Abstract
Background:
Parkinson’s disease (PD) is marked clinically by motor symptoms and pathologically by Lewy bodies and dopamine neuron loss in the substantia nigra pars compacta (SNc). Higher iron accumulation, assessed by susceptibility MRI, also is observed as PD progresses. Recently, evidence has suggested that PD affects the retina.
Objective:
To better understand retinal alterations in PD and their association to clinical and SNc iron-related imaging metrics.
Methods:
Ten PD and 12 control participants (2 eyes each) from an ongoing PD imaging biomarker study underwent enhanced depth imaging optical coherence tomography evaluation. Choroidal (vascular) thickness and nerve layers were measured in 4 subregions [superior, temporal, inferior, and nasal] and at 3 foveal distances (1, 1.5, and 3 mm). These metrics were compared between PD and control groups. For significantly different metrics, their associations with clinical [levodopa equivalent daily dosage (LEDD), motor and visuospatial function] and SNc susceptibility MRI metrics [R2* and quantitative susceptibility mapping (QSM)] were explored.
Results:
Compared to control participants, PD participants had a thicker choroid (p=0.005), but no changes in nerve layers. Higher mean choroidal thickness was associated with lower LEDD (p<0.01) and better visuospatial function (p<0.05). Subregion analyses revealed higher choroidal thickness correlated with lower LEDD and improved motor and visuospatial measures. Higher mean choroidal thickness also was associated with lower nigral iron MRI (p<0.05).
Conclusions:
A small cohort of PD research participants displayed higher choroidal thickness that was related to better clinical performance and less nigral pathology. These intriguing findings warrant further investigation.
Keywords: choroid, optical coherence tomography, Parkinson’s disease, retina, susceptibility MRI, visuospatial function
1. Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases that affects approximately 1% of people over the age of 60 [1]. Clinically, PD presents with motor (i.e., resting tremor, rigidity, bradykinesia) and non-motor (i.e., cognitive decline, depression, and autonomic dysfunction) symptoms. Pathologically, the disease process is marked by α-synuclein positive Lewy bodies and loss of dopaminergic neurons in the substantia nigra pars compacta (SNc). Beyond the key Lewy body pathology, higher iron accumulation also is observed in the SNc of PD participants. Susceptibility MRI, reflecting brain iron content, may reflect pathological processes in the SNc [2] and track PD progression [3].
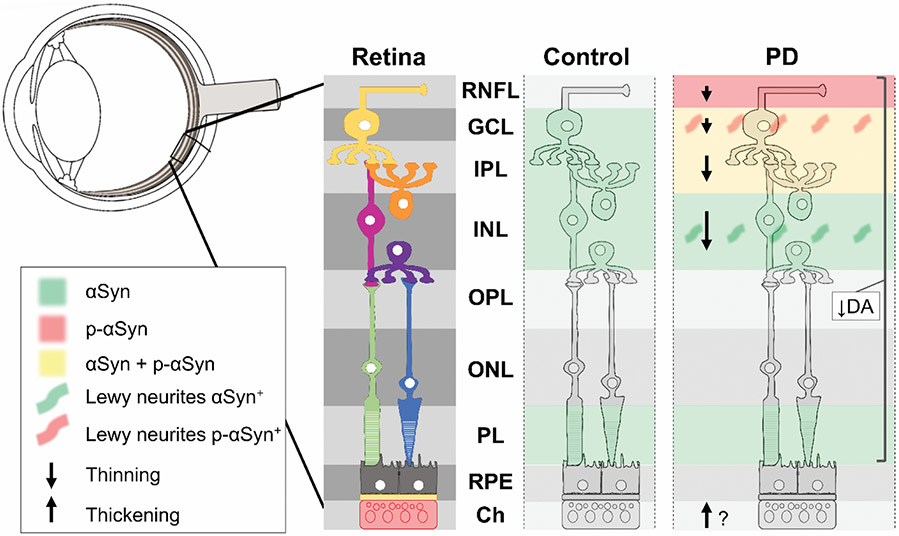
While often under-recognized, visual dysfunction is common and reported by 80% of PD participants [4]. Although the exact cause is unknown, some of the visual deficits in PD are due, at least in part, to retinal dopamine deficiency [4]. The retina is composed of several layers of neurons that use dopamine as a neurotransmitter, and alterations in these layers may have possible implications for PD pathophysiology (Fig. 1). Dopaminergic cells primarily are located in the inner layers of the macula and fovea, and post-mortem studies in PD have shown α-synuclein deposits in the inner layers and loss of dopamine neurons throughout the retina [5,6]. Many studies have investigated the retinal nerve fiber layer (RNFL), which contributes to 30-35% of retinal thickness and is thinner in all quadrants in PD participants [7]. Furthermore, Lewy bodies have been detected in the ganglion cell (GCL) and inner nuclear (INL) layers [5]. Thinning of the GCL and inner plexiform layers (IPL, collectively called the GCIP) has been associated with early progression to dementia in PD participants [8]. The outermost layers of the retina [i.e. the outer plexiform (OPL), nuclear (ONL), and photoreceptor (PL) layers and retinal pigmented epithelium (RPE)] have been less implicated in PD [5].
Fig 1. Summary of PD-related pathologies present in the retina according to previous studies.
Previous studies have identified α-synuclein deposits in the inner retinal layers, as well as thinning of inner retina layers (via OCT) and decreased dopamine throughout the retinal layers. Additionally, there are opposing results amongst studies describing choroidal thickness in PD participants, where two studies have stated there is an increase in thickness [11,16], one reported increased choroidal and lumen area [17], and one study reported a decrease in thickness [10]. Abbreviations: αSyn: alpha-synuclein, Ch: choroid, DA: dopamine, GCL: ganglion cell layer, INL: inner nuclear layer, IPL: inner plexiform layer, OCT: optical coherence tomography, ONL: outer nuclear layer, OPL: outer plexiform layer, p-αSyn: phosphorylated alpha-synuclein, PD: Parkinson’s disease, PL: photoreceptor layer, RNFL: retinal nerve fiber layer, RPE: retinal pigmented epithelium. Figure was adapted from Veys et al. [5].
