Abstract
Concentrated animal feeding operations (CAFOs) have emerged as an environmental justice issue due to disproportionate siting in low-income and minority communities. However, CAFOs’ impact on health is not fully understood. We examined risk of cause-specific mortality associated with CAFOs in North Carolina (NC) for 2000-2017 and health disparities. We obtained data on individual-level cause-specific mortality and on permitted animal facilities. We estimated associations between exposure to CAFOs and cause-specific mortality using logistic regression, controlling for demographics (e.g., age) and area-level covariates. To estimate exposure to CAFOs, we considered (1) a binary indicator (presence or absence) of CAFOs within a buffer around individual residence based on several buffer sizes, and (2) four levels of exposure (no, low, medium, and high) based on the number of CAFOs within 15 km around each residence. We considered individual-level (sex, race/ethnicity, age, education) and community-level (median household income, urbanicity, and region) factors. Under all buffer sizes used to estimate CAFO exposure, people living near CAFOs had significantly higher risk of cardiovascular mortality than other persons. Comparing those living near CAFOs to the no exposure group, odds ratios (ORs) for cardiovascular mortality were 1.01 (95% confidence interval (CI) 1.00, 1.03), 1.04 (1.03, 1.06), and 1.06 (1.05, 1.07) for low, medium, and high CAFOs exposure, respectively, indicating a trend of higher risk with higher exposure. Those in the high CAFOs exposure group had significantly higher risk of anemia and kidney disease mortality than those with no exposure. Results suggest higher mortality risk from CAFOs for some subpopulations, however differences were not statistically significant. Findings provide evidence of excess mortality risk from CAFOs in NC. These results have implications for future studies of environmental justice and CAFOs.
Keywords: CAFOs, Environmental justice, Health disparities, Mortality, Vulnerable population
Graphical abstract
1. Introduction
The number of large industrial-scale farms, including concentrated animal feeding operations (CAFOs) in the US, has substantially increased over the past few decades (USDA, 2019). These facilities produce large amounts of animal waste that have substantial negative impacts on environments and health in proximate communities. The environmental detriment from CAFOs include harmful airborne emissions such as particulates, volatile organic compounds (VOC), hydrogen sulfide, ammonia, and endotoxins, and harm to soil and water quality and other environmental systems (O’Connor et al. 2017; Schiffman et al. 2001). Through several pathways such as emitted harmful air pollutants, odor, and contaminated surface- and groundwater, CAFOs may affect human health and quality of life. For example, gaseous and particulate contaminants such as ammonia, hydrogen sulfide may cause respiratory tract, skin, or eye irritations, coughing, chronic lung disease, inflammation of the membranes, and odors (Donham et al. 1995; Nicole 2013).
Some previous studies have reported adverse health impacts of CAFOs including respiratory dysfunction, lower immune function, exacerbation of pre-existing chronic conditions, mental health, and poorer quality of life for farm workers and the nearby community, however, there remains limited evidence of CAFOs exposure on health outcomes and the results are inconclusive (Guidry et al. 2018; O’Connor et al. 2017; Schinasi et al. 2011; Schultz et al. 2019; Wing and Wolf 2000). Moreover, most studies investigating the health impacts of CAFOs-related exposure focused on specific health outcomes such as respiratory-related health effects. Studies investigating the effect of CAFOs exposure on other health outcomes are limited. A previous systematic review of associations between living near animal feeding operation and human health outcomes showed that findings for non-respiratory health outcomes were inconclusive due to the small number of studies or inconsistent results between studies (O’Connor et al. 2017).
Several epidemiological studies have reported that CAFOs are disproportionately located in disadvantaged communities with high levels of people of color or those of low socioeconomic status (SES) (Nicole 2013; Wilson et al. 2002; Wing et al. 2000; Wing et al. 2008). Those populations may be more likely to experience a disproportionate health burden from the exposures. Previous studies on environmental justice have investigated disproportionate CAFOs-related exposure and health burden among surrounding populations. Wing and Wolf (2000) found that incidence of respiratory, gastrointestinal, and mucous membrane irritation was elevated for residents living near swine CAFOs in eastern North Carolina. Some studies have reported higher prevalence of asthma associated with CAFOs exposure for susceptible populations such as children (Merchant et al. 2005; Sigurdarson and Kline 2006). These health impacts may be exacerbated by limited access to protective measures such as air conditioning as well as medical care for some populations. While attention to CAFOs has been growing, research on the disproportionate health burden among population living near CAFOs remains limited. Spatial clustering of CAFOs in low-income and minority communities in North Carolina (NC) has raised environmental justice concerns. Addressing the public health implications of environmental justice from CAFOs is critical given that livestock production is a major industry and NC has a large number and extensive history of CAFOs. Although a few studies have investigated disproportionate CAFOs exposure and associated health burdens for populations living near CAFOs (Mirabelli et al. 2006; Schultz et al. 2019), more studies considering various health outcomes and advanced CAFOs exposure assessment are needed. In particular, most previous studies used simplistic exposure methods such as the presence or absence of a CAFO within a given spatial area. We examined several cause-specific mortality risks associated with CAFOs exposure based on different CAFOs exposure approaches for NC from 2000 to 2017. We evaluated whether the association between exposure to CAFOs and risk of mortality varied by individual- and community-level characteristics.
2. Methods
Individual-level data on cause-specific mortality for NC from 2000 to 2017 were obtained from the North Carolina State Center for Health Statistics, Vital Statistics Department. Mortality data included date of death, cause of death, residential location, sex, race/ethnicity, age at death, and highest level of education. We estimated association between exposure to CAFOs and five causes of mortality: (1) cardiovascular disease (International Classification of Diseases, ICD-10, I00-I99); (2) respiratory disease (J00-J99); (3) asthma (J45); (4) anemia (D50-D53, D55-D59, D60-D64); and (5) kidney disease (N00-N19) as the primary cause of death. These causes were selected based on a previous literature review (Kravchenko et al. 2018).
