Abstract
Intratumoral heterogeneity is a negative prognostic factor for cancer and commonly attributed to microenvironment-driven genetic mutations and/or the emergence of cancer stem cells (CSCs). How aberrant extracellular matrix (ECM) remodeling regulates the phenotypic diversity of tumor cells, however, remains poorly understood due in part to a lack of model systems that allow isolating the physicochemical heterogeneity of malignancy-associated ECM for mechanistic studies. Here, we review the compositional, microarchitectural, and mechanical hallmarks of cancer-associated ECM and highlight biomaterials and engineering approaches to recapitulate these properties for in vitro and in vivo studies. Subsequently, we describe how such engineered platforms may be explored to define the spatiotemporal dynamics through which cancer-associated ECM remodeling regulates intratumoral heterogeneity and the CSC phenotype. Finally, we highlight future opportunities and technological advances to further elucidate the relationship between tumor-associated ECM dynamics and intratumoral heterogeneity.
Introduction
Intratumoral heterogeneity is a hallmark of cancer and is characterized by the presence of different cancer cell subpopulations that severely limit patient outcomes due to their varied proliferative, invasive, and therapy resistance capabilities [1,2]. Historically, tumor cell heterogeneity has been attributed to oncogenic mutations that increase cell fitness in response to environmental pressures or chemotherapy [3]. However, phenotypic differences caused by epigenetic reprogramming as well as transient changes in gene expression, phosphoproteomics, and metabolic signaling are equally important [2,4,5]. Moreover, the self-renewal and therapy resistance of cancer stem-like cells (CSC) contributes to clonal diversity within tumors [6]. Indeed, an increase in CSCs due to transformation of tumor cells or environmental selection pressures promotes tumor development, metastasis, and treatment response [6]. Which role the tumor microenvironment (TME) plays in the emergence of CSCs and which effect this has on tumor heterogeneity is not well understood.
Within the TME, cancer cell phenotypes are regulated through crosstalk with tissue-resident stromal cells including cancer-associated fibroblasts (CAFs), adipocytes, endothelial cells, and infiltrating immune cells [7]. Much emphasis has been placed on how secretory functions of these cells control tumor heterogeneity and progression. Yet their impact on the physical properties of the TME may be similarly critical [8]. In particular, CAFs are widely studied for their role in changing the quantity, biochemical composition, and mechanical properties of extracellular matrix (ECM) in tumors [8], and these alterations regulate the geno- and phenotype of tumor cells as well as CSC quantity and functions [9]. Nevertheless, CAF-dependent ECM changes are not homogeneous, but are subject to spatial and temporal variations (Figure 1). How ECM heterogeneity is functionally linked to tumor heterogeneity remains unclear due in part to the lack of relevant model systems.
Figure 1: Engineered model systems to study tumor heterogeneity.
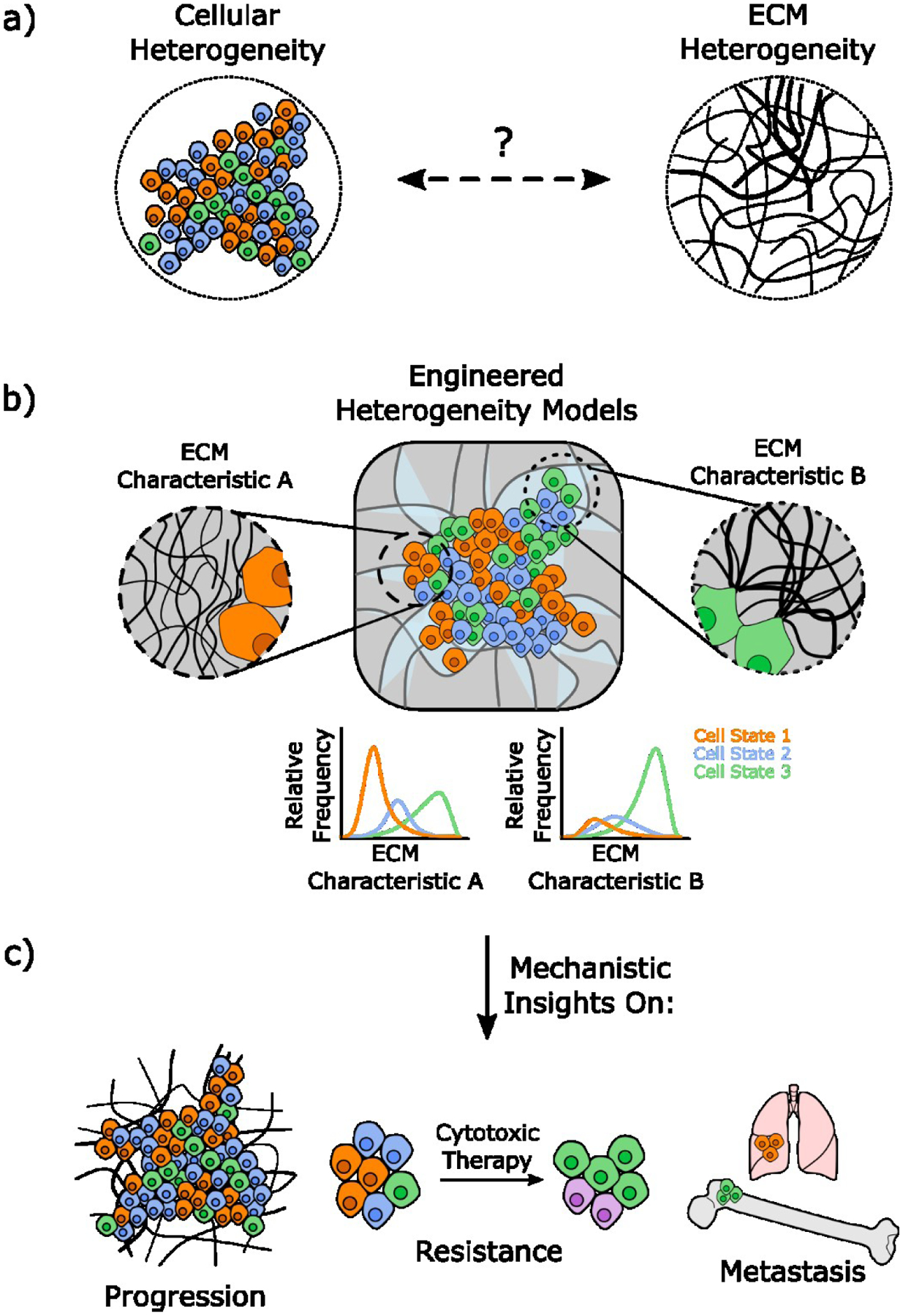
a) Tumors are characterized by both cellular and ECM heterogeneity. To understand how the interplay between both parameters affects cell fate decisions in the TME b) engineered heterogeneity models are needed that recapitulate both. c) Mechanistic studies with these models will enable new insights into tumor progression, therapy resistance, and metastasis.
