Abstract
Mutations in the rpoB gene of 90 rifampin-resistant Mycobacterium tuberculosis isolates mostly from Asian countries were analyzed. Ten distinct single-nucleotide substitutions were found among the isolates by automated sequencing. A 3-nucleotide insertion was found in two isolates, and no mutation was found in five isolates (5.6%). A reverse hybridization-based line probe assay (INNO-LiPA Rif TB) for rapid detection of the mutations was evaluated with these isolates. Concordance rates with sequencing results for five wild-type probes (S probes) and four probes for specific mutations (R probes) were 96.7 and 100%, respectively. The overall concordance rate with the in vitro susceptibility testing results was 92.2% (83 of 90 isolates). These results indicate that a commercial line probe assay kit may be useful for rapid diagnosis of rifampin-resistant tuberculosis.
Dramatic outbreaks of multidrug-resistant tuberculosis occurred in human immunodeficiency virus-infected patients in the United States (2, 7, 9, 26) and Europe (12, 21). These outbreaks had high case fatality rates. The need for rapid identification of mycobacteria and rapid drug susceptibility testing of the isolates has increased.
Multidrug-resistant tuberculosis is thought to occur either through infection with organisms already resistant to antimicrobial agents (primary drug resistance) or by treatment of a strain that was originally drug sensitive (acquired drug resistance). The latter process is believed to be a result of the selection of drug-resistant mutants of the original strain as a consequence of inadequate treatment (3, 34).
Isoniazid resistance is well known to be associated with alterations in the catalase-peroxidase gene (katG) or the inhA gene (1, 37). Pyrazinamide-resistant Mycobacterium tuberculosis strains lose their pyrazinamidase activity (18). Mutations in the pncA gene encoding the pyrazinamidase of M. tuberculosis could be involved in the loss of pyrazinamidase activity (14, 20, 25). Mutations in genes encoding the ribosomal S12 protein (rpsL) and 16S rRNA (rrs) have always been detected in streptomycin-resistant M. tuberculosis isolates (10, 17, 22). It was also reported that kanamycin resistance is associated with the rrs gene mutations (29, 30). Amino acid substitutions in EmbB alter the drug-protein interaction and thereby cause ethambutol resistance (4, 27). Missense mutations in the DNA gyrase gene (gyrA) have always been associated with increased levels of resistance to fluoroquinolones (5).
Rifampin is one of the major antituberculosis drugs (3, 34), and the mechanism of rifampin resistance was the first characterized mechanism of drug resistance in M. tuberculosis (31). The rpoB gene encodes the β-subunit of the RNA polymerase. Rifampin specifically interacts with prokaryotic RNA polymerase to inhibit transcription, which leads to and causes cell death, and specific mutations in rpoB produce drug resistance and diminishing rifampin binding affinity for the RNA polymerase (15, 16).
In this study, mutations in the rpoB genes of rifampin-resistant isolates mostly from Asian countries were analyzed, and a recently described reverse hybridization-based line probe assay for rapid detection of the mutations was evaluated with these isolates.
MATERIALS AND METHODS
M. tuberculosis isolates.
M. tuberculosis strains which were mostly isolated in Asian countries as well as Canada were used for this study (Table 1). We used 2 isolates from Bangladesh (A. M. Ishaque, Nuclear Medicine Centre, Dhaka, Bangladesh), 13 isolates from Canada (A. Laszlo, Laboratory Centre for Disease Control, Ottawa, Ontario, Canada), 6 isolates from India (P. Seth, All India Institute of Medical Science, New Dehli, India), 5 isolates from Indonesia (P. Sudarmono, University of Indonesia, Jakarta, Indonesia), 3 isolates from Korea (S.-N. Cho, Yonsei University, Seoul, Korea), 20 isolates from Malaysia (R. Soshila, Institute of Respiratory Medicine, Kuala Lumpur Hospital, Kuala Lumpur, Malaysia), 18 isolates from Myanmar (D. Ti Ti, Union Tuberculosis Institute, Yangon, Myanmar), 2 isolates from Nepal (K. Yamakami, National Tuberculosis Centre, Bhaktapum, Nepal), 4 isolates from the Philippines (T. T. Torres, Philippine General Hospital, Manila, the Philippines), 19 isolates from Thailand (S. Twmwasorn, Chulalongkorn University, Bangkok, Thailand), and 24 isolates from Yemen (M. Nakayama, Yemen Tuberculosis Control Project of Japan International Cooperation Agency, Sana’s, Yemen).
