Abstract
Background:
Polygenic risk scores (PRS) may enhance risk stratification for coronary heart disease (CHD) among young adults. Whether a CHD PRS improves prediction beyond modifiable risk factors in this population is not known.
Methods:
Genotyped adults aged 18–35 were selected from the Coronary Artery Risk Development in Young Adults (CARDIA) study (n=1,132) and Framingham Offspring Study (FOS) (n=663). Systolic blood pressure, total and HDL cholesterol, triglycerides, smoking and waist circumference or BMI were measured at the visit 1 exam of each study and coronary artery calcium (CAC), a measure of coronary atherosclerosis, was assessed at year 15 (CARDIA) or year 30 (FOS). A previously validated PRS for CHD was computed for each subject. The C-statistic and integrated discrimination improvement (IDI) were used to compare Improvements in prediction of elevated CAC between models containing the PRS, risk factors, or both.
Results:
There were 62 (5%) and 93 (14%) participants with a CAC score >20 (CARDIA) and >300 (FOS), respectively. At these thresholds, the C-statistic changes of adding the PRS to a risk factor-based model were 0.015 (0.004, 0.028) and 0.020 (0.001, 0.039) in CARDIA and FOS, respectively. When adding risk factors to a PRS-based model, the respective changes were 0.070 (0.033, 0.109) and 0.051 (0.017, 0.079). The IDI, when adding the PRS to a risk factor model, was 0.027 (−0.006, 0.054) in CARDIA and 0.039 (0.0005, 0.072) in FOS.
Conclusions:
Among young adults, a PRS improved model discrimination for coronary atherosclerosis, but improvements were smaller than those associated with modifiable risk factors.
Keywords: Genetic, Association Studies, Coronary Artery Disease
INTRODUCTION
Coronary heart disease (CHD) is a major cause of morbidity and mortality.1 Common genetic variation contributes to CHD risk, and there is considerable interest in using polygenic risk scores (PRS), which measure a portion of this genetic liability, for risk stratification.2,3 The added benefit of PRS for risk stratification is modest once individuals reach middle-age.4–6 It has been proposed that these scores be used for risk stratification earlier in life in young adults, as currently implemented risk factor-based risk assessment tools are not well-suited for young individuals who typically manifest few conventional risk factors.7,8 Whether a PRS would improve risk stratification in young adults, beyond traditional risk factors is not known.
Although 4–6% of CHD cases are early onset, the low absolute risk makes prospective studies of overt CHD events in young populations challenging.9 One potential surrogate marker for CHD in young individuals is coronary artery calcium (CAC). CAC assessments, as measured by computed tomography, identify subclinical atherosclerotic disease among both young and older adults10, and the presence of CAC is strongly associated with incident CHD risk. For example, a CAC Agatston score >20 in young adults is associated with more than a 5 fold increased incident risk of CHD events and an Agatston score >300 in middle-aged to older adults is associated with 4 to 7-fold increased incident risk.11,12 These data have fostered calls for increased surveillance for CAC in an effort to detect (and potentially initiate lipid-lowering therapies for) subclinical atherosclerosis.13–15 A CHD PRS has also been shown to strongly associate with CAC in young and middle-aged adults, independent of traditional cardiovascular risk factors.16–20 However, whether the addition of a PRS to conventional risk factors would improve risk stratification for subclinical atherosclerosis, as measured by CAC, in young adults remains unresolved.
We evaluated the incremental benefit attributable to a validated CHD PRS 2,21, beyond modifiable risk factors, to risk stratify young adults (<35 years of age) in the Coronary Artery Risk Development in Young Adults (CARDIA) study and Framingham Offspring Study (FOS) for the presence of CAC in mid-life 22–24.
METHODS
Data and materials used for these analyses are publicly available through the dbGaP resource [phs000285 and phs000007] and can be accessed at https://www.ncbi.nlm.nih.gov/gap/. Use of de-identified data made available through dbGaP was approved by the Institutional Review Board of Vanderbilt University Medical Center. Detailed methods are described in the Supplemental Materials.
RESULTS
Baseline Populations
The final CARDIA sample comprised 1,132 White participants (48% male) with a mean age of 25.6 (standard deviation, 3.3) years (Table 1 and Supplemental Tables I and II and III). The final FOS sample comprised 663 participants (47% male) with a mean age of 27.8 (s.d. 4.7) years (Table 1 and Supplemental Tables I and IV). The CHD PRS was positively nominally associated with baseline factors in both sets including LDL-C levels (p=0.0004) in CARDIA, and LDL-C (p=0.007), BMI (p=0.04) and smoking status (p=0.01) in FOS (Table 1).
Table 1.
Characteristics of the CARDIA and Framingham Offspring Cohort participants.
| Characteristic* | CARDIA CAC≤20 | CARDIA CAC>20 | Framingham CAC≤300 | Framingham CAC>300 |
|---|---|---|---|---|
| Total | 1070 | 62 | 570 | 93 |
| Males | 494 (46.2%) | 51 (82.3%) | 236 (41.4%) | 72 (77.4%) |
| Age (years) | 25.5 (3.3) | 27.2 (2.9) | 27.5 (4.7) | 30 (4) |
| Systolic blood pressure (mmHg) | 108.4 (10.8) | 115.4 (12) | 115.3 (11.3) | 122.4 (13) |
| Diastolic blood pressure (mmHg) | 67.8 (8.9) | 71.1 (11.4) | 74.8 (8.4) | 79.3 (8.8) |
| Body mass index (kg/m)†,‡ | 23.5 (3.7) | 25.5 (4.6) | 23.9 (3.7) | 26.7 (4.6) |
| Waist circumference (cm) | 76.8 (10.1) | 85.5 (10.2) | n/a | n/a |
| Total cholesterol (mg/dl) † | 175 (30.8) | 197.1 (39.5) | 178.6 (32.3) | 197.3 (32.4) |
| Triglycerides (mg/dl) | 64 (47 – 88) | 87 (54 – 118) | 207.5 (146 – 305) | 325 (224 – 492) |
| HDL cholesterol (mg/dl) ‡ | 52.1 (13) | 45.3 (10.6) | 51.7 (13) | 42.4 (11.5) |
| LDL cholesterol (mg/dl) †,‡ | 107.5 (28.5) | 131.3 (34.3) | 111.3 (29.3) | 131.2 (31.5) |
| Glucose (mg/dl) | 82 (77 – 86) | 85 (79 – 90) | 97 (91 – 103) | 100 (95 – 108) |
| Current smoker‡ | 270 (25.2%) | 28 (45.2%) | 225 (39.5%) | 55 (59.1%) |
| Anti-hypertensive medications | 6 (0.6%) | 3 (4.8%) | 3 (0.5%) | 0 (0%) |
| Type 2 diabetes | 3 (0.3%) | 1 (1.6%) | 2 (0.4%) | 1 (1.1%) |
| Mother MI Prior to Age 60 or 55§ | 24 (2.2%) | 0 (0%) | 14 (2.5%) | 3 (3.2%) |
| Father MI Prior to Age 60 or 55§ | 111 (10.4%) | 11 (17.7%) | 65 (11.4%) | 11 (11.8%) |
Footnotes
For continuous variables, values shown are mean (standard deviation). For glucose and triglycerides, values are median (interquartile range).
