Abstract
Variability in psychiatric response following stressful/traumatic life events is frequently observed. There is also variability in propensity for alcohol use disorder (AUD) such that some can consume substantial amounts and not develop AUD symptoms whereas others develop an AUD. Our group has applied discrepancy-based approaches to capture psychiatric resilience (PR) and alcohol resistance (AR), both moderately heritable. This study sought to 1) examine the genetic and environmental correlation of these constructs and 2) model qualitative and quantitative sex effects. Data came from a large twin sample (N=4500 twin pairs) with self-report measures and interviews of distress symptoms, stressful life events, alcohol use, and AUD. Correlated liability model results suggested a moderate degree of genetic correlation between PR and AR (.54) due to the same genetic factors in males and females. Findings highlight the shared genetic predisposition of these resilience/resistance constructs while emphasizing the impact of unique environmental experiences.
Keywords: resilience, addiction, alcohol, stressful life events, heritability
The wide variability in responses following stressful life events is well-documented. The occurrence of resilient responding, although defined in numerous ways, is considered to represent adaptive functioning or lack of distress in the face of adversity (Bonanno & Mancini, 2012; Luthar et al., 2000; Rutter, 2013). Resilience has been conceptualized as a trait-like capacity, as a dynamic process that occurs in the context of adversity, and as an outcome following adversity (see review by Choi et al., 2019). All three of these approaches have been the focus of efforts to better understand contributions to resilience from the perspective of environmental factors, cognitive or psychological factors, and, more recently, heritable genetic factors (Choi et al., 2019).
Resilience has been examined in a number of twin-based analyses, demonstrating that modest to moderate variance in resilience phenotypes can be explained by genetic variation (Amstadter et al., 2014; Boardman et al., 2008; Kim-Cohen et al., 2004; Waaktar and Torgersen, 2012; Wolf et al., 2018). Waaktar & Torgersen (2012) and Wolf and colleagues (2018) conceptualized resilience using a trait-based conceptualization. The remaining studies (Amstadter et al., 2014; Boardman et al., 2008, Kim-Cohen et al., 2004), including work by our group, conceptualized resilience as an outcome following adversity using a discrepancy-based approach with various measures (i.e., positive mood adjusting for life stressors; Boardman et al., 2008; psychiatric distress after adjusting for recent stressors; Amstadter et al., 2014, children’s IQ after adjusting for socio-economic status deprivation). Specifically, our group has quantified resilience based on the difference between actual and predicted psychiatric distress given the level of exposure to stressors (i.e., the standardized residuals from a linear regression; Amstadter et al., 2014) which we have termed discrepancy-based psychiatric resilience (herein referred to as PR). Phenotypically, PR has demonstrated predictive validity, with evidence that PR buffers the effects of new onset stressors on the risk of depression and generalized anxiety disorder (Sheerin et al., 2018). In twin-based studies, PR has been demonstrated to be stable and moderately heritable (50% in longitudinal models; Amstadter et al., 2014).
It has been recognized that resilience is highly multifaceted and should ideally incorporate multiple domains of functioning, supported by both twin (Sawyers et. al., 2020) and molecular genetic (Stein et al., 2019) work demonstrating variability in genetic overlap across different resilience conceptualizations. Resilience is also generally considered to represent more than simply the inverse of internalizing psychopathology. For example, if the resulting residual variable conceptualized as PR were the inverse of internalizing, then it would follow that the genetic liability to PR and internalizing disorders would be close to unity. Our analyses did not yield support for this, demonstrating moderate overlap in additive genetic heritability between PR and internalizing disorders (e.g., 42% with major depressive disorder and 61% with generalized anxiety disorder) and an even more modest overlap in environmental influences. These results suggest that although some overlap exists, there is something unique being captured by resilience beyond the lack of liability to internalizing psychopathology. Indeed, broader resilience work has conceptualized resilience as a conscious process of moving forward in a positive manner, regardless of, and separate from, lack of psychopathology (Southwick et al., 2014). Our work has also demonstrated that PR has a modest overlap with externalizing conditions (20% with AUD, 18% with antisocial personality disorder; Amstadter, Maes, et al., 2016). The multifaceted nature of resilience, along with evidence of a modest overlap of PR with AUD, suggests the need for examining the association between resilience and phenotypes of interest, such as AUD, in more detail using alternative approaches.
One such extension has been our group’s approach to quantifying resilience in the context of resistance to substance use disorders. To capture the concept that while some individuals can consume large quantities of substances (e.g., alcohol) and experience few adverse consequences, others with similar consumption patterns experience more dysfunction in their personal and social lives, the construct of addiction resistance (AR) was described and validated (Kendler & Myers, 2015). The AR phenotype was used to characterize the discrepancies between reported AUD symptoms and maximal levels of alcohol consumption. Here, a high level of AR is defined as reporting fewer symptoms of AUD than expected for increasing levels of alcohol consumption while low levels of AR would be the reverse — higher levels of AUD symptoms given lower amounts of consumed alcohol (Kendler & Myers, 2015). A longitudinal twin model was applied for AR to alcohol, and a heritability estimate of 34.8% was demonstrated. To date, one other study has constructed AR in another sample, identifying a similar amount of variance explained between alcohol use and AUD criteria as well as identifying similar factors to be predictive of AR (Hoffmeister et al., 2019).
Of interest for identifying the etiology, predictors, and impact of resilience is the examination of potential sex differences in resilience. However, to date, the literature is mixed. For example, phenotypic work found no sex differences in the relation between resilience and future internalizing disorders following new-onset stressors (Sheerin et al., 2018). Others found different patterns as a function of the type of resilience metric used, wherein women showed higher relative resilience but less perceived resilience compared to men (Nishimi et al., 2020). Within the classical twin design (Boomsma et al., 2002; Neale & Cardon, 1992), there are two general forms of sex differences that can be tested (Eaves & Heath, 1981; Neale & Cardon, 1992): qualitative sex limitation (male and female differences in the resemblance or ‘kind’ of genetic effects) and quantitative sex limitation (male and female differences in the magnitude of the effect sizes for what are presumed to be the “same” genetic and/or environmental sources). Two prior twin studies found evidence for quantitative but not qualitative sex effects on resilience defined as a trait (Waaktaar & Torgersen, 2012) and using a discrepancy-based approach (Boardman et al., 2008), with slightly greater heritability estimates in males). In contrast, prior work examining PR demonstrated that although heritability was equivalent for males and females (i.e., no quantitative sex effects), the genes affecting resilience were not identical across the sexes (i.e. qualitative sex effects; Amstadter et al., 2014). To date, sex differences in heritability for AR have not been examined.