TABLE 1.
M. tuberculosis isolates used in the study
| Isolate source | No. of isolates
|
|
|---|---|---|
| Rifampin resistant | Rifampin susceptible | |
| Bangladesh | 2 | 0 |
| Canada | 13 | 0 |
| India | 3 | 3 |
| Indonesia | 2 | 3 |
| Korea | 0 | 3 |
| Malaysia | 14 | 6 |
| Myanmar | 15 | 3 |
| Nepal | 2 | 0 |
| Philippines | 4 | 0 |
| Thailand | 14 | 5 |
| Yemen | 21 | 3 |
| Total | 90 | 26 |
Identification of mycobacterial isolates.
All isolates were differentiated and identified by an RNA-DNA hybridization assay with commercial kits for culture confirmation and identification of species belonging to the M. tuberculosis complex (Gen-Probe, San Diego, Calif.) and by conventional culturing or biochemical testing.
Drug susceptibility testing.
The proportion method with Löwenstein-Jensen egg slants was used to test susceptibility to rifampin (6, 35, 36). Organisms were grown in a Middlebrook 7H9 liquid medium at 37°C until the optical density at 540 nm reached about 0.1, which corresponds to a McFarland no. 0.5 standard. One hundred microliters of a 1:100 dilution of the bacterial cultures was inoculated onto drug-containing medium, and two dilutions (1:100 and 1:10,000) were inoculated onto drug-free control media. The slants were incubated at 37°C. A preliminary reading was made at 3 weeks, and a final reading was made at 4 weeks. The number of CFU on the rifampin-containing slant was compared with the number on control medium inoculated with a 1:10,000 dilution. The breakpoint was 1% (a value of >1% was used to classify the organisms as resistant).
PCR amplification and sequencing of the products.
Bacterial suspensions containing approximately 105 bacteria in 100 μl of distilled water were prepared from M. tuberculosis isolates grown on Ogawa egg slants for 3 to 4 weeks, and they were treated by the repetition of freezing-thawing for PCR amplification. Twenty microliters of the crude bacterial cell lysates was added to the PCR mixture. Two primers with the sequences reported by Williams et al. (33), Rif-1 (5′-CAG ACG TTG ATC AAC ATC CG-3′) and Rif-2 (5′-TAC GGC GTT TCG ATG AAC-3′) were used to amplify a 305-bp fragment of the rpoB gene of M. tuberculosis. After the reaction mixtures were incubated at 94°C for 10 min, PCR was carried out in a Perkin-Elmer 9600 thermal cycler for 40 cycles, with each cycle consisting of denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and primer extension at 72°C for 90 s. Filtration with Suprec-02 tubes (Takara, Kyoto, Japan) was used to separate the unincorporated nucleic acids and primers from amplified DNA. Sequencing reactions with a DNA sequencing kit (Dye Terminator Cycle Sequencing Ready Reaction; Applied Biosystems Inc., Foster City, Calif.) were performed with 5 μl of PCR-amplified DNA and 3.2 pmol of the primer, either Rif-1 or Rif-2. Centri-sep spin columns (Applied Biosystems Inc.) were used to separate the unincorporated dye terminators and primers from the extension products. The products were dried in a vacuum centrifuge, resuspended in loading buffer, heat denatured for 2 min at 90°C, and immediately loaded onto a 4% acrylamide gel in an Applied Biosystems Prism 377 automated DNA sequencer. Both strands were sequenced.
