Abstract
Background:
Spinal anesthesia is utilized as an alternative to general anesthesia in infants for some surgeries. After spinal anesthesia, infants often become less conscious without administration of sedative medications. The aim of this study was to assess electroencephalographic (EEG) correlates after spinal anesthesia with electroencephalography in a cohort of infants.
Methods:
This pilot study included 12 infants who underwent spinal anesthesia. Unprocessed electroencephalography was recorded. The electroencephalogram was interpreted by four neurologists. Processed analyses compared electroencephalogram changes 30 minutes after spinal anesthesia to baseline.
Results:
Following spinal anesthesia, all 12 infants became sedated. Electroencephalography in all 12 demonstrated Stage 2 sleep with the appearance of sleep spindles (12–14 Hz) in the frontal and central leads in 8/12 (67%) of subjects. The median time to onset of sleep spindles was 24.7 interquartile range (21.2, 29.9) minutes. The duration of sleep spindles was 25.1 interquartile range (5.8, 99.8) minutes. Voltage attenuation and background slowing were the most common initial changes. Compared to baseline, the electroencephalogram 30 minutes after spinal anesthesia showed significantly increased absolute delta power (p=0.02) and gamma power (p<0.0001); decreases in beta (p=0.0006) and higher beta (p<0.0001) were also observed. The Fast Fourier Transform power ratio difference for delta/beta was increased (p=0.03). Increased coherence was noted in the delta (p=0.02) and theta (p=0.04) bandwidths.
Conclusions:
Spinal anesthesia in infants is associated with increased electroencephalographic slow wave activity and decreased beta activity compared to the awake state, with appearance of sleep spindles suggestive of normal sleep. The etiology of the observed voltage attenuation and background slowing remains unclear. Our preliminary findings contribute to the understanding of the brain effects of spinal anesthesia in early development.
Keywords: infant, anesthesia, spinal, electroencephalography, sleep, anesthesia, sedation
Introduction
Infant spinal anesthesia (SA) is an alternative to general anesthesia (GA) with favorable clinical properties in some infants and neonates. Hypotension, bradycardia, hypoxemia, and apnea occur rarely in infants after SA, unlike in adults1,2, with supplemental oxygen usually not required3,4. In a retrospective review of 1,483 infants who received SA, only 3.7% required supplemental oxygen; oxygen desaturation to <90% was noted in only 10 of 1483 patients (0.6%)5. A prospective, multi-center, international trial also demonstrated the cardiopulmonary stability of SA in infants6. A depressed level of arousal, similar to sedation, is commonly observed after SA in early age. This depressed arousal seems to occur after lumbar puncture and intrathecal administration of bupivacaine alone without administration of any other sedative-hypnotic agents, either in the spinal block or intravenously. While commonly observed, its incidence has not been reported in the literature7. The etiology of this state is unknown. The objective of this study was to characterize the EEG associated with decreased arousal following spinal anesthesia in infants8. We hypothesized that SA would produce distinct, measurable changes on the EEG that are different from the awake state.
Methods
This pilot prospective, multi-center, observational study was reviewed and approved by the Institutional Review Boards of the University of Vermont and Albert Einstein College of Medicine. After written informed consent was obtained from the legal guardian, we obtained EEG data from 12 infants (all less than 12 months of age) presenting for elective infraumbilical surgery.
Spinal Anesthesia Protocol:
Local anesthetic cream was applied to the lumbar spine and covered with a clear bioocclusive dressing approximately 30 minutes prior to the SA. SA was performed in the sitting position with a 22-gauge, 1.5-inch spinal needle. After lumbar puncture and confirmation of clear CSF return through the spinal needle, 0.5% isobaric bupivacaine was administered at a dose of 1 mg/kg into the intrathecal space, maximum dose of 5 mg, maximum volume of injectate of 1 mL. Clonidine 1 mcg/kg was added to the local anesthetic at the discretion of the clinician. No systemic sedatives were necessary.
Electroencephalogram (EEG) Recording:
The EEG was recorded using a system of non-invasive scalp electrodes (microEEG®, BioSignal Group, Acton, MA, USA)9. The EEG electrodes were arranged in a single headset designed for use in infants according to the International 10–20 System (Supplemental Figure 1). EEG was continuously recorded for a minimum of 5 minutes before anesthesia, during SA placement, and through the procedure until the infant was in the postoperative care unit. All EEG analyses used the linked-ear montage. Four neurologists interpreted the recordings in the context of the age of the patients. Sixty second epochs of artifact-free EEG were analyzed during the awake state and at 10–20 min, 30 min and 60 min following the SA.
EEGs were analyzed using NeuroGuide (Applied Neuroscience, Inc., Largo, FL, USA). Frequencies from 0.5–50 Hz were analyzed using a Fast Fourier Transform (FFT) with the following parameters: epoch = 2 sec at a sample rate of 128 Hz (75% window overlap). FFT of the EEG for each of the 19 electrodes for Delta (δ) (0–4 Hz), Theta (θ) (4–8 Hz), Alpha (α) (8–12 Hz), α1 (8–10 Hz), α2 (10–12 Hz), Beta (β) (12–25 Hz), High β (25–30 Hz). β1 (12–15 Hz), β2 (15–18 Hz), β3 (18–25 Hz), Gamma (γ)(30–40 Hz), High γ (40–50 Hz), γ1 (30–35 Hz), γ2 (35–40 Hz) was obtained. To assess reliability of EEG the split-half reliability coefficient, defined by the ratio of the variance of the even and odd seconds of the selected EEG record and referred to as the proportion of the obtained variance that is true variance, was obtained. Test-retest reliability was determined by the ratio of the variance of the first 30 sec of the recording with the second 30 sec to assure there is no change in state during the 60 second epoch. Only recordings in which the split-half reliability coefficient was above 0.95 and the test-retest reliability was greater than 0.9 were used10.
