Abstract
β cells in the hyperglycemic environment of diabetes have marked changes in phenotype and function that are largely reversible if glucose levels can be returned to normal. A leading hypothesis is that these changes are caused by the elevated glucose levels leading to the concept of glucose toxicity. Support for the glucose toxicity hypothesis is largely circumstantial, but little progress has been made in defining the responsible mechanisms. Then questions emerge that are difficult to answer. In the very earliest stages of diabetes development, there is a dramatic loss of glucose-induced first-phase insulin release (FPIR) with only trivial elevations of blood glucose levels. A related question is how impaired insulin action on target tissues such as liver, muscle and fat can cause increased insulin secretion. The existence of a sophisticated feedback mechanism between insulin secretion and insulin action on peripheral tissues driven by glucose has been postulated, but it has been difficult to measure increases in blood glucose levels that might have been expected. These complexities force us to challenge the simplicity of the glucose toxicity hypothesis and feedback mechanisms. It may turn out that glucose is somehow driving all of these changes, but we must develop new questions and experimental approaches to test the hypothesis.
Keywords: insulin, beta cells, glucose toxicity, insulin secretion
Introduction
β cells in diabetes have been intensively studied (1), yet we have a very poor understanding of the β-cell changes that occur in the earliest stages of either type 1 diabetes (T1D) or type 2 diabetes (T2D). β cells normally do a spectacular job keeping blood glucose levels within a very narrow range (2), even for the large numbers of our population with insulin resistance, who usually can compensate for many years with increased insulin secretion. However, for some there comes a time when insulin secretion is insufficient and glucose levels start to rise, whereupon the elegant secretory machinery of these cells starts to falter (2–5). We have previously proposed that there are five stages that characterize progression of both T1D and T2D (3, 5). For Stage 1, blood glucose levels are normal and this can include normal compensatory insulin secretion for insulin resistance. Stage 2 is associated with mild hyperglycemia that would generally be considered glucose intolerance and typically includes the first signs of β-cell dysfunction. Then Stage 3 is when β-cell function deteriorates enough to result in an accelerated rise in glucose levels to unequivocal diabetes at Stage 4. Then Stage 5 is when severe ketosis develops. The Whitehall study group found evidence for this rapid pattern of progression seen in Stage 3 in their studies of T2D pathogenesis (6). Much has been written about the changes in β-cell secretion and phenotype and the hypothesis that glucose somehow causes these changes, which has led to the term glucose toxicity (3). While there is a great deal of circumstantial evidence in support of this hypothesis, the responsible mechanisms remain poorly defined. In this commentary, we will take a hard look at the hypothesis by focusing on the β-cell changes that take place in the earliest development of diabetes, which is how β cells compensate for insulin demand from insulin resistance in Stage 1 and how dysfunction develops in Stage 2
Gaps in our understanding of how β-cells progress from successful compensation to failure
To maintain normal plasma glucose concentrations in face of insulin resistance there is pressure for β cells to secrete more insulin and to replicate. There is every reason to think that β cells face the same pressure during the progression to either T2D or to T1D. With T2D, the demand comes from years of insulin resistance and a β-cell mass that finally becomes inadequate to provide compensation. For those headed to T1D, β cells are being killed by autoimmunity and then the time comes when their β-cell mass is also inadequate. The capacity to secrete more insulin to keep blood glucose levels from rising can be thought of as β-cell reserve (7)(2), which is helpful until it is used up. Thus, when β-cell reserve is no longer available, glucose levels must rise as insulin secretion becomes inadequate.
A loss of glucose-induced first-phase insulin release (FPIR) has been found to be a particularly sensitive marker of the onset of decompensation and risk of progression to diabetes, thus causing it to receive considerable attention (8–13). The changes in FPIR are striking but poorly understood. With the insulin resistance of obesity, FPIR is increased (14) and then it is completely lost when blood glucose levels are only slightly higher than normal (15). The abrupt loss of FPIR is depicted in Figure 1, which shows the dramatic changes that take place as fasting plasma glucose levels rise from 80 to 115 mg/dl, levels that are not even diagnostic for diabetes.
Figure 1.
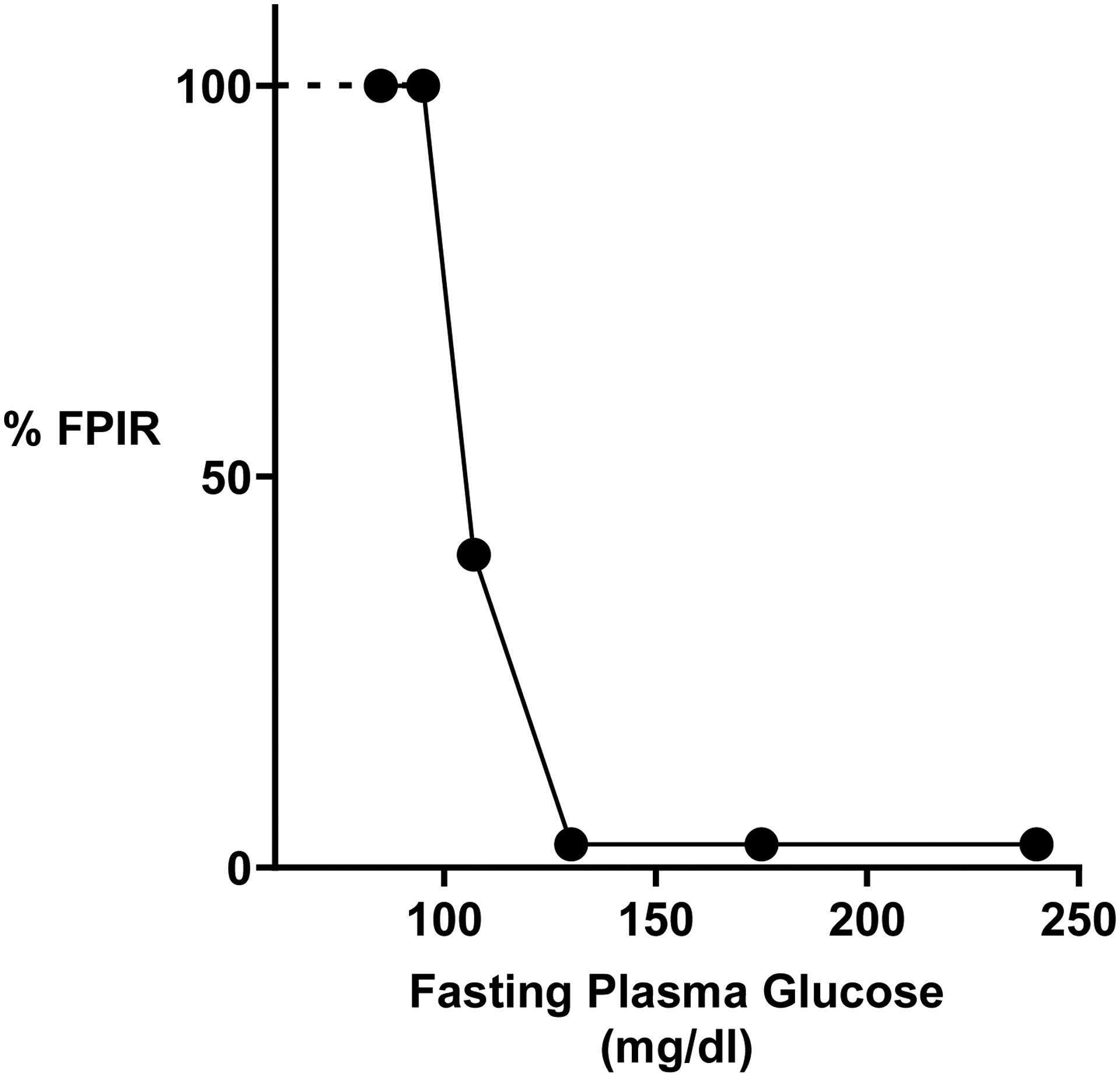
Abrupt loss of glucose-stimulated first phase insulin release (FPIR) with increasing fasting plasma glucose levels. Data are taken from the study of Brunzell et al (15). When glucose levels increased to the range of 100–114 mg/dl, FPIR is reduced to 40% of normal and with further increases in fasting glucose FPIR was absent.
