Abstract
Cognate interactions between autoreactive B and T cells promote systemic lupus erythematosus (SLE) pathogenesis by, inter alia, facilitating spontaneous germinal center (GC) formation. Whereas both myeloid and B cell antigen presenting cells (APCs) express B7 ligands (CD80 and CD86), the prevailing model holds that dendritic cell (DC) costimulation is sufficient for CD28-dependent T cell activation. Here, we report that B cell-intrinsic CD80/CD86 deletion unexpectedly abrogates GCs in murine lupus. Interestingly, absent GCs differentially impacted serum autoantibodies (autoAb). In keeping with distinct extra-follicular (EF) and GC activation pathways driving lupus autoAb, lack of GCs correlated with loss of RNA-associated autoAb but preserved anti-dsDNA and connective tissue autoAb titers. Strikingly, even heterozygous B cell CD80/CD86 deletion was sufficient to prevent autoimmune GCs and RNA-associated autoAb. Together, these findings identify a key mechanism whereby B cells promote lupus pathogenesis, by providing a threshold of costimulatory signals required for autoreactive T cell activation.
Introduction
Emerging data indicating that autoreactive B cell activation, epitope spreading, and pathogenic anti-nuclear antibodies (ANA) generation in SLE occurs within spontaneous germinal centers (GCs) (1). Thus, delineating the cellular mechanisms underlying initial breaks in B cell tolerance and spontaneous GC formation is of significant importance. During lupus pathogenesis, autoreactive B cells engage cognate T cell help and initiate breaks in T cell tolerance via B cell-intrinsic MHC Class II (MHCII)-dependent antigen presentation and pro-inflammatory cytokine production (2–4). Complete CD4+ T cell activation requires costimulatory signals, including CD28 engagement by B7 receptors CD80 (B7.1) and CD86 (B7.2) on antigen presenting cells (APCs). Animal studies have confirmed the importance of B7:CD28 signals in GC formation since both Cd28−/− and Cd80−/−/Cd86−/− mice fail to generate GCs (5, 6). Conversely, the CD28 homolog CTLA-4 inhibits pathogenic T cell activation via cell-intrinsic and cell-extrinsic mechanisms (7).
Importantly, CD4+ T cell costimulatory signals could conceivably be provided by distinct APC populations, dendritic cells (DC) and B cells. Based on our earlier identification of B cells as critical APCs in SLE (3), we predicted that B cell-derived costimulatory signals likely facilitate breaks in T cell immune tolerance during autoimmunity (1). However, Watanabe et al. reported that B cell CD80/CD86 expression is redundant for T cell activation and GC formation after NP-OVA/Alum immunization (8). An important caveat is that candidate antigen immunization models may not accurately inform the biology of autoimmune GCs given the differences in (auto)antigen abundance and affinity, adjuvant load, and the impact of B cell/T cell tolerance mechanisms. For these reasons we hypothesized that costimulatory signals might exert a more nuanced impact on GC formation in autoimmunity.
Using a well-characterized model of murine SLE, we here show that, while autoreactive CD4+ T cell priming is B cell independent, B cell-derived costimulatory signals are critical for complete T follicular helper (Tfh) cell differentiation and autoimmune GC formation. Strikingly, even heterozygous deletion of B cell CD80/CD86 recapitulated this phenotype, indicating that during initial interactions between antigen-primed autoreactive T and B cells, a threshold of B cell costimulatory signals is required for CD4+ T cell activation and spontaneous GC formation.
Materials and methods:
Murine models
Murine studies performed in specific pathogen-free (SPF) environment in accordance with IACUC approved protocols. To establish chimeras, donor BM (from femora and tibiae) was depleted of CD138+ plasma cells (Miltenyi Biotec, 130–098-257) and mixed with μMT BM (20:80 ratio, 6 × 106 total cells) and injected retro-orbitally into lethally irradiated (450cGy × 2 doses) μMT recipients. For CD28-null chimeras, Was−/−.Cd28−/− and Cd28−/−.μMT BM (20:80 ratio, 6 × 106 total cells) was transferred into Cd28−/−.μMT recipients. WAS chimeras sacrificed at 24 weeks and VLP models at 8 weeks post-transplant. For VLP experiments, CellTrace Violet-labeled 2 × 106 TCR transgenic OT-II CD4+ T cells, negatively enriched using magnetic microbeads (≥95% purity), were transferred into either WT or Cd80−/−.Cd86−/− recipients (global model) or B cell-intrinsic WT and Cd80−/−.Cd86−/− chimeras (B cell-intrinsic model). Recipient animals were immunized I.P. with 10μg Qβ-Ova VLP and splenocytes analyzed at day 3 post immunization (9).