Line probe assay.
Two primers with the indicated sequences, IP1 (5′-GGT CGG CAT GTC GCG GAT GG-3′) and IP2 (5′-GCA CGT CGC GGA CCT CCA GC-3′), were used to amplify a 256-bp fragment of rpoB (8, 24). They were biotinylated at their 5′ ends. PCR was carried out for 35 cycles, with each cycle consisting of denaturation at 94°C for 45 s, annealing at 64°C for 60 s, and primer extension at 72°C for 45 s, followed by a final cycle of 10 min at 72°C.
The line probe assay (INNO-LiPA Rif TB; Innogenetics, Zwijndrecht, Belgium) is based on the reverse hybridization principle (8, 24). Specific oligonucleotides are immobilized at known locations on membrane strips and are hybridized under strictly controlled conditions with the biotin-labeled PCR product. Five partially overlapping probes (S1 through S5) of 19 to 23 bases were designed. These S probes exclusively hybridize to the wild-type sequence. If a mutation is present in one of the five regions, the corresponding probe will be prevented from hybridizing under the stringent hybridization and washing conditions (SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate] buffer containing 0.1% sodium dodecyl sulfate at 62°C) used. In addition, four R probes (19 or 23 bases) were designed. These R probes, probes R2, R4a, R4b, and R5, hybridize with amplicons carrying the following mutations: Asp-516-Val, His-526-Tyr, His-526-Asp, and Ser-531-Leu, respectively (Fig. 1). INNO-LiPA Rif TB kits were kindly provided by Nissho Cooporation (Kusatsu, Shiga, Japan). The assay was performed by using an automated system (AUTO-LiPA; Innogenetics) according to the manufacturer’s instruction.
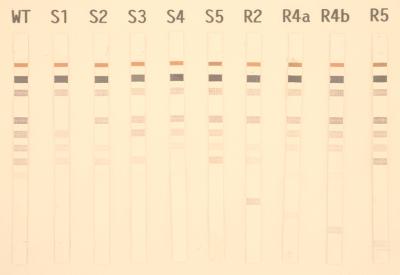
FIG. 1.
Representative hybridization patterns obtained with line probe strips. S1 through S5, probes for wild-type (WT) sequences in the 75-bp hypervariable region of rpoB gene. Probes for specific mutations were as follows: R2, Asp-516-Val; R4a, His-526-Tyr; R4b, His-526-Asp; R5, Ser-531-Leu.
RESULTS
The evaluation of 90 rifampin-resistant M. tuberculosis strains from 10 countries identified 11 mutations within a 75-bp region of the rpoB gene (Table 2).
TABLE 2.
Sequence analysis of rifampin-resistant M. tuberculosis isolates from different countries
| Rifampin susceptibility and amino acid substitution | No. (%) of isolates | Origin of isolatesa |
|---|---|---|
| Rifampin resistant | ||
| Leu 511 Pro | 1 (1.1) | Nep (1) |
| Gln 513 Pro | 5 (5.6) | Mal (1), Mya (1), Thai (2), Yem (1) |
| 514 Phe ins.b | 2 (2.2) | Mal (1), Yem (1) |
| Asp 516 Tyr | 1 (1.1) | Mal (1) |
| Asp 516 Val | 12 (13.3) | Ban (1), Mal (2), Mya (2), Nep (1), Thai (2), Yem (4) |
| Ser 522 Leu | 1 (1.1) | Yem (1) |
| His 526 Arg | 3 (3.3) | Can (1), Thai (2) |
| His 526 Asp | 4 (4.4) | Ind (1), Mya (1), Thai (2) |
| His 526 Tyr | 8 (8.9) | Indn (1), Mal (2), Mya (1), Phi (1), Thai (1), Yem (2) |
| Ser 531 Leu | 46 (51.1) | Can (12), Mal (7), Mya (9), Phi (3), Thai (4), Yem (11) |
| Ser 531 Trp | 2 (2.2) | Ind (2) |
| None | 5 (5.6) | Ban (1), Ind (1), Mya (1), Thai (1), Yem (1) |
| Rifampin susceptible, none | 26 (100) | Ind (3), Indn (3), Kor (3), Mal (6), Mya (3), Thai (5), Yem (3) |
Ban, Bangladesh; Can, Canada; Ind, India; Indn, Indonesia; Kor, Korea; Mal, Malaysia; Mya, Myanmar; Nep, Nepal; Phi, Philippines; Thai, Thailand; Yem, Yemen.
