Abstract
Introduction:
Cardiotoxicities induced by cancer therapy can negatively affect quality of life and survival. We investigated whether high-sensitivity cardiac troponin T (hs-cTnT) levels could serve as biomarker for early detection of cardiac adverse events (CAEs) after chemoradiation therapy (CRT) for non-small cell lung cancer (NSCLC).
Methods:
This study included 225 patients who received concurrent platinum and taxane– doublet chemotherapy with thoracic radiotherapy to a total dose of 60–74 Gy for NSCLC. All patients were evaluated for CAEs; 190 patients also had serial hs-cTnT measurements.
Results:
Grade ≥3 CAEs occurred in 24 patients (11%) at a median interval of 9 months after CRT. Pretreatment hs-cTnT levels were higher in men, in patients ≥64 years, and in patients with pre-existing heart disease or poor performance status (P<0.05). hs-cTnT levels increased at 4 weeks during CRT (P<0.05) and decreased after completion of CRT but did not return to pretreatment levels (P=0.002). The change (delta) in hs-cTnT levels during CRT correlated with mean heart dose (P=0.0004), the heart volumes receiving 5–55 Gy (P<0.05), and tumor location (P=0.006). Risks of severe CAEs and mortality were significantly increased if the pretreatment hs-cTnT was >10 ng/L or the delta during CRT was ≥5 ng/L.
Conclusions:
Elevation of hs-cTnT during CRT was radiation heart dose–dependent, and high hs-cTnT levels during the course of CRT were associated with CAEs and mortality. Routine monitoring of hs-cTnT could identify patients who are at high risk of CRT-induced CAEs early to guide modifications of cancer therapy and possible interventions to mitigate cardiotoxicity.
Keywords: NSCLC, troponin, cardiac adverse event, chemoradiotherapy
Introduction
Cardiac toxicity has long been observed as an adverse effect of anti-cancer therapies. Thoracic irradiation significantly increases the risk of cardiovascular disease among cancer survivors; long-term survivors of Hodgkin lymphoma and childhood cancers have 2- to >7-fold increases in risk of cardiac death after receipt of 30–40 Gy.1,2 Patients undergoing radiation therapy for breast cancer showed a 27% excess of heart disease over those who did not receive radiation.3,4 Cardiac toxicity is also well known as an adverse reaction to radiotherapy for NSCLC,5 with a reported incidence of symptomatic cardiac events as high as 28.6% that can manifest as early as 3 months after chemoradiation.6 A recent study of 748 patients with NSCLC7 showed that 2-year cumulative incidences were 5.8% for major CAEs and 23.3% for grade ≥3 CAEs, suggesting that CAEs were common in NSCLC and occurred earlier than had previously been understood. This stands in contrast to patients given radiation therapy for breast cancer or lymphoma, in whom most cardiac toxicity occurs years after treatment.8 The risk of cardiac death has been linked with mean cardiac dose and is estimated to increase by 3% per Gy administered.4 Cardiac events and overall survival (OS) are associated with the doses to the heart subvolumes9,10 and doses to specific anatomic cardiac substructures.11,6 Radiation can damage any substructure of the heart and can manifest as pericarditis, pericardial fibrosis, myocardial fibrosis, coronary artery disease, microvascular damage, and valvular stenosis,12–14 which can all eventually lead to myocardial damage.2
Lung cancer remains the leading cause of cancer death worldwide15 even though breakthrough advances have been made in the treatment of inoperable, stage II-IIIB locally advanced, non-small cell lung cancer (NSCLC) by using immune checkpoint inhibitors; the median survival time was extended to 41 months with the addition of adjuvant durvalumab (an antibody against programmed death-ligand 1 [PDL1]).16,17 As survival times become longer, manifestations of cardiac adverse events have become issues that warrant clinical and translational intervention for prevention and mitigation. To do so, risk stratification and early detection of patients at high risk of developing cardiotoxicity before the clinical manifestation of heart damage, optimally before or during treatment (to afford opportunity for therapy modification), is a first step. High-sensitivity cardiac troponin assays have been recommended for routine clinical use for the diagnosis of myocardial infarction.18–21 Cardiac troponins I and T are the preferred biomarkers for evaluating myocardial injury because they are expressed almost exclusively by the heart. Their sensitivity and specificity are higher than those of other biomarkers, e.g., creatine kinase MB isoform.21–26
In this report of a prespecified secondary subgroup analysis, we evaluated whether high-sensitivity troponin T (hsTnT) would provide prognostic value in risk stratification for toxicity and OS, and if this biomarker assessed during the course of treatment can facilitate early detection of cardiac events occurs after CRT. The aims of the current study were to (1) evaluate the incidence of symptomatic CAEs; (2) investigate the dynamics of hs-cTnT levels in serum before, during, and after CRT and potentially associated factors; and (3) assess potential associations between hs-cTnT levels and CAEs and OS.