Antibodies
Antibodies used: B220 (RA3–6B2), CD4 (RM4–5), CD138 (281–2), CXCR5 (2G8), CD86 (GL1), ICOS (7E.17G9) from BD Biosciences; CD62L (MEL-14), CD11c (N418), CD11b (M1/70), PD-1 (J43), T-bet (4B10), BCL6 (BCL-DWN) from eBioscience; PD-1 (J43) from Life Technolgoies; CD19 (ID3), CD44 (IM7), CD4 (RM4–4), CCR7 (4B12) from BioLegend; PNA (Fl-1071) from Vector Labs; and Fas (Jo2) from BD Pharmingen. Qβ-Ova and VLP-APC from Dr. Baidong Hou. Flow cytometry of splenocyte suspensions was performed as described (10).
Measurement of autoantibodies
AutoAb were measured as described (3) using the following reagents: calf thymus dsDNA (Sigma-Aldrich D3664–5X2MG); Sm/RNP (Arotec Diagnostic Limited ATR01–10); goat anti-mouse IgG-, IgG2c-, IgG3-HRP conjugated Ab (Southern Biotech). Autoantigen microarrays performed at UTSW Microarray Core (11).
Spleen immunofluorescence staining
Immunofluorescence staining of frozen splenic sections was performed as described (3).
Single cell BCR sequencing
Single-cell BCR sequencing was performed as described (12). Briefly, single B220lowCD138+ splenic plasma cells were sorted from representative WAS and B cell-intrinsic Cd80−/−.Cd86−/− WAS chimeras, and BCR κ light chain-specific transcripts amplified in separate nested PCRs prior to sequencing. Processed sequences were submitted to IMGT V-Quest for alignment and somatic hypermutation counts determined by using the IMGT mutation table output.
Results
B cell-derived costimulatory signals enhance Tfh differentiation in SLE
Using a chimeric murine lupus model, termed the Wiskott-Aldrich syndrome (WAS) model, we have shown that B cell-intrinsic TLR signals, B cell antigen presentation to cognate CD4+ T cells, and cell-intrinsic cytokine signals coordinate to orchestrate autoimmune GC formation (3, 4, 10, 13). To address the relative importance of B cell- vs. non-B cell (myeloid)-derived costimulatory signals in SLE, we used a similar strategy and generated WAS chimeras either globally Cd28 deficient or with B cell-intrinsic Cd80/Cd86 deletion. In keeping with established roles for B7:CD28 engagement in T cell activation, CD44hiCD62Llo/hi effector/memory (EM) CD4+ T cells formation was abrogated by global Cd28 deletion. Moreover, lack of CD28 costimulation prevented CXCR5 expression on developing Tfh cells. In contrast, B cell-intrinsic Cd80/Cd86 deletion exerted a limited impact on CD4+ T cell EM differentiation or CXCR5 upregulation, indicating that initial priming of autoreactive CD4+ T cells in this model is B cell independent (Fig. 1A, B; Supplemental Fig. 1).
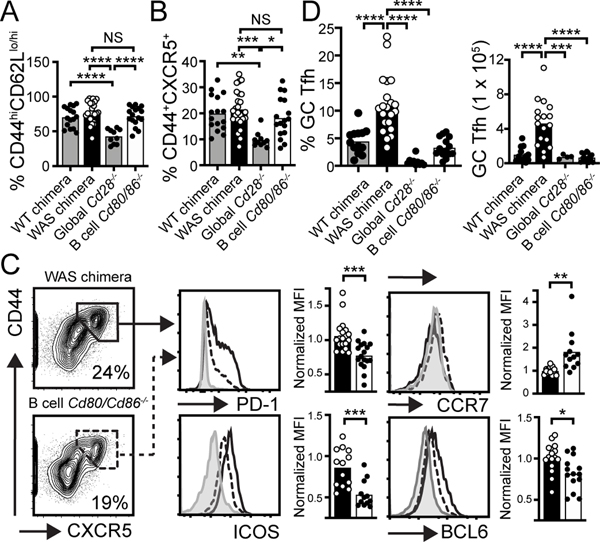
Figure 1: B cell-derived costimulatory signals promote Tfh differentiation.
(A, B) % CD44hiCD62Llo/hi EM (A) and CD44+CXCR5+ (B) within CD4+ compartment in indicated chimeras. (C) FACS plot (gated on CD4+; % in gate) show strategy to identify CD44+CXCR5+ T cells. Histograms show Tfh markers PD1, ICOS, CCR7, and BCL-6 in CD44+CXCR5+CD4+ T cells from WAS (solid line) and B cell-intrinsic Cd80−/−.Cd86−/− WAS (dashed line) chimeras. Gray histogram: CD44−CXCR5− naïve CD4+ T cells. Graphs: MFI of indicated markers (normalized to WAS chimera for each independent experiment) in WAS (open circle) and B cell-intrinsic Cd80−/−.Cd86−/− WAS (solid circle) chimeras. (D) Splenic CD44+CXCR5hiPD1hiICOShi “GC” Tfh cells (Left: % of CD4+; Right: number). (A-D) *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001, by one-way ANOVA and Tukey’s multiple comparison test (A, B, D), and by two-tailed Student’s t test (C). Each data point indicates individual animal.
