Abstract
Introduction:
Long-term effects of early hyperglycemia in VLBW infants are poorly characterized. The objective of this study was to systematically review the effect of early hyperglycemia on growth, metabolic health, and neurodevelopment after NICU discharge in VLBW infants.
Methods:
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A study protocol was registered in PROSPERO (CRD42019123335). Data sources included Ovid MEDLINE, Embase, Cochrane Library, CINAHL, and Scopus. Selected studies included infants with a blood glucose concentration >150 mg/dL (8.3 mmol/L) during the first 28 days of life, a gestational age <32 weeks and/or a birthweight <1500 grams, and longitudinal data on growth, metabolic health, or neurodevelopment outcomes. The GRADE system was used to assess quality of evidence.
Results:
Eight studies (n=987 infants) reported long-term outcomes from 4 months corrected gestational age to 7 years old. Most studies compared long-term outcomes of preterm infants with and without hyperglycemia. Two studies addressed outcomes related to interventions following early hyperglycemia. Some studies found differences in growth, metabolic health, and neurodevelopment outcomes between VLBW preterm infants with hyperglycemia and without hyperglycemia, while other studies found no differences between groups. The overall graded quality of evidence was low.
Conclusions:
Well-designed randomized controlled and prospective studies are necessary to determine the effect of early hyperglycemia and its treatment on later metabolic and neurodevelopmental outcomes in VLBW infants. Authors propose a potential study design for standardizing the assessment of long-term metabolic and neurodevelopmental outcomes following early hyperglycemia in preterm infants.
Keywords: glucose, insulin, preterm infant, prognosis, neurodevelopment, metabolic, NICU
Introduction
Very low birth weight (VLBW) infants, defined as infants born with a birthweight less than 1500 grams, frequently develop hyperglycemia soon after birth [1–7]. Studies using continuous glucose monitoring report the incidence of early hyperglycemia between 43-80% in VLBW infants [2, 8]. The prevalence of hyperglycemia is inversely correlated with birthweight and gestational age (GA) [2]. The short-term effects of hyperglycemia in VLBW infants are more frequently reported in the literature than long-term effects [4, 7, 9–11]. Long-term risks associated with hyperglycemia in VLBW infants are studied less, and therefore poorly characterized. Outcomes extrapolated from infants of diabetic mothers, a population exposed to hyperglycemia during a similar period of development, suggest that hyperglycemia may affect long-term metabolic and neurodevelopmental outcomes [12–15].
Hyperglycemia in the very preterm population is a biochemical disorder representing an imbalance between excess available glucose and decreased glucose utilization [16]. Dextrose infusion for enhanced nutrition, relative insulin resistance, defective islet β-cell function, and aberrant counter regulatory hormones are the most common etiologies of hyperglycemia in preterm infants [16, 17]. Hyperglycemia can be further induced or exacerbated in VLBW infants by common NICU morbidities such as sepsis [18]. Hyperglycemia causes increased fat deposition, reduced lean mass accretion, pancreatic β-cell toxicity, fatty infiltration of the heart and liver, and cardiac septal hypertrophy and RV dysfunction [16]. Hyperglycemia also impairs neuronal development with fewer dendrites and reduced synapse formation in animal models [16]. These physiologic complications of hyperglycemia provide biologic plausibility for the development of metabolic and neurodevelopmental complications following early hyperglycemia in the VLBW population.
The objective of this systematic analysis was to answer the following question: Is there enough evidence on long-term outcomes to guide clinical management of hyperglycemia in VLBW infants? We reviewed all relevant published studies that evaluated long-term growth, metabolic and neurodevelopmental outcomes after early hyperglycemia in VLBW infants. Following a review of the relevant articles, we assessed the quality of evidence to determine if there were associations between (1) hyperglycemia and long-term growth, metabolic, and neurodevelopment outcomes, (2) severity and duration of hyperglycemia and long-term outcomes, and (3) treatment and long-term outcomes.
Methods
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplement 1) [19]. A study protocol was registered in PROSPERO (CRD42019123335) on https://www.crd.york.ac.uk/prospero. The review questions were formatted in PICOTT format (Table 1) [20].
Table 1.
Population-Exposure-Comparison-Outcome-Time Summary Statement
| Study population | Very low birth weight (VLBW) infants (<32 weeks or <1500 grams) |
| Exposure | Hyperglycemia ± insulin treatment |
| Comparison | Normoglycemic/Standard glycemic control/Non-insulin treatment |
| Outcomes | Growth, metabolic, and neurodevelopmental outcomes |
| Time | Studies published through August 30, 2020 |
| Setting | Worldwide |
| Study design | Any study design with longitudinal data and at least 1 post-NICU follow-up |
Search Strategy
The search strategy was built and tested using Ovid MEDLINE and included both MeSH terms and keywords (Supplement 2) and translated to four other databases: Embase, Cochrane Library, CINAHL, and Scopus. No search limits were applied with respect to publication date or language. Search results were de-duplicated using EndNote. Hand searching methods, including searching references lists of articles selected for inclusion, were also used to identify relevant materials that may have been missed by the database search. Only full text publications were included in the analysis. Authors of abstracts were contacted to determine if full text publications existed prior to manuscript submission.
Inclusion Criteria
Any study design was included. The population of interest was infants admitted to the NICU worldwide that were born at less than 32 weeks GA and/or were VLBW. Criteria for study inclusion were: (1) blood glucose concentrations (BG) of greater than 150 mg/dL (8.3 mmol/L) in the first 28 days after birth and (2) a gestational age less than 32 weeks or a birthweight less than 1500 grams. Titles and abstracts of reports from the above search strategy were assessed independently by two review authors using Rayyan to determine their eligibility for inclusion [21]. Disagreements were resolved by consensus. If consensus could not be reached, reports were referred to a third author for arbitration. Outcomes were growth (weight, height, and occipital-frontal head circumference [OFC]), metabolic health (body mass index, adiposity, glucose metabolism, diabetes, hypertension, fatty liver), and neurodevelopment (intelligence, processing speed, learning, attention, executive function, behavior, cerebral palsy, vision deficits, hearing deficits) following discharge from the NICU. Subgroup analyses were completed for varying definitions of hyperglycemia, duration of hyperglycemia, and insulin treatment.
Data Extraction and Analysis
Data were extracted independently by two authors using a customized form (Supplement 3) including: study location, design, population, hyperglycemia definition, hyperglycemia assessment, hyperglycemia incidence, insulin exposure, episodes of insulin related hypoglycemia, comparator group, sample size, growth outcomes, metabolic outcomes, neurodevelopmental outcomes, sex-specific outcomes, longitudinal follow-up rates, and study confounders. Principal summary measures were differences in means and correlation coefficients. Quality assessment was independently assessed by two authors using the GRADE system [22]. Conflicts were resolved by consensus of all authors.
Results
Description of Selected Studies
Of the 6,348 records identified, 6,329 were excluded following title and abstract screening, and a further 11 were excluded following full-text review (Figure 1). A total of 8 studies involving 987 VLBW infants were included in the analysis [6, 7, 23–28]. The majority of studies focused on the long-term metabolic and neurodevelopmental outcomes of early hyperglycemia versus no hyperglycemia [1, 6, 7, 23–25]. Two studies reported long-term metabolic and neurodevelopmental outcomes following early hyperglycemia intervention (tight versus standard glycemic control [26], insulin treatment [27]). A meta-analysis of outcomes was not performed given the small number of studies and the heterogeneity of long-term outcomes.