Methods
Patients and follow-up
This report represents a secondary analysis of a completed prospective randomized trial comparing intensity-modulated (photon) radiation therapy (IMRT) with passive scattering proton therapy (PSPT) for NSCLC (NCT009105005), a trial approved by our institutional review board. Details of the trial design, inclusion criteria, treatment, and radiation dosimetric planning are reported elsewhere.27 Of the total 272 patients who signed informed consent to enroll in the prospective randomized trial, 225 patients received a radiation dose of at least 60 Gy and were included for evaluation of CAEs in this analysis. Among these 225 patients, 190 patients also signed informed consent to donate blood samples for correlative studies (Suppl. Fig. S1).
Smoking history and cardiac history were obtained as part of a comprehensive systems review at registration. Pre-existing cardiac disease was documented prospectively at enrollment and included angina, congestive heart failure, coronary bypass surgery, hypertension, myocardial infarction, stroke, or transient ischemia attack. The first follow-up visit was at 1–3 months after CRT, followed by visits every 2–4 months for the first 2 years, every 6 months for the next year, and then annually thereafter.
CAEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5, and included arrhythmia, acute coronary syndrome, pericarditis, cardiomyopathy, ventricular dysfunction, cardiac arrest, and congestive heart failure. Stable pre-existing cardiac comorbidities or CAEs attributable to other medications were not counted as cancer therapy–induced events. If several CAEs occurred in one patient, the highest-grade event was used in the analysis. All CAEs were reviewed by three independent physicians, one of whom is a cardiologist.
Treatments
Details of radiation treatment, including planning and radiation dose metrics by treatment modality, and chemotherapy were described in the primary report of the trial.27 The current secondary analysis included both randomized and non-randomized patients. Patients were treated with radiation to a dose of 60–74 Gy or Gy(RBE) [the unit of absorbed dose for protons] in daily 2-Gy(RBE) fractions to the planning target volume. All patients received platinum and taxane– based doublet chemotherapy delivered concurrently with either IMRT or PSPT to the thorax. Cardiac dose constraints were (volume [%] receiving more than a threshold dose of radiation, Vdose) V30 Gy <50%, V45Gy <40%, and V60 Gy <20%.
Cardiac contouring and dose-volume histogram variables
The heart and its substructures were contoured retrospectively on treatment-planning CT images according to the RTOG atlas28 and reviewed by board-certified radiation oncologists. Cardiac dosimetric variables for the whole heart, pericardium, and the 4 chambers (left and right atria and left and right ventricles) were extracted from the treatment planning system for each patient; these variables included mean dose, maximum dose, and V5 Gy to V70 Gy in increments of 5 Gy.
Blood samples
Blood samples for hs-cTnT analysis were collected within 2 months before CRT, 2–3 times during CRT, and at the first follow-up visit (i.e., at 4–12 weeks after CRT). Among the 190 patients who consented to provide blood samples, 158 provided samples at baseline (before treatment), 181 patients provided samples during CRT, and 143 patients provided samples after CRT (Suppl. Fig. S1). Serum was separated within 2 hours after collection and stored at –80°C for testing and long-term storage. The hs-cTnT concentrations in serum were measured in duplicate with an Elecsys® TnT Gen 5 STAT assay (Roche Diagnostics, Indianapolis, IN, USA) on a Cobas e411 analyzer.