The choroid is a complex, highly vascularized structure providing nutrients to the outer layers of the retina. Increased choroidal thickness using optical coherence tomography (OCT) was identified in several diseases, including polypoidal choroid vasculopathy and central serous chorioretinopathy [9]. To date, there have been conflicting studies regarding choroidal thickness in PD [8,10,11], although no study has yet to relate choroidal thickness to metrics of PD severity.
In this study, we obtained state-of-art enhanced depth imaging (EDI)-OCT to measure the thickness of vascular (i.e., choroidal) and neuronal layers in PD and related the measures with PD-related clinical severity (motor and non-motor) and imaging (R2*) metrics. We hypothesized that the choroid and retinal nerve layers would be altered in PD compared to control participants, and, in PD, these metrics would relate to clinical [i.e., levodopa equivalent daily dosage (LEDD), motor symptoms, and visuospatial function] and SNc MRI metrics [i.e., R2* and quantitative susceptibility mapping (QSM)]. Testing these hypotheses may help to understand retinal alterations within the context of broader PD-related processes.
2. Experimental Procedures
2.1. Study participants
The study protocol was approved by the Penn State Hershey Institutional Review Board and written informed consent was obtained from all participants. A total of 10 PD (9 Caucasian, 1 race unknown) and 12 control (8 Caucasian, 4 race unknown) participants were recruited from an ongoing cohort participating in the National Institute of Neurological Disorders and Stroke PD Biomarker Program (NINDS PDBP) at Pennsylvania State University. Diagnosis was confirmed by movement disorders specialists according to previously published criteria [12]. Other inclusion criteria were age ≥18 years, intraocular pressure 10-22 mmHg, Mini Mental Status Exam (MMSE) score >24, and refractive error <4.0 diopters. Control participants had no known neurological diseases. Exclusion criteria for the study were the presence of other ocular diseases including diabetic retinopathy, age-related macular degeneration, retinal or optic nerve abnormalities, retinal arterial or venous occlusions, uveitis, ocular hypertension, or glaucoma. Age, sex, and axial length were obtained from the same visit as the OCT measures. Behavioral metrics including Hoehn and Yahr score (H&Y), disease duration, LEDD [13], the United PD Rating Scale (UPDRS) part III score (a measure of motor severity assessed in the “on” state), and a figure-copy task of visuospatial function were obtained from a recent NINDS PDBP visit. All medications for participants are listed in Supplementary Table 1.
2.2. Ophthalmic evaluation
Forty-four eyes (10 PD and 12 control) were analyzed for this study. All participants underwent an ophthalmic evaluation including assessment of distance visual acuity via Snellen’s chart, intraocular pressure via Reichert Tonopen XL tonometer, color perception via Ishihara color plates, contrast sensitivity via Pelli-Robinson CS chart, axial length via Zeiss IOLMaster 700 (Carl Zeiss Meditec AG, Jena, Germany), and retinal and choroidal imaging with EDI-OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany). Low-quality OCT scans were excluded and repeat scans were obtained.
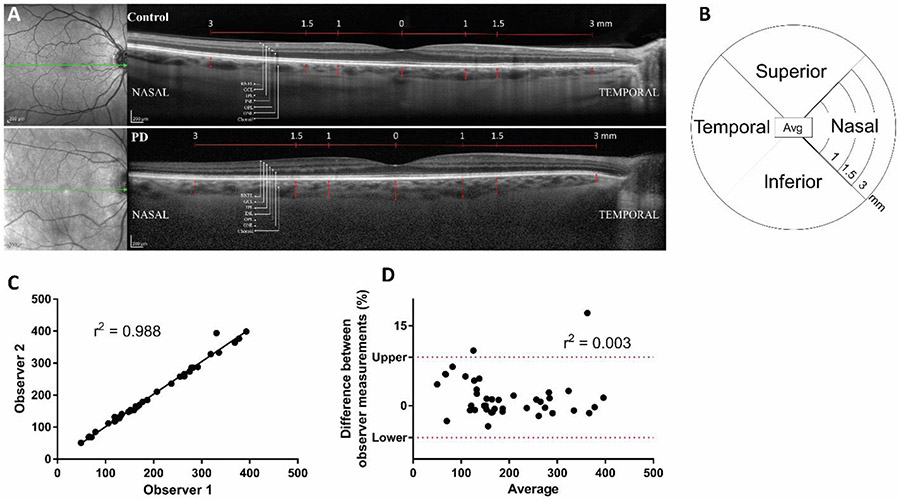
EDI-OCT scans were analyzed by one of two independent blinded investigators (either SK or MC) to obtain choroidal thickness and retinal measures. Fig. 2A shows representative images from one control and one PD participant. Fig 2B is a schematic of the 12 subregions of measurements [4 quadrants (superior, nasal, inferior, and temporal) at 3 distances (1, 1.5, and 3mm)] with the average value in the middle. To measure the thickness of the choroid layer, in-software calipers were used to draw a vertical line between the retinal pigment epithelium and choroidoscleral junction. The observers were trained by expert retinal imagers. Then, observer caliper placement was calibrated by the two observers measuring two OCT images together before actual measurements. To validate inter-rater variability, both observers analyzed images from 3 random control and 3 random PD participants. The observer measurements were plotted against each other on a linear correlation graph (Fig. 2C) and Pearson’s r2 coefficient was determined to be 0.988, suggesting a very strong correlation. Additionally, the difference between observer measurements (%) versus the average measurement was plotted on a Bland-Altman plot (Fig. 2D) where r2=0.003, indicating there is little difference between observer measurements. Combined, these measurements suggest that the interobserver variability was very low and, therefore, the measurements are reliable. With this very high interobserver validity, each image was analyzed by a single observer. The thickness of the RNFL, GCL, IPL, INL, OPL, ONL, and RPE were auto-segmented using Heidelberg Engineering Spectralis, Viewing Module 6.6.2.0. Spectralis. EDI-OCT results of each layer were obtained at 1.0 mm, 1.5 mm, and 3.0 mm from the fovea in four quadrants (inferior, nasal, superior, temporal) from orthogonal B-scans produced from each study eye.
Fig 2. Methods and validation of OCT measurements.
