Abstract
Early life experiences, including separation from caregivers, can result in substantial, persistent effects on neural, behavioral, and physiological systems as is evidenced in a long-standing literature and consistent findings across species, populations, and experimental models. In humans and other animals, differential rearing conditions can affect brain structure and function. We tested for whole brain patterns of morphological difference between 108 chimpanzees reared typically with their mothers (MR; N=54) and those reared decades ago in a nursery with peers, human caregivers, and environmental enrichment (NR; N=54). We applied support vector machine (SVM) learning to archival MRI images of chimpanzee brains to test whether we could, with any degree of significant probability, retrospectively classify subjects as MR and NR based on variation in gray matter within the entire brain. We could accurately discriminate MR and NR chimpanzee brains with nearly 70% accuracy. The combined brain regions discriminating the two rearing groups were widespread throughout the cortex. We believe this is the first report using machine language learning as an analytic method for discriminating nonhuman primate brains based on early rearing experiences. In this sense, the approach and findings are novel, and we hope they stimulate application of the technique to studies on neural outcomes associated with early experiences. The findings underscore the potential for infant separation from caregivers to leave a long-term mark on the developing brain.
Keywords: primate, brain, MRI, cortical, adversity, experience
Introduction
Early life environments have substantial effects on many neural, behavioral, and physiological systems as is evidenced in a long-standing literature that reports findings across species, populations, and experimental models(Brett, Humphreys, Fleming, Kraemer, & Drury, 2015). The effects of childhood maltreatment, neglect, and impoverishment are significant, and often—but not always— include profoundly deleterious health outcomes across the lifespan. Understanding the range, persistence, and plasticity of the effects of early life adversity is important for many reasons. Among them, such basic knowledge provides the empirical basis for assessment of the long-term consequences of decisions about the treatment and care of infants and children. Further, evidence about both the persistence of, and potential to reverse, biologically embedded effects of early life adversity can inform practices and policy to avert harm or to develop effective intervention strategies in response adversity. At the same time, identifying how different aspects of the early social and physical environment affect the brain is critically important to constructing a full account of typical neurobehavioral development.
Studies of both humans and of other animals are important to uncovering how life experiences affect acute and long-term brain development. In nonhuman animals, the manipulation of rearing experiences allows for a controlled, experimental approach that can help to disentangle confounding factors that are unavoidable in human studies. For instance, individuals differ not only in early rearing experiences but also diet, healthcare, physical environment, access to resources, and a myriad of social influences—all of which may affect health and neurobehavioral development. By contrast, nonhuman animal studies can be used to isolate the effects of specific experiences and provide more precise identification of environmental and experiential factors that may affect development.
One experimental comparison in nonhuman primates is between individuals reared typically, by their conspecific mothers compared to those reared atypically. For a variety of reasons, some captive born nonhuman primates are raised in a human nursery setting with same-aged peers. Some key features of the nonhuman primate nursery resemble what is defined as institutional care for human infants (Novak, Meyer, Lutz, & Tiefenbacher, 2006; Zeanah et al., 2003). Infants raised in institutionalized settings often lack a primary conspecific adult attachment figure, and instead are raised with age-mate peers, human caregivers, and various environmental enrichment. The results of several decades of studies in monkeys and apes have demonstrated robust consequences of disrupted attachment relationships during infancy and childhood on behavioral and, to a lesser extent, brain development (Bennett & Pierre, 2010; Dettmer & Suomi, 2014; J.A. French & S.B. Carp, 2016; Lyons, Parker, & Schatzberg, 2010; Machado & Bachevalier, 2003; Nelson & Winslow, 2009). The effects often parallel those observed in human children with adverse experiences early in life, although there are many gaps in knowledge.
In both human and nonhuman primates, brain morphology is also affected by early social rearing(Bick & Nelson, 2016; Jeffrey A French & Sarah B Carp, 2016), with differences in the volume of structures that include those involved in socioemotional processing and cognition. For the most part, however, previous studies of the neural consequences of early life experiences in humans and other primates have centered on relatively molar measures. For instance, there is evidence of group differences in three types of measures: volume of major brain regions including gray and white matter volume or their ratio, but to a lesser extent—patterns of cortical folding (Bick & Nelson, 2016; Teicher et al., 2003). In nursery-reared monkeys, there are reports of decreased corpus callosum volume, smaller cerebellum, decreased dendritic branching in motor and somatosensory regions, decreased dorsal cingulate and dorsal medial prefrontal cortical volume (Bennett & Pierre, 2010; Spinelli et al., 2009). There are very few studies of chimpanzees, but there is evidence that nursery-reared chimpanzees have lower white matter volume and exhibit differences in sulci morphology compared to mother-reared conspecifics. Specifically, nursery- and mother-reared chimpanzees differ in gray matter thickness and the depth of a number of sulci, particularly in the primary and secondary motor and somatosensory cortex(Bogart, Bennett, Schapiro, Reamer, & Hopkins, 2014). A more recent study in MR and NR chimpanzee reported significant differences in structural covariation in gray matter within the basal forebrain (Bard & Hopkins, 2018).
