Abstract
The hypothesis that the physiological response to psychological stress influences the initiation of cancer is highly controversial. The link between initiating stressors, the psychological stress response, and disease is plausible considering that the stress response is associated with defined physiological outcomes and molecular mechanisms. In light of this, we review the clinical relevance of psychological stress on the risk of cancer, and we propose potential molecular pathways that may link the stress response to early stages of malignant cell transformation.
Introduction
Exposure to psychological stressors is highly relevant amid the current COVID-19 pandemic. Increased levels of stress and anxiety have been reported globally due to social isolation, lockdowns, and job losses(1,2). Exposure to psychological stressors produces a physiological response involving the activation of the hypothalamic-pituitary axis (HPA) and the sympathetic nervous system (SNS)(3). Mediators of this stress response are cortisol, which activates downstream glucocorticoid receptor (GR) signalling pathways, and catecholamines (adrenaline and noradrenaline), that stimulate the adrenergic pathway through the β-adrenoceptors(3–5) (Fig. 1A). Stress is defined as acute or chronic depending on the length of exposure to the stressor and the response. Acute stress is short-term and activates the “fight or flight” response, increasing alertness and readiness for physical activity, whilst inhibiting functions such as feeding or reproduction(6). While the body readily adapts to acute stress, prolonged exposure to stress due to chronic stressors can cause an inappropriate basal activity or hyper-responsiveness to stressors that may lead to long-term damage to tissues such as the hippocampus and prefrontal cortex in the brain(7–9). Chronic stressors can include situations such as caregiving, bereavement, socioeconomic burden, societal micro-aggressions, and social isolation. These stressors generally result in an increased and continued release of stress hormones that may favour tumour initiation(10). However, the individual response to stressors can vary, affecting levels of stress hormones(11). For example, an individual’s perception of a stressor and appraisal of their ability to cope influences the downstream biological response(11).
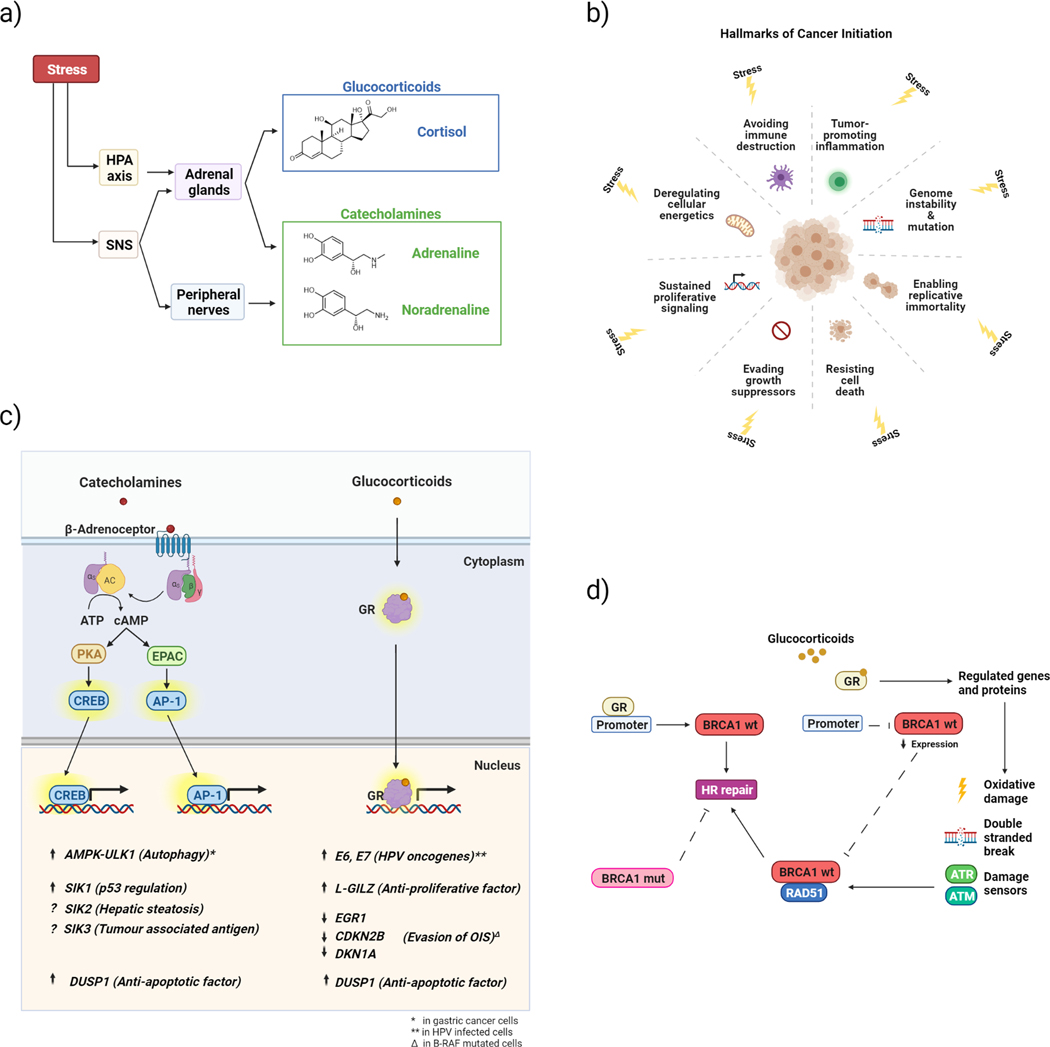
Fig 1a. Stress stimulus produces a physiological response which involves the central nervous system (CNS) and the periphery.
The stress response includes the activation of two endocrine systems: the hypothalamic-pituitary axis (HPA) and the sympathetic nervous system (SNS). The final mediators of the stress response are cortisol and catecholamines (adrenaline and noradrenaline) secreted by the adrenal cortex and medulla respectively. SNS signalling also occurs via nerves. Fig 1b. Stress regulation of tumour initiation. Reported stress effects on the hallmarks of cancer proposed by Hanahan and Weinberg(52,53). Fig 1c. Proposed mechanisms of glucocorticoids and catecholamines on tumour initiation. The adrenergic pathway mediates the effect of catecholamines by binding the β2-adrenoceptor and activating into adenylyl cyclase which converts ATP in cAMP. cAMP activates two major effectors, protein kinase A (PKA) and the exchange factor directly regulated by cAMP (EPAC) respectively leading to the activation of CREB and AP-1, transcription factors that modulate gene expression. Cortisol activates the stress pathway mediated by glucocorticoid receptors (GRs). The binding of glucocorticoids to GRs results in the translocation of the complex from the cytoplasm to the nucleus where it modulates gene expression. Stress-regulated processes include important molecular mechanisms such as proliferation, cell cycle regulation, DNA damage and repair that might contribute to cancer initiation. Arrows indicate up/down regulation. Fig 1d. Stress effects on DNA damage response Glucocorticoids bind to the GR, induce ROS/RNS and activate the DNA Damage Response (DDR). Two key signal transducing kinases are ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related (ATR) which are primarily involved in the responses to DSB’s or stalled replication, respectively. ATM and ATR activation leads to phosphorylation of effector kinases, facilitating cell cycle arrest via cyclin-dependent kinase inhibition, DNA damage repair and apoptosis, through phosphorylation of substrates like p53 and BRCA1. Stress hormones also affect DNA repair by disrupting the signalling pathways mediated by RAD51 and BRCA1, contributing to DNA damage accumulation. The unliganded GR can directly interact and positively regulate activity on the BRCA1 promoter. Binding of glucocorticoids negates this positive effect, reducing promoter activity.
Chronic psychological stress clearly influences the pathogenesis of some cardiac and dermatological diseases (12–15); therefore, it is not unreasonable to postulate that a vigorous stress response can promote cancer initiation by influencing stress-related biochemical pathways. It is widely accepted that bacterial, viral infections, autoimmune diseases, and environmental exposures can induce inflammation leading to increased cancer risk(16). Inflammation can increase mutation rates as activated inflammatory cells producing reactive oxygen species (ROS) and reactive nitrogen species (RNS) that are capable of inducing DNA damage and genomic instability(17). Additionally, psychological stress can promote negative health behaviours such as smoking, alcohol consumption, and sleep disruption that can increase the risk of developing diseases such as heart disease and cancer(18).