Because glucose plays such a dominant role in β-cell function and growth, one might expect there to be a sophisticated feedback system dominated by glucose that provides this compensation. However, this assumption is often challenged because it has been so difficult to measure or identify the changes in blood glucose that could exert this purported effect. Nonetheless, it is clear that β-cells exposed to glucose levels in the diabetic range have dramatic derangements of insulin secretion and marked changes in their phenotype as shown clearly with measurements of gene expression, which is often thought to represent dedifferentiation (16). In this situation, the effects of hyperglycemia can be considered to be toxic, justifying the concept of glucose toxicity (17).
There is so much we do not understand about the relationships that occur between glucose and β-cells as diabetes develops. Is there a way in which β- cells can be over-work?, and how might this be identified? How can FPIR be lost with normal or even slightly elevated glucose levels? Even when glucose levels are clearly elevated, how do they cause the changes that we think of as glucose toxicity? Our focus for this short commentary is on the role of glucose in causing the β-cell dysfunction of diabetes, so we will not be able to critically analyze the many other mechanisms that are being studied by others such as endoplasmic reticulum stress (ER stress), IAPP/amyloid toxicity, oxidative stress, glucose toxicity’s possible relationship to lipotoxicity, and inflammation.
Evolving understanding about how β cells respond to glucose
The conventional view of β cells has been that the rates of insulin secretion are tightly linked to glucose oxidation (18, 19). Glucose enters β cells freely via the transporters [mainly GLUT1 in humans (20) or Glut2 in rodents (21)], whereupon its metabolism is regulated by the rate-limiting effects of glucokinase on glycolysis (22). Then increases in ATP from mitochondrial oxidative phosphorylation (OxPhos) close KATP channels resulting in depolarization that opens voltage-dependent calcium channels allowing entry of calcium that stimulates exocytosis of insulin granules (23). Work by Henquin (24) and others showed there are two separate mechanisms leading to insulin secretion; the first being the KATP-dependent insulin secretion, the other being the KATP-independent secretion. These mechanisms are also described as triggering and amplification, respectively. It seems particularly important to understand the KATP-dependent mechanism because it seems very likely to be involved in the loss of FPIR.
In recent years more layers of complexity have been identified. Merrins, Kibbey and co-workers (25) have proposed revising the older model described above to one with metabolic compartmentalization. The first component, referred to as MitoSynth, is adjacent to the plasma membrane and the KATP channel. In this microenvironment, phosphoenolpyruvate (PEP) is hydrolyzed by pyruvate kinase to form pyruvate and ATP, the latter being able to close the KATP channel. The PEP can come from two sources, directly from glycolysis or from the “PEP cycle” in mitochondria which provides PEP from anaplerotic flux of carbons through pyruvate carboxylase. Because ATP is generated by the pyruvate kinase, ADP levels fall, which reduces oxidative phosphorylation by mitochondria. The first component shuts down the KATP channels leading to calcium uptake via voltage dependent-calcium channels. The calcium entering the cell stimulates a variety of reactions that consume ATP, thus providing ADP that allows mitochondria to shift to an oxidative phosphorylation mode. This second component termed MitoOx, refers to the oxidative phosphorylation by mitochondria. These two components toggle back and forth leading to oscillations that occur every few minutes.
The KATP independent (amplification) mechanism of insulin secretion can result from a number of processes that change membrane potential of β cells, such as depolarization directly by KCl or uptake of arginine that has a positive charge. Secretion is also increased by glucagon-like peptide 1 through generation of cyclic AMP and by acetyl choline working through muscarinic receptors that stimulate G proteins. Among other mechanisms glycerol-3-phosphate acting through Munc13-1 to stimulate exocytosis has emerged as a possibility (26). Yet another mechanism that could amplify insulin secretion is exerted by sentrin/SUMO-specific protease-1 (STENP1) (27).
β-cell processing, storage and secretion of insulin in diabetes
β cells have an impressive capacity to store insulin. On average, β cells contain about 20 pg of insulin, which is stored in about 10,000 granules (28). The processing of proinsulin in the normal state is efficient but not perfect in that the ratio of secreted proinsulin to total insulin immunoreactivity is about 1–2%. However, because circulating proinsulin is cleared so much more slowly than insulin, the ratio of proinsulin to insulin in plasma is much higher. In one study the ratio in non-diabetic subjects was about 15% but was considerably higher at about 30% with T2D (29). A likely explanation is that the conversion of proinsulin to insulin in the presence of hyperglycemia is less efficient leading to disproportionate secretion of proinsulin (30). This fits with the finding in diabetes of more immature granules characterized with electron microscopy by the absence of insulin crystals (31).
Questions are often raised about the vulnerability of β cells to ER stress because of their high demand for insulin secretion and because the folding requirements for proinsulin and packaging are highly restrictive (32–34). One can expect that β cells often go through a benign unfolded protein response (UPR) that presumably correlates with restoration of insulin stores after each meal. Yet, we do not have good evidence that ER stress becomes more severe enough in T2D to cause cell death. However, it was found that C/EBP homologous protein (CHOP) was more likely to have a perinuclear distribution in β cell in T2D than in controls (35). The efficiency of proinsulin folding to form three intramolecular disulfide bonds has been recently studied and found to be imperfect even in the normal state and more so as diabetes develops. It seems that the problem lies with free Cys(B19) and Cys(A20), which lead to folding abnormalities in the form of accumulated disulfide-linked complexes best shown in the LepRdb/db mouse model (34). This demonstration of abnormal folding is of considerable interest but its contribution to the problems of β-cell mass and function in T2D has not been established.
In addition to the abnormal processing of proinsulin to insulin found in the diabetic state, the insulin content of β cells has been found to be reduced by more than 50% in the db/db mouse model of diabetes (31). Data on the pancreatic insulin content of human pancreases are limited, but one study found little, if any, reduction in insulin content as a function of β-cell mass in T2D (36). Therefore, there is little to suggest that limitations of insulin content contribute to the functional defects of insulin secretion.
