Graphical abstract
Keywords: Left atrial appendage, Coronary artery aneurysm, Computed tomographic angiography
Highlights
-
•
LAA is a complex, variable anatomic structure that can be mistaken for other cardiac structures.
-
•
Color and spectral Doppler may help to discern the LAA from surrounding structures.
-
•
Cross-sectional imaging such as CTA can aid in defining the morphology of the LAA.
Introduction
The left atrial appendage (LAA) is a structure with variable morphology and position. Given this variation, it can be mistaken for other cardiac structures. In this study, we describe a case of a dilated and tortuous LAA confirmed on computed tomographic angiography (CTA) that was initially thought to represent left coronary artery (LCA) ectasia on transthoracic echocardiography.
Case Presentation
A previously healthy 3-year-old girl was found to have a heart murmur at her annual health supervision visit, for which an echocardiogram was obtained at her local hospital. This study suggested an abnormality of the LCA, describing two coronary arteries arising from the left sinus with one being very large and possibly aneurysmal. Due to this concern, she was referred for pediatric cardiology evaluation. At the time of her outpatient cardiology consultation, she was growing and developing appropriately with no symptoms of tachycardia, increased work of breathing, chest pain, syncope, or cyanosis. There was no history of prolonged febrile illness, rash, lymphadenopathy, conjunctival changes, oral mucosal changes, swelling of the hands/feet, or desquamation. On physical examination, she was noted to have an early systolic ejection click and a I/VI crescendo-decrescendo systolic ejection murmur at the base, synchronous pulses in the upper and lower extremities, and no hepatomegaly. The remainder of the physical examination was unremarkable. An electrocardiogram was normal for age. A repeat echocardiogram in our clinic suggested left main coronary artery dilation (ectasia) measuring 3.4 mm (Z score = 2.42; Figure 1, Video 1) as well as a functionally bicuspid aortic valve.
Figure 1.
Parasternal short-axis view demonstrating suspected ectasia of the LCA (A) and color Doppler with flow toward the transducer, seen as red(B).
Given the difficulty in visualizing the coronary artery anatomy, the patient underwent CTA of the coronary arteries. This study demonstrated normal origin, caliber, and course of the coronary arteries. The structure of concern on echocardiogram was found to be a dilated and tortuous LAA (Figure 2). She was followed up 1 year later for continued surveillance of the bicuspid aortic valve, and she had no cardiac symptoms or evidence of aortic valve dysfunction.
Figure 2.
CTA demonstrating normal LCA origin and tortuous LAA (A) and 3D reconstruction with LAA in purple(B).
Discussion
The LAA is a vestige of the left atrium (LA) that develops during gestation. The LAA has been well described in the adult population, primarily in the setting of atrial fibrillation and thrombus formation. Several recent studies have described the variation in LAA anatomy seen both in pathology specimens as well as on echocardiography. There have been few cases of variable LAA anatomy that have been described in the pediatric population, with these cases using cardiac catheterization or transesophageal echocardiography (TEE) to confirm the diagnosis. Based on our literature review, we have found very few cases of LAA anatomy described in the pediatric literature. We have not found other cases reported that used computed tomography (CT) to confirm the diagnosis of tortuous LAA in a pediatric patient.
The LAA is a narrow, finger-like muscular structure that arises from the LA and can have one to four lobes.1 In most patients, it lies anteriorly and superiorly to the pulmonary artery and sits along the left atrioventricular groove, atop the proximal left circumflex artery.1,2 Its position can cause it to be easily mistaken for other structures on echocardiography, which leads to difficulties in diagnosis and additional evaluation. Ramaswamy et al.3 described a series of clinical scenarios in which the LAA was misdiagnosed on initial echocardiography as a coronary aneurysm, left upper pulmonary vein stenosis, LA thrombus, organizing effusion, and the main pulmonary artery.3 In two of three patients where coronary artery aneurysm was suspected, additional testing using TEE and/or cardiac catheterization was performed.
The course of the LAA along the proximal left circumflex coronary artery can make it particularly difficult to discern on echocardiography. On echocardiography, the coronary arteries are best imaged in the parasternal short-axis view at the level of the pulmonary valve, with laminar forward flow (toward the transducer) seen as red by color Doppler in diastole.4 In most patients, the LAA covers only the circumflex artery, which is not usually visible in the standard short-axis view (Figure 3). The LAA is best visualized in the apical four-chamber views and the parasternal short-axis view when tipped inferiorly from the standard view.5,6 However, in some patients, as the one described in this report, the LAA may lie along the LCA and thus may be misinterpreted as a LCA aneurysm.3 The LAA can also have laminar flow in diastole, which can further compound this misinterpretation. In the cases described above from Ramaswamy et al., color flow through the coronary artery orifice and through the presumed coronary aneurysm were not able to be visualized in the same imaging plane. On review of our case, the finding that helps differentiate the LAA from the coronary artery is the color flow pattern. When reviewing the color Doppler flow through the structure in question, one can see positive (toward the transducer) and negative (away from the transducer) flow in subsequent frames (Figure 4, Video 2). This color flow pattern contrasts with that of a coronary artery, which typically demonstrates a continuous diastolic flow pattern. Agmon et al.7 described a TEE technique to isolate the LAA using pulse-wave Doppler, as the LAA and coronary artery patterns are different. In sinus rhythm, LAA contraction results in a late diastolic Doppler flow signal toward the LA after the onset of the electrocardiographic P wave, followed by LAA filling, which results in an early Doppler flow signal away from the LA following LA contraction. Following LAA contraction and filling, there are alternating LAA outflow and inflow signals of lower amplitude that are a result of passive flow after the initial contraction and filling. After early diastolic mitral flow (mitral E wave), there is early diastolic LAA flow, which is a low-velocity outflow signal.7 Although this article described imaging of the LAA using TEE, its principles may be applied to transthoracic echocardiography (Figure 5).
