Abstract
Osteoblasts in vivo form an epithelial-like layer with tight junctions between cells. Bone formation involves mineral transport into the matrix and acid transport to balance pH levels. To study the importance of the pH gradient in vitro, we used Transwell inserts composed of polyethylene terephthalate (PET) membranes with 0.4 μm pores at a density of (2 ± 0.4) × 106 pores per cm2. Mesenchymal stem cells (MSCs) prepared from murine bone marrow were used to investigate alternative conditions whereby osteoblast differentiation would better emulate in vivo bone development. MSCs were characterized by flow cytometry with more than 90% CD44 and 75% Sca-1 labeling. Mineralization was validated with paracellular alkaline phosphatase activity, collagen birefringence, and mineral deposition confirming MSCs identity. We demonstrate that MSCs cultured and differentiated on PET inserts form an epithelial-like layer while mineralizing. Measurement of the transepithelial resistance was ~ 1,400 ohms•cm2 at three weeks of differentiation. The pH value of the media above and under the cells were measured while cells were in proliferation and differentiation. In mineralizing cells, a difference of 0.145 pH unit was observed between the medium above and under the cells indicating a transepithelial gradient. A significant difference in pH units was observed between the medium above and below the cells in proliferation compared to differentiation. Data on pH below membranes were confirmed by pH-dependent SNARF1 fluorescence. Control cells in proliferative medium did not form an epithelial-like layer, displayed low transepithelial resistance, and there was no significant pH gradient. By transmission electron microscopy, membrane attached osteoblasts in vitro had abundant mitochondria consistent with active transport that occurs in vivo by surface osteoblasts. In keeping with osteoblastic differentiation, scanning electron microscopy identified deposition of extracellular collagen surrounded by hydroxyapatite. This in vitro model is a major advancement in modeling bone in vivo for understanding of osteoblast bone matrix production.
Keywords: MSC, epithelial-like osteoblasts, Acid transport, Mineralization
Graphical Abstract
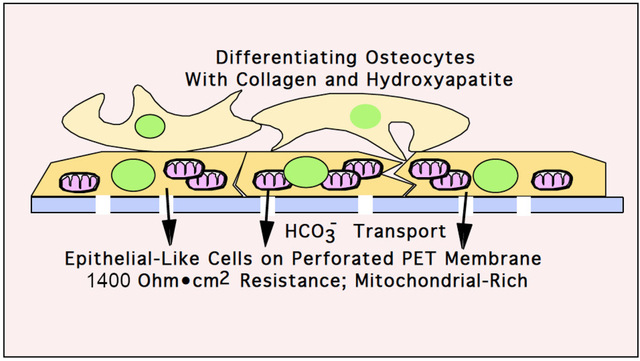
Introduction
Our work over several years has postulated that compact bone synthesis requires an epithelial-like layer of osteoblasts that transport bone mineral components, export protons and secrete proteins into the bone matrix space producing the mineralized bone matrix [1]. A major difficulty in studying this system is that, in vitro and in vivo, epithelial-like osteoblasts are microscopic and very difficult to preserve for morphology or physiologic study [2].
Here we created an epithelial-like organ in vitro by employing permeable support membranes for cell growth in medium with slightly alkaline pH, Dulbecco’s modified Essential Medium (DMEM) in 5.1% CO2. We report that murine MSCs differentiate well, producing an initial epithelial-like layer of cells on porous PET membranes. The bone differentiation correlates with a significant transmembrane pH gradient and transepithelial resistance (also called TEER).
Transepithelial electrical resistance measurements were used to assess the barrier function of marrow stem cells (MSC) grown in culture. This increased 250 fold after the cell layer was confluent and allowed to differentiate on these porous membranes. When assessing the electrical resistance, a current was passed through the Transwell membrane interrogating both transcellular and paracellular pathways. The transcellular resistance is primarily from apical and basolateral plasma membrane and paracellular resistance from cell-cell contacts. Hence, the transepithelial electrical resistance reflects physical properties of filter-grown epithelial-like cells.
Materials and Methods
MSCs isolation.
MSCs were isolated from C57Bl/6J mouse bone marrow. Epiphyses were removed from the dissected femur and tibia. Bone marrow was flushed using RPMI-1640, 10% fetal bovine serum (FBS), with antibiotic-antimycotics, with a needle 26 gauge (0.46 mm) driven through the bone. MSCs were cultured as described [3]. Briefly, from primary tissue aspirate, erythrocytes were removed by hypotonic red cell lysis. To remove fibroblasts, cells were plated in 25 cm2 flasks for overnight, after which non-adherent cells, containing the MSCs, were re-plated in supplemented RPMI at 2 × 106 cells per cm2 for 72 hours. Remaining non-adherent cells were discarded and medium was replaced with MesenCult expansion media (Stem Cell Technologies, Cambridge, MA). After passage 3–5 MesenCult expansion medium was changed to proliferation medium.
MSCs culture and material.
Proliferation medium was DMEM with1 g/l glucose, 10% FBS and an antibiotic-antimycotic. Osteogenic differentiation medium was proliferation medium supplemented with 10 mM 2-phosphoglycerol, 30 μg/ml of ascorbic acid and 0.5 mM CaCl2 (to obtain 2 mM final concentration). Cells were grown on PET membrane inserts, 4.2 cm2 surface, for 6 well plates. (Transwells, Falcon, Corning NY). The membranes were 12 μm thick with 2 ± 0.4 × 106 0.4 μm perforations per cm2 (See Fig 1C).