At the same time, chimpanzees continue to live in captive settings such as sanctuaries and zoos in the US and elsewhere. Chimpanzees and other primates continue to be bred in captivity with the justification that expanding and maintaining the populations serves educational, conservation, and species-survival goals. Given that these animals must be cared for in a manner to promote their wellbeing and long-term health, scientific knowledge about the immediate and long-term consequences of different rearing and infant care conditions can be important to inform their care and management. Behavioral studies show that individuals who are reared in a nursery or other atypical, non-maternal situations early in life, are at higher risk of atypical behavior and behavioral pathology later in life (Freeman, Weiss, & Ross, 2016; Jacobson, Ross, & Bloomsmith, 2016; Nash, Fritz, Alford, & Brent, 1999; Walsh, Bramblett, & Alford, 1982), there is a paucity of information about neural consequences in these populations.
Previous findings illustrate significant early rearing influences on brain morphology that are often parallel in human and nonhuman primates, but several crucial gaps in knowledge remain. Among these gaps is a more fine-grained—or molecular—description of rearing influences on overall organization and connectivity in the adult brain for nonhuman primates. Much of the previous monkey and ape research has focused on directly translational questions, with hypotheses drawn from findings in human children with early life adverse experiences. As a result, the organizational framework in which the findings are placed has largely been a “deficit model”, in which nursery-reared animals are expected to show negative consequences parallel to those of humans. In the translational model, the focus of hypotheses and interpretation of findings is typically on systems involved in affective, socioemotional, cognitive, and behavioral domains related to the robust deleterious clinical and health consequences associated with early life adversity. An alternative approach is to instead begin with an overall comparison of individuals who differ in early life experiences. In this case, the goal is to identify whether, and where, the brains of individuals in the two groups systematically differ. If the results of such whole brain analysis demonstrate orderly patterns of rearing group differences, they can provide valuable information that addresses a number of broad questions beyond whether there is convergence between human and nonhuman primates with respect to deficits following disruption of early attachment relationships.
The goal of the current paper was to determine if we could significantly and reliably discriminate between differentially reared chimpanzees scanned more than 15 years after being raised in these conditions. Specifically, beginning in the 1980’s, the National Institutes of Health (NIH) initiated a breeding program of captive chimpanzees to increase the number of available apes for potential future research in a variety of biomedical applications. During the duration of the breeding program although many of the female chimpanzees exhibited normal, species-typical maternal care of their offspring (herein mother-reared, MR), some neglected, or failed to exhibit adequate care, of their newborns. In these cases, the infants were separated from their social groups and were raised in a human nursery setting with same-aged peers and human caregivers (herein nursery-reared, NR), much like human children raised in institutionalized settings. We emphasize here that the infants were not separated from their mothers for the purposes of this study; rather, we took advantage of this unique and serendipitous rearing and management circumstance to test whether the effects of rearing on brain morphology persisted into adulthood.
Rather than use traditional inferential statistics, we used support vector machine (SVM) learning to test whether we could, with any degree of significant probability, retrospectively classify subjects as MR and NR based on voxel variation in gray matter within the entire brain. Gray matter voxel values were combined in a kernel-based approach, commonly applied to pattern recognition of large sample sizes (LaConte, Strother, Cherkssky, Anderson, & Hu, 2005), and binary SVM learning, when attempting to classify two groups (i.e., binary classes)(Orru, Pettersson-Yeo, Marquand, Sartori, & Mechelli, 2012). Here we sought to identify those combinations of voxels within the entirety of the brain that could significantly, and reliably, discriminate between MR and NR chimpanzees scanned more than 15 years after these rearing experiences.
Methods and Materials
Subjects
The sample was comprised of the archival magnetic resonance image (MRI) scans of 108 chimpanzees, housed at either housed at either the Yerkes National Primate Research Center (YNPRC; 29 MR, 29 NR), or the National Center for Chimpanzee Care (NCCC) or at the University of Texas MD Anderson Cancer Center (25 MR, 25 NR). In total there were 54 mother-reared (MR) and 54 nursery-reared (NR) chimpanzees matched on age, sex, and scanner magnet strength (1.5T or 3T). Ages at the time of their MRI scans ranged from 11 to 43 years with the mean age for MR and NR chimpanzees being 21.76 (SD = 6.87) and 23.39 (SD = 7.01) years, respectively.