Phe ins., Phe insertion.
Ten were point mutations, and two isolates contained a 3-base insertion that encoded a phenylalanine residue inserted between Gln-513 and Phe-514. No other missense mutations, deletion mutations, or two mutations in separate codons were found in any of the 90 rifampin-resistant M. tuberculosis isolates. Mutations of Ser-531 were present in 48 (53.3%) of 90 rifampin-resistant isolates. The second most dominant mutation was observed at His-526 (16.7%), followed by Asp-516 (14.4%), indicating that approximately 85% of rifampin-resistant isolates have mutations in one of the above three dominant amino acids between amino acid position 511 and 533 analyzed. Five rifampin-resistant isolates (5.6%) contained no mutations within the 305-bp region of the rpoB gene. None of the rifampin-susceptible strains tested had mutations within the 305-bp region of the rpoB gene.
A reverse hybridization-based line probe assay for the rapid detection of the rpoB gene mutations was evaluated with 90 rifampin-resistant and 26 rifampin-susceptible isolates. The hybridization patterns obtained with the wild-type probes correlated with the sequencing results for 87 of 90 rifampin-resistant isolates and all of the susceptible isolates. Four distinct nucleotide substitutions that accounted for resistance were correctly identified in 70 of 90 rifampin-resistant isolates (77.8%) with R probes specific for these mutations (Table 3). The rate of concordance with the phenotypic rifampin susceptibility testing results was 92.2% (83 of 90 rifampin-resistant isolates). Two isolates that possessed a 3-nucleotide insertion (TTC, which codes for Phe-514) and four isolates that had no mutations in the 75-bp region were identified as rifampin sensitive by line probe assay results. The line probe assay revealed in one instance the presence of a mixture (wild-type plus an R2 strain) which had a resistance phenotype by rifampin susceptibility testing but no mutations in the 75-bp region. All 26 strains which were sensitive to rifampin according to the susceptibility testing were correctly identified by the line probe assay.
TABLE 3.
Comparison of results obtained by line probe assay and sequencing
| Line probe profile | No. of isolates | Mutation(s) identified by sequencinga |
|---|---|---|
| ΔS1 | 6 | 511 cCg (1), 513 cCa (5) |
| ΔS2 | 1 | 516 Tac (1) |
| ΔS3 | 1 | 522 tTg (1) |
| ΔS4 | 3 | 526 cGc (3) |
| ΔS5 | 2 | 531 tGg (2) |
| R2 | 12 | 516 gTc (12) (Asp→Val) |
| R4a | 8 | 526 Tac (8) (His→Tyr) |
| R4b | 4 | 526 Gac (4) (His→Asp) |
| R5 | 46 | 531 tTg (46) (Ser→Leu) |
| WT/R2b | 1 | None |
| WT | 6 | 514 TTC ins.c (2), none (4) |
Capital letters in the codon indicate the change in nucleotide sequence. Values in parentheses are number of isolates.
Mixed culture (a wild-type [WT] plus an R2 strain).
ins., insertion.