Figure 1. MSCs characterization by flow cytometry and image of the PET membrane without cells by SEM.
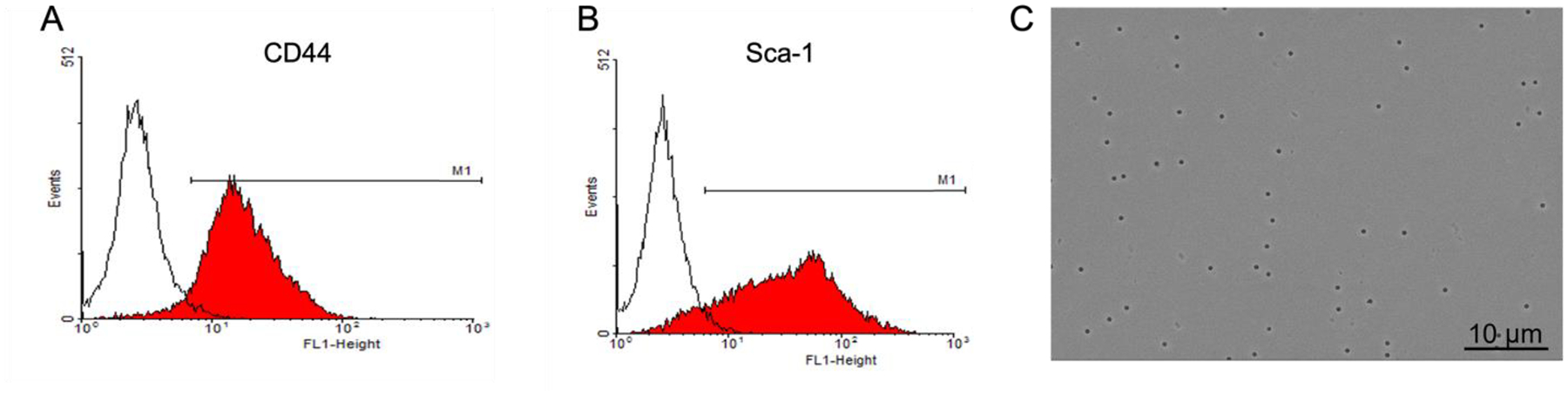
Empty histograms are isotype controls and selected MSCs antigens CD44 (A) and Sca-1(B) are shown.
C. Image of the PET membrane by SEM, shows inserts with 0.4 μm pores. Perforation area was measured as 2.3% of the total area, Bar= 10 μm.
Flow cytometry and cell labeling.
Characterization of MSCs from murine bone marrow was assessed by flow cytometry [4] using a FACScalibur instrument (Becton Dickinson, San Diego, CA). Cells were washed in Hank’s balanced salt solution containing 0.1% bovine serum albumin and 0.1% sodium azide (NaN3) and labeled with appropriately diluted antibodies (CD44, Sca-1, isotype controls) directly conjugated with fluorescein, for 30 minutes, followed by three washes and fixation in 2% paraformaldehyde. Data analysis used Cell Quest Software (Becton Dickinson). Phalloidin was used for labeling filamentous actin. Cultured cells were washed in phosphate buffered saline (PBS) and fixed in 3.7% formalin for 10 min. After three washes with PBS, the cells were permeabilized with 0.5% Triton X-100 (Sigma) for 20 min. These cells were stained in the dark with TRITC-conjugated phalloidin antibody (Molecular Probes, Eugene, OR) for 30 min, washed and mounted on microscope slides with Fluoroshield mounting medium (Abcam, Cambridge, UK). Images were acquired on a Nikon TE2000 inverted fluorescence microscope using a Spot 12-bit 1600 × 1200-pixel charge-coupled device; red fluorescence used excitation 536–556 nm, a 580 nm dichroic filter. Images were processed with ImageJ software (National Health Institute, Bethesda, USA)
Mineralization and alkaline phosphatase activity.
Alkaline phosphatase activity was determined in citrate-buffered saline at pH 8.0 using 0.01% naphthol phosphate substrate with 0.25 mg/ml of fast blue to precipitate the product as an insoluble blue adduct [5]. Mineralization was evaluated by silver nitrate (Von Kossa) stain. Cells were rinsed with deionized H2O (diH2O) and fixed with 3.7% formalin for 2 minutes. Subsequently, cultures were incubated with 2% AgNO3 under UV light for 10 min [5] and washed with diH2O multiple times. Stains were visualized by light microscopy.
Refractive index anisotropy.
Birefringence of secreted collagen was detected by setting linear polarizers perpendicular and observing the fixed cultures on the PET membranes as reported [2].
Transepithelial electrical resistance (TEER).