β-cell mass and β-cell turnover
While there is agreement that both impaired β-cell function and inadequate β-cell mass make critical contributions to the diabetic state, we have argued that inadequate mass precedes and is responsible for the problem with function (2). This concept pertains to both T1D and T2D, and presumably to most forms of monogenic diabetes. Detailed studies of cadaver pancreases found that although there was considerable variability among pancreases, β-cell mass in T2D was 40–60% lower than weight-matched controls (36–39). We cannot measure β-cell mass in living subjects, but we know that plasma insulin levels correlate with insulin sensitivity (40). Therefore, it is reasonable to conclude that β-cell mass is also correlated, such that non-diabetic individuals with insulin resistance and high blood insulin levels could be expected to have higher β-cell mass.
Although β-cell mass is lower in T2D than non-diabetic weight-matched subjects, we cannot conclude that the mass is necessarily reduced due to an increased rate of cell death, only that it is inadequate for producing the amount of insulin necessary to meet the demands of whatever degree of insulin resistance is present. To say that mass is reduced implies that it was greater previously and had accelerated loss, but mass can be inadequate due to there not being enough at birth resulting from genetic or environmental reasons, such as problems with the intrauterine environment, or to a lack of β-cell expansion early in life.
While it is tempting to assume that the pressure of insulin resistance increases the rate of β-cell loss, we do not know for certain this to be the case. In adults with or without T2D, the rate of decline of β-cell mass was estimated to be only about 1.5% per year (36), as determined by autopsy studies on subjects with T2D of known duration. Unfortunately, rates of β-cell turnover are too low to measure accurately with current techniques. It has been particularly difficult to measure rates of β-cell death. The most widely used marker of death is TUNEL (Tdt-mediated dUTP nick-end-labeling), which only marks some stages of β cell apoptotic death (37, 41). Furthermore, it can only indicate frequency but not rate since rate is a function of frequency over time. β cells can also die from necrosis, but reliable methods to measure this process are not available. More is known about the measurement of β-cell replication. Recent studies using high resolution isotope imaging indicate that some human β cells have life spans of many years (42). This concept is supported by finding that with age a large proportion of human β cells accumulate lipofuscin (43). However, studies using Ki67 as a measure of replication suggest that there may be populations of β cells that have a faster turnover (37, 44, 45).
Only a few years ago, many thought that human β-cell replication only occurred in early childhood, but that misconception was based on the failure to appreciate the loss of Ki67 positivity that can occur when fresh tissue samples are not used for pathological examination. We now know that when pancreases are properly collected, the Ki67 positivity of human β cells is in the range of 0.2–0.6% (45). Ki67 positivity means that cells have entered the cell cycle, but we do not know how often this leads to self-duplication. Nonetheless, if the replication process takes 2 days, there may be enough replication potential to exploit therapeutically.
There is still much to be learned about β-cell turnover at different ages and in the presence or absence of diabetes. While glucose drive has a stimulatory effect on replication, work in experimental models suggest that the diabetic state is associated with growth arrest of β cells whereby cells enter cell cycle but fail to divide (46). Looking at the complex changes in gene expression in hyperglycemia, one can find pathways that are both pro- and anti-apoptotic (46, 47). Evidence from several models finds activation of the HIF1alpha/PFKFB3 pathway (48, 49), and many HIF1alpha targets are associated with cell survival (50). Further complexity comes from knowing there are small populations of senescent β-cells (51), which have activated prosurvival mechanisms including HIF1alpha, and there must be some old non-senescent cells that are more vulnerable.
The concepts of β-cell compensation, reserve and inadequate mass
Somehow whole-body insulin resistance caused by changes in liver, fat and other tissues tells β cells to synthesize and secrete more insulin and to grow through replication. While it is tempting to assume that an efficient glucose feedback mechanism is responsible, it has proved difficult to find elevations in blood glucose concentrations in these situations, leading some to look for alternative explanations. Are there fuels other than glucose that are important for growth? The oxidation of fatty acids has been shown to be low in β-cells (52) and amino acid signaling through mTORC1 seems important for immature β cells but probably less so later on (53). Others have looked for non-fuel circulating factors that could stimulate replication (54, 55), but attractive candidates have not yet emerged.
Support for the concept of glucose as the key driving force comes from two studies of mice with heterozygote knockouts of glucokinase (56, 57). Normal mice receiving high-fat diets had increased β-cell replication, while those with glucokinase deficiency did not. These results tell us that insulin resistance can influence glucose metabolism in β-cells leading to growth, which can only occur if there are changes in gene expression. These findings fit with recent studies using partial pancreatectomies in rats in which many changes in gene expression can be found when blood levels are only trivially elevated (46). Thus, it appears that glucose metabolism in β cells can turn on growth pathways that in other cells are exerted by insulin, IGF-1 and other growth factors. In particular, glucose can activate the IRS-2 and AKT signaling pathway (56).
We could make a distinction between changes in β-cells phenotype that are driven by changes in flux of glucose metabolism in the apparent absence of hyperglycemia (58) and changes that take place when glucose levels are clearly elevated. The term glucose toxicity is often used to describe changes in β cells that are seen when glucose levels are clearly elevated. However, the effects of glucose might be also considered toxic when changes in β-cell glucose flux are occurring without obvious hyperglycemia, because they may somehow cause gene expression changes that adversely influence function. We must be careful about what we mean by flux, as this may be thought of as a general term that could include the rate at which various glucose metabolites are generated such that glucose carbons are oxidized or converted to something else like glycogen, fat or some other mediators. Thus, it seems very possible that the pressure of insulin resistance could have any number of effects that contribute to β-cell dysfunction in general and the loss of FPIR in particular. Yet, in the end, we return to the likelihood that glucose is the key driving factor.”
β-cell reserve is a concept that relates to compensation (2, 7). Thus, we know that when β cells are pushed to compensate for increased demand by increasing insulin resistance, they secrete more insulin from existing β cells (59) and also self-replicate, albeit to a limited extent. We know that β cells have varying levels of secretory activity. In rodents there is a population of cells described as sleeping that can be activated when needed (60), and it seems likely that the secretion of already active cells can be enhanced. However, a key point is that this reserve capacity is limited and when it is used up, any further loss of β-cells or increase in demand will result in climbing glucose levels that will lead to β-cell secretory dysfunction. Loss of this reserve can be considered a critical step in the process of β-cell breakdown.
Trying to understand β-cell changes that occur with minimal change in blood glucose
Although our understanding of the mechanisms of insulin secretion is improving, it is not easy to understand how β-cells compensate for insulin resistance when blood glucose levels seem to have little change. It is also challenging to explain the profound secretory abnormality of FPIR that also occurs with minimal glucose change. It is possible that actual changes are partially obscured by the oscillations in secretion that occur about every 5 minutes, which are thought to be due to a positive feedback circuit controlled by the bifunctional enzyme PFK2/FBPase2 (6-phosphofructo-2 kinase/fructose-2,6-bisphosphatase) (61).