To estimate CAFOs exposure for each participant, we used data on permitted animal facilities from the NC Department of Environmental Quality (NC DEQ 2016) for the year 2019 due to data availability. This dataset includes information on the operation such as facility name, permit number, and location for facilities in operation through February 2019, including facilities operating in previous years, including our study years of 2000 to 2017. We also performed sensitivity analysis using only mortality data from 2010 to 2017 to confirm the robustness of findings, given the temporal mismatch between the mortality data and the available data on CAFO operation.
To evaluate health disparities by several individual-level factors in the association between exposure to CAFOs and mortality, we used sex, race/ethnicity (Non-Hispanic White, Non-Hispanic Black, other), age at death (≤17, 18-59, 60-74, and ≥75 years), and education (middle school or less, some high school, high school, some college/associate degree, and bachelor’s degree or above).
To assess community-level effect modification, we used 2010 Census data at the census tract level including variables of median household income, as a surrogate for SES, and the Census Bureau’s urban-rural classification, which classified urbanicity as urban area (UA, ≥50,000 people), urban cluster (UC, 2,500-49,999 people), and rural area (<2,500 people). We categorized median household income as quartiles. We also included an indicator variable for region (i.e., Mountains, Piedmont, and Coastal plain) to consider NC’s physical, social, and economic regional characteristics.
To assign CAFOs exposure for each participant, we first generated buffers (5, 10, 15, and 20 km) around each participant’s residential location. We then calculated the number of CAFOs within each buffer for each participant. For each participant, we used several methods of assigning exposure to CAFOs. The first method assessed the presence (or absence) of CAFOs within each buffer around place of residence. The second method assigned exposure as no, low, medium, and high exposure groups (the latter three divided by tertiles) based on the number of CAFOs within a 15 km buffer around place of residence to account for intensity of CAFOs exposure. We also considered the number of CAFOs within a buffer. We refer to these methods as the binary indicator (i.e., presence or absence of one or more CAFOs within a specified buffer distance), level of exposure (i.e., low, medium, or high based on number of CAFOs within the buffer distance), and number of CAFOs (i.e., sum of the number of CAFOs within the buffer distance). Although we use the language “level of exposure”, we recognize that actual exposure to impacts from CAFOs (e.g., on air, water, noise, odor) are more complex than the number of CAFOs within a buffer. However, these approaches improve on earlier work. Previous studies often assessed exposure using residential proximity to the nearest CAFO, however, this exposure metric may not fully reflect the intensity of CAFO exposure especially in clustered CAFOs in NC. Thus, our approach is an advancement over previous methods to estimate exposure to CAFOs. Our earlier work on CAFO exposure metrics suggests that a more refined metric may better capture CAFOs exposure from multiple facilities beyond the boundaries than the simpler methods (Son et al. 2021).
To investigate the association between exposure to CAFOs and cause-specific mortality, we applied logistic regression models. This compares the risk of mortality across two population groups: those with the presence of a CAFO and those with absence of a CAFO. This analysis was repeated separately for each buffer size. Analysis was conducted separately for each cause of mortality.
First, we estimated the risk of each cause-specific mortality associated with presence of CAFOs exposure within each buffer. We compared effect estimates by several buffer sizes (i.e., 5, 10, 15, 20 km) and then chose a 15 km buffer for further analysis of level of CAFOs exposure. We selected a 15 km buffer based on the Akaike information criterion (AIC), distribution of the number of CAFOs by each buffer, and sample size. This distance represents the possible range of manure application and transport of emission from CAFOs. Previous studies suggested that exporting liquid manure from CAFOs is usually limited to an area within 15 km from the facility due to economic feasibility (Bergström et al. 2005; Long et al. 2018). We categorized CAFOs exposure groups as no, low, medium, and high CAFOs exposures. Models were adjusted for sex, age, race/ethnicity, education, median household income, urbanicity, year, season, and region. Presence of CAFOs (yes) and CAFOs exposure of low, medium, and high group were compared with the no CAFO exposure group, and odds ratios (ORs) and 95% confidence intervals (CIs) for each exposure group were estimated. The reference group for these analysis is that of no exposure. Stratified analyses were conducted by each buffer size, health outcome, and several individual- and community-level characteristics. SAS version 9.4 and ArcGIS Pro 10.6.1 (ESRI, Redlands, CA) were used for all statistical analyses (SAS Institute, Cary, NC, USA).
3. Results
Descriptive statistics of the study population by each buffer size and by CAFOs exposure level are provided in Table 1 with additional details in Supplemental Table 1. Death from cardiovascular disease accounted for 33.5% of all deaths, followed by respiratory disease (11.1%), kidney disease (2.4%), asthma (0.1%), and anemia (0.1%). Cause-specific mortality patterns were generally similar across all buffer sizes and the CAFOs exposure group based on the presence of CAFOs (yes/no). The majority of the study population was Non-Hispanic White (77.5%). There were slightly higher percentages of females, older persons, those with high school education, $25,000-$49,999 median household income, those living in urbanized areas, and those living in the Piedmont, compared to other persons. The CAFOs exposure group (i.e., presence of CAFO) had higher percentages of Non-Hispanic Black, adults (18-59, 60-74 years), lower education, people with low median household income, people living in rural areas, and people living in coastal plains compared to the no CAFO exposure group. These trends were similar across all buffer sizes. Also, these trends by CAFOs exposure level were distinct as exposure level increased from low CAFOs exposure to high CAFOs exposure.
Table 1.