DISCUSSION
Spontaneous mutations that lead to drug resistance occur rarely in M. tuberculosis, and multidrug regimens can prevent the emergence of clinical drug resistance. The problem of resistance results from treatment that is inadequate, often because of an irregular drug supply, inappropriate regimens, or poor compliance. Multidrug resistance appears to result from the stepwise acquisition of new mutations in the genes for different drug targets (13). Patients infected with strains resistant to multiple drugs are less likely to be cured, and their treatment is more toxic and more expensive than the treatment for patients infected with susceptible organisms (11, 19).
Rifampin resistance is observed less frequently than isoniazid and streptomycin resistance (23, 35). Resistance to rifampin, however, is usually associated with isoniazid resistance (13, 32). Therefore, these facts emphasize the need for fast and reliable methods for the detection of M. tuberculosis and the detection of its drug susceptibility both for optimal patient treatment and for control of the disease.
The nucleotide sequences of the rpoB genes of M. tuberculosis strains isolated mostly from Asian countries were analyzed. A total of 85 (94.5%) of 90 rifampin-resistant isolates had mutations in the hot-spot variable region of the gene. No mutations were observed within the 305-bp region for any of the rifampin-sensitive strains tested. These results indicate that sequencing of the DNA of the rpoB gene may result in the early detection of rifampin-resistant strains.
The most frequent mutations were Ser-531-Leu, Asp-516-Val, and His-526-Tyr. No significant differences in the distribution tendencies of the mutations were observed among the M. tuberculosis isolates from the different countries. These results are consistent with those of Telenti et al. (31), who analyzed M. tuberculosis strains isolated mostly in European and African countries, Williams et al. (33), who analyzed strains mostly isolated in the United States, and Suzuki et al. (28), who analyzed Japanese isolates (Table 4).
TABLE 4.
Frequency of mutations in rifampin-resistant isolates reported by four groupsa
| Mutation position | Frequency of codon substitution (no. [%] of isolates)a
|
|||
|---|---|---|---|---|
| Telenti et al. (n = 66) | Williams et al. (n = 110) | Suzuki et al. (n = 46) | This study (n = 90) | |
| Leu-511 | 2 (3.0) | 1 (1.1) | ||
| Gln-513 | 2 (3.0) | 1 (0.9) | 5 (5.6) | |
| 514-Phe ins.b | 1 (0.9) | 2 (2.2) | ||
| Met-515 | 1 (2.1) | |||
| Asp-516 | 6 (9.1) | 8 (7.3) | 4 (8.5) | 13 (14.4) |
| Asn-518 del.c | 1 (1.5) | 1 (0.9) | ||
| Leu-521 | 1 (0.9) | |||
| Ser-522 | 1 (1.5) | 2 (1.9) | 1 (2.1) | 1 (1.1) |
| His-526 | 18 (27.3) | 37 (33.6) | 13 (27.6) | 15 (16.7) |
| Ser-531 | 33 (50.0) | 46 (41.7) | 23 (48.9) | 48 (53.3) |
| Leu-533 | 1 (1.5) | 3 (2.7) | ||
| Ser-509, His-526d | 1 (0.9) | |||
| His-526, Lys-527d | 1 (0.9) | 1 (2.1) | ||
| None | 2 (3.0) | 8 (7.3) | 3 (6.4) | 5 (5.6) |
Five rifampin-resistant isolates (5.6%) contained no mutations within the 305-bp region of the rpoB gene. A similar tendency was reported by others (8, 24, 28, 31, 33). These results may imply a mutation in another part of the rpoB gene or the existence of at least one additional gene that participates in rifampin resistance.
INNO LiPA Rif TB based on the reverse hybridization principle is a kit that is used for the rapid identification of mutations in the rpoB gene and that is commercially available for research purposes (8, 24, 32). The susceptibility or resistance of 109 of 116 isolates that were rifampin sensitive and resistant in vitro was correctly identified with the LiPA Rif TB kit. The LiPA Rif TB kit failed to distinguish the TTC insertion mutation at position 514 from the wild-type sequence. This was not entirely unexpected since codon 514 is located close to the 3′ end of the S1 probe. This mutation occurs less frequently but is not rare; 2.5% among rifampin-resistant isolates in this study and 4% among isolates from New York City (8). Further studies for the improvement of the kit for the precise diagnosis of rifampin-resistant tuberculosis are needed.