Transepithelial electrical resistance was measured using an EndOhm chamber with the EVOM2 meter calibrated in normal saline solution to ± 1 Ω according to the manufacturer’s instructions (World Precision Instruments, Sarasota, FL). At every time point, measurements were performed prior to fixation. To eliminate the influence of temperature, measurement was performed within 10 min after taking the culture plate out of the incubator and values were recorded 4 min after placing the inserts in the Endohm chamber. Within this time frame, the transepithelial resistance was stable. Fluid levels were equalized above and below the membrane within 1 mm. In parallel, blank resistances were measured in culture insert with medium but without cells. To obtain the sample resistance, the blank value was subtracted from the total resistance. The reported TEER was calculated by multiplying the sample resistance by the effective area of the membrane which is 4.2 cm2. To strengthen our experiment, 4 inserts were used at each time point for transepithelial electrical resistance measurements.
pH Measurements
pH Measurements were performed by using two methods, semi-micro pH electrode and SNARF-1 fluorescence. Direct measurement used an Apera Instruments (Columbus, Ohio) semi-micro PH850-MS pH Meter with a 6 mm LabSen 243–6 pH/Temp. electrode. The pH meter was calibrated at pH 4.00, 7.00 and 10.00 directly before use. Directly after taking the culture plate out of the incubator, the pH electrode was safely placed in the media above the cells. When the instrument indicated stability, values were recorded by a second investigator. To measure the pH below the cells, inserts were temporarily removed. A cell-impermeant pH indicator fluorescent dye 4(5)-carboxy-seminaphtharhodafluorescein (SNARF-1) was used as a secondary pH assessment [6]. Fluorescence was read with a Beckman-Coulter plate fluorometer using excitation at 485 nm and emission read at 595 and 625 nm. Standard curve was produced with buffered samples at pH 7.00 to 8.00 in 0.20 pH increments. The concentration of bicarbonate (i.e., conjugate base) for pH transport was estimated from the Henderson-Hasselbalch equation [7]: pH = pKa + log10 ([]/[H2CO3]) where media, in calibrated 5.1% CO2 incubators. Calibration used volume change with NaOH absorption (Fyrite instrument, Bacharach Inc., Pittsburgh PA). Media were considered to be buffered by carbonate alone, with total carbonate and bicarbonate 44.0 mM in DMEM prior to equilibration with CO2, and using pKa2 for bicarbonate <–> carbonate, 10.32, as the constant. Other medium components including phosphate (700 μM in DMEM) were not considered in this estimate.
Electron microscopy.
Transwell inserts were washed with PBS twice for 5 min. Then Transwell inserts were fixed in 2.5% glutaraldehyde-0.01M PBS for 1 hour and washed with PBS three times for 5 minutes. Scanning electron microscopy (SEM) samples were post-fixed (1% osmium tetroxide) dehydrated in a graded ethanol series and coated with gold and imaged using a JEOL JSM-6335F. For transmission electron microscopy (TEM), samples were post-fixed (1% osmium tetroxide with 1% potassium ferricyanide), dehydrated in a graded ethanol series and embedded. Ultrathin sections (65 nm) were stained with uranyl acetate, and Reynold’s lead citrate. Samples were imaged using JEOL 1400 Plus with a side mount AMT 2 k digital camera (Advanced Microscopy Techniques, Danvers, MA).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 8 (GraphPad, California). Statistical significance was determined using one-way ANOVA with Dunnett Multiple comparison or Student’s unpaired T-test. Results were considered significant if *p<0.05. All figures include at least three independent experiments and are plotted as mean ± SD.
Results
Characterization of MSCs.
Osteoblast growth and differentiation were studied in cultures of MSCs in vitro. Flow cytometry for the characterization of MSCs derived from mouse bone marrow is shown in Fig 1 for selected MSC antigens, CD44 and Sca-1. Results confirm substantial elimination of contaminating cells, with over 90% and 75% of selected cells labeling with anti-CD44 and anti Sca-1, respectively (Fig 1A and 1B).
MSCs on perforated PET membranes grow in an epithelial-like pattern that develop a high resistance while mineralizing.
MSCs were grown on the PET membranes at a density of (2 ± 0.4) × 106 pores per cm2. In Fig 1C, a SEM image of the membrane is shown to demonstrate the membrane morphology. The uniformity of the size and shape of the perforations were measured as 2.3% of membrane area. MSCs grown in proliferation medium (Fig 2A) did not form an epithelial-like layer whereas in differentiation medium (Fig 2B) cells appeared to be denser with more cellular connection. Alkaline phosphatase activity of MSCs differentiated in osteoblasts is shown in Figure 2C at the same magnification; calcified matrix appears dark on the left of the frame with alkaline phosphatase labels encircling the cells. At the edges of these cells, the membranes are orthogonal to the field, demonstrating the membrane-associated enzyme, a characteristic of osteoblasts. The deposition of minerals as an extracellular matrix was confirmed with Von Kossa (AgNO3) staining (Fig 2D). Transepithelial electrical resistance for cells in proliferation and differentiation were measured at 7, 14 and 21 days (Fig 2E). An increase of 3.4, 20.8 and 258 times the average resistance (at 7, 14, and 21 days respectively) was observed in comparison to cells in proliferation medium. The resistance increased as MSCs differentiated into osteoblasts, resulting in a significant increase at 21 days (p<0.02). Due to the high-inter-individual variability between MSCs isolated from different C57Bl/6J mice, differentiation medium at 7 and 14 days are not compared to proliferation medium.
Figure 2. MSCs on PET membranes in differentiation medium grow in an epithelial-like pattern and develop high transepithelial resistance.