The primary aim of the present study was to examine the patterns of genetic and environmental correlation between these two conceptualizations of resilience/resistance. We hypothesized that the genetic correlation between PR and AR would be moderate. We also expected this relationship to be greater than that found previously between PR and AUD (20%; Amstadter, Maes, et al., 2016), as PR and AR aim to capture unique, but related, capacities for resistance to different “doses” of environmental pressure (i.e., stressor and alcohol). Thus, we hypothesized these related, but distinct, capabilities of the system to adapt to environmental forces would have a closer relationship compared to that of resilience to distress (PR) and liability for an externalizing disorder (AUD). A second aim of this work was to test whether sex differences were evident in PR and AR. Finally, the present study refines the methods of our prior work, by moving away from constructing and quantifying PR and AR using simple sum scores and count variables to using a model-based measurement framework that offers the benefit of having detailed information about how the PR and AR constructs are being defined by the indicator items (Neale et al., 2005).
Method
Sample
Participants were from the Virginia Adult Twin Studies of Psychiatric and Substance Use Disorders (VATSPSUD), a large, longitudinal twin study of Caucasian adults (Kendler & Prescott, 2006). The Institutional Review Board at Virginia Commonwealth University (VCU) approved this study, and all participants provided informed consent before participating. The current study used item-level data from the final waves for two related interview assessments. One included monozygotic and dizygotic same sex female-female (FF; FF4 wave, conducted between 1995 - 1997) twin pairs and the other sample consisted of monozygotic and dizygotic same sex male-male (MM) twin pairs as well as opposite sex dizygotic male-female (MMMF) twin pairs (MF wave 2, conducted between 1994 - 1998). Both of these interviews included comprehensive sections assessing alcohol related behaviors. The total sample size for the current study analyses was N = 4501 complete and incomplete (singleton) twin pairs. Due to the use of a conditional skip-in check-point item that determined whether the alcohol abuse and dependence symptom criteria would be administered, a portion of the twins not meeting a pre-determined minimum alcohol quantity and or frequency consumption cutoffs had missing data on the abuse and dependence items. Rather than replacing these missing values with zeros or attempting to impute them (neither option seeming to be reasonable or justifiable), we decided to handle all missingness by the use of suitable full information estimation techniques.
Measures
Psychiatric variables.
Data used to estimate the PR construct were items taken from two different instruments. A shortened version of the Symptom Checklist-90, (SCL-90; Derogatis et al., 1973) assessed past-month distress using a Likert-scale with options ranging from 0 (“not at all”) to 4 (“extremely”). The shortened version included 27 items from four of the SCL subscales: depression (10 items), somatization (5 items), anxiety (7 items), and phobic anxiety (5 items). Stressful life events (SLEs) were also assessed, during personal interview, and included 15 events that were both personal in nature (e.g., assault, marital problems, job loss) and “network” events (i.e., events that occurred primarily to, or in interaction with, an individual in the participant’s social network; e.g., death or severe illness of participant’s spouse, child, or parent, serious trouble getting along with others). These experiences were queried for occurrence over the past 90 days and past year; past 90-day ratings were used to be proximal to the past month SCL-90 distress ratings. Creation of the PR construct is described in the data analytic plan.
Alcohol variables.
Data used to estimate the AR construct were AUD symptoms and alcohol consumption. The symptoms of alcohol abuse and dependence were assessed during personal interview by trained mental health professionals using an adapted version of the SCID interview (Spitzer et al., 1992) and DSM-IV criteria (American Psychiatric Association, 1994). Symptoms were assessed from the perspective of lifetime maximal use of alcohol. The alcohol consumption variable represented maximal alcohol use, determined by the product of two questions assessing, at the time of maximal intake, the frequency of consumption and the average amount consumed in standard alcohol drinks. Creation of the AR construct using these variables is described in the data analytic plan below.
Demographic covariates.
Age at assessment was also included as a predictor in the models used to estimate the latent residuals for both PR and AR. Body weight was included as a conditioning covariate in the model used to estimate AR as it is important with regard to male and female differences in alcohol consumption quantity. Supplemental Table II includes descriptive information on items used in the measurement model and the conditioning covariates.
Data Analysis
Phenotypic Measurement Models to Estimate Discrepancy-based PR and AR
Prior work utilized the same variables as in the present study, but calculated resilience/resistance as follows: PR was calculated by regressing internalizing symptoms onto total stressor count and saving the residuals (i.e., PR is the residual of internalizing symptoms after the effect of recent number of stressors was regressed out). AR followed the same method, with number of AUD symptoms endorsed regressed onto maximal alcohol consumption, with the residuals representing the deviation from population expectation. Covariates of age, sex, and weight were also included as appropriate. In the present study’s extension of this approach, measurement models in the form of ordinal item factor analysis (Wirth & Edwards, 2007) were used to test the dimensionality of the item indicator sets for the psychiatric (PSY; comprised of abbreviated SCL and stressful life event endorsements) and alcohol (ALC; comprised of alcohol abuse/dependence symptoms and maximal alcohol consumption) risk liabilities. These latent constructs are more refined in the sense that they only reflect common shared variation among the fallibly assessed items with item specific and error variance being separated out. The full latent variable structural model creating the PSY and ALC common factor liabilities and the creation of PR and AR resilience variables is presented in Figure 1 as a path diagram (model fitting results are in Supplemental Table III. These liabilities were then used to estimate their respective residuals (PR and AR). Specifically, latent residuals were estimated by regressing the PSY and ALC common factors onto their respective exogenous predictors (see Figure 1). Thus, resilience/resistance here is operationalized as latent residuals — the conditional deviations from an optimal linear predicted regression line — when the PSY and ALC common factor risk liabilities are regressed onto their respective exogenous predictors. The full model was fit simultaneously resulting in a joint estimation of PR and AR residual factor scores and their respective measurement models. A correlation was allowed between the PR and AR residual factors when estimating the factor scores. Factor residual scores were estimated for each twin member (i.e., individual record data) using the robust weighted least squares mean and variance adjusted estimator for categorical data as implemented in the Mplus 8.1 software (Muthen & Muthen, 1998). Due to the nonindependence of the twin pair records, the complex survey sampling correction feature of Mplus was used to adjust fit indices and standard errors for the nested twin data. These estimated residual factor scores serve as the resilience outcome variables in the twin sex limitation modeling.
Figure 1: Path diagram of full measurement model to estimate AR and PR residual (resistance/resilience) factor scores.