Statistical analysis
Severe CAEs (grade ≥3) and OS were two clinical endpoints of this analysis. The Kaplan-Meier method29 was used to estimate the time to CAEs and OS, with subgroup estimates compared with log-rank tests. Linear mixed-effect regression was used to model the longitudinal measurements of hs-cTnT in individual patients and to compare differences in hs-cTnT between CAE groups, accounting for associations between repeated measurements in the same subjects. Random intercepts were included in the mixed-effect model, and timepoints were represented by indicator variables corresponding to hs-cTnT at baseline, weekly during CRT, and at follow-up; this approach made it possible to detect trends in average hs-cTnT that were nonlinear in time. The Kruskal-Wallis test was used to compare hs-cTnT baseline and delta during treatment (i.e., peak minus baseline) with clinical characteristics. Spearman correlation analysis was used to analyze correlations between heart dose variables and hs-cTnT delta. Univariate and multivariate Cox proportional hazard models30 were used to analyze potential associations of hs-cTnT with CAEs and OS. Clinical covariates underwent stepwise forward selection (P<0.1 for entry). The optimal cutpoints for hs-cTnT baseline, peak during CRT, delta, and after-treatment levels for risk of grade ≥2 CAEs were tested in the inner 90% distribution and selected by the method proposed by Contal and O’Quigley,31 which is based on the univariate log-rank test statistic. Statistical analyses were done with SAS 9.4 (SAS Institute, Cary, NC). Statistical significance for all analyses was set at a two-sided α of 0.05.
Results
Patient baseline characteristics
Baseline characteristics of the patients included in this study are listed in Table 1. The median age of the 119 men (53%) and 106 women (47%)was 66 years (interquartile range [IQR] 58–71). Most patients (91%) were white, had previously or currently smoked (92%), had stage III disease (83%), had good performance status (Karnofsky Performance Status [KPS] score of ≥80; 88%), and did not have pre-existing heart disease (81%). Forty-three patients had pre-existing heart conditions, including angina (n=5), congestive heart failure (n=3), coronary angioplasty (n=19), coronary bypass surgery (n=9), myocardial infarction (n=15), and transient ischemic attack (n=9). Radiation doses (range 60–74 Gy) were delivered with IMRT to 137 patients (61%) or with PSPT to 88 patients (39%). The median mean heart dose was 12.0 Gy (IQR 6.7–19.5 Gy). The median follow-up time was 26.2 months (IQR 12.1–53.1 months) after radiation therapy.
Table 1.
Patient and Treatment Characteristics and hs-cTnT Comparisons
| Characteristics | No. of Pts (n=225, %) | hs-cTnT baseline (n=158) |
hs-cTnT delta (n=149) |
||
|---|---|---|---|---|---|
| mean (SD) | P | mean (SD) | P | ||
| Age | <0.0001 | 0.683 | |||
| <64 years | 90 (40) | 5.1 (3.7) | 3.0 (4.7) | ||
| ≥64 years | 135 (60) | 10.2 (11.7) | 4.1 (11.3) | ||