FFT absolute and relative power and ratios of bandwidths (delta/theta, delta/alpha, delta/beta, delta/gamma, theta/alpha, theta/beta, theta/gamma, alpha/beta) for all electrodes were obtained10. FFT coherence for each of the electrode pairs were obtained including intra-hemispheric and inter-hemispheric pairwise combinations11,12.
Coherence represents the consistency of the phase difference between two EEG signals when compared over time and serves as a measure of synchronization between two EEG signals based mainly on phase consistency10. Two signals that have a consistent phase relationship with each other will have a high coherence, while two signals that are not related to each other will have a low coherence. Coherences vary from 0, where there is no consistency between phase of two EEG signals to 100, where there is perfect alignment of phase.
Coherence was defined as:
Where Gxy(f) is the cross-power spectral density and Gxx(f) and Gyy(f) (x- channel 1, y-channel 2) are the respective autopower spectral densities10,13,14. Coherences were computed for all pairwise combination of the 19 channels for each of the frequency bandwidths.
Statistical Analysis:
The paired-t test was used to compare EEG measures between the awake state and 10–20 minutes, 30 minutes and 60 minutes after SA. Percent changes in absolute, relative and spectral power ratios for each of the electrode pairs were calculated across the awake and anesthetic states for each of the 12 infants. This is a preliminary study designed to assess the feasibility of carrying out the study protocol with no formal power analysis. To provide a global assessment of power, both absolute and relative (bandwidth power divided by total power) power were calculated by averaging absolute and relative power for each electrode in each bandwidth. In addition, the paired-t test was used to compare changes in power ratios in the δ/θ, δ/α, δ/β, θ/α, θ/β, and α/β bandwidths and the mean coherence for all electrode pairs in the δ, θ, α, and β bandwidths. Data are presented as counts and percentages, means and standard deviations, or medians and interquartile ranges. The normality assumption was assessed using histograms. All tests were two tailed with a p<0.05 considered significant. Statistical analyses were performed using STATA 13.1 (College Station, TX, USA).
Results
Twelve infants were recruited for the study. Demographics for the patient cohort are seen in Table 1. The median age of the cohort was 2.8 IQR (2.1, 5.4) months and all twelve infants were males. All patients received 1 mg/kg of 0.5% isobaric bupivacaine, maximum dose 5 mg. Four infants (33%) infants received intrathecal clonidine, 1 mg/kg, in addition to bupivacaine at the discretion of the clinician. Of the 4 who received clonidine, all developed sleep spindles (100%) compared to 4 of 8 who did not receive clonidine (50%), p=0.21. No patient received systemic sedatives or opioids during the entire perioperative period.
Table 1 –
Patient Characteristics (n=12)
| Age (months) | 2.8 (2.1, 5.4) |
| Weight (kg) | 5.5 (4.9, 6.8) |
| Sex | |
| Male | 12 (100%) |
| Female | 0 (0%) |
| Full term | 9 (75%) |
| Postconceptual age of premature infants (months) | 33.8 (0.3) |
| ASA Physical Status Classification | |
| ASA I | 7 (58.3) |
| ASA II | 5 (33.3) |
| Surgery | |
| Achilles tendon lengthening | 5 (41.7%) |
| Inguinal hernia repair | 3 (25%) |
| Penis straightening | 1 (8.3%) |
| Circumcision | 1 (8.3%) |
| Orchiopexy and inguinal hernia repair | 1 (8.3%) |
| Inguinal hernia repair and circumcision | 1 (4.8%) |
| Sleep spindles | 8 (66.7%) |
| Median time to sleep spindles (minutes) | 24.7 (21.2, 29.9) |
| Median duration of sleep spindles (minutes) | 25.1 (5.8, 99.8) |
Variables reported as mean and standard deviation, median and inter-quartile range or count and percentage.
Following SA, infants became qualitatively less agitated with cessation of crying followed shortly by a global state of decreased arousal (similar to the infant pictured in Supplemental Figure 2). No infant required supplemental oxygen or airway management. Movement artifact on the EEG was improved after SA due to global reduction in movements and eye blinking. Initial qualitative EEG changes after SA consisted most commonly of increased background slowing. The majority of infants (8/12, 67%) developed sleep spindles indicative of sleep. The median time to onset of sleep spindles was 24.7 IQR (21.2, 29.9) minutes. The median duration of stage 2 of sleep was 25.1 IQR (5.8, 99.8) minutes. Figure 1a is an example of an EEG before SA and Figures 1b–1d showing diffuse slowing (more prominent in 1d) with sleep spindles either frontally (Figure 1b) or centrally (Figures 1c). Figure 2 shows a density spectral power array over an 8-minute period where the infant transitioned from the awake state to sleep. Sleep spindles emerged during sleep (asterisks).
Figure 1.
