Abstract
Basement membranes (BMs) are thin, dense forms of extracellular matrix (ECM) that underlie or surround most animal tissues. BMs are enormously complex and harbor numerous proteins that provide essential signaling, mechanical, and barrier support for tissues during their development and normal functioning. As BMs are found throughout animal tissues, cells frequently migrate, change shape, and extend processes along BMs. Although sometimes used only as passive surfaces by cells, studies in developmental contexts are finding that BMs are often actively modified to help guide cell motility and cell morphogenesis. Here, I provide an overview of recent work revealing how BMs are remodeled in remarkably diverse ways to direct cell migration, cell orientation, axon guidance, and dendrite branching events during animal development.
Keywords: Basement membrane, Cell migration, Axon pathfinding, Dendrite branching, Metalloproteinases, Matrix stiffness
Introduction
BMs are sheet-like ECMs that arose in animals at the time of multicellularity [1]. These specialized ECMs are pervasive in animals and underlie all epithelia and surround endothelial vessels, muscles, and fat tissues [2]. Two major components of BMs are laminin and type IV collagen, which form independent self-oligomerizing networks. Laminin is a heterotrimer composed of an α, β, and γ chain and assembles into a non-covalently associated network that initiates BM formation and anchors BMs to tissues through binding to cell surface integrin and dystroglycan receptors [3]. Type IV collagen is also a heterotrimer and is made up of two α1-like chains and one α2-like chain that wrap around each other into a long rigid triple helix [4]. The triple-helical structure, as well as covalent cross-linking between N-terminal 7S domains and C-terminal NC1 domains, bestow type IV collagen networks stiffness and tensile strength, which allows BMs to mechanically support tissues [4]. Type IV collagen networks can be linked to cell-associated laminin through the cross-bridging glycoprotein nidogen as well as the heparan sulfate proteoglycans perlecan and agrin [5,6]. A hallmark of BMs is their diversity, which arises from different amounts of core BM components and post-translational modifications, as well as the presence of regulatory proteins, such as matricellular proteins, proteases, and growth factors [7–10]. Proteomic studies have indicated that BMs may harbor over 100 distinct proteins [11], suggesting vast complexity.
BMs are built with distinctive compositions during animal development to serve as specialized scaffoldings that direct cell differentiation, mediate cell polarity, and ensure cell survival [5]. They are also uniquely constructed to carry out mechanical functions that shape organs, connect tissues, and filter blood [12,13]. Although BMs sometimes appear to be passive surfaces along which cells move [14,15], they also have active roles in guiding cell motility and directing cell morphogenesis events like neuronal process extension. Deciphering the function of BMs in cell migration and cell morphogenesis has been hampered by BM complexity—the numerous BM proteins and many roles of BMs in supporting cells and tissues. It is also challenging to visualize dynamic cell-BM interactions in vivo as these often occur deep in tissues beyond the reach of light microscopy. Yet, recent studies using advanced genetics, new imaging approaches, endogenously tagged BM proteins, ex vivo tissue culture, and analysis of BM physical properties are expanding our understanding of the diverse ways in which BMs are modified to specifically guide cell migration and cell morphogenesis events during development [16–21]. Here, I highlight these new insights and provide an overview of this important role of BMs, which is not only vital for understanding the functions of BMs in animal development, but also has powerful implications for human disease.
BMs direct cell movement and neuronal processes by localizing cues
The best understood role of BMs in guiding cells during development is BM’s ability to harbor localized or enriched BM components and signaling ligands (referred to generally as BM cues) that steer cells. A diverse array of ligands, BM matrix proteins, cell surface receptors, and signaling pathways have been identified that direct cell migration, axon pathfinding, and dendritic branching along BMs (Table 1). Modifications to BM matrix proteins can also help steer cells. Analysis of genetic mutants in zebrafish revealed that the ER resident glycosyltransferase Lh3, which appears to modify the BM component type XVIII collagen, is required both for the extension of motor axons from the spinal cord into the periphery and for the proper migration of the neural crest cells from the neural tube [22,23]. Gaps in our understanding of BM-associated cues still remain. One challenge is that it is often difficult to discern whether implicated BM components act directly on cells or through association with other proteins. For example, it was initially shown that localized accumulation of perlecan directs dendrite arborization of the PVD neuron in C. elegans at a hemidesmosome-like structure, the fibrous organelle, which links body wall muscles to the epidermis [24]. A recent study extended this finding by characterizing a fortuitous mutation in perlecan that removes four immunoglobulin domains, which were discovered to localize the BM protein nidogen [18]. Genetic studies further indicated that perlecan and nidogen act together to then promote netrin signaling, which mediates dendritic branching. This likely represents a common feature of how BM scaffolds function, where combinatorial interactions of proteins within BMs provide specificity to either signaling ligand localization, cue construction (the cue is composed of multiple proteins), or ligand presentation to direct cell movements. Mechanistically how perlecan and nidogen promote netrin signaling, however, is still unclear, as the C. elegans netrin ligand (UNC-6) has not been detected at sites of perlecan and nidogen enrichment at fibrous organelles. This illustrates another challenge in understanding BM directed guidance—many signaling ligands identified genetically as steering cells along BMs, such as netrin, Slit, Decapentaplegic (Dpp), and the cleaved ectodomain of the transmembrane collagen COL-99, appear to be present at low levels, as they have not been detected directly at sites of BM mediated guidance [25–28]. Instead, these ligands have required overexpression or activity sensors to implicate BM localization [25–27].
Table 1.