So far, there is a paucity of data identifying the molecular mechanisms through which stress might affect cancer initiation. Malignant tumour growth results from multiple cell type-specific processes and the contribution of psychological stress through stress hormone signalling may vary according to cancer subtype. However, in this opinion article, we discuss unifying processes underlying the influence of psychological stress on cancer initiation by analysing the classic physiological stress response involving the release of glucocorticoids and catecholamines. We present a critical analysis of the literature describing clinical aspects of the psychological stress response in cancer development, and review how stress hormone signalling can affect common pathways involved in carcinogenesis with a focus on DNA damage and repair, epigenetic and immune mechanisms.
Clinical Perspectives
The concept that psychological stress might contribute to tumour initiation was considered during the second century. The “seed and soil” hypothesis proposed by Paget specifically relates to cancer metastasis(19); however, it is feasible that repetitive stress enables a permissive environment for primary cancer initiation. Clinicians have observed that severe life disruptions often occur before the onset of cancer (20–23). However, the idea of stress as a factor contributing to cancer risk has been controversial with early evidence linking stress and cancer initiation resulting in inconsistent findings.
Here, we first discuss evidence showing a positive association between stress and cancer risk/incidence (Table 1, Case control/population studies). One of the most compelling studies was conducted in a cohort of 10,808 Finnish women aged ≥24 with a 15-year follow-up. A retrospective questionnaire detailing a range of life experiences was administered with an assessment of psychological stress including stress of daily activities, life, and satisfaction. Women with an accumulation of adverse life events (death of a spouse, relative or close friend) 5 years before a cancer diagnosis showed the strongest association with an increased risk of breast cancer(24). Evidence from a prospective study of 1,462 Swedish women (middle aged) reporting any type of stress up to 24 years previously showed that breast cancer incidence was increased 2-fold compared to women reporting no stress(25). This effect was not reduced when adjusted for family history of breast cancer or variables related to socioeconomic status. A prospective case-control study of 514 Australian women (average age 61) biopsied after routine mammographic screening examined the role of self-reported stressful life events and coping and breast cancer incidence(26). A significant association was observed between highly stressful life events and lack of social support and breast cancer incidence. Another case-control study conducted in 858 invasive breast cancer patients and matched controls in Poland(27) found that women who had breast cancer scored highest for previous stressful life events (from 0–21 years following diagnosis), after adjustment for potential risk factors, with the death of relative or spouses increasing breast cancer risk significantly. Similarly, in a prospective study of 115 Finnish women, breast cancer patients were found to have a significantly higher level of perceived stress in the 10 years prior to diagnosis than women with benign breast disease, suggesting a convincing link between stress and breast cancer incidence(28). To determine the effect of low-level chronic stress as opposed to acute high impact stress such as death, job strain stress was examined in 36,332 Swedish women working full-time or part-time. In women working full-time there was a weak correlation between low job control, high job demands and breast cancer risk, suggesting that even chronic low level daily stressors may be associated with elevated breast cancer risk(29). An Israeli cohort of bereaved parents showed increased incidence of lymphatic, hematopoietic and skin cancers(30). Interestingly, childhood physical abuse was associated with 47% higher odds of being diagnosed with a subsequent cancer(31). A recent study suggests that workplace stress exposure is associated with an increase of prostate cancer in men >65(32). In addition, persistent depression in older adults was shown to be associated with an 88% increase in cancer risk, which is important considering that chronic stress is also associated with the development of depression(33). Other studies highlight a positive correlation of stress and post-traumatic stress disorder in prostate, ovarian and breast cancer(34–36).
Table 1.
| Cancer Type | # Subjects | Stress measures | Positive association | Refs |
|---|---|---|---|---|
| Case control/population studies | ||||
| Breast | 10,808 | Questionnaire: modified standardised life event inventory | Divorce/separation, death of a husband/close relative or friend were associated with increased risk of breast cancer. | (24) |
| Breast | 1,462 | Questionnaire: stress | Self-reported stress was associated with a significant increase in breast cancer incidence. | (25) |
| Breast | 514 | Questionnaire: Brown and Harris Life Event and Difficulties Schedule & psychosocial variables | Highly stressful events and no emotional support significantly increased breast cancer risk. | (26) |
| Breast | 858 | Questionnaire: socioeconomic status and stressful life events | Death of relative or spouses significantly increased breast cancer risk. | (27) |
| Breast | 115 | Questionnaire: Beck Depression and Spielberger Trait Inventory and interview | Stressful life events significantly increased breast cancer risk. | (28) |
| Breast | 36,332 | Questionnaire: assess job demands, control and social support | Weak correlation between low job control, high job demands and breast cancer risk. | (29) |
| Many | 6,284 | Bereavement question | Bereavement correlated with an increased incidence of cancer. | (30) |
| All | 13,092 | Survey questions: childhood &adult Socioeconomic status | Childhood physical abuse was associated with increased risk of cancer. | (31) |
| Prostate | 1,933 | Questionnaire: perceived Stress | Prolonged workplace stress was associated with an increase in risk of cancer. | (32) |
| Many | 4,825 | Questionnaire: Center for Epidemiologic Studies-Depression scale | Six years of depression was associated with an increased risk of cancer. | (33) |
| Breast | 867 | Questionnaire: Life Events Scale Holmes and Rahe scale | Cumulative adverse life events perceived as stressful were associated with increased risk of breast cancer. | (34) |
| Ovarian | 54,710 | Modified version of the Brief Trauma Interview | PTSD symptoms were associated with increased risk of ovarian cancer. | (35) |
| Breast & Prostate | 991 women & 5,743 men | Questionnaire | A weak association between stress and risk of prostate risk but not breast cancer. | (36) |
| Cancer Type | N number | Stress measures | Negative association | Refs |
| Breast | 106,000 | Questionnaire: perceived frequency of stress, experience of adverse life events and bereavement | A positive association of divorce with ER-negative but not ER-positive breast cancer. No consistent evidence for an association of breast cancer risk with perceived stress levels or adverse life events, or loss of parents during childhood and adolescence. | (37) |
| Breast | 11,467 | Questionnaire: Health and Life Experiences and assessment of social & psychosocial circumstances | No evidence of social adversity correlating with cancer incidence. | (38) |
| Breast | 10,519 | Questionnaire: Stress of Daily Activities | No association between daily stress and breast cancer risk. | (39) |
| Breast | 167,368 | Nationwide cohort (Fertility Register) | No increase in breast cancer risk after the death of a child. | (40) |
| Breast | 69,886 | Questionnaire: informal caregiving | No association between higher levels of caregiving and breast cancer incidence. | (41) |
| Breast | 84,334 | Questionnaire: stressful life events, social support | No independent association between stressful life events and breast cancer risk. | (42) |
| Breast | 2,739 | Questionnaire: acute and chronic stress | No association between acute or chronic stress and breast cancer risk. | (43) |
| Breast | 6,689 | Questionnaire: perceived stress | High perceived stress resulted in a lower risk of breast cancer. | (44) |
| Meta Analyses/Reviews | ||||
| Breast | 27 studies | Questionnaire and interview | A modest association between death of spouse and breast cancer risk but no overall association between stressful life events and breast cancer risk. | (45) |
| Lung | 165 studies | Questionnaire | Stress-related psychosocial factors are associated with higher cancer incidence in initially healthy populations. | (46) |
| Breast | N = 471 | Observational studies and review | A positive association of perceived stress, together with potentially risky lifestyle behaviours with breast cancer. | (47) |
| Breast | N = 530 | Questionnaire: striking life events | A positive association between striking life events and primary breast cancer incidence. | (48) |
| Breast | 27 studies | Questionnaire | A modest association between death of spouse and breast cancer risk and no association bereavement, or other adverse life event. | (49) |
| Many | 12 studies | Questionnaire: Job Content and Demand | No association between stress and breast cancer risk. | (50) |
| Many | review | Self -reported work stress | Inconclusive data but nightshift work may affect incidence. | (51) |
In contrast, several studies show no association between stress and cancer incidence. A cohort investigation of 106,000 UK women did not show consistent evidence for an association of breast cancer risk with perceived stress levels (e.g. adverse life events or significant stress such as loss of parents) with a 5 year follow up(37). Two separate prospective studies involving 11,467 healthy UK and 10,519 Finnish women found no evidence of an association between social stress over a 10-year period and breast cancer incidence(38) or between daily stress and breast cancer risk(39). There was no correlation even with adjustment for confounding factors such as smoking status or BMI. Evidence from nationwide cohort defined by the Fertility Register also showed no association between death of a child and breast cancer risk(40). Similarly, there was no association with caregiving stress and cancer incidence(41). In the Women’s Health Initiative, 84,334 post-menopausal American women were examined for cancer incidence in relation to stressful life events (e.g., death of a family member) and social support(42). After adjusting for confounding variables there was no association between stressful life events or social support and breast cancer incidence. However, the follow-up time was only ~7 years. A 15 year prospective study in 2,739 women in Australasia also showed no association between acute and chronic stressors and breast cancer risk(43). Finally, a prospective study in 6,689 participants in Denmark showed that higher levels of perceived stress correlated to a lower risk of breast cancer(44). This was attributed to the fact that stress may impair oestrogen synthesis.