Denotes a nominal association (p<0.05) between the PRS and the characteristic in CARDIA, tested by either a logistic or linear regression model adjusting for age, sex and 5 principal components.
Denotes a nominal association (p<0.05) in the Framingham Offspring cohort.
Age 60 for CARDIA and 55 for Framingham.
Associations of CHD PRS with continuous CAC
In CARDIA, the PRS was associated with higher log-transformed Agatston scores (increase=0.16 [95% CI, 0.10 – 0.2] per s.d. increase in PRS) at year 15 of follow-up (average age 47 [s.d. 4.6] years) (Figure 1A). Larger increases were observed in FOS (increase=0.57 [95% CI, 0.40 – 0.74] per s.d. increase in PRS) where CAC was measured at a later timepoint (average age 58.7 [s.d. 4.6] years) (Figure 1A). There was a significant interaction between age at CAC assessment and the PRS in CARDIA (p=0.02), indicating that higher PRS values are associated with larger CAC increases over time (Figure 1B and Supplemental Figure I). A significant interaction was not observed in Framingham (Figure 1C and Supplemental Figure I). In both cohorts, there was a significant interaction between LDL-C and age (p<0.05) (Figure 1B and 1C and Supplemental Figure I).
Figure 1: Change in Agatston scores associated with a CHD PRS.
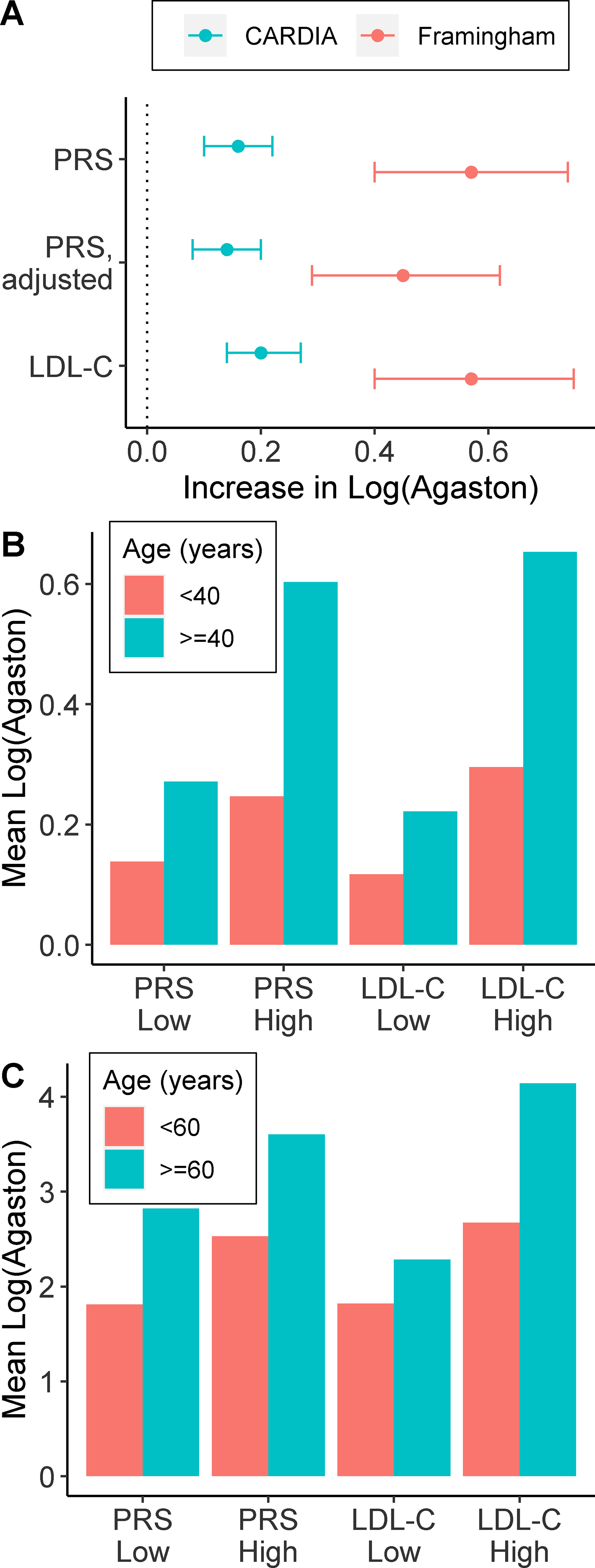
Shown is the difference (95% CI) in Agatston score per standard deviation increase in the PRS in the CARDIA and Framingham cohorts. Differences were determined by multivariable regression using Log(Agatston score+1) as the dependent variable and adjusting for age, sex and PCs. The PRS difference was also calculated after additionally adjusting for risk factors (baseline waist circumference [or BMI in Framingham], total cholesterol, HDL-C, log-transformed triglycerides, SBP and smoking status) (PRS, adjusted). Changes associated with LDL-C are shown for comparison. (B & C) Mean log(Agatston score +1) levels stratified by age (<40 or ≥40 years) and Low (bottom 50%) or high (top 50%) levels of the PRS or LDL-C in (B) CARDIA and (C) Framingham.