Characteristics of the study population by CAFOs exposure level based on the number of CAFOs within a 15 km buffer around residence
| Characteristics | Total number of deaths (n=1,195,272) |
CAFOs exposure for 15 km buffer around residence (%) | |||
|---|---|---|---|---|---|
| No CAFO exposure (n=348,394) |
Low (n=268,128) |
Medium (n=300,493) |
High (n=278,257) |
||
| Cause of Death | |||||
| Cardiovascular | 400,440 (33.5) | 112,985 (32.4) | 88,177 (32.9) | 101,359 (33.7) | 97,919 (35.2) |
| Respiratory | 133,123 (11.1) | 38,668 (11.1) | 30,224 (11.3) | 33,769 (11.2) | 30,462 (11.0) |
| Asthma | 1,667 (0.1) | 465 (0.1) | 350 (0.1) | 417 (0.1) | 435 (0.2) |
| Anemia | 1,212 (0.1) | 354 (0.1) | 251 (0.1) | 296 (0.1) | 311 (0.1) |
| Kidney | 28,126 (2.4) | 8,021 (2.3) | 6,120 (2.3) | 7,138 (2.4) | 6,847 (2.5) |
| Other | 630,704 (52.8) | 187,901 (53.9) | 143,006 (53.3) | 157,514 (52.4) | 142,283 (51.1) |
| Sex (%) | |||||
| Male | 572,949 (47.9) | 166,418 (47.8) | 127,676 (47.6) | 143,839 (47.9) | 135,016 (48.5) |
| Female | 622,303 (52.1) | 181,972 (52.2) | 140,448 (52.4) | 156,650 (52.1) | 143,233 (51.5) |
| Missing | 20 (<0.01) | 4 (0.01) | 4 (0.01) | 4 (0.01) | 8 (0.01) |
| Race/Ethnicity | |||||
| Non-Hispanic White | 925,132 (77.5) | 277,792 (79.8) | 211,299 (78.9) | 234,827 (78.2) | 201,214 (72.4) |
| Non-Hispanic Black | 244,162 (20.4) | 63,373 (18.2) | 52,370 (19.6) | 59,415 (19.8) | 69,004 (24.8) |
| Other | 24,947 (2.1) | 6,911 (2.0) | 4,218 (1.6) | 6,033 (2.0) | 7,785 (2.8) |
| Missing | 1,031 (0.09) | 318 (0.09) | 241 (0.09) | 218 (0.07) | 254 (0.09) |
| Age at death (years) | |||||
| ≤17 | 11,271 (1.0) | 3,400 (1.0) | 2,465 (0.9) | 2,726 (0.9) | 2,680 (1.0) |
| 18-59 | 186,911 (15.7) | 52,941 (15.3) | 40,621 (15.2) | 46,381 (15.5) | 46,968 (17.0) |
| 60-74 | 322,804 (27.2) | 90,588 (26.2) | 71,417 (26.8) | 80,674 (27.0) | 80,125 (29.0) |
| ≥75 | 667,240 (56.2) | 199,463 (57.6) | 152,062 (57.0) | 168,970 (56.6) | 146,745 (53.1) |
| Missing | 7,046 (0.6) | 2,002 (0.6) | 1,563 (0.6) | 1,742 (0.6) | 1,739 (0.6) |
| Education | |||||
| Middle school or less | 224,225 (19.0) | 57,595 (16.7) | 47,283 (17.9) | 57,641 (19.5) | 61,706 (22.5) |
| Some High school | 219,993 (18.7) | 55,422 (16.1) | 47,622 (18.0) | 57,050 (19.3) | 59,899 (21.8) |
| High school | 393,092 (33.3) | 113,618 (33.0) | 87,245 (33.0) | 98,309 (33.2) | 93,920 (34.2) |
| Some college/Associate Degree | 161,510 (13.7) | 52,871 (15.4) | 37,407 (14.1) | 39,624 (13.4) | 31,608 (11.5) |
| Bachelor’s Degree or above | 180,702 (15.3) | 64,733 (18.8) | 45,021 (17.0) | 43,702 (14.8) | 27,246 (9.9) |
| Missing | 15,750 (1.3) | 4,155 (1.2) | 3,550 (1.3) | 4,167 (1.4) | 3,878 (1.4) |
| Community-level actors | |||||
| Median annual household income | |||||
| < $25,000 | 73,435 (6.1) | 23,485 (6.7) | 13,902 (5.2) | 14,561 (4.9) | 21,487 (7.7) |
| $25,000-$49,999 | 765,792 (64.1) | 193,269 (55.5) | 155,728 (58.1) | 202,948 (67.5) | 213,847 (76.9) |
| $50,000-$99,999 | 336,579 (28.2) | 121,372 (34.8) | 93,399 (34.8) | 78,885 (26.3) | 42,923 (15.4) |
| > $99,999 | 19,466 (1.6) | 10,268 (3.0) | 5,099 (1.9) | 4,099 (1.4) | 0 (0.0) |
| Urbanicity | |||||
| Urbanized area | 696,516 (58.3) | 250,184 (71.8) | 171,710 (64.0) | 187,409 (62.4) | 87,213 (31.3) |
| Urban cluster | 355,776 (29.8) | 64,401 (18.5) | 77,072 (28.7) | 84,434 (28.1) | 129,869 (46.7) |
| Rural area | 142,980 (12.0) | 33,809 (9.7) | 19,346 (7.2) | 28,650 (9.5) | 61,175 (22.0) |
| Region | |||||
| Mountain | 192,820 (16.1) | 89,684 (25.7) | 45,329 (16.9) | 47,927 (16.0) | 9,880 (3.6) |
| Piedmont | 649,988 (54.4) | 203,466 (58.4) | 171,135 (63.8) | 186,061 (61.9) | 89,326 (32.1) |
| Coastal plain | 352,463 (29.5) | 55,243 (15.9) | 51,664 (19.3) | 66,505 (22.1) | 179,051 (64.4) |