Both in vivo and in vitro studies have advanced our understanding of how tumor cell interactions with the ECM affect tumor progression and therapy response. However, the high cost and species-dependent differences between humans and mouse models, as well as shortcomings associated with 2-D cell culture make it challenging to isolate mechanistic links between ECM remodeling and tumor cell state. Engineered model systems can recapitulate and isolate TME-associated ECM changes to probe their effect on tumor heterogeneity as a function of CSC enrichment. Indeed, simply switching tumor cell culture from conventional 2-D to 3-D culture impacts several hallmarks of malignancy including cellular metabolism [10], invasion [11], and therapy resistance [12]. Furthermore, 3D culturing of cancer cells enriches for CSCs in part through activation of the epithelial to mesenchymal transition and altering cytokine secretion [13]. Here, we will summarize current knowledge of ECM changes in the TME, highlight model systems to mimic these changes for mechanistic studies, and outline strategies to further improve the impact engineered ECM models have on our understanding of tumor heterogeneity and the role of CSCs in this process (Figure 1).
Extracellular Matrix Changes in the Tumor Microenvironment
Compositional Changes
Most prior research studying the role of ECM remodeling in cancer focused on the composition of the ECM and ECM-associated proteins (collectively referred to as the matrisome [14]). Changes in the matrisome relative to healthy tissue are characteristic of aggressive cancers including breast [15] and pancreatic cancer [16]. For example, fibronectin is often increased during tumorigenesis, regulates all stages of the metastatic cascade through integrin-dependent signaling, and impacts CSC marker expression [17–19]. Additionally, fibronectin provides the initial scaffolding for collagen deposition [20] and could, therefore, contribute to the elevated concentration of different collagen types in the TME (in particular, type I and VI) that ultimately promote tumor invasion and metastasis due in part to altering tumor cell stemness [9,21–23].
Stromal accumulation of glycans such as hyaluronic acid (HA) and heparan sulfate can also predict worse clinical prognosis and contributes to CSC properties [24,25]. An increase of free glycans such as heparan sulfate, due to upregulation of glycosidases, promotes tumor progression [26] possibly by perturbing the nuanced interactions between protein- and glycan-based ECM components. Indeed, disrupted biosynthesis of glycans such as HA affects ECM bulk properties by stimulating collagen and fibronectin deposition [27]. Similarly, binding of heparan sulfate to the FnIII12–14 domain of soluble fibronectin initiates deposition of fibrillar fibronectin within the ECM [28]. While these changes can indirectly promote tumor cell stemness, upregulation of glycans also increases engagement of cell surface receptors including CD44, which, in turn, activates expression of transcription factors linked to stemness [29,30]. Finally, most glycans carry a high negative charge density impacting the sequestration and bioactivity of growth factors and cytokines associated with stemness [6,31].
Physical Changes
Varied composition simultaneously alters the physical properties of the ECM (Figure 2), which independently regulate malignancy [32]. Greater ECM rigidity due to increased collagen deposition, cross-linking, and linearization is the most widely appreciated of these changes [33,34]. In fact, increased tissue density and stiffness are well known biomarkers of malignant tissue [35] and widely studied at the bulk tissue level. However, the biophysical properties of tumors vary in space and time with functional consequences on tumor heterogeneity [15,16,34]. For example, the invasive front of tumors exhibits increased ECM fiber alignment and stiffness relative to more benign tumors [34,36,37], while these features are reduced in more central regions of tumors due to a comparatively reduced ECM content [15,34]. Increased ECM fiber alignment, in turn, locally activates mechanotransduction and invasion via positive mechanical feedback and strain-dependent biochemical changes of the ECM [20,38,39]. For example, partial unfolding of fibronectin fibers due to tumor-induced stromal cell contractility increases ECM deposition and stiffness [40–42], exposes cryptic binding sites to soluble factors [43], and mechanically activates latent growth factors and cytokines stored in the ECM [44]. Such changes could increase tumor cell stemness and thus, tumor heterogeneity by modifying the signaling networks between tumor cells and the TME [45].
Figure 2: Extracellular matrix changes in the tumor microenvironment.