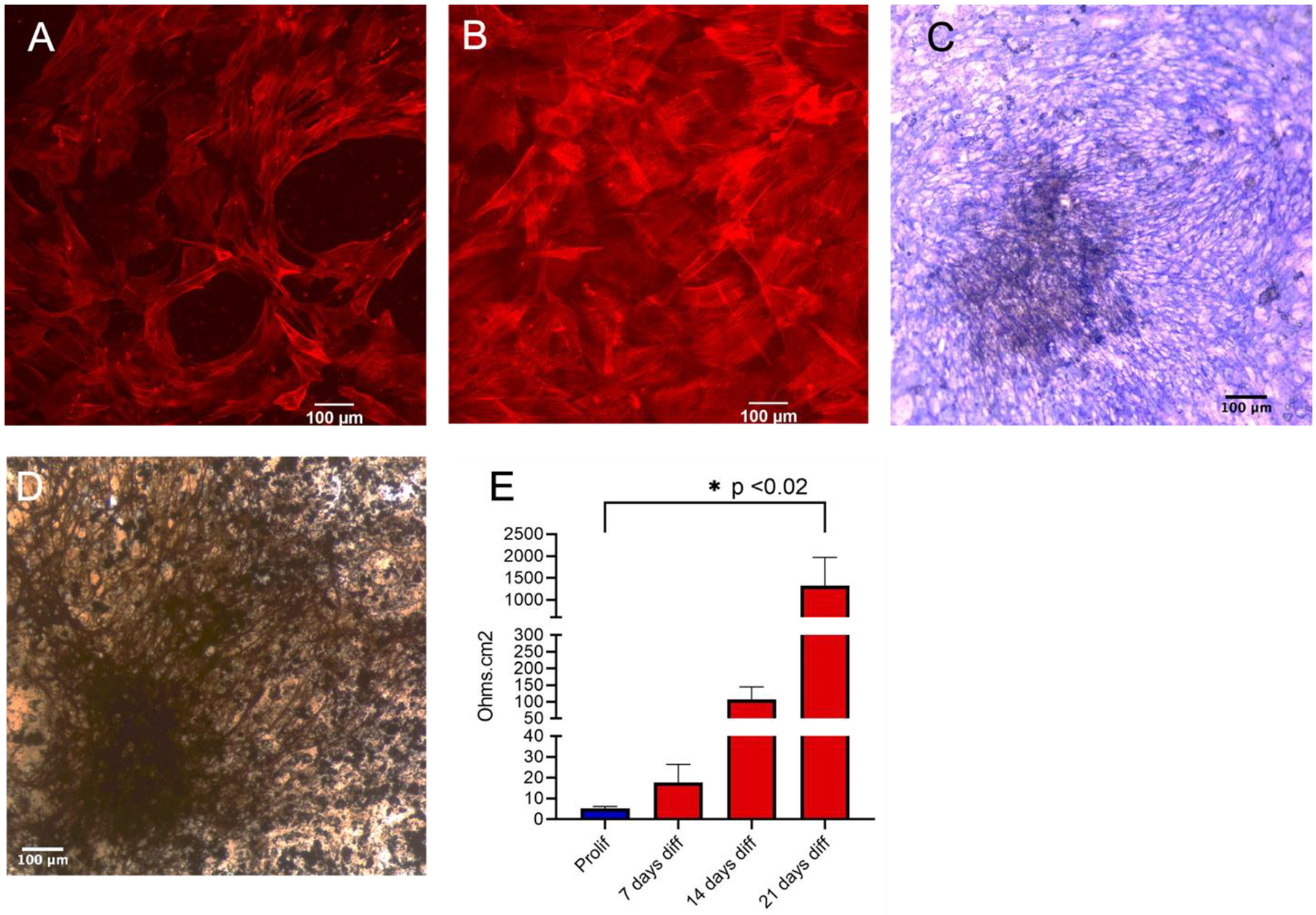
Cytoskeleton stained with phalloidin in proliferation and differentiation conditions.
A. Cells in proliferation; space between cells is noticeable. Bar=100 μm.
B. Cells in osteogenic medium; there are no visible breaks in cells and the PET membrane cannot be seen. Bar=100 μm.
C. Mineralizing osteoblasts labeled for alkaline phosphatase activity. Alkaline phosphatase labels the edges of cells, where cellular membranes are orthogonal to the field. Bar=100 μm.
D. Von Kossa staining of mineralizing osteoblasts. Bar=100 μm.
E. Transepithelial electrical resistance for cells in proliferation (blue), 7, 14 and 21 days in differentiation medium (red). Transepithelial electrical resistance increased over time. n=7, p < 0.02 between proliferation and 21 days differentiation.
MSC differentiation on PET membranes with transport of acid across membranes.
The pH was measured in medium above and below MSCs that were cultured on PET in proliferation or differentiation conditions (Fig 3). The pH value was significantly decreased (p=0.0029) above the cells in proliferation compared to differentiation media (Fig 3A). More precisely, medium above the cells acidified from 7.76 ± 0.06 in proliferation to 7.65 ± 0.03 in differentiation (0.11 pH difference), suggesting that osteoblasts on PET membranes acidify the medium above while mineralizing. The difference in pH values from the media below the cells in proliferation and in differentiation was significant (p=0.0022). The difference between the medium above the cells compared to below the cells in proliferation was significant (p=0.02) as well as in differentiation (p<0.0001), indicating the presence of a pH gradient. However, this pH gradient was 3.6 times higher in differentiation. Based on our data and calculations from the major buffer component, it is likely that the observed change in pH may be due, at least mainly, the movement of which resulted in the media below the cells to become more alkaline. According to the Henderson-Hasselbalch equation, the estimated difference from above to below the inserts was 31 μM using the carbonate PKa2 and the total carbonate (44 mM).