Note: CAFOs exposure group was based on the number of CAFOs within a 15 km buffer around residence; low, medium, and high as tertiles
Number of CAFOs based on 15km buffer: low <3, medium 3-7, and high ≥8 CAFOs
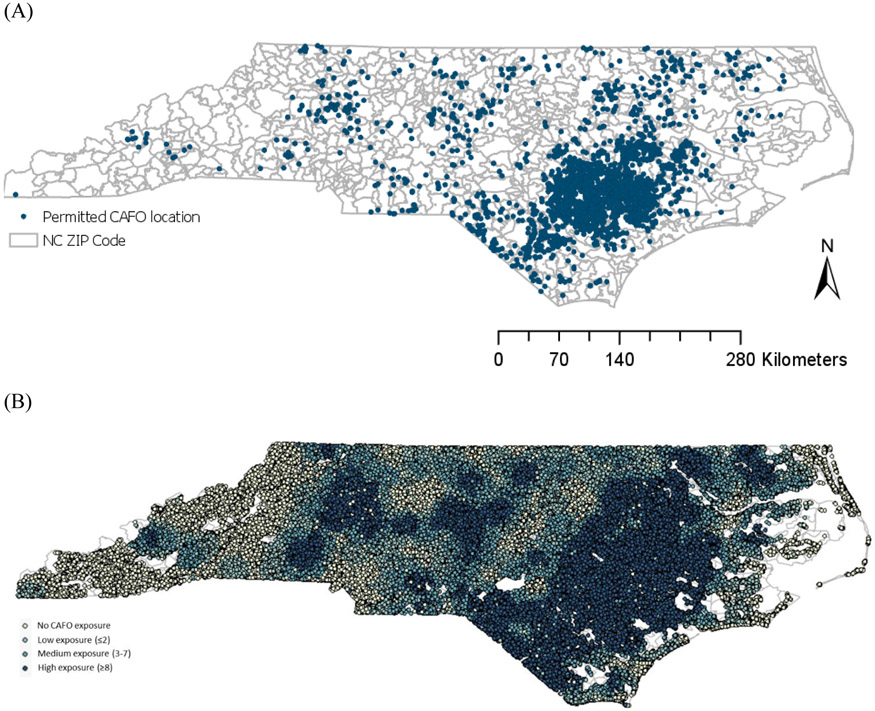
Figure 1 shows spatial distributions of CAFOs locations and each participant’s CAFOs exposure level based on the number of CAFOs within a 15 km buffer around each residence. There were a total of 2,577 CAFOs operating in NC in 2019. Most CAFOs were clustered and located in what is referred to as the Coastal Plain of NC, although some CAFOs operated in central NC and western NC as well.
Figure 1.
(A) Locations of CAFOs in NC (2019) and (B) CAFO exposure levels for each participant based on the number of CAFOs using a 15 km buffer around each residence
Distribution of the number of CAFOs within each buffer is provided in Supplemental Table 2. Average number of CAFOs within a 15 km buffer around each residence was 12, ranging from 1 to 247 CAFOs. Supplemental Table 3 shows correlations among selected variables. CAFOs exposure level was positively correlated with the indicator variable for region and urbanicity and was negatively correlated with median household income and education.
Table 2 shows the odds ratios and 95% CIs for risk of cause-specific mortality associated with CAFOs exposure, based on separate models for each cause of death. We first estimated risks based on the presence of CAFOs within various buffers around residence. Presence of CAFOs exposure was significantly associated with higher risk of cardiovascular mortality compared to absence of CAFOs for all buffer sizes (i.e., 5, 10, 15, and 20 km). For example, those within 15 km of a CAFO were 1.036 (95% CI: 1.027 to 1.046) more likely to die from cardiovascular disease than those without a CAFO within this distance. Although the associations were not statistically significant, presence of CAFOs based on a 15 km buffer was associated with higher risk of mortality from respiratory disease, asthma, anemia, and kidney disease. For example, those within 15 km of a CAFO were 1.018 (95% CI: 0.990, 1.047) more likely to die from kidney disease than those without a CAFO within this distance. We also estimated risk of cause-specific mortality by level of CAFOs exposure (i.e., low, medium, and high exposure) based on the number of CAFOs within a 15 km buffer, which showed positive associations for all mortality outcomes considered. We found significantly positive associations between cardiovascular mortality and all levels of CAFOs exposure (i.e., low, medium, and high exposure) compared to no exposure. We also found an increasing trend of mortality risk from cardiovascular disease with higher levels of CAFOs exposure. Compared with the no CAFO exposure group, the ORs for cardiovascular mortality were 1.01 (95% CI 1.00, 1.03), 1.04 (95% CI 1.03, 1.06), and 1.06 (95% CI 1.05, 1.07) for low, medium, and high CAFOs exposure group, respectively. The results of sensitivity analysis using data from 2010-2017 showed generally similar results with original findings (Supplemental Table 4). We note that using the intensity of exposure, rather than just presence or absence of exposure, helps clarify the relationship between exposure and health outcome. The ORs comparing the high CAFOs exposure group to the no exposure group was statistically significant for mortality from anemia and kidney disease.
Table 2.
Results of logistic regression analysis assessing risk of cause-specific mortality associated with different CAFOs exposure: Odds ratios (95% confidence intervals) comparing risk in CAFOs exposure group to no exposure group, using different buffer sizes to assess exposure.