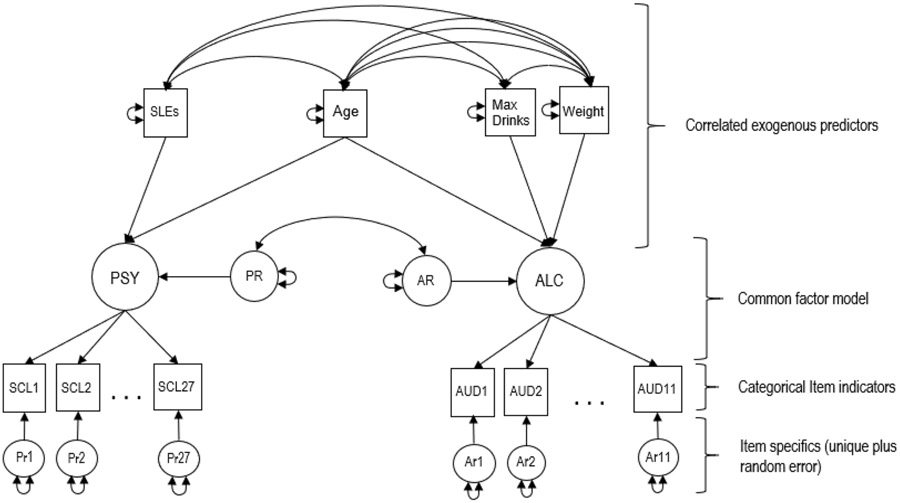
Squares = Observed item response indicator variables and covariates; Circles = unobserved common, residual and specific factors. Single headed arrows = factor loadings for the measurement models or regression coefficients for the exogenous covariate prediction; Double headed arrows = variances or covariances. SLE = count of stressful life events; SCL1…27 = symptom checklist items; AUD1…11 = alcohol abuse and dependence symptoms items; PSY unobserved psychiatric factor; ALC unobserved alcohol factor; PR = unobserved psychiatric factor residual (discrepancy-based psychiatric resilience); AR = unobserved alcohol factor residual (alcohol resistance).
The model is divided into four parts annotated by labeled brackets. The three lower brackets identify the common factor item measurement model. This model specification decomposes the item inter-correlation matrices for ALC (tetrachoric) and PSY (polychoric) into common and specific factors. The common factors are defined by the estimated item characteristics (i.e., factor loadings and thresholds) that calibrate and organize individual differences on the construct ALC and PSY continuums. The top bracket identifies the part of the model that estimates the AR and PR latent resistance/resilience variables. Resistance/resilience here is operationalized as latent residuals (conditional deviations from an optimal linear predicted regression line) when ALC and PSY common factor risk liabilities are regressed onto their respective exogenous predictors. Although the model is partitioned to facilitate description, the full model is fit simultaneously to the ordinal item data. Thus, the estimated AR and PR residual factor scores and their respective measurement models are jointly estimated within a single model-data optimization. As can be seen in the figure, a correlation is allowed between the AR and PR residual factors when estimating the factor scores. These estimated residual factor scores will serve as the resistance/resilience outcome variables in the twin sex limitation modeling. Factor residual scores are estimated for each twin member (i.e., individual record data) using the robust weighted least squares mean and variance adjusted estimator for categorical data as implemented in the Mplus 8.1 software (Muthen & Muthen, 1998).
Measurement Invariance (MI) in the Phenotypic Measurement Models.
When conducting sex limitation twin model based on item measurement models, whether the item characteristics defining the constructs are invariant for males and females is an important measurement question (Lubke et al., 2004). If MI is not supported, the interpretation of any sex differences found in the twin modeling can be compromised by confounding due to the nature of the non-equivalence of the factors in males and females. Thus, determining if MI (Meredith, 1993; Vandenberg, 2002) holds is essential to making valid inferential claims regarding sex differences. Item measurement model invariance testing was conducted for three of the four recognized levels of invariance tests (Meredith, 1993): configural (whether the number of factors and the pattern of factor loadings are the same in both sexes, typically serves as a baseline model fit for comparing the other forms of invariance), metric (restrictions imposed on the factor loadings of an essentially unidimensional (Stout, 2002) factor structure by equating loadings across the groups), and scalar (adding the additional restrictive constraint of forcing the item indicator thresholds to be equal across groups). Strict MI (i.e., forcing item residual specific variances to be equal for males and females) was not considered or tested for this application.
For both the PSY and ALC item indicator sets, confirmatory factor models were fit to the male and female item data separately to test for unidimensionality. The alcohol abuse (4) and dependence (7) items were coded as binary whereas the 27 SCL items were coded using a 4-category ordered Likert-type response scale. These different data coding schemes required different item characteristic invariance testing specifications (Millsap & Yun-Tein, 2004). For the AR factor structure, only the configural and a combined metric and scalar tests were conducted due to the necessary identification requirements needed for binary items. For the PR factor, separate configural, metric, and scalar tests were conducted and compared to evaluate MI globally across males and females. Differences between the two types of MI models (configural and metric) were evaluated based on the discussion in (Cheung & Rensvold, 2002) and the recommended changes for fit indices such as the comparative fit index (CFI), Tucker-Lewis fit index (TLI) and root mean square error of approximation (RMSEA).
Sex Limitation Modeling
A correlated factors or “liabilities” approach was used rather than a Cholesky “triangular” decomposition approach to circumvent issues of variable ordering (Neale et al., 2006). The correlated factors model was used for bivariate sex limitation models for the PR and AR factor scores. We note that univariate models were also conducted prior to completing the primary, bivariate models, and will be briefly presented in the results.
Path diagrams illustrating theoretical correlated factors/liabilities models are presented in Supplemental Figure 1. A series of increasingly restrictive models is set up to test the different A (additive genetic), C (common environment), and E (unique environment) qualitative and quantitative sex differences. A baseline comparison “saturated” model is first specified and fit. This model imposes the least number of parameter restrictions for the five-group correlated liability model. Next, several bivariate qualitative sex limitation parameter restrictions are imposed for the genetic and environmental etiological sources. These involve equating the (co)variance parameters of the same and opposite sex twin pairs. Differences in minus twice the log likelihood (−2LL) misfit and changes in the AIC information index (Akaike, 1987) are used to evaluate the statistical comparisons of models. AIC was chosen as it combines both the −2LL misfit information and model complexity, with lower AIC values indicate a more parsimonious “better” fit.
