Abstract
Extracellular vesicles (EVs) play important roles in various intercellular communication processes. The abscopal effect is an interesting phenomenon in cancer treatment, in which immune activation is generally considered a main factor. We previously developed a telomerase-specific oncolytic adenovirus, Telomelysin (OBP-301), and occasionally observed therapeutic effects on distal tumors after local treatment in immunodeficient mice. In this study, we hypothesized that EVs may be involved in the abscopal effect of OBP-301. EVs isolated from the supernatant of HCT116 human colon carcinoma cells treated with OBP-301 were confirmed to contain OBP-301, and they showed cytotoxic activity (apoptosis and autophagy) similar to OBP-301. In bilateral subcutaneous HCT116 and CT26 tumor models, intratumoral administration of OBP-301 produced potent antitumor effects on tumors that were not directly treated with OBP-301, involving direct mediation by tumor-derived EVs containing OBP-301. This indicates that immune activation is not the main factor in this abscopal effect. Moreover, tumor-derived EVs exhibited high tumor tropism in orthotopic HCT116 rectal tumors, in which adenovirus E1A and adenovirus type 5 proteins were observed in metastatic liver tumors after localized rectal tumor treatment. In conclusion, local treatment with OBP-301 has the potential to produce abscopal effects via tumor-derived EVs.
Keywords: extracellular vesicles, exosome, abscopal effect, oncolytic adenovirus, local treatment, systemic delivery, drug delivery system
Graphical abstract
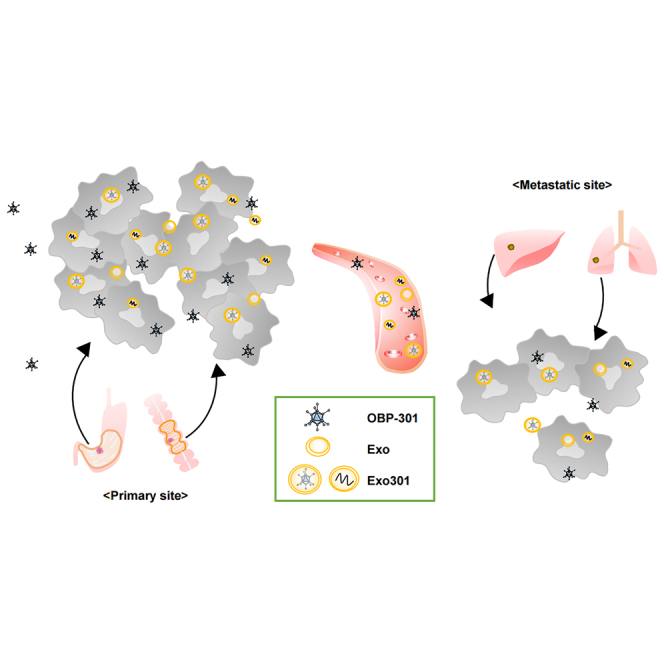
This study demonstrates a novel mechanism for the abscopal effect of oncolytic virotherapy, which differs from immune activation, that tumor-derived extracellular vesicles secreted after local treatment of a primary tumor with oncolytic viruses deliver viruses to a metastatic site and produce a direct antitumor effect on the metastatic tumor.
Introduction
Recently, extracellular vesicles (EVs) have garnered high levels of scientific interest, as they are well known to play important roles in various intercellular communication processes such as immune responses, delivery systems, and others.1,2 EVs are recognized to carry cellular components such as proteins, messenger RNA (mRNA), microRNA (miRNA), DNA, lipids, and transcriptional factors. In the field of cancer research, the involvement of cancer-derived EVs in mediating paracrine signaling in the tumor microenvironment, as well as cancer development and metastasis, is now recognized.3 Furthermore, certain molecules that are key to their functionality have been identified.4, 5, 6 However, determining the origins and functions of specific EV subsets is difficult or impossible.7,8 EVs consisting of various subtypes (in terms of size, electrical potential, density, morphology, marker expression, and dependency for a specific enzyme) are divided into several categories, including exosomes (Exos) (30–150 nm in diameter) derived from the endocytic pathway.9, 10, 11 Key enzymes for the biogenesis of Exos include NSMASE2 and RAB27A, while the secretion of Exos is blocked by Exo inhibitors such as the selective inhibitor of a neutral sphingomyelinase GW4869.6,10,12
The abscopal effect was first reported in 1953 as a phenomenon involving tumor regression at a site distal to the primary site of radiotherapy,13 and its mechanism is currently thought to involve activation of the immune system.14,15 This phenomenon has attracted more attention since the advent of immunotherapy drugs such as immune checkpoint inhibitors (ICIs).16,17 In addition to radiotherapy, other localized treatments such as oncolytic virotherapy have also been reported to induce the abscopal effect and produce potent antitumor effects on metastatic tumors, especially in combination with ICIs.18, 19, 20
We previously developed a telomerase-specific oncolytic adenovirus, Telomelysin (OBP-301), which is designed to replicate selectively in tumor cells and induce oncolytic cell death.21 The safety of intratumoral administration of Telomelysin in humans was proven in a phase I clinical trial performed in the United States.22 Based on its safety profile and promising preclinical results of combination therapy with ionizing radiation, a phase I/II clinical trial to evaluate the safety and efficacy of this combination in patients with esophageal cancer is currently underway in Japan.23,24
In the process of developing OBP-301, we occasionally observed therapeutic effects on metastatic tumors after local administration of OBP-301 to primary tumors in immunodeficient mouse models25 that could not be explained by induction of the abscopal effect via immune activation. This suggested that unknown non-immune mechanisms were responsible for the abscopal effect of oncolytic virotherapy. Interestingly, there is a previous study that reported that purified Exos from hepatitis C virus-infected cells harbored viral protein and RNA,26 and entry mechanisms of virus-containing EVs, such as clathrin-mediated endocytosis, and their intracellular dynamics have also been reported.27,28 In the present study, we hypothesized that EVs may be involved in the abscopal mechanism and investigated whether tumor-derived EVs secreted after local treatment with OBP-301 could induce antitumor effects in tumors distal to the direct administration site as well as the primary tumors.
Results
OBP-301 is present in Exo301
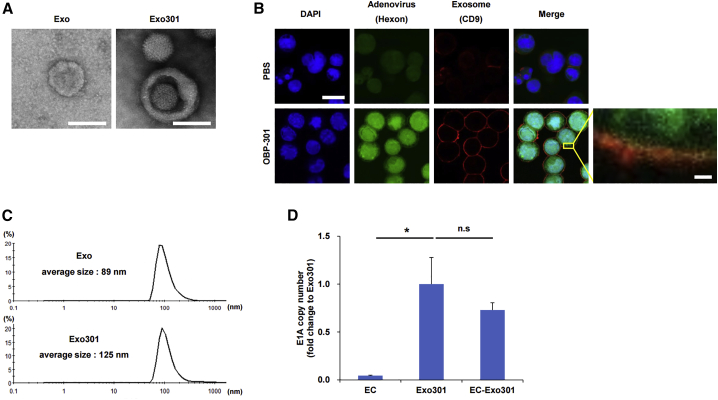
We investigated whether OBP-301 or OBP-301-related components, such as DNA, were present in Exo301 (EVs secreted from OBP-301-treated cells) by using cultured HCT116 cells in vitro. Transmission electron microscopy (TEM) demonstrated that both normal Exos and Exo301 were spherical in shape. The Exo301 preparation appeared to contain both adenovirus-containing and free adenovirus particles (Figure 1A). Immunofluorescence staining showed that adenoviruses (green) were diffusely present throughout the cell, and that some adenovirus appeared to be co-localized (yellow staining) with EVs (red) in the area surrounding the cells (Figure 1B). Dynamic light scattering (DLS) revealed that the average size of Exo301 was 125 nm in diameter, which was larger than Exos, with an average size of 89 nm in diameter (Figure 1C).
