Abstract
Introduction:
Weight loss and increased physical activity interventions are commonly recommended for individuals with type 2 diabetes (T2D) and overweight or obesity. We examined the impact of randomization to an intensive lifestyle intervention (ILI) on trajectories of cognitive function over 10 years in a cohort of participants in a randomized clinical trial who had T2D and overweight/obesity at baseline.
Methods:
Participants aged 45–76 were enrolled in 2001–2004 and were randomized to the ILI or a diabetes support and education (DSE) condition. Cognitive function was assessed in 3,938 participants at up to four time points 8–18 years after randomization. General linear mixed effects models examined cognitive trajectories over time. Subgroup analyses focused on sex, individuals with baseline BMI>30, those carrying the APOE ε4 allele, and those with a baseline history of cardiovascular disease.
Results:
Overall, there were no differences in the rate of cognitive decline by intervention arm. Subgroup analyses showed that participants who had a baseline history of CVD and were randomized to the ILI arm of the study performed significantly worse on the Stroop Color Word test compared to those in the DSE arm.
Discussion/Conclusions:
The ILI did not result in preserved cognitive function or slower rates of cognitive decline in this cohort of individuals who had T2D and were overweight or obese at baseline.
Keywords: cognitive decline, executive function, type 2 diabetes, overweight, obesity, clinical trial
Introduction
Preservation of brain health is a critical goal in the care of adults, including those with type 2 diabetes (T2D), who are at increased risk for cerebrovascular disease, brain atrophy, and cognitive impairment.[1–5] Cognitive function is also critical to diabetes care as it correlates with diabetes control.[6] People with overweight/obesity, particularly in mid-life, are at increased risk for cognitive impairment and dementia and may experience cognitive benefits from weight loss.[7] For people with T2D and overweight/obesity, weight loss and increased physical activity are commonly held as effective strategies to maintain cognitive function, but are currently unproven in any population. It is important to understand the role of lifestyle intervention for the treatment of older adults with T2D and obesity because together, these diseases nearly double one’s risk for Alzheimer’s disease and related dementias.[8–10]
Previously, Look AHEAD results suggested that the association of an intensive lifestyle intervention (ILI) with cognitive impairment is heterogeneous, depending on individuals baseline weight and/or cardiovascular disease (CVD) history; however, statistical power was limited.[11–13] Look AHEAD also reported that among those with a higher body mass index (BMI), and among those reporting a history of CVD at baseline, the ILI appeared to increase the odds of cognitive impairment, again with limited power.[13]
The Look AHEAD-MIND ancillary study was designed to determine whether interventions to induce and sustain long-term weight losses lead to cognitive benefit in some, or harm in others, and to determine the roles of baseline weight and history of CVD in modifying this association. Herein, cognitive data collected during Look AHEAD-MIND, and earlier ancillary studies: Look AHEAD Physical and Cognitive Function,[14] Look AHEAD Movement & Memory,[15] Look AHEAD Brain,[16] and Look AHEAD-Continuation,[13] are used to assess the legacy of the Look AHEAD interventions on cognitive trajectories. Our objective was to determine whether effects vary across baseline BMI groups and baseline CVD history using these additional data. We also examine potential differences by subgroups including age, APOE ε4 carrier status, and sex.
Materials and Methods
The Look AHEAD study design,[17] and CONSORT diagram[18] were published previously. Briefly, Look AHEAD was a multi-center, randomized controlled clinical trial of 5,145 participants aged 45–76, from 16 clinical centers across the US, enrolled from 2001 to 2004. Trial eligibility required that participants have a BMI>25 kg/m2 (>27 kg/m2 if on insulin), glycated hemoglobin (HbA1c) < 11%, systolic/diastolic blood pressure <160/100 mmHg, and triglycerides level <600 mg/dl. Participants were required to demonstrate over a two-week run-in period, their ability to record daily their diet and physical activity. Each participant met with a behavioral psychologist or interventionist to confirm that the requirements of the intervention were understood and that participants did not have any competing life stressors that would potentially impair adherence to the protocol. Local Institutional Review Boards approved the protocols and all participants provided written informed consent.
Participants were randomly assigned to either an Intensive Lifestyle Intervention (ILI) or a Diabetes Support and Education (DSE) condition. The ILI multidomain intervention included dietary modification and physical activity; with a goal of an average of at least 7% weight loss at one year and maintenance over the course of the study.[19] ILI participants had a daily calorie goal of 1200 to 1800 kcal/day based on initial weight. The diet specified <30% total calories from fat (<10% saturated fat) and a minimum of 15% total calories from protein. The physical activity goal was similar in intensity to brisk walking for at least 175 minutes/week. The DSE condition included inviting, but not requiring, participants to attend three group sessions/year. These sessions focused on diet, physical activity, and social support.[20] There was no specific instruction for diet modification, physical activity, or weight goals. The intervention was terminated in September 2012. At this time, all surviving participants were invited to join a follow-up observational study to determine the longer-term effects of the intervention on outcomes. The average length of the intervention for participants included in this study was 9.8 years (range 8.3–11.1 years). There was no baseline cognitive assessment.
Cognitive Function:
Cognitive assessments were conducted between August 2009 and February 2020 for various ancillary studies. These include: Look AHEAD Physical and Cognitive Function (Aug 2009-Jun 2012, n= 977); Look AHEAD M&M/Brain (Nov 2011-Aug 2013, n=601); and Look AHEAD-C (Aug 2013-Dec 2014; n=3,750). The same cognitive protocol was instituted in Look AHEAD-MIND (May 2018-Feb 2020, n=2451), for a total of 4 possible assessments which varied in the numbers of participants who completed each. For each of these cognitive assessments, staff were centrally trained and certified in administration of the standardized assessments and they were masked to participant’s randomization status.[13] The assessments comprised the Rey Auditory Verbal Learning Test (RAVLT),[21] Digit Symbol Coding (DSC),[22] the Stroop Color and Word Test (Stroop),[23] and the Trail Making Test Parts A and B (Trails A, Trails B).[24] The Modified Mini Mental Status Exam (3MS)[25] was used to assess global cognitive function. Participants scoring below pre-specified age and education-specific cut points triggered administration of the Functional Assessment Questionnaire (FAQ) to a proxy informant previously identified by the participant. The FAQ assesses functional status and performance on instrumental activities of daily living.[26]
Independent variables:
Data were collected by certified, trained staff who were blinded to intervention assignments[17] including demographic characteristics (age, sex, race/ethnicity, education), smoking, and medical histories. Depression symptoms were assessed with the Beck Depression Inventory. Prescription medications were documented and hypertension status was based on current medication use and/or measured blood pressure. CVD history included participant self-reported myocardial infarction, heart bypass surgery, coronary artery bypass graft, carotid endarterectomy, lower leg angioplasty, aortic aneurysm, congestive heart failure, or stroke. Blood samples for HbA1c were analyzed centrally. APOE ε4 was determined for participants who consented using TaqMan genotyping (rs7412 and rs429358). Height was measured at baseline and weight was measured with digital scales throughout follow-up. BMI (kg/m2) was calculated at baseline and annually.
