Abstract
Background.
Although metastatic breast cancer (MBC) remains incurable, advances in therapies have improved survival. Using a contemporary dataset of de novo MBC patients, we explore how overall (OS) and cancer-specific survival (CSS) changed over time.
Methods.
All patients with de novo MBC from 1988 to 2016 were selected from Surveillance, Epidemiology, and End Results (SEER) 18. Unadjusted OS and CSS were estimated by Kaplan–Meier method and stratified by disease characteristics. Cox proportional hazards models determined factors associated with survival.
Results.
47,034 patients were included, with median OS of 25 months and CSS of 27 months. Survival steadily improved over time (1988: 1-year OS 62%, CSS 65%; 2015: 1-year OS 72%, CSS 74%). Patients with triple-negative breast cancer (TNBC) had the worst prognosis and were most likely to die from MBC [versus human epidermal growth factor receptor 2 (HER2)+ and hormone receptor (HR)+/HER2−]. Those with ≥ 4 sites of metastatic disease were also more likely to die from MBC with nearly identical OS and CSS (5-year OS 9%, CSS 9%), when compared with those with 1 site (5-year OS 31%, CSS 35%). After adjustment, improved CSS was associated with bone-only disease [hazard ratio (HR) 0.88, 95% confidence interval (CI) 0.83–0.94], while TNBC (versus HER2+: HR 3.12, 95% CI 2.89–3.36) and > 3 sites of metastatic disease (versus 1 site: HR 3.24, 95% CI 2.68–3.91) were associated with worse CSS (all p < 0.001).
Conclusions.
Accurate prognostic estimates are essential for patient care. As treatments for patients with MBC have expanded, OS and CSS have improved, and more patients, particularly with limited distant disease or favorable tumor subtypes, are also dying from non-MBC causes.
In 2020, an estimated 276,480 women received a diagnosis of breast cancer in the USA, and approximately 6% were metastatic at presentation.1,2 Considered incurable, metastatic breast cancer (MBC) remains a devastating disease with patient goals directed towards improved quality of life and extended survival.3 However, a growing list of medical treatment options has fundamentally shifted the landscape of BC mortality.4–8 Over the past 30 years, there has been a 40% decline in all breast cancer deaths.9 While advances in breast cancer treatments have been impressive for women with early-stage disease, women diagnosed with the most advanced forms of breast cancer have also experienced survival benefits.2, 10,11
With improvements in cancer-related survival, evidence shows that noncancer deaths have proportionally increased more than cancer-related deaths in the USA;12 For example, a retrospective review of 754,270 women with breast cancer in the SEER program (all stages of disease, diagnosed in 2000–2015) demonstrated that 38.2% of patients died from breast cancer, while almost half of the cohort (48.4%) died of noncancer causes.13 In patients surviving more than 10 years, over 60% died from noncancer causes, while only 23% died from their breast cancer.13 Internationally, researchers have observed similar trends. A population-based cohort study of women in northeastern Spain diagnosed with breast cancer from 1985 to 2004 (N = 10,195) demonstrated that women who did not die from breast cancer at 10 or 20 years after diagnosis had a 20% higher risk of dying from other (non-breast-cancer) causes than women without breast cancer.14
Thus, the rate of non-cancer-related deaths for patients with breast cancer has increased over time. Most of these large survival analyses, however, either exclude those with metastatic disease or include all stages of disease (and those with MBC represent a small fraction of the population). Furthermore, most large studies specific to MBC lag behind modern datasets and likely do not reflect the benefits derived from modern therapies. While many studies recognize the heterogeneity in the MBC population, most also agree that many patients with MBC are living longer.15 Therefore, using a contemporary dataset of patients with de novo MBC, we explored how overall (OS) and cancer-specific survival (CSS) have changed over time.
METHODS
All patients diagnosed with breast cancer in 1975–2016 were selected from the April 2019 release of the SEER 18 dataset. Patients with nonmetastatic disease or unknown metastatic disease status were excluded, as were those with histology codes other than those identified by the World Health Organization classification of tumors16 and those with missing or unknown survival data. Metastatic disease was defined as having clinical or pathological M1 disease. Patients diagnosed in 1975–1987 were also excluded due to lack of reliable entry of tumor characteristics for these diagnosis years. HR+ disease was defined as being estrogen receptor (ER) positive and/or progesterone receptor (PR) positive. TNBC was defined as being ER−, PR −, and HER2−. ER, PR, and HER2 status listed as “borderline” were treated as “negative” when defining tumor phenotype.
OS was defined as time from diagnosis to death due to any cause. Patients who did not die were censored at time of last follow-up for all OS analyses. CSS was defined as time from diagnosis to death due to this cancer diagnosis. Patients who did not die were censored at time of last follow-up, and patients who died of causes other than this cancer were censored at date of death for all CSS analyses. Additionally, patients with more than one primary tumor were excluded from all CSS analyses.