Results
Twin Correlations
The bivariate twin correlations and precision of the point estimates of the correlations (standard errors) for AR and PR are shown in Table I. The sample included five twin pair types: same-sex monozygotic (MZ) females (n = 651), dizygotic (DZ) females (n = 454), same-sex MZ males (n = 964), DZ males (n = 782), and opposite-sex DZ pairs (n = 1650). The cross-twin correlations for AR were considerably higher for MZ compared to DZ females and MZ compared to DZ males. Additive genetic factors and unique-environmental influences may explain the variability in this phenotype in females and males. PR cross-twin correlations in MZ females were substantially higher than DZ females; whereas the difference in the correlation for MZ and DZ males was less pronounced. PR in females may be influenced by additive genetic and unique-environmental factors. In contrast, shared-environmental influences may also play a role in the phenotypic variation in PR in males in addition to additive genetic and unique-environmental factors. The cross-twin within-trait and cross-twin cross-trait correlations for opposite-sex twins are slightly higher than those for DZ males and DZ females, with the exception of cross-twin cross-trait correlations between DZ males and opposite-sex twins. The absence of markedly lower correlations for opposite-sex DZ twins, compared to those of same-sex DZ twins, may suggest that there is lack of support for sex-specific genetic effects (i.e., qualitative) influencing these phenotypes. The cross-twin cross-trait correlations were higher for MZ pairs compared to DZ pairs regardless of sex; nevertheless, the difference was greater for females across zygosity. This indicates that shared genetic factors may contribute to the variability in the two phenotypes for both sexes.
Table I.
Twin pair correlations (with Standard Errors given in parentheses; N = 4501 twin pairs)
| MZ female pairs (n = 651) |
MZ male pairs (n = 964) |
DZ female pairs (n = 454) |
DZ male pairs (n = 782) |
DZ opposite sex pairs (n = 1650) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AR T1 |
PR T1 |
AR T2 |
PR T2 |
AR T1 |
PR T1 |
AR T2 |
PR T2 |
AR T1 |
PR T1 |
AR T2 |
PR T2 |
AR T1 |
PR T1 |
AR T2 |
PR T2 |
AR T1 |
PR T1 |
AR T2 |
PR T2 |
|
|
AR
T1 |
1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||||||||
|
PR
T1 |
0.37 (0.06) |
1.00 | 0.25 (0.04) |
1.00 | 0.21 (0.08) |
1.00 | 0.35 (0.04) |
1.00 | 0.32 (0.03) |
1.00 | ||||||||||
|
AR
T2 |
0.27 (0.07) |
0.16 (0.06) |
1.00 | 0.28 (0.05) |
0.12 (0.05) |
1.00 | 0.05 (0.11) |
−0.06 (0.09) |
1.00 | 0.08 (0.07) |
0.09 (0.06) |
1.00 | 0.12 (0.05) |
0.12 (0.04) |
1.00 | |||||
|
PR
T2 |
0.18 (0.06) |
0.23 (0.05) |
0.44 (0.05) |
1.00 | 0.14 (0.05) |
0.31 (0.04) |
0.36 (0.04) |
1.00 | 0.05 (0.08) |
0.01 (0.06) |
0.28 (0.07) |
1.00 | 0.09 (0.06) |
0.19 (0.05) |
0.35 (0.05) |
1.00 | 0.07 (0.05) |
0.20 (0.03) |
0.34 (0.04) |
1.00 |
Note: AR = alcohol resistance; PR = psychiatric resilience; MZ = monozygotic; DZ = dizygotic; T1 = Twin 1; T2 = Twin 2.
Measurement Invariance (MI) Testing
Separate multiple-group invariance tests were conducted for the AR and PR common factor measurement models. Because the alcohol abuse and dependence items were binary, configural and scalar MI tests were performed for the AR phenotype whereas the SCL items were ordinal, configural, metric, and scalar MI were conducted for the PR phenotype. MI model comparisons for the AR configural and scalar models were Δχ2 (9) = 98.9, p < 0.001, ΔCFI = 0.004, ΔTLI = 0.003, and ΔRMSEA = 0.005. For the PR MI testing, model fit index differences comparing configural, metric, and scalar invariance constraints were (metric vs. configural: Δχ2 (26) = 366.5, p < 0.001, ΔCFI = 0.00, ΔTLI = 0.00, and ΔRMSEA = 0.002; scalar vs. configural: Δχ2 (106) = 468.3, p < 0.001, ΔCFI = 0.02, ΔTLI = 0.03, and ΔRMSEA = 0.01; and for scalar vs. metric: Δχ2 (80) = 215.3, p < 0.001, ΔCFI = 0.02, ΔTLI = 0.03, and ΔRMSEA = 0.01). Based on recommendations for interpreting differences in goodness-of-fit for MI model comparisons (Cheung & Rensvold, 2002), the statistical evidence for departures from invariance were stronger for the PR compared to the AR constructs. However, since the PR construct included 27 indicators that were ordinally coded with 5 categories (4 thresholds per item), there is much more item-level information available to detect departures from factor loading and threshold invariance with such a large measurement model.
Sex Limitation ACE Models
Univariate Sex Limitation ACE Models.
For each of the two resilience phenotypes, univariate ACE models were first fit estimating the qualitative and quantitative sex differences (see Supplemental Table Ia and Ib). In the case of PR, the scalar sex limitation quantitative differences AE model provided the best fit to the twin data (comparison to the full PR base model: AIC=4362.7363, Δχ2 (3) < −0.01; female point estimates: A=.29, C<.01, E=0.71; male point estimates: A= .33, C<.01, E= 0.67). For AR, however, the homogeneity model where the variance components predicting the phenotype are not allowed to differ for the sexes resulted in an improvement in fit for these twin data (comparison to the full AR base model: AIC=3170.7328, Δχ2 (5)= 1.16; A=27, C<.01, E=0.73).
Bivariate Sex Limitation ACE Models.
Four models were examined that included the estimation of the bivariate genetic and environmental correlated liability factors to test for qualitative and quantitative sex differences. Models were ordered from least restrictive (i.e., most heavily parameterized) to models that impose increasing levels of constraints by either dropping parameters by fixing them to zero or equating their values across twin groups. As shown in Table II, the base bivariate twin model (model 1) allows A, C, and E factors, and qualitative genetic and quantitative sex differences, as well as genetic and environmental correlated liability factors between the two traits (−2LL=28985.38; df=10940, AIC=7105.38). We compared model 1 with i) the same model but instead testing for qualitative common environment (model 2), ii) a scalar sex limitation quantitative only (no qualitative) ACE model (model 3), and iii) the no-sex-differences, or homogeneity, ACE model (model 4). Model 3, the scalar sex limitation quantitative sex differences ACE model showed the best fit and parsimony at explaining the data compared to the base model (Model 1: AIC=7105.38; Model 3: AIC=7096.93, Δχ2 (5) = 1.55). Similarly, model 3 showed better fit than model 2 (AIC=7106.23) with five fewer parameters. Further, when quantitative sex effects were not allowed to differ in the homogeneity model (model 4 ), the fit worsened significantly (AIC=7099.10, Δχ2 (7) = 16.17) compared to model 3.
Table II.