Associations of CHD PRS with CAC thresholds
In both cohorts, the odds-ratio point estimates for the association between the PRS and elevated CAC increased at more stringent CAC threshold values (Figure 2A and Supplemental Tables V and VI). In CARDIA, 62 (5%) participants had a CAC score >20 and, in Framingham, 93 (14%) had CAC >300 (Table 1). Approximately 80% of these subjects were male. The magnitude of the odds-ratio for the association between the PRS and CAC above these thresholds was comparable to those for other risk factors in each cohort (Figure 2B). After adjustment for risk factors, the odds-ratios per s.d. increase in the PRS were 1.74 (1.29 – 2.36) and 1.90 (1.42 – 2.54) in CARDIA (CAC >20) and FOS (CAC >300), respectively.
Figure 2: Association of the PRS and baseline characteristics with CAC.
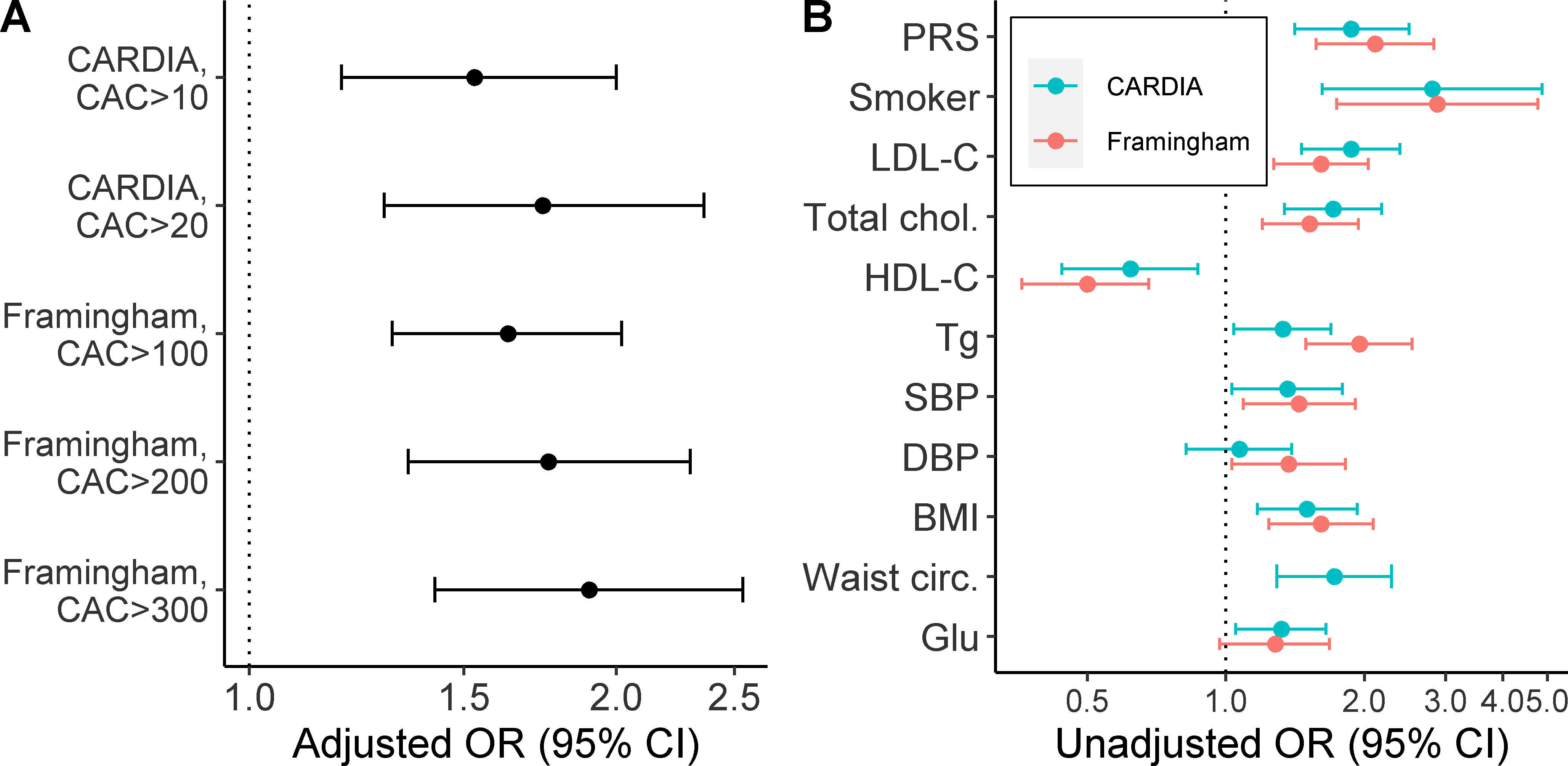
(A) Shown are the adjusted odds ratio (OR) for a CAC score above the indicated thresholds per standard deviation change in the PRS. Odd ratios were adjusted for risk factors (baseline waist circumference [or BMI in Framingham], total cholesterol, HDL-C, log-transformed triglycerides, SBP and smoking status), age, sex and PCs. (B) Association of the PRS and baseline risk factors with CAC scores>20 (CARDIA) or CAC scores>300 (Framingham). For continuous variables, odds ratios are per standard deviation change in the risk factor. All models were adjusted for age, sex and PCs.
Discrimination
Adding the PRS to a minimal model containing age, sex and ancestry (using principal components [PCs]) increased the C-statistic from 0.764 to 0.794 (difference=0.030 [0.007, 0.054]) in CARDIA and 0.759 to 0.804 (difference= 0.045 [0.010, 0.076]) in FOS (Table 2). By comparison, adding conventional risk factors (baseline waist circumference [or BMI in FOS], total cholesterol, HDL-C, triglycerides, SBP and smoking status) to the minimal model resulted in larger increases to the C-statistic in both CARDIA (0.764 to 0.849 (difference = 0.085 [0.036, 0.131]) and FOS (0.759 to 0.835 [difference= 0.076 [0.031, 0.110]]).
Table 2.
C-statistics from logistic regression models for associations with elevated CAC.
| Model | CARDIA (CAC>20) C-statistic (95% CI) | Framingham (CAC>300) C-statistic (95% CI) |
|---|---|---|
| Base* | 0.764 (0.692, 0.816) | 0.759 (0.702, 0.808) |
| Base* + PRS | 0.794 (0.728, 0.840) | 0.804 (0.751, 0.845) |
| Base* + risk factors† | 0.849 (0.789, 0.889) | 0.835 (0.781, 0.871) |
| Base* + risk factors† + PRS | 0.864 (0.807, 0.904) | 0.855 (0.805, 0.887) |
Footnotes
The base model comprised age, sex and 5 principal components.