Figure 3. The pH difference between the media above and below cells cultured in proliferation and differentiation media.
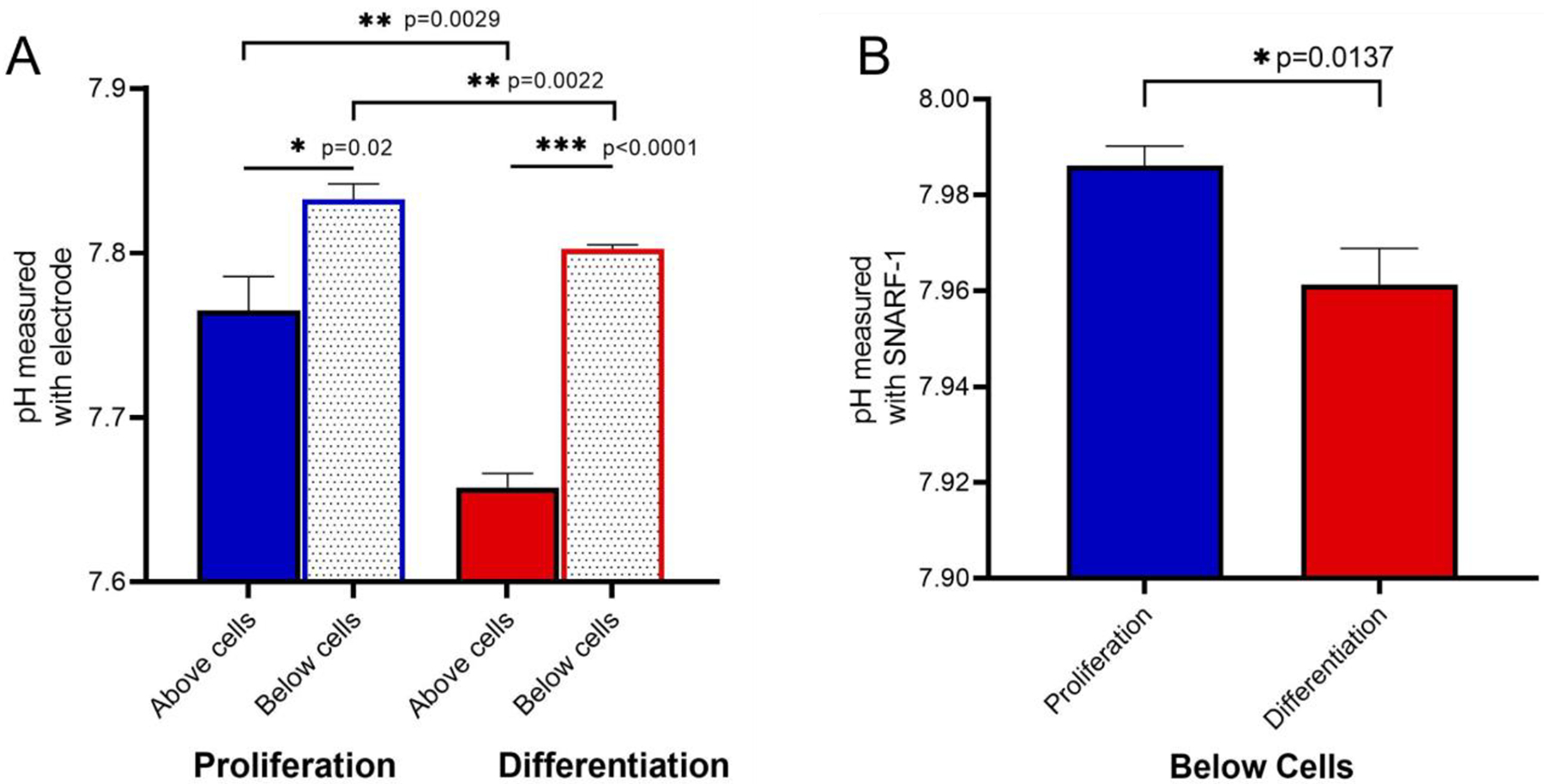
A. Direct pH measurements using semi-micro pH electrode. The medium above the differentiated osteoblasts is significantly more acidic compared to medium above cells in proliferation. N=4, p=0.0029. The pH measurements of medium below cells, after removing the inserts, in proliferation and differentiation is also more acidic in differentiation medium. N=4, p=0.0022. A significant increase of the pH value is observed in proliferation and differentiation media (p=0.02 and p<0.0001, respectively) suggesting a pH gradient.
B. pH measurements using cell-impermeant fluor SNARF-1 in proliferation and differentiation media below the cells. Significant decrease (N=4, p=0.0137) of the pH value in differentiation confirmed the acidification as observed in A. The pH was calculated based on the standard curve from the ratio of E625/595.