Unadjusted OS and CSS were estimated using the Kaplan–Meier method for all patients diagnosed in 1988–2016, and for subgroups including age group (< 40, 40–70, and > 70 years), phenotype (HER2+, HR+/HER2−, and TNBC), metastatic site for patients with a single organ system involved (bone, brain, liver, and lung), number of metastatic organ systems involved (1 to [3 metastatic sites), and year of diagnosis (1988–1995, 1996–2002, 2003–2009, and 2010–2016). All subgroup analyses other than by year of diagnosis were conducted only on patients diagnosed 2010–2016. Log-rank tests were used to compare unadjusted survival between select subgroups including phenotype and year of diagnosis. One-, three-, and five-year OS and CSS rates and 95% confidence intervals (CIs) were estimated using the Kaplan–Meier method by year of diagnosis, and these rates were plotted to visualize change in survival over time. One-year OS and CSS rates and 95% CIs were also estimated for tumor phenotype by year of diagnosis and were plotted to visualize change in survival over time.
Cox proportional hazards models were used to determine which factors were associated with OS and CSS, after adjustment for other covariates. Variables in the adjusted model included gender, age, race/ethnicity, year of diagnosis, insurance status, tumor phenotype, bone-only metastasis, number of metastatic organ systems involved (one site = one organ system involved with an unknown number of metastases at that site; two sites = two organ systems involved with an unknown number of metastases at those sites; etc.), surgery receipt, chemotherapy receipt, and radiation receipt. Additional models were conducted that included interaction terms of year of diagnosis with each subgroup variable to determine if the change over time in risk of all-cause and cancer-specific death differs based on patient and tumor characteristics.
Our group recently proposed a novel staging system for patients with de novo MBC based on data from the National Cancer Data Base (NCDB),17 which categorizes patients based on disease characteristics into three distinct groups (A, B, and C). Similar to the staging system for nonmetastatic breast cancer, this stratification system incorporates both tumor anatomy and biology. The variables in the proposed staging system include ER, PR, HER2, grade, T stage, number of metastatic sites (one site = one organ system involved; two sites = two organ systems involved; etc.), and bone-only disease. In general, those with more limited disease and either HER2+ or ER+/HER2− disease were found to have higher survival rates (thus classified as “stage IVA” or “stage IVB”), while those with ≥ 3 sites of metastatic disease had generally unfavorable outcomes (thus classified as “stage IVC”). Applying this classification system (stages IVA/B/C) to the current study population, additional exploratory analyses were performed including Kaplan–Meier survival analyses and Cox proportional hazards modeling to further investigate the potential utility of the proposed staging system. Variables in the adjusted model included gender, age, race/ethnicity, year of diagnosis, insurance status, surgery receipt, chemotherapy receipt, radiation receipt, and metastatic stage group (A/B/C).