CXCR5 upregulation, and corresponding downregulation of the T cell zone receptor CCR7, results in CXCL13-dependent migration of activated CD4+ T cells to the T-B border, where cognate interactions between primed CD4+ T cells and B cells facilitate Tfh maturation and GC formation (14). Surprisingly, whereas the proportion of CD44+CXCR5+ CD4+ T cells was similar, B cell-intrinsic CD80/CD86 deletion resulted in absent upregulation of Tfh surface receptors PD-1 and ICOS, preserved CCR7 expression, and reduced expression of the Tfh-defining transcription factor BCL-6 (Fig. 1C). Consistent with a critical role for B cells in completing Tfh differentiation, we observed a marked reduction in stringently-gated CD44+CXCR5hiPD-1hiICOShi “GC” Tfh cells in the B cell-intrinsic Cd80−/−.Cd86−/− model (Fig. 1D).
The role for B cell costimulatory signals in Tfh maturation prompted us to test whether these events were unique to autoimmune settings. B cells can function as APCs following Qβ virus-like particle (VLP) immunization, suggesting a potential role for B cell-derived costimulation in anti-viral responses (9). To test this idea, we transferred CellTrace™ Violet-labelled CD4+ TCR transgenic (OT-II) T cells into either WT and Cd80−/−.Cd86−/− recipients (global model) or B cell WT and Cd80−/−.Cd86−/− chimeras (B cell-intrinsic model), that were subsequently immunized with VLP comprising Qβ protein and ovalbumin-derived peptide (Qβ-VLP/Ova). As predicted, OT-II CD4+ T cells in global Cd80−/−.Cd86−/− mice failed to proliferate, upregulate CD44, or express BCL-6 in response to Qβ-VLP/Ova. In contrast, OT-II CD4+ T cells in B cell Cd80−/−.Cd86−/− chimeras divided and upregulated CD44, although BCL-6 expression was significantly reduced relative to WT chimera controls (Supplemental Fig. 1). Thus, whereas initial CD4+ T cell priming is B cell-independent in the setting of both humoral autoimmunity and anti-viral responses, B cell-derived costimulatory signals provide important contributions to Tfh maturation, including enhancing the expression of the Tfh master regulator BCL-6.
Loss of B cell-intrinsic costimulatory signals prevents autoimmune GC formation
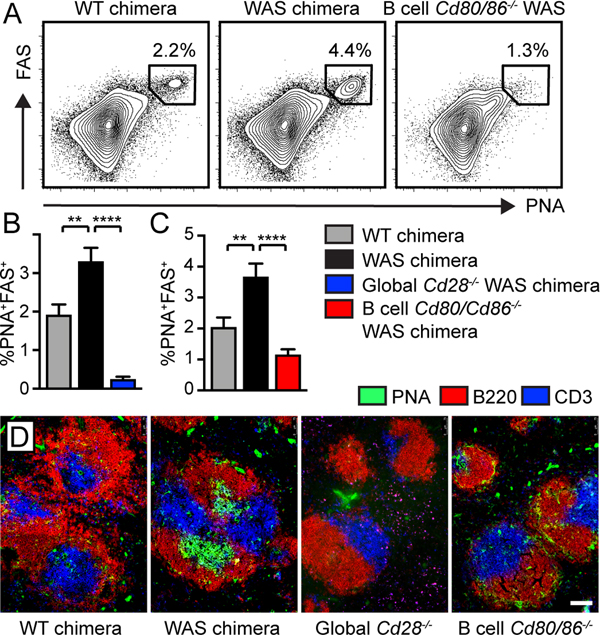
Based on this unanticipated role for B cell costimulation on Tfh differentiation, we examined the phenotype of spontaneous GCs in B cell-intrinsic Cd80−/−.Cd86−/− WAS chimeras. Strikingly, expansion of PNA+FAS+ GC B cells was abrogated in both global Cd28−/− and B cell-intrinsic Cd80−/−.Cd86−/− WAS chimeras (Fig. 2A-C). Immunofluorescence staining of splenic sections confirmed equivalent loss of autoimmune GCs in chimeras lacking global CD28 or B cell CD80/CD86 expression (Fig. 2D). Thus, in contrast with candidate antigen immunization (8), B cell-derived costimulatory signals are critical for spontaneous GCs during humoral autoimmunity.