Figure 1.
Characterization of EVs
(A) Representative TEM images of EVs isolated from HCT116 cells (Exos) and HCT116 cells treated with OBP-301 (Exo301). Scale bars, 100 nm. (B) HCT116 cells treated with PBS or OBP-301 (MOI of 50) were subjected to immunofluorescence staining for CD9 (Exo marker, red), hexon (adenovirus marker, green), and DAPI (nuclear marker, blue) 24 h after treatment. The right bottom image is a magnified view of the boxed region in the merged image. Scale bars, 20 μm (left upper) and 2 μm (right bottom). (C) Particle size of Exos and Exo301 were measured by DLS. (D) DNAs extracted from EC, Exo301, and EC-Exo301 were subjected to qRT-PCR for the adenovirus E1A gene (n = 3). E1A copy numbers are expressed as fold change relative to Exo301. ∗p < 0.05.
ExoCap (EC) was used to extract Exos from Exo301 (EC-Exo301) to avoid contamination (non-Exo substances and non-encapsulated adenovirus particles) associated with ultracentrifugation methods, and it was confirmed that EC preparations were not contaminated with non-encapsulated OBP-301 (EC-OBP-301) (Figure S1A). Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) showed that adenovirus E1A gene expression of EC-Exo301 was approximately 70% of Exo301 (Figure 1D). These findings suggest that OBP-301 or OBP-301-related components such as DNA and OBP-301-related proteins such as E1A (Figure S1B) were released from HCT116 cells, which are covered in EVs, after OBP-301 treatment.
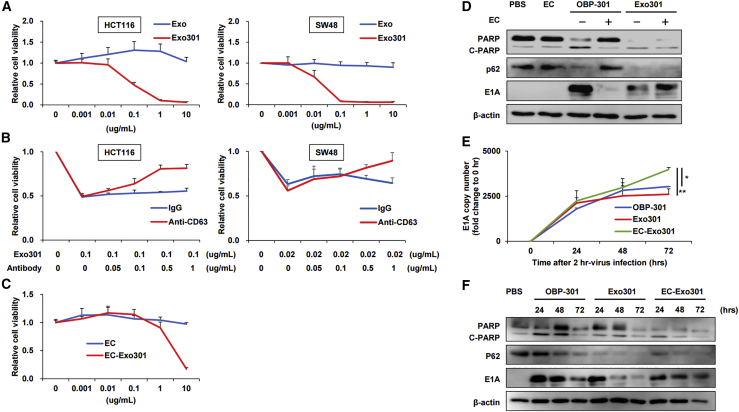
Exo301 shows cytotoxic activity by mechanisms similar to OBP-301
We determined whether Exo301, which was thought to contain various components, such as viral particles, viral proteins, and viral DNAs (based on Figure 1), produced antitumor effects, following confirmation that OBP-301 produces dose-dependent cytotoxicity toward HCT116 cells (Figure S2A) and SW48 cells (Figure S2B). The titer of Exo301 isolated from HCT116 and SW48 cells was determined as 4.2 × 107 plaque-forming unites (PFU) and 1.3 × 108 PFU, respectively, indicating that Exo301 produced functional virus progenies in both cell types (Table 1). Cell viability assays showed that Exo301 produced potent cytotoxic effects on HCT116 and SW48 cells in a dose-dependent manner while Exos were ineffective (Figure 2A). Exo301 cytotoxicity was blocked by anti-CD63 antibody in a dose-dependent manner, reaching 60%–70% inhibition on both cell lines at 1.0 μg/mL (Figure 2B), although anti-CD63 antibody itself did not significantly affect HCT116 cell growth (Figure S3). Similarly, EC-Exo301 produced a dose-dependent cytotoxic effect on HCT116 cells (Figure 2C). When Exo301 was treated with neutralizing antiserum to adenovirus type 5 (Ad5) to neutralize free OBP-301 or OBP-301 attached on the surface of EVs, cytotoxicity was inhibited approximately by half at 2.5 U of anti-Ad5 antiserum. However, cytotoxicity in the presence of 5 U was not different from baseline (0 U) values (Figure S4A), which may be due to cytotoxicity of the Ad5 neutralizing antiserum itself (Figure S4B).
Table 1.
Titer of Exo301
| Cell line from which Exo301 was isolated | Titer (PFU) |
|---|---|
| HCT116 | 4.2 × 107 |
| SW48 | 1.3 × 108 |
PFU, plaque-forming units.
Figure 2.
In vitro cytotoxic activity of Exo301
(A) Viability of HCT116 and SW48 cells 3 days after treatment with Exos or Exo301 at the indicated concentrations was assessed using an XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)2H-tetrazolium-5-carboxanilide) assay (n = 5). (B) Anti-CD63 antibody or IgG (for control) was added to the culture medium at the indicated concentrations together with Exo301 to inhibit Exo function, and the viability of HCT116 and SW48 cells was subsequently assessed using the XTT assay 3 days after treatment (n = 5). (C) Viability of HCT116 cells treated with EC or EC-Exo301 was assessed using the XTT assay 3 days after treatment (n = 5). (D) HCT116 cells were harvested 48 h after treatment with a 25% inhibitory concentration (IC25) dose of OBP-301, Exo301, or EC-Exo301, or a dose of EC that was equivalent to that used in the EC-Exo301 isolation process. Whole-cell HCT116 lysates were then subjected to western blot analysis for PARP, p62, E1A, and β-actin. (E) HCT116 cells were treated with OBP-301, Exo301, or EC-Exo301 for 2 h and were harvested at the indicated time points after removing the treatments. The extracted DNA was subjected to qRT-PCR analysis of adenovirus E1A gene levels (n = 3). E1A copy numbers are described as fold change relative to time = 0 h. ∗p < 0.05, ∗∗p < 0.01. (F) HCT116 cells treated with an IC25 dose of OBP-301, Exo301, or EC-Exo301 were harvested at the indicated post-treatment time points. Whole-cell HCT116 lysates were subjected to western blot analysis for PARP, p62, E1A, and β-actin.
Next, we compared the cytotoxic mechanisms of Exo301 and EC-Exo301 with OBP-301, which induces apoptosis and autophagy.29 Western blot analysis demonstrated that treatment with both Exo301 and EC-Exo301 caused E1A induction, poly(ADP-ribose) polymerase (PARP) cleavage, and p62 reduction (Figure 2D). These results indicate that Exo301 and EC-Exo301 produced similar effects on adenovirus replication, apoptosis, and autophagy. Determination of adenovirus replication efficacy after Exo301 or EC-Exo301 treatment of HCT116 cells (measured by qRT-PCR) indicated that adenovirus E1A gene expression after EC-Exo301 treatment increased at a constant rate until 72 h, while the replication rate after OBP-301 or Exo301 treatment slowed after 24 h (Figure 2E). This resulted in E1A gene expression after EC-Exo301 treatment being significantly higher than OBP-301 and Exo301 at 72 h (Figure 2E). Western blot analysis showed that E1A protein expression after EC-Exo301 treatment of HCT116 cells continued until 72 h while E1A expression was decreased at 72 h compared to 24 h after OBP-301 or Exo301 treatment (Figure 2F); this observation is generally consistent with the PCR findings described above. These findings suggested that Exo301 possesses antitumor activity that is mechanistically similar to OBP-301. Moreover, 60%–70% of this effect was observed following treatment with EC-Exo301, which contains only OBP-301-containing EVs or OBP-301-related components such as DNA.