We defined a nursery-reared chimpanzee as an individual that was separated from his or her mother within the first 30 days life due to unresponsive care, injury, or illness (Bard, 1994; Bard, Platzman, Lester, & Suomi, 1992). NR chimpanzees were placed in incubators, fed standard human infant formula (not supplemented with DHA to our knowledge), and cared for by humans until they could sufficiently care for themselves, at which time they were placed with other infants of the same age until they were three years of age (Bard, 1994; Bard et al., 1992). At three years of age, the nursery-reared chimpanzees were integrated into larger social groups of adult and sub-adult chimpanzees. It should be noted that a subset of NR chimpanzees participated in some type of early social stimulation program was designed to minimize the negative effect of this rearing practice on behavioral development. For the YNPRC chimpanzees, this early intervention was referred to as responsive care (RC) and included 4 hours a day, 5 days a week of extra social stimulation from human adults and same -age peers (Bard, 1996; Mason & Capitanio, 1988). In contrast, for NCCC chimpanzees, as has been in done with NR monkeys (Thompson, Bloomsmith, & Taylor, 1991), dogs were used as companion animals with the nursery-reared individuals. During the day, the NR chimpanzees raised at the NCCC were housed together in a large play pen. To facilitate social stimulation and interactions, between 6 to 8 hours each day, an adult. companion dog with a gentle temperament was housed with the chimpanzees. Mother-reared chimpanzees were not separated from their mother during at least the first 2.5 years of life and were raised in ‘nuclear’ family groups of conspecifics, with group sizes ranging from 4 to 20 individuals.
Magnetic Resonance Image Collection
All chimpanzees were scanned while sedated for one of their annual physical examinations that are performed for routine healthcare by veterinary staff. MRI scans followed standard procedures at the YNPRC and NCCC and were designed to minimize stress. Thus, the animals were first sedated with ketamine (10 mg/kg) or telazol (3–5mg/kg) and were subsequently anaesthetized with propofol (40–60 mg/(kg/h)). They were then transported to the MRI scanning facility and placed in a supine position in the scanner with their head in a human-head coil. Upon completion of the MRI, chimpanzees were briefly singly housed for 2–24 hours to permit close monitoring and safe recovery from the anesthesia prior to returning to their social group. All procedures were approved by the Institutional Animal Care and Use Committees at YNPRC and NCCC. Forty chimpanzees were scanned using a 3.0 Tesla scanner (Siemens Trio, Siemens Medical Solutions USA, Inc., Malvern, Pennsylvania, USA). T1-weighted images were collected using a three-dimensional gradient echo sequence (pulse repetition = 2300 ms, inversion time = 1100 ms, echo time = 4.4 ms, number of signals averaged = 3, matrix size = 320 × 320, with slice thickness = 0.6 mm). The remaining 72 chimpanzees were scanned using a 1.5T GE echo-speed Horizon LX MR scanner (GE Medical Systems, Milwaukee, WI). T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, number of signals averaged = 8, matrix size = 256 × 256, with 0.7 × 0.7 × 1.2 resolution). Two different scanners were used to produce the archival MRI data, thus for the purpose of this study the MR and NR chimpanzees were matched on the scanner and image acquisition protocol so as to control for this potential confound when comparing the MR and NR groups.
Post-Image Processing
Consistent with previous studies with chimpanzees, prior to the machine language learning analysis, the MRI scans were imported and resampled at .625 mm isotropic voxels, aligned in the AC-PC axis, skull-stripped and processed though the FSL VBM pipeline procedures as described elsewhere (Bianchi, Reyes, Hopkins, Taglialatela, & Sherwood, 2016) (fsl.fmrib.ox.ac.uk/fsl/fslwiki/fslvbm/userguide). This included the initial segmentation of the T1-weighted scan into gray and white matter, registration to a chimpanzee standard template (W.D. Hopkins & Avants, 2013) and creation of a study-specific template brain. Each segmented gray matter volume was then linearly and subsequent non-linear registered to the study-specific template brain. To compensate for the expansion or enlargement due to the non-linear component of the spatial transformation, the non-linear registered brains were multiplied by the Jacobian warping field and appended to create a 4-D modulated gray matter volume. The images were then smoothed with an isotropic Gaussian kernel (sigma=2 mm, FHWM = ~ 4.7 mm).
Machine Language Learning Classification Methods
Kernel-based SVM was performed using customized Matlab® scripts and standard protocols of the Pattern Recognition for Neuroimaging Toolbox 2 (PRoNTo 2) for Matlab® (Schrouff et al., 2013). First, the individual modulated gray matter volumes derived from the FSL preprocessing steps were placed into separate directories corresponding to the MR and NR groups. We then performed a SVM analysis using the whole brain gray matter mask to determine the accuracy in group classification based on information provided from all gray matter voxels in the brain. Each SVM analysis included one volume of brain data per subject in a one-fold, leave one subject per group out, cross-validation scheme. Within this scheme, iterative cross-validation folds relied on 50% of the data for training and 50% of the left-out data for testing. For each analysis, the omnibus SVM model was generated by averaging model parameters from each cross-validation, and model accuracy was calculated as follows:
where TC and FC are the number of true classifications and false classifications, respectively, considering the classification outcome of each cross-validation fold. An omnibus SVM model was considered significant if it outperformed identically designed models, each generated from randomized group (i.e., class) assignment more than 950 out of 1000 times (e.g., p<.05). For SVM models that significantly distinguished between MR and NR groups, model weights were obtained and displayed in standardized chimpanzee template space (W.D. Hopkins & Avants, 2013).