In a laboratory that used the proportion method with Löwenstein-Jensen medium (6, 35, 36), resistance was defined as at least 1% colony growth at critical concentrations of the drugs (40 μg/ml for rifampin). One isolate that was resistant in vitro (isolate R2) had a line probe assay profile for a mixed culture: a wild-type and a resistant organism. On the other hand, sequencing analysis did not reveal mutations within the 305-bp region of the gene of the isolate. These discrepant results indicate that the DNA sequence of a more dominant population with a mixture of two strains may be analyzed by automated sequencing. Although the pattern may be indistinguishable from that for a wild-type strain for mixtures of two strains in which a mutant is not recognized by one of the R probes (ΔS) or strains with a ΔS and a wild-type profile, which were infrequent, the LiPA Rif TB would also have clinical usefulness for the detection of mutations in mixed cultures.
AUTO-LiPA is a fully automated test from hybridization to color development. Results of tests for susceptibility to rifampin were obtained within 2 h after the addition of amplicons to the tray of the AUTO-LiPA. In addition, the rate of concordance of the results of the line probe assay with the results of the in vitro susceptibility test was high (92.2%). Although the kit could not detect insertion mutations and in vitro resistant isolates with the wild-type sequence in the hot-spot region of the rpoB gene, it may be useful for the rapid diagnosis of rifampin-resistant tuberculosis.
ACKNOWLEDGMENTS
This work was partly supported by the Health Sciences Research Grants, Ministry of Health and Welfare of Japan (Research on Emerging and Re-emerging Infectious Diseases), and the Tuberculosis and Leprosy Panel, US-Japan Cooperative Medical Science Program.
REFERENCES
- 1.Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Sun Um K, Wilson T, Collins D, de Lisle G, Jacobs W R., Jr inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263:227–230. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- 2.Barnes P F, Bloch A B, Davidson P T, Snider D E., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 3.Bass J B, Jr, Farer L S, Hopewell P C, O’Brien R, Jacobs R F, Ruben F, Snider D E, Jr, Thornton G. Treatment of tuberculosis and tuberculosis infection in adults and children. Am J Respir Crit Care Med. 1994;149:1359–1374. doi: 10.1164/ajrccm.149.5.8173779. [DOI] [PubMed] [Google Scholar]
- 4.Belanger A E, Besra G S, Ford M E, Mikusova K, Belisle J T, Brennan P J, Inamine J M. The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc Natl Acad Sci USA. 1996;93:11919–11924. doi: 10.1073/pnas.93.21.11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cambau E, Sougakoff W, Besson M, Truffot-Pernot C, Grosset J, Jarlier V. Selection of a gyrA mutant of Mycobacterium tuberculosis resistant to fluoroquinolones during treatment with ofloxacin. J Infect Dis. 1994;170:479–483. doi: 10.1093/infdis/170.2.479. [DOI] [PubMed] [Google Scholar]
- 6.Canetti G. Present aspects of bacterial resistance in tuberculosis. Am Rev Respir Dis. 1965;92:687–703. doi: 10.1164/arrd.1965.92.5.687. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. Nosocomial transmission of multidrug-resistant tuberculosis among HIV-infected persons—Florida and New York, 1988–1991. Morbid Mortal Weekly Rep. 1991;40:585–591. [PubMed] [Google Scholar]