Cell Migration and Neuronal Processes Guided by Localized or Enriched BM Cues
| Guided cell process/Animal | Localized or enriched BM component/Tissue BM where enriched | Receptor or molecular pathway activated to guide cell | References |
|---|---|---|---|
| Cell invasion through BM/C. elegans larva | UNC-6 (netrin)/uterine vulval connection site | UNC-40 (DCC) receptor | [17,51] |
| Longitudinal neuronal axon pathfinding/C. elegans larva | Nidogen (may localize ectodomain of transmembrane collagen COL-99)/Sublateral nerves and edges of body wall muscles | Discoidin domain receptor | [20,25,52] |
| Halting somatic gonad precursor migration/C. elegans embryo | Laminin/Posterior endoderm cells | pathway unknown | [21] |
| PVD neuron dendrite branching/C. elegans larva | Perlecan and nidogen (may localize UNC-6(netrin))/Fibrous organelles (muscle linkage site to epidermis) | SAX-7 (L1CAM) adhesion molecule and UNC-40 (DCC) receptor | [15,16] |
| Sematosensory neuron peripheral axon pathfinding/Zebrafish embryo | Heparan sulfate proteoglycan/Skin | LAR receptor tyrosine phosphatase | [22] |
| Commissural neuron axon crossing/Chick embryo | Cleaved F-spondin/Spinal cord floor plate | pathway unknown | [24] |
| Commissural neuron axon crossing/Mouse embryo | Glycosylated dystroglycan binding Slit/, Spinal cord floor plate | Robo receptor | [28] |
| Retinal ganglion cell axon projections/Zebrafish larva | Type IV collagen binding Slit/Surface of optic tectum | Robo receptor | [18] |
| Renal tubule elongation pathfinding/Drosophila embryo | Type IV collagen (may localize Decapentaplegic (Dpp))/Leading anterior tubule cells | BMP receptor | [19] |
| Motor axon pathfinding in embryonic ventral nerve cord/Drosophila embryo | Perlecan secreted by motor neurons/Enriched along motor axon trajectories and pathway choice points | Semaphorin-Plexin | [29] |
| Enteric neural crest-derived cell (ENCDCs) migration/Mouse, chick, zebrafish embryos | Collagen XVIII secreted by ENCDCs at wavefront and agrin secreted by trailing cells/Gut blood vessels | Collagen XVIII pathway unknown, Agrin may signal through dystroglycan receptor | [23,26] |
| Ventral spinal motor neuron migration/Mouse embryo | Slit gene expressed by spinal cord motor neurons/Spinal cord floor plate | Robo receptor | [27,28] |
| Neural crest mid-segmental migration & Motor axon outgrowth/Zebrafish embryo | Lh3 glycosyl transferase and presumptive substrate collagen XVIII / Adaxial muscle cells | Pathway unknown | [53,54] |
In many cases, cells respond to cues within BMs that are deposited by other cells or the tissue on which the BM resides [22,23,25,26,29–33]. However, a recent study examining migration of enteric neural crest-derived cells (ENCDCs) within the developing chick and mouse gut revealed how migrating cells lay down their own BM migration cues (Figure 1) [31]. The ENCDC’s are predominantly derived from multipotent neural crest cells that delaminate from the vagal neural tube, enter the foregut, and migrate proximally-to-distally along the gut blood vessel BM to populate the midgut and then hindgut to form the neurons and glia of the enteric nervous system [34]. Notably, as the ENCDCs reach the hindgut, the migratory wavefront cells secrete type XVIII collagen, which promotes rapid directional migration of ENCDCs. As the ENCDCs stop migrating, they secrete the BM component agrin, which inhibits ENCDC movement [31]. Other cells also appear to secrete their own directional