A) Representative images of one PD participant OCT and one control participant OCT showing the nerve layers measured. The red arrows indicate the choroidal thickness. B) A representative plot of the 12 subregions measured [4 quadrants (superior, nasal, inferior, and temporal) at 3 distances (1, 1.5, and 3mm)] with the average value in the middle. Interobserver variability was determined by plotting C) observer 1 versus 2 measurements on a linear scatter plot where r2=0.988, indicating a very strong agreement between observer measurements; or D) the difference between observer measurements (%) versus the average measurement on a Bland-Altman plot where r2=0.003, indicating there is little difference between observer measurements.
2.3. Substantia nigra MRI acquisition and analysis
Of the 22 total participants, 14 participants (7 PD and 7 control participants) had brain MRIs obtained for the PD biomarker study. All participants were scanned using a 3.0 Tesla MR scanner (Trio, Siemens Magnetom, Erlangen, Germany, with an 8-channel phased-array head coil). T2*-weighted images were acquired using a multi-gradient-echo sequence with 8 echoes (echo times ranging from 6.2 to 49.6 milliseconds with equal spacing), and repetition time, 55 milliseconds; flip angle, 15 °; field of view, 240 × 240; matrix, 256 × 256; slice thickness, 2 mm; slice number, 64; and voxel size, 0.9 × 0.9 × 2 mm3. All images were inspected offline and deemed free of severe motion artifacts or any major structural abnormalities. Transverse relaxation rate (R2*) images were generated by nonlinear curve fitting of a mono-exponential equation (S(TE)=S0e−R2*TE), using the Levenberg-Marquardt approach with an in-house MATLAB program. QSM images were generated using the morphology-enabled dipole inversion method with a nonlinear formulation of the magnetic field to the source.11 The substantia nigra pars compacta (SNc) was segmented using automatic atlas-based parcellation, followed by manual correction as described in our previous work [3]. SNc R2* and QSM metrics have been used previously to reflect higher nigral iron in PD [2] and have been associated with worse clinical metrics [3].
2.4. Statistical analysis
Statistical analyses were performed using Matlab (version R2020a; MathWorks, Natick, Massachusetts). Demographic information between PD and control participants was compared using 2-sided Student’s t-tests. To compare PD and control OCT metrics, the effect of PD status was analyzed using a mixed-effects general linear model (GLM) that also included age, sex, and axial-length as covariates. Average values were corrected for the distance from the fovea and quadrant.
Within PD, no effect of side of disease onset was observed (Supplementary Fig. 1). Therefore, choroidal thickness was averaged between both eyes for each participant and correlated using a bootstrapped partial Pearson’s correlation with behavioral and MRI metrics correcting for age, sex, and axial-length using 10,000 repetitions for PD and control participants. The 95% confidence intervals were determined using the 2.5 and 97.5 percentile values. Exact p-values are not reported for correlations, since this would be statistically inappropriate for bootstrapped correlations and confidence intervals are more informative of spread. No correction for multiple comparisons was applied in this pilot study.
3. Results
3.1. Demographics
PD participants tended to be younger and more likely female than control participants, although the group comparisons were not significant. PD participants had a shorter axial length (p=0.006), but no other ophthalmic metrics were significantly different between the groups. For PD participants, the average H&Y score was 2.0 ± 1.2, disease duration was 13.7 ± 5.3 years, and LEDD was 796 ± 298. When correcting for age, sex, and axial length, motor [i.e., UPDRS III (p=0.006)] and MRI [i.e., SNc R2* (p=0.047) and SNc QSM (p=0.043)] metrics of PD severity were different between PD and control participants, while figure-copy performance was not (p=0.884). The summary of demographic, clinical, and MRI information is shown in Table 1.
Table 1.
Demographic and clinical information. Data represent mean ± standard deviation, except sample size which represents number (Male/Female). P-values were derived from a 2-sided t-test. Best corrected visual acuity (BCVA) is presented as logMAR for statistical analysis with mean logMAR converted to closest Snellen visual acuity ratio for interpretation and presented in parentheses.
| Control | PD | p-value | |
|---|---|---|---|
| N (M/F) | 12 (9/3) | 10 (4/6) | 0.097 |
| Age, years | 75.2 ± 7.9 | 70.6 ± 9.0 | 0.218 |
| H&Y | N/A | 2.0 ± 1.2 | N/A |
| Disease Duration | N/A | 13.7 ± 5.3 | N/A |
| Ophthalmic metrics | |||
| AL, mm | 24.3 ± 0.8 | 23.5 ± 0.9 | 0.006 |
| BCVA, logMAR | 0.07 ± 0.11 (20/20) | 0.15 ± 0.13 (20/25) | 0.137 |
| Spherical Equivalence | 0.7 ± 0.8 | 1.1 ± 0.9 | 0.205 |
| Contrast | 1.5 | 1.5 ± 0.1 | 0.081 |
| Pressure | 15.4 ± 2.1 | 13.9 ± 2.1 | 0.137 |
| R/G Colorblind (#) | 0/12 | 1/10 | 0.263 |
| Clinical metrics | |||
| LEDD | N/A | 796 ± 298 | N/A |
| UPDRS III | 10.2 ± 8.3 | 21.8 ± 10.4 | 0.006* |
| Figure-Copy | 48.2 ± 14.6 | 42.8 ± 12.6 | 0.884* |
| SNc MRI metrics | |||
| R2* | 24.0 ± 2.4 | 28.4 ± 5.2 | 0.047* |
| QSM | 536.8 ± 32.6 | 570.1 ± 40.8 | 0.043* |
Indicates corrected for age, sex, and axial length. Abbreviations: AL: axial length, F: female, H&Y: Hoehn and Yahr score, LEDD: levodopa equivalent daily dosage, N: sample size, PD: Parkinson’s disease, QSM: quantitative susceptibility mapping, SNc: Substantia nigra pars compacta, UPDRS: United Parkinson’s Disease Rating Scale, UPSIT: University of Pennsylvania Smell Identification Test.