Figure 2: B cell costimulatory signals are required for autoimmune GC formation.
(A) FACS plots (gated on CD19+ B cells; % in gate) showing PNA+FAS+ GC B cells. (B,C) % PNA+FAS+ GC B cells (of total CD19+ B cells) in global Cd28−/− WAS (B) and B cell-intrinsic Cd80−/−.Cd86−/− WAS chimeras (C). Error bars indicate SEM. **, P<0.01; ****, P<0.0001, by one-way ANOVA, followed by Tukey’s multiple comparison test. (D) Splenic sections stained with B220 (red), PNA (green) and CD3 (blue); bars, 100μm.
Divergent impacts of B cell-intrinsic CD80/CD86 deletion on the serum autoAb repertoire
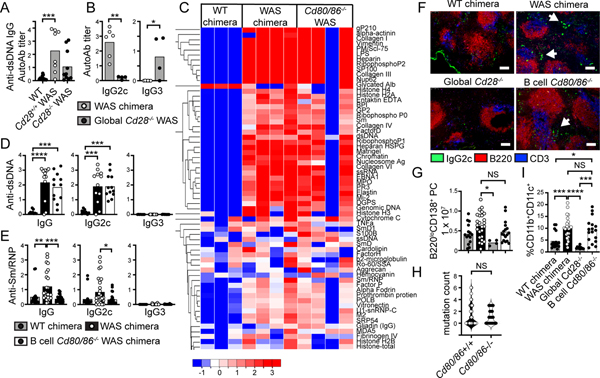
Both Was−/− mice and WAS patients develop diverse serum autoAb (10, 15), although in Was−/− animals these are predominantly T cell-independent IgG3 subclass, with WT CD4+ T cell help required for IgG2c autoAb generation (10). Consistent with these data, global Cd28 deletion limited class-switched autoAb targeting dsDNA, although a subset of global Cd28−/− WAS chimeras exhibited low-titer anti-dsDNA IgG autoAb (Fig. 3A). However, these were predominantly IgG3, with no IgG2c autoAb detected (Fig. 3B); findings consistent with our previous data in which interventions disrupting B cell:T cell cross-talk, including CD4+ T cell depletion and B cell-intrinsic MHC Class II deletion, abrogate IgG2c autoAb (3, 10).
Figure 3: Distinct EF and GC-dependent pathways contribute to lupus autoAb.
(A, B) Anti-dsDNA IgG (A) and Ig subclass amongst DNA-reactive samples (B) in global Cd28−/− WAS model. (C) Serum IgG2c autoAb by autoantigen microarray. Each column represents pooled sera from an independent chimera cohort. (D, E) Serum anti-dsDNA (D) and anti-Sm/RNP (E) autoAb. (F) Splenic sections stained with B220 (red), IgG2c (green) and CD3 (blue), showing expansion of IgG2c+ foci in splenic red pulp in WAS and B cell Cd80/Cd86-deficient WAS chimeras, but not Cd28−/− chimeras; bars, 100μm. (G) Splenic B220loCD138+ plasma cell number. (H) SHM analysis: mutation count in κ light chains from sorted splenic B220loCD138+ plasma cells. (I) CD11b+CD11c+ ABCs (% of CD19+ B cells). (A,B,D,E,G,H,I) *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001; NS, not-significant; by one-way ANOVA and Tukey’s multiple comparison test (A,D,E,G,I), and by two-tailed Student’s t test (B, H). Each data point equals individual animal.
Thus, we predicted that absent GCs in B cell Cd80−/−.Cd86−/− WAS chimeras would be accompanied by loss of pathogenic IgG2c autoAb. However, significant complexity underlies plasma cell generation in SLE, with both GC and EF pathways contributing to the autoAb repertoire (16). Strikingly, despite absent GCs, we observed widespread IgG2c self-reactivity by autoantigen microarray in B cell Cd80/Cd86−/− chimeras, that was enriched for autoAb binding diverse connective tissue proteins such as alpha-actinin, vimentin, and collagen proteins (Fig. 3C). Moreover, B cell Cd80−/−.Cd86−/− WAS chimeras also developed high-titer anti-dsDNA autoAb, including the pathogenic IgG2c subclass, whereas T-independent anti-dsDNA IgG3 were absent (Fig. 3D). Surprisingly, lack of autoimmune GCs exerted only a limited impact on the autoAb repertoire. Whereas anti-dsDNA titers were unaffected, RNA-associated anti-Sm/RNP autoAb were lost in the absence of B cell costimulation, implicating spontaneous GCs as the likely source for this specificity (Fig. 3E). Anti-Sm/RNP autoAb were similarly reduced in Cd28−/− WAS chimeras (Supplemental Fig. 2).