Model fit comparisons within the full bivariate ACE model examining qualitative and quantitative sex differences.
| Model | Model Description | Estimated Parameters |
−2LL | df | AIC | Δ(−2LL) | Δdf | p | Comparison Model |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Sex limitation, qual. on A and quant. sex-differences | 25 | 28985.38 | 10940 | 7105.38 | NA | NA | NA | NA |
| 2 | Sex limitation, qual. on C and quant. sex-differences | 25 | 28986.23 | 10940 | 7106.23 | 0.85 | 0 | NA | 1 |
| 3 | Scalar sex limitation, quant. sex-differences | 20 | 28986.93 | 10945 | 7096.93 | 1.55 | 5 | 0.91 | 1 |
| 4 | Homogeneity, no qual. nor quant. sex-differences | 13 | 29003.10 | 10952 | 7099.10 | 17.72 | 12 | 0.12 | 1 |
| 4 | Homogeneity, no qual. nor quant. sex-differences | 13 | 29003.10 | 10952 | 7099.10 | 16.17 | 7 | 0.02 | 3 |
Note: Models 1-4 include common paths for correlated factors a, c, and e. A=Additive genetic component, C=Common/Shared environmental component, E=Unique environmental component, qual.=qualitative, quant.=quantitative. Best fitting model is designated in bold text. All five twin groups are included in the modeling (MZ female, MZ male, DZ female, DZ male, DZ opposite sex). Best fitting model denoted in bold.
Bivariate Sex Limitation Submodels.
As seen in Table III, we then conducted model fit comparisons to determine whether A or C could be dropped from the best fitting model (scalar sex limitation quantitative sex differences ACE model; model 3). We constrained independently the A, C, and AC parameters to zero, and compared the fit of model 3 to its nested models. The nested AE model (model 5) had a better fit in terms of parsimony (fewer estimated parameters; AIC=7089.08, Δχ2 (5) = 2.15). The nested CE (model 6; AIC=7104.57, Δχ2 (5) = 17.64) and E only models (model 7; AIC=7253.422, Δχ2 (10) = 176.49) had significantly greater misfits (Δχ2) and poorer (larger) AIC values. We next constrained the correlated liability of common-environmental factors (Fc) to zero, keeping the additive genetic (Fa) and unique environmental (Fe) correlated liability factors of the scalar sex limitation quantitative sex differences AE model (model 8). Model 8 explained the data with higher parsimony (AIC=7089.08, Δχ2 (10) = 3.7) while minimizing the reduction of information from model 1. Thus, using model 8 as comparison, we then tested the correlated liability factors for the two traits, constraining to zero i) Fa (model 9, freely estimating Fe) and then ii) Fe (model 10, freely estimating Fa) for males and females. Table III shows that the likelihood and fit of model 9 and 10 deteriorated, supporting the AE scalar sex limitation quantitative sex differences model with additive genetic (Fa) and unique environmental (Fe) correlated liability factors (model 8) as the best fitting model (AIC=7089.08, Δχ2 (10) = 3.7).
Table III.
Model fit comparisons in the best-fitting bivariate scalar sex limitation ACE model to determine if C or A can be dropped.
| Model | Model Description | Estimated Parameters |
−2LL | df | AIC | Δ(−2 LL) |
Δdf | p | Comparison Model |
|---|---|---|---|---|---|---|---|---|---|
| 5 | AE, quant. sex-differences, commPath (a, c and e) | 15 | 28989.08 | 10950 | 7089.08 | 2.15 | 5 | 0.828 | 3 |
| 6 | CE, quant. sex-differences, commPath (a, c and e) | 15 | 29004.57 | 10950 | 7104.57 | 17.64 | 5 | 0.00343 | 3 |
| 7 | E, quant. sex-differences, commPath (a, c and e) | 10 | 29163.42 | 10955 | 7253.42 | 176.49 | 10 | 1.25 × 10−32 | 3 |
| 8 | AE, quant. sex-differences, commPath (a and e) | 15 | 28989.08 | 10950 | 7089.08 | 3.7 | 10 | 0.96 | 1 |
| 9 | AE, quant. sex-differences, commPath (e) | 14 | 29026.47 | 10951 | 7124.47 | 37.4 | 1 | 9.66 × 10−10 | 8 |
| 10 | AE, quant. sex-differences, commPath (a) | 13 | 29043.07 | 10952 | 7139.07 | 54 | 2 | 1.89 × 10−12 | 8 |
Note: All five twin groups are included in the modeling (MZ female, MZ male, DZ female, DZ male, DZ opposite sex).
This table presents the model fit comparisons of the scalar sex limitation quantitative sex differences model (model 3 from the Table 2) and nested models (5-7). Models 8-10 represent fit comparisons of scalar sex limitation quantitative sex differences AE with common paths for correlated factors a and e (model 8) with model 5, model 1 (base model), and nested models AE with common paths for correlated factors e and a (model 9 and 10, respectively). CommPath: Common paths for correlated factors a, c and e; A=Additive genetic component, C=Common/Shared environmental component, E=Unique environmental component, qual.=qualitative, quant.=quantitative, a=Additive genetic, c=Common/Shared environmental, e=Unique environmental. Best fitting models are designated in bold text.
Figure 2 presents the parameter point estimates and 95% confidence intervals from the best fitting model (model 8). Additive genetic factors for each phenotype accounted for 25% and 26% of the total variance for AR across females and males, respectively and 29% and 33% of the total variance for PR across females and males, respectively. Both phenotypes markedly shared quantitative additive genetic factors, resulting in a genetic correlation of .54 for females and males. Results also indicate that E factors accounted for a higher proportion of the variability specific to the two phenotypes for females (75% for AR, 71% for PR) and males (74% for AR and 67% for PR) compared to A factors. Finally, the joint liability for PR and AR from E factors in females (.26) and in males (.24) was moderate.
Figure 2. Bivariate, sex-limitation model of AR and PR results.