p value < 0.05 was considered statistically significant. No adjustments were made for multiple comparisons. Only patients with available data were included in each analysis, and effective sample sizes are included for all tables and figures. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary NC) or R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Due to use of deidentified data, our institutional review board granted the study exempt status.
RESULTS
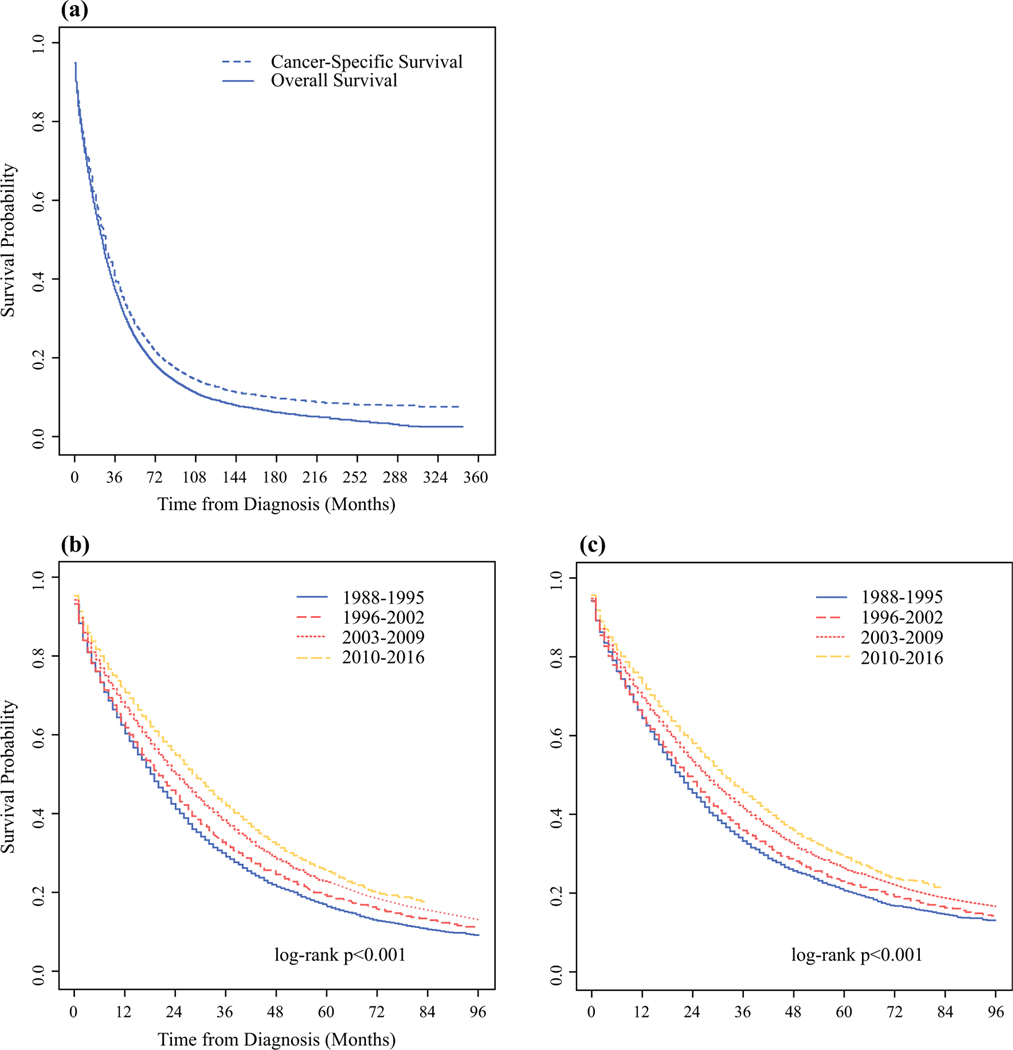
Of the 1,402,959 patients in the SEER 18 database with breast cancer, there were 47,034 patients with de novo MBC diagnosed in 1988–2016, meeting the inclusion and exclusion criteria, 19,444 of whom were diagnosed between 2010 and 2016 (Supplementary Fig. 1). The median follow-up time was 91 months with an overall mortality rate of 76% and cancer-specific mortality rate of 68.8%. Most patients were aged 40–70 years (64.7%), female (99%), non-Hispanic White (67.2%), and insured (73.9%). Most tumors were grade 3 (43.5%), ER+ (62.4%), PR+ (49.2%), and HER2− (67.8%). At initial diagnosis, most patients had one organ system involved (68.8% with one metastatic site = one organ system involved with an unknown number of metastases at that site), and 37.4% had bone-only metastasis. Patients largely received chemotherapy (54.3%), but the majority did not receive surgery or radiation therapy (Supplementary Table 1). The median OS and CSS were 25 and 27 months, respectively. The unadjusted 5-year OS and CSS rates were 22.8% (95% CI 22.4–23.2%) and 26.4% (95% CI 25.9–26.9%), respectively (Fig. 1a).
FIG. 1.
Unadjusted OS and CSS for patients with metastatic breast cancer diagnosed in 1988–2016 from the SEER Program: a for the entire cohort, b OS stratified by year of diagnosis, and ≥ CSS stratified by year of diagnosis
Survival Changes over Time
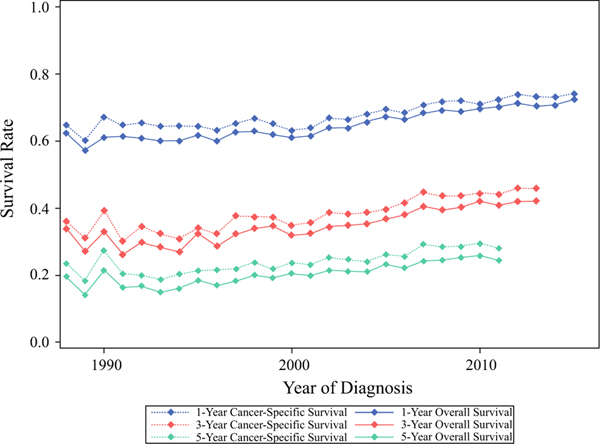
When stratified by year of diagnosis, patients diagnosed in the most recent years had the best survival outcomes (Fig. 1b, c). For example, the median OS improved from 19 months for those diagnosed in 1988–1995 to 29 months for those diagnosed in 2010–2016; similar trends were observed in CSS, which increased from 21 to 32 months for the same time periods. Furthermore, these trends in improvement were also noted in the unadjusted 1-, 3-, and 5-year survival rates (Fig. 2). At 1 year, the OS rate increased from 62.3% in 1988 to 72.4% in 2015, while the CSS rate increased from 64.7 to 74.1%. At 3 years, the OS rate increased from 33.8% in 1988 to 42.1% in 2013, while the CSS rate increased from 36 to 45.9%. At 5 years, the OS rate increased from 19.4% in 1988 to 24.3% in 2011, while the CSS rate increased from 23.4% to 28%.
FIG. 2.