In keeping with EF B cell activation driving diverse autoAb, we observed GC-independent accumulation of IgG2c+ plasma cell foci in the EF red pulp of B cell-intrinsic Cd80−/−.Cd86−/− WAS chimeras, but not global Cd28−/− mice (Fig. 3F). Splenic B220loCD138+ plasma cells were also expanded in WAS and B cell Cd80−/−.Cd86−/− WAS chimeras, but reduced in the absence of T cell CD28 signals (Fig. 3G). Moreover, whereas the GC is the primary site for antigen-driven selection, we observed equivalent B cell receptor (BCR) mutational frequencies in sorted splenic plasma cells from B cell CD80/CD86-sufficient and -deficient chimeras (Fig. 3H), in keeping with prior evidence for B cell somatic mutation within EF foci in murine SLE (17). Finally, conflicting EF and GC-dependent models have also been proposed for the generation CD11b+CD11c+ age/autoimmunity-associated B cells (ABCs) that act as precursors for pathogenic ASCs in SLE (18–20). Interestingly, we observed preserved expansion of T-bet-expressing ABCs in B cell-intrinsic Cd80/Cd86−/− WAS chimeras, but not global Cd28−/− models, indicating that T cell-dependent EF activation can support development of this lupus-associated memory B cell subset (Fig. 3I; Supplemental Fig. 2).
Together, these data support a model in which both EF and GC activation pathways facilitate autoreactive B cell class-switch recombination, SHM, ABC formation, and differentiation into pathogenic plasma cells, with each pathway providing distinct contributions to the lupus autoAb repertoire. Loss of B cell costimulatory signals uniquely dissociates these events, by abrogating the formation of autoimmune GCs without preventing T cell-dependent EF B cell activation.
B cell-intrinsic CD80/CD86 haploinsufficiency limits autoimmune GC formation
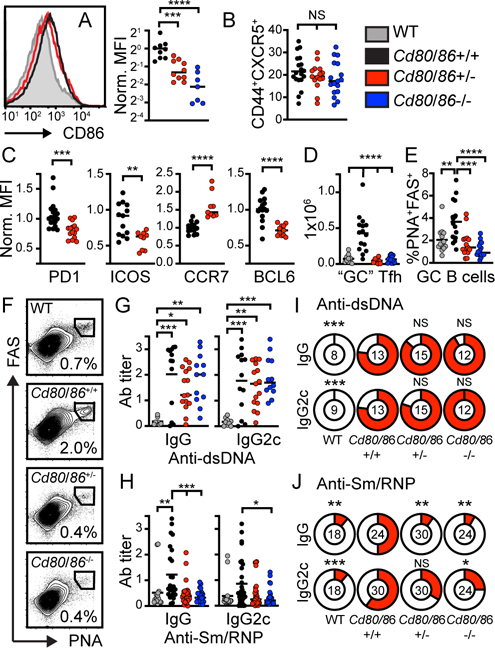
The strength of T cell costimulation needs to be tightly regulated to prevent systemic autoimmunity. Heterozygous mutations in CTLA4 or its regulator LRBA (lipopolysaccharide-responsive and beige-like anchor protein) promote spontaneous humoral autoimmunity in humans (21–23). Thus, we examined whether reduced surface CD80/CD86 expression on B cells is sufficient to limit autoimmune GC formation in SLE. To do this, we generated parallel cohorts of WAS chimeras in which B cells expressed 0–2 copies of CD80/CD86. Strikingly, despite a relatively modest reduction in B cell costimulatory molecule expression (Fig. 4A), heterozygous CD80/CD86 deletion recapitulated the phenotype of the B cell-intrinsic Cd80−/−.CD86−/− model. Similar to biallelic CD80/CD86 deletion, we observed no change in CXCR5 upregulation on CD4+ T cells in B cell Cd80/Cd86-haploinsufficient chimeras (Fig. 4B). Rather, reduced B cell CD80/CD86 expression correlated with the failure of CXCR5+CD4+ T cells to upregulate ICOS, PD-1, and BCL-6, and downregulate CCR7 expression (Fig. 4C). This resulted in a corresponding lack of “GC” Tfh cells (Fig. 4D). In keeping with Tfh maturation being required for GC formation, we observed a parallel loss of splenic GC B cells in heterozygous B cell Cd80+/−.CD86+/− chimeras (Fig. 4E, F). Finally, heterozygous CD80/CD86 deletion exerted no impact on the generation of IgG / IgG2c anti-dsDNA titers but reduced anti-Sm/RNP autoAb (Fig. 4G-J).
Figure 4: B cell-intrinsic CD80/CD86 heterozygosity prevents spontaneous GC formation.