Figure 1.
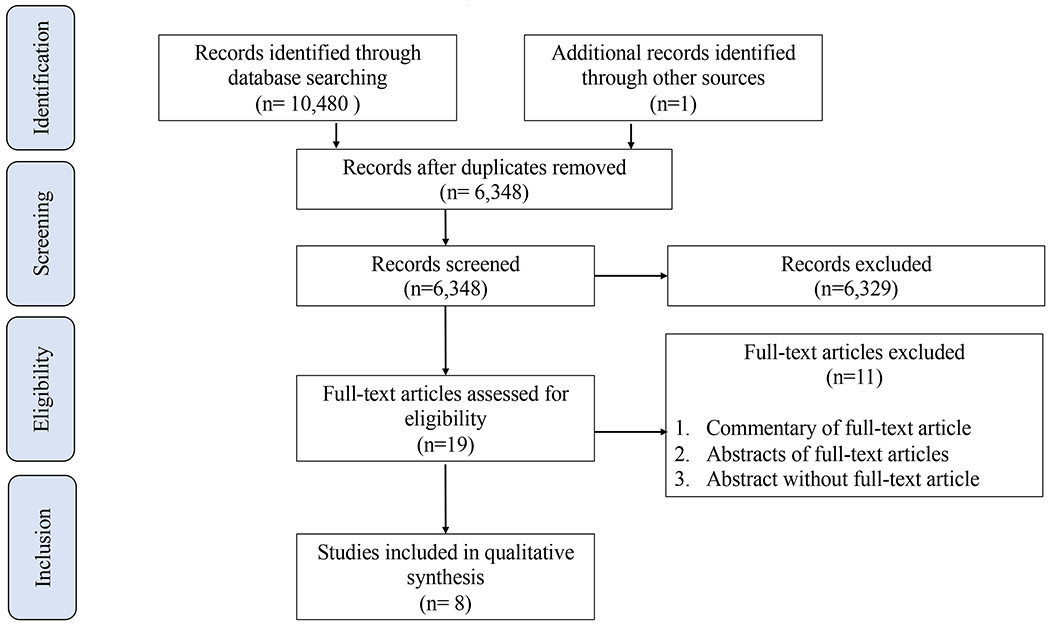
Flow diagram of study identification and selection
Normoglycemia vs. Hyperglycemia
The design and results of these studies are summarized in Table 2. Hyperglycemia was defined differently between studies using threshold values between 150 mg/dL (8.3 mmol/L) and 182 mg/dL (10.1 mmol/L) [7, 23–25, 27, 28]. Zamir et al. reported three tiers of BG thresholds: > 180 mg/dL (10.0 mmol/L), >216 mg/dL (12.0 mmol/L), and >252 mg/dL (14.0 mmol/L)[28]. Three studies reported outcomes following varying duration (number of days) of hyperglycemia [23, 25, 28]. The incidence of hyperglycemia was 45-77% in studies using a BG threshold of 150 mg/dL (8.3 mmol/L) [23–25] and 8-17.5% in studies using a BG threshold of 180 mg/dL (10.0 mmol/L) [7, 27, 28].
Table 2.
Summary of Results
| van der Lugt, 20107 | Heald, 201227 | Ramel, 201324 | Scheurer, 201625 | Tottman, 20176 | Tottman, 201826 | Zamir, 201928 | Gonzalez Villamizar, 202023 | |
|---|---|---|---|---|---|---|---|---|
| Location | Leiden University Medical Center, The Netherlands | Australian National University Medical School, Canberra Hospital, Australia | University of MN Children’s Hospital, Minneapolis, MN, USA | University of MN Children’s Hospital, Minneapolis, MN, USA | National Women’s Hospital; Auckland, New Zealand | National Women’s Hospital; Auckland, New Zealand | Sweden (Lund, Umea, Stockholm) | University of MN Children’s Hospital, Minneapolis, MN, USA |
| Study design | Retrospective follow-up | Retrospective | Retrospective | Prospective observational | Retrospective observational cohort | Randomized control trial, follow-up | Prospective, population-based cohort | Retrospective post-hoc analysis from prospective study |
| Population | 24-32 weeks GA, Jan 2002 - Dec 2006 | 25-28 weeks GA, Jan 2006 - Dec 2008 | 22-30 weeks GA, AGA infants, Jan 2003 - July 2007 | AGA VLBW infants, April 2011 to Nov 2012 | 23-35 weeks GA, July 2005 - Oct 2008 | <1500 grams or <30 weeks GA, HINT 2005-2008 | <27 weeks GA, April 2004 - March 2007 | 22-32 weeks GA, Feb 2012 - June 2016 |
| Exposure | Hyperglycemia | Hyperglycemia treated with insulin | Hyperglycemia | Hyperglycemia | Hyperglycemia | Hyperglycemia | Hyperglycemia | Hyperglycemia |
| Definition (hyperglycemia) | ≥2 BG 180 mg/dL (≥10.0 mmol/L) during a 12-hour period | ≥2 BG of 180 mg/dL (≥10.0 mmol/L) during a 12-hour period | BG >150 mg/dL (>8.3 mmol/L) | BG >150 mg/dL (>8.3 mmol/L); Moderate (1-5) and severe (>5) hyperglycemia days | ≥ 2 measures of BG ≥155 mg/dL (≥8.6 mmol/L) >1 hour apart or any BG ≥182 mg/dL (≥10.1 mmol/L) | 2 consecutive measures of BG≥153 mg/dL (≥8.5 mmol/L) at least ≥4 hour apart | Tiers of assessment: BG >180 mg/dL (>10.0 mmol/L), >216 mg/dL (>12.0 mmol/L), 252 mg/dL (>14.0 mmol/L) | BG >150 mg/dL (>8.3 mmol/L) |
| Comparator | Age matched controls without hyperglycemia | No insulin treatment | Normoglycemia | Normoglycemia | Normoglycemia (study included 2 additional groups: hypoglycemia and unstable glycemia) | Hyperglycemic neonates randomized to tight (treated with insulin) vs. standard (+/− insulin treatment) glycemic control | Normoglycemia | 3 groups: normoglycemia; 1-4 days of hyperglycemia; >5 days of hyperglycemia |
| Sample size * | N=96(n=33 hyperglycemia; n=63 without hyperglycemia) | N=97 (n=80 no insulin, n=17 insulin) | N=80 | N=50 (n=30 normoglycemia, n=12 1-5 days of hyperglycemia, n=8 >5 days of hyperglycemia) | N=360 (n=287 normoglycemic, n=73 hyperglycemic) | N=88 (n=43 tight glycemic control, n=45 standard glycemic control) | N=171 | N=97 |
| Incidence (hyperglycemia) * | 8% (25% of ELBW infants) | 17.5% hyperglycemia + insulin drip | 77% | 45% (26% moderate, 19% severe) | 16% | All infants in cohort | 47% | 49% (27% <5 days; 22%≥5 days) |
| Assessment (glucose) | Whole blood | Not reported | Not reported | Not reported | Whole blood (blood gas analyzer) | Whole blood (glucose oxidase method) | Plasma | Not reported |
| Treatment (hyperglycemia) | Insulin (0.05 U/kg/hr) was started when hyperglycemia persisted >12 hrs despite reduction of GIR of 5-6 mg/kg/min | Insulin (0.01 u/kg/hr) was started when BG>180-216 mg/dL + glycosuria on 10% dextrose | At discretion of neonatologist, general guideline: limit GIR to 7 mg/kg/min followed by insulin bolus/infusion | At discretion of neonatologist, general guideline: limit GIR to 7 mg/kg/min followed by insulin bolus/infusion | Reducing GIR or initiation of insulin infusion to maintain BG of 72-180 mg/dL (4-10 mmol/L) | •Standard glycemic control: BG maintained <180 mg/dL (<10.0 mmol/L). Insulin treatment when BG>180 mg/dL (>10.0 mmol/L) or persistent glycosuria >2+; tolerating < 100 kcal/kg/day; >72 hours of age, and not acutely stressed. •Tight control: BG maintained <155 mg/dL (<8.6 mmol/L) with insulin treatment •Insulin treatment: Insulin (0.05 u/kg/hr) was infused to maintain BG in standard group 144-180 mg/dL (8-10 mmol/L) or in tight group 72-108 mg/dL (4-6 mmol/L) |
At the discretion of neonatologist | At discretion of neonatologist, general guideline: limit GIR to 7 mg/kg/min followed by insulin bolus/infusion |
| Age (outcome measurement) | 2 years (±3 months) CGA | 12 months CGA | Discharge, 4 months, 12 months, and 24 months CGA | Term CGA, 4 months CGA | 2 years CGA | 7 years CGA (±6 months) | 6.5 years (±3 months) | Discharge, 4 months CGA (metabolic outcomes), 12 months CGA (neurodevelopment outcomes) |
| Growth outcomes | No difference in weight, length, OFC | No difference in <10th percentile weight, length, OFC | •Decrease in weight •No difference in length or OFC •Decrease in growth velocity correlated with >5 days of hyperglycemia |
≥5 days of hyperglycemia associated with: •Increase in weight •Decrease in length and OFC |
Not reported | •Decrease in height, leg-length, and growth velocity between 36 weeks CGA to 7 years CA in tight control group •No differences in weight, sitting height, OFC, or BMI |
Not reported | Not reported |