As a confirmatory method, fluor SNARF-1 pH indicator dye was used. Because of technical limitations to read fluorescence inside Transwells, inserts were removed, and the pH was only assessed for the media below the cells. Consistent with pH electrode measurements, the pH below the cells decreased from 7.99 ± 0.01 in proliferation to 7.96 ± 0.01 in differentiation (0.03 difference) confirming the gradient (Fig 3B).
Morphology of osteoblasts.
Cross-section of osteoblasts mineralizing on inserts were processed for TEM (Fig 4A). Multiple layers of cells are visible. Osteoblasts attached to the PET membrane (amorphous material, bottom of the frame) are highly enriched in mitochondria (white arrow) and rough endoplasmic reticulum (RER) suggesting increased metabolic activity during osteoblast differentiation and extracellular matrix production. SEM images of early osteocytes are shown in figures 4B–D. Fig 4B shows, at low magnification, layers of flattened early osteocytes above the cells adhered to the PET membrane. Attachments between cells are mostly broad, although narrow attachments are developing. At higher magnification, the collagen fibers can be distinguished from the extracellular matrix produced by the osteocytes (Fig 4C and 4D). In Figure 4C, the collagen is deposited in multiple layers at roughly 90-degree orientation to each other, characteristic of bone collagen [1]. The dotted square is magnified in figure 4D, revealing dense 40–80 nm hydroxyapatite (HAP) attached to fibrils. To show the abundant collagen more clearly, Fig 4E depicts a low power light micrograph with collagen birefringence using crossed polarizers. The image is 600 μm wide. Collagen is the only significantly birefringent biological material, displayed as abundant white material (for appearance of collagen within the cell culture, compare with Fig 2C and 2D above). The background is blue birefringence of the PET membrane.
Figure 4. Cultured osteoblasts form a cellular network and produce mineralized extracellular matrix.
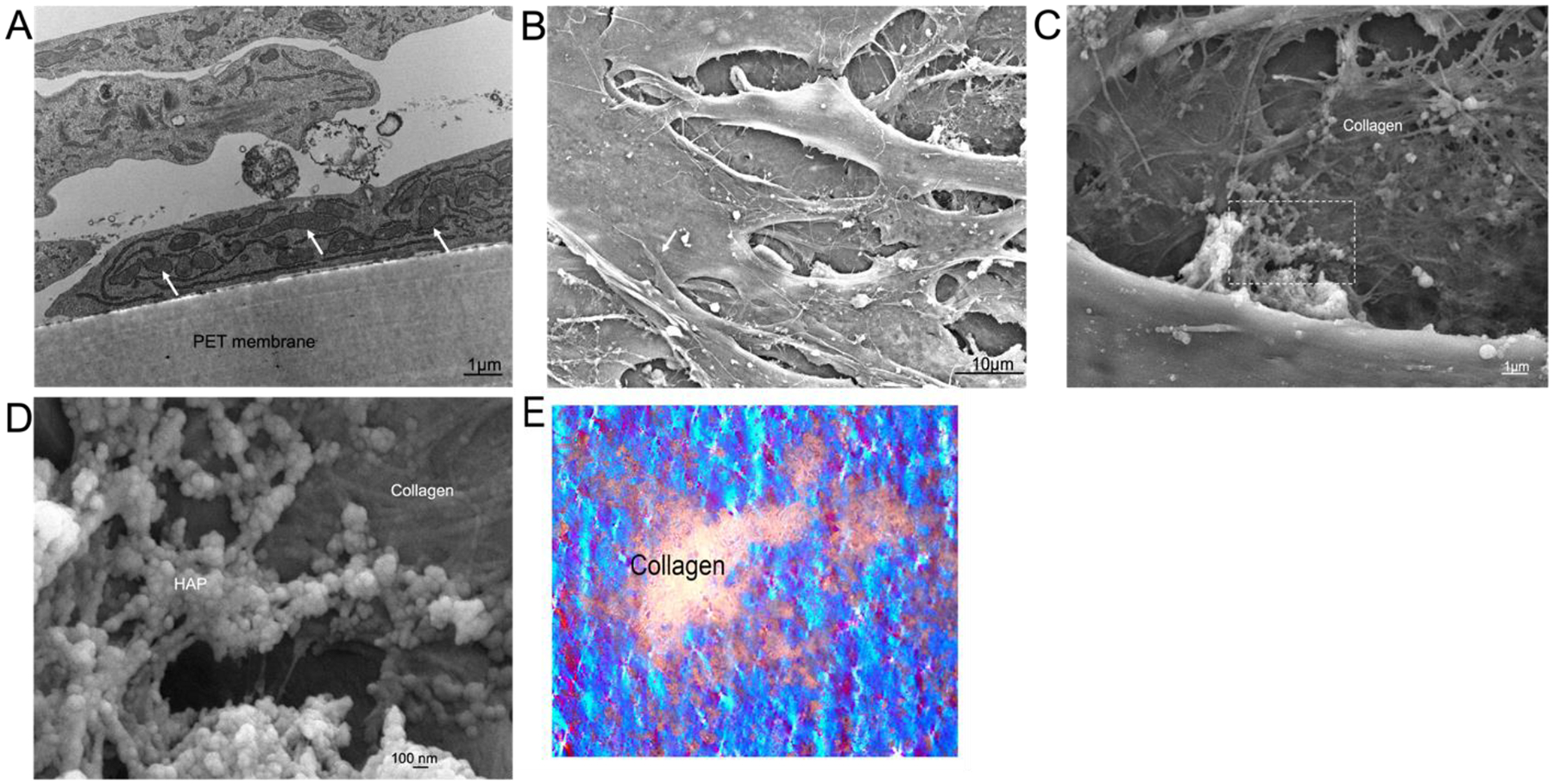
A. TEM showing osteoblasts attached to the PET membrane which are highly enriched in mitochondria and RER. Selected mitochondria are indicated (white arrows). Bar= 1 μm.