3.2. Comparison of retinal metrics between PD and control participants
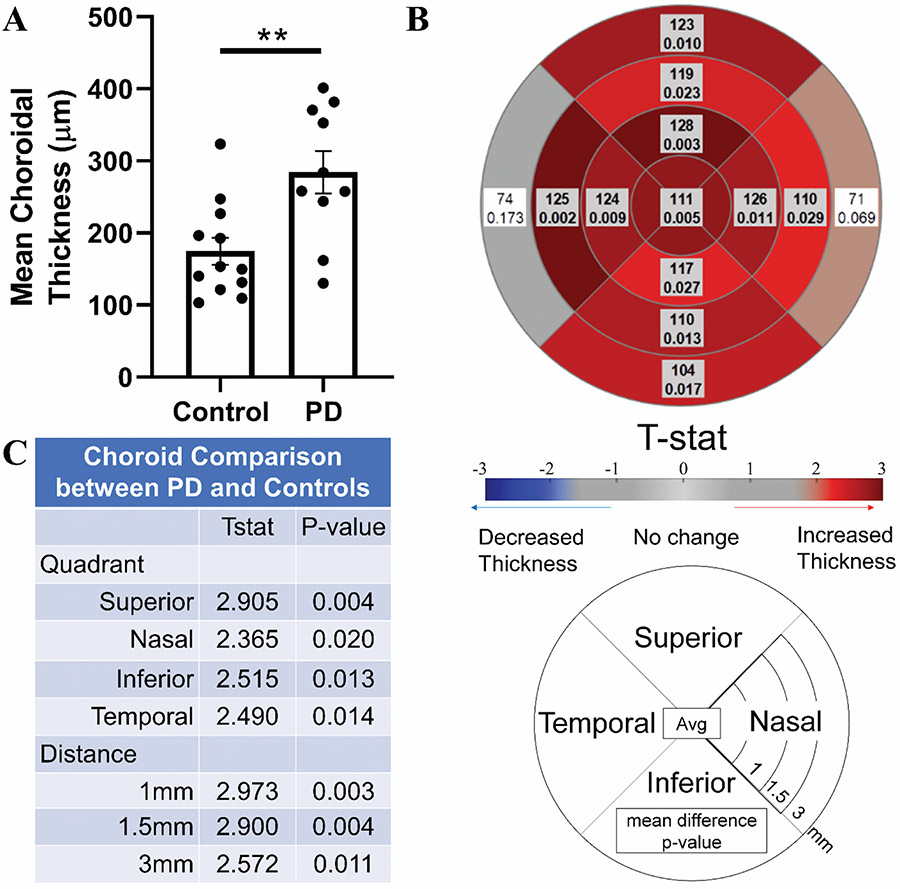
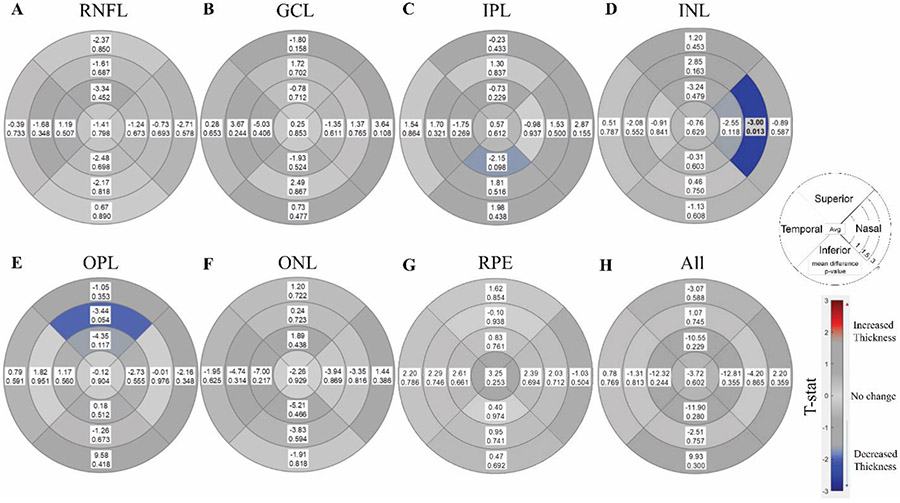
Mean choroidal thickness (p=0.005, Fig. 3A) was significantly higher in PD participants compared to control participants. Subregion analyses showed choroidal thickness was higher in all quadrants and regions except the temporal and nasal quadrants at 3mm (0.002<p<0.029, Fig. 3B). Comparing quadrants regardless of distance, PD participants’ choroids were thicker in all four quadrants, particularly in the superior quadrant (p=0.004, Fig. 3C). Comparing distances regardless of the quadrant, PD participants had thicker choroids at all three distances, with the strongest effects occurring closest to the fovea (p=0.003, 0.004, and 0.011 for 1mm, 1.5mm, and 3mm, respectively, Fig. 3C). There were no differences in the mean values of any retinal nerve layer (p>0.602, Fig. 4). Only one subregion was significant, the nasal quadrant of the INL at 1.5mm (p=0.012, Fig. 4D).
Fig 3. Comparison of choroidal thickness between PD and control participants.
A) Comparison of mean choroidal thickness between control and PD participants, correcting for age, sex, axial length, distance from fovea, and quadrant. B) Subregion analysis comparing control and PD participants using a mixed effect model correcting for age, sex, and axial length. Shaded/Bolded boxes represent significance (p<0.05). C) Effect of PD status on choroid thickness in each quadrant (or distance) using a mixed effects model correcting for distance (or quadrant), age, sex, and axial length.
Fig 4. Comparison of retinal layers between PD and controls participants.
Subregion analysis comparing nerve layer thickness between control and PD participants using a mixed effect model correcting for age, sex, and axial length for A) retinal nerve fiber layer (RNFL), B) ganglion cell layer (GCL), C) inner plexiform layer (IPL), D) inner nuclear layer (INL), E) outer plexiform layer (OPL), F) outer nuclear layer (ONL), G) retinal pigmented epithelium (RPE) and H) all layers combined. Circle plot represents the 4 quadrants (superior, nasal, inferior, and temporal) and 3 distances (1,1.5, and 3mm), with average displayed in the center. Each box represents the mean difference (PD - Control) on top and p-value below. Shaded/Bolded boxes represent significance (p<0.05).