While we can postulate the presence of a very efficient feedback mechanism between β cells and insulin sensitive target tissues, we must acknowledge that our understanding of this is very limited. For example, an obese insulin resistant individual with increased β-cell mass and secretion may not have a discernable increase in blood glucose levels in either the fasting or postprandial state. For discussion’s sake, if plasma glucose levels were increased by 5 mg/dl at certain points during the day, could this provide more PEP than could come from directly from glycolysis or indirectly from anapleurosis and the PEP cycle? The small increase in glucose could also influence mitochondrial oxidative phosphorylation. One can perhaps also ask whether these shifts in metabolism influence gene expression, which could lead to a variety of outcomes. Data obtained from rats with surgical reduction of β-cell mass (partial pancreatectomy) indicate that very small increases of glucose levels are associated with, and may cause, many changes in gene expression (46, 62), suggesting a variety of possible mechanisms that should be explored. Another study with a similar rat partial pancreatectomy model (63) found increased activity of glucokinase, which means there should be increased glucose flux at whatever glucose level might be present. A change that merits a close look is lactate dehydrogenase which is normally suppressed in β cells but becomes activated with even very minor increases in blood glucose (16, 46). This could lead to leakage of carbons as lactate is formed that would otherwise have been oxidized by mitochondria.
Making things more complicated is the problem of defining what is a normal level of blood glucose. The road to diabetes must begin at some “normal” glucose level, but what does it mean when fasting glucose concentrations rise from 80 to 90 mg/dl and then on to 100 mg/dl? Perhaps there is a spectrum of glucose levels that on the lower end are associated with healthy adaptation of β cells to increased demand and on the upper end, represent signs of breakdown. But, again, there must be genetic variation, such that fasting value of 80 may be normal for one family while 90 or 100 might be normal for others. Even within an individual, there must be differences that are driven by countless variables including diet, age, activity, etc.
Reduction of glucose-induced first-phase insulin release (FPIR) is an early sensitive sign of β-cell dysfunction
Perhaps the earliest indication of β-cell damage when they are pushed to compensate is the striking loss of glucose-induced first phase insulin secretion (FPIR). When β cells are compensating effectively for the demands of obesity-driven insulin resistance, first-phase glucose-induced insulin release (FPIR) is higher than normal (14). However, in adult subjects studied with varying levels of plasma glucose after an overnight fast, FPIR remained intact as long as fasting blood glucose levels remained below 100 mg/dl, but at higher levels the response was markedly reduced, (Figure 1) and when glucose levels exceeded 114 mg/dl, the response was completely gone (impaired fasting glucose levels are defined as being between 100–125 mg/dl) (15).
There has been interest in the loss of FPIR as risk factor for T2D for many years and even a suggestion that it could be a primary genetic risk factor, preceding the development of obesity and insulin resistance (64), however, the weight of evidence indicates that β cells of animals or humans will always lose their FPIR when β-cell mass is insufficient and hyperglycemia occurs.
Another example of impaired FPIR are the individuals who have undergone a 50% pancreatectomy as donors for islet transplantation (65) or for neoplasms (7, 8, 66) Not only were these donors found to have increased risk of developing either impaired glucose tolerance or diabetes, but a recent study by Mezza et al (8) found that reduction of FPIR in particular was a strong predictor of progression to diabetes. The variability among these subjects was not surprising because many observations in both humans and animals indicate that a 50% reduction of β-cell mass is a tipping point for progression to diabetes (2, 66–71). Therefore, as might have been predicted for this population, some would have enough β-cell mass and functional reserve to maintain completely normal glucose levels for years while others over time would advance toward glucose intolerance or diabetes. The rate of progression might resemble that of adults with impaired glucose tolerance who develop diabetes at the rate of about 10% per year (72).
Based on data from studies of subjects with insulin resistance, preclinical T1D, and partial pancreatectomies in humans and animals, it appears that β cells do an impressive job of compensating for the increased demands of insulin resistance and inadequate β-cell mass. However, some develop a loss of FPIR while glucose levels remain in the normal or near-normal range. This marked reduction in FPIR indicates that a profound abnormality of the secretory machinery has developed, but interestingly, β-cell function can still keep such individuals from progressing to frank diabetes because at least some of the so-called second phase of glucose stimulated insulin secretion(GSIS) remains active and β cells can still respond at least reasonably well to gut hormones (incretins), amino acids and parasympathetic stimulation. It may be that the triggering (KATP-dependent) mechanism for insulin secretion is more impaired than the amplification mechanisms (KATP-independent). This could explain why both FPIR and glucose potentiation of non-glucose secretagogues are severely impaired.
How long does it take for hyperglycemia to induce a change in β-cell phenotype and what is known about reversibility?
There has been skepticism about the glucose toxicity concept because infusions of glucose have not been found to reproduce the same secretory and phenotypic abnormalities that are found in diabetes. There have been a number of studies in which rats have been infused with glucose to maintain hyperglycemia for 2–4 days and some disruption of secretion has been found (73–76). Infusions of glucose into humans for 48 hours have also been carried out (77). A particularly valuable study found that after a 90% partial pancreatectomy in rats a marked reduction in FPIR was only found after 2–3 weeks (78). The question needs more study, but it appears that the defects that have been found with glucose intolerance and diabetes evolve over a period of weeks.
Then comes the important question of whether and to what extent the secretory abnormalities can be reversed. Partial improvement can be found by simply lowering glucose levels with insulin overnight (79). Similar improvement could be found when glucose levels in T2D were lowered with fish insulin resulting in an increased secretion of human insulin (80). Another example is seen with T1D; those who have a remission shortly after diagnosis have been found to have increased insulin secretion (81). Restoration to essentially normal FPIR can be seen in people with T2D who have a full remission after bariatric surgery (82, 83) or with effective glucose lowering treatments (84). When subjects with T2D were treated with somatostatin overnight, there was a return of secretory pulsatility and normalization of the proinsulin/insulin ratio even though hyperglycemia was maintained (85). There is a paucity of information about the timing of the induction and reversal of the secretory abnormalities that are so well characterized in the presence of diabetes. It is possible that the abnormal insulin secretion of T2D is completely reversible as is suggested by the impressive improvement seen after bariatric surgery, but this needs further study.
The particular importance of loss of FPIR in type 1 diabetes
Very similar changes in β-cell dysfunction occur during the development of T1D (9, 10, 86–89). Thus, in individuals with active autoimmunity as determined by the presence of islet and β-cell autoantibodies, a marked and often a complete loss of FPIR can be found even when fasting glucose and hemoglobin A1c values are still in the normal range. This loss of FPIR can now be considered an indicator of imminent decompensation as frank diabetes will typically occur within a matter of months (9). The immunology community has sometimes blamed cytokines or other immune factors for the loss of FPIR in T1D, but this does not fit with the observation that when pancreases are studied shortly after diagnosis most islets have no evidence of lymphocytic infiltration (81, 90). Based upon the known occurrence of remission (81) and a limited number of available pathology studies, β-cell mass at the time of diagnosis can be estimated to be in the range of 30% or even higher. Thus, it is difficult to see how inflammation affecting only some islets or circulating cytokine levels could explain the complete loss of FPIR. We know that loss of FPIR is associated with many changes of the β-cell phenotype, including increased expression of genes that promote the appearance of both MHC class I and II antigens (46, 91). The appearance of these antigens may contribute to the accelerated rate of autoimmune killing and the more rapid development of frank diabetes. Other evidence pointing to this acceleration is the finding during this time period of increased circulating unmethylated insulin DNA (92) that is thought to reflect β-cell death.