Analytic Design
Participants who completed any cognitive assessment were eligible for inclusion. Cognitive assessments were grouped according to time frame as the cognitive protocols for the ancillary studies were identical and all were observational (i.e., they did not involve any interventions). The four periods included years from randomization: 8–9, 10–11, 12–14, and 15–18. Those without cognitive assessments or missing primary covariates were excluded. We compared participants who were included versus excluded, and we compared included participants across intervention arms on baseline demographic and health characteristics. Chi-square tests were used for categorical variables and t-tests were used for continuous variables. Cognitive scores were standardized (z-scores) by ordering them so that positive scores reflect better performance and then subtracting scores from the overall cohort-wide mean of the initial assessments and dividing by their standard deviation (SD).[11] The primary cognitive measure was a composite average of these scores.[27] Mixed-effects models were fitted using restricted maximum likelihood to longitudinal scores. Inferences and confidence intervals were based on average scores over repeat assessments, and separately on their slope over time since randomization, with covariate adjustment for fixed effects including time from randomization, baseline age, baseline BMI, sex, education, race/ethnicity, clinic site, and repetition (i.e, whether the first, second, third, or fourth test administration). Using data from baseline (to maintain randomization) and cognitive assessments from post-randomization years 8–10, 10–11, 12–14, and 15–18, we evaluated whether the intervention groups were characterized by different trajectories. Based on prior findings, we evaluated whether there were any differences between participants by baseline history of CVD, sex, BMI, and APOE ε4. We also evaluated potential differences based on whether participants baseline age was age 65 or older vs. under age 65. Inverse probability weighting was used to evaluate the impact of potential bias due to differential attrition.
Results
Of 5,145 randomized participants, a total of 3,938 contributed cognitive data and were included in this analysis. The cohort was roughly equally distributed by intervention arm (DSE=49.6%; ILI=50.4%). At baseline, the mean age was 58 years, 61% were female, and the racial/ethnic distribution was 62% White, 17% African American, 5% American Indian, 1% Asian/Pacific Islander, 13% Hispanic, and 2% other. Baseline demographic, health, and lifestyle indicators (Table 1) did not differ between intervention arms with the exception of baseline systolic blood pressure (SBP), which was slightly higher on average in the DSE group (p<0.001). The ILI was successful in inducing and maintaining greater weight losses from baseline throughout follow-up (Fig. 1).
Table 1.
Baseline Characteristics of Participants by Randomization Group
| Characteristic | DSE (n=1954) | ILI (n=1984) | P-value |
|---|---|---|---|
| Age, mean ± SD, years | 58.3 ± 6.7 | 58.0 ± 6.5 | 0.180 |
| Age Category (years), No. (%) | 0.376 | ||
| 45 – 55 | 646 (33.1%) | 673 (33.9%) | |
| 56 – 65 | 1019 (52.1%) | 1048 (52.8%) | |
| 66 – 76 | 289 (14.8%) | 263 (13.3%) | |
| Sex, No. (%) | 0.277 | ||
| Male | 745 (38.1%) | 790 (39.8%) | |
| Female | 1209 (61.9%) | 1194 (60.2%) | |
| Race, No. (%) | 0.859 | ||
| African American / Black (not Hispanic) | 331 (16.9%) | 319 (16.1%) | |
| American Indian / Native American / Alaskan Native | 107 ( 5.5%) | 108 ( 5.4%) | |
| Asian/Pacific Islander | 16 ( 0.8%) | 23 ( 1.2%) | |
| White | 1208 (61.8%) | 1225 (61.7%) | |
| Hispanic | 250 (12.8%) | 267 (13.5%) | |
| Other/Mixed | 42 ( 2.1%) | 42 ( 2.1%) | |
| Years of Education, No. (%) | 0.187 | ||
| < 13 years | 382 (20.1%) | 388 (20.0%) | |
| 13 – 16 years | 748 (39.4%) | 716 (36.9%) | |
| > 16 years | 768 (40.5%) | 839 (43.2%) | |
| Body Mass Index, mean ± SD, kg/m2 | 36.0 ± 5.8 | 35.7 ± 5.9 | 0.118 |
| SBP, mean ± SD, mmHg | 129.1 ± 16.7 | 127.1 ± 16.9 | <0.001 |
| DBP, mean ± SD, mmHg | 70.3 ± 9.5 | 69.9 ± 9.5 | 0.157 |
| Baseline Smoking, No. (%) | 0.497 | ||
| Never | 1023 (52.4%) | 1010 (51.0%) | |
| Past | 852 (43.7%) | 882 (44.5%) | |
| Present | 76 ( 3.9%) | 89 ( 4.5%) | |
| Diabetes Duration (years), No. (%) | 0.522 | ||
| < 5 years | 898 (46.3%) | 932 (47.3%) | |
| 5+ years | 1041 (53.7%) | 1037 (52.7%) | |
| Baseline Insulin Use, No. (%) | 0.963 | ||
| No | 1592 (84.7%) | 1624 (84.7%) | |
| Yes | 287 (15.3%) | 294 (15.3%) | |
| Baseline Hypertension, No. (%) | 0.445 | ||
| No | 355 (18.2%) | 342 (17.2%) | |
| Yes | 1599 (81.8%) | 1642 (82.8%) | |
| Baseline CVD History, No. (%) | 0.393 | ||
| No | 1729 (88.5%) | 1738 (87.6%) | |
| Yes | 225 (11.5%) | 246 (12.4%) | |
| BDI>24 Severe Depression, No. (%) | 0.143 | ||
| No | 1940 (99.7%) | 1971 (99.4%) | |
| Yes | 5 ( 0.3%) | 11 ( 0.6%) | |
| APOE ε4 carrier status, No. (%) | 0.584 | ||
| No | 1228 (77.4%) | 1278 (76.6%) | |
| Yes | 358 (22.6%) | 390 (23.4%) |
Abbreviations: APOE ε4=Apolipoprotein E gene, ε4 carrier status; BDI=Beck Depression Inventory; CVD=cardiovascular disease; DBP=diastolic blood pressure; DSE=diabetes support and education; ILI=intensive lifestyle intervention; SPB= systolic blood pressure; SD=standard deviation
Fig. 1.

Weight changes and sample sizes from baseline through Look AHEAD-MIND assessments for 3,938 participants.
Abbreviations: LAC= Look AHEAD Continuation; LAE1= Look AHEAD Extension Visit 1; LAE2= Look AHEAD Extension Visit 2; Rand=randomization
Numbers represent sample size contributing to each time point from each treatment arm.
Comparison between those who were included versus excluded (n=1,207) showed that the latter were significantly different in that they were slightly older, included more males, fewer African Americans, American Indians, and more Whites. They also had had higher baseline BMI and SBP, more of them were current or past smokers, more were insulin users and antidepressant users at baseline, more of them had hypertension and a baseline history of CVD, and they a longer diabetes duration at baseline (Supplemental Table 1). There were no significant differences between the groups in randomization to treatment arm, education, depression, and APOE ε4 status.
The mean age at first cognitive assessment was 69.4 years (standard deviation 6.5). Approximately 82% of participants (n=3,223) had an HbA1c measure within 6 months of their first cognitive assessment, and there were no differences in HbA1c levels across treatment arms. There was a mean of 2 cognitive assessments for each participant (range 1–4). A total of 1,052 participants (26.7%) had a cognitive assessment prior to the end of the intervention. Assessments took place at years 8–9 (n=978), 10–11 (n=2,655), 12–14 (n=1,738), and 15–18 (n=2,408). Trajectories of mean cognitive scores for the intervention groups are portrayed in Figure 2. The figure is based on linear mixed-effects models by intervention arm, adjusted for covariates. There were no differences between intervention groups in the mean cognitive scores over time.
Fig. 2.

Cognitive trajectories over time by intervention assignment.
Abbreviations: AVLT=Rey Auditory Verbal Learning Test; DSC=Digit Symbol Coding; 3MS=Modified Mini Mental State Exam; Trails A=Trail Making Test Part A; Trails B=Trail Making Test Part B
Sample size at each assessment period: years 8–9 (n=978), 10–11 (n=2,655), 12–14 (n=1,738), and 15–18 (n=2,408).