3.3. Association between choroidal thickness and clinical metrics within PD participants
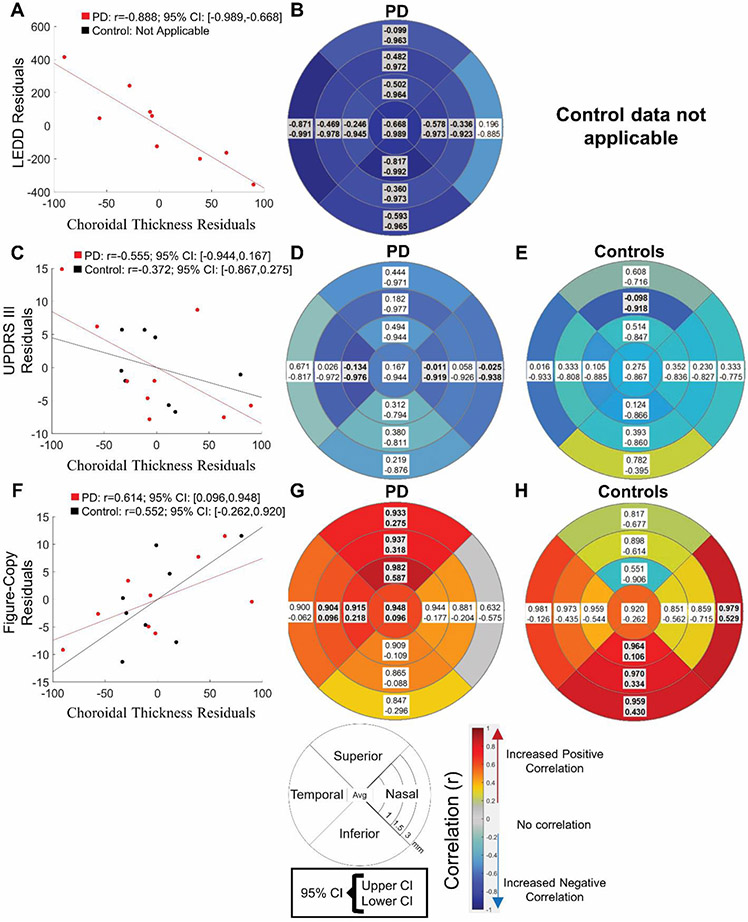
A thicker mean choroid was associated significantly with better performance on a figure copy task in PD but not control participants (PD: r= 0.614, 95% CI: 0.096<r<0.948, control: 0.552, 95% CI: −0.262<r<0.920, Fig. 5A). Subregion analysis revealed, for PD participants, a thicker choroid in the temporal and superior quadrant at 1 and 1.5 mm and the superior quadrant at 3mm was correlated with better figure-copy performance (0.580<r<0.862, Fig. 5B). In control participants, a thicker choroid in the inferior quadrant at 1, 1.5 mm and 3mm and the nasal quadrant at 3mm was correlated with better figure-copy performance (0.106<r<0.979, Fig. 5C). Higher mean choroidal thickness was not associated significantly with better motor function in either PD or control participants (PD: r=−0.555, 95% CI: −0.944<r< 0.167, control: r=−0.372, 95% CI: −0.867<r< 0.275, Fig. 5D), but subregion analysis revealed that, for PD participants, a thicker choroid in the temporal and nasal quadrants at 1mm and the nasal quadrant at 3mm was correlated with better motor function (UPDRS-III score, −0.712<r<−0.576, Fig. 5E). For control participants, a thicker choroid in the superior quadrant at 1mm was correlated with better motor function (UPDRS-III score, −0.918<r<−0.098, Fig. 5F). A thicker mean choroid was associated significantly with lower LEDD in PD participants (r= −0.888, 95% CI: −0.989<r<−0.668, Fig. 5G). Subregion analysis for PD participants revealed a thicker choroid in all regions except the nasal quadrant at 3 mm was correlated with lower LEDD (−0.925<r<−0.731, Fig. 5H). LEDD analyses are not applicable for control participants.
Fig 5. Relationship between choroidal thickness and clinical metrics in PD and control participants.
Bootstrapped partial correlations in Parkinson’s disease (PD) between average choroidal thickness between both eyes and clinical variables of A) mean and subregion analysis of visuospatial function (figure-copy task) in B) PD and C) control participants, D) mean and subregion analysis of motor (Unified Parkinson’s Disease Rating Scale-III, UPDRS III) in E) PD and F) control participants, G) mean and H) subregion analysis of levodopa equivalent daily dosage (LEDD) in PD participants. Control participants are not on dopaminergic therapies so this last metric is not applicable. Covariates are age, sex, and axial length, and 10,000 samples were used. Boxes represent 95% confidence interval and the color represents the mean correlation coefficient (r). Boxes represent 95% confidence interval (CI) and the color represents the mean correlation coefficient (r). Shaded/Bolded boxes represent significance (p<0.05).