To further examine the specific regions which contribute to the accurate classification of MR and NR chimpanzees, we followed the overall SVM analysis with region-specific analyses. To do so, we utilized eight gray matter components that differed between mother-, nursery-, and responsive care program-reared chimpanzees in a previous source-based morphometry study (Bard & Hopkins, 2018). We created masks of each component, masked the gray matter volumes for each of our subjects (once for each component), and subsequently ran eight separate SVM analyses to determine if gray matter variation in these particular components/regions could be used to accurately classify mother- and nursery-reared chimpanzees in our sample.
Results
With subjects matched on sex, age at scan, and colony/scanner magnet, an overall significant proportion of chimpanzee brains were correctly classified as MR and NR (Percent correct = 68.52%, p < .01, after 1000 permutation tests) by SVM analysis. Moreover, the proportion of correctly classified chimpanzee brains was significant for both MR (Percent correct = 72%, p < .001) and NR (Percent correct = 66%, p < .001) subjects. Thus, misclassification of NR or MR subjects was not significantly skewed or overrepresented in one group. The weight map for each voxel for the whole brain analysis is shown in Figure 1. As can be seen, the brain regions that contributed greatest to the decision function were widespread and throughout the cortex. The regions contributing the most to the decision function (at the extreme ends of the color spectrum) were superior temporal cortex, anterior and posterior parietal region, prefrontal cortex (left hemisphere), superior frontal gyrus (right hemisphere), cingulate cortex and portions of the cerebellum.
Figure 1:
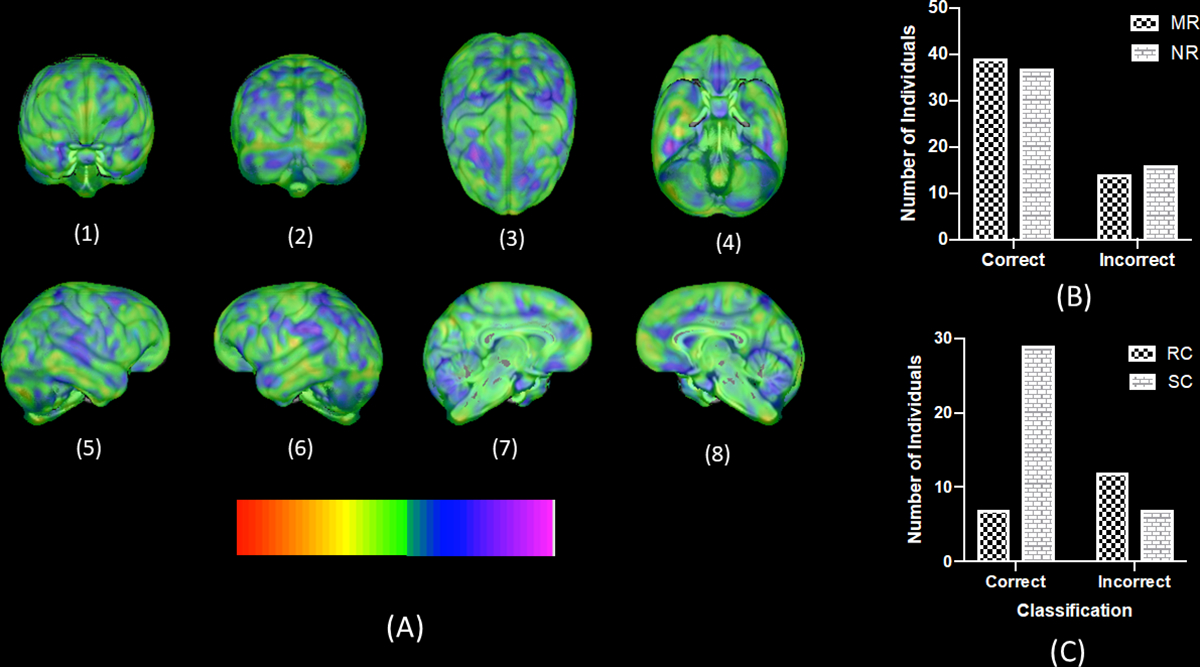
(A) 3D rendering of voxel intensity map indicating the weighted contributions of whole brain gray matter voxels to the decision function of NR and MR chimpanzees. Color bar indicates the intensity values with colors at the extreme ends reflecting increased contribution to the classification function. (B) Number of MR and NR chimpanzees correctly and incorrectly classified based on the SVM algorithm (C) Number of standard nursery-reared chimpanzees (SR) and those that received some early social stimulation (RC) classified correctly or incorrectly as nursery-reared.