We determined a priori to examine models stratified by previously indicated group differences, i.e., baseline BMI, history of CVD, age, APOE ε4, and sex.[11, 12, 27–30] Models stratified by baseline BMI levels ≥30 versus <30 revealed no significant differences on test performance (data not shown). Results stratified by CVD history are shown in Table 2 and Figure 3. There was some evidence that performance on the Stroop test was modified by CVD history (difference [95% confidence interval] in least squares means [lsmeans] between ILI and DSE −0.23(−0.42, −0.04), interaction p=0.02) such that individuals with a baseline CVD history, randomized to ILI, performed significantly worse than those assigned to DSE. Those without a CVD history did not differ by randomization assignment. DSC performance among those with a CVD history was similarly worse in ILI participants compared to DSE, although the difference was not statistically significant (ILI-DSE lsmeans: −0.13 [−0.29, 0.02], interaction p=0.07). Overall, participants with a baseline history of CVD who were randomized to ILI tended to perform worse than similarly affected participants randomized to DSE on all tests including the composite, with the exception of the 3MS and RAVLT.
Table 2.
Mean standardized cognitive score across follow-up by intervention group and baseline CVD history adjusted for repeated measures, order of cognitive test, visit category, age, sex, race, education, baseline BMI, and systolic blood pressure
| Randomization Group | Difference | ||||
|---|---|---|---|---|---|
| ILI | DSE | ILI-DSE | Randomization *CVD History | ||
| Cognitive Test | CVD History | LSMEAN ± SE | LSMEAN ± SE | LSMEAN (95% CI) | P-value |
| Cognitive Composite | No | −0.18±0.02 | −0.19±0.02 | 0.01(−0.04,0.06) | 0.253 |
| Yes | −0.48±0.04 | −0.41±0.05 | −0.07(−0.19,0.06) | ||
| DSC | No | −0.25±0.02 | −0.27±0.02 | 0.02(−0.03,0.08) | 0.068 |
| Yes | −0.62±0.06 | −0.49±0.06 | −0.13(−0.29,0.02) | ||
| 3MS | No | −0.14±0.02 | −0.15±0.02 | 0.01(−0.05,0.07) | 0.853 |
| Yes | −0.40±0.06 | −0.39±0.06 | −0.01(−0.18,0.16) | ||
| Rey AVLT Delayed | No | −0.02±0.02 | −0.04±0.02 | 0.02(−0.04,0.08) | 0.568 |
| Yes | −0.21±0.06 | −0.18±0.06 | −0.03(−0.20,0.13) | ||
| Rey AVLT Immed | No | −0.17±0.02 | −0.15±0.02 | −0.01(−0.07,0.05) | 0.452 |
| Yes | −0.35±0.06 | −0.41±0.06 | 0.06(−0.11,0.22) | ||
| Stroop | No | −0.23±0.02 | −0.24±0.02 | 0.01(−0.05,0.08) | 0.015 |
| Yes | −0.62±0.07 | −0.38±0.07 | −0.23(−0.42,−0.05) | ||
| Trails A | No | −0.12±0.02 | −0.14±0.02 | 0.02(−0.04,0.08) | 0.135 |
| Yes | −0.45±0.06 | −0.33±0.06 | −0.11(−0.29,0.06) | ||
| Trails B | No | −0.18±0.02 | −0.17±0.02 | −0.01(−0.07,0.05) | 0.281 |
| Yes | −0.53±0.06 | −0.42±0.07 | −0.11(−0.29,0.06) | ||
Abbreviations: AVLT=Auditory Verbal Learning Test; BMI=body mass index; CVD=cardiovascular disease; DSC=Digit Symbol Coding; DSE=diabetes support and education; Hx=history; ILI=intensive lifestyle intervention; Immed= immediate recall; LSMEAN=least squares mean; 3MS= Modified Mini Mental State Exam; SE=standard error; Trails A=Trail Making Test Part A; Trails B=Trail Making Test Part B
Fig. 3.
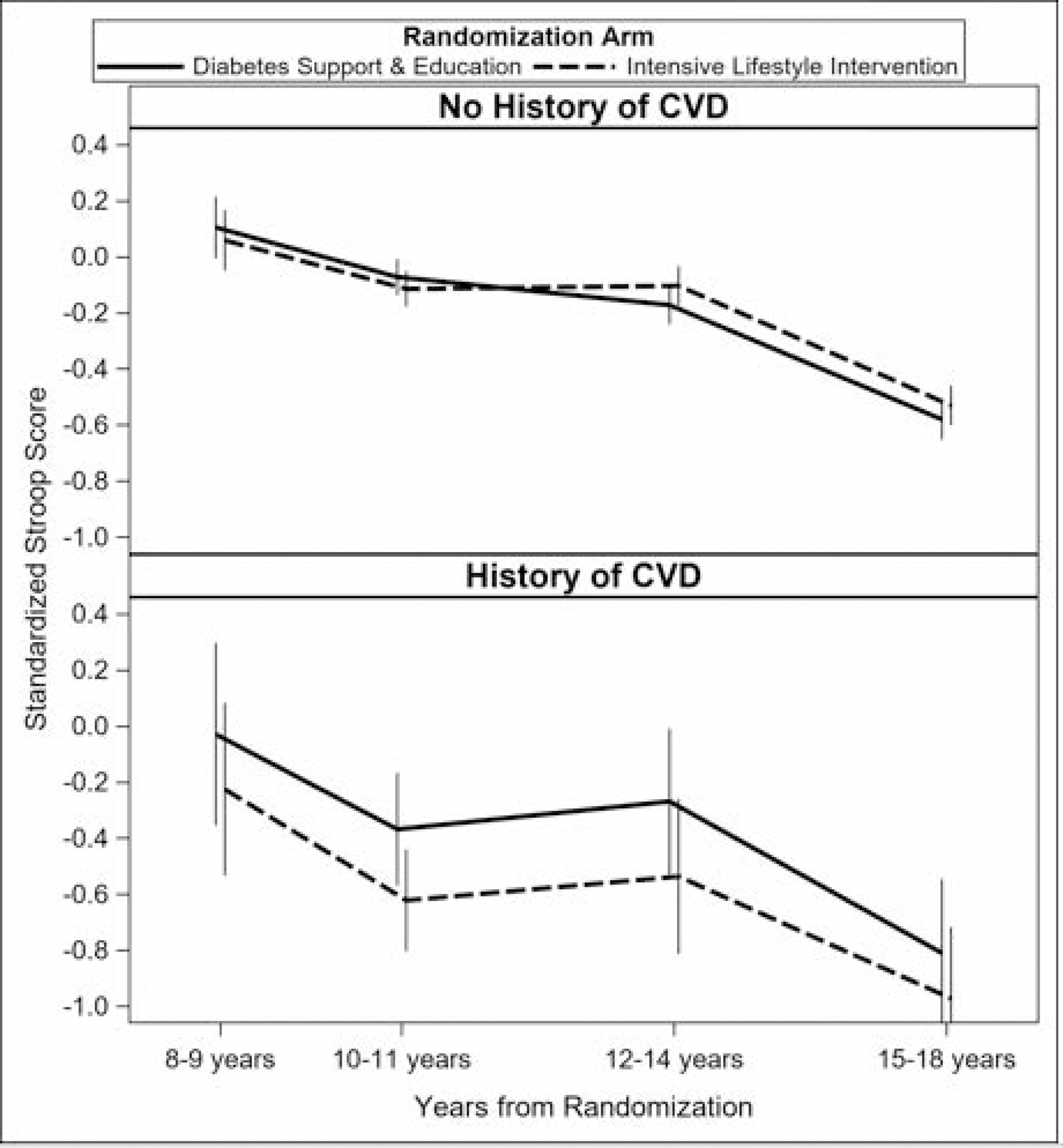
Trajectory Models Stratified by Intervention Arm and Baseline CVD Status.