Meta-analyses on this topic have generally shown small effect sizes (Table 1, Meta Analyses/Reviews). For example, a meta-analysis of 27 breast cancer studies showed a modest association between death of spouse and breast cancer risk, although there was no overall association between stressful life events and breast cancer risk(45). However, a larger meta-analysis of 165 longitudinal studies showed that psychosocial factors and stressful life experiences were associated with higher cancer incidence, poorer cancer survival, and higher mortality(46). Two other meta analyses on breast cancer also report positive associations with breast cancer incidence(47,48). In contrast, other studies in breast cancer yielded opposite results. An epidemiological study of the link between stress and breast cancer examined 29 studies of adverse life events and breast cancer risk. No link was found between breast cancer and bereavement, or any other adverse life event(49). A meta-analysis from 12 studies on work stress (50), on 116,056 participants was studied to ascertain if high job strain correlates to an increased risk of breast cancer, and there was no association even after adjustment for various risk factors e.g. BMI and smoking. However, interestingly, there is evidence that night shift work may affect breast cancer occurrence(51).
The irregularity in findings of case control/ population and meta-analysis studies reflects the complexity of tumour initiation studies. Study design and control for confounding variables such as socioeconomic status, smoking, drinking alcohol, BMI etc. are not always recorded. Furthermore, the type and timing of stress exposure, and follow-up times all vary between studies, making direct comparisons challenging. Prospective studies can be influenced by recall bias as patients may interpret stressful events differently following a diagnosis. However, there are considerably more population and meta-analyses studies showing a positive association between high stress and breast cancer incidence.
Stress and common carcinogenesis pathways
The process of tumour initiation involves multiple events whereby cells acquire malignant characteristics. These hallmarks of cancer include increased proliferation, evasion of cell death, deregulating cellular energetics, induction of angiogenesis, and evasion/editing of the immune system(52,53). In the following sections, we discuss pathways that are activated in response to psychological stress, and which are also involved in tumour initiation (Summarised in Fig 1b).
A more prominent role of psychological stress has been demonstrated in the initiation of cancers with a viral aetiology (e.g. cervical carcinoma, lymphoma, and hepatocarcinoma)(11). Early work suggested that oncogenic transformation of primary cells with a combination of HPV-16 DNA and the activated form of the human H-ras oncogene occurred only in the presence of the glucocorticoid, dexamethasone(54). Indeed, human papillomavirus type 16 glucocorticoid response elements have been proven functional for cell transformation, transient expression, and DNA-protein interactions(55). Steroid hormones are thought to increase the expression of the E6 and E7 HPV 16 oncogenes, which bind to and degrade p53 leading to tumour initiation(55). Cortisol can also regulate the HPV-E6-p53-miR-145 pathway(56). Interestingly, hepatocarcinoma is closely linked to chronic viral hepatitis infection which is affected by chronic stress. In mice, chronic restraint stress promoted hepatocarcinoma growth through β-adrenergic signalling. This was mediated by the CXCR2-CXCL2/CXCL3 axis and recruitment of myeloid cells, which are thought to be immune suppressive(57).
One of the main characteristics that normal cells acquire during malignant transformation is inappropriate proliferation. Although the role of psychological stress on proliferation has been studied in cancer progression, less is known about the mechanisms affecting cancer initiation(58–60). A role in mediating tumourigenesis in B-RAF mutated cells is exerted by the long glucocorticoid-induced leucine zipper (L-GILZ). Blockade of BRAF activity in thyroid cell line (8505C) carrying BRAFV600E mutation led to the inhibition of proliferation, an effect mediated by the upregulation of L-GILZ expression(61,62). This suggests that BRAF mutations can promote proliferation by downregulating L-GILZ expression and contribute to tumour initiation. Glucocorticoids also play a role in the escape from the oncogene-induced senescence (OIS), a quiescence mechanism. BRAFV600E expression in non-cancerous cells triggers two opposing responses. Neural stem cells expressing BRAFV600E showed signs of transformation by growing in an anchorage-independent manner, a hallmark of cell transformation, and forming colonies in soft agar(63). However, proliferation was blocked by entering OIS(63,64). Cells in OIS can remain quiescent for long periods of time before progressing to malignancy(64). These two opposite responses activated by B-Raf signalling create a balance between a pro-oncogenic signal and a senescent proliferative arrest. The switch towards tumour initiation might be sustained by other important tumourigenic stimuli. BRAFV600E-induced senescence was also bypassed by the addition of glucocorticoids, albeit at pharmacological doses, in human fibroblasts allowing for cancer transformation. Furthermore, growth arrest was mediated by BRAFV600E via the activation of the early growth response protein (EGR1) which stimulates the expression of tumour suppressor genes such as the cyclin-dependent kinase 4 inhibitor B (CDKN2B) and cyclin-dependent kinase inhibitor 1 (CDKN1A). Treatment with clobetasol, a synthetic corticosteroid, decreased EGR1, CDKN2B, and CDKN1a levels consistent with allowing evasion from senescence and promoting cancer initiation(65).
It is noteworthy that there is a role of the outgrowth of nerves (axonogenesis) and SNS signalling in cancer initiation(66). Novel work in a pancreatic cancer mouse model showed active bidirectional communication between the pancreas and sensory neurons (which interact with parasympathetic neurons) prior to the establishment of tumours. Ablation of sensory nerves in mouse models of pancreatic adenocarcinoma slowed cancer initiation(67). Although it can be argued that these mice are genetically engineered to develop cancer it still highlights the importance of the nervous system in supporting inflammation associated with oncogenic Kras-induced neoplasia(67). Catecholamines can also mediate tumour proliferation and alter gene expression pathways associated with carcinogenesis. Ovarian and fallopian tube surface epithelial immortalized cells show a differential gene expression when treated with noradrenaline compared to controls(68). These genes include salt-Inducible kinases 1–3 (SIKs) each having different functions in cancer and are candidates for initiation(69). SIK1 works as a p53 regulator promoting anoikis and protecting from cancer initiation. SIK1 suppression in mammary epithelial cells enhances cell transformation by expressing cancer related mutations (such as PIK3-H1047R) and promoting anchorage-independent growth(70). Noradrenaline upregulates SIK1(68), highlighting a possible protective effect of the stress hormone. However, long-term effects of noradrenaline on SIK1 remain unknown. SIK2 and SIK3 may also contribute to tumour development due to their roles in cell cycle regulation(69). The role of SIK2 in cancer initiation has emerged because it is a key factor in hepatic steatosis which increases the chance of developing hepatocarcinoma(71,72).
Another interesting protein is the anti-apoptotic Dual Specificity Phosphatase 1 (DUSP1), which is upregulated by stress hormones(68). DUSP1 encodes a Serine/Threonine specific protein phosphatase (MPK-1) involved in the MAP kinase dephosphorylation, and highly expressed in many epithelial tumours including breast cancer(73–78). Interestingly, DUSP1/MPK-1 expression is lower in hepatocarcinoma and head and neck cancer compared to normal tissues(79). Noradrenaline upregulates DUSP-1 in normal ovarian and fallopian tube epithelial cells and is overexpressed in ovarian cancer cells(68). Noradrenaline-induced DUSP1 protects ovarian cancer cells from apoptosis and impairs chemotherapy-induced cell death(80). DUSP1 is also upregulated by GR activation in ovarian tissues(81,82) suggesting that both glucocorticoids and catecholamines may decrease chemotherapy-induced apoptosis in human epithelial cancers through the activation of anti-apoptotic signalling. Mechanisms underlying DUSP1/MKP-1 expression in cancers are complex as they are regulated by environmental factors such as oxidative stress, DNA damage, and hypoxia(79) (Fig. 1C). In summary, the observation that stress hormones can regulate molecular mediators of tumour formation such as SIKs and anti-apoptotic genes such as DUSP-1 suggests that these genes may contribute to tumour initiation.