| CAFOs exposure |
Cause of death | ||||
|---|---|---|---|---|---|
| Cardiovascular | Respiratory | Asthma | Anemia | Kidney | |
| Presence of CAFOs within each buffer | |||||
| 5 km | 1.022 (1.011, 1.032) | 0.988 (0.972, 1.003) | 1.037 (0.914, 1.175) | 1.066 (0.916, 1.241) | 1.020 (0.988, 1.053) |
| 10 km | 1.040 (1.031, 1.048) | 0.989 (0.977, 1.001) | 0.989 (0.892, 1.097) | 0.977 (0.866, 1.103) | 1.031 (1.005, 1.058) |
| 15 km | 1.036 (1.027, 1.046) | 1.007 (0.994, 1.020) | 1.017 (0.907, 1.140) | 1.003 (0.880, 1.143) | 1.018 (0.990, 1.047) |
| 20 km | 1.039 (1.027, 1.050) | 1.002 (0.986, 1.019) | 0.966 (0.839, 1.111) | 1.155 (0.975, 1.367) | 1.017 (0.983, 1.053) |
| CAFOs exposure based on the number of CAFOs within a 15 km buffer | |||||
| Low | 1.014 (1.002, 1.025) | 1.017 (1.000, 1.033) | 0.996 (0.865, 1.147) | 0.914 (0.775, 1.077) | 0.984 (0.951, 1.018) |
| Medium | 1.044 (1.033, 1.055) | 1.002 (0.986, 1.018) | 1.043 (0.910, 1.195) | 0.988 (0.843, 1.157) | 1.021 (0.988, 1.055) |
| High | 1.060 (1.047, 1.073) | 1.000 (0.981, 1.018) | 1.005 (0.863, 1.171) | 1.203 (1.006, 1.438) | 1.073 (1.033, 1.114) |
Note: CAFO category was based on the presence of CAFOs within each buffer around residence or tertiles group (low, medium, and high) based on the number of CAFOs within a 15 km buffer. Models were adjusted for sex, age, race/ethnicity, education, median household income, urbanicity, year, season, and region.
Each cause of death was analyzed separately.
Figure 2 and Supplemental Table 5 show ORs and 95% CIs for cardiovascular mortality stratified by individual- and community-level characteristics, whereas unstratified results are shown in Table 2. Figure 2 compares estimates for the high CAFOs exposure group with no CAFO exposure group. The ORs for CAFOs exposure of the low, medium, and high groups compared with no CAFO exposure group are provided in Supplemental Table 4. Although the ORs for high CAFOs exposure group compared with no CAFO exposure were slightly higher in females, non-Hispanic Whites, people ≤17 years, those with high education level, people with high median household income, residents of urban areas, and those in the Piedmont compared to other persons, they were not statistically different.
Figure 2.
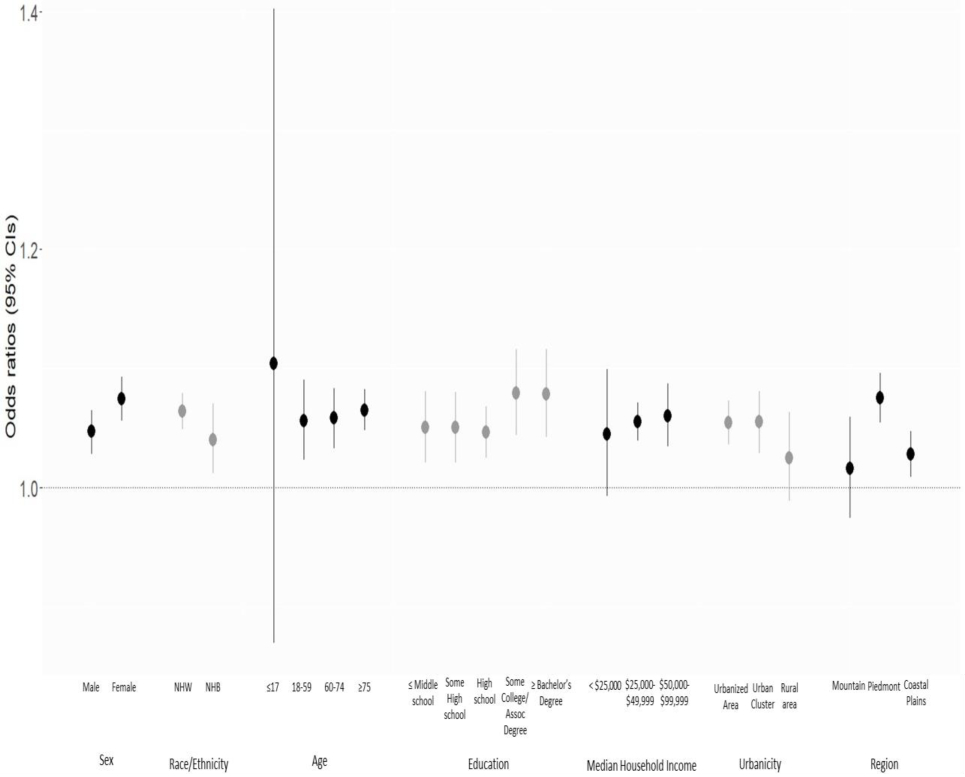
Results of logistic regression analysis assessing the odds ratio for risk of cardiovascular mortality for the high exposure CAFOs group compared to the no CAFO exposure group, by individual- and community-level characteristics.
Note: NHW = non-Hispanic White. NHB = non-Hispanic Black. There was no estimate in > $99,999 median household income category due to no Census tracts with median income in the high exposure category.
4. Discussion
We evaluated the risk of cause-specific mortality, including health disparities, associated with CAFOs exposure based on different CAFOs exposure approaches (e.g., various buffer sizes, exposure level) in NC. We found that presence of CAFOs was associated with higher risk of mortality. Especially, for cardiovascular mortality, those living near CAFOs had significantly higher risk of mortality than those with no CAFO exposure for all buffer sizes. We also found an increasing trend of higher risk of cardiovascular mortality with higher levels of CAFOs exposure. The risk for the high CAFOs exposure group was significantly higher than for the no CAFO exposure group for mortality from anemia and kidney disease. Findings by individual- and community-level characteristics showed that the ORs for the high exposure group compared with no CAFO exposure were higher in some subpopulations, but these differences were not statistically different.