Unadjusted OS (solid lines) and CSS (dotted lines) rates at 1-year (blue lines), 3-years (red lines), and 5-years (green lines) for patients with metastatic breast cancer from the SEER Program diagnosed in 1988–2016, stratified by year of diagnosis
When stratified by tumor phenotype for those diagnosed in 2010–2016, the unadjusted 1-year survival rates for each phenotype increased minimally (1–2%) over the most recent timeframe. For those with HER2+ disease, the 1-year OS and CSS rates were 74.3% and 77.3% for those diagnosed in 2010, compared with 77.3% and 78.9% in 2015. For those with HR+/HER2− disease, the 1-year OS and CSS rates were 77.0% and 77.2% for those diagnosed in 2010, compared with 76.8% and 78.6% in 2015. For those with TNBC, the 1-year OS and CSS rates were 44.0% and 48.4% for those diagnosed in 2010, compared with 48.3% and 50.1% in 2015.
Patient Factors Impacting Survival
Stratified Analysis
When stratified by patient age, those aged < 40 years had the best survival outcomes (unadjusted median OS 43, CSS 46 months), particularly compared with those > 70 years (median OS 18, CSS 21 months). Similar to the overall cohort and patients of all ages, those aged > 70 years were most likely to die from breast cancer; however, patients aged > 70 years had the lowest survival rates. However, other causes of death became more common as these older patients lived longer. When stratified by patient gender, men had better survival outcomes in the unadjusted analysis (median OS and CSS: men 33 and 40 months versus women 29 and 32 months), although non-cancer causes of death were more common among men.
Multivariable Model
In the adjusted analysis, gender was not associated with survival, while age was significantly associated with survival outcomes (Table 1). More specifically, age > 70 years was associated with worse survival (OS: HR 1.96, 95% CI 1.78–2.16); CSS: HR 1.78, 95% CI 1.60–1.98; reference age < 40 years). In the adjusted analysis, an interaction was noted between year of diagnosis and age, suggesting that the impact of age on survival varied by year of diagnosis (Supplementary Table 2).
TABLE 1.
Adjusted OS and CSS for patients with metastatic breast cancer from the SEER Program, diagnosed 2010–2016
| OS (N = 17,740) |
CSS (N = 14,429) |
|||||
|---|---|---|---|---|---|---|
| HR (95% CI) |
p value | Overall p value | HR (95% CI) |
p value | Overall p value | |
|
| ||||||
| Age (years) | < 0.001 | < 0.001 | ||||
| < 40 | REF | REF | ||||
| 40–70 | 1.26 (1.15–1.38) | < 0.001 | 1.27 (1.15–1.40) | < 0.001 | ||
| > 70 | 1.96 (1.78–2.16) | < 0.001 | 1.78 (1.60–1.98) | < 0.001 | ||
| Gender | 0.68 | 0.82 | ||||
| Female | REF | REF | ||||
| Male | 1.04 (0.86–1.26) | 0.68 | 0.97 (0.78–1.22) | 0.82 | ||
| Tumor phenotype | < 0.001 | < 0.001 | ||||
| HER2+ | REF | REF | ||||
| HR+/HER2- | 0.96(0.91–1.01) | 0.12 | 0.99(0.93–1.05) | 0.75 | ||
| TNBC | 2.95 (2.77–3.15) | < 0.001 | 3.12(2.89–3.36) | < 0.001 | ||
| Bone-only metastasis | < 0.001 | < 0.001 | ||||
| No/unknown | REF | REF | ||||
| Yes | 0.89 (0.84–0.94) | < 0.001 | 0.88 (0.83–0.94) | < 0.001 | ||
| Number of metastatic sites | < 0.001 | < 0.001 | ||||
| 1 | REF | REF | ||||
| 2 | 1.44 (1.36–1.52) | < 0.001 | 1.49 (1.40–1.59) | < 0.001 | ||
| 3 | 2.20 (2.04–2.38) | < 0.001 | 2.41 (2.20–2.63) | < 0.001 | ||
| ≥ 4 | 3.06 (2.58–3.64) | < 0.001 | 3.24 (2.68–3.91) | < 0.001 | ||
Model adjusted for gender, age, race/ethnicity, year of diagnosis, tumor phenotype, bone-only metastasis, number of metastatic sites, surgery receipt, chemotherapy receipt, and radiation receipt