We also considered what factors may or may not have contributed to the misclassification of MR and NR chimpanzees. We identified the individual chimpanzee brains that were misclassified and failed to find a significant association between classification success (yes, no) and either sex X2 (1,112) =0.86, p=0.268, scanner magnet X2 (1,112) =0.87, p=0.351 or rearing history X2(1,112) =1.09, p=0.296. However, upon further inspection of the early rearing histories of NR chimpanzees, we found a significant association between their classification success and whether they received any early social intervention experiences X2 (1,56) =6.94, p=0.008. Specifically, 12 of the 19 NR chimpanzees that were misclassified as MR, received some form of social stimulation intervention early in life. In contrast, of the 37 correctly classified NR chimpanzees, only 7 were individuals that received this same early social intervention compared to 29 that had not. In short, the SVM algorithm disproportionally classified those NR chimpanzees that received some type of social intervention program as MR compared to chimpanzees that did not received the intervention.
For our region-specific SVM analyses, we found that gray matter variation in a single component (Component 8, comprised of the parietal cortex, precuneus, and pre- and post-central cortex; Bard & Hopkins, 2018) could be used to classify mother- and nursery-reared chimpanzees with 66.67% accuracy (p =.001; after 1000 permutations; see Figure 2). The proportion of correctly classified chimpanzee brains was significant for both MR (percent correct = 68.52%; p = 0.003), and NR (percent correct = 64.81%; p = 0.016) subjects. No other regional components were able to accurately classify a significant proportion of MR and NR brains (p > 0.05).
Figure 2:
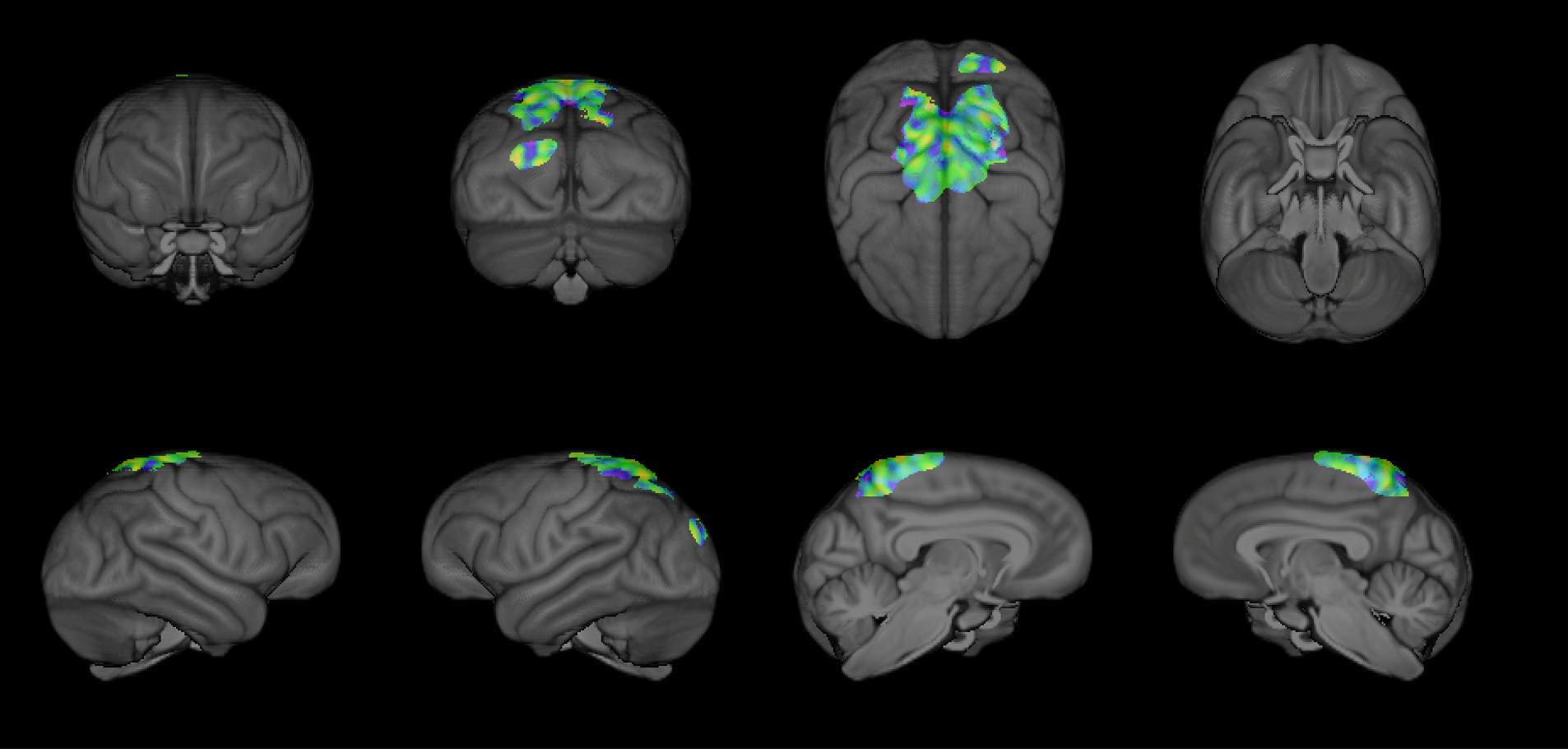
3D rendering of the voxel intensity map indicating the weighted contributions of the regional gray matter voxels (within component 8 from Bard & Hopkins, 2018) used to accurately classify MR and NR chimpanzees.