| Sex | <0.0001 | 0.455 | |||
| Female | 106 (47) | 6.3 (9.1) | 3.1 (6.5) | ||
| Male | 119 (53) | 9.9 (10.0) | 4.3 (11.6) | ||
| Race | 0.848 | 0.282 | |||
| White | 204 (91) | 8.0 (9.5) | 3.8 (9.4) | ||
| Other | 21 (9) | 10.1 (12.6) | 2.5 (9.1) | ||
| Disease Stage | 0.902 | 0.902 | |||
| II | 18 (8) | 10.7 (11.4) | 3.7 (6.1) | ||
| IIIA | 91 (40) | 7.6 (8.9) | 2.2 (3.9) | ||
| IIIB | 92 (41) | 8.5 (10.1) | 4.6 (12.8) | ||
| IV | 10 (5) | 11.3 (16.8) | 7.3 (14.6) | ||
| Recurrent | 14 (6) | 5.8 (3.5) | 3.9 (7.3) | ||
| Tumor Histology | 0.065 | 0.428 | |||
| Adeno | 118 (52) | 7.4 (8.3) | 4.5 (11.6) | ||
| Squamous | 76 (34) | 7.9 (9.0) | 3.2 (3.7) | ||
| Other | 31 (14) | 12.5 (15.2) | 1.3 (8.1) | ||
| Gross Tumor Volume* | 0.191 | 0.651 | |||
| ≤126.5 cm3 | 147 (65) | 7.4 (7.6) | 4.2 (10.6) | ||
| >126.5 cm3 | 77 (34) | 10.0 (13.1) | 2.5 (6.1) | ||
| Tumor Location | 0.999 | 0.006 | |||
| Left/ Mediastinal | 90 (40) | 7.0 (6.2) | 5.9 (12.1) | ||
| Right | 130 (58) | 9.1 (11.7) | 2.1 (6.6) | ||
| Other | 5 (2) | 5.6 (1.5) | 2.3 (2.7) | ||
| KPS | 0.025 | 0.762 | |||
| <80 | 26 (12) | 17.1 (20.2) | 1.8 (9.4) | ||
| ≥80 | 199 (88) | 7.2 (7.3) | 3.9 (9.3) | ||
| Smoking Status | 0.334 | 0.889 | |||
| Never | 18 (8) | 7.0 (5.9) | 4.0 (6.5) | ||
| Previous | 159 (71) | 8.7 (9.6) | 3.3 (6.1) | ||
| Current | 48 (21) | 7.3 (11.5) | 4.6 (16.3) | ||
| Pre-existing heart disease | 0.0008 | 0.554 | |||
| No | 182 (81) | 7.6 (9.7) | 3.8 (10.0) | ||
| Yes | 43 (19) | 11.1 (9.8) | 2.8 (5.7) | ||
| Modality | 0.622 | 0.088 | |||
| PSPT† | 88 (39) | 9.3 (11.6) | 3.5 (13.0) | ||
| IMRT | 137 (61) | 7.5 (8.3) | 3.8 (5.8) | ||
Abbreviations: Pts, patients; hs-cTnT, high-sensitivity cardiac troponin T; KPS, Karnofsky performance status score; PSPT, passively-scattered proton therapy; IMRT, intensity-modulated (photon) radiation therapy.
One patient had no information on gross tumor volume.
One patient was grouped in PSPT but was treated with 34 Gy PSPT first and 38 Gy IMRT later
Cardiac events
Among all 225 patients evaluated for CAEs, such CAEs occurred in 57 patients (25%): 24 (10.7%) experienced grade 1 CAEs, 9 (4%) experienced grade 2 CAEs, and 24 (10.7%) experienced grade ≥3 CAEs. Among the 24 patients with grade ≥3 CAEs, 10 (4.4%) were grade 3, 5 (2.2%) were grade 4, and 9 (4.0%) were grade 5 (Table 2). The most common grade ≥3 CAEs were atrial fibrillation/flutter (n=9), followed by cardiac arrest (n=5), ischemia/myocardial infarction (n=4), and others. One event of grade 3 cardiomyopathy reported at 10 months after radiation therapy was attributed to the use of a MEK/AKT inhibitor. The cumulative incidences of CAEs and mortality are shown in Figure 1. The median time to grade ≥3 CAEs was 9 (IQR 3.2–18.8) months after CRT. By 24 months after the start of CRT, 80% of the grade ≥3 CAEs had occurred, with an incidence rate of 8%. No difference in incidence of CAEs was found between IMRT- and PSPT-treated patients (p=0.20, Suppl. Figure S2).
Table 2.