The risk factors were SBP, total cholesterol, HDL-C, triglycerides, current smoker and either waist circumference (CARDIA) or BMI (Framingham).
In both cohorts, an increase in the C-statistic was observed after the addition of the PRS to the risk factor model (difference= 0.015 [0.004, 0.028] in CARDIA; difference= 0.02 [0.001, 0.039] in Framingham). The absolute increase to the full model was greater for risk factors than for the PRS (i.e. addition of risk factors to the PRS model resulted in larger increases to the C-statistic than adding the PRS to risk factor models). In CARDIA, these respective changes were 0.070 (0.033, 0.109) and 0.015 (0.004, 0.028), and in Framingham they were 0.051 (0.017, 0.079) and 0.020 (0.001, 0.039) (Table 2 and Figures 3A and 3B). Similar results were observed when using other CAC cut-off values (Figure 3D and 3C and Supplemental Tables V and VI).
Figure 3. Discrimination characteristics for the PRS and risk factors.
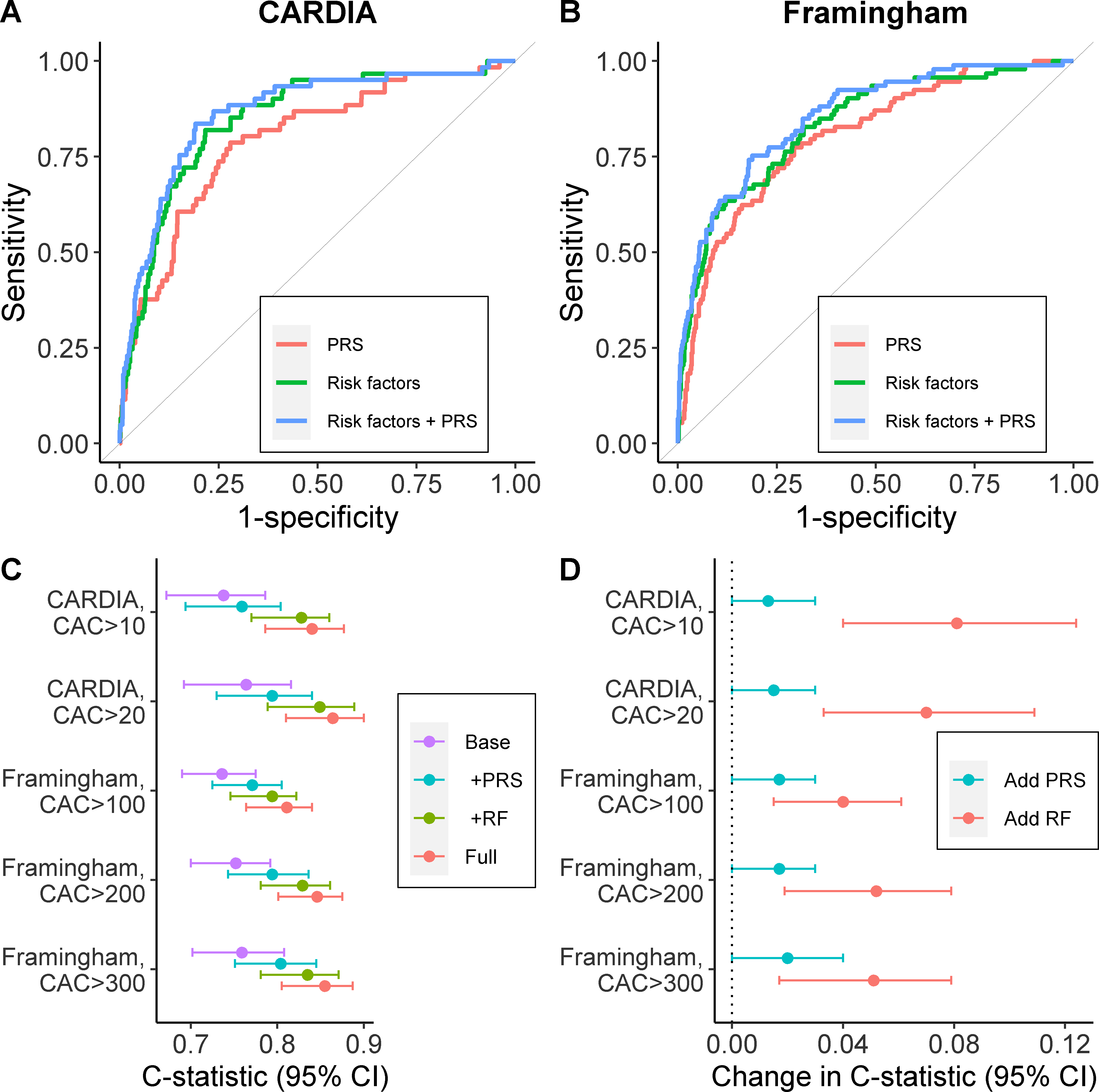
Receiver Operating Characteristic curves for risk models in (A) CARDIA for CAC scores>20 and (B) Framingham for CAC scores>300. Logistic regression models included the PRS, risk factors (baseline waist circumference [or BMI in Framingham], total cholesterol, HDL-C, log-transformed triglycerides, SBP and smoking status) or both. (C) C-statistics associated with logistic regression models adjusted for: age and sex (Base); Base + PRS (PRS); Base + risk factors (RF); and Base + PRS + risk factors (Full). Models using multiple CAC score cut-offs are shown. 95% CI were computed by bootstrapping. D) Change in C-statistics when: the PRS was added to logistic model with age, sex and risk factors; the risk factors were added to a model that included age and the PRS.
In CARDIA, all risk models demonstrated a good fit to the data (slope=1.0) (Supplemental Figure II). In FOS, a minimal model that further included only the PRS demonstrated a poorer fit than models that also included risk factors (slope=1.1 vs 1.0) (Supplemental Figure III).
Reclassification, as measured continuously by the IDI, was not significantly improved using the full model compared to models incorporating either risks factors or PRS. For example, when the PRS was added to the risk factor model (difference in IDI=0.027 [−0.006, 0.054] or when risk factors were added to the PRS model (difference in IDI=0.070 [−0.005, 0.111]) in CARDIA. In FOS, there were significant improvements in both instances: 0.039 (0.0005, 0.072) and 0.111 (0.036, 0.158), respectively. Similar results were seen at lower CAC cut-offs, except that the IDI was significant in CARDIA when risk factors were added to the PRS model at CAC>10 (IDI=0.070 [0.0001, 0.106], but not vice versa (IDI=0.016 [−0.009, 0.0373]) (Supplemental Table V). Overall, changes in predicted risk estimates were modest when the PRS was added to the risk factor models in both cohorts (Figure 4).