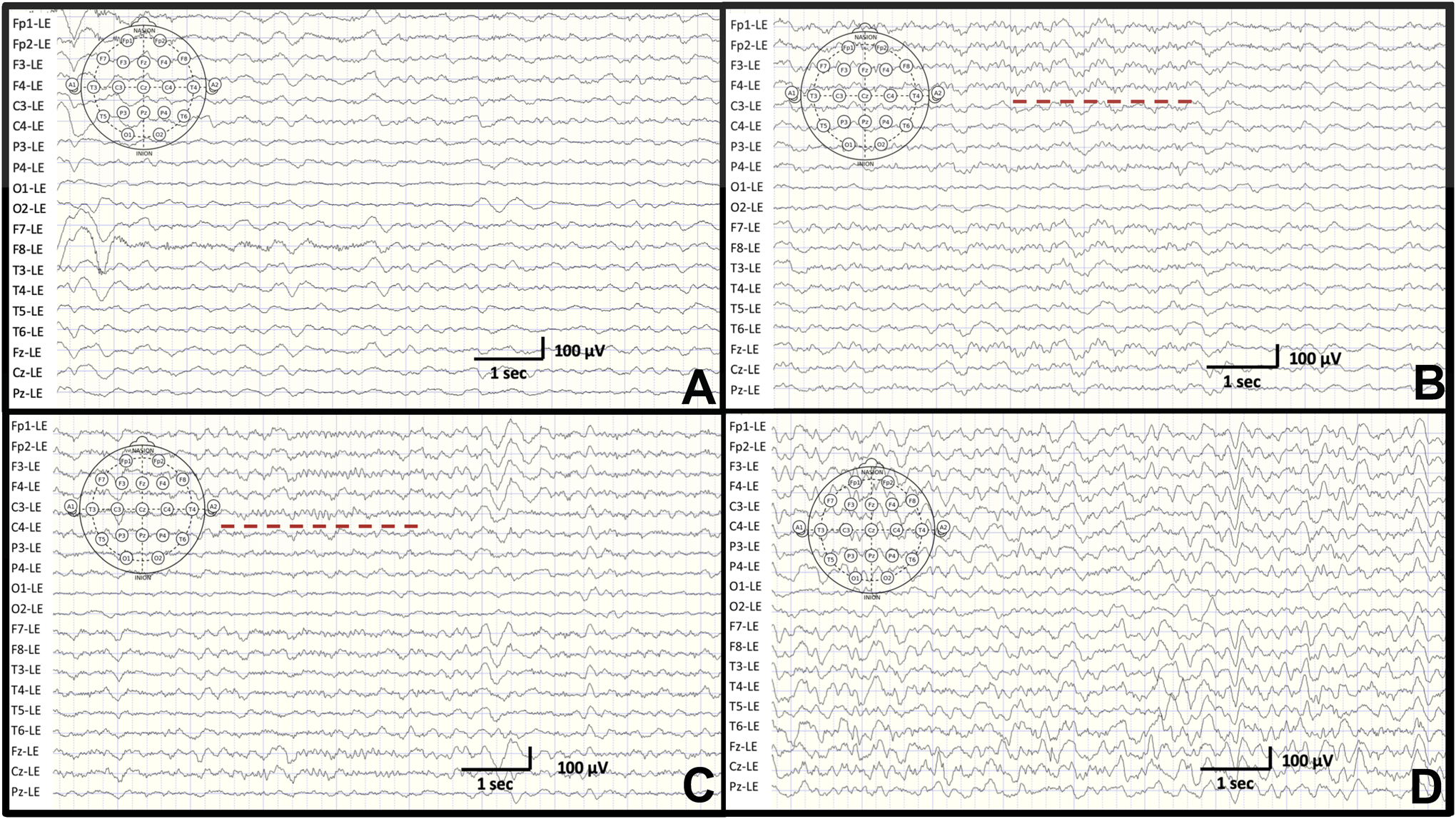
Unprocessed EEG tracings. A. An example of an EEG during the awake state in a 3 month old male infant. B. Diffuse slowing with sleep spindles in frontal leads (dashed red line) in a 3 month old male. C. Diffuse slowing with sleep spindles in central leads (dashed red lines) in a 4 month old male. D. As sleep continues, diffuse delta activity indicative of stage III of sleep is seen in a 7 month old male.
Figure 2.
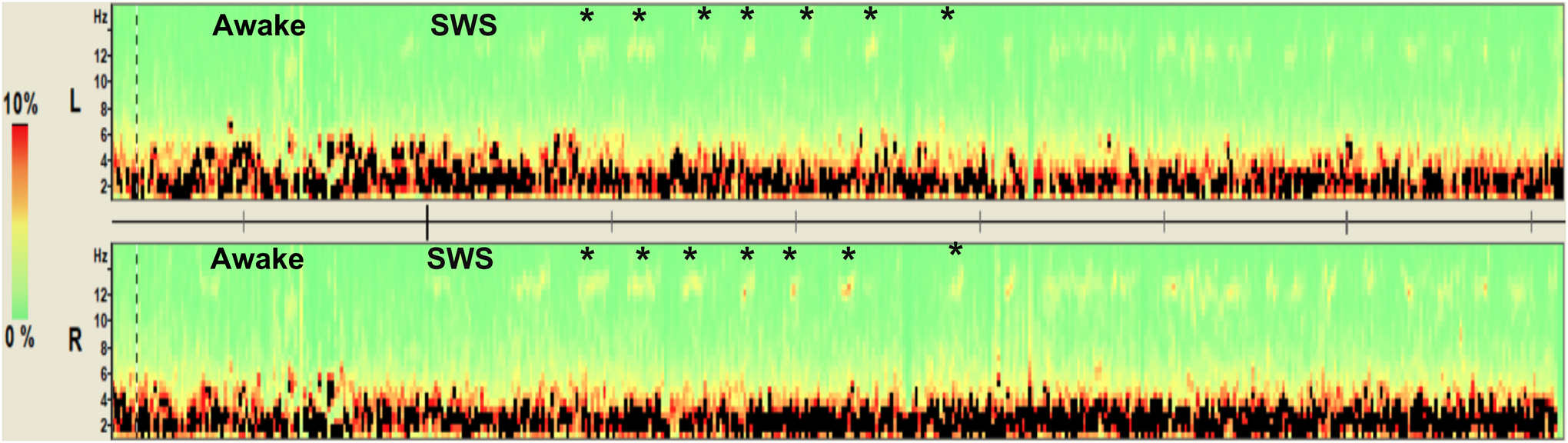
Density Spectral Array. Density of spectral power (color coded for percentage of total power as a function of frequency) over an 8-minute period showing transition from the awake state to stage 2 of sleep. Central electrodes from the left (L) and right (R) hemispheres are displayed. Sleep spindles (12–14 Hz) are denoted by the asterisks and the faint yellow density measures.