B. Layers of flattened cells above the attachment layer by SEM at low power. Cells are overlapping in irregular shapes, consistent with early osteocytes. One cell-cell attachment is indicated (white arrow); cell attachments at this stage are narrowing. The fibrillar material is extracellular collagen and hydroxyapatite. (Bar= 10 μm).
C. An intermediate power SEM of a matrix region with mineralization. Collagen is deposited in layers at rough 90-degree orientation. (Bar= 1 μm).
D. Extracellular matrix produced by the osteoblasts at high power in SEM. The fibrillar material is collagen, with dense 40–80 nm mineral attached to the fibrils. Dotted square magnified from C. (Bar= 0.1 μm).
E. Low power (20x) light micrograph showing collagen birefringence with crossed polarizers. The only biological material that is birefringent is collagen, which appears as abundant white material. The PET membranes have blue birefringence (background), and the field is 0.6 mm wide.
Discussion
Osteoblasts form an epithelial-like layer that separates the extracellular fluid from bone.
Using preparations of mouse MSCs isolated from bone marrow (validated by flow cytometry) allowed us to study osteoblast growth and mineralization in vitro by alkaline phosphatase activity and Von Kossa staining of mineral. Differentiating these cells on porous PET membranes (Fig 1C, 2A and 2B) produced an epithelial-like layer which was confirmed by a transepithelial electrical resistance greater than ~ 1,400 ohms•cm2 and a transmembrane pH gradient (Fig 2E and Fig 3). Osteoblasts express gap junctions and form tight junctions which play an important role in the regulation of ion transport across the epithelial-like layer [2]. Osteoblasts synthesize a predominately type I collagen matrix, which is mineralized by calcium and phosphate precipitation (Fig 4).
The epithelial-like nature of the surface osteoblasts has been discussed [1,2]. However, there is limited information on osteoblasts differentiating as an epithelial-like organ in vitro. To our knowledge, only one group, Wongdee et al., in 2008 showed osteoblasts as a surface membrane (on Snapwell inserts) in Boyden chambers [8]. In that case, isolated primary rat osteoblasts formed a layer with a transepithelial electrical resistance of ~100 Ω•cm2. Other results with differentiation from mesenchymal stem cells were, however, consistent with our work, although in a different context than bone differentiation [9], discussed below.
It is established that pH above 7 is necessary for hydroxyapatite precipitation [10]. Later, Chakkalakal et al. [11] demonstrate that during the earlier stages of fracture healing, tissue pH is acidic (in keeping with removal of acid produced) but later becomes more alkaline, which is positively correlated to an increase in calcium content. Galow et al. investigated the effect of alkaline pH medium on MC3T3-E1 murine osteoblasts in vitro and the maximum pH for osteoblast-like cells to proliferate was within the pH range of 8.0 to 8.4. They observed that elevated pH improved differentiation, however, mineralization was only assessed by alizarin red and extracellular mineralized matrix was not documented. Congruent with the literature, our results indicate that active osteoblasts in Transwells acidify the media due to the production of hydroxyapatite crystals (medium above the cells). Interestingly, medium below the osteoblasts on PET membranes was alkalinized, consistent with vectorial bicarbonate transport (Fig 3 and 4). This is novel and will be a subject for further study.
The mechanism of protons transported through the Transwell is not completely understood. Possible reasons for this finding include that the media used, DMEM with 1 g/l of glucose at pCO2 of 5.1%, has a high pH (between 7.8 and 8.0); the media in which mineralization occurs remains at pH ~7.6. In other words, further removal of acid would not be needed. While osteoclast acid production is well known [12], the mechanisms supporting pH gradients in osteoblasts in vitro is not established. Others noted in MC3T3-E1 cells that alkaline media promote proliferation and differentiation of osteoblast-like cells [13], consistent with our results. In addition to the report of epithelial layers of rat osteoblasts with transepithelial electrical resistance by Wongdee et al. [8], in the context of blood brain-barrier characterization, not related to osteoblast formation, epithelial differentiation from pluripotent stem cells on Transwell membranes with high transepithelial electrical resistance has been reported recently by Lu et al. [9].
Osteoblasts on the membrane retained high numbers of large mitochondria (Fig 4A), in tandem with active transport, and with synthesis of bone components including collagen. The pH gradient of the media across the membrane suggest that osteoblasts may release carbonate under the conditions studied. In the media used, osteoblast differentiation, including alkaline phosphatase activity, collagen and hydroxyapatite production, were uniformly excellent, although the pH was above physiological; in future work effect of medium pH will be studied.