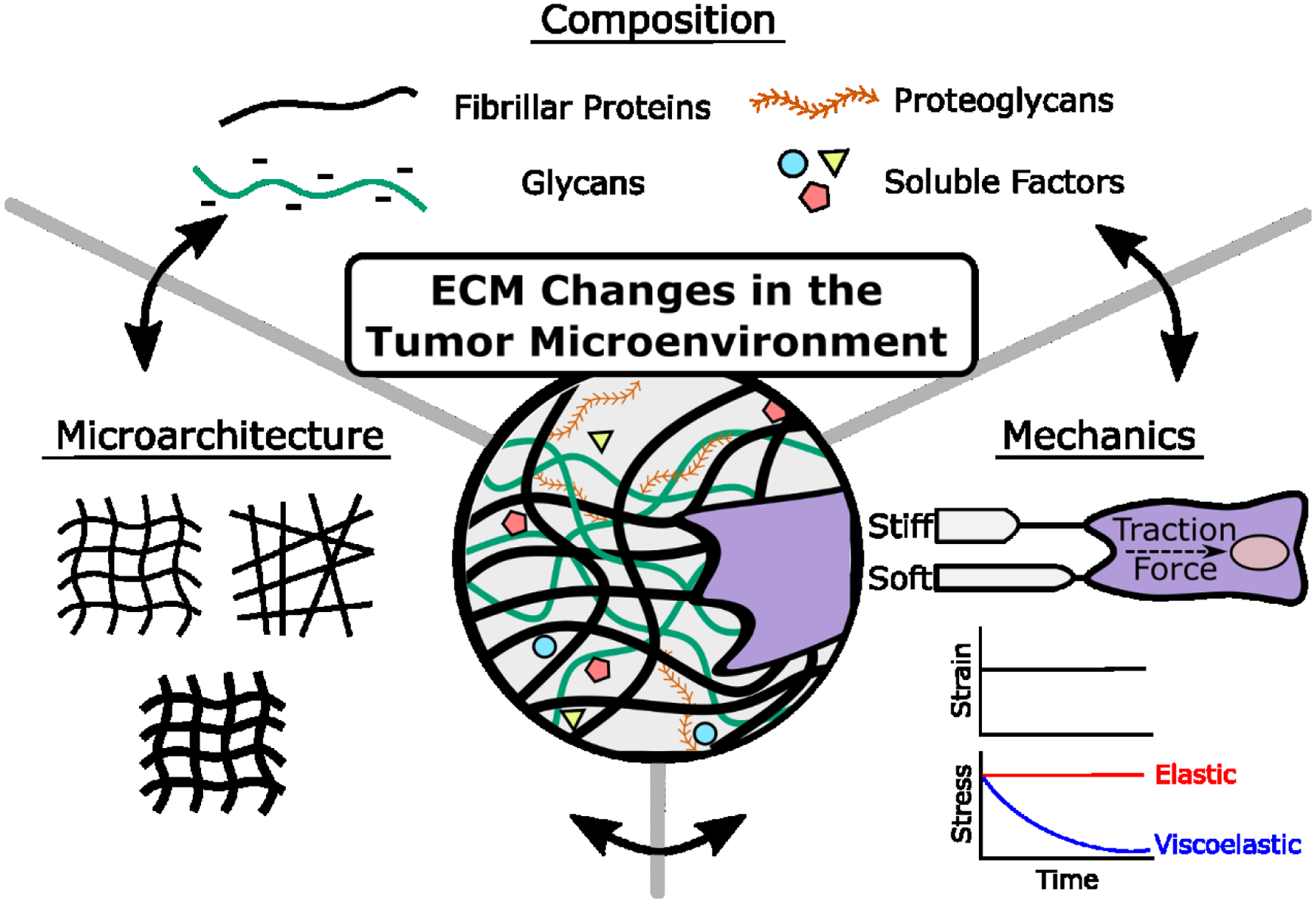
Tumors are characterized by changes in ECM composition including increased levels of fibrillar proteins (e.g. collagen and fibronectin), glycans (e.g. hyaluronic acid, proteoglycans), and soluble factors (e.g. growth factors, cytokines). Compositional changes entail physical ECM changes including microarchitectural (e.g. ECM fiber linearization and thickness) and mechanical changes (e.g. ECM elasticity [time-independent] or viscoelasticity [time-dependent, e.g. stress relaxation]). Compositional and physical changes are interdependent and lead to changes in cell phenotype.
Glycan-protein interactions also contribute to microarchitectural changes of the ECM by either cross-linking individual protein fibers or physically interweaving with them to increase fiber diameter [27,43,46]. Importantly, the high negative charge density associated with increased glycan content increases tumor osmotic pressure, which can promote tumor heterogeneity by altering interstitial fluid flow to direct migration [47] or by impairing drug delivery [25,31,32]. As compositional and physical ECM changes are closely interconnected in the TME, engineered model systems are necessary to deconvolve how their individual and combined effects promote heterogeneity through altering CSCs.
Model Systems of the Extracellular Matrix
Decellularized scaffolds generated from tissue, patient samples, or deposited by cells in culture (cell-derived matrices, CDMs) mimic the native biochemical and physical properties of the ECM (Figure 3a) [23,48–50]. In particular, CAF-derived CDMs are often used to recapitulate tumor-associated ECM and promote the malignant potential of both tumor and stromal cells by activating mechanosignaling [49,50]. Despite their obvious benefits, the complexity of decellularized scaffolds and inability to selectively control substrate mechanics and architecture make it challenging to delineate mechanistic details of how ECM compositional and physical parameters impact tumor heterogeneity.
Figure 3: Examples of engineered model systems with controlled ECM properties.
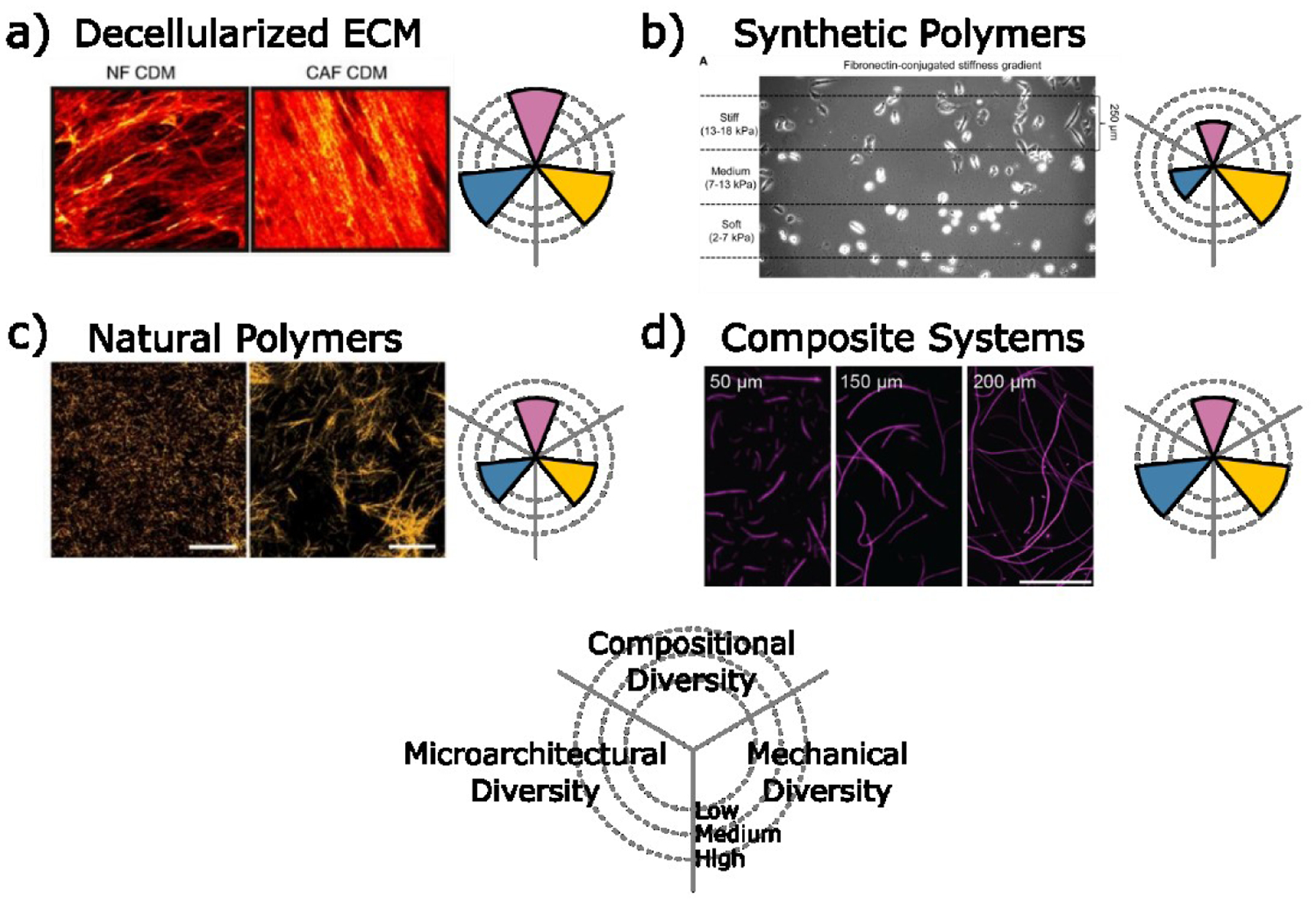
a) Decellularized ECM from normal (NF) and cancer associated fibroblasts (CAF) visualized by collagen I staining [46]. Reproduced with permission of the Nature Publishing Group. b) Synthetic polymers such as polyacrylamide gels with stiffness gradients provide a range of mechanical diversity [47]. Reproduced with permission of Elsevier. c) Natural polymers such as collagen I provide control over ECM structure by adjusting the gelation parameters [51]. Reproduced with permission of the National Academy of Science. d) Composite systems of fibrous polymers encapsulated within hydrogels such as electrospun dextran vinyl sulfone fibers encapsulated in methacrylated gelatin enable microarchitectural and mechanical control [55]. Reproduced with permission of the American Chemical Society.