Compared to the baseline prior to anesthesia, quantitative EEG changes 30 minutes post-SA demonstrated a significant increase in FFT absolute δ power (t=2.30, df=215, p=0.02) and significant decreases in beta power (t=3.49, df=215, p=0.0006), high beta power (t=6.42, df=215, p<0.0001) and γ power (t=7.30, df=215, p<0.0001) (Figure 3). Relative power was significantly higher in the delta band (t=7.22, df=215, p<0.0001) and lower in the β (t=3.59, df=215, p=0.0006) and higher β (t=6.37, df=215, p<0.0001) bands. This figure demonstrates that post-SA is associated with increased slow wave and decreased β activity compared to the baseline awake state, similar to normal sleep.
Figure 3.
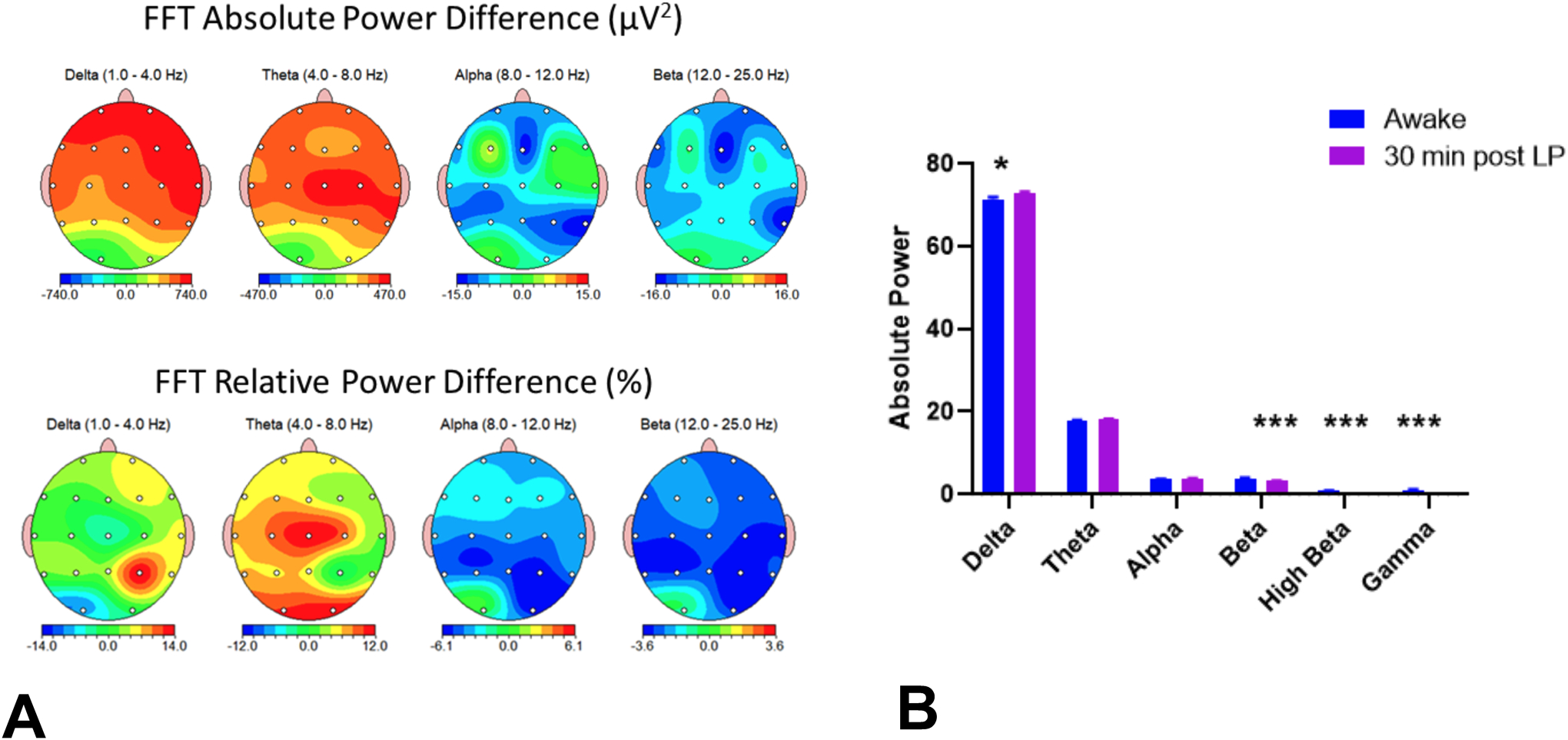
Absolute and relative power percent differences between awake and post-SA EEG. A Graphic of group differences in the major bandwidths between the awake and post-SA (30 min) recording. The top panel shows absolute power differences and the bottom panel relative power differences. The figures show the changes in absolute and relative power between the awake and post-SA states. Note increases in slow frequencies and decreases in fast frequencies with SA. B. Graphs of total absolute power comparing the awake and post-SA EEG. High beta and gamma bands are included in the graphs.