| Metabolic outcomes | Not reported | Not reported | Not reported | ≥5 days of hyperglycemia is associated with increases in fat free mass, fat mass, and percent body fat | Not reported | •Lower fasting glucose in tight group •Lower height adjusted lean mass in tight group •No differences in abdominal circumference, blood pressure, fasting insulin concentration, glucose effectiveness, glucose disappearance constant, acute insulin response to glucose, insulin sensitivity, fat mass, height-adjusted fat mass, android/gynoid fat ratio, lean mass, bone mineral density, height-adjusted bone mineral density |
•Increased daily carbohydrate intake was associated with increased z-score for both SBP and DBP •BG>216 mg/dL (12 mmol/L) >2 days was associated with higher SBP z-scores •BG>180 mg/DL (10 mmol/L) for >3 days or >216 mg/dL (12 mmol/L) for >2 days was associated with higher DBP •Duration of BG>216 mg/dL (12 mmol/L) was positively associated with DBP z-score •BG>252 mg/dL (14 mmol/L was positively associated with both SBP and DBP z-scores |
Hyperglycemia ≥5 days was negatively associated with fat free mass z score a 4 months’ PMA |
| Neurodevelopment outcomes | •Increased abnormal neurological examination with hyperglycemia •Increased abnormal Child Behavior Checklist/2-3 with hyperglycemia •Insulin treatment did not affect outcomes |
•No differences in GQ/MDI <-2SD on either GMDS or BSID-II, CP incidence, need for walking aids, bilateral blindness, or bilateral hearing loss | •Increase in cognitive BSID-III scores •No difference in language or motor BSID-III scores |
Not reported | Decreased OR of survival without neurodevelopment impairment on BSID-II or BSID-III | •No differences in neurodevelopment impairment (WISC-IV full scale IQ<85, MABC-2 total score <5th percentile, CP, blind, deaf) between tight and standard glycemic control in hyperglycemic infants •No difference in cognitive (WISC-IV), motor (MABC-2), or executive function (BRIEF) between groups |
Not reported | Hyperglycemia for ≥5 days was negatively associated with cognition, language, and motor scores on the BSID-III |
| Sex-specific outcomes | Not reported (63% male, no differences between groups) | Not reported | Not reported (50% male) | Not reported (51% male, no differences between groups) | Not reported (55% male, no differences between groups) | Not reported (48% male, no differences between groups) | Not reported (56% male, no differences between groups) | Not reported (52% male, no differences between groups) |
| Follow-up rate | 87% | 95% | 100% at 1-year follow-up; 78% at 2-year follow-up | 95% at discharge from NICU; 89% at 4-month CGA follow-up | 65% at 2-year follow-up | 78% for primary outcome (survival without neurodevelopment impairment); 65% follow-up at 7 years | 35% of initial population-based cohort | 78% at 4 months; 68% at 12 months |
| Confounders | • Gestational age, gender, birth weight, length of stay, exposure to steroids, presence of severe RDS, sepsis, PROM, chorioamnionitis, NEC, BPD, PVL, and IVH. •Confounding was minimized by matching and using multivariable regression analysis |
• Gestational age, birth weight, illness. •No age-matching. •Confounding was minimized by multivariable regression analysis. |
• Caloric deficit and insulin use. •Confounding was minimized by linear mixed effects regression. |
• None listed. •Confounding was minimized by linear regression. |
Gestational age, birth weight z-score, socioeconomic quintile at birth, and neurodevelopment assessment type at 2 years PMA | Sex, deprivation index, SGA, multiple births, protein intake in first 14 days of life. | •Gestational age and heart rate at follow up •Results not adjusted for steroid treatment, weight, body mass index |
• Gestational age, average first week kilocalorie deficit, average first week protein deficit, SNAP-II score, IVH, OFC z score at discharge. •Confounding was minimized by linear regression analysis. Modeling used to account for nutrition and illness severity. |
Abbreviations: AGA: Average weight for gestational age, BG: Blood glucose, BMI: Body mass index, BPD: Bronchopulmonary dysplasia, BRIEF: Behavior Rating Inventory for Executive Function, BSID: Bayley Scales of Development, CGA: Corrected gestational age, CP: Cerebral palsy, DBP: diastolic blood pressure, ELBW: extremely low birthweight, GA: Gestational age, GIR: glucose infusion rate, GMDS: Griffith Mental Development Scales, GQ: Griffiths General Quotient, HINT: Hyperglycemia and Insulin in Neonates Trial, IQ: Intelligence quotient, IVH: Intraventricular hemorrhage, MABC: Movement Assessment Battery for Children, MDI: Bayley’s Mental Developmental Index, NEC: Necrotizing enterocolitis, NICU: neonatal intensive care unit, OFC: Occipital frontal circumference, OR: odds ratio; PMA: postmenstrual age, PROM: Premature rupture of membranes, PVL: Periventricular leukomalacia, RDS: Respiratory Distress Syndrome, SBP: systolic blood pressure, SGA: Small weight for gestational age, SNAP: Score for Neonatal Acute Physiology, SD: standard deviation, VLBW: Very low birthweight infant, WISC: Wechsler Intelligence Scale for Children
The sample size reported in this table reflects the sample size of the cohort studied at the long-term timepoint. The incidence of hyperglycemia reported in this table reflects the incidence reported by the authors in each study which in some cases reflects the incidence of hyperglycemia in the larger early neonatal cohort.6,7
Six studies compared long-term outcomes following early hyperglycemia. Growth was reported by weight, height, or OFC percentiles, z-scores, or velocity. Three studies reported associations between hyperglycemia in VLBW infants and long-term growth [7, 24, 25]. Metabolic outcomes studied were defined as body composition or blood pressure. Two studies evaluated body composition in VLBW infants with hyperglycemia [23, 25]. One study evaluated blood pressure in VLBW infants with hyperglycemia at 6.5 years [28]. Collectively, these outcomes were studied between 4 months corrected gestational age (CGA) and 6.5 years [7, 24, 25]. Four studies evaluated neurodevelopment between 4 months CGA and 2 years of age [7, 23–25]. The location of the study, population, study design, comparator group, age the outcome measurement was assessed, and method of assessment of long-term outcome differed between studies.