(A) Left: Histogram showing modest reduction in B cell CD86 in B cell-intrinsic Cd80/Cd86+/− (red) vs. Cd80/Cd86+/+ (black) WAS chimeras. Gray histogram: Cd80/Cd86−/− control. Right: CD86 MFI (normalized to WAS chimera) on CD19+ B cells from indicated models. (B) % CD44+CXCR5+ (of total CD4+ T cells). (C) Normalized PD1, ICOS, CCR7, and BCL-6 MFI on CD4+CD44+CXCR5+ T cells from Cd80/Cd86+/+ (black) and B cell-intrinsic Cd80/Cd86+/− heterozygous (red) WAS chimeras. (D) Splenic CD4+CXCR5hi CD44hiPD1hiICOShi “GC” Tfh cells. (E) %PNA+FAS+ GC B cells (of total CD19+ B cells). (F) FACS plots (gated on CD19+ B cells) showing loss of PNA+FAS+ GC B cells with homo- and heterozygous Cd80/Cd86 deletion. (G, H) Anti-dsDNA (G) and anti-Sm/RNP (H) IgG and IgG2c autoAb. (I, J) Pie chart showing percentage of individual animals positive for anti-dsDNA (I) and anti-Sm/RNP (J) IgG and IgG2c autoAb (positive cut-off = mean + 3 S.D. for WT chimera control; number indicates animals analyzed per genotype). (A, C, D, F, G, H, I, J) *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001; NS, not significant; by one-way ANOVA and Tukey’s multiple comparison test (A, C, D, F, G, H), and Fisher’s exact test (I, J). Each data point equals individual animal.
Discussion
In summary, our work defines a new role for B cells during the pathogenesis of SLE, namely the provision of costimulatory signals above a threshold required for Tfh maturation and autoimmune GC formation. These data are consistent with lack of class-switched autoAb and systemic inflammation in Cd28−/−.MRL/lpr mice (24), and with delayed disease onset in NZB/NZW F1 mice treated with either CD28 blockade or combined CD80/CD86 inhibition and cytotoxic immunosuppression (25, 26). Mechanistically, while both EF and GC pathways have been described in human SLE and murine lupus models, it has been technically challenging to define the source of individual autoreactive plasma cells. However, since plasma cells can be functionally divided into short-lived EF and long-lived GC-derived subsets, variable autoAb persistence following B cell depletion provides indirect evidence for the cellular source for individual specificities. In this context, our data are strikingly consistent with the decline in anti-dsDNA IgG, but durable persistence of RNA-associated autoAb, following B cell ablation in SLE (16).
Our study also informs the understanding of autoimmunity in CTLA4 and LRBA haploinsufficiency (21–23). CTLA-4 regulates surface B7 ligand levels by capturing CD80/CD86 molecules from the surface of APCs via trans-endocytosis, thereby limiting available costimulatory signals for T cell activation (27). Since CTLA4 and LRBA haploinsufficiency is accompanied by histologic GC formation and the expansion of circulating CXCR5+ Tfh cells (28, 29), these data suggest that modest B7 elevation via reduced trans-endocytosis is sufficient to drive CD28-dependent Tfh differentiation. Based on this model, we performed the converse experiment and confirmed that even modest reductions in B cell CD80/CD86 surface expression is sufficient to abrogate autoimmune GCs.
The ubiquitous presence of self-antigens and stochastic nature of lupus pathogenesis have each complicated the characterization of underlying immune events. Self-reactive B cell transgenic models have demonstrated that initial dual BCR/TLR-dependent activation of autoreactive B cells occurs in a T cell-independent manner, but that provision of CD4+ T cell at EF sites enhances B cell proliferation and differentiation into autoAb-producing plasmablasts (30). Although dendritic cells (DCs) are classically considered the primary APC driving adaptive T cell responses, T cell activation is preserved in DC-deficient MRL.Faslpr mice (31) and B cell antigen presentation promotes murine SLE (2, 10). Moreover, autoreactivity in SLE converges on a relatively limited subset of nucleic acid-containing autoantigens able to engage B cell endosomal TLRs. Thus, this B cell-centric model for lupus pathogenesis raises the important countervailing question as to whether myeloid cells are redundant for T cell activation during humoral autoimmunity. Here, we show that the expansion of CD44hiCD62Llo/hi EM T cells and CD44+CXCR5+ Tfh precursors requires initial CD80/CD86 costimulatory signals provided by non-B cell lineages. In this context, we predict that B cell propensity for activation by dual BCR/TLR signals focuses anti-nuclear autoreactivity in SLE, both by enhancing B cell presentation of nuclear self-antigens to cognate T cells, and, indirectly, via the generation of circulating immune complexes which enhance delivery of specific self-antigens to myeloid APCs. Taken together, our data highlight how myeloid lineages and B cells co-ordinate to drive autoreactive T cell activation and emphasize how tight regulation of costimulatory signals is required to maintain immune tolerance.