Stroop test score trajectories (z-scores) interaction by intervention arm and baseline history of CVD (p=0.02). Sample size at each assessment period: years 8–9 (n=978), 10–11 (n=2,655), 12–14 (n=1,738), and 15–18 (n=2,408).
Abbreviations: CVD=Cardiovascular disease
Table 3 displays results of interactions between study arm and age. There were no significant interactions but results trended toward better performance among younger (baseline age<65 ) compared to older participants. Older DSE participants performed worse than the ILI group on RAVLT immediate recall, although this was not statistically significant (ILI-DSE lsmeans: 0.11 [−0.03, 0.25], interaction p=0.077). There were no significant interactions found by APOE ε4 status (data not shown). Table 4 shows interactions in cognitive performance by study arm and sex. Although there were no significant interactions, the results trended toward worse performance in men compared with women in all tests. Finally, to account for any potential sources of selection bias that may have been caused by differential dropout or death, a conditional probability was calculated based on baseline characteristics (age, sex, race/ethnicity, BMI, clinic site, CVD history, and hypertension) and its inverse was used to weight models. This yielded no material changes to our results.
Table 3.
Standardized cognitive score by randomization group and age adjusted for repeated measures, order of cognitive test, CVD history, age, sex, race, education, baseline BMI, systolic blood pressure, years from randomization, interaction between age and years from randomization.
| Randomization Group | Difference | ||||
|---|---|---|---|---|---|
| ILI | DSE | ILI-DSE | Randomization *Age | ||
| Cognitive Test | Age | LSMEAN ± SE | LSMEAN ± SE | LSMEAN (95% CI) | P-value |
| Cognitive Composite | <65 years | −0.12±0.02 | −0.12±0.02 | −0.01(−0.05,0.04) | 0.246 |
| 65+ years | −0.67±0.04 | −0.73±0.04 | 0.06(−0.04,0.16) | ||
| DSC | <65 years | −0.21±0.02 | −0.20±0.02 | −0.00(−0.06,0.06) | 0.605 |
| 65+ years | −0.77±0.05 | −0.81±0.05 | 0.04(−0.10,0.17) | ||
| 3MS | <65 years | −0.09±0.02 | −0.09±0.02 | −0.00(−0.06,0.06) | 0.649 |
| 65+ years | −0.63±0.05 | −0.66±0.05 | 0.04(−0.11,0.18) | ||
| Rey AVLT Delayed | <65 years | 0.03±0.02 | 0.04±0.02 | −0.01(−0.07,0.05) | 0.131 |
| 65+ years | −0.42±0.05 | −0.52±0.05 | 0.11(−0.03,0.24) | ||
| Rey AVLT Immed | <65 years | −0.10±0.02 | −0.08±0.02 | −0.02(−0.09,0.04) | 0.077 |
| 65+ years | −0.66±0.05 | −0.77±0.05 | 0.11(−0.03,0.25) | ||
| Stroop | <65 years | −0.19±0.02 | −0.16±0.02 | −0.03(−0.09,0.04) | 0.464 |
| 65+ years | −0.75±0.06 | −0.78±0.06 | 0.04(−0.12,0.20) | ||
| Trails A | <65 years | −0.07±0.02 | −0.07±0.02 | 0.00(−0.06,0.07) | 0.996 |
| 65+ years | −0.67±0.05 | −0.67±0.05 | 0.00(−0.14,0.14) | ||
| Trails B | <65 years | −0.12±0.02 | −0.11±0.02 | −0.02(−0.08,0.05) | 0.722 |
| 65+ years | −0.72±0.05 | −0.68±0.05 | −0.04(−0.19,0.10) | ||
Abbreviations: AVLT=Auditory Verbal Learning Test; BMI=body mass index; CVD=cardiovascular disease; DSC=Digit Symbol Coding; DSE=diabetes support and education; ILI=intensive lifestyle intervention; Immed=immediate recall; LSMEAN=least squares mean; 3MS= Modified Mini Mental State Exam; SE=standard error; Trails A=Trail Making Test Part A; Trails B=Trail Making Test Part B
Table 4.
Standardized cognitive score by randomization group and sex adjusted for repeated measures, order of cognitive test, CVD history, age, sex, race, education, baseline BMI, systolic blood pressure, years from randomization, and interaction between age and years from randomization
| Randomization Group | Difference | ||||
|---|---|---|---|---|---|
| ILI | DSE | ILI-DSE | Randomization *Gender | ||
| Cognitive Test | Gender | LSMEAN ± SE | LSMEAN ± SE | LSMEAN (95% CI) | P-value |
| Cognitive Composite | Female | −0.11±0.02 | −0.09±0.02 | −0.02(−0.08,0.03) | 0.204 |
| Male | −0.36±0.02 | −0.39±0.03 | 0.04(−0.03,0.10) | ||
| DSC | Female | −0.19±0.03 | −0.18±0.03 | −0.01(−0.08,0.06) | 0.489 |
| Male | −0.46±0.03 | −0.49±0.03 | 0.03(−0.06,0.11) | ||
| 3MS | Female | −0.07±0.03 | −0.05±0.03 | −0.02(−0.09,0.05) | 0.307 |
| Male | −0.32±0.03 | −0.36±0.03 | 0.04(−0.05,0.13) | ||
| Rey AVLT Delayed | Female | 0.19±0.03 | 0.19±0.03 | −0.00(−0.08,0.07) | 0.524 |
| Male | −0.39±0.03 | −0.42±0.03 | 0.03(−0.06,0.12) | ||
| Rey AVLT Immed | Female | 0.06±0.03 | 0.09±0.03 | −0.03(−0.10,0.04) | 0.167 |
| Male | −0.56±0.03 | −0.61±0.03 | 0.05(−0.04,0.14) | ||
| Stroop | Female | −0.25±0.03 | −0.20±0.03 | −0.05(−0.13,0.03) | 0.144 |
| Male | −0.30±0.04 | −0.35±0.04 | 0.04(−0.06,0.14) | ||
| Trails A | Female | −0.11±0.03 | −0.11±0.03 | −0.00(−0.07,0.07) | 0.734 |
| Male | −0.22±0.03 | −0.24±0.03 | 0.02(−0.07,0.11) | ||
| Trails B | Female | −0.19±0.03 | −0.15±0.03 | −0.04(−0.12,0.03) | 0.380 |
| Male | −0.27±0.03 | −0.27±0.04 | 0.01(−0.08,0.10) | ||
Abbreviations: AVLT=Auditory Verbal Learning Test; BMI=body mass index; CVD=cardiovascular disease; DSC=Digit Symbol Coding; DSE=diabetes support and education; ILI=intensive lifestyle intervention; Immed=immediate recall; LSMEAN=least squares mean; 3MS= Modified Mini Mental State Exam; SE=standard error; Trails A=Trail Making Test Part A; Trails B=Trail Making Test Part B
Discussion/Conclusion
Our goal was to assess the legacy of Look AHEAD interventions on cognitive trajectories and to determine whether effects varied across baseline BMI groups, baseline CVD history, and subgroups defined by age (+/− 65), APOE ε4, and sex. Prior analyses of cognitive data in Look AHEAD did not detect significant group differences, although those analysis did not have a very large longitudinal component. This analysis expands on prior work and uses Look AHEAD MIND data, adding additional cognitive assessments for over ~2400 participants and extending cognitive follow up by 4 years. Our primary finding is that the ILI was not related to better cognitive trajectories ascertained beginning 8 years after randomization. Our secondary finding is that participants with a history of CVD who were randomized to the ILI arm of the study, performed significantly worse on the Stroop compared to those in the DSE arm. It is unlikely that this result would be maintained after correction for multiple comparisons. However, DSC and Trails B scores trended in the same direction, i.e., those with baseline CVD and randomized to ILI performed worse, although these differences were not statistically significant. There were no significant interactions with intervention arms in 3MS or RAVLT scores; additionally, there was no evidence that differential attrition biased our results.