Treatment of Hyperglycemia: Tight Glycemic Control
Alsweiler et al. (2012) performed a randomized controlled trial randomizing preterm infants to tight glycemic control with insulin (target BG 72-108 mg/dL, 4-6 mmol/L) or standard practice (restrictive insulin use, target BG 144-180 mg/dL, 8-10 mmol/L) for neonatal hyperglycemia [29]. Outcomes from this study showed that preterm infants treated with tight glycemic control (BG 72-108 mg/dL, 4-6 mmol/L) were exposed to more insulin, had similar carbohydrate intake, had improved weight gain without change in linear growth at 36 weeks PMA, and a 3-fold increase in the incidence of hypoglycemia, compared with the standard practice group [29]. Tottman et al. (2018) studied growth, metabolic outcomes, and neurobehavior of this cohort at 7 years of age [26]. These results are summarized in Table 2.
Treatment of Hyperglycemia: Insulin Treatment
Insulin treatment was discussed in 7 of the 8 studies [6, 7, 23–27]. In 5 studies, use of insulin was at the discretion of the neonatologist [6, 7, 23–27]. Four studies, 3 of which were from the same center, provided general guidelines that the glucose infusion rate (GIR) was initially limited followed by insulin treatment if hyperglycemia persisted [6, 23–25]. Five studies provided descriptive information and addressed complications related to, or outcomes associated with insulin treatment [7, 24–27].
The remaining 4 studies described insulin use in the hyperglycemia group [7, 24–26]. No study found associations between insulin use and growth, metabolic, or neurodevelopment outcomes [7, 24–26]. Van der lugt et al. reported the mean duration of insulin infusion as 129 hours with a mean infusion rate of 0.06 U/kg/h [7]. Scheurer et al. reported increased use of insulin with longer duration of hyperglycemia [25]. VLBW infants with 1-5 days of hyperglycemia had a mean of 2.6 days of insulin treatment compared with infants with 5 or more days of hyperglycemia who had a mean of 15.7 days of insulin treatment [25]. Ramel et al. reported both duration of insulin treatment and its association with outcome measurements [24]. The authors described a mean of 2.14 insulin days for treatment of hyperglycemia and reported no association between insulin treatment and growth or neurodevelopment outcomes [24]. Two of these studies reported 0.3 and 0.65 mean number of episodes of hypoglycemia but did not report associations between hypoglycemia and outcomes [7, 24].
Heald et al. [27] aimed to study the effects of insulin infusion for the treatment of early hyperglycemia in preterm infants on long-term neurodevelopmental outcome. Infants with hyperglycemia (BG>180 mg/dL, 10 mmol/L) met criteria for insulin treatment when treatment for hyperglycemia limited nutrition to 10% dextrose infusion and the infant had persistent glucosuria [27]. Of the infants who received insulin, the median blood glucose prior to insulin infusion was 223 mg/dL (12.4 mmol/L) [27]. The median length of insulin treatment was 41 hours. The authors reported 1.3% episodes of hypoglycemia with insulin treatment which occurred in 2 of 17 infants in the insulin treated group [27]. These researchers found no significant differences in infant growth failure, Bayley Scales of Infant Development (BSID-II) motor scores, incidence of cerebral palsy, need for walking aids, blindness, or hearing loss in VLBW infants with hyperglycemia with or without insulin treatment at 12 months CGA [27].
Quality of Evidence
The overall quality of evidence, using established GRADE criteria, is summarized in Table 3 [22]. Overall, the quality of evidence to characterize associations between hyperglycemia and long-term outcomes was low. The quality of evidence demonstrating an association between duration of early hyperglycemia and long-term outcomes was moderate. The quality of evidence that a BG threshold of 150 mg/dL (8.3 mmol/L), a surrogate definition for severity of hyperglycemia, in VLBW infants is associated with worse long-term outcomes was moderate. Lastly, the quality of evidence reporting both complications and effects of insulin treatment for early hyperglycemia on long-term outcomes of VLBW infants was low.
Table 3.