Stress mediators on DNA damage and repair
Damage to DNA is understood to be one of the major events in cancer initiation. In response to DNA damage, pathways are activated to identify and repair the damage. Stress hormones have the propensity to induce DNA damage and modulate the transcription of DNA damage related genes(83) (Fig. 1D). This was demonstrated in 3T3 fibroblasts exposed to cortisol, adrenaline, and noradrenaline which induced significant DNA damage and inhibited DNA repair through modulation of DNA damage sensors and cell cycle progression genes(83). However, there is conflicting evidence of noradrenaline on DNA damage. In a non-tumourigenic ovarian epithelial cell line (IOSE-29), noradrenaline significantly reduced constitutive levels of DNA breaks and attenuated DNA damage induced by treatment with bleomycin. This effect was mediated by the anti-oxidant properties of noradrenaline which prevented Reactive Oxygen species (ROS)-induced DNA damage(84). In contrast, using the β-adrenergic agonist, isoproterenol, adrenergic signalling led to accumulation of DNA damage(85). Chronic stimulation of β2-adrenoceptor with isoproterenol, adrenaline, or noradrenaline in mice or in cultured U2OS cells, led to decreased levels of p53 and accumulation of DNA damage. Isoproterenol activated the murine double minute 2 (MDM2) via beta-arrestin-mediated PI3K/AKT MDM2 phosphorylation and promoted p53 degradation(86). The accumulation of DNA damage was a result of a decreased repair. There is emerging data showing that stress hormones can reduce p53 function through the activation of MDM2(86). Similarly, a study in mice showed that restraint stress, via release of glucocorticoids results in decreased p53 and tumourigenesis in heterozygous p53+/− irradiated mice(87). This evidence highlights the possibility that a pharmacological blockade of these pathways should be further investigated in tumour initiation can represent a therapeutic option in managing the negative effects of chronic stress(88). Other studies have shown that stress hormones can induce DNA damage through the production of ROS and reactive nitrogen species (RNS) capable of interacting with DNA, causing base changes and strand breaks(89). We can conclude that stress hormones can elicit damaging effects on DNA and impact the repair processes, known contributors to tumour initiation.
Given the highly mutagenic landscape of cancer cells, subtle and transient increases in DNA damage because of psychological stress are hard to observe in tumour samples. It is therefore prudent to highlight the limitations of DNA damage studies which have been confined to cancer cell lines, which allow controlled exposure and quantification of any stress hormone-induced damage. Whilst there is limited evidence of the direct effect of stress hormones on DNA damage in patient tumours, Gidron et al. summarise literature relating to psychological factors and DNA damage in a range of animal models and human studies(90,91). Meta-analysis of rodent studies (up to the year 2006), indicates that psychological stressors such as sensory stress can elevate the oxidative DNA damage marker 8-hydroxy-2’ -deoxyguanosine (8-OHdG). Furthermore, several studies have linked psychological perturbations such as perceived stress, depression and anxiety to elevated levels of 8-OHdG(92–94). Excreted 8-OHdG has been shown to be a reliable biomarker of risk in colon and breast cancer risk indicating an association between stress hormones, oxidative DNA damage and cancer(95–97).
Dysregulation of DNA damage response and repair pathways are key in cancer initiation, as the ability to maintain genomic integrity is compromised in favour of proliferation. In certain types of tumours, DNA repair capacity and tumourigenesis are closely linked because of mutations in breast cancer type 1 and 2 susceptibility (BRCA1 and BRCA2) genes, encoding for proteins involved in DNA repair. Mutations in BRCA genes increase the risk of breast, ovarian, and to a lesser extent other types of cancer such as prostate, pancreatic, and melanoma(98). As already discussed, cortisol can induce DNA damage and alter DNA repair mechanisms in cancer cells through the production of ROS/RNS, known contributors to carcinogenesis(99,100). Indeed, BRCA deficient cells are more sensitive to the effects of oxidative stressors and in BRCA1 deficient breast epithelial cells, a number of ROS inducing compounds promoted an increase in DNA damage(101,102). This suggests that stress-mediated DNA damage through the production of ROS/RNS together with an impaired DNA repair mechanism exerted by mutated BRCA1 can contribute to an accumulation of genomic alterations that are crucial for tumour initiation. Although the effects of stress can contribute to carcinogenesis, the concurrent impairment of other molecular mechanisms implies that stress may not necessarily be the cause but may be a contributing factor to tumour initiation. These considerations also suggest that stress signalling and BRCA1 function might have reciprocal interactions.
There is compelling evidence of the effects of glucocorticoids on BRCA1 expression and vice versa. Hydrocortisone and dexamethasone can downregulate expression of the BRCA1 gene in a non-malignant mouse mammary cell line(103). Further investigation of the mechanism revealed that glucocorticoids specifically repress BRCA1 promoter activity. Hydrocortisone decreased BRCA1 luciferase gene reporter expression in non-malignant mammary cell lines, but not in malignant lines. Furthermore, long-term repression of the BRCA1 promoter was only achieved in the continuous presence of hydrocortisone(103). This suggests that stress hormones may contribute to breast cancer initiation via reducing BRCA1 expression in normal breast epithelium, allowing DNA damage to occur without effective repair.
Manipulation of unliganded GR expression was observed to upregulate activity on the BRCA1 promoter(104). Binding of glucocorticoids negated this positive effect, reducing promoter activity. Whilst this study supports the hypothesis that an increased risk of breast cancer could be associated with psychological stress, it’s possible that basal levels of GR may be linked to BRCA1 expression. Indeed, the unliganded GR may act as a cooperative transcription factor for BRCA1. Expression of GR varies across tissues, as well as in certain subtypes of breast cancer, with increased expression of GR associated with shorter relapse-free survival in the aggressive ER-negative subtype(105). The liganded-GR dampens oestrogen receptor mediated breast cancer cell proliferation, and decreases ER occupancy at proliferative gene enhancer sites, as well as inhibiting recruitment of crucial protein complexes to the ER-bound enhancer(106,107). However in ER-negative breast cancer, GR-regulated genes involved in proliferation and cell survival were associated with poor prognosis and relapse(108). Further studies reveal that the GR and ER can work in a complex and taken together these results suggest ER status may be an important factor in the GR transcriptional activity among breast cancer subtypes(109). Furthermore, whilst high basal expression of the GR may promote BRCA1 activity, a sustained increase in its ligand - as seen during prolonged periods of psychological stress - could feasibly suppress BRCA1 expression more so than in GR-low tissues, leading to an increased risk. Interestingly, methylation of the GR promoter resulting in downregulation of GR expression was more frequently observed in breast tumours, compared to normal tissue samples(110). In a follow up study, knockdown of the GR in mammary epithelial cells by transfection with GR directed short-hairpin RNA (shRNA) generating a stable shGR line also demonstrated positive regulation of BRCA1 by GR(111). Microarray analysis of the shGR line also identified several pro-apoptotic genes regulated by the unliganded GR in a similar manner to BRCA1(111). This may suggest an anti-tumourigenic role for the unliganded GR in non-cancerous cells, although in breast cancer this depends on the ER status, and that the release of glucocorticoids from psychological stress could abolish this and promote tumour initiation.
The crosstalk between the GR and BRCA1 in mammary epithelial cells (Fig. 1D) points towards potential implications for patients with breast cancers driven by hereditary BRCA1-mutations that are prone to ER-independent breast cancers. Chronic elevation of cortisol because of continued stressors such as cancer diagnosis and treatment, which have been reported as major stress-inducing factors for women with breast cancer, can lead to increased stimulation of the GR. In turn this could place additional burden on DNA repair mechanisms in BRCA1-deficient cells, leading to an accumulation of DNA damage. Combined with the increase in oxidative DNA damage also triggered by stress hormones, this has the potential for an additional risk for women with BRCA1 mutations.
Stress hormones and epigenetics
Dysregulated epigenetic mechanisms as a non-genomic risk in cancer initiation have received increased attention. GR activation causes epigenetic modifications via methylation in cytosine-guanine dinucleotides of the DNA; changes in histone methylation and acetylation and regulation of microRNAs (miRNAs), such as miR-708 in breast cancer(112). Recent work in neurons has demonstrated that stress exposure and glucocorticoids alter N6-methyladenosine and N6,2′-O-dimethyladenosine mRNA methylation that regulates transcript processing and translation(113). Glucocorticoids can also increase the expression of enzymes involved in active demethylation, e.g. the Tet family of 5-methylcytosine dioxygenases, and decrease the expression and activity of the maintenance DNA methyltransferases DNMT1 and DNMT2 and de novo methyltransferase DNMT3(114). GR activation can promote dynamic chromatin remodelling that may alter accessibility of GR-binding sites to the transcriptional machinery(113–115).