Three of the tests that were administered: Stroop, DSC, and Trails B are all dependent upon executive function, requiring task switching and a high level of executive control.[31–33] All three are also timed tests so processing speed is an important component. Because CVD has been associated with executive function deficits in a number of studies,[34] and obesity has been associated with processing speed as well as cognitive flexibility[35], our findings might be expected. Indeed, on average, Look AHEAD participants with a history of CVD tended to perform worse on cognitive assessments than those without CVD. What is unexpected is that participants randomized to an intervention that would presumably have had a positive impact, did not demonstrate improvements in cognition and instead showed a modest negative impact compared to usual diabetes care. These participants performed worse than their DSE counterparts on tests of executive function. The explanation for this anomaly may lie in the heterogeneity of effects that have been noted in Look AHEAD participants with a history of CVD[36]; or perhaps the DSE arm of the study was simply a more appropriate intervention for these participants.
Analyses of data collected prior to randomization showed that the 14% of participants with a history of CVD, had significantly reduced exercise capacity compared to participants without CVD.[37] These participants tended to be older, White, and male; they had longer diabetes duration, and were more likely to be on insulin.[36] The intervention did not have any effect on the incidence of newly diagnosed atrial fibrillation (p=0.94). However, those with a history of coronary heart disease had a significantly higher incidence of atrial fibrillation (HR 1.75, 95% CI 1.27–2.39) in Look AHEAD[38] and atrial fibrillation is a risk factor for cognitive impairment.[39, 40] Similarly, the ILI intervention did not have the anticipated effect of lowering the prevalence of left ventricular hypertrophy,[41] another risk factor for cognitive impairment.[42] However, these findings of little intervention impact do not explain why participants randomized to the ILI, with a history of CVD, would perform worse on cognitive testing than their counterparts in DSE.
The key factors that distinguish intervention arms are increased physical activity and weight loss in the ILI group. ILI participants eventually regained much of their weight (~half) and this loss and re-gain may have had a negative impact on cognition. We did not assess cognitive function in the first 8 years post-randomization, when the benefits of the ILI intervention on adiposity, physical fitness, and glycemia were more pronounced.[18] Thus, we cannot address whether there were short term cognitive benefits during the most impactful period of the intervention, and whether cognitive benefits dissipated as the benefits of the ILI on adiposity and other parameters also dissipated. Participants were queried about cognitive abilities (memory, problem-solving, decision-making) at baseline and during intervention delivery.[43] For these, ILI was found to provide relative benefit in decision-making beginning at year 1 and extending throughout 8-years of follow-up. It also was associated with better self-reported problem-solving for participants without baseline obesity. However, ILI participants reporting baseline problem-solving deficits and a CVD history, showed relatively worse self-reported abilities through year 8.
In Look AHEAD and other studies testing lifestyle interventions such as the Diabetes Prevention Program,[44] the impact of the ILI on weight loss is most pronounced 12 months after randomization and is attenuated thereafter, when weight is progressively regained for several years. Both weight loss and gain are associated with changes in hormones, which may serve to alter homeostasis in energy sources for the brain. Future work will examine weight loss and regain as well as hormones, as contributing factors in the differences in cognitive performance.
We note some potential limitations. Our findings are potentially generalizable to only a high-risk subset of the population, i.e., older adults with T2D and overweight/obesity. Cognitive function was not measured at baseline and was not the primary focus of the trial. Thus, no participants were excluded at baseline based on cognitive impairment, although rigorous screening procedures would have effectively excluded those with clear impairment. Randomization facilitated comparable demographic and health characteristics across arms at baseline, and there is no reason to suspect that the two groups would have differed in cognitive performance had it been measured. Although most weight loss occurred among those randomized to ILI, unintentional weight loss (in either arm) related to other health factors, may have adversely affected cognitive functioning. Even though our primary finding was not significant, we performed additional subgroup analyses and did not correct for multiple comparisons. As a result, our subgroup analyses would likely not be significant with corrections. Our analysis also has notable strengths, including the fact that Look AHEAD was a randomized controlled clinical trial and as such was conducted using rigorous methods. Participants have been closely followed for nearly twenty years, providing deep phenotyping and very well-characterized outcomes. This rich source of data will support future detailed investigations into the impacts of the intervention on cognitive function.
Supplementary Material
Funding Sources
The Action for Health in Diabetes is supported through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The Look AHEAD Brain MRI ancillary study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services: DK092237–01 and DK092237–02S2. The Look AHEAD Movement and Memory ancillary study was supported by the National Institute on Aging, National Institutes of Health, Department of Health and Human Services, R01AG03308701. The Look AHEAD Mind ancillary study was funded by AG058571. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women’s Health; the Centers for Disease Control and Prevention; the National Institute on Aging; and the Department of Veterans Affairs. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); Frederic C. Bartter General Clinical Research Center (M01RR01346); and the Wake Forest Alzheimer’s Disease Core Center (P30AG049638–01A1).