cues into BMs. For example, expression of the Slit2 gene by spinal cord motor neurons in mice has recently been discovered to halt the transmigration of these motor neurons across the spinal cord BM [35], where the Slit protein is thought to accumulate [36]. Furthermore, motor neurons of the Drosophila embryonic ventral nerve cord secrete perlecan along motor axon trajectories and branching points, where perlecan promotes semaphorin-plexin mediated repulsive guidance [37]. Thus, the secretion of matrix components and signaling ligands by migrating cells and process extending neurons themselves into the BMs they encounter or move on, appears to be a common way that cells regulate their own navigation.
Figure 1. Neural crest cells secrete matrix components to control their own migration.
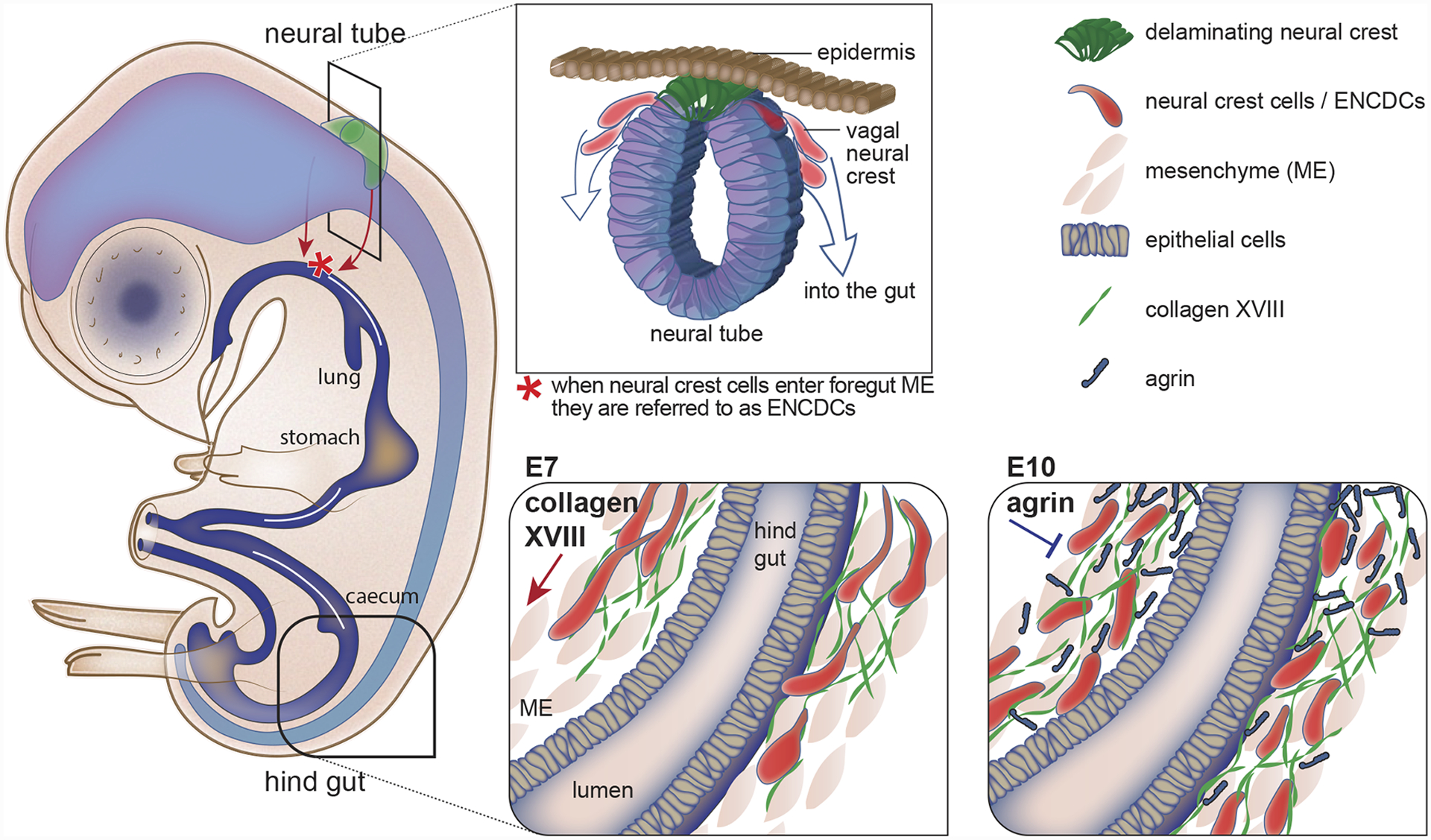
Most of the enteric nervous system of the gut is derived from vagal neural crest cells that undergo an epithelial-to-mesenchymal transition (EMT) at the dorsal neural tube, delaminate, and then migrate and colonize the mesenchyme of the foregut. Once in the gut, the neural crest cells are referred to as enteric neural crest-derived cells (ENCDCs) and they undergo a long migration along the basement membrane (BM) of gut blood vessel (not shown) to populate the entire gut. At embryonic day 7 (E7) the wave front of the ENCDCs reaches the distal hind gut where they secrete collagen XVIII, which promotes ENCDC migration. Later, at E10, the ENCDCs secrete agrin, which inhibits ENCDC migration.
BM physical properties control cell morphogenesis and cell invasion
Recent studies have discovered that the physical properties of BMs can also help direct cells. The Drosophila ovarian follicle (egg chamber) has emerged as a powerful model for understanding how dynamic alterations in BM stiffness and physical features guide cellular morphogenetic behaviors. Each Drosophila ovarian follicle is composed of a germ cell cluster surrounded by a follicular epithelium, which secretes a BM that localizes to their basal side and encircles the follicle. The egg chamber starts as a small sphere, but then during 14 stages of development undergoes dramatic growth and a 3-fold elongation along the anterior-posterior (AP) axis (Figure 2) to transform its size and shape [38]. Advances in ex vivo culture and live imaging revealed that the follicle epithelium cells collectively crawl along the inside of the stationary BM and rotate the follicle along the AP axis during stages 1–8 of development [20]. Visualization of GFP-tagged collagen, laminin, and perlecan have shown that follicle rotation helps form and polarize BM fibrils that align perpendicular to the AP axis and embed within a planar BM [39,40]. In addition, examination of BM levels and development of atomic force microscopy approaches revealed that overall BM deposition and stiffness increase during follicle cell rotation. Furthermore, a gradient of type IV collagen levels forms with higher collagen in the central follicle that tapers at the both poles. This asymmetry in collagen deposition generates a BM stiffness gradient along the AP axis by stage 8 [16,39], with highest stiffness at the center of the egg chamber and softer BM at the poles. Recent studies have uncovered how these dynamic physical BM properties direct several cellular behaviors that contribute to follicle elongation.
Figure 2. Basement membrane (BM) physical properties help drive Drosophila follicle elongation.