HR+ estrogen receptor (ER) and/or progesterone receptor (PR) positive, TNBC triple-negative breast cancer (ER-/PR-/HER2−), HR hormone receptor, HER2 human epidermal growth factor receptor 2
Disease Factors Impacting Survival
Stratified Analysis
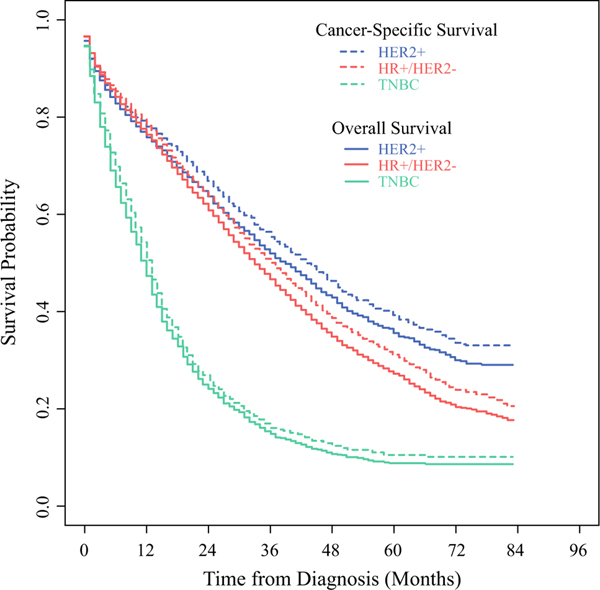
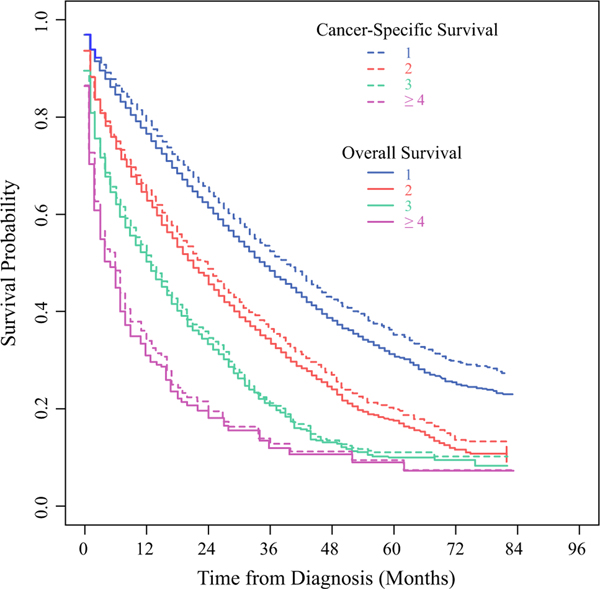
When stratified by tumor phenotype, limited to those diagnosed in 2010–2016, patients with TNBC had the worst survival outcomes (median OS 12, CSS 13 months), compared with those with HR+/HER2disease (median OS 33, CSS 36 months) and those with HER2+ disease (median OS 39, CSS 43 months) (Supplementary Fig. 2). Although breast cancer was the most common cause of death for all tumor phenotypes (Fig. 3), those with HER2+ disease were increasingly likely to die of noncancer causes with longer survival, as indicated by the increasing separation of the OS and CSS curves, as compared with those with TNBC who had little divergence of the survival curves over time. For those with a single site of metastasis, the median OS and CSS were 35 and 39 months, respectively; survival decreased steadily with increasing number of metastatic sites ([ 3 sites: median OS 5, CSS 6 months). When stratified by the number of metastatic sites (Fig. 4), those with only one site of metastasis were noted to have increasing rates of noncancer deaths with longer survival. For those with one metastatic site, patients with brain-only metastasis had the worst survival outcomes (median OS 10, CSS 11 months), while those with bone-only metastases had the best survival outcomes (median OS 37, CSS 42 months). With longer survival, patients with bone-only, lung-only, or liver-only metastases had increasing rates of noncancer deaths.
FIG. 3.
Unadjusted OS (solid lines) and CSS (dotted lines) for patients with metastatic breast cancer from the SEER Program, diagnosed 2010–2016, stratified by tumor phenotype: HER2+ (blue lines), HR+/HER2− (red lines), and TNBC (green lines). HR+ estrogen receptor (ER) and/or progesterone receptor (PR) positive, TNBC ER-/PR-/HER2−.HR: hormone receptor. HER2: humanepidermal-growth-factor-receptor-2. TNBC: triple negative breast cancer
FIG. 4.
Unadjusted OS (solid lines) and CSS (dotted lines) for patients with metastatic breast cancer from the SEER Program, diagnosed 2010–2016, stratified by the number of metastatic sites: 1 (blue lines), 2 (red lines), 3 (green lines), and ≥ 4 (purple lines). one site = one organ system involved, irrespective of the number of the metastases within that organ system; two sites = two organ systems involved; etc.
Multivariable Model
After adjustment, there was no significant difference in survival outcomes for those with HER2+ or HR+/HER2− disease, while those with TNBC had worse outcomes (OS: HR 2.95, 95% CI 2.77–3.15; CSS: HR 3.12, 95% CI 2.89–3.36; reference HER2+) (Table 1). Not surprisingly, an increasing number of metastatic sites was also associated with worse survival outcomes, while having bone-only metastases was associated with improved survival (OS: HR 0.89, 95% CI 0.84–0.94; CSS: HR 0.88, 95% CI 0.83–0.94) (Table 1). In the adjusted analysis, an interaction was noted between year of diagnosis and bone-only metastasis, and between year of diagnosis and number of metastatic sites, suggesting that the impact of bone-only metastasis and number of metastatic sites on survival varied by year of diagnosis (Supplementary Table 2).