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Statements
Clinical Sites
The Johns Hopkins Medical Institutions Frederick L. Brancati, MD, MHS1*; Lee Swartz2; Lawrence Cheskin, MD3; Jeanne M. Clark, MD, MPH3; Kerry Stewart, EdD3; Richard Rubin, PhD3*; Jean Arceci, RN; Suzanne Ball; Jeanne Charleston, RN; Danielle Diggins; Mia Johnson; Joyce Lambert; Kathy Michalski, RD; Dawn Jiggetts; Chanchai Sapun
Pennington Biomedical Research Center George A. Bray, MD1; Allison Strate, RN2; Frank L. Greenway, MD3; Donna H. Ryan, MD3; Donald Williamson, PhD3; Timothy Church, MD3; Catherine Champagne, PhD, RD; Valerie Myers, PhD; Jennifer Arceneaux, RN; Kristi Rau; Michelle Begnaud, LDN, RD, CDE; Barbara Cerniauskas, LDN, RD, CDE; Crystal Duncan, LPN; Helen Guay, LDN, LPC, RD; Carolyn Johnson, LPN, Lisa Jones; Kim Landry; Missy Lingle; Jennifer Perault; Cindy Puckett; Marisa Smith; Lauren Cox; Monica Lockett, LPN
The University of Alabama at Birmingham Cora E. Lewis, MD, MSPH1; Sheikilya Thomas MPH2; Monika Safford, MD3; Stephen Glasser, MD3; Vicki DiLillo, PhD3; Charlotte Bragg, MS, RD, LD; Amy Dobelstein; Sara Hannum, MA; Anne Hubbell, MS; Jane King, MLT; DeLavallade Lee; Andre Morgan; L. Christie Oden; Janet Raines, MS; Cathy Roche, RN, BSN; Jackie Roche; Janet Turman
Harvard Center
Massachusetts General Hospital. David M. Nathan, MD1; Enrico Cagliero, MD3; Kathryn Hayward, MD3; Heather Turgeon, RN, BS, CDE2; Linda Delahanty, MS, RD3; Ellen Anderson, MS, RD3; Laurie Bissett, MS, RD; Valerie Goldman, MS, RD; Virginia Harlan, MSW; Theresa Michel, DPT, DSc, CCS; Mary Larkin, RN; Christine Stevens, RN; Kylee Miller, BA; Jimmy Chen, BA; Karen Blumenthal, BA; Gail Winning, BA; Rita Tsay, RD; Helen Cyr, RD; Maria Pinto
Joslin Diabetes Center: Edward S. Horton, MD1; Sharon D. Jackson, MS, RD, CDE2; Osama Hamdy, MD, PhD3; A. Enrique Caballero, MD3; Sarah Bain, BS; Elizabeth Bovaird, BSN, RN; Barbara Fargnoli, MS,RD; Jeanne Spellman, BS, RD; Kari Galuski, RN; Ann Goebel-Fabbri, PhD; Lori Lambert, MS, RD; Sarah Ledbury, MEd, RD; Maureen Malloy, BS; Kerry Ovalle, MS, RCEP, CDE
Beth Israel Deaconess Medical Center: George Blackburn, MD, PhD1*; Christos Mantzoros, MD, DSc3; Ann McNamara, RN; Kristina Spellman, RD
University of Colorado Anschutz Medical Campus James O. Hill, PhD1; Holly Wyatt, MD3; Marsha Miller, MS RD2; Brent Van Dorsten, PhD3; Judith Regensteiner, PhD3; Debbie Bochert; Ligia Coelho, BS; Paulette Cohrs, RN, BSN; Susan Green; April Hamilton, BS, CCRC; Jere Hamilton, BA; Eugene Leshchinskiy; Lindsey Munkwitz, BS; Loretta Rome, TRS; Terra Thompson, BA; Kirstie Craul, RD, CDE; Sheila Smith, BS; Cecilia Wang, MD
Baylor College of Medicine John P. Foreyt, PhD1; Rebecca S. Reeves, DrPH, RD2; Molly Gee, MEd, RD2; Henry Pownall, PhD3; Ashok Balasubramanyam, MBBS3; Chu-Huang Chen, MD, PhD3; Peter Jones, MD3; Michele Burrington, RD, RN; Allyson Clark Gardner,MS, RD; Sharon Griggs; Michelle Hamilton; Veronica Holley; Sarah Lee; Sarah Lane Liscum, RN, MPH; Susan Cantu-Lumbreras; Julieta Palencia, RN; Jennifer Schmidt; Jayne Thomas, RD; Carolyn White
The University of Tennessee Health Science Center
University of Tennessee East. Karen C. Johnson, MD, MPH1; Carolyn Gresham, RN2; Mace Coday, PhD; Lisa Jones, RN; Lynne Lichtermann, RN, BSN; J. Lee Taylor, MEd, MBA
University of Tennessee Downtown. Abbas E. Kitabchi, PhD, MD1*; Helmut Steinberg, MD1;Ebenezer Nyenwe, MD3; Helen Lambeth, RN, BSN2; Moana Mosby, RN; Amy Brewer, MS, RD, LDN; Debra Clark, LPN; Andrea Crisler, MT; Debra Force, MS, RD, LDN; Donna Green, RN; Robert Kores, PhD; Renate Rosenthal, Ph.D.
University of Minnesota Robert W. Jeffery, PhD1; Tricia Skarphol, MA2; John P. Bantle, MD3; J. Bruce Redmon, MD3; Richard S. Crow, MD3; Cindy Bjerk, MS, RD; Kerrin Brelje, MPH, RD; Carolyne Campbell; Melanie Jaeb, MPH, RD; Philip Lacher, BBA; Patti Laqua, RD; Therese Ockenden, RN; Birgitta I. Rice, MS, RPh, CHES; Carolyn Thorson, CCRP; Ann D. Tucker, BA; Mary Susan Voeller, BA St. Luke’s Roosevelt Hospital Center Xavier Pi-Sunyer, MD1; Jennifer Patricio, MS2; Carmen Pal, MD3; Lynn Allen, MD;Janet Crane, MA, RD, CDN; Lolline Chong, BS, RD; Diane Hirsch, RNC, MS, CDE; Mary Anne Holowaty, MS, CN; Michelle Horowitz, MS, RD.
University of Pennsylvania Thomas A. Wadden, PhD1; Barbara J Maschak-Carey, MSN, CDE 2; Robert I. Berkowitz, MD3; Gary Foster, PhD 3; Henry Glick, PhD 3; Shiriki Kumanyika, PhD, RD, MPH3; Brooke Bailer, PhD; Yuliis Bell; Chanelle Bishop-Gilyard, Psy.D; Raymond Carvajal, Psy.D; Helen Chomentowski; Renee Davenport; Lucy Faulconbridge, PhD; Louise Hesson, MSN, CRNP; Robert Kuehnel, PhD; Sharon Leonard, RD; Caroline Moran, BA; Monica Mullen, RD, MPH; Victoria Webb, BA.; Marion Vetter, MD, RD
University of Pittsburgh John M. Jakicic, PhD1, David E. Kelley, MD1; Jacqueline Wesche-Thobaben, RN, BSN, CDE2; Lewis H. Kuller, MD, DrPH3; Andrea Kriska, PhD3; Amy D. Rickman, PhD, RD, LDN3, Lin Ewing, PhD, RN3, Mary Korytkowski, MD3, Daniel Edmundowicz, MD3; Rebecca Danchenko, BS; Tammy DeBruce; Barbara Elnyczky; David O. Garcia, MS; Patricia H. Harper, MS, RD, LDN; Susan Harrier, BS; Dianne Heidingsfelder, MS, RD, CDE, LDN; Diane Ives, MPH; Juliet Mancino, MS, RD, CDE, LDN; Lisa Martich, MS, RD; Tracey Y. Murray, BS; Karen Quirin; Joan R. Ritchea; Susan Copelli, BS, CTR The Miriam Hospital/Alpert Medical School of Brown University Providence, RI Rena R. Wing, PhD1; Caitlin Egan, MS2; Vincent Pera, MD3; Jeanne McCaffery, PhD3; Jessica Unick, PhD3; Ana Almeida; Kirsten Annis, BA; Barbara Bancroft, RN; April Bernier, BS; Sara Cournoyer, BA; Lisa Cronkite, BS; Jose DaCruz; Michelle Fisher, RN, CDOE; Linda Gay, MS, RD, CDE; Stephen Godbout, BS, BSN; Jacki Hecht, RN, MSN; Marie Kearns, MA; Deborah Maier-Fredey, MS, RD; Heather Niemeier, PhD; Suzanne Phelan, PhD; Angela Marinilli-Pinto, PhD; Deborah Ranslow-Robles; Hollie Raynor, PhD; Erica Robichaud, MSW, RD; Jane Tavares, BA; Kristen Whitehead
The University of Texas Health Science Center at San Antonio Steven M. Haffner, MD1; Helen P. Hazuda, PhD1; Maria G. Montez, RN, MSHP, CDE2; Carlos Lorenzo, MD3; Charles F. Coleman, MS, RD; Domingo Granado, RN; Kathy Hathaway, MS, RD; Juan Carlos Isaac, RC, BSN; Nora Ramirez, RN, BSN; Ronda Saenz, MS, RD
VA Puget Sound Health Care System / University of Washington Steven E. Kahn MB, ChB1; Brenda Montgomery, RN, MS, CDE2; Robert Knopp, MD3; Edward Lipkin, MD, PhD3; Dace Trence, MD3; Elaine Tsai, MD3; Valerie Baldisserotto, RD; Linda Castine, RN, BSN, CDE; Basma Fattaleh, BA; Kathy Fitzpatrick, RN; Diane Greenberg, PhD; Sukwan Nhan Jolley, RD; Hailey Mack, RD, MS, CDE; Ivy Morgan-Taggart; Anne Murillo, BS; Gretchen Otto, BS; Betty Ann Richmond, MEd; Jolanta Socha, BS; April Thomas, MPH, RD; Alan Wesley, BA; Diane Wheeler, RD, CDE
Southwestern American Indian Center, Phoenix, Arizona and Shiprock, New Mexico William C. Knowler, MD, DrPH1; Paula Bolin, RN, MC2; Tina Killean, BS2; Cathy Manus, LPN3; Jonathan Krakoff, MD3; Jeffrey M. Curtis, MD, MPH3; Sara Michaels, MD3; Paul Bloomquist, MD3; Bernadita Fallis RN, RHIT, CCS; Diane F. Hollowbreast; Ruby Johnson; Maria Meacham, BSN, RN, CDE; Christina Morris, BA; Julie Nelson, RD; Carol Percy, RN; Patricia Poorthunder; Sandra Sangster; Leigh A. Shovestull, RD, CDE; Miranda Smart; Janelia Smiley; Teddy Thomas, BS; Katie Toledo, MS, LPC
University of Southern California Anne Peters, MD1; Siran Ghazarian, MD2
Coordinating Center