Our results provide consistent evidence that CAFOs exposure was associated with harmful effects on health. All the causes of mortality that we investigated were positively associated with presence of CAFOs exposure based on a 15 km buffer, although results were not statistically significant except for cardiovascular mortality. Also, for analysis by CAFOs exposure level, we found an increasing trend with higher risk of cardiovascular mortality as the level of CAFOs exposure increased. Although we did not observe significant positive effect of CAFOs exposure with respiratory outcomes, other studies have suggested that people living near CAFOs had significantly higher risk of respiratory-related health outcomes such as asthma (Pavilonis et al. 2013; Borlée et al. 2017). A recent study suggested that CAFOs may be an important source of adverse air quality associated with reduced respiratory and allergic health among rural residents living near CAFOs (Schultz et al. 2019). Another study (Domingo et al. 2021) estimated the air quality-related health impacts of agriculture in the United States and found that food production results in 15,900 PM2.5-related annual deaths. Kravchenko et al. (2018) found that residents living near hog CAFOs in NC had higher risks for various health outcomes including mortality due to infections, anemia, kidney disease, perinatal conditions, and hospital admissions and emergency department visits for low birth weight infants. Other studies reported that odor from CAFOs may affect quality of life for people living near CAFOs such as socializing with others, working outside, and opening windows for ventilation (Wing and Wolf 2000; Tajik et al. 2008). However, some studies reported opposite or null effects for people living near confined livestock operations (Radon et al. 2007). Hooiveld et al. (2016) examined the association between the CAFOs exposure and respiratory and gastrointestinal conditions. They reported that no association was found between swine, cattle, and poultry CAFOs and respiratory, allergic or gastrointestinal conditions, although pneumonia and unspecified infectious diseases were positively associated with the number of goat CAFOs near residents’ homes. Population risk by health outcomes may differ by several factors and depend on intensity and duration of exposure, individual susceptibility, acute vs. chronic exposure (e.g., high exposure for short time, low exposure for long time). More studies considering various health outcomes, populations, and accurate exposure assessment are needed to understand the health effects associated with CAFOs exposure, as well as work to disentangle the impacts of the various pathways through which CAFOs can impact health (e.g., water quality, air pollution), and how impacts may differ by type of CAFO and health outcome, including cause of death.
CAFOs emissions from several sources including the animal themselves, their manure, manure applied to fields, and waste lagoons may cause harmful effects to humans and environments. Animal wastes emit harmful pollutants as they decompose and are dispersed in the atmosphere and deposited to the land surface. Some of these pollutants may travel several miles (Wing et al. 2008) and cause negative health effects to people far from the CAFO. Contaminated surface and ground water pollution from CAFOs lagoons can also affect other areas by transporting wastes during rainfall or hurricane (Heaney et al. 2015; Wing et al. 2002). These complex mixtures of CAFOs air emissions (e.g., particulate matter (PM), hydrogen sulfide, ammonia), deterioration of water quality, and soil contamination can affect human health via several mechanisms. Health effects associated with inhalation of toxins and bioaerosols include respiratory diseases such as asthma, bronchitis, cardiovascular events, and neuropsychiatric conditions. Some possible mechanisms include interactive effect or synergism between PM and/or endotoxin and/or ammonia with regard to respiratory effect and declines in lung function; ammonia can damage clearance mechanisms (cilia) in the upper respiratory tract thereby increasing inhalation of particles (Iowa working report 2002). Bacterial and fungal bioaerosols of PM from CAFOs include endotoxins, exotoxins, lipoteichoic acids, which is a potent inflammatory agent that produce systemic effects and lung obstruction and has been associated with lung inflammation (Iowa working report 2002). Hazardous gases, including volatile organic compounds (VOC) emitted from manure storage piles and lagoons especially from microbial degradation of liquid manure, may act as sensory and respiratory irritants. These pollutants are associated with nasal, sinus, and eye irritation, wheezing, odors (Hribar and Schultz 2010). Previous study suggested that airborne contaminants (e.g., hazardous gases, dusts, bioaerosols, odors), soil transport of microbes and contaminated water sources from wastes and leaking lagoons from swine CAFOs can adversely affect health through several pathways such as infectious agents, direct irritant and psychophysiologic mechanisms (Cole et al. 2000).
Inconsistency in findings across studies could be due to differences in CAFOs characteristics such as management system of manure and various CAFOs exposure metrics applied across studies. Intensity of emissions may differ by CAFO size, animal type, management practices and controls. Also, although many studies have used several exposure metrics such as distance to the nearest CAFO or density of CAFOs, there is no consistent and comprehensive exposure metric to assess CAFOs exposure, thus we applied multiple metrics based on our earlier work (Son et al. 2021). Differences in the impacts of CAFOs on water, noise, odor, and air, and the influence of characteristics of CAFOs on these impacts, are not fully disentangled by the current methods to assess exposure to CAFOs, including the approach used in this study and the more simplistic methods such as binary indicators of the presence or absence of a CAFO within a specified buffer distance. Furthermore, CAFOs tend to cluster in communities with higher percentage of minorities and in low-income communities. Thus, multiple factors including SES, baseline health conditions, and community-level resources may play an important role in these associations.
Previous work has raised concerns about disproportionate exposure to environmental hazards for low income and minority communities. Our work indicates two pathways of disparities in relation to CAFOs: first, we found higher exposure for minority and low-income communities, and second, we found suggestive evidence of higher risk of mortality associated with CAFO exposure, although some of findings were not statistically significant and warrant further investigation. In this study and our earlier work (Son et al. 2021), we found disproportionate siting of CAFOs in areas with higher percentages of Non-Hispanic Black, those with low education, and people with low median household income. This indicates higher levels of exposure for those vulnerable populations. Further, we found suggestive evidence of higher risk of mortality associated with CAFOs for some populations such as younger persons and people living in urban areas. Consistent with our findings, previous studies showed disproportionate distributions of CAFO locations in relation to race and SES. They reported that CAFOs were located and clustered disproportionately in areas with high percentage of minorities, persons with low educated, and people living in poverty (Lenhardt and Ogneva-Himmelberger 2013; Wing et al. 2000). In addition to the environmental disparities from CAFOs exposures, there may be disparities in the health responses for a given level of exposure to CAFOs. Children, the elderly, and people with pre-existing conditions were reported to be at higher risk of health outcomes (e.g., asthma) associated with CAFOs exposure (Hribar and Schultz 2010; Merchant et al. 2005; Sigurdarson and Kline 2006). Sneeringer et al. (2009) found that increased risk of infant mortality was associated with air pollution in the proximity of livestock farming operations. Another study reported that adolescents attending public schools near CAFOs (within 3 miles) had increased rates of asthma symptoms compared to those attending schools beyond 3 miles (Mirabelli et al. 2006). In this study, we found that the ORs for the high CAFOs exposure group compared with no CAFO exposure were slightly higher in those with high education level, although they were not statistically different. Education is often used as one of indicators of SES, however, it can relate to several factors that affect health such as access to health care, presence of health insurance, baseline health status, and health behaviors (e.g., smoking, exercise). To better understand environmental health disparities associated with CAFOs exposure, further studies considering complex exposure to CAFOs for vulnerable populations are needed, with attention to disparities both by exposure and by health response.