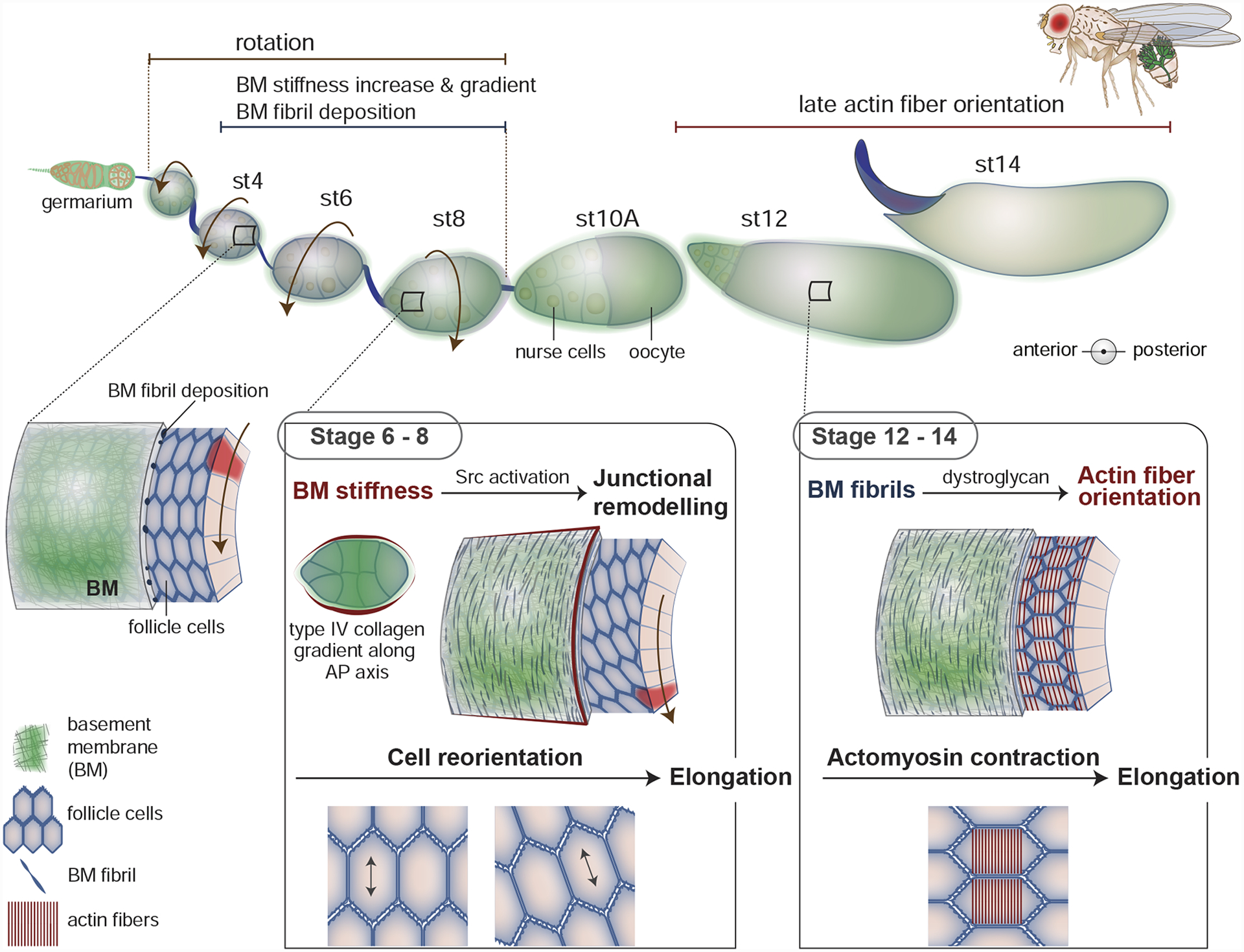
The Drosophila follicle (egg chamber) is initially small and spherical, but dramatically expands in volume and elongates along the anterior-posterior (AP) axis during its 14 stages of development. A key driver of elongation is the BM surrounding the follicle. During stages 1–8 the follicle cells collectively migrate, causing the egg chamber to rotate within its encasing BM. As the chamber rotates, the follicular epithelium deposits more planar BM as well as BM fibrils (first seen at stage 4) that embed within the planar BM and orient perpendicular to the AP axis. Also, a gradient of type IV collagen forms along the AP axis, with increased levels in the central region that tapper at both poles. The overall increase in BM deposition, fibril formation, and the gradient of type IV collagen increases BM stiffness and creates a BM stiffness gradient. During stages 6–8 the softer BM in the anterior region of the follicle is translated into appropriate Src activation, which alters junctional E-cadherin trafficking and facilitates the reorientation cells such that their long axis is more parallel to the AP axis. This reorientation helps promote follicle elongation. In addition, during stages 12–14, the follicle cells, through the dystroglycan matrix receptor, use the orientation of the BM fibrils to guide the alignment of F-actin stress fibers, which promotes later follicle elongation.
Morphometric analysis of follicle cell behavior during elongation has revealed a fascinating cell orientation shift that contributes to follicle elongation. During stages 4–8 follicle cells in the anterior region of the egg chamber reorient such that their long axis shifts from being perpendicular to the AP axis to running more parallel to AP axis—a reorientation that collectively helps elongate the follicle [21]. Through genetic screens, BM stiffness manipulations, immunohistochemistry, and fluorescence recovery after photobleaching (FRAP) experiments, it was discovered that BM stiffness modulates Src kinase activation, which alters junctional E-cadherin trafficking and facilitates the change in orientation of anterior follicle cells [21]. Although it is unclear how BM stiffness modifies Src activity and why the posterior follicle cells are not similarly reoriented by soft BM (Figure 2), these studies are amongst the first to link BM stiffness to directing specific cellular behaviors. Interestingly, new findings in the Drosophila ovary have further implicated type IV collagen deposition and expression of the nuclear lamin, LamC, a marker of mechanical constraint, in promoting cell intercalation of stalk cells—the cells that link adjacent follicles [41]. In addition, a recent study that pioneered BM stiffness analysis in mice through pressure myography, AFM, and the stiffness-sensitive structure of caveolae, found that netrin-4, which disrupts laminin networks, softens BMs in vivo and decreases the ability of cancer cells to invade through BMs in vitro. Consistent with a role in cancer progression, increased netrin-4 expression is associated with improved patient prognosis in breast, kidney, and melanoma patients [17]. While the mechanisms through which BM stiffness promotes stalk cell intercalation and cancer cell invasion are unknown, these studies suggest that BM stiffness might guide many different cellular behaviors.
In addition to regional stiffness, evidence has emerged through recent studies in the Drosophila egg chamber that BM topography—the oriented fibrils embedded within the BM—can also guide cells. The ECM receptor Dystroglycan and the cytosolic protein Dystrophin are part of a complex that links the ECM to the F-actin cytoskeleton. Live imaging, mutant analysis, and spatiotemporal knockout and rescue experiments, demonstrated that Dystroglycan and Dystrophin translate the perpendicular BM fibril orientation established early in egg chamber development into perpendicularly oriented basal F-actin stress fibers within follicle cells during the last stages of elongation—stage 12 and onward [38]. This F-actin alignment allows anisotropic myosin contractions that promote late stages of elongation [42]. How Dystroglycan and Dystrophin translate the orientation of BM fibrils to F-actin stress fibers is unclear, but might involve the higher density of binding sites for Dystroglycan [38]. Collagen and laminin based BM fibrils have not yet been observed in other BMs, but the BM-associated matrix proteins hemicentin and CPSG4 form into tracks and fibrils linked with cell migration and morphogenesis events during C. elegans, mouse, and sea urchin development [43–45]. Thus, as more imaging approaches are developed to examine individual BM components, it seems likely that additional examples of BM-associated fibrils guiding cells will be discovered.