Wake Forest University Mark A. Espeland, PhD1; Judy L. Bahnson, BA, CCRP3; Lynne E. Wagenknecht, DrPH3; David Reboussin, PhD3; W. Jack Rejeski, PhD3; Alain G. Bertoni, MD, MPH3; Wei Lang, PhD3; Michael S. Lawlor, PhD3; David Lefkowitz, MD3*; Gary D. Miller, PhD3; Patrick S. Reynolds, MD3; Paul M. Ribisl, PhD3; Mara Vitolins, DrPH3; Daniel Beavers, PhD3; Haiying Chen, PhD, MM3; Delia S. West, PhD3; Lawrence M. Friedman, MD3; Ron Prineas, MD3; Tandaw Samdarshi, MD3; Kathleen M. Hayden, PhD3; Kathy M. Dotson, BA2; Amelia Hodges, BS, CCRP2; Dominique Limprevil-Divers, MA, MEd2; Karen Wall2; Carrie C. Williams, MA, CCRP2; Andrea Anderson, MS; Jerry M. Barnes, MA; Mary Barr; Tara D. Beckner; Cralen Davis, MS; Thania Del Valle-Fagan, MD; Melanie Franks, BBA; Candace Goode; Jason Griffin, BS; Lea Harvin, BS; Mary A. Hontz, BA; Sarah A. Gaussoin, MS; Don G. Hire, BS; Patricia Hogan, MS; Mark King, BS; Kathy Lane, BS; Rebecca H. Neiberg, MS; Julia T. Rushing, MS; Valery S. Effoe, MD, MS; Michael P. Walkup, MS; Terri Windham
Central Resources Centers
DXA Reading Center, University of California at San Francisco Michael Nevitt, PhD1; Ann Schwartz, PhD2; John Shepherd, PhD3; Michaela Rahorst; Lisa Palermo, MS, MA; Susan Ewing, MS; Cynthia Hayashi; Jason Maeda, MPH
Central Laboratory, Northwest Lipid Metabolism and Diabetes Research
Laboratories Santica M. Marcovina, PhD, ScD1; Jessica Chmielewski2; Vinod Gaur, PhD4
ECG Reading Center, EPICARE, Wake Forest University School of Medicine
Elsayed Z. Soliman MD, MSc, MS1; Charles Campbell 2; Zhu-Ming Zhang, MD3; Mary Barr; Susan Hensley; Julie Hu; Lisa Keasler; Yabing Li, MD
Diet Assessment Center, University of South Carolina, Arnold School of Public Health, Center for Research in Nutrition and Health Disparities Elizabeth J Mayer-Davis, PhD1; Robert Moran, PhD1 Hall-Foushee Communications, Inc.
Richard Foushee, PhD; Nancy J. Hall, MA
Federal Sponsors
National Institute of Diabetes and Digestive and Kidney Diseases Mary Evans, PhD; Barbara Harrison, MS; Van S. Hubbard, MD, PhD; Susan Z. Yanovski, MD
National Heart, Lung, and Blood Institute Lawton S. Cooper, MD, MPH; Peter Kaufman, PhD, FABMR
Centers for Disease Control and Prevention Edward W. Gregg, PhD; Ping Zhang, PhD
______________________________
1 Principal Investigator
2 Program Coordinator
3 Co-Investigator
All other Look AHEAD staffs are listed alphabetically by site.
*Deceased
Footnotes
Statement of Ethics
This study was conducted ethically, in accordance with the World Medical Association Declaration of Helsinki. The study protocol was approved by the Wake Forest School of Medicine (Look AHEAD study Coordinating Center) Institutional Review Board (IRB #BG99-042) as well as the Institutional Review Boards of all the data collection sites. All participants provided written informed consent to participate. The ClinicalTrials.gov identifier is NCT00017953.
Conflict of Interest Statement
The authors report no conflicts of interest.
References
- 1.Maggi S, Limongi F, Noale M, Romanato G, Tonin P, Rozzini R, et al. Diabetes as a risk factor for cognitive decline in older patients. Dement Geriatr Cogn Disord 2009;27(1):24–33. [DOI] [PubMed] [Google Scholar]
- 2.The Emerging Risk Factors C. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. The Lancet 2010. 2010/06/26/;375(9733):2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callisaya ML, Beare R, Moran C, Phan T, Wang W, Srikanth VK. Type 2 diabetes mellitus, brain atrophy and cognitive decline in older people: a longitudinal study. Diabetologia 2019. March;62(3):448–58. [DOI] [PubMed] [Google Scholar]
- 4.Moran C, Beare R, Wang W, Callisaya M, Srikanth V. Type 2 diabetes mellitus, brain atrophy, and cognitive decline. Neurology 2019. February 19;92(8):e823–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang R, Pedersen NL, Bao C, Xu W, Xu H, Song R, et al. Type 2 diabetes in midlife and risk of cerebrovascular disease in late life: a prospective nested case-control study in a nationwide Swedish twin cohort. Diabetologia 2019. August;62(8):1403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munshi M, Grande L, Hayes M, Ayres D, Suhl E, Capelson R, et al. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care 2006. August;29(8):1794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siervo M, Arnold R, Wells JC, Tagliabue A, Colantuoni A, Albanese E, et al. Intentional weight loss in overweight and obese individuals and cognitive function: a systematic review and meta-analysis. Obes Rev 2011. November;12(11):968–83. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Jr., et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol 2009. March;66(3):336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee S, Peters SA, Woodward M, Mejia Arango S, Batty GD, Beckett N, et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared With Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care 2016. February;39(2):300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hugenschmidt CE. Type 2 diabetes, obesity, and risk for dementia: recent insights into brain insulin resistance and hypometabolism. Curr Behav Neurosci Rep 2016;3:293–300. [Google Scholar]
- 11.Espeland MA, Rapp SR, Bray GA, Houston DK, Johnson KC, Kitabchi AE, et al. Long-term impact of behavioral weight loss intervention on cognitive function. J Gerontol A Biol Sci Med Sci 2014. September;69(9):1101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espeland MA, Luchsinger JA, Baker LD, Neiberg R, Kahn SE, Arnold SE, et al. Effect of a long-term intensive lifestyle intervention on prevalence of cognitive impairment. Neurology 2017. May 23;88(21):2026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapp SR, Luchsinger JA, Baker LD, Blackburn GL, Hazuda HP, Demos-McDermott KE, et al. Effect of a Long-Term Intensive Lifestyle Intervention on Cognitive Function: Action for Health in Diabetes Study. J Am Geriatr Soc 2017. May;65(5):966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beavers KM, Leng I, Rapp SR, Miller ME, Houston DK, Marsh AP, et al. Effects of Longitudinal Glucose Exposure on Cognitive and Physical Function: Results from the Action for Health in Diabetes Movement and Memory Study. J Am Geriatr Soc 2017. January;65(1):137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houston DK, Leng X, Bray GA, Hergenroeder AL, Hill JO, Jakicic JM, et al. A long-term intensive lifestyle intervention and physical function: the Look AHEAD Movement and Memory Study. Obesity (Silver Spring) 2015. January;23(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espeland MA, Erickson K, Neiberg RH, Jakicic JM, Wadden TA, Wing RR, et al. Brain and White Matter Hyperintensity Volumes After 10 Years of Random Assignment to Lifestyle Intervention. Diabetes Care 2016. May;39(5):764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Look Ahead Research Group. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled Clinical Trials 2003. 2003/10/01/;24(5):610–28. [DOI] [PubMed] [Google Scholar]
- 18.Look Ahead Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013. July 11;369(2):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Look Ahead Research Group, Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006. May;14(5):737–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wesche-Thobaben JA. The development and description of the comparison group in the Look AHEAD trial. Clin Trials 2011. June;8(3):320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rey A L’examen clinique en psychologie 1958.