Tumor-derived materials are delivered to distal non-tumor sites via tumor-derived EVs
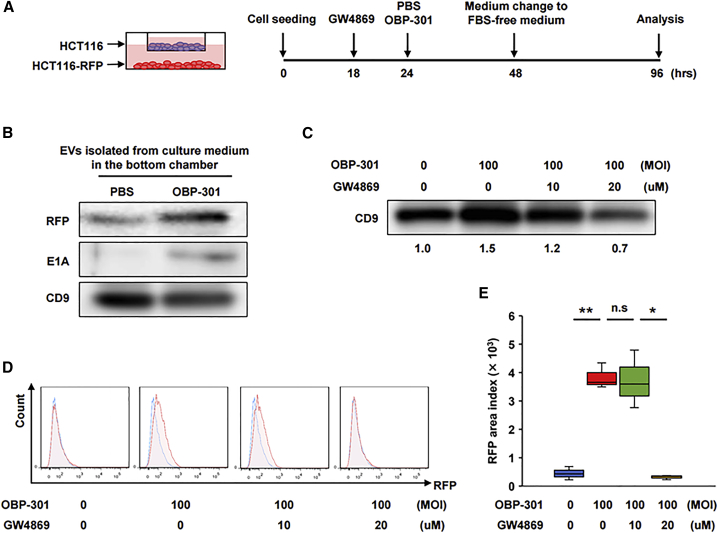
Initial evaluation of the effect of the Exo secretion inhibitor GW4869 on cell growth and viral replication indicated that GW4869 produced cytotoxicity in a dose-dependent manner (Figure S5A), but it did not cause apoptosis even at a high dose (Figure S5B) and did not affect viral replication (Figure S5C). We used an in vitro co-culture model to determine whether red fluorescent protein (RFP) (representative tumor-derived material) expressed in HCT116-RFP cells seeded on the bottom chamber were delivered to HCT116 cells seeded on the upper chamber via EVs (Figure 3A). Western blot analysis of EVs isolated from the supernatant of HCT116-RFP cells (bottom chamber) treated with either OBP-301 or PBS showed that OBP-301 treatment increased RFP secretion compared to PBS (Figure 3B). Western blot analysis also showed that the Exo marker CD9 was increased by treatment with a multiplicity of infection (MOI) of 100 for OBP-301, which was dose-dependently inhibited by GW4869 (Figure 3C). Flow cytometry analysis of RFP uptake in HCT116 cells in the upper chamber showed that RFP uptake was increased by treatment with an MOI of 100 for OBP-301 on the bottom chamber, which was significantly inhibited by addition of 20 μM GW4869; however, no significant difference was observed in the presence of 10 μM GW4869 (Figures 3D and 3E). These findings suggest that RFPs secreted from primary HCT116-RFP cells were delivered to distal HCT116 cells via EVs, whose secretion was strongly increased by OBP-301 treatment.
Figure 3.
In vitro model of delivery of tumor-derived substances to distal tumor sites via tumor-derived EVs
(A) Experimental design for the co-culture model. Briefly, HCT116-RFP cells and HCT116 cells were seeded in the lower and upper chambers, respectively. HCT116-RFP cells were treated with PBS or OBP-301 (MOI of 100) and the culture medium was changed to FBS-free medium 24 h after treatment. Cells were incubated for another 48 h and then harvested for analysis. GW4869 was added to the culture medium at 6 h prior to PBS or OBP-301 treatment to block Exo secretion. (B) EVs isolated from culture medium in the bottom chamber after PBS or OBP-301 treatment of HCT116-RFP cells were subjected to western blot analysis for RFP, E1A, and CD9. (C) EVs isolated after GW4869 treatment at the indicated doses in addition to OBP-301 treatment were subjected to western blot analysis for CD9. Relative intensities to non-treatment condition (measured by ImageJ) are provided beneath the blot. (D and E) HCT116 cells in the upper chamber after treatment of HCT116-RFP cells in the bottom chamber with OBP-301 and GW4869 were subjected to flow cytometry to measure RFP uptake (n = 3). Representative data for each treatment are shown in (D), and statistical assessments of RFP amounts in each treatment are shown in (E). ∗p < 0.05, ∗∗p < 0.01.
Localized treatment with OBP-301 produces abscopal effects via tumor-derived EVs by a non-immune activation mechanism
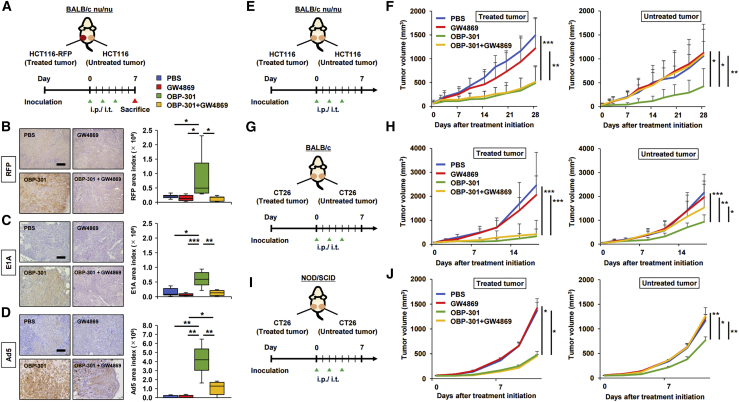
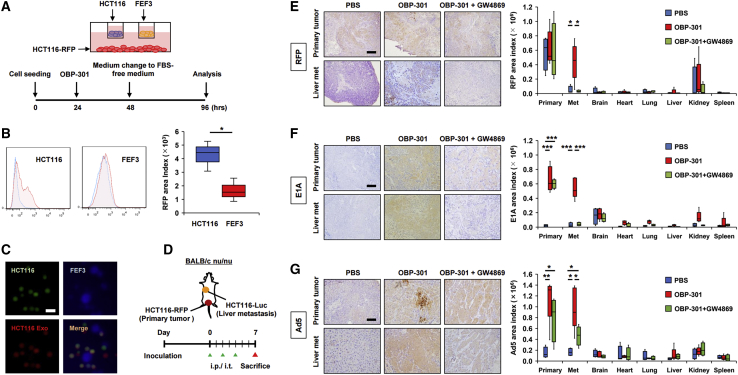
We performed in vivo experiments to determine whether tumor-derived materials (OBP-301 or OBP-related components) were delivered to distal tumors via EVs after local treatment of primary tumors with OBP-301, and whether the EVs produced an abscopal effect on the distal tumors. A bilateral subcutaneous tumor model using HCT116-RFP and HCT116 was established in BALB/c nu/nu mice. The HCT116-RFP tumors received three intratumoral treatments with OBP-301 or PBS, with or without concurrent intraperitoneal administration of GW4869. In contrast, the HCT116 tumors did not receive intratumoral treatments with either OBP-301 or PBS (Figure 4A). Immunohistochemistry (IHC) staining of HCT116 tumors demonstrated that RFP, E1A, and Ad5 including capsid proteins were strongly expressed after OBP-301 treatment of the HCT116-RFP tumors, effects that were blocked by GW4869 treatment (Figures 4B–4D). In a bilateral HCT116 tumor model using BALB/c nu/nu mice, the antitumor effects of three intratumoral administrations of OBP-301 or PBS, with or without concurrent intraperitoneal GW4869 administration, were measured in the OBP-301-treated tumor (treated tumor) and the tumors not directly treated with OBP-301 (untreated tumor) (Figure 4E). While OBP-301 treatment produced potent antitumor effects on treated tumors, which was not blocked by GW4869, OBP-301 treatment also produced strong antitumor effects on the untreated tumors, which was almost completely nullified by GW4869 (Figure 4F; Figure S6). Following confirmation of replication capability and dose-dependent cytotoxic activity of OBP-301 on CT26 cells in vitro (Figures S2C and S2D), the previous experiment was repeated in a CT26 bilateral tumor model using immunocompetent BALB/c mice (Figure 4G). Consistent with results in the HCT116 tumors, the antitumor effects of OBP-301 on untreated CT26 tumors were significantly blocked by GW4869, although not to the extent observed in the HCT116 tumors (Figure 4H). Furthermore, when the same experiment was conducted using immunodeficient non-obese diabetic (NOD)/severe combined immunodeficiency (SCID) mice, which have a low natural killer (NK) cell activity (Figure 4I), the antitumor effects of OBP-301 on untreated tumors were also significantly inhibited by GW4869, which indicates that NK cell-mediated effects were not significant (Figure 4J). These findings suggest that OBP-301 or OBP-301-related components were delivered to distal tumors via tumor-derived EVs and produced an abscopal effect. Interestingly, the underlying mechanism of action does not appear to require systemic immune activation, which is currently thought to be the primary mechanism of the abscopal effect.