GRADE summary of quality of evidence for effect of neonatal hyperglycemia on long-term outcomes
| Outcome | Agreement between studies | Findings | Participants (Studies) | Risk of bias | Certainty/Quality of evidence |
|---|---|---|---|---|---|
| Growth | |||||
| Overall impairment | No | Conflicting results from studies reporting weight, height, OFC outcomes | 356 (5)7,24–27 | Higha,b,c,d,e | Low |
| Weight | No | Decrease or no change in weight | 356 (5)7,24–27 | Higha,b,c,d,e | Low |
| Length/height | No | Decrease or no change in length/height; tight glycemia control associated with decreased growth velocity and height | 356 (5)7,24–27 | Higha,b,c,d,e | Low |
| Head circumference | No | Decrease or no change in OFC | 356 (5)7,24–27 | Higha,b,c,d,e | Low |
| Metabolic | |||||
| Overall impairment | No | Studies that compared hyperglycemia and normoglycemia groups reported metabolic differences; no differences reported between tight and standard glycemia groups | 375 (4)23,25–26,28 | Higha,b,c,d,e | Low |
| Body composition | Yes | Decrease in fat-free mass | 204 (3)23,25–26 | Higha,b,c,d,e | Moderate |
| Blood pressure | No | Increase in blood pressure; there was no difference between tight and standard glycemia groups | 228 (2)26,28 | Higha,b,c,d,e | Low |
| Glucose metabolism | N/A | No effect reported between tight and standard glycemia groups | 57 (1)26 | Higha,b,c,d,e,f | Low |
| Neurodevelopment | |||||
| Overall impairment | No | Studies that compared hyperglycemia and normoglycemia groups reported conflicting results; no differences reported between tight and standard glycemia groups | 763 (6)6–7,23–24,26–27 | Higha,b,c,d,e,f | Low |
| Cognitive impairment | No | Increase, decrease, or no change in BSID cognitive scores | 594 (4)6,23–24,27 | Higha,b,c,d,e,f | Low |
| Language impairment | No | Decrease or no change in BSID language scores | 594 (4)6,23–24,27 | Higha,b,c,d,e | Low |
| Motor impairment | No | Decrease or no change in BSID motor scores; there was no difference between tight and standard glycemia groups | 763 (6) 6–7,23–24,26–27 | Higha,b,c,d,e,f | Low |
| Blindness | Yes | No blindness associated with hyperglycemia; no differences between tight and standard glycemia treatment | 490 (3)6,26,–27 | Higha,b,d,e | Moderate |
| Deafness | Yes | No deafness associated with hyperglycemia; no differences between tight and standard glycemia treatment | 490 (3)6,26–27 | Higha,b,c,d,e | Moderate |
| Cerebral palsy | Yes | No CP associated with hyperglycemia; no differences between tight and standard glycemia treatment | 130 (2)7,26 | Higha,b,d,e,f | Low |
| Intelligence quotient | N/A | No effect reported between tight and standard glycemia groups | 57 (1)26 | Higha,b,d,e | Low |
| Visual-motor impairment | N/A | No effect reported between tight and standard glycemia groups | 57 (1)26 | Higha,b,d,e | Low |
| Behavior | No | Abnormal behavior reported by parents/teachers; no effect reported between tight and standard glycemia groups | 153 (2)7,26 | Higha,b,d,e,f | Very low |
| Executive function | N/A | No effect reported between tight and standard glycemia groups | 57 (1)26 | Higha,b,d,e | Low |
| Insulin Treatment | |||||
| Hypoglycemia | Yes | Reported hypoglycemia; no reports of comparator group episodes of hypoglycemia | 269 (3)7,24,27 | Higha,b,c,e | Low |
| Association with outcomes | Yes | No association reported between growth, metabolic, or neurodevelopment outcomes | 359 (5)7,24–27 | Higha,b,c,d,e | Low |
| Glucose Threshold | |||||
| >150 mg/dL | Yes | Outcomes varied; associations found with growth, metabolic, and neurodevelopment outcomes; no difference in outcomes between tight and standard glycemic control | 747 (5)6,23–26 | Higha,b,c,e | Moderate |
| >180 mg/dL | Yes | Outcomes varied; associations found with growth, metabolic, and neurodevelopment outcomes; no difference in outcomes between tight and standard glycemic control | 364 (4)6–7,27–28 | Higha,b,c,e | Moderate |
| >220 mg/dL | N/A | Associated with higher diastolic and systolic blood pressure | 171 (1)28 | Higha,b,c,e | Low |
| Duration of hyperglycemia | Yes | Longer duration of hyperglycemia is associated with worse metabolic and neurodevelopment outcomes | 387 (4)6,23,25,28 | Higha,b,c,e | Moderate |
The Risk of Bias in Non-randomized Studies (ROBINS-I)
Confounding
Selection of participants
Classification of interventions
Deviations from intended interventions
Missing data
Measurement of outcomes
Section of the reported result
Evidence reported as very low- or low-grade quality were limited by the number of studies reporting an outcome or reported conflicting results. Collectively, studies were underpowered across growth, metabolic, and neurodevelopment outcomes to interpret findings confidently. All studies showed a risk of bias limiting the quality of evidence. Some studies did not adjust for confounders. All studies adjusting for confounders did not assess the same confounders. Additionally, there was variation in blood glucose threshold to define hyperglycemia among the studies as well as variation in the treatment of hyperglycemia both within and between studies. There was an overlap between studies from the same centers, potentially limiting generalizability of the results. Lastly, studies did not account for sex-specific outcomes. There was one follow up study from a randomized control trial that initially was considered high-quality due to its randomized study design; however, this study received a lower quality grade given its small sample size and lack of a normoglycemia comparator group [26].
Discussion
Best management practices for hyperglycemia in the VLBW preterm infant remain unknown despite its common occurrence in this population. Our findings report the results of a systematic search for all relevant studies on long-term growth, metabolic health, and neurodevelopmental outcomes after early neonatal hyperglycemia in the VLBW preterm infant. The primary objective of this study was to determine if there was enough evidence to guide clinical management of early hyperglycemia in preterm infants. A meta-analysis, which would have guided interpretation of the existing findings, was not conducted because of the clinical and methodologic heterogeneity in a small number of published studies. Additionally, the quality of evidence presented in the collective studies is low. Therefore, currently, there is not enough high-quality evidence of long-term outcomes to guide clinical management of early hyperglycemia in the preterm infant.
We critically evaluated the quality of evidence to determine if: (1) hyperglycemia affects growth, metabolic health, and neurodevelopmental outcomes following NICU discharge of VLBW preterm infants; (2) the severity and duration of hyperglycemia affects long-term outcomes in VLBW preterm infants after hyperglycemia; and (3) treatment of hyperglycemia attenuated adverse long-term outcomes. The results are conflicting. Some studies found differences in long-term outcomes between VLBW preterm infants with hyperglycemia and without hyperglycemia, while other studies found no differences between groups.
Studies were heterogeneous and underpowered to adequately determine differences between groups. There appears to be a trend among the studies that the severity and duration of hyperglycemia is associated with worse metabolic or neurodevelopmental outcomes. However, without adequately powered studies aimed at investigating this trend, clinicians are left with a hypothesis rather than relevant conclusions to inform clinical decision making. Treatment of hyperglycemia with insulin was not associated with a change in long-term outcomes. The threshold for treatment, the infant’s nutritional status, and protocols for insulin use varied among the studies or were not reported, thus confounding interpretation to guide evidence-based practices. In addition to standardized experimental designs with consistent variables, we recommend the following considerations in hyperglycemia assessment, outcome analysis, and treatment in future research.
Hyperglycemia Assessment
The assessment of hyperglycemia was not standardized within or between institutions. In future study development we recommend standardized hyperglycemia screening, evaluation, and definition. First, infants who are sicker or diagnosed to have glucose disturbances generally have more frequent glucose measurements than clinically stable infants with euglycemia. The differences in frequency of glucose evaluation may confound long-term outcomes therefore frequency should be determined a priori. Second, details of how blood glucose concentration is measured should be reported in each study. Blood glucose values can vary as much as 20% depending on the specimen (i.e., whole blood vs plasma) and assessment method thereby confounding potential conclusions regarding early hyperglycemia and long-term outcome associations [30]. Third, we recommend a unified definition of hyperglycemia. There was moderate quality of evidence to suggest that a BG of greater than 150 mg/dL (>8.3 mmol/L) was associated with long-term adverse metabolic and neurodevelopmental outcomes and there was no difference in outcomes whether hyperglycemia was defined as 150 mg/dL (8.3 mmol/L) or 180 mg/dL (10.0 mmol/L). Therefore, our recommendation is to use a blood glucose threshold of 150 mg/dL (8.3 mmol/L).
Outcome Analysis
The impact of preterm birth, postnatal growth velocity, exposure to over/under nutrition, insulin treatment, and sex are potential drivers of developmental programming of metabolic disease and neurodevelopmental impairment in adulthood. For example, male preterm infants are at a higher risk for mortality and morbidity compared with female infants [31]. Increased attention to metabolic and neurodevelopment outcomes, both of which are especially susceptible to hyperglycemia-mediated programming in adults born preterm, and sex differences within outcomes will inform current treatment practices.