There is increasing evidence that glucocorticoids induce long lasting epigenetic modifications in many tissues and these modifications are tissue-type specific. For example, an interesting study in mice showed that cortisol exposure decreases DNA methylation of the GR chaperone FKBP5 in the hippocampus and hypothalamus and also causes CPG demethylation of FKBP5(116). Others have shown that stress and ageing synergistically decrease DNA methylation in FKBP5 CpGs in immune cells prompting NF-kB mediated inflammation(117). Epigenetic upregulation of the GR chaperone FKPP5 may therefore induce inflammation and cancer risk. It is important to note that glucocorticoid-induced changes in DNA methylation last many weeks even following removal of glucocorticoids. Stress exposure has been shown to induce lasting epigenetic modifications through DNA methylation, histone modification etc. throughout the lifespan(115,118–120). The epigenome is sensitive to stressors across all periods of mammalian life, but it may be particularly susceptible during periods of rapid epigenetic remodelling or deficient functioning of epigenetic maintenance systems, such as in early development or older age, which may be important with regards to tumour initiation(115).
There is evidence linking stress exposure in early development to an increased cancer risk via epigenetic mechanisms. A study in Israel on 152,622 Holocaust survivors who were followed for over 45 years showed that Holocaust survivors have a small but consistent increase in the risk of developing cancer. Intergenerational effects of maternal Holocaust exposure on FKBP5 methylation have also been reported(121). A large British birth cohort study also showed that cancer risk may be influenced by exposure to psychosocial adversity in childhood. Women who experienced two or more adverse events doubled their risk of having a cancer before 50 compared to women who had had no childhood adversities(122). Stress exposure during childhood can lead to long term epigenetic modifications e.g. persistent demethylation of CPGs located near the glucocorticoid response elements of the FKBP5 gene(123). A model whereby exposure to life stress via glucocorticoid signaling may alter the epigenetic landscape across the lifespan impacting genomic regulation and function has been proposed(124). These are important aspects of the transgenerational stress response and potential non-genomic effects of the activated neuroendocrine system on cancer susceptibility.
Stress, cell mediated immunity and inflammation.
We have discussed how stress can contribute to tumour initiation by acting on specific carcinogenic pathways. However, a dysregulation of immune and inflammatory processes can also contribute to carcinogenesis(125). In principle, tumour initiation can be controlled by innate and adaptive immune cells; additionally, these cells express β2-adrenoceptors and GR indicating that they can be regulated by stress. Stress hormones can directly modulate multiple aspects of the immune response including cell-mediated immunity, humoral immunity, lymphocyte proliferation, macrophage response and polarization, NK cell function and immune cell trafficking(125,126). Stress can suppress immune surveillance mechanisms, enhance inflammation, and upregulate immunosuppressive signals(127–129), thus impairing an individual’s ability to recognise and destroy transformed cells. A human study demonstrated that laboratory stressors increase levels of circulating and stimulated cytokines(130). It is possible that these responses contribute to associations between exposure to life challenges and vulnerability to diseases such as cancer. The role of stress on immune cell trafficking, suppression and inflammation is key to tumour initiation. Immune cells constantly traffic between the blood and various lymphoid and non-lymphoid organs and studies in rodents suggest that behavioural stress induces a significant redistribution of T cells in the body(131). Similarly, SNS innervation of the spleen and lymph nodes also modulates the progression of peripheral immune responses by dampening lymphocyte trafficking to tissue. Activation of lymphocyte β2-adrenoceptor enhances the responsiveness of chemokine receptors (CCR7 and CXCR4) that promote lymph node retention of lymphocytes, and consequently inhibits their lymph node emergence(132).
Stress hormone signalling can upregulate immunosuppressive signals. A mouse study showed that chronic stress increased the susceptibility of UV-induced melanoma through shifting the balance of protective immune cells towards suppressive (T regulatory cells) immunity(133). Interestingly, studies in rats showed that social isolation increased the risk of spontaneous mammary tumours(134). A vast body of literature highlights the anti-inflammatory properties of glucocorticoids and how they can affect immune signalling pathways and inhibit inflammatory mediators(135). The SNS also regulates inflammation and immunity(136). Catecholamines influence inflammation by increasing the recruitment of hematopoietic stem and progenitor cells (HSPCs) to the spleen supporting long-lasting splenic myelopoiesis(137). Furthermore, β-adrenergic up-regulation of myelopoiesis is one molecular mechanism by which chronic stress may result in a pro-inflammatory shift(138). A recent study showed that stress hormones, via β2-adrenoceptor can cause the release of pro-inflammatory S100A8/A9 complexes by neutrophils. This led to the release of oxidized lipids directly activating proliferation of dormant tumour cells through an upregulation of the fibroblast growth factor pathway(139). Fibroblasts can also secrete lipids leading to activation of mitogenic pathways. Indeed, fatty acids secreted into the microenvironment can impact infiltrating immune cell function and phenotype(140). With their role in suppressing host immunity, promotion of inflammation and release of DNA damaging ROS and oxidized lipids, it is likely that stress hormones can promote a favourable niche sustaining malignant cell transformation.
Conclusions
The research outlined in this review supports the notion that persistent and chronic stress exposure might contribute to tumour initiation in specific cancers. This research is still in its early stages compared to research on stress and tumour progression, and evaluation of the physiological stress response and cancer initiation mechanisms is greatly needed. A more detailed examination of biomolecular mechanisms that underlie physiological stress responsiveness and how stress-hormones contribute to tumour-initiating pathways in susceptible patients is warranted. In light of the COVID-19 pandemic, with increased reports of chronic stress, social isolation, and reluctance to visit the General Practitioner, the aforementioned studies should encourage clinicians and cancer biologists alike to consider psychological stress as a synergistic risk factor to inherited genetic and environmental factors that increase cancer risk. These findings also raise the importance of addressing resilience in response to psychological stress e.g., in high-risk patients such as those with germline mutations in DNA repair mechanisms and other cancer susceptibility pathways.
Acknowledgments:
This work has been partially supported by R01CA246540 and R01CA193249 (SL).
Premal Thaker reports personal fees from Stryker, Astra Zeneca, grants and personal fees from Glaxo Smith Kline, grants and personal fees from Merck, personal fees from Iovance, Novocure, Celsion, Aravive, outside the submitted work. Suzanne Conzen receives patent licensing royalties from Corcept Therapeutics.
Footnotes
COI
All other authors have no COI to declare.