Figure 4.
Abscopal effects of OBP-301 mediated by tumor-derived EVs in vivo
(A) Experimental design for (B)–(D). In brief, in a bilateral subcutaneous tumor model with HCT116-RFP and HCT116 using BALB/c nu/nu mice, HCT116-RFP tumors were treated with intratumoral administration of PBS or OBP-301 (1 × 108 PFU) and/or intraperitoneal administration of GW4869 (2.5 μg/g) three times every 2 days, and harvested 7 days after treatment initiation (n = 8). (B) Representative images of IHC staining for RFP in HCT116 tumors in each treatment are shown on the left, and statistical assessment of RFP staining intensity (measured by ImageJ) is shown on the right. Scale bar, 200 μm. ∗p < 0.05. (C) Representative images of IHC staining for adenovirus E1A in HCT116 tumors in each treatment are shown on the left, and statistical assessment of E1A staining intensity (measured by ImageJ) is shown on the right. Scale bar, 200 μm. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (E) Experimental design for (F). Briefly, in a HCT116 bilateral subcutaneous tumor model using BALB/c nu/nu mice, larger HCT116 tumors were treated with intratumoral administration of PBS or OBP-301 (1 × 108 PFU) and/or intraperitoneal administration of GW4869 (2.5 μg/g) three times every 2 days, and both tumors were monitored until 28 days after treatment initiation to evaluate antitumor effects of the treatments (n = 9–10). (F) Volumes of both HCT116 tumors were monitored twice a week until 28 days after treatment initiation. “Treated tumor” indicates the tumor directly treated with OBP-301, and “Untreated tumor” indicates the tumor not directly treated with OBP-301. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (G) Experimental design for (H). Briefly, in a CT26 bilateral subcutaneous tumor model using BALB/c mice, larger CT26 tumors were treated with intratumoral administration of PBS or OBP-301 (1 × 108 PFU) and/or intraperitoneal administration of GW4869 (2.5 μg/g) three times every 2 days, and both tumors were monitored until 18 days after treatment initiation to evaluate antitumor effects of the treatments (n = 9–10). (H) Volumes of both CT26 tumors were monitored twice a week until 18 days after treatment initiation. “Treated tumor” indicates the tumor directly treated with OBP-301, and “Untreated tumor” indicates the tumor not directly treated with OBP-301. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (I) Experimental design for (J). Briefly, in a CT26 bilateral subcutaneous tumor model using NOD/SCID mice, larger CT26 tumors were treated with intratumoral administration of PBS or OBP-301 (1 × 108 PFU) and/or intraperitoneal administration of GW4869 (2.5 μg/g) three times every 2 days, and both tumors were monitored until 11 days after treatment initiation to evaluate antitumor effects of the treatments (n = 4). (J) Volumes of both CT26 tumors were monitored twice a week until 11 days after treatment initiation. “Treated tumor” indicates the tumor directly treated with OBP-301, and “Untreated tumor” indicates the tumor not directly treated with OBP-301. ∗p < 0.05, ∗∗p < 0.01.
Tumor-derived EVs exhibit tumor tropism
The possibility of tumor-derived EVs exhibiting tumor tropism was investigated in in vitro and in vivo experiments. An in vitro triple co-culture model, in which HCT116-RFP cells were seeded on the bottom chamber and HCT116 and FEF3 cells were separately seeded on upper chambers, was used to evaluate tumor tropism. In this study, FEF cells were used as non-transformed control cells, and RFP uptake in HCT116 or FEF3 cells after OBP-301 treatment of HCT116-RFP cells was evaluated (Figure 5A). Flow cytometry showed that accumulation of RFP protein secreted from HCT116-RFP cells after OBP-301 treatment was significantly greater in HCT116 cells than in FEF3 cells (Figure 5B). In contrast, a triple co-culture model, in which FEF3 cells stained with CellTracker Green (FEF3-Green) were seeded on the bottom chamber and treated with OBP-301, was used to show greater accumulation of green fluorescence secreted from FEF3-Green cells in FEF3 cells than in HCT116 cells (Figure S7). Moreover, addition of EVs isolated from the supernatant of OBP-301-treated HCT116 cells (HCT116 Exos) to a co-culture system of HCT116 and FEF3 cells resulted in co-localization of HCT116-derived EVs with HCT116 cells but not FEF3 cells (Figure 5C).
Figure 5.