When power ratios in the delta/theta, theta/alpha, delta/beta, theta/alpha, theta/beta, and alpha/beta were compared between the baseline and at 30 minutes post-SA, there were increases in all ratios at the 30-minute timepoint, with the δ/β difference reaching significance (t=2.6, df=11, p=0.03) (Figure 4).
Figure 4.
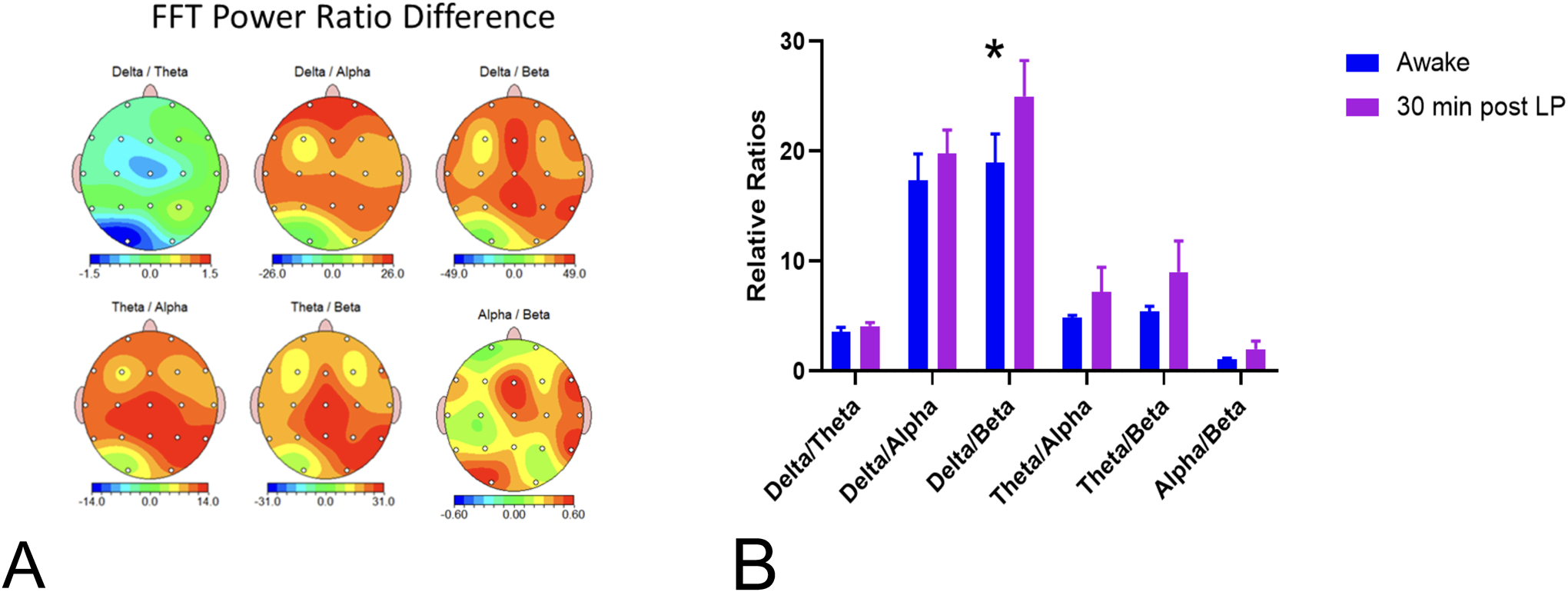
FFT power ratio differences from awake to 30 min following SA. A. Delta/theta, theta/alpha, theta/beta and alpha/beta power ratios 30 minutes post-SA, as compared to the awake recording. B. Compared to the awake state, the delta/beta ratio was significantly higher 30 minutes post-SA than during the awake state.
Coherence, a measure of waveform coupling and functional connectivity, increases during sleep11. Following SA, significant increases in coherence were noted in the δ (t=2.87, f=11, p=0.02) and Θ (t=2.46, df=11, p=0.04) bandwidths (Figure 5).
Figure. 5.