Our study has several limitations. We considered cause-specific mortality based on the primary cause of death. While this approach is commonly used across studies, future work could investigate additional causes due to the multiple possible contributors to mortality and multiple co-morbidities. Although we controlled for many confounding factors in the model, confounding by other factors that may affect the association between CAFOs exposure and mortality may remain. For example, we could not consider individual-level information on the history of chronic disease or smoking status, which may affect risk, especially for respiratory health outcomes. Weather conditions, topography, wind direction or speed could affect exposure. Our CAFOs exposure was based on participants’ residential locations, and while our approach improves on earlier studies, this method does not fully capture exposure to pollutants and conditions from CAFOs, such as occupational exposure. Further, people do not spend the whole day at home so exposure misclassification may exist. Assessing accurate exposure to CAFOs is difficult due to complexities of CAFOs exposure, with multiple pathways through which CAFOs could impact health. We used the number of CAFOs within buffers as an indicator of CAFOs exposure level, which assigns a large number of CAFOs to higher exposure levels. However, this approach may not fully reflect exposure intensity as several characteristics such as CAFO size, type and number of animals, and manure production may affect the intensity of CAFOs exposure.
To better capture CAFOs exposure, future research should consider more advanced exposure assessment such as direct measures of exposure or exposure intensity incorporating the type and size of CAFOs, manure management and control systems, with detailed data on CAFO characteristics and consider using different buffer sizes and operation history to assess exposure. Our dataset from the NC DEQ has some limitations. The dataset does not capture most facilities operating with dry waste management, most notable poultry CAFOs (88.3% of the included CAFOs were for swine, 10.6% were for cattle and 0.74% were for poultry). Also, we only included regulated CAFOs with provided information due to data availability. We do not have information on facilities such as smaller facilities with fewer animals, which are not regulated. Future work is needed to consider multiple data sources to include other types of animals or additional facilities.
5. Conclusion
Our study adds to the literature on health outcomes associated with CAFOs, indicating that proximity to these facilities increases risk of mortality. We considered multiple causes of mortality and various individual- and community-level characteristics such as race/ethnicity, SES, and urbanicity. We also compared mortality risks based on several CAFOs exposure using different buffer sizes and exposure level. We found that CAFOs exposure was associated with increased risk of mortality in nearby communities. These results suggest the need to address potentially harmful impacts from CAFOs and can inform future study on health disparities associated with CAFOs exposure. To further understand the health implications of CAFOs, more research considering accurate exposure assessment, various health outcomes, and multiple disparity factors is needed. Such work can aid policy makers in establishing appropriate interventions.
Supplementary Material
Highlights.
Presence of CAFOs was associated with higher risk of mortality.
People living near CAFOs had significantly higher risk of cardiovascular mortality than other persons.
We found increasing trend of higher risk of cardiovascular mortality with higher levels of CAFOs exposure.
Findings have implications for future studies of environmental justice and CAFOs.
Acknowledgements
The authors thank Rebecca L. Muenich and Danica Schaffer-Smith for guidance on data used to estimate exposure to CAFOs.
Funding Sources
This publication was developed under Assistance Agreement No. RD835871 awarded by the U.S. Environmental Protection Agency to Yale University. It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency
Research reported in this publication was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD012769. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CAFOs
Concentrated animal feeding operations
- CI
confidence interval
- OR
odds ratio
- SES
socioeconomic status
Footnotes
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergström L, Bowman BT, Sims JT. 2005. Definition of sustainable and unsustainable issues in nutrient management of modern agriculture. Soil Use and Management 21:76–81. [Google Scholar]
- Borlée F, Yzermans CJ, Aalders B, Rooijackers J, Krop E, Maassen CBM et al. 2017. Air pollution from livestock farms is associated with airway obstruction in neighboring residents. Am J Respir Crit Care Med 196:1152–1161. [DOI] [PubMed] [Google Scholar]
- Cole D, Todd L, Wing S. 2000. Concentrated swine feeding operations and public health: A review of occupational and community health effects. Environ Health Perspect 108:685–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo NGG, Balasubramanian S, Thakrar SK, Clark MA, Adams PJ, Marshall JD et al. 2021. Air quality-related health damages of food. PNAS 118(20):e2013637118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donham K, Reynolds S, Whitten P, Merchant J, Burmeister L, Popendorf W. 1995. Respiratory dysfunction in swine production facility workers: dose-response relationships of environmental exposures and pulmonary function. Am J Ind Med 27:405–418. [DOI] [PubMed] [Google Scholar]
- Guidry VT, Rhodes SM, Woods CG, Hall DJ, Rinsky JL. 2018. Connecting environmental justice and community health Effects of hog production in North Carolina. NCMJ 79(5):324–328. [DOI] [PubMed] [Google Scholar]
- Heaney CD, Myers K, Wing S, Hall D, Baron D, Stewart JR. 2015. Source tracking swine fecal waste in surface water proximal to swine concentrated animal feeding operations. Sci Total Environ 511:676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooiveld M, Smit LAM, van der Sman-de Beer F, Wouters IM, van Dijk CE, Spreeuwenberg P et al. 2016. Doctor-diagnosed health problems in a region with a high density of concentrated animal feeding operations: a cross-sectional study. Environmental Health 15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hribar C, Schultz M. 2010. Understanding concentrated animal feeding operations and their impact on communities. National association of local boards of health: Bowling Green, KY, USA. Available at: https://www.cdc.gov/nceh/ehs/Docs/Understanding_CAFOs_NALBOH.pdf Accessed on 20 November 2020. [Google Scholar]