Supplementary Material
Key points:
B cell-derived costimulation facilitates autoimmune germinal center (GC) formation
Distinct extra-follicular (EF) vs. GC contributions to autoantibody repertoire
Age-associated B cells (ABCs) develop via a GC-independent EF pathway
Acknowledgements:
This work was supported by the National Institutes of Health (K08AI112993 (SWJ), R01AR073938 (SWJ) and R01AR075813 (SWJ)); ACR REF Rheumatology Scientist Development Award (SWJ); American College of Rheumatology (ACR) Rheumatology Research Foundation (RRF) Career Development K Supplement (SWJ); Arthritis National Research Foundation (ANRF) Eng Tan Scholar Award (SWJ); and Lupus Research Alliance, Novel Research Grant (SWJ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations used:
- μMT
B cell-deficient
- autoAb
autoantibody
- ANA
antinuclear antibody
- EM
effector/memory
- GC
germinal center
- MFI
mean fluorescence intensity
- SLE
systemic lupus erythematosus
- TFH
T follicular helper
- WAS
Wiskott-Aldrich syndrome
- EF
extrafollicular
- DC
dendritic cell
- VLP
virus-like particle
- ABCs
age/autoimmunity-associated B cells
- ASCs
antigen secreting cells
- SHM
somatic hypermutation
- DC
dendritic cells
Footnotes
The authors have declared that no conflict of interest exists.
References
- 1.Rawlings DJ, Metzler G, Wray-Dutra M, and Jackson SW. 2017. Altered B cell signalling in autoimmunity. Nature reviews. Immunology 17: 421–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giles JR, Kashgarian M, Koni PA, and Shlomchik MJ. 2015. B Cell-Specific MHC Class II Deletion Reveals Multiple Nonredundant Roles for B Cell Antigen Presentation in Murine Lupus. J Immunol 195: 2571–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson SW, Jacobs HM, Arkatkar T, Dam EM, Scharping NE, Kolhatkar NS, Hou B, Buckner JH, and Rawlings DJ. 2016. B cell IFN-gamma receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J Exp Med 213: 733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arkatkar T, Du SW, Jacobs HM, Dam EM, Hou B, Buckner JH, Rawlings DJ, and Jackson SW. 2017. B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J Exp Med 214: 3207–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, and Sharpe AH. 1997. B7–1 and B7–2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 6: 303–313. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson SE, Han S, Kelsoe G, and Thompson CB. 1996. CD28 is required for germinal center formation. J Immunol 156: 4576–4581. [PubMed] [Google Scholar]
- 7.Topalian SL, and Sharpe AH. 2014. Balance and imbalance in the immune system: life on the edge. Immunity 41: 682–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe M, Fujihara C, Radtke AJ, Chiang YJ, Bhatia S, Germain RN, and Hodes RJ. 2017. Co-stimulatory function in primary germinal center responses: CD40 and B7 are required on distinct antigen-presenting cells. J Exp Med 214: 2795–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong S, Zhang Z, Liu H, Tian M, Zhu X, Zhang Z, Wang W, Zhou X, Zhang F, Ge Q, Zhu B, Tang H, Hua Z, and Hou B. 2018. B Cells Are the Dominant Antigen-Presenting Cells that Activate Naive CD4(+) T Cells upon Immunization with a Virus-Derived Nanoparticle Antigen. Immunity 49: 695–708 e694. [DOI] [PubMed] [Google Scholar]
- 10.Becker-Herman S, Meyer-Bahlburg A, Schwartz MA, Jackson SW, Hudkins KL, Liu C, Sather BD, Khim S, Liggitt D, Song W, Silverman GJ, Alpers CE, and Rawlings DJ. 2011. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J Exp Med 208: 2033–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, Mohan C, Wakeland EK, and Olsen NJ. 2007. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol 147: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs HM, Thouvenel CD, Leach S, Arkatkar T, Metzler G, Scharping NE, Kolhatkar NS, Rawlings DJ, and Jackson SW. 2016. Cutting Edge: BAFF Promotes Autoantibody Production via TACI-Dependent Activation of Transitional B Cells. J Immunol 196: 3525–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson SW, Scharping NE, Kolhatkar NS, Khim S, Schwartz MA, Li QZ, Hudkins KL, Alpers CE, Liggitt D, and Rawlings DJ. 2014. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J Immunol 192: 4525–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, and Cyster JG. 2007. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol 179: 5099–5108. [DOI] [PubMed] [Google Scholar]
- 15.Dupuis-Girod S, Medioni J, Haddad E, Quartier P, Cavazzana-Calvo M, Le Deist F, de Saint Basile G, Delaunay J, Schwarz K, Casanova JL, Blanche S, and Fischer A. 2003. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics 111: e622–627. [DOI] [PubMed] [Google Scholar]