Synthetic polyacrylamide (PAA) gels are widely used to achieve tunable control of substrate stiffness. These gels can be functionalized with different adhesion ligands or full-length proteins (e.g. fibronectin, laminin) to promote cell adhesion and determine the effect of ECM mechanics in the context of varied adhesion receptor engagement (Figure 3b). Furthermore, PAA-based systems are easy to implement and can be tailored to mimic the spatial heterogeneity of ECM stiffness in tumors. Investigations of cancer cell migration on PAA gels with stiffness gradients, for example, have revealed that durotaxis of cancer cells requires an optimal stiffness [51,52]. However, time-dependent mechanical properties or viscoelasticity (e.g. strain-stiffening) are critical features of the native ECM that linearly elastic (i.e. time-independent) PAA gels cannot recapitulate [53].
Collagen type I-based hydrogels capture the viscoelastic and fibrillar nature of ECM in the TME (Figure 3c) [53]. Manipulation of collagen fibrillogenesis (e.g. by adjusting gelation temperature, collagen cross-linking agents, or the presence of a macromolecular crowding agent) enables selective control over collagen fiber structure and thus, scaffold microarchitecture and mechanics [39,54,55]. For example, lower casting temperature increases fiber diameter, scaffold pore size, and shear modulus independent of collagen concentration [39,55]. These differences have phenotypic consequences as stromal cells seeded into cold- versus warm-cast collagen hydrogels assume CAF-like characteristics enabled by localized strain-stiffening of the surrounding matrix [39,55]. The physical properties of collagen gels can be further adjusted by incorporating additional ECM components. For example, combining collagen and a dynamically crosslinked-HA hydrogel as a viscoelastic interpenetrating network (IPN) increases the compositional complexity and enables precise control over stress relaxation [56]. This toolbox can be additionally expanded by tuning HA crosslinker affinities, molecular weight, and concentration to generate faster stress relaxing gels that increase cell-mediated collagen fiber alignment, focal adhesion formation [56] and may affect cell cycle progression [57].
While collagen-based hydrogels permit a certain level of control over scaffold microarchitecture, collagen fibers in vitro differ significantly from those in vivo. Furthermore, isolating effects mediated by individual fiber properties (e.g. rigidity, thickness, and length) from bulk properties (e.g. scaffold porosity) is challenging, but can be accomplished with composite fibrous gel systems (Figure 3d). For example, electrospun methacrylated dextran (or dextran vinyl sulfone) fibers enable precise control over both bulk and individual fiber architecture and mechanics, while limiting fiber biochemical activity such as collagen degradation to influence cell phenotypes [54,58,59]. Softer fibers in these systems are more readily recruited by cells to promote focal adhesion formation and cell proliferation, a phenotype typically seen on stiffer hydrogels [58]. Encapsulating electrospun fibers in a hydrogel furthermore revealed that fiber density can regulate fibroblast mechanosignaling independently of bulk stiffness [59]. Together, these results suggest that the physical properties of ECM fibers can influence cell phenotypes independent of ECM bulk and biochemical properties.