Type and Severity of Cardiac Adverse Events*
| Cardiac adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 3–5 |
|---|---|---|---|---|---|---|
| Atrial fibrillation/flutter | 1 | 3 | 7 | 2 | 9 | |
| Myocardial infarction | 4 | 2 | 1 | 1 | 4 | |
| Cardiomyopathy | 1 | 1 | 1 | |||
| Cardiac arrest | 1 | 4 | 5 | |||
| Congestive heart failure | 2 | 1 | 3 | |||
| Pericarditis | 1 | 1 | 1 | |||
| Tachycardia or bradycardia | 23 | 0 | ||||
| Left ventricular systolic dysfunction | 1 | 1 | ||||
|
| ||||||
| Subtotal, no. (%) | 24 (10.7) | 9 (4) | 10 (4.4) | 5 (2.2) | 9 (4) | 24 (10.7) |
A total of 57 events occurred among 225 patients.
Fig. 1.
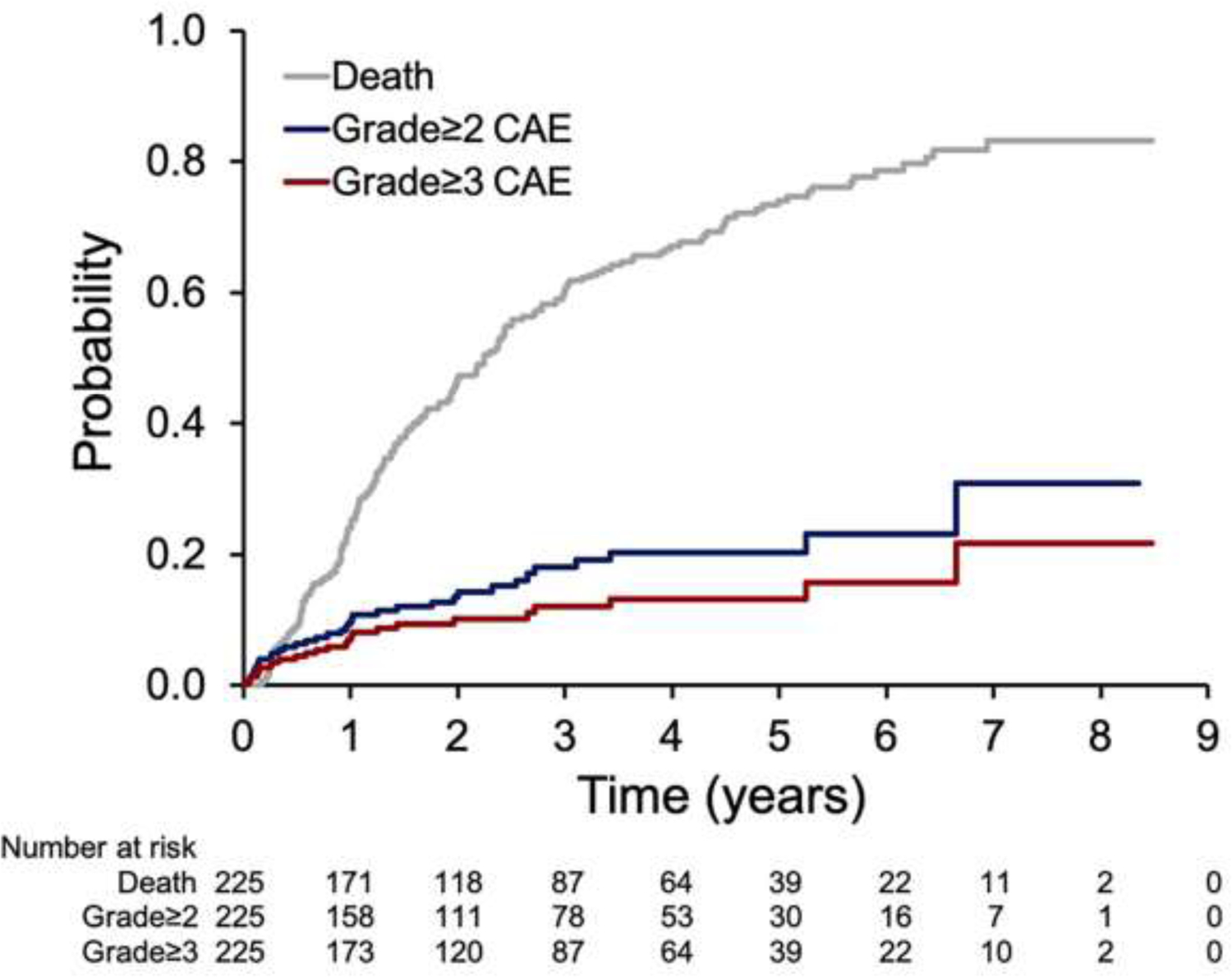
Cumulative incidences of death and cardiac adverse events (CAEs).