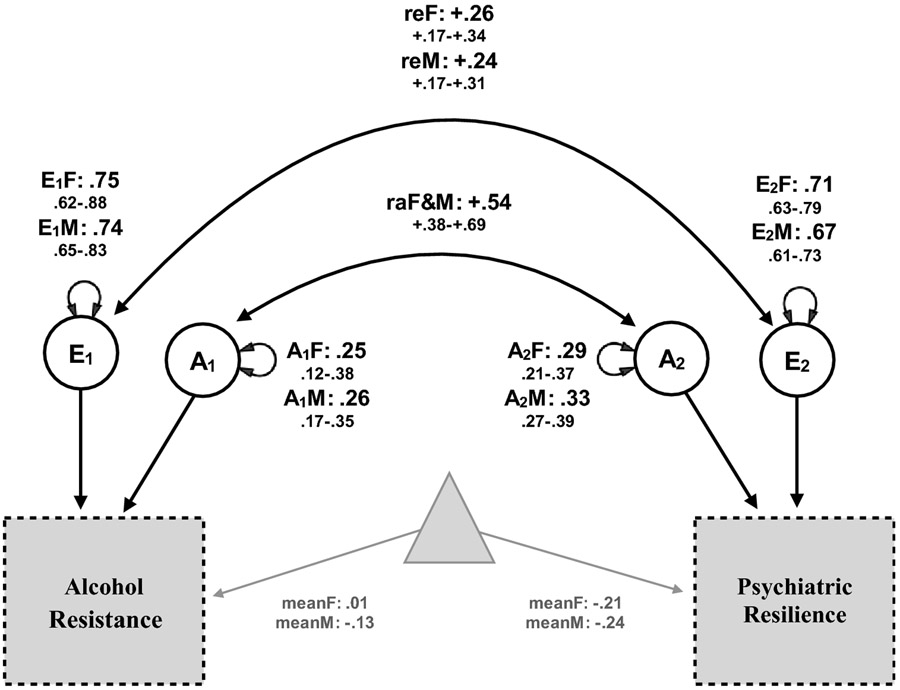
Standardized additive genetic (A) and unique environmental (E) variance components, genetic (a) and unique environmental (e) correlations (r), and means including 95% confidence intervals for females (F) and males (M) from the bivariate twin model assessing alcohol resistance (AR) and psychiatric resilience (PR).
Discussion
Using discrepancy-based psychiatric resilience and addiction resistance constructs based on an item-level measurement modeling framework, this study aimed to clarify patterns of genetic and environmental variance and covariance and utilize all five types of twin pairs (i.e., same sex male and female MZ, DZ, and opposite sex DZ twins) to test for potential sex differences in PR and AR and their correlation. Both sets of findings will be discussed in turn.
Univariate modeling results were broadly consistent with prior work using these constructs (i.e., AE models were the best fit, moderate heritability estimates; e.g., Amstadter et al., 2014; Kendler & Meyers, 2015). These findings, however, were determined via two approaches, a measurement model (current work) and a sum score approach (previous work), suggesting consistency across the two methods of creating PR and AR constructs. The correlation of the genetic factors for PR and AR may be due to a broader level of resilience that, in part, influences one’s likelihood of resistance to different “doses” (i.e., stressor, substance). Further, the finding that more of the genetic propensity for PR is related to AR, as compared to alcohol abuse/dependence (Amstadter, Maes, et al., 2016), as hypothesized, may hint at the presence of a modest core set of “resilience genes” that predispose individuals towards being able to overcome mental health or alcohol use consequences, given “dose” stressful events and alcohol exposure, respectively. Cutting-edge, molecular genetic work is needed to determine what these specific markers might be. Alternatively, findings of correlated genetic liability between these two constructs may also indicate related genetic propensity for a third variable relevant to both resilience and resistance outcomes. Notably, a recent study has examined predictors of the AR construct in a community sample of young adults who all consumed some alcohol and found that behavioral and mood regulation factors were the strongest predictors of AR (Hoffmeister et al., 2019). There is a large body of literature on the relevance of emotion regulation strategies with regard to psychiatric resilience, as well as the importance of problem-focused coping strategies (see review by Mancini et al., 2009). Thus, factors such as mastery, which have been associated with AR (Kendler & Myers, 2015) may also be relevant for PR.
The evidence of genetic correlation should be viewed in the context of individual additive genetic (A) and unique environmental (E) effects. Between 29-33% of the variability in PR was attributable to genetic effects whereas 67-71% was accounted for by nonshared unique environmental sources. For AR, between 25-26% of the variability was found to have an additive genetic basis while 74-75% of the phenotypic individual differences in resilience were attributable to unique environment. Further, the environmental correlation between AR and PR was less than the genetic correlation at .24 for males and .26 for females. In line with prior work, notable environmental effects were evident (i.e., explaining more variance than genetic effects; Amstadter et al., 2014; Sawyers et al., 2020). Thus, although genetic sources are involved in the predispositions for resilience and resistance, as well as their relationship, environmental experiences and their resulting learning processes specific to individuals play an important role. Such unique environmental events may include differences in various trauma/stressor exposures, peer influences related to alcohol use, community and social resources, or countless other aspects in which twins may encounter and be confronted with different challenges and experiences as they develop. Although examination of such environmental factors is beyond the scope of this work, this represents an important area for future investigation. For example, the benefit of studies incorporating both genomic and environmental data has been highlighted (Choi et al., 2019) as they may shed light on gene-environment interplay by examining genetic influences in combination with nongenetic risk and protective factors over time (Choi et al., 2019).
Another goal of the present study was to test for sex differences in PR and AR and their covariance. The best-fitting model was one with quantitative, but not qualitative, sex differences. This finding suggests that that although common additive genetic influences are involved in PR and AR, the magnitude of these similar genetic effects may vary slightly in males and females. While significance tests support that genetic effects on PR and AR were stronger for males than females, and that unique environment effects on PR and AR were stronger for females than males, both sets of estimates displayed overlapping confidence intervals. Thus, although there is some preliminary evidence to suggest that genetic and environmental influences on PR and AR vary by sex, these modest effects are likely not meaningful. Present findings add to the mixed literature and further suggest that the investigation of possible sex differences in resilience are likely nuanced. Examination of PR and AR in different samples will be important to determine the consistency of these findings. As previously noted, two prior twin studies have also found evidence for slightly greater heritability estimates in males (Boardman et al., 2008; Waaktaar & Torgersen, 2012). These findings are all in contrast to prior univariate work that found evidence for qualitative sex differences for the PR conceptualization, indicating that different genetic factors influence liability to PR for males and females (Amstadter et al., 2014). A number of possible explanations may explicate this discrepancy. First, it may be that while different genetic effects influence PR, the same genetic effects to varying degrees influence the association between PR and AR for males and females. This discrepancy may also be due to the differences in how resilience was measured between the two sets of analyses (e.g., manifest residual scores versus latent residual factors) and the present study’s approach of using all available item-level data. Additional work is needed to further explore potential sex differences in PR and AR.