Biomaterials Systems to Elucidate the Interplay between Tumor and ECM Heterogeneity
Biomaterials models have expanded our understanding of how biophysical alterations of the ECM influence tumor heterogeneity and stemness. For example, studies with PAA and fibrillar collagen gels suggest that ECM stiffness and microarchitecture synergize to increase CSC numbers and tumor metastatic burden [60]. Tumors cells cultured on stiff versus soft PAA gels increase stem cell marker expression and invasiveness, and hypoxia, an independent inducer of tumor cell stemness, further elevates these differences ultimately promoting tumor invasion and metastasis [61]. Comparing tumor cell migration in isotropic and anisotropic collagen gels additionally revealed that CSCs respond to collagen alignment with increased motility relative to their differentiated counterparts due to greater morphological plasticity and protrusion frequency [62]. Consistent with these findings, more weakly adherent cells migrate faster due in part to more labile focal adhesions, and these changes are predictive of metastatic potential [63]. While altered focal adhesion dynamics can be intrinsic to a specific cell phenotype, they are further regulated by collagen fiber structure. Adjusting collagen microarchitecture by altering gelation temperatures, for example, suggested that adhesion lifetimes depend on the balance between fiber mechanical properties and cell contractility [64]. While these connections have been elucidated with fibroblasts, fiber mechanical properties may also affect tumor cell stemness as focal adhesion-dependent cell signaling varies between CSCs and differentiated tumor cells [64,65].
Biomaterials systems have also increased understanding of how ECM-dependent changes of metabolism and DNA damage regulate tumor heterogeneity. Metabolic flexibility is a hallmark of cancer enabling tumor cells to produce energy and building blocks for growth. Furthermore, it maintains redox homeostasis by balancing reactive oxygen species (ROS) that influence tumor cell responses to DNA damage and thus, genomic instability as well as stem-like characteristics [66,67]. Interestingly, all of these mechanisms depend on the specific ECM environment in which tumor cells are located. Exposure of tumor cells to confining collagen microarchitectures (i.e. small pore sizes and short fiber length), for example, increases oxidative stress and ROS-responsive gene expression [54], which has been shown independently to promote the transition of quiescent CSCs to more proliferative CSCs [67]. In addition to affecting tumor cell phenotype through ROS-dependent changes in DNA damage, ECM-mediated cell confinement can alter DNA damage mechanisms directly. Indeed, tumor cell migration in confining ECM microarchitectures mechanically induces DNA damage due to nuclear deformation or rupture [68,69]. As CSCs are softer and have lower ROS levels as well as increased antioxidant defenses relative to more differentiated tumor cells, they may be less susceptible to nuclear rupture-induced DNA damage under confining ECM conditions [67,70,71]. Collectively, these results imply that the ECM microenvironment impacts tumor heterogeneity by altering their metabolism and DNA damage response with functional consequences on tumor pheno- and genotype.
Conclusion and Future Perspectives
Studies with engineered ECM models suggest that CSCs interpret biophysical changes in the ECM differently than their differentiated counterparts. These differences may contribute to the pheno- and genotypic heterogeneity of tumors by inducing the transformation (e.g. through altered mechanotransduction) and selection (e.g. by affecting DNA-damage mechanisms) of tumor cells with stem-like properties. While these examples highlight how ECM changes alter cell behavior, ECM and cellular heterogeneity are reciprocally linked and it is the interplay between both that drives the evolution of the TME.
Although biomaterials approaches have advanced knowledge of how ECM biophysical properties regulate tumor cell heterogeneity, the opposite is much less clear; i.e., how specific tumor cell subpopulations affect ECM heterogeneity (Figure 1). Cells under nutrient-rich conditions, for example, increase ECM deposition and stiffness, while nutrient deprivation causes ECM degradation possibly explaining varied abundancy of ECM at the invasive front versus central regions of a tumor [72]. Furthermore, cells exposed to a specific ECM or biomaterial deposit new ECM, which, in turn, influences cell adhesion and proliferation independent of the initial materials properties [55,73]. Such changes can, for example, affect mechanosignaling-dependent, long-range interactions between distant cells [39,74]. Model systems that incorporate both cellular and ECM heterogeneity will be essential to better understand how the reciprocal links between both parameters affect tumor progression, therapy resistance, and metastasis (Figure 1).
Engineered tumor models conventionally use cell lines, which are intrinsically homogeneous and do not accurately reflect properties of the original cell source making it difficult to assign a specific result to a particular cancer [75]. Patient-derived organoids (PDOs) can mimic the intratumoral heterogeneity of a specific patient [76,77], but are challenging to expand and thus, have not been widely adapted in engineered model systems yet. Furthermore, their formation typically relies on the use of Matrigel, which is problematic given the poorly defined nature and batch-to-batch variability of Matrigel. Microengineered cell culture arrays can facilitate the large-scale formation of organoids at significantly reduced Matrigel concentrations, which has the potential to provide scalable methods to generate PDOs for mechanistic studies of ECM-dependent tumor heterogeneity [77].
Monitoring cellular responses to ECM heterogeneity in a spatiotemporally controlled manner is equally critical to delineate the functional and mechanistic relationships between cells and ECM in engineered tumor models. Highly, multiplexed imaging [78] as well as high-throughput image acquisition and analysis pipelines [77] permit maximizing the amount of information that can be extracted from precious samples. In addition, advances in spatial transcriptomics provide molecular information at near cellular resolution [79]. When combined with computational analysis tools of single-cell sequencing these methods yield spatially resolved information to better understand the mechanisms of ECM-dependent tumor heterogeneity [79].
In conclusion, compositional and physical changes of the ECM and their spatiotemporal variations synergistically contribute to tumor heterogeneity by guiding cell fate decisions in the TME. Engineered model systems that can recapitulate both cellular and ECM heterogeneity are critical to elucidate the mechanisms through which ECM characteristics and different cellular states are linked. When combined with enabling technologies to spatiotemporally profile cell states as a function of the ECM new insights into tumor progression, metastasis, and therapeutic resistance will emerge that have the potential to inform more efficacious anti-cancer therapies.
Acknowledgements
The work described was supported by the Center on the Physics of Cancer Metabolism through Award Number 1U54CA210184-01 from the National Cancer Institute and an NSF Graduate Research Fellowship (DGE-1650441) to A. Shimpi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclosure
The authors declare no conflict of interest.