Tumor tropism of tumor-derived EVs
(A) Experimental design of the triple co-culture model used for (B). Briefly, HCT116-RFP cells were seeded in the bottom chamber, and HCT116 cells and FEF3 cells were individually seeded in the upper chambers. HCT116-RFP cells were treated with OBP-301 (MOI of 100) and the culture medium was changed to FBS-free medium 24 h after treatment initiation. Cells were incubated for another 48 h and then harvested for analysis. (B) HCT116 cells and FEF3 cells in the upper chamber were subjected to flow cytometry for RFP uptake (n = 3). Representative data for each cell line are shown on the left, and statistical assessments of RFP amounts in each cell line are shown on the right. ∗p < 0.05. (C) EVs isolated from culture medium of OBP-301-treated HCT116 cells (HCT116 Exos) were added to a co-culture system of HCT116 cells and FEF3 cells. The cells were observed by fluorescence microscopy after 48-h incubation. HCT116 cells, FEF3 cells, and HCT116 Exos were stained green with CellTracker Green, blue with CellTracker Blue, and red with PKH26, respectively. Scale bar, 100 nm. (D) Experimental design for (E)–(G). Briefly, in an orthotopic model of rectal tumors (HCT116-RFP) with liver metastasis (HCT116-Luc) using BALB/c nu/nu mice, HCT116-RFP tumors were treated with intratumoral administration of PBS or OBP-301 (1 × 108 PFU) and/or intraperitoneal administration of GW4869 (2.5 μg/g) three times every 2 days, and primary rectal tumors and metastatic liver tumors along with major organs (brain, heart, lung, liver, kidney, and spleen) were harvested 7 days after treatment initiation (n = 4–5). (E) Representative images of IHC staining for RFP in the primary rectal tumor and metastatic liver tumor in each treatment are shown on the left and statistical assessment of RFP staining intensity (measured by ImageJ) in primary rectal tumors, metastatic liver tumors, and major organs are shown on the right. Scale bar, 200 μm. ∗p < 0.05. (F) Representative images of IHC staining for E1A in primary rectal tumors and metastatic liver tumors in each treatment are shown on the left, and statistical assessment of E1A staining intensity (measured by ImageJ) in primary rectal tumors, metastatic liver tumors, and major organs are shown on the right. Scale bar, 200 μm. ∗∗∗p < 0.001. (G) Representative images of IHC staining for Ad5 in primary rectal tumors and metastatic liver tumors in each treatment are shown on the left, and statistical assessment of E1A staining intensity (measured by ImageJ) in primary rectal tumors, metastatic liver tumors, and major organs are shown on the right. Scale bar, 200 μm. ∗p < 0.05, ∗∗p < 0.01.
An orthotopic rectal tumor (HCT116-RFP) model with liver metastasis (HCT116-Luc) was used to evaluate tumor tropism of tumor-derived EVs in vivo. In this model, rectal HCT116-RFP tumors received intratumoral administration of OBP-301 or PBS, with and without concurrent intraperitoneal administration of GW4869, and primary rectal tumors, metastatic liver tumors, and major organs were harvested to evaluate the biodistribution of RFP, E1A, and Ad5 (Figure 5D). IHC staining of RFP demonstrated that significant levels of RFP protein were detected in metastatic liver tumors after OBP-301 treatment, which was blocked by GW4869, whereas GW4869 did not affect RFP expression in the primary rectal tumors (Figure 5E; Figure S8A). In contrast, IHC analysis demonstrated that E1A and Ad5 proteins were significantly detected in both the primary rectal tumors and the metastatic liver tumors after OBP-301 treatment, which was blocked by GW4869, especially in the metastatic tumors (Figures 5F and 5G; Figures S8B and S8C). Uptake of RFP, E1A, and Ad5 in major organs was relatively weak, surprisingly even in liver, compared to the primary tumor and metastatic tumors. These findings suggested that tumor-derived EVs exhibit strong tumor tropism, which can contribute to the abscopal effect of OBP-301.
Discussion
The abscopal effect is widely thought to be caused by systemic activation of a cancer immune response, although the precise mechanisms remain unknown. As with radiotherapy, oncolytic virotherapy has been recently recognized as a promising treatment strategy that induces an abscopal effect in addition to its strong local effects.30 We recently reported that our telomerase-specific oncolytic adenovirus produced an abscopal effect on murine cancer cells in in vivo experiments using immunocompetent mice, and it synergistically enhanced antitumor effects with ICIs.20 We characterized the immune modulation produced by the oncolytic adenovirus as a mechanism for the abscopal effect and observed significant recruitment of CD8-positive T lymphocytes in distal tumors, as well as the primary tumors to which the oncolytic adenovirus had been directly administered.20 Notably, we observed that this effect was occasionally observed in in vivo experiments using immunodeficient mice, which prompted us to examine additional mechanisms for the abscopal effect.
While EVs have recently become a highly topical research field, we are not aware of any previous reports describing the involvement of EVs in the biology of adenoviruses, especially in the process of spreading viral progeny from infected cells to surrounding cells or tissues. Although we hypothesized that OBP-301 may be delivered to distal sites via EVs, one of the biggest challenges in this study was to conclusively demonstrate that OBP-301 was present within tumor-derived EVs secreted after OBP-301 treatment. This is because EVs and adenovirus particles are very similar in size and are very difficult to separate with the commonly used ultracentrifugation method. Thus, EVs prepared by ultracentrifugation are expected to contain free OBP-301 particles that are present in the culture medium, which was shown in TEM images (Figure 1A). Because of this, there was a concern that the cytotoxic effects of Exo301 would be unrelated to the EVs and attributable to free OBP-301. We addressed this concern in the following two ways. We used the magnetic-bead-based EC system to extract purified EVs, as well as an antibody toward the Exo surface marker CD63 to physically block cellular uptake of EVs.31 A cell viability assay showed that EC-purified Exo301 (EC-Exo301) produced a potent cytotoxic effect against HCT116 cells (Figure 2C). Moreover, anti-CD63 blocked 60%–70% of the cytotoxicity of Exo301 (Figure 2B), which was consistent with PCR data that demonstrated the adenovirus E1A copy number of EC-Exo301 was approximately 70% of Exo301 (Figure 1D). Western blot analysis was used to examine the cytotoxic mechanisms and demonstrated that both Exo301 and EC-Exo301 showed induction of autophagy and apoptosis in HCT116 cells, which was mechanistically similar to OBP-301 (Figures 2D and 2F). This suggests that Exo301 contained viable OBP-301 or DNA producing viable OBP-301. Although the particle size of Exo301 was slightly larger than normal Exos, this is considered reasonable if EVs contained an adenovirus particle of similar size to that of EVs (Figure 1C). Based on these findings, we were confident that tumor-derived EVs contained OBP-301 or OBP-301-related components and were responsible for the observed cytotoxic activity. However, EV production by cells is unstable and varies depending on cell types, culture conditions, hypoxia, and treatment, which makes EV-related research challenging.32
While the in vivo evidence that local treatment with OBP-301 produced potent antitumor activity on distal tumors via EVs as well as on the primary tumor was very definitive, another intriguing observation in this study was the tumor tropism of tumor-derived EVs. There appears to be no further reports regarding tumor tropism of tumor-derived EVs, with respect to key tropism-mediating molecules, beyond a 2015 report describing a possible mechanism for metastatic organotropism via tumor Exo integrins.6 Although we have not been able to identify a mechanism in this study, we confirmed the phenomenon of tumor-derived EVs having tumor tropism, as has been previously described.33, 34, 35 Most notably, EVs secreted from primary rectal tumors showed surprisingly high tumor tropism in an orthotopic rectal tumor model with liver metastasis. In this study, tumor tropism was confirmed by the high uptake of tumor-derived RFP and adenovirus E1A in metastatic liver tumors after OBP-301 treatment of the primary rectal tumor. In comparison, other major organs (liver and spleen) exhibited little to no uptake of RFP, E1A and Ad5 (Figures 5E–5G). Typically, when oncolytic adenoviruses such as OBP-301 are systemically administered in vivo they are non-specifically trapped in the reticuloendothelial system of the liver and spleen.36,37 Thus, the results of the present study suggest that tumor-derived EVs imparted a masking property upon OBP-301. This further suggests that tumor-derived EVs offer great promise as a drug carrier for cancer therapeutics, which is expected to become an intriguing topic in the field of drug delivery systems.
In the present study, we have revealed a novel aspect of EVs, namely that tumor-derived EVs secreted following OBP-301 treatment can play an important role in the abscopal effect of OBP-301, which appears to be mediated through the direct delivery of OBP-301 to the metastatic site, rather than by indirectly activating a systemic immune response. Although a lot of work is needed to clarify the detailed mechanisms, the observed abscopal effect represents a therapeutic mechanism that can be widely applied to other oncolytic viruses.38 Therefore, this study represents an important step toward initiating subsequent breakthroughs in the development of cancer therapeutics.