Figure 4. Scatterplot of predicted probabilities for prediction models in CAC in CARDIA and Framingham.
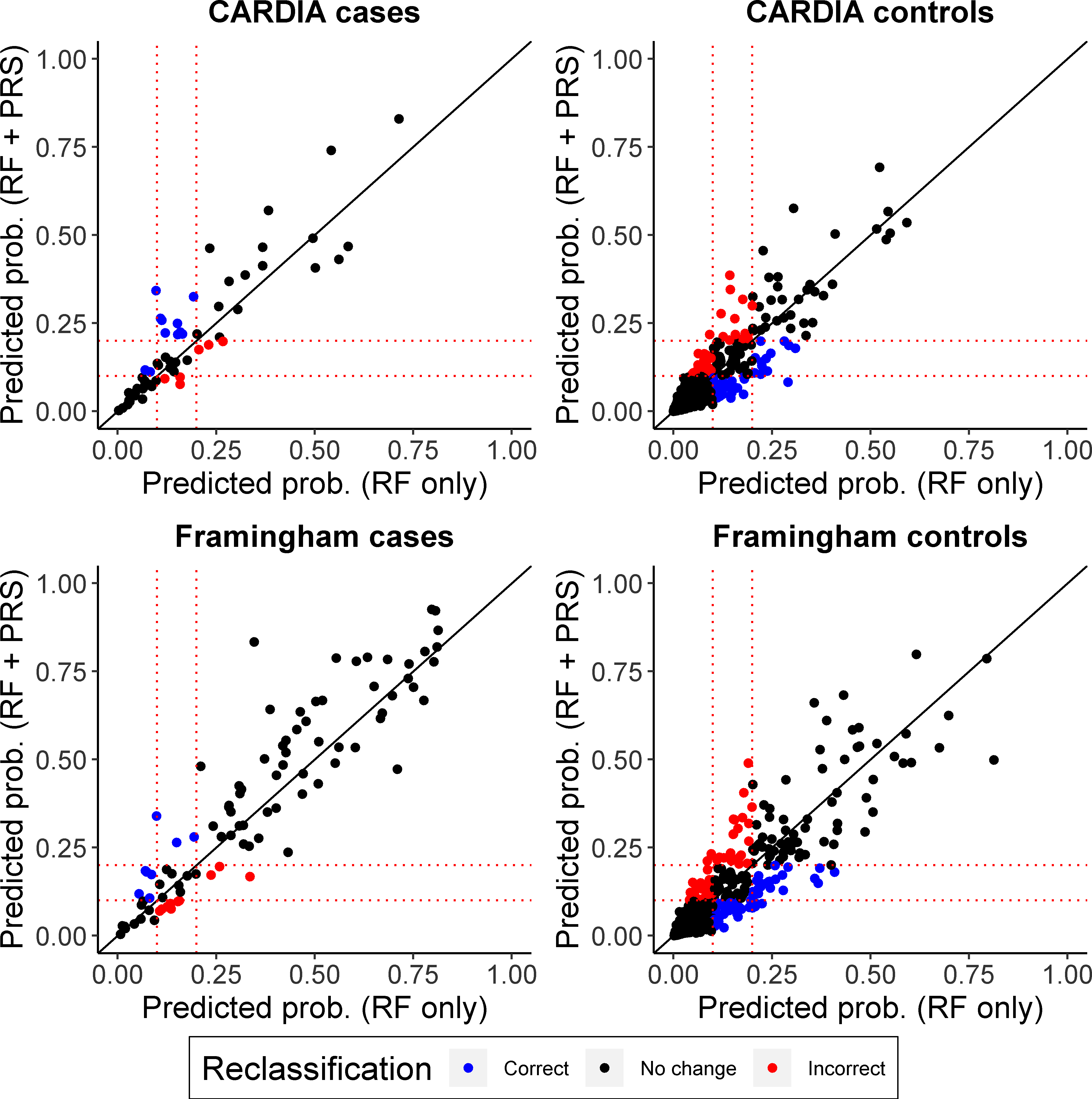
Predicted probabilities are based on logistic regression models for CAC>20 (CARDIA) or CAC>300 (Framingham) that include either: risk factors (baseline waist circumference [or BMI in Framingham], total cholesterol, HDL-C, log-transformed triglycerides, SBP and smoking status) (RF); or risk factors + PRS. All models were adjusted for age, sex and PCs. Predicted probability scatterplots are shown separately for Framingham (A) cases and (B) controls and CARDIA (C) cases and (D) controls. Blue and red dots highlight participants for whom addition of the PRS causes their probability estimates to cross the 10% or 20% probability thresholds (shown by dotted lines). Blue dots highlight individuals whose probability is correctly reclassified (i.e. is higher for cases or lower for controls) across the thresholds and red dots highlight incorrect reclassifications across the thresholds.
PRS thresholds
It has been proposed that young adults whose PRS value falls within the upper tail of the CHD PRS distribution be identified as high risk.21 The point estimates for performance characteristics of such a PRS-based classifier at different risk thresholds is shown in Table 3. For instance, among CARDIA participants whose PRS value fell within the top 10% of the distribution, 11% had CAC>20, which accounted for 19% of all individuals with CAC>20. By comparison, among participants whose measured LDL-C fell within the top 10% of the distribution, 15% had CAC>20, and this accounted for 29% of all individuals with CAC>20. Similar results are seen in the FOS (Table 3).
Table 3.
Performance features of a PRS or measured LDL-C risk classifier.
| Classifier | Top risk percentile* | CARDIA Sensitivity for CAC>20 (%)† | CARDIA Positive predictive value for CAC>20 (%)‡ | Framingham Sensitivity for CAC>300 (%)† | Framingham Positive predictive value for CAC>300 (%)‡ |
|---|---|---|---|---|---|
| PRS | |||||
| 5 | 13 | 14 | 13 | 35 | |
| 10 | 19 | 11 | 17 | 24 | |
| 15 | 27 | 10 | 25 | 23 | |
| 20 | 37 | 10 | 30 | 21 | |
| LDL-C | |||||
| 5 | 19 | 20 | 14 | 38 | |
| 10 | 29 | 15 | 24 | 33 | |
| 15 | 39 | 14 | 30 | 28 | |
| 20 | 45 | 12 | 39 | 27 |
Footnotes
The percentile threshold used to select high risk individuals based on the upper tails of the distribution for either the PRS or LDL-C.