- 8.Cooksey R C, Morlock G P, Glickman S, Crawford J T. Evaluation of a line probe assay kit for characterization of rpoB mutations in rifampin-resistant Mycobacterium tuberculosis isolates from New York City. J Clin Microbiol. 1997;35:1281–1283. doi: 10.1128/jcm.35.5.1281-1283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dooley S W, Jarvis W R, Marlone W J, Snider D E. Multidrug-resistant tuberculosis. Ann Intern Med. 1992;117:257–259. doi: 10.7326/0003-4819-117-3-257. [DOI] [PubMed] [Google Scholar]
- 10.Finken M, Kirschner P, Meier A, Wrede A, Bottger E C. Molecular basis of streptomycin resistance in Mycobacterium tuberculosis alterations of the ribosomal protein S12 gene and point mutations within a functional 16S ribosomal RNA pseudoknot. Mol Microbiol. 1993;9:1239–1246. doi: 10.1111/j.1365-2958.1993.tb01253.x. [DOI] [PubMed] [Google Scholar]
- 11.Goble M, Iseman M D, Madsen L A, Waite D, Ackerson L, Horsburgh C R. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993;328:527–532. doi: 10.1056/NEJM199302253280802. [DOI] [PubMed] [Google Scholar]
- 12.Herrera D, Cano R, Godoy P, Peiro E F, Castell J, Ibanez C, Martinez Navarro F. Multidrug-resistant tuberculosis outbreak on an HIV ward—Madrid, Spain, 1991–1995. Morbid Mortal Weekly Rep. 1996;45:330–333. [PubMed] [Google Scholar]
- 13.Heym B, Honore N, Truffot-Pernot C, Banerjee A, Schurra C, Jacobs W R, Jr, van Embden J D A, Grosset J H, Cole S T. Implications of multidrug resistance for the future of short-course chemotherapy of tuberculosis: a molecular study. Lancet. 1994;344:293–298. doi: 10.1016/s0140-6736(94)91338-2. [DOI] [PubMed] [Google Scholar]
- 14.Hirano K, Takahashi M, Kazumi Y, Fukasawa Y, Abe C. Mutation in pncA is a major mechanism of pyrazinamide resistance in Mycobacterium tuberculosis. Tubercle Lung Dis. 1998;78:117–122. doi: 10.1016/s0962-8479(98)80004-x. [DOI] [PubMed] [Google Scholar]
- 15.Jin D J, Gross C A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988;202:45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- 16.Jin D J, Gross C A. Characterization of the pleiotrophic phenotypes of rifampin-resistant rpoB mutants of Escherichia coli. J Bacteriol. 1989;171:5229–5231. doi: 10.1128/jb.171.9.5229-5231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsukawa C, Tamaru A, Miyata Y, Abe C, Makino M, Suzuki Y. Characterization of the rpsL and rrs genes of streptomycin-resistant clinical isolates of Mycobacterium tuberculosis in Japan. J Appl Microbiol. 1997;83:634–640. doi: 10.1046/j.1365-2672.1997.00279.x. [DOI] [PubMed] [Google Scholar]
- 18.Konno K, Feldmann F M, McDermott W. Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am Rev Respir Dis. 1967;95:461–469. doi: 10.1164/arrd.1967.95.3.461. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoudi A, Iseman M D. Pitfalls in the care of patients with tuberculosis. JAMA. 1993;270:65–68. [PubMed] [Google Scholar]
- 20.McClatchy J K, Tsang A Y, Cernich M S. Use of pyrazinamidase activity in Mycobacterium tuberculosis as a rapid method for determination of pyrazinamide susceptibility. Antimicrob Agents Chemother. 1981;20:556–557. doi: 10.1128/aac.20.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monno L, Angarano G, Carbonara S, Coppola S, Costa D, Quarto M, Pastore G. Emergence of drug-resistant Mycobacterium tuberculosis in HIV-infected patients. Lancet. 1991;337:852. doi: 10.1016/0140-6736(91)92559-k. [DOI] [PubMed] [Google Scholar]
- 22.Nair J, Rouse D A, Bai G-H, Morris S L. The rpsL gene and streptomycin resistance in single and multiple drug-resistant strains of Mycobacterium tuberculosis. Mol Microbiol. 1993;10:521–527. doi: 10.1111/j.1365-2958.1993.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 23.Pablos-Mendez A, Raviglione M C, Laszlo A, Binkin N, Rieder H L, Bustreo F, Cohn D L, Lambregts-van Weezenbeek C S B, Kim S J, Chaulet P, Nunn P. Global surveillance for antituberculosis-drug resistance, 1994–1997. N Engl J Med. 1998;338:1641–1649. doi: 10.1056/NEJM199806043382301. [DOI] [PubMed] [Google Scholar]