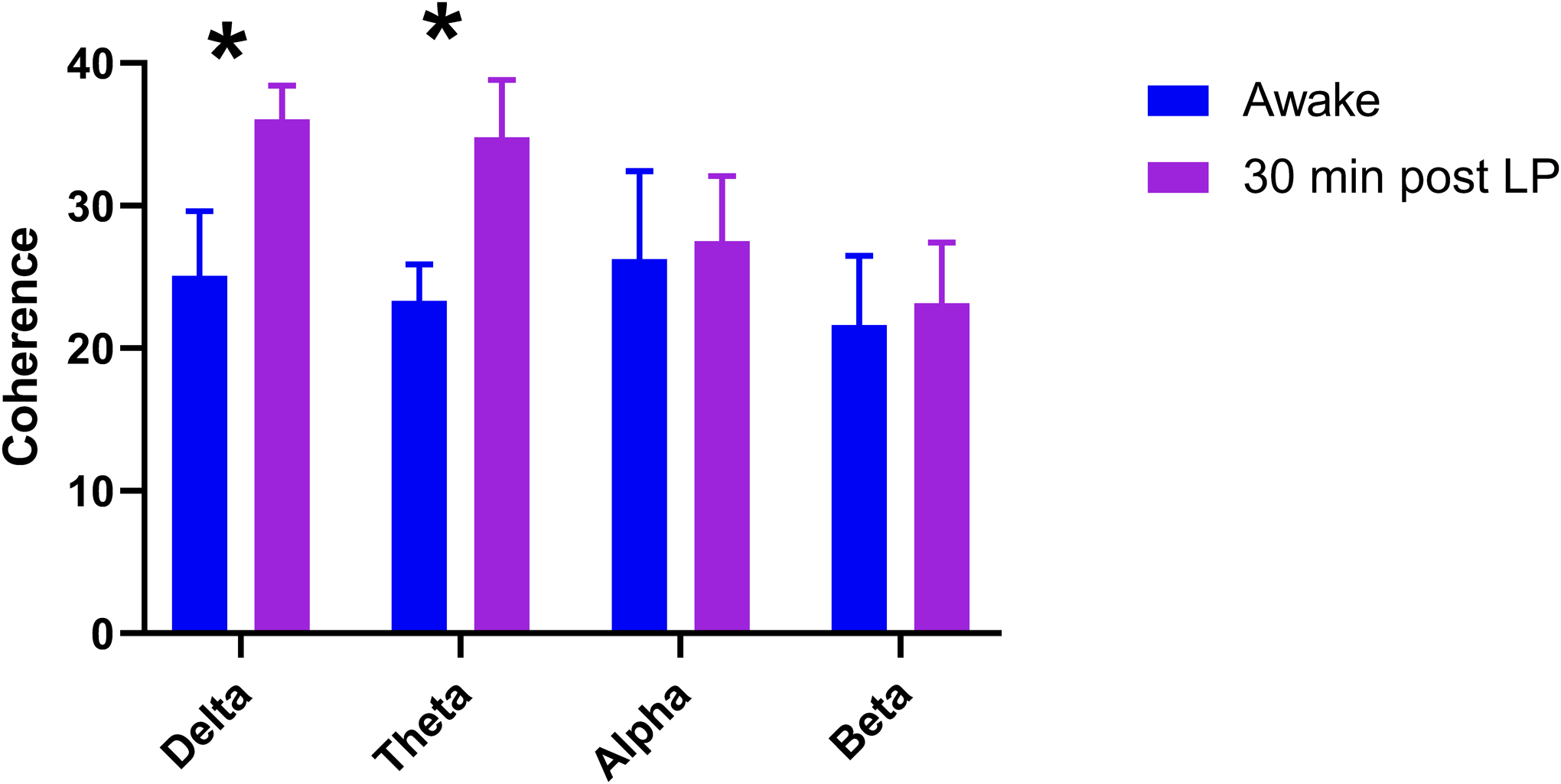
Comparison of coherences between bandwidths during the awake and post-SA EEG. There was an increase in delta (t=2.870, df=11, p=0.024) and theta (t=2.462, df=11, p=0.043) coherence in the post-SA EEG compared to the awake state.
Discussion
The present study was designed to better characterize brain activity after SA in infants. While it is clear that infant SA causes decreased arousal in the majority of patients, it remains unclear why this occurs. Our study has 4 main findings: 1) SA in infants is associated with global slowing consistent with normal Stage 2 sleep; 2) in many patients global slowing is accompanied by sleep spindles; 3) SA is associated with increased absolute power in the delta bandwidth and decreased power in the β bandwidth, similar to physiologic sleep; 4) SA in infants is associated with increased δ and Θ coherence.
Although there is now a significant body of knowledge regarding EEG signatures of different general anesthetics15, the effects of neuraxial anesthesia on the brain remain understudied, particularly during infant SA. The combination of neuraxial blockade and decreased arousal seen with SA is typically sufficient to allow surgery to proceed without the use of general anesthetic agents, exogenous sedatives, or opioids. The reduction in opioid use is typically extended into the postoperative recovery period, consistent with prior work which demonstrated significantly lower postoperative pain scores in infants receiving awake regional anesthesia compared to GA16. Similarly, none of the patients in this trial were exposed to general anesthesia, sedatives or opioids at any point. The sedated state induced by infant SA appears similar to normal sleep and not a typical anesthetized condition. SA, with typical duration lasting between 60 up to 90 minutes, is not a viable option for all types of surgery; it is limited by the site of surgery as well as surgical duration. However, the anesthetic state induced by SA in infants is, in many ways, ideal and may serve as an aspirational endpoint for the development of newer anesthetic agents and methodologies for both general and regional techniques. In this regard, understanding the details of the EEG and consequently beginning to understand the function and connectivity of the brain during this state is an important first step in this process.
Burst suppression on the EEG has been reported after spinal anesthesia in infants17. Burst suppression is a stereotypic pattern of activity in the brain that presents as continuous alternation between two states, high-voltage waves (burst) and isoelectric epoch (suppression) and is fundamentally different from the slow wave activity observed during sleep18. Burst suppression is not required to ensure lack of awareness, prevent patient movement, or provide adequate analgesia during anesthesia. Several studies in older adults have shown that intraoperative burst suppression under GA is associated with an increased risk of postoperative cognitive dysfunction18–20. Though burst suppression occurs in children under multiple modalities of GA21 at a rate of approximately 50–60%22–24, potential consequences of this remain unknown. Our data collection to date does not show any evidence of burst suppression after administration of SA in infants, suggesting SA may be associated with less risk for this pattern of brain activity and any potential morbidities therefrom. GA, particularly with sevoflurane, has been associated with cortical epileptiform EEG discharges, usually without clinical manifestation25, and this has been observed in infants26. Although epileptiform activity is more common during sleep than during the awake state27,28, in our cohort of infants, no epileptiform activity was noted.