BM Proteolysis Guides Cell Migration and Dendrite Reshaping
Matrix metalloproteases of the ADAMTS and MMP families cleave BM components and can both degrade BMs and generate bioactive ECM fragments that have unique signaling activities [46]. Determining whether MMP-mediated functions are carried out by ECM fragments has been challenging, as it is not yet possible to visualize the precise localization of ECM fragments or eliminate ECM fragments without perturbing the parent BM protein. Thus, while addition of BM-derived ECM fragments to cells and tissues in culture can regulate branching morphogenesis and epithelial-to-mesenchymal transitions (EMTs) [47,48], determining their localization and function in vivo has not yet been possible.
Despite experimental challenges, elegant studies are beginning to establish that metalloproteases guide cells through their localized action on BMs in vivo. For example, the Drosophila ADAMTS family member AdamTS-A is expressed by surface glial cells of the developing Drosophila central nervous system (CNS). The surface glial cells sit under the BM that enwraps the CNS. Reduction of AdamTS-A activity causes a mass exodus of neuronal and glial cells from the CNS, which then invade other tissues [49]. Loss of AdamTS-A function leads to a buildup of type IV collagen within the CNS BM and reducing collagen suppresses the AdamTS-A migration and invasion phenotype. As increased collagen leads to greater BM stiffness, these observations suggest that AdamTS-A might counteract a BM stiffness signal that triggers neuronal and glial migration [49]. Consistent with a possible shared function in precisely tuning type IV collagen levels within BM to regulate cell migration, the C. elegans ortholog of AdamTS-A, GON-1, is secreted by the BM-encased migrating distal tip cell in C. elegans, where it functions to lower BM type IV collagen levels and promote distal tip cell movement [50,51].
There is also evidence that matrix metalloprotease MMP family members guide cells by degrading and removing BM in specific locations. A study in Drosophila examining sensory neuron dendrites in the abdomen of young adults found that MMP-2, a plasma membrane tethered MMP, is crucial for dendrite reshaping [52]. MMP-2 is transiently expressed in the epidermis of early adults and degrades the underlying BM between the epidermis and musculature. The BM that rests in the grooves between muscle fibers, however, is not in contact with epidermal cells and is thus protected from MMP-2 mediated degradation. The sensory neuron dendrites initially adhere to the intact BM near the epidermis, but BM degradation is thought to loosen this dendrite-BM attachment and allow dendrites to move between the muscle fibers and bind to the remnant BM in the channels between the muscle fibers. This movement remodels the dendrites into a lattice-like pattern that follows the grooves between the muscle fibers [52]. Localized removal of BM by MMPs also appears to guide cells during mouse gastrulation, where the primordial germ layers of ectoderm, mesoderm, and endoderm are established. In the early mouse embryo, the expression of several MMPs correlates with the formation of holes within the epiblast BM (Figure 3) [19]. These BM holes presage the path of the primitive streak—a progressive EMT of epiblast cells that initiates in the proximal posterior region of the embryo and moves to the distal anterior zone (Figure 3) [53]. Cells undergoing EMT transmigrate the BM and ingress into the space between the epiblast and embryonic visceral endoderm and give rise to mesoderm and endoderm. Culturing embryos in the presence of MMP inhibitors prior to primitive streak formation, led to a loss of BM holes, defective primitive streak extension, and a failure to properly gastrulate [19]. These results suggest that the generation of holes in the BM via MMP activity may facilitate EMT and ingression of the mesoderm and endoderm. Importantly, it is unknown whether MMP activity instructs EMT and primitive streak formation, or simply acts permissively to allow BM transmigration during EMT. Furthermore, in all of these examples of matrix protease activity guiding cells, their direct BM substrates are unknown, and it is possible that bioactive ECM fragments generated by protease activity contribute to their mechanism of action.
Figure 3. Basement membrane (BM) perforations presage primitive streak extension during mouse gastrulation.