3.4. Association between choroidal thickness and MRI metrics
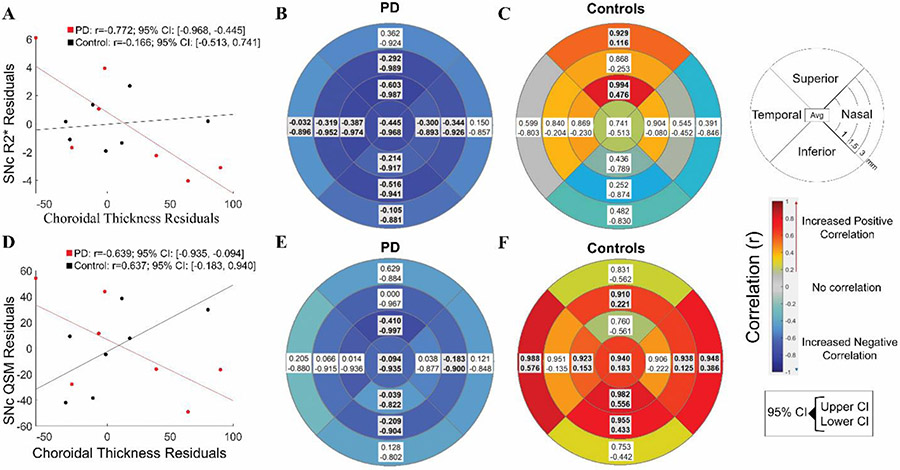
A thicker mean choroid correlated with lower R2* in the SNc in PD but not control participants (PD: r=0.772, 95% CI: −0.968<r<−0.445, control: r=0.166, 95% CI: −0.513<r<−0.741, Fig. 6A). Subregion analyses for PD participants revealed choroidal thickness in all regions except at 3mm in the superior and nasal quadrants correlated negatively with SNc R2* values (−0.844<r<−0.493, Fig. 6B). For control participants, thicker choroid correlated with increased R2* in the superior quadrant at 1mm and 3mm (0.116<r<0.994, Fig. 6C). Thicker mean choroid measurements also correlated with lower QSM in the SNc for PD and higher QSM in the SNc in control participants (PD: r=−0.639, 95% CI: −0.935<r<−0.094, control: r=0.637, 95% CI: 0.183<r<0.940, Fig. 6D). Subregion analysis revealed choroidal thickness in the superior and inferior quadrants at 1mm, and the nasal and inferior quadrants at 1.5mm, correlated negatively with SNc QSM values for PD participants (−0.773<r<−0.613 Fig. 6E). For control participants, choroidal thickness in the inferior and temporal quadrants at 1mm; the superior, nasal, and inferior quadrants at 1.5mm; and nasal and temporal quadrants at 3mm correlated positively with SNc QSM values (0.125<r<−0.988 Fig. 6F).
Fig 6. Association between choroidal thickness and SNc MRI metrics in PD and control participants.
Bootstrapped partial correlations between average choroidal thickness of both eyes and MRI metrics A) mean, subregion analysis of R2* in B) Parkinson’s Disease (PD) and C) control participants and D) mean and subregion analysis of quantitative susceptibility mapping (QSM) in the substantia nigra pars compacta (SNc) in E) PD and F) control participants. Covariates are age, sex, and axial length, and 10,000 samples were used. Boxes represent 95% confidence interval (CI) and the color represents the mean correlation coefficient (r). Shaded/Bolded boxes represent significance (p<0.05).
4. Discussion
In this pilot study, we found that a small cohort of PD participants of ongoing biomarker research had increased choroidal thickness compared to control participants. A thicker choroid was present in all quadrants, with the effects appearing strongest in the superior quadrant and closer to the fovea. Despite the large effect in the choroid, we did not identify a consistent pattern of nerve layer changes in PD. Most intriguingly, there was a significant association between a thicker choroid and better visuospatial and motor function and lower LEDD. These results warrant additional studies that may shed light on vascular changes in PD-related pathophysiological processes.
4.1. Choroidal thickness is higher in a small cohort of PD participants
We identified a thicker choroid in our small cohort of PD research participants, even after correcting for age, sex, and axial length as potential confounders (Fig. 3). The mean choroidal thickness measured 1mm from the fovea in PD was greater than the 75th percentile of previously reported choroidal thickness measurements in healthy participants between 70-79 years of age [14]. Furthermore, an observed difference of 111μm between PD and controls is clinically quite large. We suspect that the thicker retinal choroid is probably systemic, because the choroid was thicker in PD participants in all four quadrants and at all three distances (Fig. 3) and there were no differences between ipsi- and contralateral eye to disease onset (Supplementary Fig. 1). PD is known to be an asymmetric illness [15], which may stem from differences in neuronal loss in the SNc. Our findings suggest that increased choroidal thickness may not be directly related to SNc neuronal loss, but instead be a parallel or compensatory pathophysiological process.
To date, only a few studies have compared choroidal metrics in PD and control participants [10,11,16,17]. The results have been mixed, with two studies reporting increased choroidal thickness [11,16], one reporting increased choroidal and luminal area [17], and another reporting decreased choroidal thickness [10] in PD participants. Our results agree with the findings that choroidal thickness is significantly increased in the PD retina. Eraslan et al. (2016) reported that choroid thickness is decreased in PD, but they also had participants with more severe PD [8]. Our study revealed that, in our research participants with PD, a thinner choroid is associated with worse motor severity, more dopaminergic therapy usage, and increased MRI pathology in the SNc. It is interesting to note that, whereas choroid thickness is increased in most regions in PD participants, the most prominent location is in the superior quadrant and closer to the fovea. Choroidal thickness decreases throughout aging and most rapidly in the inferior quadrant [18], which suggests that our findings may be specific to PD pathology and warrant further investigation.
4.2. Diverse retinal nerve layer findings may reflect PD heterogeneity
Despite the large effect in choroidal thickness, we did not find significant differences in the nerve layers (Fig. 4). Post-mortem studies totaling 27 PD and 24 control eyes have identified α-synuclein deposits in the inner retinal layers, and decreased dopamine throughout the retinal layers [5] (Fig. 1). In vivo OCT studies in PD are equivocal, with some identifying alterations in the nerve layers, mostly in the inner layers, but others did not find differences [19]. Contrary to our original hypothesis, we found no consistent pattern of differences between PD and control participants in our small cohort. Although, notably, our observed mean difference in the RNFL (~1-3 μm) is similar to differences identified by meta-analysis [20]. The only subregion to show a difference was in the INL, but this should be interpreted cautiously due to the large number of comparisons.
It is important to note that our cohort was selected from an ongoing longitudinal biomarker study that we are conducting, and the PD participants seemed to display a relatively benign clinical disease course, as determined by an intermediate average H&Y score (2.0 ± 1.2) despite a longer disease duration (13.7 ± 5.3). This is uncommon and may reflect a self-selection bias that only healthier participants would opt-in for the supplemental study protocol [21]. Thus, the lack of changes in the nerve layers may reflect the unique signature of PD participants with a relatively “benign course,” where pathology may be more limited and not spread beyond the basal ganglia to the retina. Consistent with this reasoning, a previous study found that the inferior quadrant of the RNFL is thinner in the faster-progressing akinetic/rigid subtype of PD compared to the slower-progressing tremor dominant subtype [22]. This is an intriguing hypothesis that needs to be tested in a larger sample size study that includes participants with diverse presentations, disease stages, and clinical courses and may provide insight into PD subtypes.