Survival Based on Staging System (IVA/B/C)
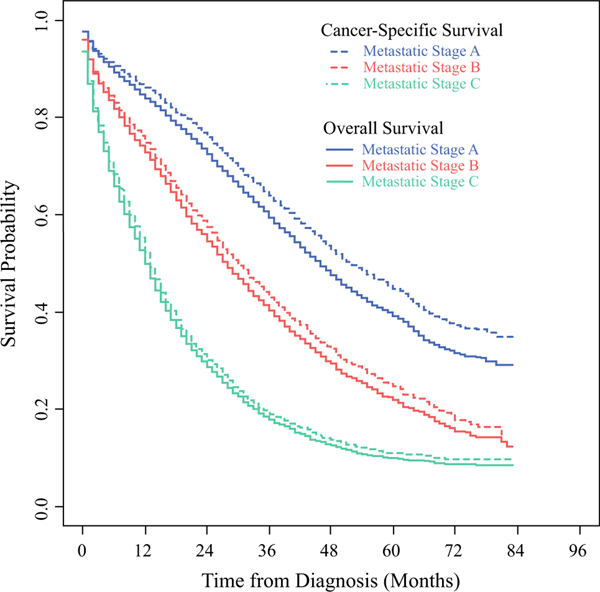
After applying the proposed MBC staging guidelines described by Plichta et al.17 to the entire cohort, patient survival outcomes separated in accordance with their staging patterns (Supplementary Fig. 3), with those classified as stage IVA having the best outcomes (median OS 46, CSS 52 months) and those with stage IVC disease having the worst outcomes (median OS 12, CSS 13 months). When stratified by metastatic stage group IVA/B/≥ (Fig. 5), the proportion of noncancer deaths increased over time for those with stage IVA disease and longer follow-up. After adjustment, increasing stage category was associated with increasingly worse survival (stage IVB— OS: HR 1.55, 95% CI 1.46–1.64; CSS: HR 1.65, 95% CI 1.55–1.76; stage IVC—OS: HR 3.35, 95% CI 3.18–3.52; CSS: 3.67, 95% CI 3.46–3.90; reference stage IVA) (Table 2). Furthermore, an interaction was noted between year of diagnosis and metastatic stage group (IVA/B/C), suggesting that the impact of stage group on survival varied by year of diagnosis (Supplementary Table 3).
FIG. 5.
Unadjusted OS (solid lines) and CSS (dotted lines) for patients with metastatic breast cancer from the SEER Program, diagnosed 2010–2016, stratified by proposed prognostic stage group: metastatic stage IVA (blue lines), IVB (red lines), and IVC (green lines)
TABLE 2.
Adjusted OS and CSS for patients with metastatic breast cancer from the SEER Program, diagnosed 2010–2016
| OSS (N = 15,743) |
CSS (N = 12,923) |
|||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | Overall p value | HR (95% CI) | p value | Overall p value | |
|
| ||||||
| Metastatic stage group | < 0.001 | < 0.001 | ||||
| IVA | REF | REF | ||||
| IVB | 1.55 (1.46–1.64) | < 0.001 | 1.65 (1.55–1.76) | < 0.001 | ||
| IVC | 3.35 (3.18–3.52) | < 0.001 | 3.67 (3.46–3.90) | < 0.001 | ||
| Gender | 0.79 | 0.65 | ||||
| Female | REF | REF | ||||
| Male | 0.97 (0.80–1.19) | 0.79 | 0.95 (0.75–1.20) | 0.65 | ||
| Age (years) | < 0.001 | < 0.001 | ||||
| < 40 | REF | REF | ||||
| 40–70 | 1.22 (1.11–1.34) | < 0.001 | 1.24 (1.12–1.38) | < 0.001 | ||
| > 70 | 1.89 (1.71–2.10) | < 0.001 | 1.74 (1.55–1.95) | < 0.001 | ||
DISCUSSION
This study explored survival outcomes for > 47,000 breast cancer patients with de novo metastatic disease in the SEER database. While our analysis demonstrated the expected improvements in survival, it is noteworthy that the increase in CSS resulted in a nearly equivalent increase in OS, suggesting that these women are likely otherwise healthy with few competing morbidities. In addition, we also demonstrated compelling differences in OS and CSS trends based on select stratifications of patient and tumor characteristics with longer follow-up. Breast cancer was clearly the most common cause of death for all groups regardless of stratification; however, select populations (e.g., HER2+, HR+/HER2−, and limited metastatic disease) were noted to have increasing rates of noncancer deaths with longer follow-up.
Consistent with previous studies, we show that a number of patient and disease characteristics were associated with OS and CSS.18–20 While year of diagnosis, age < 40 years at diagnosis, and HR+ disease were associated with decreasing risks of death and improved survival in our study, worse survival was experienced by patients with increasing age at diagnosis, TNBC, and increasing number of metastatic sites. Dawood et al.18 reviewed de novo MBC patients in SEER from 1988 to 2003 and found that earlier year of diagnosis, grade 3 disease, increasing age, and HR- disease were all independently associated with worse survival. A later study by Di Meglio et al. reviewed 10,000 patients in SEER from 1990 to 2011 and found that year of diagnosis, age at diagnosis, and HR status were associated with OS.19 Consistent with prior findings, we also report a steady rate of improvement in 1- and 5-year OS and CSS rates. Furthermore, we showed that improved survival is significantly associated with bone-only metastasis and one site of metastasis.