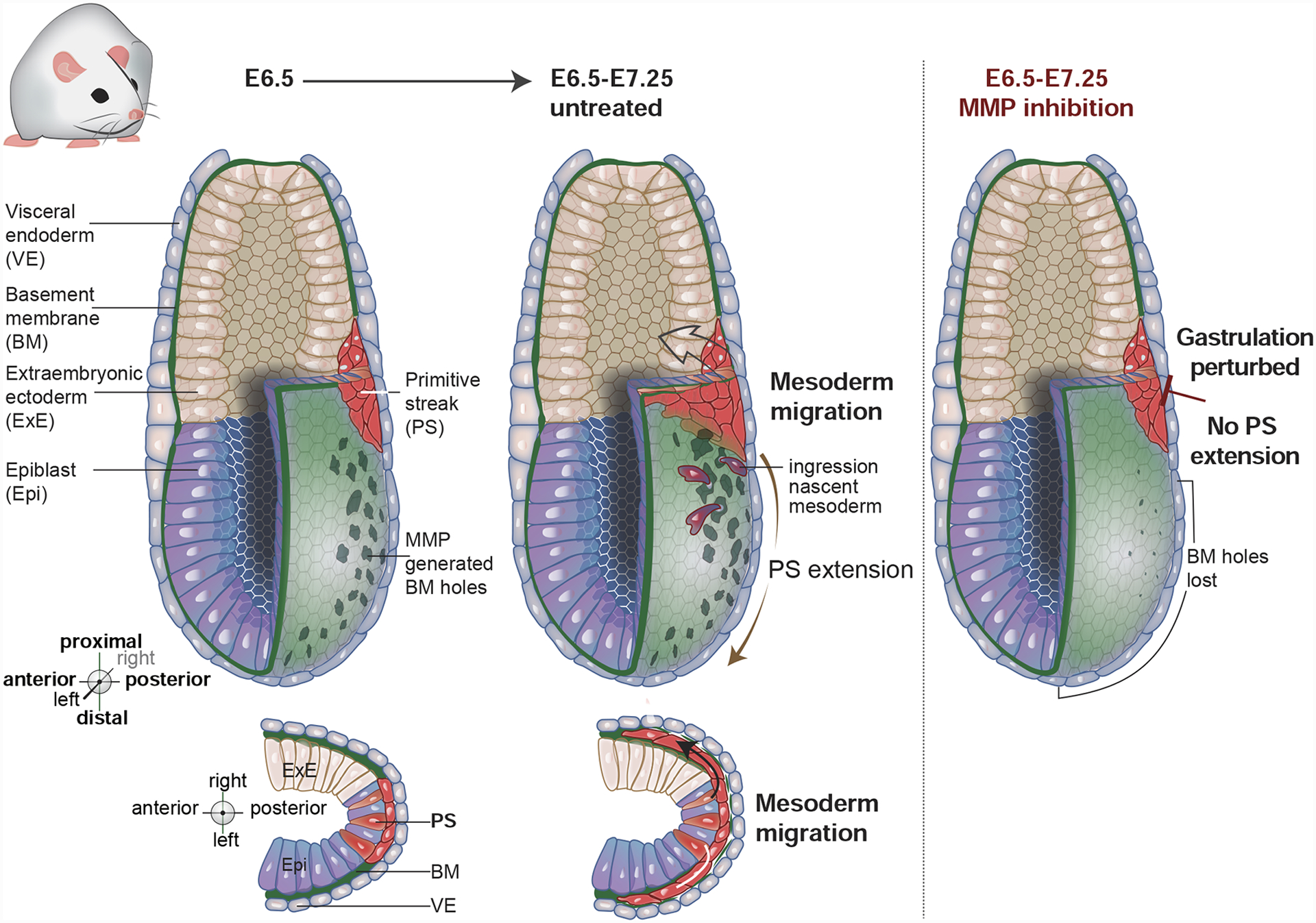
During early mouse development, the embryo consists of the abutting epiblast and extraembryonic ectoderm, which is enveloped by a BM and the visceral endoderm. By embryonic day 6.5 (E6.5) matrix metalloproteinase (MMP) activity generates perforations within the BM on the posterior side of the embryo. Gastrulation initiates at the most distal region of these BM perforations at the extraembryonic/embryonic boundary in a region called the primitive streak, where epiblast cells undergo an epithelial-to-mesenchymal transition (EMT), move through the BM gaps and ingress into the space between the epiblast and extraembryonic ectoderm. The first ingressing cells are nascent mesoderm, which then migrate as a sheet between the visceral endoderm and epiblast to create a layer of mesoderm around the embryo. The primitive streak extends through a progressive wave of EMT that follows the BM perforations. Primitive streak extension helps to complete gastrulation by giving rise to additional mesoderm and the definitive endoderm of the embryo. Inhibiting MMP activity blocks BM perforations, prevents primitive streak extension, and disrupts gastrulation.
Outlook
BM complexity provides a seemingly infinite reservoir of mechanical and chemical cues to guide cells. As outlined in this review, recent studies indicate that BMs are deposited and modified in diverse ways to steer cells throughout animal development. Elucidating the role of BMs in directing cell migration and morphogenesis in dynamic and complex native settings, however, remains a challenge. While new adaptive light-sheet microscopy and computational image analysis methods in mice and zebrafish are allowing single cell analysis of cellular behaviors in living embryos [54,55], BM matrix components have not yet been endogenously tagged with genetically encoded fluorophores in vertebrates, limiting our understanding of dynamic cell-BM interactions in these animals. Recent advances in comprehensive tagging of endogenous BM components with genetically encoded fluorophores in C. elegans [8], should help provide genome editing strategies to fill this gap in vertebrate experimental systems. In addition, new methods to assess BM mechanical properties in vivo in the Drosophila egg chamber and adult mouse tissues, [16,17] can now be adopted in other developing animals and tissues to explore the recently recognized role of BM stiffness in guiding cells. As cell migration and changes in cell morphogenesis play critical roles in the pathogenesis of cancer, autoimmune disease, and neurological disorders, studies in dynamic and experimentally accessible developmental contexts will not only help to reveal how animals develop, but also provide important insights into human disease.
Acknowledgements
We thank S. Horne-Badovinac, H. Bülow, A. Sutherland, N. Nagy, M. Granato, and J. Rivera for helpful discussions, S. Payne, C. Gianakas, and A. Garde for comments on the manuscript, and A. Kawska (info@illuscientia) for all figure illustrations. D.R.S. was supported by R35GM118049 and R21OD028766.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The author declares no competing interests.