As a noted extension to prior work with these resilience constructs, in the present study we used measurement models to construct our resilience measures. As previously stated, it is noteworthy that the current findings are largely consistent with prior work, which employed manifest residual scores as the measure of discrepancy-based resilience, while current analyses utilized factor scores as the resilience measures. However, it is important to note that AR and PR heritability point estimates found here are smaller than what was found in two previous twin studies, one using a somewhat similar metric of resilience (positive affect controlling for interpersonal stressors) in adults with additive genetic factors explaining 52% of the variance in males and 38% in females (Boardman et al., 2008) and another using a trait-based resilience factor in adolescents finding even higher rates, at 78% in males and 70% in females (Waaktaar & Torgersen, 2012). Compared to these studies, but broadly similar to prior work with PR, a predominate portion of PR and AR variability was attributed to nonshared unique environmental sources. While the reasons for these different estimates are unknown, findings do highlight the relevance of genetic factors in underlying a wide range of resilience approaches while also highlighting the importance of a wide range of individual experiences and environmental contexts in these resilience conceptualizations. Thus, although genetic sources are involved in the predispositions for developing resilience, environmental experiences and their resulting learning processes and interpretations specific to individuals play a proportionately large role.
This study has a number of limitations that should be mentioned. First, participants included were all White twins born in Virginia, which may limit the generalizability of findings. Second, while the use of latent variables takes into account measurement error and restricts the construct variation to only the shared covariance among the item sets, all study variables were collected via self-report, so there is some concern for reporting bias, especially for the more sensitive issues associated with substance use problems. Third, to receive the alcohol abuse and dependence symptom items, each twin was required to have met specified male and female cutoffs for quantity and/or frequency of alcohol consumed which resulted in missing data for a portion of the twin samples. However, to retain information on the PR items, all twin records were retained when estimating the measurement models. Finally, in terms of PR, we did not assess for each participant’s personal reaction to the individual stressful life events, or the severity of the stressors. While extant work has demonstrated that emotional reactions to stressors/traumas are risk factors for psychiatric outcomes (e.g., PTSD risk; Karam et al., 2018), other work has demonstrated that the presence or absence of peritraumatic reactions do not have a substantial impact on post-trauma psychopathology (O’Donnell et al., 2010). We also note that the majority of available measures of stressors/traumas assess only the experience of the event, not the reaction to the event itself. Regardless, it is still possible that those who were estimated to be “less resilient” may have been the individuals who experienced the more severe events or more severe reactions to those events. Thus, severity of stressors and one’s personal reaction to them are unknown in the present study and future work could benefit from examining stressor/trauma severity or reactions to create a more nuanced picture of resilience.
Despite these limitations, the current study adds to the current literature and has some important implications. Specifically, this is the first twin study, to our knowledge, to employ a bivariate twin model to 2 different forms of resilience (PR and AR) and also test for sex differences. We also tested these questions using psychometrically rigorous measurement models that jointly estimated both the AR and PR liabilities and their respective residuals (the resilience phenotypes) using latent variables. Findings suggest that both phenotypes are modestly heritable, and are moderately correlated, suggesting a relationship between the genetic factors of PR and AR. Downstream molecular genetic efforts related to these types of outcomes (i.e., variants that enable individuals to adapt more efficiently than predicted) have yet to be investigated, but should be approached with the acknowledgement that environmental sources of variance continue to appear more strongly relevant for resilience. Further, results highlight that molecular genetic efforts into resilience should seek to incorporate various conceptualizations of resilience. Summarizing the directions for future genomic research on resilience, Choi and colleagues (2019) suggested focusing on emerging genetic evidence associated with resilience as defined in three ways: as factors underlying capacity for resilience, genetic and environmental factors that contribute to resilience processes, and genetic factors associated with resilience outcomes (of which the current approach falls) noting that together these definitions of resilience may provide complementary or distinct insights into adaptation. Finally, as noted, a substantial portion of the AR and PR phenotypic variability was attributed to nonshared unique environmental sources.
The contribution of unique experiences to resilience remains notable, which is a common pattern found in the resilience literature, and appears to differ between males and females. Thus, there is a need for further research to identify these unique environmental and psychological factors that are modifiable and may be intervened upon to foster resilience.
Supplementary Material
Funding
The dataset in this paper comes from the Mid-Atlantic Twin Registry, previously supported by NIH grant UL1RR031990. Dr. Sheerin’s time is funded by grant K01 AA025692 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA); Dr. Bountress’s time is funded by K01 AA028058-01 from NIAAA; Ms. Cusack’s time during the course of this work was supported by grant F31AA027703 from the NIAAA; Dr. Amstadter’s time is partially funded by grants K02 AA023239 and R01 AA020179 from NIAAA.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors declare they have no conflicts of interest.
Ethics Approval
Approval was obtained from the ethics committee (Institutional Review Board) of Virginia Commonwealth University. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
References
- Akaike H (1987). [Prediction of Future Observations in Growth Curve Models]: Comment. Statistical Science, 2(4), 464–465. 10.1214/ss/1177013124 [DOI] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). American Psychiatric Association. [Google Scholar]
- Amstadter AB, Maes HH, Sheerin CM, Myers JM, & Kendler KS (2016). The relationship between genetic and environmental influences on resilience and on common internalizing and externalizing psychiatric disorders. Social Psychiatiy and Psychiatric Epidemiology, 51(5), 669–678. 10.1007/s00127-015-1163-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Moscati A, Maes HH, Myers JM, & Kendler KS (2016). Personality, cognitive/psychological traits and psychiatric resilience: A multivariate twin study. Personality and Individual Differences, 91, 74–79. 10.1016/j.paid.2015.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Myers JM, & Kendler KS (2014). Psychiatric resilience: Longitudinal twin study. The British Journal of Psychiatry, 205(4), 275–280. 10.1192/bjp.bp.113.130906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Blalock CL, & Button TMM (2008). Sex Differences in the Heritability of Resilience. Twin Research and Human Genetics, 11(1), 12–27. 10.1375/twin.11.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno GA, & Mancini AD (2012). Beyond resilience and PTSD: Mapping the heterogeneity of responses to potential trauma. Psychological Trauma: Theory, Research, Practice, and Policy, 4(1), 74–83. 10.1037/a0017829 [DOI] [Google Scholar]