The EEG demonstrated voltage attenuation and increased background slowing as the most common initial changes. These findings may be due to the deafferentation of ascending sensory-proprioceptive transmission that occurs as a result of the spinal block29–33. Sleep spindles appeared frontally or centrally in 8 of 12 subjects (67%). These findings are consistent with EEG patterns seen during normal, stage 2 non-rapid eye movement (NREM) sleep. Stage 2 sleep typically begins approximately 10 minutes after a sleep episode commences, lasts approximately 10–25 minutes, and constitutes 45–55% of a total sleep cycle. The physiologic importance of stage 2 sleep is not fully understood, but it likely plays an important role in memory consolidation by transmitting information from the hippocampus to the neocortex34.
Technical challenges in the clinical care of younger patients also apply to the application of EEG electrodes. A strength of this study is the use of a full head montage, unprocessed EEG recording. However, the EEG recordings were subject to artifact due to patient movement and positioning for the placement of the SA, as well as surgery. Nevertheless, this preliminary data had epochs of clear, artifact-free recordings that were interpretable. Qualitative assessment of the unprocessed EEG by neonatal and pediatric neurologists and electroencephalographers are another strength of the study, which allowed for age-specific interpretation.
This pilot study has a number of limitations including recruitment of a relatively small number of infants with a strong preponderance of male gender. In addition, although the dose of bupivacaine in this study was standardized, the use of intrathecal clonidine likely contributed to more sedation in 4 infants (though EEG data were similar in both groups). However, the prevalence of sleep spindles was not significantly different between those who received clonidine in the SA (4/4, 100%) compared to those who did not (4/8, 50%), p=0.2.
Here, we report findings that provide the electroencephalographic correlate of decreased arousal seen after induction of infant SA. SA in infants produces a rapid onset of physiologically normal stage 2 sleep different from general anesthesia, sedative or opioid effects. Further work is required to understand the mechanism(s) of SA-induced sleep and the potential implications of these findings.
Supplementary Material
Supplemental Figure 1. A. Stock image of an infant with International 10–20 System of electrodes (microEEG) http://biosignalgroup.com/product-service/hydrodot-biosensors/ B. Schematic of International 10–20 System.
Supplemental Figure 2. Infant in the operating room wearing microEEG headset.
Clinical Implications:
What is already known about this topic: Spinal anesthesia in infants causes a state of apparent sedation, even in infants that have not received any oral or intravenous sedatives.
What this study adds: Spinal anesthesia in early infant development is associated with increased background slowing and the appearance of sleep spindle complexes on the EEG that demonstrate sleep.
Acknowledgements:
We thank all patients and family members who engaged in conversation about participation in this clinical study. The authors wish to acknowledge Max Breidenstein and Alex Friend for their invaluable assistance with subject recruitment and data collection. The authors would also like to acknowledge Samah Baki, MD and James Henson, Eng. of Bio-Signal Group.
Funding:
The research was supported by the Departments of Anesthesiology at the University of Vermont Larner College of Medicine and Montefiore Medical Center, Albert Einstein College of Medicine as well as NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Numbers UL1TR001073 and KL2 TR002558 (JC).
Footnotes
Data Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest/Financial Disclosures: The authors report no conflicts of interest.
References
- 1.Ing C, Sun LS, Friend AF, et al. Adverse Events and Resource Utilization After Spinal and General Anesthesia in Infants Undergoing Pyloromyotomy. Reg Anesth Pain Med. 2016;41(4):532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams RK, Abajian JC. High spinal anaesthesia for repair of patent ductus arteriosus in neonates. Paediatr Anaesth. 1997;7(3):205–209. [DOI] [PubMed] [Google Scholar]
- 3.Ebert KM, Jayanthi VR, Alpert SA, et al. Benefits of spinal anesthesia for urologic surgery in the youngest of patients. J Pediatr Urol. 2019;15(1):49 e41–49 e45. [DOI] [PubMed] [Google Scholar]
- 4.Whitaker EE, Wiemann BZ, DaJusta DG, et al. Spinal anesthesia for pediatric urological surgery: Reducing the theoretic neurotoxic effects of general anesthesia. J Pediatr Urol. 2017;13(4):396–400. [DOI] [PubMed] [Google Scholar]
- 5.Williams RK, Adams DC, Aladjem EV, et al. The safety and efficacy of spinal anesthesia for surgery in infants: the Vermont Infant Spinal Registry. Anesth Analg. 2006;102(1):67–71. [DOI] [PubMed] [Google Scholar]
- 6.Ing C, Sun LS, Friend AF, et al. Differences in intraoperative hemodynamics between spinal and general anesthesia in infants undergoing pyloromyotomy. Paediatr Anaesth. 2017;27(7):733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roeggen II, Olischar M, Davidson A, Disma N. Sleep and the EEG in infants. Paediatr Anaesth. 2010;20(4):368–369. [DOI] [PubMed] [Google Scholar]
- 8.Antognini JF, Jinks SL, Atherley R, Clayton C, Carstens E. Spinal anaesthesia indirectly depresses cortical activity associated with electrical stimulation of the reticular formation. Br J Anaesth. 2003;91(2):233–238. [DOI] [PubMed] [Google Scholar]