References
- 1.Xiong J, Lipsitz O, Nasri F, Lui LMW, Gill H, Phan L, et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020; 277:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serafini G, Parmigiani B, Amerio A, Aguglia A, Sher L, Amore M. The psychological impact of COVID-19 on the mental health in the general population. Qjm. 2020; 113:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrousos GP. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009; 5:374–81. [DOI] [PubMed] [Google Scholar]
- 4.Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am. 2001;30:695–728. [DOI] [PubMed] [Google Scholar]
- 5.Andersson K, Fuxe K, Agnati LF. Determinations of catecholamine half-lives and turnover rates in discrete catecholamine nerve terminal systems of the hypothalamus, the preoptic region and the forebrain by quantitative histofluorimetry. Acta Physiol Scand. 1985;123:411–26. [DOI] [PubMed] [Google Scholar]
- 6.Black PH. Central nervous system-immune system interactions: Psychoneuroendocrinology of stress and its immune consequences. Antimicrob. Agents Chemother. 1994; 38:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bremner JD. Does stress damage the brain. Biol. Psychiatry. 1999; 45:797–805. [DOI] [PubMed] [Google Scholar]
- 8.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–48. [DOI] [PubMed] [Google Scholar]
- 9.Gold PW, Machado-Vieira R, Pavlatou MG. Clinical and biochemical manifestations of depression: Relation to the neurobiology of stress. Neural Plast. 2015; 2015:581976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deary IJ. Measuring stress: A guide for health and social scientists. J Psychosom Res. 1996;41:186. [Google Scholar]
- 11.Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, et al. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nat. Rev. Cancer. 2006; 6:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. J. Am. Med. Assoc. 2007; 298:1685–7. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S, Gianaros PJ, Manuck SB. A Stage Model of Stress and Disease. Perspect Psychol Sci. 2016;11:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer SE, Prather AA, Puterman E, Lin J, Arenander J, Coccia M, et al. Cumulative lifetime stress exposure and leukocyte telomere length attrition: The unique role of stressor duration and exposure timing. Psychoneuroendocrinology. 2019;104:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010; 140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer. 2013; 13:759–71. [DOI] [PubMed] [Google Scholar]
- 18.Dimsdale JE. Psychological Stress and Cardiovascular Disease. J. Am. Coll. Cardiol. 2008; 51:1237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fidler IJ, Poste G. The “seed and soil” hypothesis revisited. Lancet Oncol. 2008; 9:808. [DOI] [PubMed] [Google Scholar]
- 20.Antoni MH, Pereira DB, Marion I, Ennis N, Andrasik MP, Rose R, et al. Stress management effects on perceived stress and cervical neoplasia in low-income HIV-infected women. J Psychosom Res. 2008;65:389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira DB, Antoni MH, Danielson A, Simon T, Efantis-Potter JN, Carver CS, et al. Life stress and cervical squamous intraepithelial lesions in women with human papillomavirus and human immunodeficiency virus. Psychosom Med. 2003;65:427–34. [DOI] [PubMed] [Google Scholar]
- 22.Goodkin K, Antoni MH, Blaney PH. Stress and hopelessness in the promotion of cervical intraepithelial neoplasia to invasive squamous cell carcinoma of the cervix. J Psychosom Res. 1986;30:67–76. [DOI] [PubMed] [Google Scholar]
- 23.Fife A, Beasley PJ, Fertig DL. Psychoneuroimmunology and cancer: Historical perspectives and current research. Adv Neuroimmunol. 1996;6:179–90. [DOI] [PubMed] [Google Scholar]
- 24.Lillberg K, Verkasalo PK, Kaprio J, Teppo L, Helenius H, Koskenvuo M. Stressful life events and risk of breast cancer in 10,808 women: A cohort study. Am J Epidemiol. 2003;157:415–23. [DOI] [PubMed] [Google Scholar]
- 25.Helgesson Ö, Cabrera C, Lapidus L, Bengtsson C, Lissner L. Self-reported stress levels predict subsequent breast cancer in a cohort of Swedish women. Eur J Cancer Prev. 2003;12:377–81. [DOI] [PubMed] [Google Scholar]
- 26.Price MA, Tennant CC, Butow PN, Smith RC, Kennedy SJ, Kossoff MB, et al. The role of psychosocial factors in the development of breast carcinoma: Part II - Life event stressors, social support, defense style, and emotional control and their interactions. Cancer. 2001;91:686–97. [PubMed] [Google Scholar]
- 27.Kruk J. Self-reported psychological stress and the risk of breast cancer: A case-control study. Stress. 2012;15:162–71. [DOI] [PubMed] [Google Scholar]
- 28.Ollonen P, Lehtonen J, Eskelinen M. Stressful and adverse life experiences in patients with breast symptoms; a prospective case-control study in Kuopio, Finland. Anticancer Res. 2005;25:531–6. [PubMed] [Google Scholar]
- 29.Kuper H, Yang L, Theorell T, Weiderpass E. Job strain and risk of breast cancer. Epidemiology. 2007;18:764–8. [DOI] [PubMed] [Google Scholar]
- 30.Levav I, Kohn R, Iscovich J, Abramson JH, Tsai WY, Vigdorovich D. Cancer incidence and survival following bereavement. Am J Public Health. 2000;90:1601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller-Thomson E, Brennenstuhl S. Making a link between childhood physical abuse and cancer: Results from a regional representative survey. Cancer. 2009;115:3341–50. [DOI] [PubMed] [Google Scholar]
- 32.Blanc-Lapierre A, Rousseau MC, Parent ME. Perceived workplace stress is associated with an increased risk of prostate cancer before age 65. Front Oncol. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penninx BWJH, Guralnik JM, Pahor M, Ferrucci L, Cerhan JR, Wallace RB, et al. Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst. 1998;90:1888–93. [DOI] [PubMed] [Google Scholar]
- 34.Fischer A, Ziogas A, Anton-Culver H. Perception matters: Stressful life events increase breast cancer risk. J Psychosom Res. 2018;110:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts AL, Huang T, Koenen KC, Kim Y, Kubzansky LD, Tworoger SS. Posttraumatic stress disorder is associated with increased risk of ovarian cancer: A prospective and retrospective longitudinal cohort study. Cancer Res. 2019;79:5113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metcalfe C, Smith GD, Macleod J, Hart C. The role of self-reported stress in the development of breast cancer and prostate cancer: A prospective cohort study of employed males and females with 30 years of follow-up. Eur J Cancer. 2007;43:1060–5. [DOI] [PubMed] [Google Scholar]
- 37.Schoemaker MJ, Jones ME, Wright LB, Griffin J, McFadden E, Ashworth A, et al. Psychological stress, adverse life events and breast cancer incidence: A cohort investigation in 106,000 women in the United Kingdom. Breast Cancer Res. 2016;18:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Surtees PG, Wainwright NWJ, Luben RN, Khaw KT, Bingham SA. No evidence that social stress is associated with breast cancer incidence. Breast Cancer Res Treat. 2010;120:169–74. [DOI] [PubMed] [Google Scholar]
- 39.Lillberg K, Lillberg K, Verkasalo PK, Kaprio J, Teppo L, Helenius H, et al. Stress of daily activities and risk of breast cancer: A prospective cohort study in Finland. Int J Cancer. 2001;91:888–93. [DOI] [PubMed] [Google Scholar]
- 40.Lambe M, Cerrato R, Askling J, Hsieh CC. Maternal breast cancer risk after the death of a child. Int J Cancer. 2004;110:763–6. [DOI] [PubMed] [Google Scholar]
- 41.Kroenke CH, Hankinson SE, Schernhammer ES, Colditz GA, Kawachi I, Holmes MD. Caregiving stress, endogenous sex steroid hormone levels, and breast cancer incidence. Am J Epidemiol. 2004;159:1019–27. [DOI] [PubMed] [Google Scholar]
- 42.Michael YL, Carlson NE, Chlebowski RT, Aickin M, Weihs KL, Ockene JK, et al. Influence of Stressors on Breast Cancer Incidence in the Women’s Health Initiative. Heal Psychol. 2009;28:137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butow P, Price M, Coll J, Tucker K, Meiser B, Milne R, et al. Does stress increase risk of breast cancer? A 15-year prospective study. Psychooncology. 2018;27:1908–14. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen NR, Zhang ZF, Kristensen TS, Netterstrøm B, Schnohr P, Grønbæk M. Self reported stress and risk of breast cancer: Prospective cohort study. Br Med J. 2005;331:548–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duijts SFA, Zeegers MPA, Borne B Vd. The association between stressful life events and breast cancer risk: A meta-analysis. Int J Cancer. 2003;107:1023–9. [DOI] [PubMed] [Google Scholar]
- 46.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008; 5:466–75. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Liao WC, Tsai CJ, Wang LR, Mao IF, Chen CC, et al. The Effects of Perceived Stress and Life Style Leading to Breast Cancer. Women Heal. 2013;53:20–40. [DOI] [PubMed] [Google Scholar]
- 48.Lin Y, Wang C, Zhong Y, Huang X, Peng L, Shan G, et al. Striking life events associated with primary breast cancer susceptibility in women: A meta-analysis study. J Exp Clin Cancer Res. 2013;32:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petticrew M, Fraser JM, Regan MF. Adverse life-events and risk of breast cancer: A meta-analysis. Br J Health Psychol. 1999;4:1–17. [Google Scholar]