The most common metabolic outcome in individuals born preterm is higher blood pressure [32]. Prematurity also increases the risk of short stature [33], insulin resistance and diabetes [34, 35], increased visceral fat mass [36], and cardiac hypertrophy [37]. Adult dyslipidemia, coronary artery, and cerebrovascular diseases in infants born preterm have shown mixed findings across studies [32]. Pre-clinical studies evaluating hyperglycemia induced programming of adult metabolic disease provide support that early metabolic stress permanently alters tissue and organ function and predisposes to metabolic disease [38, 39]. Important metabolic outcomes should include linear growth/height, body composition (% fat mass, fat-free mass), fasting glucose, and blood pressure.
Preterm birth increases the risk for psychiatric disorders, social-emotional challenges, and cognitive impairments [32]. Research reveals a multifactorial psychopathology for these associations [32]. Impaired postnatal growth is associated with delayed brain development and diminished cortical connections with persistent structural and functional changes on neuroimaging in adulthood [40, 41]. Additionally, both clinical and pre-clinical studies have shown that hyperglycemia alters brain structure and is associated with worse neurodevelopmental outcomes [42, 43]. Key neurodevelopmental outcomes should focus on determinants of disability, including motor, vision, cognitive, and social-emotional assessments.
None of the studies in this analysis reported sex-specific outcomes. All but one study reported no significant differences in males in the control and hyperglycemia groups [6, 7, 23–26, 28]. The Fenton growth chart accounts for sex differences in growth of preterm infants [44]. Variation in growth rates of males and females may suggest that nutritional requirements, and therefore the incidence of hyperglycemia, may be sex-specific. Research on sex-specific nutritional guidelines is sparse in preterm infants [45]. Studies have shown that early nutritional interventions have a pronounced neurodevelopmental benefit in preterm males compared to female infants [46, 47]. Additionally one study has shown that female preterm infants have higher insulin secretion at similar BG concentrations than male preterm infants [48]. Collectively, these studies suggest that incidence of early hyperglycemia and the effect of its treatment on long-term outcomes differ between male and female infants. Such sex-specific results should be reported in future studies.
Treatment of Hyperglycemia
There are variations in management practices of hyperglycemia. The most widely practiced treatment strategy is a reduction in GIR. Although this practice is effective in achieving euglycemia [49] it can lead to lower energy intake early in the NICU course, which is associated with worse metabolic and neurodevelopmental outcomes [6, 17, 24, 26, 50, 51]. Insulin administration is also frequently used for treating hyperglycemia and is effective in lowering blood glucose. Insulin treatment, in contrast to GIR reduction, also promotes weight gain in VLBW infants [49]. The use of insulin in the treatment of hyperglycemia in VLBW infants is controversial because of its potential to cause hypoglycemia and impact on long-term metabolic outcomes. To date there have been no randomized controlled trials that have investigated long-term growth, metabolic, or neurodevelopment outcomes in preterm infants with early hyperglycemia treated with or without insulin.
There were only two studies aimed at investigating the effect of intervention for early hyperglycemia (tight glycemic control and insulin treatment) on long-term metabolic and neurodevelopmental outcomes in preterm infants [26, 27]. Tottman et al. (2018) longitudinally followed a cohort of previously randomized preterm infants to tight versus standard glycemic control [26]. The study included all surviving infants from the Hyperglycemia in Neonates Trial which was powered to detect difference in outcome (linear growth rate) between infants randomized to standard or tight glycemic control at 36 weeks PMA rather than 7-year outcomes [26, 29]. We estimate a sample size of 775 is necessary to detect a difference of 10% effect size between groups in neurodevelopmental impairment at 80% power and an alpha level of 0.05. An example of this potential limitation is that the adjusted odds ratio (OR) for mean full-scale IQ less than 85 was 0.75 (0.25-2.21 95% CI) and for motor impairment 0.74 (0.25-2.20 95% CI) if treated with tight glycemic control [26]. These findings were not statistically significant but may be clinically significant if adequately powered to detect differences between groups.
Heald et al. (2012) retrospectively studied treatment-associated hypoglycemia and neurodevelopmental outcomes at 12 months PMA in preterm infants with hyperglycemia treated with insulin. This study has several factors limiting its generalizability. First, the study is retrospective. Second, the study is limited by unequal and small sample sizes (n=64 no treatment, n=9 insulin treatment) and not powered to detect differences in outcomes between the groups. For example, there was > 10% difference in the number of infants with weight and height < 10th percentile in no treatment and insulin treatment groups. These differences were not significant potentially due to the small sample size. Third, infants who received insulin were of lower birthweight and gestational age.
All studies reporting insulin use in the treatment of hyperglycemia did not show associations between insulin treatment and long-term growth, metabolic, or neurodevelopmental outcomes [6, 7, 23, 24, 27]. Given that less mature and more critically ill infants are more likely to have hyperglycemia, and thus more likely to be treated with insulin, the collective finding showing no difference in long-term outcomes between infants treated or not treated with insulin may suggest a protective effect of insulin treatment on long-term metabolic and neurodevelopmental outcomes. None of these studies, however, were adequately powered to provide clinical direction regarding insulin treatment in early hyperglycemia for preterm infants.
An established concern of insulin treatment is its association with hypoglycemia in the VLBW population, which has previously been reported to occur more than 25% of the time [49]. The results from the studies in this analysis, however, would suggest this is an overestimation of hypoglycemia episodes associated with insulin treatment [7, 24, 27]. None of the studies in this analysis included insulin-associated hypoglycemia as a mediator of long-term outcomes. Improved reporting of insulin-associated hypoglycemia, as well as including hypoglycemia episodes as a confounder in analyses, are necessary, given the evidence that persistent hypoglycemia is associated with adverse neurodevelopmental outcomes [52].
Caloric deficit, a potential result of decreased GIR as treatment of hyperglycemia, was associated with long-term growth, metabolic, and neurodevelopmental compromise [1, 23–25]. Most recently, Gonzalez Villamizar et al., assessed the potential influence of limiting early nutrition as a treatment of hyperglycemia in VLBW infants [23]. This group reported lower BSID-III cognition, language, and motor scores in VLBW infants exposed to hyperglycemia (BG>150 mg/dL, 8.3 mmol/L). However, when the analysis model was adjusted for average first-week kilocalorie and protein deficits, the association between hyperglycemia and BSID-III language and cognitive scores resolved [23]. This study emphasizes the importance of providing optimal early nutrition even in the setting of hyperglycemia. Future studies that are powered to evaluate different treatment strategies (i.e., reduction in GIR and/or insulin treatment) are needed to inform best practice in the treatment of hyperglycemia.
Conclusions
Current evidence of long-term health outcomes following early hyperglycemia in VLBW infants does not provide high-quality recommendations to guide assessment and treatment of early hyperglycemia. There is a critical need, as well as clinical equipoise, for well-designed, large randomized controlled trials and prospective studies that address the following questions: (1) Who is at risk for long-term metabolic and neurodevelopmental compromise after hyperglycemia? (2) What BG threshold and duration predisposes VLBW infants to worse metabolic and neurodevelopmental outcomes? and (3) Which interventions decrease the risk of long-term metabolic and neurodevelopmental compromise after hyperglycemia in VLBW infants? Prospective studies using standardized approaches to early nutrition, evaluation and treatment of hyperglycemia, and outcome assessment will improve the quality of evidence to inform clinical practice for management of hyperglycemia. We propose, and have included, an example of such a study (Table 4). We invite content experts and clinicians worldwide to collaborate on such studies and a prospective meta-analysis.