Proportion of individuals with CAC>cut-off whose value above the risk threshold.
Proportion of individuals classified as high risk who have CAC>cut-off.
DISCUSSION
Consistent with observations from prior studies, higher CHD PRS scores were associated with increased burden of coronary atherosclerosis, as measured by CAC scoring, in early and later adulthood.16–19 Addition of the PRS to a model comprising modifiable CHD risk factors improved discrimination for CAC elevations across a range of thresholds, comparable to changes in studies that have examined CHD as the primary outcome.25 The magnitude of these improvements was less than those seen when adding modifiable risk factors to a PRS-based model. Collectively, these results suggest that a PRS-based risk stratification approach in a young adult population would not be superior to a risk-factor based strategy, but addition of the PRS to a risk factor-based risk model may modestly improve risk stratification in young adults.
Recent studies have shown that a PRS may offer only modest improvements in CHD risk stratification among Whites over 40 years old, as compared to measuring traditional cardiovascular risk factors including smoking, blood pressure, cholesterol and diabetes.4,5 Thus, by age 40, risk may be better characterized by direct assessment of modifiable risk factors, which has the secondary benefit of providing specific targets for risk reduction. An important question is whether the performance of a PRS would be significantly improved if implemented prior to age 40.
Our results extend the current literature by evaluating the performance of a PRS in younger adults. Whether a PRS-based or PRS-augmented strategy would improve risk stratification in this group has been of considerable interest, in part due to the poor performance of current risk models in young populations and the potential to target high-risk individuals for preventive therapies. One challenge of evaluating CHD risk prediction in younger populations is the overall low event rate. Accordingly, we examined CAC, a well validated marker of early coronary atherosclerosis, as a surrogate outcome. Thus, while our analysis does not directly address the ability of PRS to predict hard CHD events in young individuals, the use of a validated biomarker of atherosclerosis and CHD risk provides important insight into an otherwise difficult to study phenotype.
We observed that among young adults, a model incorporating risk factors demonstrated better discrimination (measured by C-statistic) with respect to the presence of CAC in mid-life compared to a model incorporating PRS. Moreover, the magnitude of the improvements in the C-statistic (0.015 to 0.02) when a PRS was added to risk factors were modest and comparable to those that have been reported when CHD is the outcome in older adults.6,25–27 Overall, a change in C-statistic of 0.02 is small though, in large data sets, this change may be associated with a very low p-values. Changes of this magnitude are seen with other validated CHD risk factors not used in clinical practice, such as B-type natriuretic peptide and high-sensitivity C-reactive protein levels.28,29 These results suggest that the discriminative performance of a PRS may not be better among young adults than in middle-aged adults. Furthermore, risk stratification based on modifiable risk factors is apt to perform better in this population than a stand-alone PRS-based predictor.
One proposed clinical implementation strategy for a PRS is to identify individuals whose PRS value falls within the high-risk tail of the distribution, as these individuals are at increased relative risk of disease compared to those with lower PRS scores.30,31 This is similar to what is done for disease mediators such LDL-C and systolic blood pressure. One reason a PRS may not be optimally suited for this purpose is that it is a calculated value that partially captures an individual’s genetic potential to manifest risk. Its value is constant and does not modulate when risk has been attenuated. This can lead to misclassification for individuals who have attenuated their genetic risk by optimizing risk factors through lifestyle measures or other approaches.18
An important consideration when identifying young adults at elevated polygenic risk for CHD is what prevention strategies should be offered, especially in the absence of levels of traditional risk factors currently regarded as warranting intervention. Retrospective studies have shown that adherence to a healthy lifestyle is associated with larger risk reductions among individuals at elevated polygenic risk.18 Lifestyle interventions are safe to implement, so targeting high polygenic risk individuals to lifestyle interventions is appropriate. Whether high genetic risk populations sustain healthy behavioral changes is not clear.32,33 However, low-risk interventions (smoking cessation, healthy lifestyle behaviors) benefit all individuals, regardless of genetic risk, and would have a greater epidemiological impact if targeted broadly.18 Furthermore, the value of knowing PRS risk with respect to motivating cardiovascular risk modification or behavioral changes is not well-established.33–35
Re-analyses of statin treatment trials have shown that individuals in the top 20% of the PRS distribution have larger decreases in absolute risk of incident CHD associated with statin therapy, as compared to the lower PRS percentiles.17 For instance, in a re-analysis of the JUPITER trial, which randomized individuals with LCL-C<130 mg/dl, there was a nonsignificant 32% risk reduction associated with rosuvastatin in the lower genetic risk group versus a nonsignificant 59% risk reduction in the higher genetic risk (top 20%) group.36 Based on these observations, some have suggested treating individuals with high PRS scores with lipid-lowering statin medications even in the absence of conventional risk factors. However, it is notable that in the re-analysis of the JUPITER trial statin treatment actually prevented larger numbers of events in the lower genetic risk groups, which represented the majority of the study participants. This would suggest that ubiquitous statin therapy would have been a more effective primary prevention strategy than a PRS-based strategy in that patient population. At present, it is unknown whether statins meaningfully reduce CHD events among young individuals with high PRS scores, and whether treatment benefits outweigh risks (such as increased risk of type 2 diabetes). Determining treatment efficacy would be important, as implementing statin therapy at a young age will result in individuals taking medications for up to half a century or more, with associated increased costs and adverse drug events.
In our analyses, the PRS was associated with some baseline measures including LDL-C and smoking, indicating that a portion of its predictive ability derives from capturing variation in these risk factors. Consistently, the association with CAC was attenuated when adjusting for these risk factors, confirming the shared risk mechanisms. However, it is important to highlight that the PRS remained strongly associated with higher CAC scores, even after adjusting for risk factors. This indicates that the PRS is capturing risk mechanisms not measured by traditional risk factors. These additional mechanisms must be identified so that biomarkers that capture the fuller genetic and environmental influences modulating them can be measured in order to further improve risk stratification and potentially identify new therapeutic targets.