Loss of FPIR is associated with major changes in β-cell gene expression that become more severe as glucose levels rise
While derangements in β-cell phenotype and function can be found when glucose levels are still in the normal range (Stage 2), they become much more disrupted when glucose levels climb into stages 3–5. For example, glucose potentiation of arginine-stimulated insulin secretion in full-blown T2D was found to be about 15% of normal (93). Knowing that the β-cell mass in such subjects with T2D is roughly 50% that of normal suggests that β-cells are probably functioning at about only 30% of their capacity throughout the day. These findings have important implications for therapy because based upon functional improvements seen with bariatric surgery in T2D (82, 83) and various treatments (84, 94), lowering glucose levels with treatment of any kind should restore an important proportion of β-cell function.
The changes in β-cell phenotype seen in the presence of chronic hyperglycemia state have been described as dedifferentiation (16), which partially fits with the increased expression of many genes that are suppressed during the course of normal β-cell development. Remarkably, in our study of gene expression of islets obtained 4 weeks after partial pancreatectomy in rats, with very mild glucose elevations, 2313 of 13,971 (17%, adjusted P value less than 0.01) of the analyzed genes were differentially expressed, and in the group with higher glucose levels studied at 10 weeks after surgery 7844 of 15207 genes (52%) were differentially expressed (46). Many of the changes fit with what was already known (32), others open up new avenues.
As mentioned, a leading hypothesis is that the marked abnormalities of insulin secretion are caused by β cells being exposed to higher concentrations of glucose than they are accustomed to, hence the use of the term glucose toxicity. We know that isolated islets cultured in elevated concentrations of glucose will lose some their secretory responses to glucose (95, 96). While we must be careful about making too much of the tissue culture results, studies in humans with T2D or T1D and multiple animal models in which β-cells mass is reduced either by surgery or by the beta cell toxin streptozocin (97) have remarkably similar findings of tightly correlated elevations in plasma glucose and impairment of GSIS. These changes in function correlate very well with changes in the gene expression. Importantly, the secretory abnormality and gene expression abnormalities are reversed when glucose values are normalized (16, 62).
There are a growing number of studies measuring gene expression in β cells and islets of humans with T2D and animal models of diabetes. Similarities in the findings are becoming more and more evident. Our studies of gene expression of islets in rats with surgical reduction of β-cell mass using partial pancreatectomy (46) provide data that complement what is found in other systems (98). As expected, there was marked reduction of key β-cell genes including insulins 1 and 2, and major transcription factors important for β-cell identity including Pdx1, Nkx6-1, and MafA. In addition, there were marked changes in the genes responsible for the machinery that facilitates the metabolism of glucose to produce ATP. The marked impairment of GSIS in the diabetic state fits well with the disrupted expression of genes important for metabolism in β cells. There were marked decreases in the expression of key genes including glut2 (Slc2a2), glucokinase, the sulfonylurea receptor, the potassium channel, mitochondrial shuttles, glycolysis and the Krebs cycle (enolase 2, phosphofructokinase, pyruvate carboxylase as well as notable upregulation of the disallowed genes lactate dehydrogenase, monocarboxylate transporter 1and aldolase B. Thus, the elegant ways in which β-cells link glucose metabolism to insulin secretion are markedly torn apart. These findings fit very well with studies of islets obtained from cadaver donors with T2D in which generation of ATP from glucose was markedly impaired (19).
Major defects in the distal steps of insulin secretion cannot explain the loss of FPIR by glucose
The complicated process of exocytosis has been thoroughly studied leading to a good understanding of how insulin-containing granules are transferred from the Golgi to the plasma membrane to be docked and then released (99). The key point is that in T2D FPIR to a variety of secretory agents other than glucose remains intact, including to arginine, glucagon, GLP1, sulfonylureas and isoproterenol (3), but the responses to these agents are very poorly enhanced by glucose potentiation. To clarify, subjects with T2D will have completely absent FPIR to glucose but will have FPIR responses to arginine or isoproterenol that are very similar to control non-diabetic subjects (93). However, when glucose levels are increased by glucose infusions, there is a large potentiation of the responses to these two agents in the non-diabetic subjects but almost none in those with T2D (100). Therefore, one can conclude that in T2D the granules are well positioned for exocytosis and can response to signals such as cyclic AMP or depolarization, but the first-phase signals from glucose are unable to stimulate insulin release by itself or through potentiation.
How does the heterogeneity of T2D relate to β-cell dysfunction?
There is great interest in the heterogeneity of T2D (101) and even in T1D. It is clear that there are a wide variety of genetic and environmental factors that lead to the outcome of T2D. Some exert their major influence by increasing insulin resistance while others no doubt exert their effects mainly on β cells, but then in virtually all of these situations β-cells function becomes inadequate to maintain normoglycemia and then the β cells become dysfunctional, as evidenced by loss of FPIR and changes of their identity with many changes in gene expression. The main point is that there are many paths to diabetes but when β-cells start to falter and then continue the process of failure, the changes that occur in β cells are very similar whether in T1D or T2D, or in young, old, obese, or lean individuals.
Posing some questions that should be examined
We predict that that more sharply defining the progression from normal to diabetes will provide very valuable information. There are four conditions that, if studied carefully, could provide important insights because the β-cell phenotype of each as determined by gene expression or other measurements should be very distinct in each case. It might be best to carry out these studies first in rodent models because they can provide more precise data, which can then be used to guide experiments with human β cells. The conditions are:
First: Normal controls
Second: β cells compensating to increased demand with increased insulin output and fully intact FPIR. We know that there are important changes in gene expression at this stage but have little understanding of how this works.
Third: β cells with minimal hyperglycemia and loss of FPIR. This would provide insights into the state of impaired glucose tolerance. It seems there is a problem with the triggering phase of GSIS that should be explored.
Fourth: Frank diabetes with the combination of loss of FPIR and hyperglycemia. These are likely to provide new insights on mechanisms of β-cell vulnerability.
It would also be instructive to determine the extent to which these changes are reversible. These types of experiment and others can also address the fundamental question of what is the difference between beta cells that are “working” with increased flux of glucose through metabolic pathways in the absence of hyperglycemia as compared to what is happening in the presence of hyperglycemia? Recent experiments have found evidence in a phenotypic change in the β cells of mice with an activating mutation in glucokinase but no hyperglycemia (58).
The importance of recognizing how much we do not understand
At this point we are probably safe is concluding that β-cells exposed to high glucose levels develop severe abnormalities of insulin secretion and major changes in β-cell gene expression, and that these changes are largely reversible. However, we have little understanding as to how glucose exerts these effects. We know that glucose can influence growth and various other pathways; surely its metabolism in β cells must be able to influence gene expression. Therefore, we suggest that the problem begins when increased flux of glucose causes changes in gene expression that lead to alterations in the elegant machinery that links glucose metabolism to insulin secretion. In its early stages the damage shows up as reduced FPIR but then as it becomes more severe, β-cell identify is dramatically altered, which leads to major changes in secretory function and factors that influence survival. Our commentary has not touched on many areas of intense investigation, but we can expect that the relative importance these processes and how they relate to the complex effects of glucose metabolism in β cells will be sorted out with time.
