese F., Mehta A., et al. Plasma ACE2 predicts outcome of COVID-19 in hospitalized patients. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0252799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hariyanto T.I., Kurniawan A. Thyroid disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(5):1429–1430. doi: 10.1016/j.dsx.2020.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koch L. Thyroid gland: TSH reference limits specific for age, sex and ethnicity. Nat Rev Endocrinol. 2011;7(2):61. doi: 10.1038/nrendo.2010.218. [DOI] [PubMed] [Google Scholar]
- 51.Alqahtani A.M., AlMalki Z.S., Alalweet R.M., Almazrou S.H., Alanazi A.S., Alanazi M.A., et al. Assessing the severity of illness in patients with coronavirus disease in Saudi Arabia: a retrospective descriptive cross-sectional study. Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.593256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almazeedi S., Al-Youha S., Jamal M.H., Al-Haddad M., Al-Muhaini A., Al-Ghimlas F., et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baldelli R., Nicastri E., Petrosillo N., Marchioni L., Gubbiotti A., Sperduti I., et al. Thyroid dysfunction in COVID-19 patients. J Endocrinol Invest. 2021:1–5. doi: 10.1007/s40618-021-01599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brix T.H., Hegedüs L., Hallas J., Lund L.C. Risk and course of SARS-CoV-2 infection in patients treated for hypothyroidism and hyperthyroidism. Lancet Diabetes Endocrinol. 2021;9(4):197–199. doi: 10.1016/S2213-8587(21)00028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao M., Zhang D., Wang Y., Lu Y., Zhu X., Li Y., et al. 2020. Clinical features of patients infected with the 2019 Novel coronavirus (COVID-19) in Shanghai, China. medRxiv. [Google Scholar]
- 56.Daraei M., Hasibi M., Abdollahi H., Mirabdolhagh Hazaveh M., Zebaradst J., Hajinoori M., et al. Possible role of hypothyroidism in the prognosis of COVID-19. Intern Med J. 2020;50(11):1410–1412. doi: 10.1111/imj.15000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao W., Guo W., Guo Y., Shi M., Dong G., Wang G., et al. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinol Invest. 2021;44(5):1031–1040. doi: 10.1007/s40618-020-01460-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong J., Wang D.K., Dong H., Xia Q.S., Huang Z.Y., Zhao Y., et al. Prognostic significance of low TSH concentration in patients with COVID-19 presenting with non-thyroidal illness syndrome. BMC Endocr Disord. 2021;21(1):111. doi: 10.1186/s12902-021-00766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Güven M., Gültekin H. The prognostic impact of thyroid disorders on the clinical severity of COVID-19: results of single-centre pandemic hospital. Int J Clin Pract. 2021;75(6) doi: 10.1111/ijcp.14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khoo B., Tan T., Clarke S.A., Mills E.G., Patel B., Modi M., et al. Thyroid function before, during, and after COVID-19. J Clin Endocrinol Metab. 2021;106(2):e803–e811. doi: 10.1210/clinem/dgaa830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lang S., Liu Y., Qu X., Lu R., Fu W., Zhang W., et al. Association between thyroid function and prognosis of COVID-19: a retrospective observational study. Endocr Res. 2021:1–8. doi: 10.1080/07435800.2021.1924770. [DOI] [PubMed] [Google Scholar]
- 62.Lui D.T.W., Lee C.H., Chow W.S., Lee A.C.H., Tam A.R., Fong C.H.Y., et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab. 2021;106(2):e926–e935. doi: 10.1210/clinem/dgaa813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muller I., Cannavaro D., Dazzi D., Covelli D., Mantovani G., Muscatello A., et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8(9):739–741. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen C., Yale K., Ghigi A., Zheng K., Mesinkovska N.A., Wambier C.G., et al. SARS-CoV-2 infection in patients with thyroid disease: a cross-sectional study. Ann Thyroid. 2022;6 doi: 10.21037/aot-21-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sen K., Sinha A., Sen S., Chakraborty S., Alam M.S. Thyroid function test in COVID-19 patients: a cross-sectional study in a tertiary care hospital. Indian J Endocrinol Metab. 2020;24(6):532–536. doi: 10.4103/ijem.IJEM_779_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shabrawishi M., Al-Gethamy M.M., Naser A.Y., Ghazawi M.A., Alsharif G.F., Obaid E.F., et al. Clinical, radiological and therapeutic characteristics of patients with COVID-19 in Saudi Arabia. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siso-Almirall A., Kostov B., Mas-Heredia M., Vilanova-Rotllan S., Sequeira-Aymar E., Sans-Corrales M., et al. Prognostic factors in Spanish COVID-19 patients: a case series from Barcelona. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Gerwen M., Alsen M., Little C., Barlow J., Naymagon L., Tremblay D., et al. Outcomes of patients with hypothyroidism and COVID-19: a retrospective cohort study. Front Endocrinol. 2020;11:565. doi: 10.3389/fendo.2020.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang W., Su X., Ding Y., Fan W., Zhou W., Su J., et al. Thyroid function abnormalities in COVID-19 patients. Front Endocrinol. 2020;11 doi: 10.3389/fendo.2020.623792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y., Lin F., Tu W., Zhang J., Choudhry A.A., Ahmed O., et al. Thyroid dysfunction may be associated with poor outcomes in patients with COVID-19. Mol Cell Endocrinol. 2021;521:111097. doi: 10.1016/j.mce.2020.111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.