Table 4.
Proposed study design: Long-term metabolic and neurodevelopmental outcomes following early hyperglycemia treatment in preterm infants receiving standardized nutrition
| Study population | Very low birth weight (VLBW) infants (<32 weeks or <1500 grams) |
| Study design | Multi-center prospective international cohort and randomized control trial |
| Study groups | (1) Normoglycemia (2) Hyperglycemia |
| Treatment Randomization | Reduction of GIR to basal metabolic caloric needs followed by insulin versus no nutritional modification with insulin |
| Hyperglycemia | Blood glucose over 150 mg/dL (>8.3 mmol/L) ≥4 hours during the first two weeks of life |
| Treatment threshold | Blood glucose over 180 mg/dL (>10 mmol/L) ≥4 hours during the first two weeks of life |
| Assessment (glucose) | Continuous glucose monitoring |
| Nutrition | Standardized parenteral and enteral nutrition and vitamin supplementation during the first 2 weeks of life |
| Estimated sample size | N=750 (normoglycemia=250, hyperglycemia=500; 250 randomized to GIR reduction with or without insulin, 250 randomized to insulin) calculated by assumption of a 65% follow-up rate to detect a 15% difference in primary outcome between groups at 80% power at an alpha level of 0.05. |
| Assessment (time) | 12-months PMA, 24-months PMA, 5-years of age, 15-years of age |
| Primary outcome | Survival without significant metabolic or neurodevelopmental impairment at 5-years of age*. |
| Secondary outcomes | Individual components of primary outcome, anthropometrics, BSID-III, need for glasses, body composition, glucose metabolism, quality of life measurements |
| Modifying variables | Insulin treatment, sex, duration/severity of hyperglycemia |
| Confounding variables | Maternal characteristics, demographic characteristics, infant characteristics, morbidities associated with preterm birth, food consumption, social determinants of health, hypoglycemia |
Any of the following: BMI≥85 percentile, fasting glucose≥100 mg/dL, HbA1c≥5.7%, sBP or dBP>95 percentile, cerebral palsy, Movement Assessment Battery for Children-2 (MABC-2) total score ≤ 5th percentile, hearing impairment requiring hearing aids, visual acuity of 6/60, full scale IQ standard score > 1 standard deviation below the mean, visual acuity of 6/60 or worse in best eye, ADHD/Mental health diagnosis requiring behavior/psychological therapy, early intervention, or medication.
Supplementary Material
Funding Sources:
Dr. Paulsen was supported by the National Institute of Health Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) grant HD055887. The NIH had no role in the design and conduct of the study.
Footnotes
Statement of Ethics: An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
References
- 1.Zamir I, Tornevi A, Abrahamsson T, Ahlsson F, Engstrom E, Hallberg B, et al. Hyperglycemia in Extremely Preterm Infants-Insulin Treatment, Mortality and Nutrient Intakes. J Pediatr. 2018;200:104–10 e1. [DOI] [PubMed] [Google Scholar]
- 2.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, Ong K, et al. Prevalence and determinants of hyperglycemia in very low birth weight infants: cohort analyses of the NIRTURE study. J Pediatr. 2010;157(5):715–9 e1-3. [DOI] [PubMed] [Google Scholar]
- 3.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, van Weissenbruch M, et al. Early insulin therapy in very-low-birth-weight infants. N Engl J Med. 2008;359(18):1873–84. [DOI] [PubMed] [Google Scholar]
- 4.Blanco CL, Baillargeon JG, Morrison RL, Gong AK. Hyperglycemia in extremely low birth weight infants in a predominantly Hispanic population and related morbidities. J Perinatol. 2006;26(12):737–41. [DOI] [PubMed] [Google Scholar]
- 5.Yoon JY, Chung HR, Choi CW, Yang SW, Kim BI, Shin CH. Blood glucose levels within 7 days after birth in preterm infants according to gestational age. Ann Pediatr Endocrinol Metab. 2015;20(4):213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tottman AC, Alsweiler JM, Bloomfield FH, Pan M, Harding JE. Relationship between Measures of Neonatal Glycemia, Neonatal Illness, and 2-Year Outcomes in Very Preterm Infants. J Pediatr. 2017;188:115–21. [DOI] [PubMed] [Google Scholar]
- 7.van der Lugt NM, Smits-Wintjens VE, van Zwieten PH, Walther FJ. Short and long term outcome of neonatal hyperglycemia in very preterm infants: a retrospective follow-up study. BMC Pediatr. 2010;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szymonska I, Jagla M, Starzec K, Hrnciar K, Kwinta P. The Incidence of Hyperglycaemia in Very Low Birth Weight Preterm Newborns. Results of a Continuous Glucose Monitoring Study--Preliminary Report. Dev Period Med. 2015;19(3 Pt 1):305–12. [PubMed] [Google Scholar]
- 9.Alexandrou G, Skiold B, Karlen J, Tessma MK, Norman M, Aden U, et al. Early hyperglycemia is a risk factor for death and white matter reduction in preterm infants. Pediatrics. 2010;125(3):e584–91. [DOI] [PubMed] [Google Scholar]
- 10.Hays SP, Smith EO, Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics. 2006; 118(5):1811–8. [DOI] [PubMed] [Google Scholar]
- 11.Kao LS, Morris BH, Lally KP, Stewart CD, Huseby V, Kennedy KA. Hyperglycemia and morbidity and mortality in extremely low birth weight infants. J Perinatol. 2006;26(12):730–6. [DOI] [PubMed] [Google Scholar]
- 12.Georgieff MK. The effect of maternal diabetes during pregnancy on the neurodevelopment of offspring. Minn Med. 2006;89(3):44–7. [PubMed] [Google Scholar]
- 13.Adane AA, Mishra GD, Tooth LR. Diabetes in Pregnancy and Childhood Cognitive Development: A Systematic Review. Pediatrics. 2016;137(5). [DOI] [PubMed] [Google Scholar]
- 14.Landi SN, Radke S, Engel SM, Boggess K, Sturmer T, Howe AS, et al. Association of Long-term Child Growth and Developmental Outcomes With Metformin vs Insulin Treatment for Gestational Diabetes. JAMA Pediatr. 2019;173(2):160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan KM, Gale C, Hyde MJ, Santhakumaran S, Modi N. Diabetes in pregnancy and infant adiposity: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2017;102(1):F65–F72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hay WW Jr. Nutritional Support Strategies for the Preterm Infant in the Neonatal Intensive Care Unit. Pediatr Gastroenterol Hepatol Nutr. 2018;21(4):234–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hay WW Jr. Aggressive Nutrition of the Preterm Infant. Curr Pediatr Rep. 2013;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramel S, Rao R. Hyperglycemia in Extremely Preterm Infants. Neoreviews. 2020;21(2):e89–e97. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- 20.Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123(3):A12–3. [PubMed] [Google Scholar]