Materials and methods
Reagents and oncolytic adenovirus
A PKH26 red fluorescent cell linker mini kit (catalog no. [cat.] MINI26-1KT; Sigma-Aldrich, St. Louis, MO, USA) was used to stain EVs. CellTracker Green CMFDA (5-chloromethylfluorescein diacetate) (cat. C7025; Invitrogen, Waltham, MA, USA) and CellTracker Blue CMAC (7-amino-4-chloromethylcoumarin) (cat. C2110; Invitrogen) were used to stain HCT116 and FEF3 cells. An EC streptavidin CD9/CD63/CD81 set (cat. MEX-SA123; MBL International, Woburn, MA, USA) was used to extract pure EVs. GW4869 (Sigma-Aldrich) was used to inhibit Exo secretion, which has been utilized in both in vitro and in vivo studies.39,40 An Adeno-X rapid titer kit (cat. 632250; Clontech Laboratories, CA, USA) was used for titer determination. Telomelysin (OBP-301) is a telomerase-specific oncolytic adenovirus in which the human telomerase reverse transcriptase (hTERT) promoter drives the E1A and E1B genes linked with an internal ribosome entry site.21 OBP-301 is designed to replicate in tumors, many of which have high telomerase activity, and lead to oncolytic cell death; however, OBP-301 does not replicate in normal tissues.
Cell lines and culture
The human colon carcinoma cell lines HCT116 and SW48, HCT116 expressing RFP (HCT116-RFP), HCT116 expressing firefly luciferase (HCT116-Luc), the murine colon carcinoma cell line CT26, and normal human fibroblast cells (FEF3) were used in this study. HCT116 and CT26 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA), HCT116-RFP and SW48 were obtained from AntiCancer (San Diego, CA, USA), HCT116-Luc was purchased from the Japanese Collection of Research Bioresources (JCRB) Cell Bank (Japan), SW48 was purchased from Horizon Discovery (UK), and FEF3 was obtained from the Wister Institute (Philadelphia, PA, USA). HCT116 and HCT116-Luc were cultured in McCoy’s 5A medium, HCT116-RFP and SW48 were cultured in RPMI 1640 medium, and CT26 and FEF3 were cultured in DMEM medium. All culture media were supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (100 U/mL).
Isolation and characterization of EVs
Normal EVs were isolated by ultracentrifugation of supernatants collected after cell culture in FBS-free medium for 48 h (Exos). EVs were also isolated by the same process following OBP-301 treatment for 24 h (Exo301) of HCT116 cells at an MOI of 35 and SW48 cells at an MOI of 5. Briefly, the ultracentrifugation method involved centrifuging the collected supernatants at 2,000 × g for 10 min to remove cells and debris, followed by another centrifugation at 100,000 × g for 70 min at 4°C after filtration through a 0.22-μm filter. The pellets were then rinsed with phosphate-buffered saline (PBS), ultra-centrifuged again with the same conditions, and dispersed in PBS.
Protein concentrations were measured using the bicinchoninic acid (BCA) assay according to the manufacturer’s protocol (Thermo Scientific, Waltham, MA, USA). Exo sizes were measured using a DLS method with a Zetasizer Nano ZSP (Malvern Instruments, Malvern, UK). Exo morphology and structure were visualized using TEM (H-7560, Hitachi, Japan).
Cell viability assay
HCT116 and SW48 cells (3.0 × 103 cells/well in a 96-well plate) were treated with various concentrations of Exos, Exo301, or OBP-301 (n = 5). Cell viability was determined 3 days after treatment using a cell proliferation kit II (XTT) (Roche, Basel, Switzerland) according to the manufacturer’s protocol. The inhibition assay involved adding anti-CD63 antibody (1:1,000, cat. 10628D, Invitrogen) to the culture medium to inhibit Exo function. The percentage of viable cells relative to untreated cells (0 μg/mL) was plotted. All error bars indicate 95% confidence intervals.
Western blot analysis
Proteins (20 μg) extracted from cells or EVs were electrophoresed on 10% SDS-polyacrylamide gels and transferred to Hybond-polyvinylidene fluoride membranes (GE Healthcare UK, Amersham, UK). The membranes were incubated overnight at 4°C with primary antibodies against E1A (1:500, cat. 554155; BD Pharmingen, San Jose, CA, USA), PARP (1:1,000, cat. 9542; Cell Signaling Technology, Danvers, MA, USA), p62 (1:1,000, cat. 5114; Cell Signaling Technology), β-actin (1:5,000, cat. A-5441; Sigma-Aldrich), CD9 (1:1,000, cat. 10626D; Invitrogen), and RFP (1:1,000, cat. R10367; Invitrogen), followed by incubation with secondary antibodies for 1 h at room temperature. The Amersham enhanced chemiluminescence (ECL) system (GE Healthcare UK) was used to detect the peroxidase activity of the bound antibody.
Immunofluorescence staining
HCT116 cells treated with an MOI of 50 for OBP-301 or PBS were incubated with anti-CD9 antibody (1:500, cat. 10626D; Invitrogen) overnight at 4°C after blocking with Blocking-One (Nacalai Tesque, Kyoto, Japan), followed by incubation with fluorescein isothiocyanate (FITC)-conjugated anti-adenovirus antibody (Hexon) (1:200, cat. AB1056F; Chemicon, Temecula, CA, USA) or anti-mouse immunoglobulin (Ig)G Alexa Fluor 647 secondary antibody (1:1,000, cat. A21237; Invitrogen). DAPI (4′,6-diamidino-2-phenylindole) (1:1,000, cat. D1306; Invitrogen) was used for DNA staining. Colocalization of CD9 and adenovirus was observed using an LMS 780 laser-scanning confocal microscope (Carl Zeiss, Tokyo, Japan).
Flow cytometry
To evaluate cellular uptake of EVs, a non-contact coculture system was used, in which HCT116-RFP cells were seeded in the lower chamber, and HCT116 and/or FEF3 cells were individually seeded in the upper chambers. HCT116-RFP cells were treated with OBP-301 or PBS, and the culture medium was changed to FBS-free medium 24 h after treatment. HCT116 cells and/or FEF3 cells in the upper chambers were subjected to flow cytometry (BD FACSLyric, BD Biosciences, San Jose, CA, USA) 48 h after the medium change.
qRT-PCR analysis
Total RNA was extracted from cells using the miRNeasy mini kit (QIAGEN, Valencia, CA, USA) after cell lysis, and from EVs using the a total Exo RNA & protein isolation kit (Invitrogen). Reverse transcription reactions were performed at 22°C for 10 min, 42°C for 15 min, 95°C for 5 min, and 4°C for 5 min. PCR amplification was conducted with 40 cycles of denaturation at 95°C for 20 s, annealing at 60°C for 20 s. Data analysis was performed using a StepOnePlus real-time PCR system (Applied Biosystems, Waltham, MA, USA). The relative gene expression was determined using the ΔΔCT method. Primers and probes were predesigned by the manufacturer (Applied Biosystems): TaqMan E1A probe, 5′-CTGTGTCTAGAGAATGC-MGB-3′; TaqMan E1A primers, forward, 5′-CCTGAGACGCCCGACATC-3′, reverse, 5′-GGACCGGAGTCACAGCTATCC-3′.