- 24.Rossau R, Traore H, de Beenhouwer H, Mijs W, Jannes G, de Rijk P, Portaels F. Evaluation of the INNO-LiPA Rif.TB assay, a reverse hybridization assay for the simultaneous detection of Mycobacterium tuberculosis complex and its resistance to rifampin. Antimicrob Agents Chemother. 1997;41:2093–2098. doi: 10.1128/aac.41.10.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scorpio A, Zhang Y. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat Med. 1996;2:662–667. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- 26.Snider D E, Roper W L. The new tuberculosis. N Engl J Med. 1992;326:703–705. doi: 10.1056/NEJM199203053261011. [DOI] [PubMed] [Google Scholar]
- 27.Sreevatsan S, Stochbauer K E, Pan X, Kreiswirth B N, Moghazen S L, Jacobs W R, Jr, Telenti A, Musser J M. Ethambutol resistance in Mycobacterium tuberculosis: critical role of embB mutations. Antimicrob Agents Chemother. 1997;41:1677–1681. doi: 10.1128/aac.41.8.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki Y, Katsukawa C, Inoue K, Yin Y P, Tasaka H, Ueda N, Makino M. Mutations in rpoB gene of rifampicin resistant clinical isolates of Mycobacterium tuberculosis in Japan. J Jpn Assoc Infect Dis. 1995;69:413–419. doi: 10.11150/kansenshogakuzasshi1970.69.413. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki Y, Katsukawa C, Tamaru A, Abe C, Makino M, Mizuguchi Y, Taniguchi H. Detection of kanamycin-resistant Mycobacterium tuberculosis by identifying mutations in the 16S rRNA gene. J Clin Microbiol. 1998;36:1220–1225. doi: 10.1128/jcm.36.5.1220-1225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taniguchi H, Chang B, Abe C, Nikaido Y, Mizuguchi Y, Yoshida S. Molecular analysis of kanamycin and viomycin resistance in Mycobacterium smegmatis by use of the conjugation system. J Bacteriol. 1997;179:4795–4801. doi: 10.1128/jb.179.15.4795-4801.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S T, Colston M J, Matter L, Schoolfer K, Bodmer T. Detection of rifampicin-resistant mutations in Mycobacterium tuberculosis. Lancet. 1993;341:647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- 32.Watterson S A, Wilson S M, Yates M D, Drobniewski F A. Comparison of three molecular assays for rapid detection of rifampin resistance in Mycobacterium tuberculosis. J Clin Microbiol. 1998;36:1969–1973. doi: 10.1128/jcm.36.7.1969-1973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams D L, Waguespack C, Eisenach K, Crawford J T, Portaels F, Salfinger M, Nolan C M, Abe C, Sticht-Groh V, Gillis T P. Characterization of rifampin resistance in pathogenic mycobacteria. Antimicrob Agents Chemother. 1994;38:2380–2386. doi: 10.1128/aac.38.10.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Tuberculosis programme: framework for effective tuberculosis control. Publication no. WHO/TB/94.179. Geneva, Switzerland: World Health Organization; 1994. [Google Scholar]
- 35.World Health Organization/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Anti-tuberculosis drug resistance in the world. Publication no. WHO/TB/97.229. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 36.World Health Organization/IUATLD Global Working Group on Antituberculosis Drug Resistance Surveillance. Guidelines for surveillance of drug resistance in tuberculosis. Publication no. WHO/TB/96.216. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 37.Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]