References
- 1.Fidler AL, Darris CE, Chetyrkin SV, Pedchenko VK, Boudko SP, Brown KL, Gray Jerome W, Hudson JK, Rokas A, Hudson BG: Collagen IV and basement membrane at the evolutionary dawn of metazoan tissues. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayadev R, Sherwood DR: Basement membranes. Curr. Biol 2017, 27:R207–R211. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Qi Y, McKee K, Liu J, Hsu J, Yurchenco PD: Integrin and dystroglycan compensate each other to mediate laminin-dependent basement membrane assembly and epiblast polarization. Matrix Biol 2017, 57–58:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fidler AL, Boudko SP, Rokas A, Hudson BG: The triple helix of collagens - an ancient protein structure that enabled animal multicellularity and tissue evolution. J. Cell Sci 2018, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pozzi A, Yurchenco PD, Iozzo RV: The nature and biology of basement membranes. Matrix Biol 2017, 57–58:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hohenester E, Yurchenco PD: Laminins in basement membrane assembly. Cell Adh Migr 2013, 7:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glentis A, Gurchenkov V, Matic Vignjevic D: Assembly, heterogeneity, and breaching of the basement membranes. Cell Adh Migr 2014, 8:236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keeley DP, Hastie E, Jayadev R, Kelley LC, Chi Q, Payne SG, Jeger JL, Hoffman BD, Sherwood DR: Comprehensive Endogenous Tagging of Basement Membrane Components Reveals Dynamic Movement within the Matrix Scaffolding. Dev. Cell 2020, 54:60–74.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastor-Pareja JC: Atypical basement membranes and basement membrane diversity - what is normal anyway? J. Cell Sci 2020, 133. [DOI] [PubMed] [Google Scholar]
- 10.Jayadev R, Chi Q, Keeley DP, Hastie EL, Kelley LC, Sherwood DR: α-Integrins dictate distinct modes of type IV collagen recruitment to basement membranes. J. Cell Biol 2019, 218:3098–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randles MJ, Humphries MJ, Lennon R: Proteomic definitions of basement membrane composition in health and disease. Matrix Biol 2017, 57–58:12–28. [DOI] [PubMed] [Google Scholar]
- 12.Keeley DP, Sherwood DR: Tissue linkage through adjoining basement membranes: The long and the short term of it. Matrix Biol 2019, 75–76:58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrissey MA, Sherwood DR: An active role for basement membrane assembly and modification in tissue sculpting. J. Cell Sci 2015, 128:1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown NH: Extracellular matrix in development: insights from mechanisms conserved between invertebrates and vertebrates. Cold Spring Harb. Perspect. Biol 2011, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gritsenko P, Leenders W, Friedl P: Recapitulating in vivo-like plasticity of glioma cell invasion along blood vessels and in astrocyte-rich stroma. Histochem. Cell Biol 2017, 148:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crest J, Diz-Muñoz A, Chen D-Y, Fletcher DA, Bilder D: Organ sculpting by patterned extracellular matrix stiffness. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuten R, Zendehroud S, Nicolau M, Fleischhauer L, Laitala A, Kiderlen S, Nikodemus D, Wullkopf L, Nielsen SR, McNeilly S, et al. : Basement membrane stiffness determines metastases formation. Nat. Mater 2021, 10.1038/s41563-020-00894-0. [DOI] [PubMed] [Google Scholar]
- 18.Celestrin K, Díaz-Balzac CA, Tang LTH, Ackley BD, Bülow HE: Four specific immunoglobulin domains in UNC-52/Perlecan function with NID-1/Nidogen during dendrite morphogenesis in Caenorhabditis elegans. Development 2018, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyprianou C, Christodoulou N, Hamilton RS, Nahaboo W, Boomgaard DS, Amadei G, Migeotte I, Zernicka-Goetz M: Basement membrane remodelling regulates mouse embryogenesis. Nature 2020, 582:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cetera M, Lewellyn L, Horne-Badovinac S: Cultivation and live imaging of drosophila ovaries. Methods Mol. Biol 2016, 1478:215–226. [DOI] [PubMed] [Google Scholar]
- 21.Chen D-Y, Crest J, Streichan SJ, Bilder D: Extracellular matrix stiffness cues junctional remodeling for 3D tissue elongation. Nat. Commun 2019, 10:3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider VA, Granato M: The myotomal diwanka (lh3) glycosyltransferase and type XVIII collagen are critical for motor growth cone migration. Neuron 2006, 50:683–695. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee S, Isaacman-Beck J, Schneider VA, Granato M: A novel role for Lh3 dependent ECM modifications during neural crest cell migration in zebrafish. PLoS One 2013, 8:e54609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang X, Dong X, Moerman DG, Shen K, Wang X: Sarcomeres Pattern Proprioceptive Sensory Dendritic Endings through UNC-52/Perlecan in C. elegans. Dev. Cell 2015, 33:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziel JW, Hagedorn EJ, Audhya A, Sherwood DR: UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat. Cell Biol 2009, 11:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao T, Staub W, Robles E, Gosse NJ, Cole GJ, Baier H: Assembly of lamina-specific neuronal connections by slit bound to type IV collagen. Cell 2011, 146:164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunt S, Hooley C, Hu N, Scahill C, Weavers H, Skaer H: Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev. Cell 2010, 19:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor J, Unsoeld T, Hutter H: The transmembrane collagen COL-99 guides longitudinally extending axons in C. elegans. Mol. Cell. Neurosci 2018, 89:9–19. [DOI] [PubMed] [Google Scholar]