In vivo experiments
To evaluate the delivery of tumor-derived RFP via EVs, a bilateral subcutaneous tumor model was established by implanting 2 × 106 HCT116-RFP cells in one flank and 2 × 106 HCT116 cells in the other flank of 5-week-old female immunodeficient BALB/c nude mice. When HCT116-RFP tumors reached ≈200 mm3 in volume, HCT116-RFP tumors were treated with intratumoral (i.t.) administration of PBS, intraperitoneal (i.p.) administration of GW4869 (2.5 μg/g), OBP-301 (i.t., 1 × 108 PFU), or OBP-301 (i.t., 1 × 108 PFU) + GW4869 (2.5 μg/g) (i.p. 6 h before OBP-301 treatment) three times every 2 days, whereas HCT116 tumors were not treated directly with OBP-301. Three days after the last treatment, the mice were sacrificed and all tumors were harvested and fixed in formalin (n = 8).
To evaluate the antitumor effects of OBP-301, a bilateral subcutaneous tumor model was established by implanting 2 × 106 HCT116 cells in both flanks of 5-week-old female immunodeficient BALB/c nude mice (n = 9-10), or implanting 1 × 106 CT26 cells in both flanks of 5-week-old female immunocompetent BALB/c mice (n = 9–10) or NOD/SCID mice (n = 4). When the larger of the two tumors reached 50–100 mm3, the larger tumors were treated with PBS (i.t.), GW4869 (i.p., 2.5 μg/g), OBP-301 (i.t., 1 × 108 PFU), and OBP-301 (i.t., 1 × 108 PFU) + GW4869 (2.5 μg/g) (i.p. 6 h before OBP-301 treatment) three times every 2 days. The smaller tumors were not treated directly with OBP-301, and tumors were monitored until 28 days after the initiation of treatment or when tumors exceeded 4,000 mm3. The perpendicular diameter of each tumor was measured twice a week until 28 days after the initiation of treatment, and tumor volume was calculated using the following formula: tumor volume (mm3) = a × b2 × 0.5, where a is the longest diameter, b is the shortest diameter, and 0.5 is a constant used to calculate the volume of an ellipsoid.
For the orthotopic rectal tumor model with liver metastasis, 2 × 106 HCT116-RFP cells were implanted into the rectum and 4 × 106 HCT116-Luc cells were injected into the superior mesenteric vein of 5-week-old female BALB/c nude mice. When the luminescence intensity after luciferin injection of the upper abdominal region, measured using an IVIS Imaging System (Xenogen, Alameda, CA, USA), reached 1.0 E+04, the HCT116-RFP rectal tumors were treated with PBS (i.t.), GW4869 (i.p., 2.5 μg/g), OBP-301 (i.t., 1 × 108 PFU), and OBP-301 (i.t., 1 × 108 PFU) + GW4869 (2.5 μg/g) (i.p. 6 h before OBP-301 treatment) three times every 2 days. Three days after the last treatment, the mice were sacrificed, and all main organs (primary rectal tumor, metastatic liver tumor, brain, heart, lung, liver, kidney, and spleen) were harvested and subjected to IHC staining to determine the biodistribution of tumor-derived RFP, adenoviral E1A, and Ad5 proteins (n = 4–5).
Mice were maintained in specific pathogen-free conditions in the animal laboratory of Okayama University. All animal protocols were approved by the Ethics Review Committee for Animal Experimentation of Okayama University.
IHC staining
Formalin-fixed, paraffin-embedded tissue samples cut at 2 μm were deparaffinized and soaked in 0.3% H2O2 for 10 min at room temperature to extinguish endogenous peroxidase activity. After antigen retrieval by heating in 10 mM sodium citrate (pH 6.0) buffer solution or 10% EDTA using a microwave for 14 min, the samples were incubated with primary antibodies against RFP (1:1,000, cat. R10367; Invitrogen), E1A (1:200, cat. bs-7697R; Bioss, Woburn, MA, USA), and Ad5 (1:200, cat. ab6982; Abcam, Cambridge, UK) overnight at 4°C, and then with peroxidase-linked secondary antibody for 30 min at room temperature. After washing, the samples were stained with 3,3′-diaminobenzidine (Dako, Santa Clara, CA, USA) for visualization and counterstained with Meyer’s hematoxylin.
Statistical analysis
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander that was designed to add frequently used biostatistical functions. The Pearson chi-square test or the Fisher exact test was used for categorical variables, and the Mann-Whitney U test was used for continuous variables. A p value less than 0.05 was considered statistically significant.
Acknowledgments
The authors would like to thank Tomoko Sueishi, Tae Yamanishi, and Yuko Hoshijima for excellent technical support. This work was supported by JSPS KAKENHI (grant no. JP16K19893 to S. Kuroda) and JSPS KAKENHI (grant no. JP20K17617 to Y.K.).
Author contributions
Y.K. and S. Kuroda designed the experiments and wrote the manuscript. Y.K., N.K., K.K., T.T., M.H., C.Y., R.S., and Y.H. conducted the experiments. S. Kuroda, S. Kikuchi, M.N., S. Kagawa, H.T., and T.F. assisted in interpretation of the results. Y.U. provided reagents, and all authors reviewed the manuscript.
Declaration of interests
Y.U. is the President and CEO of Oncolys BioPharma, Inc., the manufacturer of OBP-301 (Telomelysin). H.T. and T.F. are consultants of Oncolys BioPharma, Inc. The remaining authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.05.015.