- 9.Grant AC, Abdel-Baki SG, Omurtag A, et al. Diagnostic accuracy of microEEG: a miniature, wireless EEG device: Epilepsy Behav 2014; 34:81–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NeuroGuide Help Manual. 2002–2018. Applied Neuroscience, Inc. (https://www.appliedneuroscience.com/PDFs/NeuroGuide_Manual.pdf) [Google Scholar]
- 11.Buckley AW, Scott R, Tyler A, et al. State-Dependent Differences in Functional Connectivity in Young Children With Autism Spectrum Disorder. EBioMedicine. 2015;2(12):1905–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy FH, Als H. A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls - a large case control study. BMC Med. 2012;10:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thatcher RW. Coherence, phase differences, phase shift, and phase lock in EEG/ERP analyses. Dev Neuropsychol. 2012;37(6):476–96. [DOI] [PubMed] [Google Scholar]
- 14.Thatcher RW, North DM, Biver CJ. Development of cortical connections as measured by EEG coherence and phase delays. Hum Brain Mapp. 2008;29(12):1400–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelissen L, Kim SE, Purdon PL, Brown EN, Berde CB. Age-dependent electroencephalogram (EEG) patterns during sevoflurane general anesthesia in infants. Elife. 2015;4:e06513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Disma N, Withington D, McCann ME, et al. Surgical practice and outcome in 711 neonates and infants undergoing hernia repair in a large multicenter RCT: Secondary results from the GAS Study. J Pediatr Surg. 2018;53(9):1643–1650. [DOI] [PubMed] [Google Scholar]
- 17.Disma N, Tuo P, Astuto M, Davidson AJ. Depth of sedation using Cerebral State Index in infants undergoing spinal anesthesia. Paediatr Anaesth. 2009;19(2):133–137. [DOI] [PubMed] [Google Scholar]
- 18.Steriade M, Amzica F, Contreras D. Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr Clin Neurophysiol. 1994;90(1):1–16. [DOI] [PubMed] [Google Scholar]
- 19.Soehle M, Dittmann A, Ellerkmann RK, Baumgarten G, Putensen C, Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiol. 2015;15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritz BA, Kalarickal PL, Maybrier HR, et al. Intraoperative Electroencephalogram Suppression Predicts Postoperative Delirium. Anesth Analg. 2016;122(1):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rigouzzo A, Khoy-Ear L, Laude D, et al. EEG profiles during general anesthesia in children: A comparative study between sevoflurane and propofol. Paediatr Anaesth. 2019;29(3):250–257. [DOI] [PubMed] [Google Scholar]
- 22.Koch S, Stegherr AM, Rupp L, et al. Emergence delirium in children is not related to intraoperative burst suppression - prospective, observational electrography study. BMC Anesthesiol. 2019;19(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornelissen L, Bergin AM, Lobo K, Donado C, Soul JS, Berde CB. Electroencephalographic discontinuity during sevoflurane anesthesia in infants and children. Paediatr Anaesth. 2017;27(3):251–262. [DOI] [PubMed] [Google Scholar]
- 24.Yuan I, Landis WP, Topjian AA, et al. Prevalence of isoelectric electroencephalography events in infants and young children undergoing general anesthesia. Anesth Analg 2020;130(2):462–71. [DOI] [PubMed] [Google Scholar]
- 25.Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Pediatr Anesth. 2005;15(4):266–274. [DOI] [PubMed] [Google Scholar]
- 26.Chao JY, Legatt AD, Yozawitz EG, Adams DC, Delphin ES, Shinnar S. Electroencephalographic Findings and Clinical Behavior During Induction of Anesthesia With Sevoflurane in Human Infants: A Prospective Observational Study. Anesth Analg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halasz P, Bodizs R, Ujma PP, Fabo D, Szucs A. Strong relationship between NREM sleep, epilepsy and plastic functions - A conceptual review on the neurophysiology background. Epilepsy Res. 2019;150:95–105. [DOI] [PubMed] [Google Scholar]
- 28.Jain SV, Kothare SV. Sleep and Epilepsy. Semin Pediatr Neurol. 2015;22(2):86–92. [DOI] [PubMed] [Google Scholar]
- 29.Aguilar J, Humanes-Valera D, Alonso-Calviño E, et al. Spinal cord injury immediately changes the state of the brain. J Neurosci. 2010;30(22):7528–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran Y, Boord P, Middleton J, Craig A. Levels of brain wave activity (8–13 Hz) in persons with spinal cord injury. Spinal Cord. 2004;42(2):73–9. [DOI] [PubMed] [Google Scholar]
- 31.Davidson AJ, Ironfield CM, Skinner AV, Frawley GP. The effects of caudal local anesthesia blockade on the Bispectral Index during general anesthesia in children. Paediatr Anaesth. 2006;16(8):828–33. [DOI] [PubMed] [Google Scholar]
- 32.Doufas AG, Wadhwa A, Shah YM, Lin C-M, Huagh GS, Sessler DI. Block-dependent sedation during epidural anaesthesia is associated with delayed brainstem conduction. Br J Anaesth. 2004;93(2):228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tinazzi M, Rosso T, Zanette G, Fiaschi A, Aglioti SM. Rapid modultation of cortical proprioceptive activity induced by transient cutaneous deafferentation: neurophysioloigcal evidence of short-term plasticity across different somtosensory modalities in humans. Eur J Neurosci. 2003;18(11):3053–60. [DOI] [PubMed] [Google Scholar]
- 34.Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22(15):6830–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. A. Stock image of an infant with International 10–20 System of electrodes (microEEG) http://biosignalgroup.com/product-service/hydrodot-biosensors/ B. Schematic of International 10–20 System.
Supplemental Figure 2. Infant in the operating room wearing microEEG headset.