References
- 1.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, et al. : Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N Engl J Med 2012, 366:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinohara K, Polyak K: Intratumoral Heterogeneity: More Than Just Mutations. Trends Cell Biol 2019, 29:569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casasent AK, Schalck A, Gao R, Sei E, Long A, Pangburn W, Casasent T, Meric-Bernstam F, Edgerton ME, Navin NE: Multiclonal Invasion in Breast Tumors Identified by Topographic Single Cell Sequencing. Cell 2018, 172:205–210.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaffer SM, Emert BL, Reyes Hueros RA, Cote C, Harmange G, Schaff DL, Sizemore AE, Gupte R, Torre E, Singh A, et al. : Memory Sequencing Reveals Heritable Single-Cell Gene Expression Programs Associated with Distinct Cellular Behaviors. Cell 2020, 182:947–959.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using MemorySeq, a technique combining Luria and Delbrück’s fluctuation analysis with population-based RNA sequencing, this study revealed that otherwise homogeneous cell populations contain rare cells whose phenotype is determined by heritable, non-genetic changes in gene expression. This suggests that intratumoral heterogeneity is due in part to a new form of cell memory that is independent of genetic alterations.
- 5.Vasaikar S, Huang C, Wang X, Petyuk VA, Savage SR, Wen B, Dou Y, Zhang Y, Shi Z, Arshad OA, et al. : Proteogenomic Analysis of Human Colon Cancer Reveals New Therapeutic Opportunities. Cell 2019, 177:1035–1049.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibue T, Weinberg RA: EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat Rev Clin Oncol 2017, 14:611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balkwill FR, Capasso M, Hagemann T: The tumor microenvironment at a glance. J Cell Sci 2012, 125:5591–5596. [DOI] [PubMed] [Google Scholar]
- 8.Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, et al. : A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 2020, 20:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cazet AS, Hui MN, Elsworth BL, Wu SZ, Roden D, Chan C, Skhinas JN, Collot R, Yang J, Harvey K, et al. : Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat Commun 2018, 9:2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DelNero P, Lane M, Verbridge SS, Kwee B, Kermani P, Hempstead B, Stroock A, Fischbach C: 3D culture broadly regulates tumor cell hypoxia response and angiogenesis via pro-inflammatory pathways. Biomaterials 2015, 55:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baskaran JP, Weldy A, Guarin J, Munoz G, Shpilker PH, Kotlik M, Subbiah N, Wishart A, Peng Y, Miller MA, et al. : Cell shape, and not 2D migration, predicts extracellular matrix-driven 3D cell invasion in breast cancer. APL Bioeng 2020, 4:026105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz AD, Barney LE, Jansen LE, Nguyen TV., Hall CL, Meyer AS, Peyton SR: A biomaterial screening approach reveals microenvironmental mechanisms of drug resistance. Integr Biol 2017, 9:912–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Xiao Z, Meng Y, Zhao Y, Han J, Su G, Chen B, Dai J: The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials 2012, 33:1437–1444. [DOI] [PubMed] [Google Scholar]
- 14.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO: The matrisome: In silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics 2012, 11:M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desa DE, Strawderman RL, Wu W, Hill RL, Smid M, Martens JWM, Turner BM, Brown EB: Intratumoral heterogeneity of second-harmonic generation scattering from tumor collagen and its effects on metastatic risk prediction. BMC Cancer 2020, 20:1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drifka CR, Tod J, Loeffler AG, Liu Y, Thomas GJ, Eliceiri KW, Kao WJ: Periductal stromal collagen topology of pancreatic ductal adenocarcinoma differs from that of normal and chronic pancreatitis. Mod Pathol 2015, 28:1470–1480. [DOI] [PubMed] [Google Scholar]
- 17.Hamidi H, Ivaska J: Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer 2018, 18:533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barney LE, Hall CL, Schwartz AD, Parks AN, Sparages C, Galarza S, Platt MO, Mercurio AM, Peyton SR: Tumor cell-organized fibronectin maintenance of a dormant breast cancer population. Sci Adv 2020, 6:eaaz4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordahl S, Solorio L, Neale DB, McDermott S, Jordahl JH, Fox A, Dunlay C, Xiao A, Brown M, Wicha M, et al. : Engineered Fibrillar Fibronectin Networks as Three-Dimensional Tissue Scaffolds. Adv Mater 2019, 31:1904580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubow KE, Vukmirovic R, Zhe L, Klotzsch E, Smith ML, Gourdon D, Luna S, Vogel V: Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat Commun 2015, 6:8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nissen NI, Karsdal M, Willumsen N: Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J Exp Clin Cancer Res 2019, 38:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wishart AL, Conner SJ, Guarin JR, Fatherree JP, Peng Y, McGinn RA, Crews R, Naber SP, Hunter M, Greenberg AS, et al. : Decellularized extracellular matrix scaffolds identify full-length collagen VI as a driver of breast cancer cell invasion in obesity and metastasis. Sci Adv 2020, 6:eabc3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, Mohanan S, Morris PG, Du B, Zhou XK, et al. : Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med 2015, 7:301ra130–301ra130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auvinen P, Rilla K, Tumelius R, Tammi M, Sironen R, Soini Y, Kosma V-M, Mannermaa A, Viikari J, Tammi R: Hyaluronan synthases (HAS1–3) in stromal and malignant cells correlate with breast cancer grade and predict patient survival. Breast Cancer Res Treat 2014, 143:277–286. [DOI] [PubMed] [Google Scholar]
- 25.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, et al. : Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013, 62:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond E, Khurana A, Shridhar V, Dredge K: The Role of Heparanase and Sulfatases in the Modification of Heparan Sulfate Proteoglycans within the Tumor Microenvironment and Opportunities for Novel Cancer Therapeutics. Front Oncol 2014, 4:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evanko SP, Potter-Perigo S, Petty LJ, Workman GA, Wight TN: Hyaluronan Controls the Deposition of Fibronectin and Collagen and Modulates TGF-β1 Induction of Lung Myofibroblasts. Matrix Biol 2015, 42:74–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raitman I, Huang ML, Williams SA, Friedman B, Godula K, Schwarzbauer JE: Heparin-fibronectin interactions in the development of extracellular matrix insolubility. Matrix Biol 2018, 67:107–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourguignon LYW, Peyrollier K, Xia W, Gilad E: Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem 2008, 283:17635–17651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitale D, Kumar Katakam S, Greve B, Jang B, Oh E, Alaniz L, Götte M: Proteoglycans and glycosaminoglycans as regulators of cancer stem cell function and therapeutic resistance. FEBS J 2019, 286:2870–2882. [DOI] [PubMed] [Google Scholar]