- Iowa concentrated animal feeding operations air quality study Final report. 2002. Iowa State University and the University of Iowa Study Group. Available at: Iowa concentrated animal feeding operations air quality study; (oregonlegislature.gov) Accessed on 20 November 2020. [Google Scholar]
- Kravchenko J, Rhew SH, Akushevich I, Agarwal P, Lyerly HK. 2018. Mortality and health outcomes in North Carolina communities located in close proximity to hog concentrated animal feeding operations. North Carolina Medical Journal 79(5):278–288. [DOI] [PubMed] [Google Scholar]
- Lenhardt J, Ogneva-Himmelberger. 2013. Environmental injustice in the spatial distribution of concentrated animal feeding operations in Ohio. Environmental Justice 6(4):133–139. [Google Scholar]
- Long CM, Muenich RL, Kalcic MM, Scavia D. 2018. Use of manure nutrients from concentrated animal feeding operations. J Gt Lakes Res 44:245–252. [Google Scholar]
- Merchant JA et al. 2005. Asthma and farm exposures in a cohort of rural Iowa children. Environ Health Perspect 113(3):350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabelli MC, Wing S, Marshall SW, Wilcosky TC. 2006. Race, poverty, and potential exposure of middle-school students to air emissions from confined swine feeding operations. Environ Health Perspect 114(4):591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabelli MC, Wing S, Marshall SW, Wilcosky TC. 2006. Asthma symptoms among adolescents who attend public schools that are located near confined swine feeding operations. Pediatrics 118(1):e66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicole W. 2013. CAFOs and environmental justice: The case of North Carolina. Environ Health Prospect 121:A182–A189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NC Department of Environmental Quality (DEQ). 2016. NC Dept. of Environmental Quality Online GIS. https://data-ncdenr.opendata.arcgis.com/ [accessed 1 April 2019].
- Schiffman SS, Bennett JL, Raymer JH. 2001. Quantification of odors and odorants from swine operations in North Carolina. Agric. For. Meteorol 108:213–240. [Google Scholar]
- Schinasi L, Horton RA, Guidry VT, Wing S, Marshall SW, Morland KB. 2011. Air pollution, lung function, and physical symptoms in communities near concentrated swine feeding operations. Epidemiology 22(2):208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz AA, Peppard P, Gangnon RE, Malecki KM. 2019. Residential proximity to concentrated animal feeding operations and allergic and respiratory disease. Environ Int. 130:104911. [DOI] [PubMed] [Google Scholar]
- Schulze A, Rommelt H, Ehrenstein V, van Strien R, Praml G, Kuchenhoff H et al. 2011. Effects on pulmonary health of neighboring residents of concentrated animal feeding operations: exposure assessed using optimized estimation technique. Arch Environ Occup Health 66:146–154. [DOI] [PubMed] [Google Scholar]
- Sigurdarson ST, Kline JN. 2006. School proximity to concentrated animal feeding operations and prevalence of asthma in students. Chest 129:1486–1491. [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Auvermann BW, Dzikamunhenga RS, Glanville JM, Higgins JP, Kirychuk SP, Sargeant JM, Totton SC, Wood H, Von Essen SG. 2017. Updated systematic review: associations between proximity to animal feeding operations and health of individuals in nearby communities. Systematic Reviews 6:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavilonis BT, Sanderson WT, Merchant JA. 2013. Relative exposure to swine animal feeding operations and childhood asthma prevalence in an agricultural cohort. Environ Res 122:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radon K, Schulze A, Ehrenstein V, van Strien RT, Praml G, Nowak D. 2007. Environmental exposure to confined animal feeding operations and respiratory health of neighboring residents. Epidemiology 18:300–308. [DOI] [PubMed] [Google Scholar]
- Schultz AA, Schauer JJ, Malecki KM. 2017. Allergic disease associations with regional and localized estimates of air pollution. Environ Res. 155:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdarson ST, Kline JN. 2006. School proximity to concentrated animal feeding operations and prevalence of asthma in students. CHEST Journal 129(6):1486–1491. [DOI] [PubMed] [Google Scholar]
- Sneeringer S. 2009. Does animal feeding operation pollution hurt public health? A national longitudinal study of health externalities identified by geographic shifts in livestock production. Am J Agric Econ 91(1):124–137. [Google Scholar]
- Son JY, Muenich RL, Schaffer-Smith D, Miranda ML, Bell ML. 2021. Distribution of environmental justice metrics for exposure to CAFOs in North Carolina, USA. Environ Res 195:110862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajik M, Muhammad N, Lowman A, Thu K, Wing S, Grant G. 2008. Impact of odor from industrial hog operations on daily living activities. New Solut 18(2):193–205. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture (USDA). National Agricultural Statistics Service. https://www.nass.usda.gov/ [accessed 7 June 2021].
- Wilson SM, Howell F, Wing S, Sobsey M. 2002. Environmental injustice and the Mississippi hog industry. Environ Health Perspect 110(suppl 2):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing S, Cole D, Grant G. 2000. Environmental injustice in North Carolina’s hog industry. Environ Health Perspect 108:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing S, Wolf S. 2000. Intensive livestock operations, health, and quality of life among eastern North Carolina residents. Environ Health Perspect 108(3): 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing S, Freedman S, Band L. 2002. The potential impact of flooding on confined animal feeding operations in eastern North Carolina. Environ Health Perspect 110(4):387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing S, Horton RA, Marshall SW et al. 2008. Air pollution and odor in communities near industrial swine operations. Environ Health Perspect 116(10):1362–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.