Discussion
To our knowledge, the results presented here are the first demonstration that the persistent effects of infant rearing on nonhuman primate brain morphology can be detected with high accuracy using machine learning. The results of this study are straightforward. Using SVM learning applied to smoothed modulated gray matter volumes, we were able to accurately discriminate MR and NR chimpanzee brains with nearly 70% accuracy. The combined brain regions discriminating the two rearing groups were widespread throughout the cortex and persisted into adulthood. Follow-up analyses using regions of interest, found that MR and NR chimpanzee brains could be accurately classified (67%) by gray matter variation in the parietal cortex, precuneus, and pre- and post-central cortex. Bard and Hopkins (2018) previously found that NR chimpanzees had significantly lower gray matter covariation scores in these regions compared to both MR and responsive-care program chimpanzees. However, none of the other source-based morphometry components which differed across rearing condition in the previous study could be used to accurately classify MR and NR chimpanzees via machine learning.
We also found that the SVM algorithm misclassified a significantly higher proportion of NR chimpanzees as MR if they received some type of social intervention program early in life. The misclassification of the NR chimpanzees that received either form of early social stimulation is of interest for at least two reasons. First, these findings suggest that the SVM algorithm essentially generated a third group of chimpanzees, who had somewhat similar rearing histories, and did not resemble the remaining MR and NR apes. Second, at least for the YNPRC chimpanzees, previous work demonstrates that NR chimpanzees differ in gray matter variation within the basal forebrain compared to MR and responsive-care (RC) chimpanzees, who did not differ significantly from each other (Bard & Hopkins, 2018). These findings are consistent with the results reported here in that the RC chimpanzees represent a distinct group from the NR individuals. Importantly, based on these collective results, it appears that early socialization and stimulation, broadly speaking, enhances a more typical pattern of brain development in NR chimpanzees.
Although federal policy in the US impedes many forms of scientific research with living chimpanzees, including MRI, the results of this study underscore the value of archival analysis to better understand how the animals’ care conditions can affect their long-term development. Indeed, previous studies have reported that lesions and tumors have been identified during MRI scans obtained for scientific purposes and, in turn, the clinically-relevant information has resulted in changes in housing and enrichment activities for the affected individuals (Hopkins & Latzman, 2017). Furthermore, elderly chimpanzees express both neurofibrillary tangles and amyloid plaques, the two neuropathological markers of Alzheimer’s disease in humans (Edler et al., 2017; Rosen et al., 2008), the onset of which could be potentially predicted from neuroimaging data. Thus, the continued collection of MRI scans of research-retired chimpanzees could be a useful way to assist in the responsible management and care of captive chimpanzee populations.
In summary, the demonstration of persistent, long-term changes in gray matter evidenced by comparison of MR and NR individuals in such a closely related species are truly unique and has profound implications for our understanding of human brain development. There are, worldwide, an estimated more than 150 million children currently orphaned and another 2.7 million currently in institutionalized care (Petrowski, Cappa, & Gross, 2017). Our findings add to a long-standing and robust literature that provides evidence of persistent consequences of these experiences to permanently, and possibly irreversibly, alter their brains in ways that can affect social, emotional, and cognitive health and development. Our findings are also consistent with a recent report using machine learning to discriminate between the brain structure of humans with and without childhood experience of maltreatment (Price, Albaugh, Hahn, Juliano, Fani, Brier, et al., 2021). Finally, our results and those of others (Simpson et al., 2019), suggest that early social stimulation either through cross-fostering or other intervention efforts may provide a buffer against the negative and deleterious consequences of traditional nursery rearing practices on the brain and behavior.
Highlights.
Machine language learning was applied to archival neuroimages from 108 chimpanzees who were either reared typically with their mothers or, decades ago, in a nursery.