In spite of these remarkable changes, β-cell mass can be remarkably well-maintained even for decades in the face of continuing hyperglycemia. Knowing that at least some of the dysfunction is reversible provides further impetus to continue our efforts to fill these many gaps in our knowledge.
Highlights.
β-cell mass and glucose-induced first phase insulin release (FPIR) are increased by insulin resistance, but with slight increases in fasting plasma glucose levels, FPIR is abolished. This loss of FPIR is associated with increased risk of progressing to diabetes. The most attractive way to explain these changes is that they are driven by glucose. However, while the circumstantial evidence supporting the relationship is strong, the responsible mechanisms have not been found. The complexities of addressing this challenge are discussed and suggestions for experiments are proposed.
Acknowledgements
Funding for this study was supported by grants from the NIH (RO1 DK110390 [S.B-W) and the Joslin Diabetes Research Center (P30 DK036836), JDRF (2-SRA-2018-527-S-B [GCW]), the Diabetes Research and Wellness Foundation. We thank Jennifer Hollister-Lock for help with making the figure. All of the authors contributed to the writing of this manuscript and none of the authors have any conflicts of interest.
Abbreviations:
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- FPIR
first phase insulin release
- GSIS
glucose-stimulated insulin secretion
- KATP channels
ATP-sensitive potassium channel
- PEP
phosphoenolpyruvate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, et al. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37(6):1751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weir GC, Gaglia J, Bonner-Weir S. Inadequate beta-cell mass is essential for the pathogenesis of type 2 diabetes. The lancet Diabetes & endocrinology. 2020;8(3):249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weir GC, Bonner-Weir S. Islet beta cell mass in diabetes and how it relates to function, birth, and death. Ann N Y Acad Sci. 2013;1281:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bensellam M, Jonas JC, Laybutt DR. Mechanisms of beta-cell dedifferentiation in diabetes: recent findings and future research directions. J Endocrinol. 2018;236(2):R109–r43. [DOI] [PubMed] [Google Scholar]
- 5.Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53 Suppl 3:S16–21. [DOI] [PubMed] [Google Scholar]
- 6.Tabak AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimaki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373(9682):2215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weir GC, Bonner-Weir S. Reduced glucose-induced first-phase insulin release is a danger signal that predicts diabetes. J Clin Invest. 2021;131(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mezza T, Ferraro PM, Di Giuseppe G, Moffa S, Cefalo CM, Cinti F, et al. Duodenopancreatectomy as a model to demonstrate the fundamental role of dysfunctional β cell in predicting diabetes. J Clin Invest. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sosenko JM, Skyler JS, Beam CA, Krischer JP, Greenbaum CJ, Mahon J, et al. Acceleration of the loss of the first-phase insulin response during the progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes. 2013;62(12):4179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godsland IF, Jeffs JAR, Johnston DG. Loss of beta cell function as fasting glucose increases in the non-diabetic range. Diabetologia. 2004;47(7):1157–66. [DOI] [PubMed] [Google Scholar]
- 11.van Haeften TW, Pimenta W, Mitrakou A, Korytkowski M, Jenssen T, Yki-Jarvinen H, et al. Relative conributions of beta-cell function and tissue insulin sensitivity to fasting and postglucose-load glycemia. Metabolism. 2000;49(10):1318–25. [DOI] [PubMed] [Google Scholar]
- 12.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104(6):787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. 2004;47(1):31–9. [DOI] [PubMed] [Google Scholar]
- 14.Karam JH, Grodsky GM, Forsham PH. Excessive insulin response to glucose in obese subjects as measured by immunochemical assay. Diabetes. 1963;12:196–204. [DOI] [PubMed] [Google Scholar]
- 15.Brunzell JD, Robertson RP, Lerner RL, Hazzard WR, Ensinck JW, Bierman EL, et al. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. JClinEndocrinolMetab. 1976;42:222–9. [DOI] [PubMed] [Google Scholar]
- 16.Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, et al. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999;274(20):14112–21. [DOI] [PubMed] [Google Scholar]
- 17.Weir GC. Glucolipotoxicity, beta-Cells, and Diabetes: The Emperor Has No Clothes. Diabetes. 2020;69(3):273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meglasson MD, Matschinsky FM. New perspectives on pancreatic islet glucokinase. AmJPhysiol. 1984;246:E1–E13. [DOI] [PubMed] [Google Scholar]
- 19.Doliba NM, Qin W, Najafi H, Liu C, Buettger CW, Sotiris J, et al. Glucokinase activation repairs defective bioenergetics of islets of Langerhans isolated from type 2 diabetics. Am J Physiol Endocrinol Metab. 2012;302(1):E87–e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Vos A, Heimberg H, Quartier E, Huypens P, Bouwens L, Pipeleers D, et al. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest. 1995;96(5):2489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorens B, Weir GC, Leahy JL, Lodish HF, Bonner-Weir S. Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats. ProcNatlAcadSciUSA. 1990;87:6492–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matschinsky FM, Glaser B, Magnuson MA. Pancreatic beta-cell glucokinase: closing the gap between theoretical concepts and experimental realities. Diabetes. 1998;47:307–15. [DOI] [PubMed] [Google Scholar]
- 23.Ashcroft FM, Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell. 2012;148(6):1160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52(5):739–51. [DOI] [PubMed] [Google Scholar]
- 25.Lewandowski SL, Cardone RL, Foster HR, Ho T, Potapenko E, Poudel C, et al. Pyruvate Kinase Controls Signal Strength in the Insulin Secretory Pathway. Cell Metab. 2020;32(5):736–50.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poursharifi P, Madiraju SRM, Prentki M. Monoacylglycerol signalling and ABHD6 in health and disease. Diabetes Obes Metab. 2017;19 Suppl 1:76–89. [DOI] [PubMed] [Google Scholar]
- 27.Ferdaoussi M, Dai X, Jensen MV, Wang R, Peterson BS, Huang C, et al. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional beta cells. J Clin Invest. 2015;125(10):3847–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pisania A, Weir GC, O’Neil JJ, Omer A, Tchipashvili V, Lei J, et al. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab Invest. 2010;90(11):1661–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward WK, LaCava EC, Paquette TL, Beard JC, Wallum BJ, Porte D Jr. Disproportionalte elevation of immunoreactive proinsulin in type 2 (noninsulin-dependent) diabetes mellitus and in experimental insulin resistance. Diabetologia. 1987;30:698–702. [DOI] [PubMed] [Google Scholar]
- 30.Rhodes CJ, Alarcon C. What beta cell defect could lead to hyperproinsulinemia in NIDDM? Diabetes. 1994;43:511–7. [DOI] [PubMed] [Google Scholar]