- 50.Heikkila K, Nyberg ST, Theorell T, Fransson EI, Alfredsson L, Bjorner JB, et al. Work stress and risk of cancer: Meta-analysis of 5700 incident cancer events in 116 000 European men and women. BMJ. 2013;346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang XS, Armstrong MEG, Cairns BJ, Key TJ, Travis RC. Shift work and chronic disease: The epidemiological evidence. Occup. Med. (Chic. Ill). 2011; 61:8–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000; 100:57–70. [DOI] [PubMed] [Google Scholar]
- 53.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011; 144:646–74. [DOI] [PubMed] [Google Scholar]
- 54.Pater MM, Hughes GA, Hyslop DE, Nakshatri H, Pater A. Glucocorticoid-dependent oncogenic transformation by type 16 but not type 11 human papilloma virus DNA. Nature. 1988;335:832–5. [DOI] [PubMed] [Google Scholar]
- 55.Moodley M, Moodley J, Chetty R, Herrington CS. The role of steroid contraceptive hormones in the pathogenesis of invasive cervical cancer: A review. Int. J. Gynecol. Cancer. 2003;13:103–10. [DOI] [PubMed] [Google Scholar]
- 56.Shi M, Du L, Liu D, Qian L, Hu M, Yu M, et al. Glucocorticoid regulation of a novel HPV-E6-p53- MiR-145 pathway modulates invasion and therapy resistance of cervical cancer cells. J Pathol. 2012;228:148–57. [DOI] [PubMed] [Google Scholar]
- 57.Jiang W, Li Y, zhen Li Z, Sun J, wei Li J, Wei W, et al. Chronic restraint stress promotes hepatocellular carcinoma growth by mobilizing splenic myeloid cells through activating β-adrenergic signaling. Brain Behav Immun. 2019;80:825–38. [DOI] [PubMed] [Google Scholar]
- 58.Bernabé DG, Tamae AC, Biasoli ÉR, Oliveira SHP. Stress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cells. Brain Behav Immun. 2011;25:574–83. [DOI] [PubMed] [Google Scholar]
- 59.Tian D, Tian M, Han G, Li JL. Increased glucocorticoid receptor activity and proliferation in metastatic colon cancer. Sci Rep. 2019;9:11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhi X, Li B, Li Z, Zhang J, Yu J, Zhang L, et al. Adrenergic modulation of AMPK-dependent autophagy by chronic stress enhances cell proliferation and survival in gastric cancer. Int J Oncol. 2019;54:1625–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ayroldi E, Zollo O, Bastianelli A, Marchetti C, Agostini M, Di Virgilio R, et al. GILZ mediates the antiproliferative activity of glucocorticoids by negative regulation of Ras signaling. J Clin Invest. 2007;117:1605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ayroldi E, Petrillo MG, Marchetti MC, Cannarile L, Ronchetti S, Ricci E, et al. Long glucocorticoid-induced leucine zipper regulates human thyroid cancer cell proliferation. Cell Death Dis. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raabe EH, Lim KS, Kim JM, Meeker A, Mao XG, Nikkhah G, et al. BRAF activation induces transformation and then senescence in human neural stem cells: A pilocytic astrocytoma model. Clin Cancer Res. 2011;17:3590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michaloglou C, Vredeveld LCW, Soengas MS, Denoyelle C, Kuilman T, Van Der Horst CMAM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–4. [DOI] [PubMed] [Google Scholar]
- 65.Carvalho C, L’Hôte V, Courbeyrette R, Kratassiouk G, Pinna G, Cintrat JC, et al. Glucocorticoids delay RAF-induced senescence promoted by EGR1. J Cell Sci. 2019; 132(16):jcs230748. [DOI] [PubMed] [Google Scholar]
- 66.Faulkner S, Jobling P, March B, Jiang CC, Hondermarck H. Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 2019; 9:702–10. [DOI] [PubMed] [Google Scholar]
- 67.Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA, et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A. 2016;113:3078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gjyshi A, Dash S, Cen L, Cheng CH, Zhang C, Yoder SJ, et al. Early transcriptional response of human ovarian and fallopian tube surface epithelial cells to norepinephrine. Sci Rep. 2018; 8(1):8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Du WQ, Zheng JN, Pei DS. The diverse oncogenic and tumor suppressor roles of salt-inducible kinase (SIK) in cancer. Expert Opin. Ther. Targets. 2016; 20:477–85. [DOI] [PubMed] [Google Scholar]
- 70.Cheng H, Liu P, Wang ZC, Zou L, Santiago S, Garbitt V, et al. SIK1 couples LKB1 to p53-dependent anoikis and suppresses metastasis. Sci Signal. 2009;2:80ra35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bricambert J, Miranda J, Benhamed F, Girard J, Postic C, Dentin R. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J Clin Invest. 2010;120:4316–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smedile A, Bugianesi E. Steatosis and hepatocellular carcinoma risk. Eur Rev Med Pharmacol Sci. 2005;9:291–3. [PubMed] [Google Scholar]
- 73.Liao Q, Guo J, Kleeff J, Zimmermann A, Büchler MW, Korc M, et al. Down-regulation of the dual-specificity phosphatase MKP-1 suppresses tumorigenicity of pancreatic cancer cells. Gastroenterology. 2003;124:1830–45. [DOI] [PubMed] [Google Scholar]
- 74.Denkert C, Schmitt WD, Berger S, Reles A, Pest S, Siegert A, et al. Expression of mitogen-activated protein kinase phosphatase-1 (MKP-1) in primary human ovarian carcinoma. Int J Cancer. 2002;102:507–13. [DOI] [PubMed] [Google Scholar]
- 75.Bang YJ, Kwon JH, Kang SH, Kim JW, Yang YC. Increased MAPK activity and MKP-1 overexpression in human gastric adenocarcinoma. Biochem Biophys Res Commun. 1998;250:43–7. [DOI] [PubMed] [Google Scholar]
- 76.Vicent S, Garayoa M, López-Picazo JM, Lozano MD, Toledo G, Thunnissen FBJM, et al. Mitogen-activated protein kinase phosphatase-1 is overexpressed in non-small cell lung cancer and is an independent predictor of outcome in patients. Clin Cancer Res. 2004;10:3639–49. [DOI] [PubMed] [Google Scholar]
- 77.Wang HY, Cheng Z, Malbon CC. Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Lett. 2003;191:229–37. [DOI] [PubMed] [Google Scholar]
- 78.Shipp LE, Lee JV, Yu CY, Pufall M, Zhang P, Scott DK, et al. Transcriptional regulation of human dual specificity protein phosphatase 1 (DUSP1) gene by glucocorticoids. PLoS One. 2010;5:10e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shen J, Zhang Y, Yu H, Shen B, Liang Y, Jin R, et al. Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy. Cancer Med. 2016; 5:2061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang Y, Nagaraja AS, Armaiz-Pena GN, Dorniak PL, Hu W, Rupaimoole R, et al. Adrenergic stimulation of DUSP1 impairs chemotherapy response in ovarian cancer. Clin Cancer Res. 2016;22:1713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melhem A, Yamada SD, Fleming GF, Delgado B, Brickley DR, Wu W, et al. Administration of glucocorticoids to ovarian cancer patients is associated with expression of the anti-apoptotic genes SGK1 and MKP1/DUSP1 in ovarian tissues. Clin Cancer Res. 2009;15:3196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tchen CR, Martins JRS, Paktiawal N, Perelli R, Saklatvala J, Clark AR. Glucocorticoid regulation of mouse and human dual specificity phosphatase 1 (DUSP1) genes: Unusual cis-acting elements and unexpected evolutionary divergence. J Biol Chem. 2010;285:2642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flint MS, Baum A, Chambers WH, Jenkins FJ. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology. 2007;32:470–9. [DOI] [PubMed] [Google Scholar]
- 84.Patel PR. Norepinephrine Reduces Reactive Oxygen Species (ROS) and DNA Damage in Ovarian Surface Epithelial Cells. J Bioanal Biomed. 2015;7(3):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, et al. A stress response pathway regulates DNA damage through β2- adrenoreceptors and β-arrestin-1. Nature. 2011;477:349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu W, Feng Z, Levine AJ. The Regulation of Multiple p53 Stress Responses is Mediated through MDM2. Genes and Cancer. 2012; 3:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feng Z, Liu L, Zhang C, Zheng T, Wang J, Lin M, et al. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci U S A. 2012;109:7013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hara MR, Sachs BD, Caron MG, Lefkowitz RJ. Pharmacological blockade of a β2AR-β-arrestin-1 signaling cascade prevents the accumulation of DNA damage in a behavioral stress model. Cell Cycle. 2013;12:219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flaherty RL, Owen M, Fagan-Murphy A, Intabli H, Healy D, Patel A, et al. Glucocorticoids induce production of reactive oxygen species/reactive nitrogen species and DNA damage through an iNOS mediated pathway in breast cancer. Breast Cancer Res. 2017; 19(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gidron Y, Russ K, Tissarchondou H, Warner J. The relation between psychological factors and DNA-damage: A critical review. Biol Psychol. 2006;72:291–304. [DOI] [PubMed] [Google Scholar]
- 91.Jenkins FJ, Van Houten B, Bovbjerg DH. Effects on DNA damage and/or repair processes as biological mechanisms linking psychological stress to cancer risk. J Appl Biobehav Res. 2014;19:3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Irie M, Miyata M, Kasai H. Depression and possible cancer risk due to oxidative DNA damage. J Psychiatr Res. 2005;39:553–60. [DOI] [PubMed] [Google Scholar]
- 93.Shimanoe C, Hara M, Nishida Y, Nanri H, Horita M, Yamada Y, et al. Perceived Stress, Depressive Symptoms, and Oxidative DNA Damage. Psychosom Med. 2018;80:28–33. [DOI] [PubMed] [Google Scholar]