- 21.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez Villamizar JH JL; Scheurer JM; Rao R; Ramel SE. Relationships between early nutrition, illness, and later outcomes among infants born preterm with hyperglycemia The Journal of Pediatrics 2020:1–5. [DOI] [PubMed] [Google Scholar]
- 24.Ramel SE, Long JD, Gray H, Durrwachter-Erno K, Demerath EW, Rao R. Neonatal hyperglycemia and diminished long-term growth in very low birth weight preterm infants. J Perinatol. 2013;33(11):882–6. [DOI] [PubMed] [Google Scholar]
- 25.Scheurer JM, Gray HL, Demerath EW, Rao R, Ramel SE. Diminished growth and lower adiposity in hyperglycemic very low birth weight neonates at 4 months corrected age. J Perinatol. 2016;36(2):145–50. [DOI] [PubMed] [Google Scholar]
- 26.Tottman AC, Alsweiler JM, Bloomfield FH, Gamble G, Jiang Y, Leung M, et al. Long-Term Outcomes of Hyperglycemic Preterm Infants Randomized to Tight Glycemic Control. J Pediatr. 2018;193:68–75 e1. [DOI] [PubMed] [Google Scholar]
- 27.Heald A, Abdel-Latif ME, Kent AL. Insulin infusion for hyperglycaemia in very preterm infants appears safe with no effect on morbidity, mortality and long-term neurodevelopmental outcome. J Matern Fetal Neonatal Med. 2012;25(11):2415–8. [DOI] [PubMed] [Google Scholar]
- 28.Zamir I, Stoltz Sjostrom E, Edstedt Bonamy AK, Mohlkert LA, Norman M, Domellof M. Postnatal nutritional intakes and hyperglycemia as determinants of blood pressure at 6.5 years of age in children born extremely preterm. Pediatr Res. 2019;86(1): 115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alsweiler JM, Harding JE, Bloomfield FH. Tight glycemic control with insulin in hyperglycemic preterm babies: a randomized controlled trial. Pediatrics. 2012;129(4):639–47. [DOI] [PubMed] [Google Scholar]
- 30.Kim HS. Blood Glucose Measurement: Is Serum Equal to Plasma? Diabetes Metab J. 2016;40(5):365–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boghossian NS, Geraci M, Edwards EM, Horbar JD. Sex Differences in Mortality and Morbidity of Infants Born at Less Than 30 Weeks’ Gestation. Pediatrics. 2018;142(6). [DOI] [PubMed] [Google Scholar]
- 32.Raju TNK, Pemberton VL, Saigal S, Blaisdell CJ, Moxey-Mims M, Buist S, et al. Long-Term Healthcare Outcomes of Preterm Birth: An Executive Summary of a Conference Sponsored by the National Institutes of Health. J Pediatr. 2017;181:309–18 e1. [DOI] [PubMed] [Google Scholar]
- 33.Saigal S, Stoskopf B, Streiner D, Paneth N, Pinelli J, Boyle M. Growth trajectories of extremely low birth weight infants from birth to young adulthood: a longitudinal, population-based study. Pediatr Res. 2006;60(6):751–8. [DOI] [PubMed] [Google Scholar]
- 34.Kajantie E, Strang-Karlsson S, Hovi P, Wehkalampi K, Lahti J, Kaseva N, et al. Insulin sensitivity and secretory response in adults born preterm: the Helsinki Study of Very Low Birth Weight Adults. J Clin Endocrinol Metab. 2015;100(1):244–50. [DOI] [PubMed] [Google Scholar]
- 35.Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM, et al. Premature birth and later insulin resistance. N Engl J Med. 2004;351(21):2179–86. [DOI] [PubMed] [Google Scholar]
- 36.Thomas EL, Parkinson JR, Hyde MJ, Yap IK, Holmes E, Dore CJ, et al. Aberrant adiposity and ectopic lipid deposition characterize the adult phenotype of the preterm infant. Pediatr Res. 2011;70(5):507–12. [DOI] [PubMed] [Google Scholar]
- 37.Lewandowski AJ, Augustine D, Lamata P, Davis EF, Lazdam M, Francis J, et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation. 2013;127(2):197–206. [DOI] [PubMed] [Google Scholar]
- 38.Paulsen ME, Rosario FJ, Wesolowski SR, Powell TL, Jansson T. Normalizing adiponectin levels in obese pregnant mice prevents adverse metabolic outcomes in offspring. FASEB J. 2019;33(2):2899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaughan OR, Rosario FJ, Powell TL, Jansson T. Normalisation of circulating adiponectin levels in obese pregnant mice prevents cardiac dysfunction in adult offspring. Int J Obes (Lond). 2020;44(2):488–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauml JG, Daamen M, Meng C, Neitzel J, Scheef L, Jaekel J, et al. Correspondence Between Aberrant Intrinsic Network Connectivity and Gray-Matter Volume in the Ventral Brain of Preterm Born Adults. Cereb Cortex. 2015;25(11):4135–45. [DOI] [PubMed] [Google Scholar]
- 41.Jurcoane A, Daamen M, Scheef L, Bauml JG, Meng C, Wohlschlager AM, et al. White matter alterations of the corticospinal tract in adults born very preterm and/or with very low birth weight. Hum Brain Mapp. 2016;37(1):289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramel SE, Haapala J, Super J, Boys C, Demerath EW. Nutrition, Illness and Body Composition in Very Low Birth Weight Preterm Infants: Implications for Nutritional Management and Neurocognitive Outcomes. Nutrients. 2020;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satrom KM, Ennis K, Sweis BM, Matveeva TM, Chen J, Hanson L, et al. Neonatal hyperglycemia induces CXCL10/CXCR3 signaling and microglial activation and impairs long-term synaptogenesis in the hippocampus and alters behavior in rats. J Neuroinflammation. 2018; 15(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alur P Sex Differences in Nutrition, Growth, and Metabolism in Preterm Infants. Front Pediatr. 2019;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res. 2010;67(4):357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poindexter BB, Langer JC, Dusick AM, Ehrenkranz RA, National Institute of Child H, Human Development Neonatal Research N. Early provision of parenteral amino acids in extremely low birth weight infants: relation to growth and neurodevelopmental outcome. J Pediatr. 2006;148(3):300–5. [DOI] [PubMed] [Google Scholar]
- 48.Dickson JL, Chase JG, Pretty CG, Gunn CA, Alsweiler JM. Hyperglycaemic Preterm Babies Have Sex Differences in Insulin Secretion. Neonatology. 2015;108(2):93–8. [DOI] [PubMed] [Google Scholar]
- 49.Bottino M, Cowett RM, Sinclair JC. Interventions for treatment of neonatal hyperglycemia in very low birth weight infants. Cochrane Database Syst Rev. 2011(10):CD007453. [DOI] [PubMed] [Google Scholar]
- 50.Stephens BE, Walden RV, Gargus RA, Tucker R, McKinley L, Mance M, et al. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics. 2009;123(5):1337–43. [DOI] [PubMed] [Google Scholar]
- 51.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253–61. [DOI] [PubMed] [Google Scholar]
- 52.Boluyt N, van Kempen A, Offringa M. Neurodevelopment after neonatal hypoglycemia: a systematic review and design of an optimal future study. Pediatrics. 2006;117(6):2231–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