- 22.Wechsler D Wechsler Adult Intelligence Scale-III (WAIS-III), The psychological corporation San Antonio TX; 1997. [Google Scholar]
- 23.Stroop JR. Studies of interference in serial verbal reactions. Journal of experimental psychology 1935;18(6):643. [Google Scholar]
- 24.Reitan RM. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Perceptual and Motor Skills 2016;8(3):271–76. [Google Scholar]
- 25.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987. August;48(8):314–8. [PubMed] [Google Scholar]
- 26.Pfeffer RI, Kurosaki TT, Harrah CH Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol 1982. May;37(3):323–9. [DOI] [PubMed] [Google Scholar]
- 27.Espeland MA, Carmichael O, Hayden K, Neiberg RH, Newman AB, Keller JN, et al. Long-term Impact of Weight Loss Intervention on Changes in Cognitive Function: Exploratory Analyses from the Action for Health in Diabetes Randomized Controlled Clinical Trial. J Gerontol A Biol Sci Med Sci 2018. March 14;73(4):484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Espeland MA, Carmichael O, Yasar S, Hugenschmidt C, Hazzard W, Hayden KM, et al. Sex-related differences in the prevalence of cognitive impairment among overweight and obese adults with type 2 diabetes. Alzheimers Dement 2018. September;14(9):1184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayden KM, Baker LD, Bray G, Carvajal R, Demos-McDermott K, Hergenroeder AL, et al. Long-term impact of intensive lifestyle intervention on cognitive function assessed with the National Institutes of Health Toolbox: The Look AHEAD study. Alzheimers Dement (Amst) 2018;10:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espeland MA, Hayden KM, Lockhart SN, Yassine HN, Hoscheidt S, Yasar S, et al. Sex-Related Differences in Brain Volumes and Cerebral Blood Flow Among Overweight and Obese Adults With Type 2 Diabetes: Exploratory Analyses From the Action for Health in Diabetes Brain Magnetic Resonance Imaging Study. J Gerontol A Biol Sci Med Sci 2020. March 9;75(4):771–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stuss DT, Shallice T, Alexander MP, Picton TW. A multidisciplinary approach to anterior attentional functions. Ann N Y Acad Sci 1995. December 15;769:191–211. [DOI] [PubMed] [Google Scholar]
- 32.Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: Validation using a set-switching paradigm. Journal of Clinical and Experimental Neuropsychology 2000. 2000/08/01;22(4):518–28. [DOI] [PubMed] [Google Scholar]
- 33.Baudouin A, Clarys D, Vanneste S, Isingrini M. Executive functioning and processing speed in age-related differences in memory: contribution of a coding task. Brain Cogn 2009. December;71(3):240–5. [DOI] [PubMed] [Google Scholar]
- 34.Eggermont LH, de Boer K, Muller M, Jaschke AC, Kamp O, Scherder EJ. Cardiac disease and cognitive impairment: a systematic review. Heart 2012. September;98(18):1334–40. [DOI] [PubMed] [Google Scholar]
- 35.Ihle A, Mons U, Perna L, Oris M, Fagot D, Gabriel R, et al. The Relation of Obesity to Performance in Verbal Abilities, Processing Speed, and Cognitive Flexibility in Old Age: The Role of Cognitive Reserve. Dement Geriatr Cogn Disord 2016;42(1–2):117–26. [DOI] [PubMed] [Google Scholar]
- 36.Look Ahead Research Group, Lewis CE, Bantle JP, Bertoni AG, Blackburn G, Brancati FL, et al. History of Cardiovascular Disease, Intensive Lifestyle Intervention, and Cardiovascular Outcomes in the Look AHEAD Trial. Obesity (Silver Spring) 2020. February;28(2):247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribisl PM, Lang W, Jaramillo SA, Jakicic JM, Stewart KJ, Bahnson J, et al. Exercise capacity and cardiovascular/metabolic characteristics of overweight and obese individuals with type 2 diabetes: the Look AHEAD clinical trial. Diabetes Care 2007. October;30(10):2679–84. [DOI] [PubMed] [Google Scholar]
- 38.Alonso A, Bahnson JL, Gaussoin SA, Bertoni AG, Johnson KC, Lewis CE, et al. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the Look AHEAD randomized trial. Am Heart J 2015. October;170(4):770–77 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thacker EL, McKnight B, Psaty BM, Longstreth WT Jr., Sitlani CM, Dublin S, et al. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology 2013. July 9;81(2):119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alosco ML, Spitznagel MB, Sweet LH, Josephson R, Hughes J, Gunstad J. Atrial fibrillation exacerbates cognitive dysfunction and cerebral perfusion in heart failure. Pacing Clin Electrophysiol 2015. February;38(2):178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brinkley TE, Anderson A, Soliman EZ, Bertoni AG, Greenway F, Knowler WC, et al. Long-Term Effects of an Intensive Lifestyle Intervention on Electrocardiographic Criteria for Left Ventricular Hypertrophy: The Look AHEAD Trial. Am J Hypertens 2018. April 13;31(5):541–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahinrad S, Vriend AE, Jukema JW, van Heemst D, Sattar N, Blauw GJ, et al. Left Ventricular Hypertrophy and Cognitive Decline in Old Age. J Alzheimers Dis 2017;58(1):275–83. [DOI] [PubMed] [Google Scholar]
- 43.Espeland MA, Dutton GR, Neiberg RH, Carmichael O, Hayden KM, Johnson KC, et al. Impact of a Multidomain Intensive Lifestyle Intervention on Complaints About Memory, Problem-Solving, and Decision-Making Abilities: The Action for Health in Diabetes Randomized Controlled Clinical Trial. J Gerontol A Biol Sci Med Sci 2018. October 8;73(11):1560–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diabetes Prevention Program Research G, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009. November 14;374(9702):1677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