- 29.Rohrschneider MR, Nance J: The union of somatic gonad precursors and primordial germ cells during Caenorhabditis elegans embryogenesis. Dev. Biol 2013, 379:139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Wolfson SN, Gharib A, Sagasti A: LAR receptor tyrosine phosphatases and HSPGs guide peripheral sensory axons to the skin. Curr. Biol 2012, 22:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagy N, Barad C, Hotta R, Bhave S, Arciero E, Dora D, Goldstein AM: Collagen 18 and agrin are secreted by neural crest cells to remodel their microenvironment and regulate their migration during enteric nervous system development. Development 2018, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zisman S, Marom K, Avraham O, Rinsky-Halivni L, Gai U, Kligun G, Tzarfaty-Majar V, Suzuki T, Klar A: Proteolysis and membrane capture of F-spondin generates combinatorial guidance cues from a single molecule. J. Cell Biol 2007, 178:1237–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S, Wadsworth WG: Positioning of longitudinal nerves in C. elegans by nidogen. Science 2000, 288:150–154. [DOI] [PubMed] [Google Scholar]
- 34.Nagy N, Mwizerwa O, Yaniv K, Carmel L, Pieretti-Vanmarcke R, Weinstein BM, Goldstein AM: Endothelial cells promote migration and proliferation of enteric neural crest cells via beta1 integrin signaling. Dev. Biol 2009, 330:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim M, Lee CH, Barnum SJ, Watson RC, Li J, Mastick GS: Slit/Robo signals prevent spinal motor neuron emigration by organizing the spinal cord basement membrane. Dev. Biol 2019, 455:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright KM, Lyon KA, Leung H, Leahy DJ, Ma L, Ginty DD: Dystroglycan organizes axon guidance cue localization and axonal pathfinding. Neuron 2012, 76:931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho JY, Chak K, Andreone BJ, Wooley JR, Kolodkin AL: The extracellular matrix proteoglycan perlecan facilitates transmembrane semaphorin-mediated repulsive guidance. Genes Dev. 2012, 26:2222–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerqueira Campos F, Dennis C, Alégot H, Fritsch C, Isabella A, Pouchin P, Bardot O, Horne-Badovinac S, Mirouse V: Oriented basement membrane fibrils provide a memory for F-actin planar polarization via the Dystrophin-Dystroglycan complex during tissue elongation. Development 2020, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chlasta J, Milani P, Runel G, Duteyrat J-L, Arias L, Lamiré L-A, Boudaoud A, Grammont M: Variations in basement membrane mechanics are linked to epithelial morphogenesis. Development 2017, 144:4350–4362. [DOI] [PubMed] [Google Scholar]
- 40.Isabella AJ, Horne-Badovinac S: Rab10-Mediated Secretion Synergizes with Tissue Movement to Build a Polarized Basement Membrane Architecture for Organ Morphogenesis. Dev. Cell 2016, 38:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van De Bor V, Loreau V, Malbouyres M, Cerezo D, Placenti A, Ruggiero F, Noselli S: A Dynamic and Mosaic Basement Membrane controls cell intercalation in Drosophila ovaries. Development 2021, 10.1242/dev.195511. [DOI] [PubMed] [Google Scholar]
- 42.Qin X, Park BO, Liu J, Chen B, Choesmel-Cadamuro V, Belguise K, Heo WD, Wang X: Cell-matrix adhesion and cell-cell adhesion differentially control basal myosin oscillation and Drosophila egg chamber elongation. Nat. Commun 2017, 8:14708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodor PG, Illies MR, Broadley S, Ettensohn CA: Cell-substrate interactions during sea urchin gastrulation: migrating primary mesenchyme cells interact with and align extracellular matrix fibers that contain ECM3, a molecule with NG2-like and multiple calcium-binding domains. Dev. Biol 2000, 222:181–194. [DOI] [PubMed] [Google Scholar]
- 44.Lin M-H, Pope BD, Sasaki T, Keeley DP, Sherwood DR, Miner JH: Mammalian hemicentin 1 is assembled into tracks in the extracellular matrix of multiple tissues. Dev. Dyn 2020, 249:775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel BE, Hedgecock EM: Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development 2001, 128:883–894. [DOI] [PubMed] [Google Scholar]
- 46.Ricard-Blum S, Vallet SD: Fragments generated upon extracellular matrix remodeling: Biological regulators and potential drugs. Matrix Biol 2019, 75–76:170–189. [DOI] [PubMed] [Google Scholar]
- 47.Karihaloo A, Karumanchi SA, Barasch J, Jha V, Nickel CH, Yang J, Grisaru S, Bush KT, Nigam S, Rosenblum ND, et al. : Endostatin regulates branching morphogenesis of renal epithelial cells and ureteric bud. Proc. Natl. Acad. Sci. USA 2001, 98:12509–12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horejs C-M, Serio A, Purvis A, Gormley AJ, Bertazzo S, Poliniewicz A, Wang AJ, DiMaggio P, Hohenester E, Stevens MM: Biologically-active laminin-111 fragment that modulates the epithelial-to-mesenchymal transition in embryonic stem cells. Proc. Natl. Acad. Sci. USA 2014, 111:5908–5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skeath JB, Wilson BA, Romero SE, Snee MJ, Zhu Y, Lacin H: The extracellular metalloprotease AdamTS-A anchors neural lineages in place within and preserves the architecture of the central nervous system. Development 2017, 144:3102–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherwood DR, Plastino J: Invading, Leading and Navigating Cells in Caenorhabditis elegans: Insights into Cell Movement in Vivo. Genetics 2018, 208:53–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imanishi A, Aoki Y, Kakehi M, Mori S, Takano T, Kubota Y, Kim H-S, Shibata Y, Nishiwaki K: Genetic interactions among ADAMTS metalloproteases and basement membrane molecules in cell migration in Caenorhabditis elegans. PLoS One 2020, 15:e0240571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yasunaga K, Kanamori T, Morikawa R, Suzuki E, Emoto K: Dendrite reshaping of adult Drosophila sensory neurons requires matrix metalloproteinase-mediated modification of the basement membranes. Dev. Cell 2010, 18:621–632. [DOI] [PubMed] [Google Scholar]
- 53.Williams M, Burdsal C, Periasamy A, Lewandoski M, Sutherland A: Mouse primitive streak forms in situ by initiation of epithelial to mesenchymal transition without migration of a cell population. Dev. Dyn 2012, 241:270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDole K, Guignard L, Amat F, Berger A, Malandain G, Royer LA, Turaga SC, Branson K, Keller PJ: In Toto Imaging and Reconstruction of Post-Implantation Mouse Development at the Single-Cell Level. Cell 2018, 175:859–876.e33. [DOI] [PubMed] [Google Scholar]
- 55.Pang M, Bai L, Zong W, Wang X, Bu Y, Xiong C, Zheng J, Li J, Gao W, Feng Z, et al. : Light-sheet fluorescence imaging charts the gastrula origin of vascular endothelial cells in early zebrafish embryos. Cell Discov. 2020, 6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]