Some limitations should also be acknowledged. The study populations were relatively small, which decreased the precision of performance estimates for the PRS and can lead to wide confidence intervals. However, the performance estimates reported here were consistent across cohorts and are similar to those observed in other studies of CHD.3,37 The FOS also contributed cases and controls to the CARDIOGRAM-C4D GWAS, which could lead to overestimation of the performance of the PRS in that cohort. As would be expected in a young population, there were low numbers of hard CHD events which prohibited an examination of that end-point. While CAC is a measure of coronary atherosclerosis and is associated with elevated CHD risk, these results may not inform how well the PRS predicts early onset CHD. There were also few women with marked CAC score elevations, which limits inferences about the PRS in this group. There were modest differences among the excluded subjects in CARDIA who did not have CAC measurements, which may have biased these findings. In CARDIA, analyses were not adjusted for field center, which may have biased results. All participants included in this analysis were of white European ancestry, and it is unknown whether findings would be similar in other ancestral groups.
In summary, a CHD PRS improved discrimination for coronary atherosclerosis beyond traditional cardiovascular risk measures. These improvements were smaller than the collective effects of modifiable risk factors. These results suggest that PRS-based risk stratification approach in a young adult population would be not be superior to a risk-factor based strategy, but addition of the PRS to a risk factor-based predictor may improve risk stratification. The clinical and translational utility of inclusion of a PRS for CHD risk stratification among young adults remains undefined.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the CARDIA and Framingham studies for their contributions.
FUNDING
This work was supported by funding from the American Heart Association 16FTF30130005 and R01 GM130791 (JDM); R01 HL140074R35 (QSW); K12 HL133117 (AWA); R35 GM131770 (CMS); U01 HG8672 and P50 GM115305 (DMR). Framingham Heart Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (Contract No. N01-HC-25195, HHSN268201500001I and 75N92019D00031). This manuscript was not prepared in collaboration with investigators of the Framingham Heart Study and does not necessarily reflect the opinions or views of the Framingham Heart Study, Boston University, or NHLBI.
Nonstandard Abbreviations and Acronyms
- PRS
Polygenic risk scores
- CHD
Coronary heart disease
- CAC
Coronary artery calcium
- CARDIA
Coronary Artery Risk Development in Young Adults
- FOS
Framingham Offspring Study
- PCs
Principal components
- IDI
Integrated discrimination improvement
Footnotes
Disclosures: The Authors declare no competing interests.
Supplemental Materials:
REFERENCES
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT, Kathiresan S. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inouye M, Abraham G, Nelson CP, Wood AM, Sweeting MJ, Dudbridge F, Lai FY, Kaptoge S, Brozynska M, Wang T, Ye S, Webb TR, Rutter MK, Tzoulaki I, Patel RS, Loos RJF, Keavney B, Hemingway H, Thompson J, Watkins H, Deloukas P, Di Angelantonio E, Butterworth AS, Danesh J, Samani NJ, UK Biobank CardioMetabolic Consortium CHD Working Group. Genomic Risk Prediction of Coronary Artery Disease in 480,000 Adults: Implications for Primary Prevention. J Am Coll Cardiol. 2018;72:1883–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosley JD, Gupta DK, Tan J, Yao J, Wells QS, Shaffer CM, Kundu S, Robinson-Cohen C, Psaty BM, Rich SS, Post WS, Guo X, Rotter JI, Roden DM, Gerszten RE, Wang TJ. Predictive Accuracy of a Polygenic Risk Score Compared With a Clinical Risk Score for Incident Coronary Heart Disease. JAMA. 2020;323:627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott J, Bodinier B, Bond TA, Chadeau-Hyam M, Evangelou E, Moons KGM, Dehghan A, Muller DC, Elliott P, Tzoulaki I. Predictive Accuracy of a Polygenic Risk Score-Enhanced Prediction Model vs a Clinical Risk Score for Coronary Artery Disease. JAMA. 2020;323:636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mars N, Koskela JT, Ripatti P, Kiiskinen TTJ, Havulinna AS, Lindbohm JV, Ahola-Olli A, Kurki M, Karjalainen J, Palta P, FinnGen, Neale BM, Daly M, Salomaa V, Palotie A, Widén E, Ripatti S. Polygenic and clinical risk scores and their impact on age at onset and prediction of cardiometabolic diseases and common cancers. Nat Med. 2020; [DOI] [PubMed] [Google Scholar]
- 7.Wray NR, Goddard ME, Visscher PM. Prediction of individual genetic risk to disease from genome-wide association studies. Genome Res. 2007;17:1520–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet. 2018;19:581–590. [DOI] [PubMed] [Google Scholar]
- 9.Shah N, Kelly A-M, Cox N, Wong C, Soon K. Myocardial Infarction in the “Young”: Risk Factors, Presentation, Management and Prognosis. Heart Lung Circ. 2016;25:955–960. [DOI] [PubMed] [Google Scholar]
- 10.Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, Williams OD, Bild DE, Detrano R. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2007;49:2013–2020. [DOI] [PubMed] [Google Scholar]
- 11.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. [DOI] [PubMed] [Google Scholar]
- 12.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 13.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr JJ, Jacobs DR, Terry JG, Shay CM, Sidney S, Liu K, Schreiner PJ, Lewis CE, Shikany JM, Reis JP, Goff DC. Association of Coronary Artery Calcium in Adults Aged 32 to 46 Years With Incident Coronary Heart Disease and Death. JAMA Cardiol. 2017;2:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poon M, Lesser JR, Biga C, Blankstein R, Kramer CM, Min JK, Noack PS, Farrow C, Hoffman U, Murillo J, Nieman K, Shaw LJ. Current Evidence and Recommendations for Coronary CTA First in Evaluation of Stable Coronary Artery Disease. J Am Coll Cardiol. 2020;76:1358–1362. [DOI] [PubMed] [Google Scholar]