4.3. Choroidal thickness correlates with clinical and imaging metrics of PD
To our knowledge, despite a number of studies examining the choroid in PD [10,11,16,17], no study to date has related choroidal thickness to PD severity. We identified that increased choroid thickness was related to less clinical disease severity, in terms of less visuospatial and motor metrics and less daily dopamine dosage in PD (Fig. 5). Our results revealed an intriguing contrast between a thicker choroid in PD compared to control participants and a thinner choroid with increased disease severity. These findings may suggest that choroid thickness represents a compensatory mechanism in PD. Furthermore, our subregion analysis revealed visuospatial function was associated with superior/temporal quadrants in PD, but inferior quadrants in control participants. Aging has been shown primarily to affect the inferior quadrant of the choroid [18], which may indicate separate processes affecting the choroid in aging and PD. Furthermore, visuospatial disfunction is not specific to PD and occurs in many forms of aging. Likely multiple processes are occurring that affect the relationship between choroid thickness and visuospatial function. Future studies may attempt to identify the progression of choroid thickness in aging as well as pre-symptomatic people with evidence of dopamine deficiency or drug naïve PD participants to interrogate choroidal thickness during aging and disease progression.
Of the previous studies investigating the choroid in PD, the participant demographics of previously published studies were varied [10,11,16]. The study that reported a thinner choroid in PD participants included participants with worse motor function (higher UPDRS-III scores) [10] than the two studies showing increased choroid thickness [11,16]. These results are congruent with the notion that the choroid may be thicker in less severe PD participants and then become thinner as the disease progresses.
We also provide initial evidence that suggests that a thicker choroid may be related to lower nigral (Fig. 6) pathological features in PD. In the substantia nigra, QSM was shown to reflect iron (Fe) content, whereas R2* may capture other aspects of PD-related pathologies, such as α-synuclein or neuronal cell loss [2], and their progression [3]. Although the underlying mechanism is unknown, our finding of choroidal thickness-nigral iron association (Fig. 6) suggests that the vasculature changes may be an integral part of PD-related pathophysiology. Furthermore, our MRI findings mirror the associations found between clinical metrics of disease severity and choroidal thickness. Future studies are needed to determine if these findings are due to primary or compensatory processes.
4.5. Limitations and future directions
There are a number of limitations for our study. As a pilot study, the sample size is small, but we used rigorous methodology to add insights regarding choroid involvement in the context of PD clinical and imaging metrics. The data provides initial evidence for the exciting future possibility that retinal neurovasculature may provide a promising window into PD-related pathophysiology or perhaps compensatory mechanisms. Our limitations as detailed below also offer lessons in designing future studies.
First, PD and control participants were not perfectly matched. However, we did account for axial length, age, and sex in our statistical analyses, and future work should investigate further how these and other factors affect the retina in PD. Second, our participants were tested while taking dopaminergic therapies, and dopamine has been found to dilate choroidal vessels [23]. However, if the effect on the choroid was purely due to dopaminergic therapy, then we would expect the choroid to increase with increased LEDD. Instead, we found a negative relationship. Still, future studies should investigate the pharmacodynamics of dopamine effects on choroidal thickness while PD participants are in the ‘off’ therapy state, ‘on’ therapy state, and during therapy washout. Lastly, the study is cross-sectional and biased toward healthier participants. Future longitudinal studies with larger sample sizes and diverse clinical presentations are needed to confirm these results and determine how the overall PD process relates to alterations in the retina.
In conclusion, we have identified that choroidal thickness was increased in a small cohort of PD participants with a benign course of disease, and is correlated to visuospatial dysfunction, motor severity, and SNc MRI alterations. These promising results warrant further investigation. OCT may provide a simple and cost-effective method to study neurovascular changes that occur in the CNS during neurological disease progression, and also could be a promising non-invasive marker of PD in living participants.
Supplementary Material
Supplementary Table 1. Medication lists for participants. List of all medications for participants at the time of the study. Abbreviations: BID: twice per day, PRN: as needed, QD: daily, QHS: daily at bedtime, QID: 4 times per day, TID: 3 times per day.
Supplementary Fig 1. Comparison of choroidal thickness between side of eye in PD. Comparison of choroidal thickness between ipsi- and contralateral eye to disease onset in PD participants for each subregion using mixed effects model with age, sex, and axial length as covariates. Each box represents mean difference (ipsilateral – contralateral) on top and p-value on the bottom.
Acknowledgments including sources of support
This study was funded by the following sources: National Institute of Neurological Disorders and Stroke (NS060722, NS082151, NS112008 to XH), National Institute of Aging (F30AG067651 to GB), the Hershey Medical Center Clinical Research Center (National Center for Research Resources, Grant UL1 RR033184 that is now at the National Center for Advancing Translational Sciences, Grant UL1 TR0002014), the PA Department of Health Tobacco CURE Funds, the Penn State Translational Brain Research Center, the Michael J. Fox Foundation for Parkinson’s Research, Alzheimer’s Association, Alzheimer’s Research UK, and the Weston Brain Institute.
Footnotes
Conflict of Interest
The authors have no conflict of interest to report.
Supplementary Material
We have included the medications for participants at the time of the study (Supplementary Table 1) and a subregion comparison of choroidal thickness between ipsi- and contralateral eye to disease onset in PD participants (Supplementary Fig 1).