The aforementioned characteristics and their associations with survival may come as no surprise, given their similar associations with prognosis for other stages of breast cancer.21–24 However, our contemporary analysis is one of the only of this magnitude to compare OS and CSS, and to demonstrate the association of certain characteristics with non-cancer-related mortality. More specifically, patients with stage IVA disease, one site of metastasis, and bone-only metastasis are increasingly likely to die of noncancer causes with longer follow-up. However, patients with TNBC, more than one site of metastasis, or brain-only metastasis were the least likely to die of noncancer causes. This supports findings in other literature, which suggests an increase in non-cancer-related deaths over time for patients with MBC.12,13 The reality that more women are surviving one of the most lethal forms of breast cancer only to die of other causes suggests that more attention may need to be directed to other comorbidities amongst patients with MBC.
A previous study by Colzani et al. assessed the cause of death among 12,850 women in the Stockholm Breast Cancer Registry; however, it excluded all women with MBC.25 Despite this exclusion, they identified circulatory system disorders as an important cause of death and concluded that many breast cancer survivors die of nononcologic causes.25 Most recently, a retrospective review of 754,270 women in the SEER database with all stages of BC diagnosed between 2000 and 2015 identified heart and cerebrovascular diseases as significant causes of mortality.13 While MBC remains the most common cause of mortality among these patients, it is important that future studies continue exploring other causes of mortality based on prognostic factors that may suggest a longer survival. More specifically, examining groups with promising prognostic characteristics, such as those with stage IVA disease, could reveal opportunities to address deficits in patient care and monitoring.
The significant variations in survival among patients with de novo MBC in our study underscore the importance and urgent need to adopt refined prognostic estimates. Our analysis based on the novel staging system for de novo MBC published by our group17 successfully reproduced our proposed three-tiered stratification. Similar to our initial report, results presented here show that patients with stage IVA disease experienced a nearly 3-year improvement in median OS between 1988 and 2016 when compared with patients with stage IVC disease. The dramatic difference observed in the survival outcomes serves as strong evidence for the need to incorporate similar guidelines into clinical practice. In a disease where primary treatment goals are traditionally palliative, prognostic estimates that differ on the scale of years may fundamentally change our patient discussions and recommendations.
Although our study was based on a large, diverse, and population-based cohort, it also had several limitations. While the SEER registries cover a limited portion of the US population (currently * 35%26), studies suggest that it is generally representative of the greater population and thus generalizable to other populations.27 Unfortunately, data missingness is also problematic in these databases,28 which may have introduced bias into our study that we cannot quantify. It is also important to recognize that SEER may have expanded over the years to capture more of the cancer population, which could have introduced selection bias into the study. While lead-time bias may have impacted our findings as screening increased over the years, the proportion of patients diagnosed with de novo MBC in SEER remained fairly stable (ranging from 5% to 6%) over the study period, suggesting that these biases (selection and lead time bias) are likely limited. In accordance with the guidelines followed by US tumor registrars, the tumor biomarkers (ER/PR/HER2) documented in SEER are based on the primary tumor. Lastly, our study was limited to those with MBC at initial presentation, and it is unclear how our findings might apply to those who were diagnosed with earlier-stage BC with progression to metastatic disease.
In conclusion, while outcomes for patients with de novo MBC continue to improve, we have shown significant improvements in survival within a contemporary dataset. We identified that those with limited distant disease and HER2+ or HR+/HER2− tumors are dying from non-MBC causes more than ever before. While there is marked heterogeneity among patients with de novo MBC, these findings suggest that greater attention to non-breast-cancer comorbidities and treatment-related side effects will be increasingly important in this patient population.
Supplementary Material
ACKNOWLEDGEMENTS
This study used the SEER database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute and the SEER Program tumor registries in the creation of the SEER **database.
FUNDING Dr. O. Fayanju is supported by the National Institutes of Health (NIH) under Award Number 1K08CA241390 (PI: Fayanju). This work was in part supported by Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan) for the Biostatistics Core.
Podium presentation at the SSO Annual Meeting in March 2021.