- 31.Lohmann N, Schirmer L, Atallah P, Wandel E, Ferrer RA, Werner C, Simon JC, Franz S, Freudenberg U: Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci Transl Med 2017, 9:eaai9044. [DOI] [PubMed] [Google Scholar]
- 32.Nia HT, Munn LL, Jain RK: Physical traits of cancer. Science (80-) 2020, 370:eaaz0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossman M, Ben-Chetrit N, Zhuravlev A, Afik R, Bassat E, Solomonov I, Yarden Y, Sagi I: Tumor cell invasion can be blocked by modulators of collagen fibril alignment that control assembly of the extracellular matrix. Cancer Res 2016, 76:4249–4258. [DOI] [PubMed] [Google Scholar]
- 34.Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, Chen YY, Liphardt J, Hwang ES, Weaver VM: Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol 2015, 7:1120–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang JM, Park IA, Lee SH, Kim WH, Bae MS, Koo HR, Yi A, Kim SJ, Cho N, Moon WK: Stiffness of tumours measured by shear-wave elastography correlated with subtypes of breast cancer. Eur Radiol 2013, 23:2450–2458. [DOI] [PubMed] [Google Scholar]
- 36.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ: Aligned Collagen Is a Prognostic Signature for Survival in Human Breast Carcinoma. Am J Pathol 2011, 178:1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ: Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med 2006, 4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oudin MJ, Jonas O, Kosciuk T, Broye LC, Guido BC, Wyckoff J, Riquelme D, Lamar JM, Asokan SB, Whittaker C, et al. : Tumor Cell–Driven Extracellular Matrix Remodeling Drives Haptotaxis during Metastatic Progression. Cancer Discov 2016, 6:516–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall MS, Alisafaei F, Ban E, Feng X, Hui CY, Shenoy VB, Wu M: Fibrous nonlinear elasticity enables positive Mechanical feedback between cells and ECMs. Proc Natl Acad Sci U S A 2016, 113:14043–14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Attieh Y, Clark AG, Grass C, Richon S, Pocard M, Mariani P, Elkhatib N, Betz T, Gurchenkov B, Vignjevic DM: Cancer-associated fibroblasts lead tumor invasion through integrin-β3–dependent fibronectin assembly. J Cell Biol 2017, 216:3509–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandler EM, Saunders MP, Yoon CJ, Gourdon D, Fischbach C: Adipose progenitor cells increase fibronectin matrix strain and unfolding in breast tumors. Phys Biol 2011, 8:015008. [DOI] [PubMed] [Google Scholar]
- 42.Wang K, Andresen Eguiluz RC, Wu F, Seo BR, Fischbach C, Gourdon D: Stiffening and unfolding of early deposited-fibronectin increase proangiogenic factor secretion by breast cancer-associated stromal cells. Biomaterials 2015, 54:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martino MM, Hubbell JA: The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J 2010, 24:4711–4721. [DOI] [PubMed] [Google Scholar]
- 44.Hinz B: The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol 2015, 47:54–65. [DOI] [PubMed] [Google Scholar]
- 45.Korkaya H, Liu S, Wicha MS: Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest 2011, 121:3804–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouhrira N, Galie PA, Janmey PA: Hyaluronan Disrupts Cardiomyocyte Organization within 3D Fibrin-Based Hydrogels. Biophys J 2019, 116:1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kingsmore KM, Logsdon DK, Floyd DH, Peirce SM, Purow BW, Munson JM: Interstitial flow differentially increases patient-derived glioblastoma stem cell invasion via CXCR4, CXCL12, and CD44-mediated mechanisms. Integr Biol 2016, 8:1246–1260. [DOI] [PubMed] [Google Scholar]
- 48.Park D, Wershof E, Boeing S, Labernadie A, Jenkins RP, George S, Trepat X, Bates PA, Sahai E: Extracellular matrix anisotropy is determined by TFAP2C-dependent regulation of cell collisions. Nat Mater 2020, 19:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franco-Barraza J, Francescone R, Luong T, Shah N, Madhani R, Cukierman G, Dulaimi E, Devarajan K, Egleston BL, Nicolas E, et al. : Matrix-regulated integrin αvβ5 maintains α5β1-dependent desmoplastic traits prognostic of neoplastic recurrence. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaukonen R, Mai A, Georgiadou M, Saari M, De Franceschi N, Betz T, Sihto H, Ventelä S, Elo L, Jokitalo E, et al. : Normal stroma suppresses cancer cell proliferation via mechanosensitive regulation of JMJD1a-mediated transcription. Nat Commun 2016, 7:12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DuChez BJ, Doyle AD, Dimitriadis EK, Yamada KM: Durotaxis by Human Cancer Cells. Biophys J 2019, 116:670–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bangasser BL, Shamsan GA, Chan CE, Opoku KN, Tüzel E, Schlichtmann BW, Kasim JA, Fuller BJ, McCullough BR, Rosenfeld SS, et al. : Shifting the optimal stiffness for cell migration. Nat Commun 2017, 8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB: Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Velez DO, Ranamukhaarachchi SK, Kumar A, Modi RN, Lim EW, Engler AJ, Metallo CM, Fraley SI: 3D collagen architecture regulates cell adhesion through degradability, thereby controlling metabolic and oxidative stress. Integr Biol 2019, 11:221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using collagen scaffolds with confining microarchitectures this study revealed that small ECM pore size can reduce cancer cell adhesion by inhibiting matrix degradation. Furthermore, these conditions induced metabolic and oxidative stress similar to suspension culture, but simultaneously upregulated Snail1 and Notch signaling.
- 55.Seo BR, Chen X, Ling L, Song YH, Shimpi AA, Choi S, Gonzalez J, Sapudom J, Wang K, Eguiluz RCA, et al. : Collagen microarchitecture mechanically controls myofibroblast differentiation. Proc Natl Acad Sci U S A 2020, 117:11387–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]; By controlling collagen microarchitecture through adjusting the casting temperature, this study found that collagen fiber thickness and pore size regulate the viscoelastic behavior of collagen gels with functional consequences on cellular mechanosignaling, subsequent ECM deposition, and activation of stromal cells into CAF-like myofibroblasts.