- Boomsma D, Busjahn A, & Peltonen L (2002). Classical twin studies and beyond. Nature Reviews. Genetics, 3(11), 872–882. 10.1038/nrg932 [DOI] [PubMed] [Google Scholar]
- Cheung GW, & Rensvold RB, (2002). Evaluating Goodness-of-Fit Indexes for Testing Measurement Invariance. Structural Equation Modeling, 9, (2), pp. 233–255 [Google Scholar]
- Choi KW, Stein MB, Dunn EC, Koenen KC, & Smoller JW (2019). Genomics and psychological resilience: A research agenda. Molecular Psychiatry, 24(12), 1770–1778. 10.1038/s41380-019-0457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LJ, & Heath AC (1981). Sex-limitation and “asymmetric” assortative mating. Progress in Clinical and Biological Research, 69 Pt B, 73–86. [PubMed] [Google Scholar]
- Hoffmeister JR, Cohoon AJ, Sorocco KH, Acheson A, & Lovallo WR (2019). Addiction resistance to alcohol: What about heavy drinkers who avoid alcohol problems?. Drug and alcohol dependence, 204, 107552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam E, Bromet E, Ruscio A, Stein D, & Kessler R (2018). The Role of Criterion A2 in the DSM-IV Diagnosis of Posttraumatic Stress Disorder. In Bromet E, Karam E, Koenen K, & Stein D (Eds.), Trauma and Posttraumatic Stress Disorder: Global Perspectives from the WHO World Mental Health Surveys (pp. 263–272). Cambridge: Cambridge University Press. doi: 10.1017/9781107445130.019 [DOI] [Google Scholar]
- Kim-Cohen J, Moffitt TE, Caspi A, & Taylor A (2004). Genetic and environmental processes in young children’s resilience and vulnerability to socioeconomic deprivation. Child Development, 75(3), 651–668. 10.1111/j.1467-8624.2004.00699.x [DOI] [PubMed] [Google Scholar]
- Kendler KS, & Myers J (2015). Addiction resistance: Definition, validation and association with mastery. Drug and Alcohol Dependence, 154, 236–242. 10.1016/j.drugalcdep.2015.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, & Prescott CA (2006). Genes, environment, and psychopathology: Understanding the causes of psychiatric and substance use disorders. Guilford Press. [Google Scholar]
- Lubke GH, Dolan CV, & Neale MC (2004). Implications of absence of measurement invariance for detecting sex limitation and genotype by environment interaction. Twin Research : The Official Journal of the International Society for Twin Studies, 7(3), 292–298. 10.1375/136905204774200578 [DOI] [PubMed] [Google Scholar]
- Luthar SS, Cicchetti D, & Becker B (2000). The Construct of Resilience: A Critical Evaluation and Guidelines for Future Work. Child Development, 71(3), 543–562. 10.1111/1467-8624.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini AD, & Bonanno GA (2009). Predictors and parameters of resilience to loss: Toward an individual differences model. Journal of personality, 77(6), 1805–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith W (1993). Measurement invariance, factor analysis and factorial invariance. Psychometrika, 58(4), 525–543. 10.1007/BF02294825 [DOI] [Google Scholar]
- Michell J (2003). Epistemology of Measurement: The Relevance of its History for Quantification in the Social Sciences. Social Science Information, 42(4), 515–534. 10.1177/0539018403424004 [DOI] [Google Scholar]
- Millsap RE, & Yun-Tein J (2004). Assessing Factorial Invariance in Ordered-Categorical Measures. Multivariate Behavioral Research, 39(3), 479–515. 10.1207/S15327906MBR3903_4 [DOI] [Google Scholar]
- Muthen, & Muthen. (1998). Mplus User’s Guide. (8th ed.). Muthen & Muthen. [Google Scholar]
- Neale MC, Aggen SH, Maes HH, Kubarych TS, & Schmitt JE (2006). Methodological issues in the assessment of substance use phenotypes. Addictive Behaviors, 31(6), 1010–1034. 10.1016/j.addbeh.2006.03.047 [DOI] [PubMed] [Google Scholar]
- Neale MC, & Cardon LR (1992). Sex-limitation and G × E Interaction. In Neale MC & Cardon LR (Eds.), Methodology for Genetic Studies of Twins and Families (pp. 211–229). Springer Netherlands. 10.1007/978-94-015-8018-2_11 [DOI] [Google Scholar]
- Neale MC, Lubke G, Aggen SH, & Dolan CV (2005). Problems with using sum scores for estimating variance components: Contamination and measurement noninvariance. Twin Research and Human Genetics : The Official Journal of the International Society for Twin Studies, 8(6), 553–568. 10.1375/183242705774860231 [DOI] [PubMed] [Google Scholar]
- Nishimi K, Choi KW, Cerutti J, Powers A, Bradley B, & Dunn EC (2020). Measures of adult psychological resilience following early-life adversity: how congruent are different measures?. Psychological medicine, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell ML, Creamer M, McFarlane AC, Silove D, & Bryant RA (2010). Should A2 be a diagnostic requirement for posttraumatic stress disorder in DSM-V?. Psychiatry Research, 176(2-3), 257–260. [DOI] [PubMed] [Google Scholar]
- Rutter M (2013). Annual Research Review: Resilience – clinical implications. Journal of Child Psychology and Psychiatry, 54(4), 474–487. 10.1111/j.1469-7610.2012.02615.x [DOI] [PubMed] [Google Scholar]
- Sawyers C, Kurtz ED, Sheerin C, Maes HH, Kendler KS, & Amstadter AB (2020). A behavioral genetic investigation of conceptualizations of resilience in a female twin sample. Depression and anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheerin CM, Lind MJ, Brown EA, Gardner CO, Kendler KS, & Amstadter AB (2018). The impact of resilience and subsequent stressful life events on MDD and GAD. Depression and Anxiety, 35(2), 140–147. 10.1002/da.22700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Bonanno GA, Masten AS, Panter-Brick C, & Yehuda R (2014). Resilience definitions, theory, and challenges: interdisciplinary perspectives. European journal of psychotraumatology, 5(1), 25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, & First MB (1992). The Structured Clinical Interview for DSM-III-R (SCID): I: History, Rationale, and Description. Archives of General Psychiatry, 49(8), 624–629. 10.1001/archpsyc.1992.01820080032005 [DOI] [PubMed] [Google Scholar]
- Stein MB, Choi KW, Jain S, Campbell-Sills L, Chen CY, Gelernter J, … & Nock MK (2019). Genome-wide analyses of psychological resilience in US Army soldiers. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 180(5), 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout W (2002). Psychometrics: From practice to theory and back. Psychometrika, 67(4), 485–518. 10.1007/BF02295128 [DOI] [Google Scholar]
- Vandenberg RJ (2002). Toward a Further Understanding of and Improvement in Measurement Invariance Methods and Procedures. Organizational Research Methods, 5(2), 139–158. 10.1177/1094428102005002001 [DOI] [Google Scholar]
- Waaktaar T, & Torgersen S (2012). Genetic and Environmental Causes of Variation in Trait Resilience in Young People. Behavior Genetics, 42(3), 366–377. 10.1007/s10519-011-9519-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth RJ, & Edwards MC (2007). Item factor analysis: Current approaches and future directions. Psychological Methods, 12(1), 58–79. 10.1037/1082-989X.12.1.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Miller MW, Sullivan DR, Amstadter AB, Mitchell KS, Goldberg J, & Magruder KM (2018). A classical twin study of PTSD symptoms and resilience: Evidence for a single spectrum of vulnerability to traumatic stress. Depression and anxiety, 35(2), 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