- 31.Alarcon C, Boland BB, Uchizono Y, Moore PC, Peterson B, Rajan S, et al. Pancreatic β-Cell Adaptive Plasticity in Obesity Increases Insulin Production but Adversely Affects Secretory Function. Diabetes. 2016;65(2):438–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rege NK, Liu M, Yang Y, Dhayalan B, Wickramasinghe NP, Chen YS, et al. Evolution of insulin at the edge of foldability and its medical implications. Proc Natl Acad Sci U S A. 2020;117(47):29618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu M, Weiss MA, Arunagiri A, Yong J, Rege N, Sun J, et al. Biosynthesis, structure, and folding of the insulin precursor protein. Diabetes Obes Metab. 2018;20 Suppl 2(Suppl 2):28–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arunagiri A, Haataja L, Pottekat A, Pamenan F, Kim S, Zeltser LM, et al. Proinsulin misfolding is an early event in the progression to type 2 diabetes. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56(8):2016–27. [DOI] [PubMed] [Google Scholar]
- 36.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10 Suppl 4:32–42. [DOI] [PubMed] [Google Scholar]
- 37.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–10. [DOI] [PubMed] [Google Scholar]
- 38.Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88(5):2300–8. [DOI] [PubMed] [Google Scholar]
- 39.Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45(1):85–96. [DOI] [PubMed] [Google Scholar]
- 40.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42(11):1663–72. [DOI] [PubMed] [Google Scholar]
- 41.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36(1):111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arrojo EDR, Lev-Ram V, Tyagi S, Ramachandra R, Deerinck T, Bushong E, et al. Age Mosaicism across Multiple Scales in Adult Tissues. Cell Metab. 2019;30(2):343–51.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cnop M, Hughes SJ, Igoillo-Esteve M, Hoppa MB, Sayyed F, van de Laar L, et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia. 2010;53(2):321–30. [DOI] [PubMed] [Google Scholar]
- 44.Caballero F, Siniakowicz K, Hollister-Lock J, Duran L, Katsuta H, Yamada T, et al. Birth and death of human beta-cells in pancreases from cadaver donors, autopsies, surgical specimens, and islets transplanted into mice. Cell Transplant. 2014;23(2):139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan BA, Hollister-Lock J, Bonner-Weir S, Weir GC. Reduced Ki67 Staining in the Postmortem State Calls Into Question Past Conclusions About the Lack of Turnover of Adult Human beta-Cells. Diabetes. 2015;64(5):1698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ebrahimi AG, Hollister-Lock J, Sullivan BA, Tsuchida R, Bonner-Weir S, Weir GC. Beta cell identity changes with mild hyperglycemia: Implications for function, growth, and vulnerability. Mol Metab. 2020;35:100959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laybutt DR, Kaneto H, Hasenkamp W, Grey S, Jonas JC, Sgroi DC, et al. Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to beta-cell survival during chronic hyperglycemia. Diabetes. 2002;51(2):413–23. [DOI] [PubMed] [Google Scholar]
- 48.Nomoto H, Pei L, Montemurro C, Rosenberger M, Furterer A, Coppola G, et al. Activation of the HIF1α/PFKFB3 stress response pathway in beta cells in type 1 diabetes. Diabetologia. 2020;63(1):149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montemurro C, Nomoto H, Pei L, Parekh VS, Vongbunyong KE, Vadrevu S, et al. IAPP toxicity activates HIF1α/PFKFB3 signaling delaying β-cell loss at the expense of β-cell function. Nature communications. 2019;10(1):2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JW, Ko J, Ju C, Eltzschig HK. Hypoxia signaling in human diseases and therapeutic targets. Exp Mol Med. 2019;51(6):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aguayo-Mazzucato C, Andle J, Lee TB Jr., Midha A, Talemal L, Chipashvili V, et al. Acceleration of beta Cell Aging Determines Diabetes and Senolysis Improves Disease Outcomes. Cell Metab. 2019;30(1):129–42.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuok IT, Rountree AM, Jung SR, Sweet IR. Palmitate is not an effective fuel for pancreatic islets and amplifies insulin secretion independent of calcium release from endoplasmic reticulum. Islets. 2019;11(3):51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helman A, Cangelosi AL, Davis JC, Pham Q, Rothman A, Faust AL, et al. A Nutrient-Sensing Transition at Birth Triggers Glucose-Responsive Insulin Secretion. Cell metabolism. 2020;31(5):1004–16.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El Ouaamari A, Dirice E, Gedeon N, Hu J, Zhou JY, Shirakawa J, et al. SerpinB1 Promotes Pancreatic β Cell Proliferation. Cell Metab. 2016;23(1):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yi P, Park JS, Melton DA. Betatrophin: a hormone that controls pancreatic beta cell proliferation. Cell. 2013;153(4):747–58. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, et al. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest. 2007;117(1):246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, Schyr-Ben-Haroush R, Hija A, Stolovich-Rain M, et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 2011;13(4):440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tornovsky-Babeay S, Weinberg-Corem N, Ben-Haroush Schyr R, Avrahami D, Lavi J, Feleke E, et al. Biphasic dynamics of beta cell mass in a mouse model of congenital hyperinsulinism: implications for type 2 diabetes. Diabetologia. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Camastra S, Manco M, Mari A, Baldi S, Gastaldelli A, Greco AV, et al. beta-cell function in morbidly obese subjects during free living: long-term effects of weight loss. Diabetes. 2005;54(8):2382–9. [DOI] [PubMed] [Google Scholar]
- 60.Olsson R, Carlsson PO. A low-oxygenated subpopulation of pancreatic islets constitutes a functional reserve of endocrine cells. Diabetes. 2011;60(8):2068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merrins MJ, Bertram R, Sherman A, Satin LS. Phosphofructo-2-kinase/fructose-2,6-bisphosphatase modulates oscillations of pancreatic islet metabolism. PLoS One. 2012;7(4):e34036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laybutt DR, Glandt M, Xu G, Ahn YB, Trivedi N, Bonner-Weir S, et al. Critical reduction in beta-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J Biol Chem. 2003;278(5):2997–3005. [DOI] [PubMed] [Google Scholar]
- 63.Chen C, Hosokawa H, Bumbalo L, Leahy JL. Mechanism of compensatory hyperinsulinemia innormoglycemic insulin-resistant spontaneously hypertensive rats. JClinInvest. 1994;94:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerich JE. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes. 2002;51 Suppl 1:S117–21. [DOI] [PubMed] [Google Scholar]
- 65.Seaquist ER, Robertson RP. Effects of hemipancreatectomy on pancreatic alpha and beta cell function in healthy human donors. J Clin Invest. 1992;89(6):1761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menge BA, Schrader H, Breuer TG, Dabrowski Y, Uhl W, Schmidt WE, et al. Metabolic consequences of a 50% partial pancreatectomy in humans. Diabetologia. 2009;52(2):306–17. [DOI] [PubMed] [Google Scholar]