- 16.Hale M, Rawlings DJ, and Jackson SW. 2018. The long and the short of it: insights into the cellular source of autoantibodies as revealed by B cell depletion therapy. Curr Opin Immunol 55: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.William J, Euler C, Christensen S, and Shlomchik MJ. 2002. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science 297: 2066–2070. [DOI] [PubMed] [Google Scholar]
- 18.Hao Y, O’Neill P, Naradikian MS, Scholz JL, and Cancro MP. 2011. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 118: 1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, and Marrack P. 2011. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood 118: 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du SW, Arkatkar T, Al Qureshah F, Jacobs HM, Thouvenel CD, Chiang K, Largent AD, Li QZ, Hou B, Rawlings DJ, and Jackson SW. 2019. Functional Characterization of CD11c(+) Age-Associated B Cells as Memory B Cells. J Immunol 203: 2817–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, Schickel JN, Tran DQ, Stoddard J, Zhang Y, Frucht DM, Dumitriu B, Scheinberg P, Folio LR, Frein CA, Price S, Koh C, Heller T, Seroogy CM, Huttenlocher A, Rao VK, Su HC, Kleiner D, Notarangelo LD, Rampertaap Y, Olivier KN, McElwee J, Hughes J, Pittaluga S, Oliveira JB, Meffre E, Fleisher TA, Holland SM, Lenardo MJ, Tangye SG, and Uzel G. 2014. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 345: 1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, Bulashevska A, Petersen BS, Schaffer AA, Gruning BA, Unger S, Frede N, Baumann U, Witte T, Schmidt RE, Dueckers G, Niehues T, Seneviratne S, Kanariou M, Speckmann C, Ehl S, Rensing-Ehl A, Warnatz K, Rakhmanov M, Thimme R, Hasselblatt P, Emmerich F, Cathomen T, Backofen R, Fisch P, Seidl M, May A, Schmitt-Graeff A, Ikemizu S, Salzer U, Franke A, Sakaguchi S, Walker LS, Sansom DM, and Grimbacher B. 2014. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med 20: 1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo B, Zhang K, Lu W, Zheng L, Zhang Q, Kanellopoulou C, Zhang Y, Liu Z, Fritz JM, Marsh R, Husami A, Kissell D, Nortman S, Chaturvedi V, Haines H, Young LR, Mo J, Filipovich AH, Bleesing JJ, Mustillo P, Stephens M, Rueda CM, Chougnet CA, Hoebe K, McElwee J, Hughes JD, Karakoc-Aydiner E, Matthews HF, Price S, Su HC, Rao VK, Lenardo MJ, and Jordan MB. 2015. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science 349: 436–440. [DOI] [PubMed] [Google Scholar]
- 24.Tada Y, Nagasawa K, Ho A, Morito F, Koarada S, Ushiyama O, Suzuki N, Ohta A, and Mak TW. 1999. Role of the costimulatory molecule CD28 in the development of lupus in MRL/lpr mice. J Immunol 163: 3153–3159. [PubMed] [Google Scholar]
- 25.Laurent L, Le Fur A, Bloas RL, Neel M, Mary C, Moreau A, Poirier N, Vanhove B, and Fakhouri F. 2017. Prevention of lupus nephritis development in NZB/NZW mice by selective blockade of CD28. Eur J Immunol 47: 1368–1376. [DOI] [PubMed] [Google Scholar]
- 26.Daikh DI, and Wofsy D. 2001. Cutting edge: reversal of murine lupus nephritis with CTLA4Ig and cyclophosphamide. J Immunol 166: 2913–2916. [DOI] [PubMed] [Google Scholar]
- 27.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, and Sansom DM. 2011. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332: 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kucuk ZY, Charbonnier LM, McMasters RL, Chatila T, and Bleesing JJ. 2017. CTLA-4 haploinsufficiency in a patient with an autoimmune lymphoproliferative disorder. J Allergy Clin Immunol 140: 862–864 e864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alroqi FJ, Charbonnier LM, Baris S, Kiykim A, Chou J, Platt CD, Algassim A, Keles S, Al Saud BK, Alkuraya FS, Jordan M, Geha RS, and Chatila TA. 2018. Exaggerated follicular helper T-cell responses in patients with LRBA deficiency caused by failure of CTLA4-mediated regulation. J Allergy Clin Immunol 141: 1050–1059 e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sweet RA, Ols ML, Cullen JL, Milam AV, Yagita H, and Shlomchik MJ. 2011. Facultative role for T cells in extrafollicular Toll-like receptor-dependent autoreactive B-cell responses in vivo. Proceedings of the National Academy of Sciences of the United States of America 108: 7932–7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, and Shlomchik MJ. 2010. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity 33: 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.