Mother-reared and nursery-reared chimpanzee brains were discriminated with nearly 70% accuracy. The combined brain regions discriminating the groups were widespread throughout the cortex.
Social intervention in infancy was significantly associated with cases of misclassification of nursery-reared chimpanzees by the machine language learning analysis.
The findings underscore the potential for infant separation from caregivers to leave a long-term mark on the developing brain and persistent effects of early intervention.
Acknowledgements:
This research was supported in part by NIH grants NS-42867, NS-73134, HD-60563, and NSF INSPIRE grant 1542848. The NCCC chimpanzees are supported by Cooperative Agreement U42-OD011197.The Yerkes Center and NCCC are fully accredited by the AAALAC International. American Psychological Association guidelines for the ethical treatment of animals were adhered to during all aspects of this study. W.D.H. collected neuroimaging data from chimpanzees, with assistance from S.J.S. and M.C.M. M.M.M. processed MRI images. M.J.W. designed and performed the machine learning analysis. W.D.H. performed all other statistical analyses. A.J.B, P.J.P., R.L., C.C.S., B.J.B., and W.D.H. designed the study, interpreted the results, and wrote the paper.
Data availability statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Financial Disclosures and Conflicts of Interest: The authors reported no biomedical financial interests or potential conflicts of interest.
References
- Bard KA (1994). Evolutionary roots of intuitive parenting: Maternal competence in chimpanzees. Early Development and Parenting, 3(1), 19–28. doi: 10.1002/edp.2430030104 [DOI] [Google Scholar]
- Bard KA (1996). Responsive care: behavioral intervention for nursery reared chimpanzees. Ridgefield, CT: Jane Goodall Institute. [Google Scholar]
- Bard KA, & Hopkins WD (2018). Early Socioemotional Intervention Mediates Long-Term Effects of Atypical Rearing on Structural Covariation in Gray Matter in Adult Chimpanzees. Psychological Science, 29(4), 594–603. doi: 10.1177/0956797617740685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard KA, Platzman KA, Lester BM, & Suomi SJ (1992). Orientation to social and nonsocial stimuli in neonatal chimpanzees and humans. Infant Behavior and Development, 15(1), 43–56. doi: 10.1016/0163-6383(92)90005-q [DOI] [Google Scholar]
- Bennett AJ, & Pierre PJ (2010). Nonhuman primate research contributions to understanding genetic and environmental influences on phenotypic outcomes across development. In Hood KE, Halpern CT, Greenberg G, & Lerner RM (Eds.), Handbook of Developmental Science, Behavior, and Genetics (pp. 353–359). Oxford, UK: John Wiley & Sons. [Google Scholar]
- Bianchi S, Reyes LD, Hopkins WD, Taglialatela JP, & Sherwood CC (2016). Neocortical grey matter distribution underlying voluntary, flexible vocalizations in chimpanzee. Scientific Reports, 6, 34733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, & Nelson CA (2016). Early adverse experiences and the developing brain. Neuropsychopharmacology, 41(1), 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogart SL, Bennett AJ, Schapiro SJ, Reamer LA, & Hopkins WD (2014). Different early rearing experiences have long term effects on cortical organziation in captive chimpanzees (Pan troglodytes) Developmental Science, 17(2), 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett ZH, Humphreys KL, Fleming AS, Kraemer GW, & Drury SS (2015). Using cross-species comparisons and a neurobiological framework to understand early social deprivation effects on behavioral development. Developmental Psychopathology, 27(2), 347–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, & Suomi SJ (2014). Nonhuman primate models of neuropsychiatric disorders: Influences of early rearing, genetics and epigenetics. ILAR journal, 55(2), 361–371. [DOI] [PubMed] [Google Scholar]
- Edler MK, Sherwood CC, Meindl RS, Hopkins WD, Ely JJ, Erwin JM, … Raghanti MA (2017). Aged chimpanzees exhibit pathologic hallmarks of Alzheimer’s disease. Neurobiol Aging, 59, 107–120. doi: 10.1016/j.neurobiolaging.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman HD, Weiss A, & Ross SR (2016). Atypical early histories predict lower extraversion in captive chimpanzees. Developmental psychobiology, 58(4), 519–527. doi: 10.1002/dev.21395 [DOI] [PubMed] [Google Scholar]
- French JA, & Carp SB (2016). Early-life social adversity and developmental processes in nonhuman primates. Current Opinion in Behavioral Science, 7, 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, & Carp SB (2016). Early-life social adversity and developmental processes in nonhuman primates. Current opinion in behavioral sciences, 7, 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, & Avants BB (2013). Regional and hemispheric variation in cortical thickness in chimpanzees (Pan troglodytes). Journal of Neuroscience, 33, 5241–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, & Latzman RD (2017). Future research with captive chimpanzees in the USA: Integrating scientific program with behavioral management. In Schapiro SJ (Ed.), Handbook of Primate Behavioral Management (pp. 445–470). Boca Raton, FL: Taylor and Francis Group. [Google Scholar]