- 67.Matveyenko AV, Butler PC. Relationship between beta-cell mass and diabetes onset. Diabetes Obes Metab. 2008;10 Suppl 4(0 4):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC. Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes care. 2006;29(3):717–8. [DOI] [PubMed] [Google Scholar]
- 69.Leahy JL, Bonner-Weir S, Weir GC. Minimal chronic hyperglycemia is a critical determinant of impaired insulin secretion after an incomplete pancreatectomy. J Clin Invest. 1988;81(5):1407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matveyenko AV, Veldhuis JD, Butler PC. Mechanisms of impaired fasting glucose and glucose intolerance induced by an approximate 50% pancreatectomy. Diabetes. 2006;55(8):2347–56. [DOI] [PubMed] [Google Scholar]
- 71.McCulloch DK, Koerker DJ, Kahn SE, Bonner-Weir S, Palmer JP. Correlations of in vivo B-cell function tests with B-cell mass and pancreatic insulin content in streptozocin-administered baboons. Diabetes. 1991;40:673–9. [DOI] [PubMed] [Google Scholar]
- 72.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steil GM, Trivedi N, Jonas JC, Hasenkamp WM, Sharma A, Bonner-Weir S, et al. Adaptation of beta-cell mass to substrate oversupply: enhanced function with normal gene expression. Am J Physiol Endocrinol Metab. 2001;280(5):E788–96. [DOI] [PubMed] [Google Scholar]
- 74.Leahy JL, Cooper HE, Deal DA, Weir GC. Chronic hyperglycemia is associated with impaired glucose influence on insulin secretion. A study in normal rats using chronic in vivo glucose infusions. J Clin Invest. 1986;77(3):908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leahy JL, Weir GC. Evolution of abnormal insulin secretory responses during 48-h in vivo hyperglycemia. Diabetes. 1988;37(2):217–22. [DOI] [PubMed] [Google Scholar]
- 76.Bolaffi JL, Heldt A, Lewis LD, Grodsky GM. The third phase of in_vitro insulin secretion: evidence for glucose insensitivity. Diabetes. 1986;35:370–3. [DOI] [PubMed] [Google Scholar]
- 77.Boden G, Ruiz J, Kim CJ, Chen X. Effects of prolonged glucose infusion on insulin secretion, clearance, and action in normal subjects. Am J Physiol. 1996;270(2 Pt 1):E251–8. [DOI] [PubMed] [Google Scholar]
- 78.Leahy JL, Bumbalo LM, Chen C. Diazoxide causes recovery of beta-cell glucose responsiveness in 90% pancreatectomized diabetic rats. Diabetes. 1994;43:173–9. [DOI] [PubMed] [Google Scholar]
- 79.Vague P, Moulin JP. The defective glucose sensitivity of the B-cell in noninsulin dependent diabetes: improvement after twenty hours of normoglycemia. Metabolism. 1982;31:139–42. [DOI] [PubMed] [Google Scholar]
- 80.Turner RC, McCarthy ST, Holman RR, Harris E. beta-cell function imporved by supplementing basal insulin secretion in mild diabetes. British Medical Journal. 1976;1:1252–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301(15):1573–9. [DOI] [PubMed] [Google Scholar]
- 82.Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32(3):375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, Alexandrides TK. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes. 2003;52(5):1098–103. [DOI] [PubMed] [Google Scholar]
- 84.Zhyzhneuskaya SV, Al-Mrabeh A, Peters C, Barnes A, Aribisala B, Hollingsworth KG, et al. Time Course of Normalization of Functional β-Cell Capacity in the Diabetes Remission Clinical Trial After Weight Loss in Type 2 Diabetes. Diabetes Care. 2020;43(4):813–20. [DOI] [PubMed] [Google Scholar]
- 85.Laedtke T, Kjems L, Pørksen N, Schmitz O, Veldhuis J, Kao PC, et al. Overnight inhibition of insulin secretion restores pulsatility and proinsulin/insulin ratio in type 2 diabetes. Am J Physiol Endocrinol Metab. 2000;279(3):E520–8. [DOI] [PubMed] [Google Scholar]
- 86.Srikanta S, Ganda OP, Rabizadeh A, Soeldner JS, Eisenbarth GS. First-degree relatives of patients with type I diabetes mellitus: Islet-cell antibodies and abnormal insulin secretion. NEnglJMed. 1985;313:461–4. [DOI] [PubMed] [Google Scholar]
- 87.Vardi P, Crisa L, Jackson RA. Predictive value of intravenous glucose tolerance test insulin secretion less than or greater than the first percentile in islet cell antibody positive relatives of type 1 (insulin-dependent) diabetic patients. Diabetologia. 1991;34(2):93–102. [DOI] [PubMed] [Google Scholar]
- 88.Dayan CM, Korah M, Tatovic D, Bundy BN, Herold KC. Changing the landscape for type 1 diabetes: the first step to prevention. Lancet (London, England). 2019;394(10205):1286–96. [DOI] [PubMed] [Google Scholar]
- 89.Weir GC, Bonner-Weir S. Glucose Driven Changes in Beta Cell Identity Are Important for Function and Possibly Autoimmune Vulnerability during the Progression of Type 1 Diabetes. Frontiers in genetics. 2017;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Campbell-Thompson ML, Atkinson MA, Butler AE, Chapman NM, Frisk G, Gianani R, et al. The diagnosis of insulitis in human type 1 diabetes. Diabetologia. 2013;56(11):2541–3. [DOI] [PubMed] [Google Scholar]
- 91.Russell MA, Redick SD, Blodgett DM, Richardson SJ, Leete P, Krogvold L, et al. HLA Class II Antigen Processing and Presentation Pathway Components Demonstrated by Transcriptome and Protein Analyses of Islet beta-Cells From Donors With Type 1 Diabetes. Diabetes. 2019;68(5):988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herold KC, Usmani-Brown S, Ghazi T, Lebastchi J, Beam CA, Bellin MD, et al. beta cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest. 2015;125(3):1163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. JClinInvest. 1984;74:1318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222–34. [DOI] [PubMed] [Google Scholar]
- 95.Andersson A, Hellerstrom C. Metabolic characteristics of isolated pancreatic islets in tissue culture. Diabetes. 1972;21(Suppl 2):546–54. [DOI] [PubMed] [Google Scholar]
- 96.Henquin JC, Dufrane D, Kerr-Conte J, Nenquin M. Dynamics of glucose-induced insulin secretion in normal human islets. Am J Physiol Endocrinol Metab. 2015;309(7):E640–50. [DOI] [PubMed] [Google Scholar]
- 97.Weir GC, Clore ET, Zmachinski CJ, Bonner-Weir S. Islet secretion in a new experimental model for non-insulin-dependent diabetes. Diabetes. 1981;30(7):590–5. [DOI] [PubMed] [Google Scholar]
- 98.Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, et al. Inactivation of specific beta cell transcription factors in type 2 diabetes. J Clin Invest. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arvan P Secretory protein trafficking: genetic and biochemical analysis. Cell Biochem Biophys. 2004;40(3 Suppl):169–78. [DOI] [PubMed] [Google Scholar]
- 100.Shankar SS, Vella A, Raymond RH, Staten MA, Calle RA, Bergman RN, et al. Standardized Mixed-Meal Tolerance and Arginine Stimulation Tests Provide Reproducible and Complementary Measures of beta-Cell Function: Results From the Foundation for the National Institutes of Health Biomarkers Consortium Investigative Series. Diabetes Care. 2016;39(9):1602–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. The lancet Diabetes & endocrinology. 2018;6(5):361–9. [DOI] [PubMed] [Google Scholar]