References
- [1].Samii A, Nutt JG, Ransom BR (2004) Parkinson’s disease. The Lancet 363, 1783–1793. [DOI] [PubMed] [Google Scholar]
- [2].Lewis MM, Du G, Baccon J, Snyder AM, Murie B, Cooper F, Stetter C, Kong L, Sica C, Mailman RB, Connor JR, Huang X (2018) Susceptibility MRI captures nigral pathology in patients with parkinsonian syndromes: R2* and QSM Reflect Pathology in Parkinsonism. Mov Disord 33, 1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Du G, Lewis MM, Sica C, He L, Connor JR, Kong L, Mailman RB, Huang X (2018) Distinct progression pattern of susceptibility MRI in the substantia nigra of Parkinson’s patients: Longitudinal R2* and QSM Progression in PD. Mov Disord 33, 1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weil RS, Schrag AE, Warren JD, Crutch SJ, Lees AJ, Morris HR (2016) Visual dysfunction in Parkinson’s disease. Brain 139, 2827–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Veys L, Vandenabeele M, Ortuño-Lizarán I, Baekelandt V, Cuenca N, Moons L, De Groef L (2019) Retinal α-synuclein deposits in Parkinson’s disease patients and animal models. Acta Neuropathol (Berl) 137, 379–395. [DOI] [PubMed] [Google Scholar]
- [6].Tsironi EE, Dastiridou A, Katsanos A, Dardiotis E, Veliki S, Patramani G, Zacharaki F, Ralli S, Hadjigeorgiou GM (2012) Perimetric and retinal nerve fiber layer findings in patients with Parkinson’s disease. BMC Ophthalmol 12, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yu J, Feng Y, Xiang Y, Huang J, Savini G, Parisi V, Yang W, Fu X (2014) Retinal Nerve Fiber Layer Thickness Changes in Parkinson Disease: A Meta-Analysis. PLoS ONE 9, e85718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Leyland L-A, Bremner FD, Mahmood R, Hewitt S, Durteste M, Cartlidge MRE, Lai MM-M, Miller LE, Saygin AP, Keane PA, Schrag AE, Weil RS (2020) Visual tests predict dementia risk in Parkinson disease. Neurol Clin Pract 10, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim S-W, Oh J, Kwon S-S, Yoo J, Huh K (2011) Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina 31, 1904–1911. [DOI] [PubMed] [Google Scholar]
- [10].Eraslan M, Cerman E, Yildiz Balci S, Celiker H, Sahin O, Temel A, Suer D, Tuncer Elmaci N (2016) The choroid and lamina cribrosa is affected in patients with Parkinson’s disease: enhanced depth imaging optical coherence tomography study. Acta Ophthalmol (Copenh) 94, e68–e75. [DOI] [PubMed] [Google Scholar]
- [11].Garcia-Martin E, Pablo LE, Bambo MP, Alarcia R, Polo V, Larrosa JM, Vilades E, Cameo B, Orduna E, Ramirez T, Satue M (2017) Comparison of peripapillary choroidal thickness between healthy subjects and patients with Parkinson’s disease. PLOS ONE 12, e0177163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease: MDS-PD Clinical Diagnostic Criteria. Mov Disord 30, 1591–1601. [DOI] [PubMed] [Google Scholar]
- [13].Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease: Systematic Review of LED Reporting in PD. Mov Disord 25, 2649–2653. [DOI] [PubMed] [Google Scholar]
- [14].Abbey AM, Kuriyan AE, Modi YS, Thorell MR, Nunes RP, Goldhardt R, Yehoshua Z, Gregori G, Feuer W, Rosenfeld PJ (2015) Optical Coherence Tomography Measurements of Choroidal Thickness in Healthy Eyes: Correlation With Age and Axial Length. Ophthalmic Surg Lasers Imaging Retina 46, 18–24. [DOI] [PubMed] [Google Scholar]
- [15].Djaldetti R, Ziv I, Melamed E (2006) The mystery of motor asymmetry in Parkinson’s disease. Lancet Neurol 5, 796–802. [DOI] [PubMed] [Google Scholar]
- [16].Satue M, Obis J, Alarcia R, Orduna E, Rodrigo MJ, Vilades E, Gracia H, Otin S, Fuertes MI, Polo V, Larrosa JM, Pablo LE, Garcia-Martin E (2018) Retinal and Choroidal Changes in Patients with Parkinson’s Disease Detected by Swept-Source Optical Coherence Tomography. Curr Eye Res 43, 109–115. [DOI] [PubMed] [Google Scholar]
- [17].Robbins CB, Thompson AC, Bhullar PK, Koo HY, Agrawal R, Soundararajan S, Yoon SP, Polascik BW, Scott BL, Grewal DS, Fekrat S (2020) Characterization of Retinal Microvascular and Choroidal Structural Changes in Parkinson Disease. JAMA Ophthalmol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wakatsuki Y, Shinojima A, Kawamura A, Yuzawa M (2015) Correlation of Aging and Segmental Choroidal Thickness Measurement using Swept Source Optical Coherence Tomography in Healthy Eyes. PLOS ONE 10, e0144156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Indrieri A, Pizzarelli R, Franco B, De Leonibus E (2020) Dopamine, Alpha-Synuclein, and Mitochondrial Dysfunctions in Parkinsonian Eyes. Front Neurosci 14,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huang L, Wang C, Wang W, Wang Y, Zhang R (2020) The specific pattern of retinal nerve fiber layer thinning in Parkinson’s disease: a systematic review and meta-analysis. J Neurol. [DOI] [PubMed] [Google Scholar]
- [21].Søgaard AJ, Selmer R, Bjertness E, Thelle D (2004) The Oslo Health Study: The impact of self-selection in a large, population-based survey. Int J Equity Health 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rohani M, Langroodi AS, Ghourchian S, Falavarjani KG, SoUdi R, Shahidi G (2013) Retinal nerve changes in patients with tremor dominant and akinetic rigid Parkinson’s disease. Neurol Sci 34, 689–693. [DOI] [PubMed] [Google Scholar]
- [23].Reitsamer HA, Zawinka C, Branka M (2004) Dopaminergic Vasodilation in the Choroidal Circulation by D1/D5 Receptor Activation. Investig Opthalmology Vis Sci 45, 900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Medication lists for participants. List of all medications for participants at the time of the study. Abbreviations: BID: twice per day, PRN: as needed, QD: daily, QHS: daily at bedtime, QID: 4 times per day, TID: 3 times per day.
Supplementary Fig 1. Comparison of choroidal thickness between side of eye in PD. Comparison of choroidal thickness between ipsi- and contralateral eye to disease onset in PD participants for each subregion using mixed effects model with age, sex, and axial length as covariates. Each box represents mean difference (ipsilateral – contralateral) on top and p-value on the bottom.