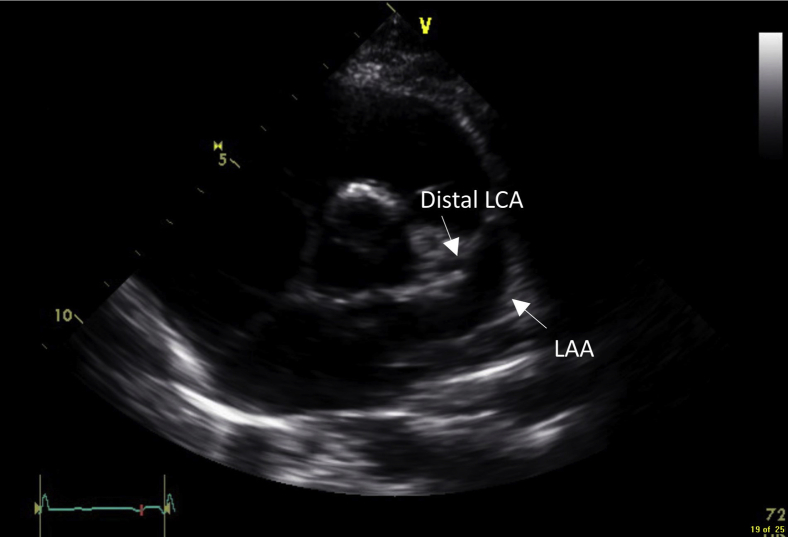
Figure 3.
Parasternal short-axis view demonstrating a normal LAA and distal LCA.
Figure 4.
LAA with flow away the transducer, seen as blue.
Figure 5.
(A) Spectral Doppler of LAA demonstrating phases of LAA flow. 1, LAA contraction; 2, LAA filling, 3, passive LAA flow; 4, early diastolic LAA outflow. (B) Spectral Doppler of LCA demonstrating diastolic flow above the baseline (arrow).
Computed tomography and magnetic resonance imaging have become important modalities for evaluating the LAA. The advantages of CT over echocardiography are increased spatial and temporal resolution. In addition, CT can generate a three-dimensional (3D) rendering of the heart and provide more accurate and detailed assessment of the LAA anatomy.1 In our patient, the CT was able to detail the tortuous course of the LAA and provide a 3D reconstruction demonstrating the similar path of the LCA and LAA.
Coronary artery ectasia in a healthy pediatric patient is concerning for vascular inflammation, such as that found in Kawasaki disease. Although this patient was not reported to have previous clinical features of a systemic inflammatory process, coronary artery ectasia may suggest a missed case of Kawasaki disease. The degree of ectasia and progression to aneurysm is significant as it directs pharmacologic therapy as well as follow-up and diagnostic testing.5 In this patient, further interrogation of the suspected coronary arteries may have helped as it would have demonstrated a different spectral Doppler pattern than expected. However, coronary artery and LAA spectral Doppler had not typically been performed in our laboratory for evaluation of the coronary arteries. Therefore, in our patient, the CT was an integral part of the diagnosis of a tortuous LAA.
Conclusion
The LAA is a small unique structure that has variations in morphology and position. Due to its location and course, it may be misdiagnosed as other, more concerning, lesions such as a LCA aneurysm. It is important to understand its anatomy and relationship to surrounding structures to better prevent diagnostic errors.
Footnotes
Conflicts of Interest: None.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2021.07.004.
Supplementary Data
Parasternal short-axis view demonstrating suspected left main coronary artery ectasia.
Parasternal short-axis view with color Doppler demonstrating to-and-fro flow in the LAA, which was initially suspected as LCA ectasia.
References
- 1.Beigel R., Wunderlich N.C., Ho S.Y., Arsanjani R., Siegel R.J. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging. 2014;7:1251–1265. doi: 10.1016/j.jcmg.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Veinot J.P., Harrity P.J., Gentile F., Khandheria B.K., Bailey K.R., Eickholt J.T. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997;96:3112–3115. doi: 10.1161/01.cir.96.9.3112. [DOI] [PubMed] [Google Scholar]
- 3.Ramaswamy P., Lytrivi I.D., Srivastava S., Sharma S., Ko H.H., Parness I.A. Left atrial appendage: variations in morphology and position causing pitfalls in pediatric echocardiographic diagnosis. J Am Soc Echocardiogr. 2007;20:1011–1016. doi: 10.1016/j.echo.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Jureidini S.B., Marino C.J., Waterman B., Syamasundar Rao P., Balfour I.C., Chen S.C. Transthoracic Doppler echocardiography of normally originating coronary arteries in children. J Am Soc Echocardiogr. 1998;11:409–420. doi: 10.1016/s0894-7317(98)70019-7. [DOI] [PubMed] [Google Scholar]
- 5.Allen H.D. Wolters Kluwer; Philadelphia: 2016. Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult. [Google Scholar]
- 6.Lai W.W., Mertens L.L., Cohen M.S., Geva T. Wiley-Blackwell; Hoboken, NJ: 2009. Echocardiography in pediatric and congenital heart disease from fetus to adult. [Google Scholar]
- 7.Agmon Y., Khandheria B.K., Gentile F., Seward J.B. Echocardiographic assessment of the left atrial appendage. J Am Coll Cardiol. 1999;34:1867–1877. doi: 10.1016/s0735-1097(99)00472-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parasternal short-axis view demonstrating suspected left main coronary artery ectasia.
Parasternal short-axis view with color Doppler demonstrating to-and-fro flow in the LAA, which was initially suspected as LCA ectasia.