DISCLOSURES The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. Dr. J. Plichta is a recipient of research funding by the Color Foundation (PI: Plichta). She serves on the NCCN Breast Cancer Screening Committee and the ASCO Clinical Practice Guideline Committee for the Management of Male Breast Cancer. Dr. E.S. Hwang serves on the NCI Breast Cancer Steering Committee and the NCCN Breast Cancer Prevention Committee. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Samantha Thomas had a consulting relationship with Abbvie, Inc. on work in bioequivalence. This relationship ended in January 2019 and was unrelated to this work. Cancer Center Support Grant P30 from NCI provided funding for statistical support. Sarah L. Sammons reports research funding from Abbvie, Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Sermonix. Advisory to Astra Zeneca, Daiichi Sankyo, Foundation Medicine, Novartis.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1245/s10434021–10227-3.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85. [DOI] [PubMed] [Google Scholar]
- 3.Beslija S, Bonneterre J, Burstein HJ, et al. Third consensus on medical treatment of metastatic breast cancer. Ann Oncol. 2009;20(11):1771–85. [DOI] [PubMed] [Google Scholar]
- 4.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. [DOI] [PubMed] [Google Scholar]
- 5.Barinoff J, Schmidt M, Schneeweiss A, et al. Primary metastatic breast cancer in the era of targeted therapy—prognostic impact and the role of breast tumour surgery. Eur J Cancer. 2017;83:116–24. [DOI] [PubMed] [Google Scholar]
- 6.Daniels B, Kiely BE, Lord SJ, et al. Long-term survival in trastuzumab-treated patients with HER2−positive metastatic breast cancer: real-world outcomes and treatment patterns in a whole-of-population Australian cohort (2001–2016). Breast Cancer Res Treat. 2018;171(1):151–9. [DOI] [PubMed] [Google Scholar]
- 7.Kaczmarek E, Saint-Martin C, Pierga JY, et al. Long-term survival in HER2−positive metastatic breast cancer treated with first-line trastuzumab: results from the french real-life curie database. Breast Cancer Res Treat. 2019;178(3):505–12. [DOI] [PubMed] [Google Scholar]
- 8.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. [DOI] [PubMed] [Google Scholar]
- 9.DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics,2019. CA Cancer J Clin. 2019;69(6):438–51. [DOI] [PubMed] [Google Scholar]
- 10.Caswell-Jin JL, Plevritis SK, Tian L, et al. Change in survival in metastatic breast cancer with treatment advances: meta-analysis and systematic review. JNCI Cancer Spectrum. 2018;2(4):pky062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(6):809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaorsky NG, Churilla TM, Egleston BL, et al. Causes of death among cancer patients. Ann Oncol. 2017;28(2):400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afifi AM, Saad AM, Al-Husseini MJ, Elmehrath AO, Northfelt DW, Sonbol MB. Causes of death after breast cancer diagnosis: A US population-based analysis. Cancer. 2020;126(7):1559–67. [DOI] [PubMed] [Google Scholar]
- 14.Ameijide A, Clèries R, Carulla M, et al. Cause-specific mortality after a breast cancer diagnosis: a cohort study of 10,195 women in Girona and Tarragona. Clin Transl Oncol. 2019;21(8):1014–25. [DOI] [PubMed] [Google Scholar]
- 15.Akay CL, Ueno NT, Chisholm GB, et al. Primary tumor resection as a component of multimodality treatment may improve local control and survival in patients with stage IV inflammatory breast cancer. Cancer. 2014;120(9):1319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO/IARC Classification of Tumours. Vol 4. 4 ed: World Health Organization; 2012. [Google Scholar]
- 17.Plichta JK, Thomas SM, Sergesketter AR, et al. A novel staging system for de novo metastatic breast cancer refines prognostic estimates. Ann Surg. 2020. 10.1097/SLA.0000000000004231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawood S, Broglio K, Gonzalez-Angulo AM, Buzdar AU, Hortobagyi GN, Giordano SH. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol. 2008;26(30):4891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Meglio A, Freedman RA, Lin NU, et al. Time trends in incidence rates and survival of newly diagnosed stage IV breast cancer by tumor histology: a population-based analysis. Breast Cancer Res Treat. 2016;157(3):587–96. [DOI] [PubMed] [Google Scholar]
- 20.Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in1,038 women with metastatic breast cancer. Ann Oncol. 2008;19(12):2012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge QD, Lv N, Kong YN, et al. Clinical characteristics and survival analysis of breast cancer molecular subtypes with hepatic metastases. Asian Pac J Cancer Prev. 2012;13(10):5081–6. [DOI] [PubMed] [Google Scholar]
- 22.Klar N, Rosenzweig M, Diergaarde B, Brufsky A. Features associated with long-term survival in patients with metastatic breast cancer. Clin Breast Cancer. 2019;19(4):304–10. [DOI] [PubMed] [Google Scholar]
- 23.Ren Z, Li Y, Hameed O, Siegal GP, Wei S. Prognostic factors in patients with metastatic breast cancer at the time of diagnosis. Pathol Res Pract. 2014;210(5):301–6. [DOI] [PubMed] [Google Scholar]
- 24.Xiao W, Zheng S, Liu P, et al. Risk factors and survival outcomes in patients with breast cancer and lung metastasis: a population-based study. Cancer Med. 2018;7(3):922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colzani E, Liljegren A, Johansson AL, et al. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol. 2011;29(30):4014–21. [DOI] [PubMed] [Google Scholar]
- 26.SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. SEER https://seer.cancer.gov/csr/1975_2014/. Updated April 2017. Accessed 12/11/2017, 2017.
- 27.Kuo TM, Mobley LR. How generalizable are the SEER registries to the cancer populations of the USA+ Cancer Causes Control. 2016;27(9):1117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallin K, Palis BE, Watroba N, et al. Completeness of American Cancer Registry Treatment Data: implications for quality of care research. J Am Coll Surg. 2013;216(3):428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.