- 94.Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2′-deoxyguanosine in clinical depression. Psychosom Med. 2006;68:1–7. [DOI] [PubMed] [Google Scholar]
- 95.Guo C, Li X, Wang R, Yu J, Ye M, Mao L, et al. Association between Oxidative DNA Damage and Risk of Colorectal Cancer: Sensitive Determination of Urinary 8-Hydroxy-2′-deoxyguanosine by UPLC-MS/MS Analysis. Sci Rep. 2016; 6:32581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bayo J, Castaño MA, Rivera F, Navarro F. Analysis of blood markers for early breast cancer diagnosis. Clin Transl Oncol. 2018;20:467–75. [DOI] [PubMed] [Google Scholar]
- 97.Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta. 2004; 399:1–9. [DOI] [PubMed] [Google Scholar]
- 98.Mersch J, Jackson MA, Park M, Nebgen D, Peterson SK, Singletary C, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer. 2015;121:269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reeder A, Attar M, Nazario L, Bathula C, Zhang A, Hochbaum D, et al. Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. Br J Cancer. 2015;112:1461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flaherty RL, Intabli H, Falcinelli M, Bucca G, Hesketh A, Patel BA, et al. Stress hormone-mediated acceleration of breast cancer metastasis is halted by inhibition of nitric oxide synthase. Cancer Lett. 2019;459:59–71. [DOI] [PubMed] [Google Scholar]
- 101.Fridlich R, Annamalai D, Roy R, Bernheim G, Powell SN. BRCA1 and BRCA2 protect against oxidative DNA damage converted into double-strand breaks during DNA replication. DNA Repair (Amst). 2015;30:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kang HJ, Bin Hong Y, Weon Yi Y, Cho CH, Wang A, Bae I. The correlations between BRCA1 defect and environmental factors in the risk of breast cancer. J. Toxicol. Sci. 2013; 38:355–61. [DOI] [PubMed] [Google Scholar]
- 103.Antonova L, Mueller CR. Hydrocortisone down-regulates the tumor suppressor gene BRCA1 in mammary cells: A possible molecular link between stress and breast cancer. Genes Chromosom Cancer. 2008;47:341–52. [DOI] [PubMed] [Google Scholar]
- 104.Ritter HD, Antonova L, Mueller CR. The unliganded glucocorticoid receptor positively regulates the tumor suppressor gene BRCA1 through GABP beta. Mol Cancer Res. 2012;10:558–69. [DOI] [PubMed] [Google Scholar]
- 105.Pan D, Kocherginsky M, Conzen SD. Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancer. Cancer Res. 2011;71:6360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tonsing-Carter E, Hernandez KM, Kim CR, Harkless R V., Oh A, Bowie KR, et al. Glucocorticoid receptor modulation decreases ER-positive breast cancer cell proliferation and suppresses wild-type and mutant ER chromatin association. Breast Cancer Res. 2019;21:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang F, Ma Q, Liu Z, Li W, Tan Y, Jin C, et al. Glucocorticoid Receptor:MegaTrans Switching Mediates the Repression of an ERα-Regulated Transcriptional Program. Mol Cell. 2017;66:321–331.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.West DC, Kocherginsky M, Tonsing-Carter EY, Dolcen DN, Hosfield DJ, Lastra RR, et al. Discovery of a glucocorticoid receptor (gr) activity signature using selective gr antagonis in er-negative breast cancer. Clin Cancer Res. 2018;24:3433–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.West DC, Pan D, Tonsing-Carter EY, Hernandez KM, Pierce CF, Styke SC, et al. GR and ER coactivation alters the expression of differentiation genes and associates with improved ER+ breast cancer outcome. Mol Cancer Res. 2016;14:707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nesset KA, Perri AM, Mueller CR. Frequent promoter hypermethylation and expression reduction of the glucocorticoid receptor gene in breast tumors. Epigenetics. 2014;9:851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ritter HD, Mueller CR. Expression microarray identifies the unliganded glucocorticoid receptor as a regulator of gene expression in mammary epithelial cells. BMC Cancer. 2014;14:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Senthil Kumar KJ, Gokila Vani M, Hsieh HW, Lin CC, Liao JW, Chueh PJ, et al. MicroRNA-708 activation by glucocorticoid receptor agonists regulate breast cancer tumorigenesis and metastasis via downregulation of NF-κB signaling. Carcinogenesis. 2019;40:335–48. [DOI] [PubMed] [Google Scholar]
- 113.Engel M, Eggert C, Kaplick PM, Eder M, Röh S, Tietze L, et al. The Role of m6A/m-RNA Methylation in Stress Response Regulation. Neuron. 2018;99:389–403.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bartlett AA, Lapp HE, Hunter RG. Epigenetic Mechanisms of the Glucocorticoid Receptor. Trends Endocrinol. Metab. 2019; 30:807–18. [DOI] [PubMed] [Google Scholar]
- 115.Zannas AS, Chrousos GP. Epigenetic programming by stress and glucocorticoids along the human lifespan. Mol. Psychiatry. 2017; 22:640–6. [DOI] [PubMed] [Google Scholar]
- 116.Lee RS, Tamashiro KLK, Yang X, Purcell RH, Harvey A, Willour VL, et al. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. 2010;151:4332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zannas AS, Jia M, Hafner K, Baumert J, Wiechmann T, Pape JC, et al. Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-κB-driven inflammation and cardiovascular risk. Proc Natl Acad Sci U S A. 2019;166:11370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ewald ER, Wand GS, Seifuddin F, Yang X, Tamashiro KL, Potash JB, et al. Alterations in DNA methylation of Fkbp5 as a determinant of blood-brain correlation of glucocorticoid exposure. Psychoneuroendocrinology. 2014;44:112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bose R, Spulber S, Kilian P, Heldring N, Lönnerberg P, Johnsson A, et al. Tet3 mediates stable glucocorticoid-induced alterations in DNA methylation and Dnmt3a/Dkk1 expression in neural progenitors. Cell Death Dis. 2015;6:e1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cox OH, Song HY, Garrison-Desany HM, Gadiwalla N, Carey JL, Menzies J, et al. Characterization of glucocorticoid-induced loss of DNA methylation of the stress-response gene Fkbp5 in neuronal cells. Epigenetics. 2021;12:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bierer LM, Bader HN, Daskalakis NP, Lehrner A, Provençal N, Wiechmann T, et al. Intergenerational effects of maternal holocaust exposure on FKBP5 methylation. Am J Psychiatry. 2020;177:744–53. [DOI] [PubMed] [Google Scholar]
- 122.Kelly-Irving M, Lepage B, Dedieu D, Lacey R, Cable N, Bartley M, et al. Childhood adversity as a risk for cancer: Findings from the 1958 British birth cohort study. BMC Public Health. 2013;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: Preliminary findings in healthy adults. PLoS One. 2012;7(1):e30148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gassen NC, Chrousos GP, Binder EB, Zannas AS. Life stress, glucocorticoid signaling, and the aging epigenome: Implications for aging-related diseases. Neurosci. Biobehav. Rev. 2017; 74:356–65. [DOI] [PubMed] [Google Scholar]
- 125.Antoni MH, Dhabhar FS. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer. 2019; 125:1417–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.De Lorenzo BHP, de Oliveira Marchioro L, Greco CR, Suchecki D. Sleep-deprivation reduces NK cell number and function mediated by β-adrenergic signalling. Psychoneuroendocrinology. 2015;57:134–43. [DOI] [PubMed] [Google Scholar]
- 127.Dhabhar FS. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014; 58:193–210. [DOI] [PubMed] [Google Scholar]
- 128.Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004; 5:617–25. [DOI] [PubMed] [Google Scholar]
- 129.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer. 2015; 15:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav Immun. 2017;64:208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells-From barracks to boulevards to battlefields: A tale of three hormones - Curt Richter Award Winner. Psychoneuroendocrinology. 2012;37:1345–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J Exp Med. 2014;211:2583–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97:1760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hermes GL, Delgado B, Tretiakova M, Cavigelli SA, Krausz T, Conzen SD, et al. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc Natl Acad Sci U S A. 2009;106:22393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017; 17:233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim TH, Rowat AC, Sloan EK. Neural regulation of cancer: From mechanobiology to inflammation. Clin. Transl. Immunol. 2016; 5:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McKim DB, Yin W, Wang Y, Cole SW, Godbout JP, Sheridan JF. Social Stress Mobilizes Hematopoietic Stem Cells to Establish Persistent Splenic Myelopoiesis. Cell Rep. 2018;25:2552–2562.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Powell ND, Sloan EK, Bailey MT, Arevalo JMG, Miller GE, Chen E, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110:16574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Perego M, Tyurin VA, Tyurina YY, Yellets J, Nacarelli T, Lin C, et al. Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Sci Transl Med. 2020; 12:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Corn KC, Windham MA, Rafat M. Lipids in the tumor microenvironment: From cancer progression to treatment. Prog. Lipid Res. 2020; 80:101055. [DOI] [PMC free article] [PubMed] [Google Scholar]