- Jacobson SL, Ross SR, & Bloomsmith MA (2016). Characterizing abnormal behavior in a large population of zoo-housed chimpanzees: Prevalence and potential influencing factors. PeerJ, 4, e2225. doi: 10.7717/peerj.2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaConte S, Strother S, Cherkssky V, Anderson J, & Hu X (2005). Support vector machines for temporal classification of block design fMRI data. NeuroImage, 26(2), 317–329. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, & Schatzberg AF (2010). Animal models of early life stress: implications for understanding resilience. Dev Psychobiol, 52(7), 616–624. doi: 10.1002/dev.20500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, & Bachevalier J (2003). Non-human primate models of childhood psychopathology: the promise and the limitations. J Child Psychol Psychiatry, 44(1), 64–87. [DOI] [PubMed] [Google Scholar]
- Mason WA, & Capitanio JP (1988). Formation and expression of filial attachment in rhesus monkeys raised with living and inanimate mother substitutes. Developmental Psychobiology, 21(5), 401–430. [DOI] [PubMed] [Google Scholar]
- Nash LT, Fritz J, Alford P, & Brent L (1999). Variables influencing the origins of diverse abnormal behaviors in a large sample of captive chimpanzees (Pan troglodytes). American Journal of Primatology, 48, 15–29. [DOI] [PubMed] [Google Scholar]
- Nelson EE, & Winslow JT (2009). Non-human primates: model animals for developmental psychopathology. Neuropsychopharmacology, 34(1), 90–105. doi: 10.1038/npp.2008.150 [DOI] [PubMed] [Google Scholar]
- Novak MA, Meyer JS, Lutz C, & Tiefenbacher S (2006). Deprived Environments: Developmental Insights from Primatology. [Google Scholar]
- Orru G, Pettersson-Yeo W, Marquand AF, Sartori G, & Mechelli A (2012). Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: A critical review. Neurosci Biobehav Rev, 36(4), 1140–1152. doi: 10.1016/j.neubiorev.2012.01.004 [DOI] [PubMed] [Google Scholar]
- Petrowski N, Cappa C, & Gross P (2017). Estimating the number of children in formal alternative care: Challenges and results. Child Abuse Negl, 70, 388–398. doi: 10.1016/j.chiabu.2016.11.026 [DOI] [PubMed] [Google Scholar]
- Price M, Albaugh M, Hahn S, Juliano AC, Fani N, Brier Z, Legrand AC, van Stolk-Cooke K, Chaarani B, Potter A, Peck K, Allgaier N, Banaschewski T, Bokde A, Quinlan EB, Desrivières S, Flor H, Grigis A, Gowland P, Heinz A, … Garavan H (2021). Examination of the association between exposure to childhood maltreatment and brain structure in young adults: a machine learning analysis. Neuropsychopharmacology. Advance online publication. 10.1038/s41386-021-00987-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Farberg AS, Gearing M, Dooyema J, Long PM, Anderson DC, … Walker LC (2008). Tauopathy with paired helical filaments in an aged chimpanzee Journal of Comparative Neurology, 509, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrouff J, Rosa MJ, Rondina M, Marquand AF, Chu C, Ashburner J, … Moutao-Miranda J (2013). PRoNTo: pattern recognition for neuroimaging toolbox. Neuroinformatics, 11(3), 319–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Sclafani V, Paukner A, Kaburu SSK, Suomi SJ, & Ferrari PF (2019). Handling newborn monkeys alters later exploratory, cognitive, and social behaviors. Dev Cogn Neurosci, 35, 12–19. doi: 10.1016/j.dcn.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, & Stein E (2009). Early-life stress induces long-term morphologic changes in primate brain. Archives of General Psychiatry, 66(6), 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, & Kim DM (2003). The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews, 27(1–2), 33–44. doi: 10.1016/s0149-7634(03)00007-1 [DOI] [PubMed] [Google Scholar]
- Thompson MA, Bloomsmith MA, & Taylor LL (1991). A canine companion for nursery-reared infant chimpanzee. Laboratory Primate Newsletter, 30(2), 1–4. [Google Scholar]
- Walsh S, Bramblett CA, & Alford PL (1982). A vocabulary of abnormal behaviors in restrictively reared chimpanzees. American Journal of Primatology, 3, 315–319. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Nelson CA, Fox NA, Smyke AT, MArchall P, Parker SW, & Koga S (2003). Designing research to study the effects of institututionalization on brain and behavioral development: The Bucharest Early Intervention Project. Development and psychopathology, 15, 885–907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