Supplemental information
References
- 1.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 2.Urabe F., Kosaka N., Ito K., Kimura T., Egawa S., Ochiya T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 2020;318:C29–C39. doi: 10.1152/ajpcell.00280.2019. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T., Kosaka N., Ochiya T. Latest advances in extracellular vesicles: From bench to bedside. Sci. Technol. Adv. Mater. 2019;20:746–757. doi: 10.1080/14686996.2019.1629835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujita Y., Yoshioka Y., Ochiya T. Extracellular vesicle transfer of cancer pathogenic components. Cancer Sci. 2016;107:385–390. doi: 10.1111/cas.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu R., Rai A., Chen M., Suwakulsiri W., Greening D.W., Simpson R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018;15:617–638. doi: 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- 6.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales-Kastresana A., Telford B., Musich T.A., McKinnon K., Clayborne C., Braig Z., Rosner A., Demberg T., Watson D.C., Karpova T.S. Labeling extracellular vesicles for nanoscale flow cytometry. Sci. Rep. 2017;7:1878. doi: 10.1038/s41598-017-01731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morales-Kastresana A., Musich T.A., Welsh J.A., Telford W., Demberg T., Wood J.C.S., Bigos M., Ross C.D., Kachynski A., Dean A. High-fidelity detection and sorting of nanoscale vesicles in viral disease and cancer. J. Extracell. Vesicles. 2019;8:1597603. doi: 10.1080/20013078.2019.1597603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poggio M., Hu T., Pai C.C., Chu B., Belair C.D., Chang A., Montabana E., Lang U.E., Fu Q., Fong L., Blelloch R. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177:414–427.e13. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 12.Catalano M., O’Driscoll L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles. 2019;9:1703244. doi: 10.1080/20013078.2019.1703244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mole R.H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 14.Postow M.A., Callahan M.K., Barker C.A., Yamada Y., Yuan J., Kitano S., Mu Z., Rasalan T., Adamow M., Ritter E. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Twyman-Saint Victor C., Rech A.J., Maity A., Rengan R., Pauken K.E., Stelekati E., Benci J.L., Xu B., Dada H., Odorizzi P.M. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngwa W., Irabor O.C., Schoenfeld J.D., Hesser J., Demaria S., Formenti S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer. 2018;18:313–322. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks E.D., Chang J.Y. Time to abandon single-site irradiation for inducing abscopal effects. Nat. Rev. Clin. Oncol. 2019;16:123–135. doi: 10.1038/s41571-018-0119-7. [DOI] [PubMed] [Google Scholar]
- 18.Havunen R., Santos J.M., Sorsa S., Rantapero T., Lumen D., Siurala M., Airaksinen A.J., Cervera-Carrascon V., Tähtinen S., Kanerva A., Hemminki A. Abscopal effect in non-injected tumors achieved with cytokine-armed oncolytic adenovirus. Mol. Ther. Oncolytics. 2018;11:109–121. doi: 10.1016/j.omto.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longo V., Brunetti O., Azzariti A., Galetta D., Nardulli P., Leonetti F., Silvestris N. Strategies to improve cancer immune checkpoint inhibitors efficacy, other than abscopal effect: A systematic review. Cancers (Basel) 2019;11:539. doi: 10.3390/cancers11040539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanaya N., Kuroda S., Kakiuchi Y., Kumon K., Tsumura T., Hashimoto M., Morihiro T., Kubota T., Aoyama K., Kikuchi S. Immune modulation by telomerase-specific oncolytic adenovirus synergistically enhances antitumor efficacy with anti-PD1 antibody. Mol. Ther. 2020;28:794–804. doi: 10.1016/j.ymthe.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawashima T., Kagawa S., Kobayashi N., Shirakiya Y., Umeoka T., Teraishi F., Taki M., Kyo S., Tanaka N., Fujiwara T. Telomerase-specific replication-selective virotherapy for human cancer. Clin. Cancer Res. 2004;10:285–292. doi: 10.1158/1078-0432.ccr-1075-3. [DOI] [PubMed] [Google Scholar]
- 22.Nemunaitis J., Tong A.W., Nemunaitis M., Senzer N., Phadke A.P., Bedell C., Adams N., Zhang Y.A., Maples P.B., Chen S. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol. Ther. 2010;18:429–434. doi: 10.1038/mt.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuroda S., Fujiwara T., Shirakawa Y., Yamasaki Y., Yano S., Uno F., Tazawa H., Hashimoto Y., Watanabe Y., Noma K. Telomerase-dependent oncolytic adenovirus sensitizes human cancer cells to ionizing radiation via inhibition of DNA repair machinery. Cancer Res. 2010;70:9339–9348. doi: 10.1158/0008-5472.CAN-10-2333. [DOI] [PubMed] [Google Scholar]
- 24.Fujiwara T. Multidisciplinary oncolytic virotherapy for gastrointestinal cancer. Ann. Gastroenterol. Surg. 2019;3:396–404. doi: 10.1002/ags3.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojima T., Watanabe Y., Hashimoto Y., Kuroda S., Yamasaki Y., Yano S., Ouchi M., Tazawa H., Uno F., Kagawa S. In vivo biological purging for lymph node metastasis of human colorectal cancer by telomerase-specific oncolytic virotherapy. Ann. Surg. 2010;251:1079–1086. doi: 10.1097/SLA.0b013e3181deb69d. [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishnaiah V., Thumann C., Fofana I., Habersetzer F., Pan Q., de Ruiter P.E., Willemsen R., Demmers J.A., Stalin Raj V., Jenster G. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc. Natl. Acad. Sci. USA. 2013;110:13109–13113. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Dongen H.M., Masoumi N., Witwer K.W., Pegtel D.M. Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiol. Mol. Biol. Rev. 2016;80:369–386. doi: 10.1128/MMBR.00063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costafreda M.I., Abbasi A., Lu H., Kaplan G. Exosome mimicry by a HAVCR1-NPC1 pathway of endosomal fusion mediates hepatitis A virus infection. Nat. Microbiol. 2020;5:1096–1106. doi: 10.1038/s41564-020-0740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto Y., Watanabe Y., Shirakiya Y., Uno F., Kagawa S., Kawamura H., Nagai K., Tanaka N., Kumon H., Urata Y., Fujiwara T. Establishment of biological and pharmacokinetic assays of telomerase-specific replication-selective adenovirus. Cancer Sci. 2008;99:385–390. doi: 10.1111/j.1349-7006.2007.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kepp O., Marabelle A., Zitvogel L., Kroemer G. Oncolysis without viruses—Inducing systemic anticancer immune responses with local therapies. Nat. Rev. Clin. Oncol. 2020;17:49–64. doi: 10.1038/s41571-019-0272-7. [DOI] [PubMed] [Google Scholar]
- 31.Nishida-Aoki N., Tominaga N., Takeshita F., Sonoda H., Yoshioka Y., Ochiya T. Disruption of circulating extracellular vesicles as a novel therapeutic strategy against cancer metastasis. Mol. Ther. 2017;25:181–191. doi: 10.1016/j.ymthe.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludwig N., Whiteside T.L., Reichert T.E. Challenges in exosome isolation and analysis in health and disease. Int. J. Mol. Sci. 2019;20:4684. doi: 10.3390/ijms20194684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai R.C., Yeo R.W., Tan K.H., Lim S.K. Exosomes for drug delivery—A novel application for the mesenchymal stem cell. Biotechnol. Adv. 2013;31:543–551. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Kim S.M., Yang Y., Oh S.J., Hong Y., Seo M., Jang M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J. Control. Release. 2017;266:8–16. doi: 10.1016/j.jconrel.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Kooijmans S.A.A., Schiffelers R.M., Zarovni N., Vago R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment. Pharmacol. Res. 2016;111:487–500. doi: 10.1016/j.phrs.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Kuroda S., Kagawa S., Fujiwara T. In: Third Edition. Lattime E.C., Gerson S.L., editors. Elsevier; 2014. Selectively replicating oncolytic adenoviruses combined with chemotherapy, radiotherapy, or molecular targeted therapy for treatment of human cancers; pp. 171–183. (Gene Therapy of Cancer). [Google Scholar]
- 37.Aoyama K., Kuroda S., Morihiro T., Kanaya N., Kubota T., Kakiuchi Y., Kikuchi S., Nishizaki M., Kagawa S., Tazawa H., Fujiwara T. Liposome-encapsulated plasmid DNA of telomerase-specific oncolytic adenovirus with stealth effect on the immune system. Sci. Rep. 2017;7:14177. doi: 10.1038/s41598-017-14717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garofalo M., Villa A., Crescenti D., Marzagalli M., Kuryk L., Limonta P., Mazzaferro V., Ciana P. Heterologous and cross-species tropism of cancer-derived extracellular vesicles. Theranostics. 2019;9:5681–5693. doi: 10.7150/thno.34824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y., Li C.W., Chan L.C., Wei Y., Hsu J.M., Xia W., Cha J.H., Hou J., Hsu J.L., Sun L., Hung M.C. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28:862–864. doi: 10.1038/s41422-018-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iguchi Y., Eid L., Parent M., Soucy G., Bareil C., Riku Y., Kawai K., Takagi S., Yoshida M., Katsuno M. Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain. 2016;139:3187–3201. doi: 10.1093/brain/aww237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.