- 56.Lou J, Stowers R, Nam S, Xia Y, Chaudhuri O: Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials 2018, 154:213–222. [DOI] [PubMed] [Google Scholar]
- 57.Nam S, Gupta VK, Lee H, Lee JY, Wisdom KM, Varma S, Flaum EM, Davis C, West RB, Chaudhuri O: Cell cycle progression in confining microenvironments is regulated by a growth-responsive TRPV4-PI3K/Akt-p27 Kip1 signaling axis. Sci Adv 2019, 5:eaaw6171. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using alginate hydrogels and tuning the ionic cross-link density to generate hydrogels with varying stress relaxation rates, this study found that fast stress relaxing hydrogels promote cell cycle progression.
- 58.Baker BM, Trappmann B, Wang WY, Sakar MS, Kim IL, Shenoy VB, Burdick JA, Chen CS: Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat Mater 2015, 14:1262–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matera DL, Wang WY, Smith MR, Shikanov A, Baker BM: Fiber Density Modulates Cell Spreading in 3D Interstitial Matrix Mimetics. ACS Biomater Sci Eng 2019, 5:2965–2975. [DOI] [PubMed] [Google Scholar]; Combining electrospun dextran vinylsulfone fibers with hydrogels such as methacrylated gelatin this study shows that increased fiber density independent of bulk hydrogel elastic modulus and fiber diameter increase cell spreading and mechanosignaling.
- 60.Shea MP, O’Leary KA, Wegner KA, Vezina CM, Schuler LA: High collagen density augments mTOR-dependent cancer stem cells in ERα+ mammary carcinomas, and increases mTOR-independent lung metastases. Cancer Lett 2018, 433:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pang MF, Siedlik MJ, Han S, Stallings-Mann M, Radisky DC, Nelson CM: Tissue stiffness and hypoxia modulate the integrin-linked kinase ilk to control breast cancer stem-like cells. Cancer Res 2016, 76:5277–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ray A, Slama ZM, Morford RK, Madden SA, Provenzano PP: Enhanced Directional Migration of Cancer Stem Cells in 3D Aligned Collagen Matrices. Biophys J 2017, 112:1023–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beri P, Popravko A, Yeoman B, Kumar A, Chen K, Hodzic E, Chiang A, Banisadr A, Placone JK, Carter H, et al. : Cell adhesiveness serves as a biophysical marker for metastatic potential. Cancer Res 2020, 80:901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doyle AD, Carvajal N, Jin A, Matsumoto K, Yamada KM: Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat Commun 2015, 6:8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo M, Zhao X, Chen S, Liu S, Wicha MS, Guan JL: Distinct FAK activities determine progenitor and mammary stem cell characteristics. Cancer Res 2013, 73:5591–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vander Heiden MG, Cantley LC, Thompson CB: Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science (80-) 2009, 324:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luo M, Shang L, Brooks MD, Jiagge E, Zhu Y, Buschhaus JM, Conley S, Fath MA, Davis A, Gheordunescu E, et al. : Targeting Breast Cancer Stem Cell State Equilibrium through Modulation of Redox Signaling. Cell Metab 2018, 28:69–86.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah P, Hobson CM, Cheng S, Colville MJ, Paszek MJ, Superfine R, Lammerding J: Nuclear Deformation Causes DNA Damage by Increasing Replication Stress. Curr Biol 2020, 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xia Y, Pfeifer CR, Zhu K, Irianto J, Liu D, Pannell K, Chen EJ, Dooling LJ, Tobin MP, Wang M, et al. : Rescue of DNA damage after constricted migration reveals a mechano-regulated threshold for cell cycle. J Cell Biol 2019, 218:2542–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lv J, Liu Y, Cheng F, Li J, Zhou Y, Zhang T, Zhou N, Li C, Wang Z, Ma L, et al. : Cell softness regulates tumorigenicity and stemness of cancer cells. EMBO J 2021, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al. : Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458:780–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Romani P, Valcarcel-Jimenez L, Frezza C, Dupont S: Crosstalk between mechanotransduction and metabolism. Nat Rev Mol Cell Biol 2021, 22:22–38. [DOI] [PubMed] [Google Scholar]
- 73.Loebel C, Mauck RL, Burdick JA: Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat Mater 2019, 18:883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that cells cultured within degradable and viscoelastic hydrogels readily deposit new ECM proteins with which they subsequently interact in a manner that affects cell spreading and mechanosignaling. These results suggest that nascent proteins can mask signals from the initial ECM or cell culture matrix.
- 74.Wang H, Abhilash AS, Chen CS, Wells RG, Shenoy VB: Long-range force transmission in fibrous matrices enabled by tension-driven alignment of fibers. Biophys J 2015, 107:2592–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gillet JP, Calcagno AM, Varma S, Marino M, Green LJ, Vora MI, Patel C, Orina JN, Eliseeva TA, Singal V, et al. : Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc Natl Acad Sci U S A 2011, 108:18708–18713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharick JT, Walsh CM, Sprackling CM, Pasch CA, Pham DL, Esbona K, Choudhary A, Garcia-Valera R, Burkard ME, McGregor SM, et al. : Metabolic Heterogeneity in Patient Tumor-Derived Organoids by Primary Site and Drug Treatment. Front Oncol 2020, 10:553. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using label-free optical metabolic imaging to measure the redox state of individual cells within patient-derived organoids this study shows that intratumoral heterogeneity of cellular redox state can predict patient therapeutic response.
- 77.Brandenberg N, Hoehnel S, Kuttler F, Homicsko K, Ceroni C, Ringel T, Gjorevski N, Schwank G, Coukos G, Turcatti G, et al. : High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat Biomed Eng 2020, 4:863–874. [DOI] [PubMed] [Google Scholar]; This study developed of a high-throughput method to reproducibly generate patient-derived organoids using poly(dimethylsiloxan) (PDMS) microwell and polyethylene glycol (PEG) hydrogel arrays. When combined with a robotic system and automated image analysis, this platform enabled drug testing in precision medicine settings.
- 78.Lin J-R, Fallahi-Sichani M, Sorger PK: Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method. Nat Commun 2015, 6:8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stickels RR, Murray E, Kumar P, Li J, Marshall JL, Di Bella DJ, Arlotta P, Macosko EZ, Chen F: Highly sensitive spatial transcriptomics at near-cellular resolution with Slide-seqV2. Nat Biotechnol 2020, doi: 10.1038/s41587-020-0739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