In summary, our new in vitro model of osteoblasts differentiating on perforated PET membranes allows robust differentiation of osteoblasts in vitro to form bone and will be useful in understanding transport during bone matrix production. Furthermore, this model shows unequivocally the epithelial-like nature of osteoblasts and pH gradient consistent with transport of by epithelial-like layers of the cells.
Mesenchymal stem cells isolated from murine long bones in special media differentiate to form osteoblasts on porous polyethylene terphthalate membranes.
Differentiation is promoted by Dubelco’s Modified Essential Medium (DMEM) with 44 mM sodium carbonate, and pH ~7.8 over cells.
Osteoblasts form an epithelial-like layer with trans-epithelial electrical resistance of ~ 1400 Ω•cm2.
During differentiation, medium pH is alkalinized below the epithelial-like layer consistent with vectorial bicarbonate transport.
Acknowledgements
Supported in part by R01 AR076146-01 from the National Institutes of Health, USA, and by BX002490-06A1 from the Department of Veteran’s Affairs, USA and by the Center for Biological Imaging, University of Pittsburgh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].H Schlesinger P, Braddock DT, Larrouture QC, Ray EC, Riazanski V, Nelson DJ, Tourkova IL, Blair HC HC. Phylogeny and chemistry of biological mineral transport. Bone. 141 (2020): 115621. doi: 10.1016/j.bone.2020.115621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Blair HC, Larrouture QC, Li Y, Lin H, Beer-Stoltz D, Liu L, Tuan RS, Robinson LJ, Schlesinger PH, Nelson DJ. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng. B 23 (2017) 268–280. 10.1089/ten.TEB.2016.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dobrowolski SF, Tourkova IL, Robinson LJ, Secunda C, Spridik K, Blair HC. A bone mineralization defect in the Pahenu2 model of classical phenylketonuria involves compromised mesenchymal stem cell differentiation. Mol Genet Metab. 125 (2018): 193–199. 10.1016/j.ymgme.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yaroslavskiy BB, Zhang Y, Kalla SE, García Palacios V, Sharrow AC, Li Y, Zaidi MM, Wu C, Blair HC. NO-dependent osteoclast motility: reliance on cGMP-dependent protein kinase I and VASP. J. Cell Sci 118 (2005): 5479–5487. 10.1242/jcs.02655. [DOI] [PubMed] [Google Scholar]
- [5].Tourkova IL, Liu L, Sutjarit N, Larrouture QC, Luo L, Robinson LJ, Blair HC. Adrenocorticotropic hormone and 1,25-dihydroxyvitamin D3 enhance human osteogenesis in vitro by synergistically accelerating the expression of bone-specific genes. Lab Invest. 97 (2017): 1072–1083. doi: 10.1038/labinvest.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maouyo D, Chu S, Montrose MH. pH heterogeneity at intracellular and extracellular plasma membrane sites in HT29-C1 cell monolayers. Am. J. Physiol. Cell Physiol 278(2000): C973–C981. 10.1152/ajpcell.2000.278.5.C973. [DOI] [PubMed] [Google Scholar]
- [7].Howorth PJ. The physiological assessment of acid-base balance. Br. J. Dis. Chest 69 (1975): 75–102. no doi available. [PubMed] [Google Scholar]
- [8].Wongdee K, Pandaranandaka J, Teerapornpuntakit J, Tudpor K, Thongbunchoo J, Thongon N, Jantarajit W, Krishnamra N, Charoenphandhu N. Osteoblasts express claudins and tight junction-associated proteins. Histochem. Cell Biol 130 (2008): 79–90. 10.1007/s00418-008-0419-6. [DOI] [PubMed] [Google Scholar]
- [9].Strates BS, Neuman WF, Levinskas GJ, The Solubility of Bone Mineral. II. Precipitation of Near-Neutral Solutions of Calcium and Phosphate, J. Phys. Chem 61 (1957) 279–282. 10.1021/j150549a005. [DOI] [Google Scholar]
- [10].Chakkalakal DA, Mashoof AA, Novak J, Strates BS, McGuire MH, Mineralization and pH relationships in healing skeletal defects grafted with demineralized bone matrix, J. Biomed. Mater. Res 28 (1994) 1439–1443. 10.1002/jbm.820281209. [DOI] [PubMed] [Google Scholar]
- [11].Blair HC, Teitelbaum SL, Tan HL, Koziol CM, Schlesinger PH. Passive chloride permeability charge coupled to H(+)-ATPase of avian osteoclast ruffled membrane. Am. J. Physiol 260 (1991): C1315–C1324. 10.1152/ajpcell.1991.260.6.C1315. [DOI] [PubMed] [Google Scholar]
- [12].Galow AM, Rebl A, Koczan D, Bonk SM, Baumann W, Gimsa J. Increased osteoblast viability at alkaline pH in vitro provides a new perspective on bone regeneration. Biochem Biophys Rep. 10 (2017): 17–25. doi: 10.1016/j.bbrep.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lu TM, Houghton S, Magdeldin T, Durán JGB, Minotti AP, Snead A, et al. Pluripotent stem cell-derived epithelium misidentified as brain microvascular endothelium requires ETS factors to acquire vascular fate. Proc Natl Acad Sci U S A. 118 (2021): e2016950118. doi: 10.1073/pnas.2016950118. [DOI] [PMC free article] [PubMed] [Google Scholar]