- 16.Thanassoulis G, Peloso GM, Pencina MJ, Hoffmann U, Fox CS, Cupples LA, Levy D, D’Agostino RB, Hwang S-J, O’Donnell CJ. A genetic risk score is associated with incident cardiovascular disease and coronary artery calcium: the Framingham Heart Study. Circ Cardiovasc Genet. 2012;5:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natarajan P, Young R, Stitziel NO, Padmanabhan S, Baber U, Mehran R, Sartori S, Fuster V, Reilly DF, Butterworth A, Rader DJ, Ford I, Sattar N, Kathiresan S. Polygenic Risk Score Identifies Subgroup With Higher Burden of Atherosclerosis and Greater Relative Benefit From Statin Therapy in the Primary Prevention Setting. Circulation. 2017;135:2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, Chasman DI, Baber U, Mehran R, Rader DJ, Fuster V, Boerwinkle E, Melander O, Orho-Melander M, Ridker PM, Kathiresan S. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. N Engl J Med. 2016;375:2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Severance LM, Contijoch FJ, Carter H, Fan CC, Seibert TM, Dale AM, McVeigh ER. Using a genetic risk score to calculate the optimal age for an individual to undergo coronary artery calcium screening. J Cardiovasc Comput Tomogr. 2019;13:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Severance LM, Carter H, Contijoch FJ, McVeigh ER. Targeted Coronary Artery Calcium Screening in High-Risk Younger Individuals Using Consumer Genetic Screening Results. JACC Cardiovasc Imaging. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aragam KG, Dobbyn A, Judy R, Chaffin M, Chaudhary K, Hindy G, Cagan A, Finneran P, Weng L-C, Loos RJF, Nadkarni G, Cho JH, Kember RL, Baras A, Reid J, Overton J, Philippakis A, Ellinor PT, Weiss ST, Rader DJ, Lubitz SA, Smoller JW, Karlson EW, Khera AV, Kathiresan S, Do R, Damrauer SM, Natarajan P. Limitations of Contemporary Guidelines for Managing Patients at High Genetic Risk of Coronary Artery Disease. J Am Coll Cardiol. 2020;75:2769–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 23.Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, Jacobs DR, Liu K, Orden S, Pirie P. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials. 1987;8:68S–73S. [DOI] [PubMed] [Google Scholar]
- 24.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. [DOI] [PubMed] [Google Scholar]
- 25.Hindy G, Aragam KG, Ng K, Chaffin M, Lotta LA, Baras A, Regeneron Genetics Center, Drake I, Orho-Melander M, Melander O, Kathiresan S, Khera AV. Genome-Wide Polygenic Score, Clinical Risk Factors, and Long-Term Trajectories of Coronary Artery Disease. Arterioscler Thromb Vasc Biol. 2020;40:2738–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun L, Pennells L, Kaptoge S, Nelson CP, Ritchie SC, Abraham G, Arnold M, Bell S, Bolton T, Burgess S, Dudbridge F, Guo Q, Sofianopoulou E, Stevens D, Thompson JR, Butterworth AS, Wood A, Danesh J, Samani NJ, Inouye M, Di Angelantonio E. Polygenic risk scores in cardiovascular risk prediction: A cohort study and modelling analyses. PLoS Med. 2021;18:e1003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isgut M, Sun J, Quyyumi AA, Gibson G. Highly elevated polygenic risk scores are better predictors of myocardial infarction risk early in life than later. Genome Med. 2021;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837–842. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D’Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. [DOI] [PubMed] [Google Scholar]
- 30.Khera AV, Chaffin M, Wade KH, Zahid S, Brancale J, Xia R, Distefano M, Senol-Cosar O, Haas ME, Bick A, Aragam KG, Lander ES, Smith GD, Mason-Suares H, Fornage M, Lebo M, Timpson NJ, Kaplan LM, Kathiresan S. Polygenic Prediction of Weight and Obesity Trajectories from Birth to Adulthood. Cell. 2019;177:587–596.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham G, Malik R, Yonova-Doing E, Salim A, Wang T, Danesh J, Butterworth AS, Howson JMM, Inouye M, Dichgans M. Genomic risk score offers predictive performance comparable to clinical risk factors for ischaemic stroke. Nat Commun. 2019;10:5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollands GJ, French DP, Griffin SJ, Prevost AT, Sutton S, King S, Marteau TM. The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ. 2016;352:i1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kullo IJ, Jouni H, Austin EE, Brown S-A, Kruisselbrink TM, Isseh IN, Haddad RA, Marroush TS, Shameer K, Olson JE, Broeckel U, Green RC, Schaid DJ, Montori VM, Bailey KR. Incorporating a Genetic Risk Score Into Coronary Heart Disease Risk Estimates: Effect on Low-Density Lipoprotein Cholesterol Levels (the MI-GENES Clinical Trial). Circulation. 2016;133:1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knowles JW, Zarafshar S, Pavlovic A, Goldstein BA, Tsai S, Li J, McConnell MV, Absher D, Ashley EA, Kiernan M, Ioannidis JPA, Assimes TL. Impact of a Genetic Risk Score for Coronary Artery Disease on Reducing Cardiovascular Risk: A Pilot Randomized Controlled Study. Front Cardiovasc Med. 2017;4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Läll K, Mägi R, Morris A, Metspalu A, Fischer K. Personalized risk prediction for type 2 diabetes: the potential of genetic risk scores. Genet Med. 2017;19:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mega JL, Stitziel NO, Smith JG, Chasman DI, Caulfield M, Devlin JJ, Nordio F, Hyde C, Cannon CP, Sacks F, Poulter N, Sever P, Ridker PM, Braunwald E, Melander O, Kathiresan S, Sabatine MS. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385:2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wünnemann F, Lo KS, Langford-Alevar A, Busseuil D, Dubé M-P, Tardif J-C, Lettre G. Validation of Genome-wide Polygenic Risk Scores for Coronary Artery Disease in French Canadians. Circ Genom Precis Med. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 39.Zheng X, Levine D, Shen J, Gogarten SM, Laurie C, Weir BS. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics. 2012;28:3326–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vilhjálmsson BJ, Yang J, Finucane HK, Gusev A, Lindström S, Ripke S, Genovese G, Loh P-R, Bhatia G, Do R, Hayeck T, Won H-H, Schizophrenia Working Group of the Psychiatric Genomics Consortium, Discovery, Biology, and Risk of Inherited Variants in Breast Cancer (DRIVE) study, Kathiresan S, Pato M, Pato C, Tamimi R, Stahl E, Zaitlen N, Pasaniuc B, Belbin G, Kenny EE, Schierup MH, De Jager P, Patsopoulos NA, McCarroll S, Daly M, Purcell S, Chasman D, Neale B, Goddard M, Visscher PM, Kraft P, Patterson N, Price AL. Modeling Linkage Disequilibrium Increases Accuracy of Polygenic Risk Scores. Am J Hum Genet. 2015;97:576–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol. 2008;102:1136–1141, 1141.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