Elevation of cardiac troponin T
Mean (±SEM) serum hs-cTnT level was 8.2±0.96 ng/L at baseline, 9.4±0.43 ng/L during CRT, and 10.4±0.74 ng/L at first follow-up. hs-cTnT baseline and delta values (from baseline to during treatment) are listed by patient characteristics in Table 1. hs-cTnT baseline levels were higher in men (P<0.0001), in patients ≥64 years (P<0.0001), and in patients with pre-existing heart disease (P=0.0008) or poor KPS (P=0.025). The hs-cTnT delta during CRT was related to tumor location (right vs. left): on average, hs-cTnT level increased by 2 ng/L during CRT for patients with right-sided tumors and increased by 6 ng/L for patients with mediastinal or left-sided tumors (P=0.006). Changes in serum hs-cTnT levels over time from before CRT to the first follow-up after CRT were analyzed by linear mixed-effects regression [Suppl. Figure S3A]. Mean hs-cTnT levels were significantly increased from baseline starting at week 4 of CRT to the end of CRT (P<0.05) but did not return to pretreatment levels at the first follow-up visit (P=0.002). At the end of CRT and at the first follow-up, hs-cTnT levels were higher in patients who developed grade ≥3 CAEs relative to patients with grade <3 CAEs with P values of 0.019 (Suppl. Figure S3B). The hs-cTnT delta during CRT was correlated with mean heart dose (P=0.0004) and with heart volumes receiving 5–55 Gy (V5–V55, P<0.05), as shown by Spearman correlation (Suppl. Table S1). Similar correlations were found between hs-cTnT delta and dose-volume variables for the pericardium, left atrium, left ventricle, and right ventricle. However, for the volumes that received high doses (V60-V70), only the left atrium or left ventricle correlated with hs-cTnT delta (Suppl. Table S1).
Association between hs-cTnT and cardiac events and survival
Univariate Cox regression analysis (Suppl. Table S2) showed that hs-cTnT levels (as a continuous variable) before CRT, and peak level during CRT, were significantly associated with grade ≥3 CAEs and OS. Optimal cutpoints for hs-cTnT baseline, peak, delta, and after CRT were then identified for grade ≥3 CAEs. The cutpoints were 10 ng/L for baseline hs-cTnT, 16 ng/L for peak hs-cTnT, 12 ng/L for hs-cTnT after CRT, and 5 ng/L for hs-cTnT delta.
Multivariate Cox regression analysis was then used to assess associations between hs-cTnT levels at all timepoints (categorized by the optimal cutpoints) and CAEs and OS, with adjustment for clinical covariates. The clinical covariates considered in multivariate models and subjected to stepwise selection included age, sex, race, disease stage, tumor histology, gross tumor volume, tumor location, KPS, smoking status, pre-existing heart disease, receipt of induction or adjuvant chemotherapy, radiation modality, total delivered radiation dose, mean heart dose, and mean lung dose. As shown in Table 3, hs-cTnT baseline and delta were independent risk factors for grade ≥3 CAEs and mortality. According to this analysis, the risk of grade ≥3 CAEs was increased, with a hazard ratio (HR) of 4.06 (P=0.021), if hs-cTnT baseline was >10 ng/L; risk of grade ≥3 CAEs was also increased, with a HR of 3.57 (P=0.009), if the hs-cTnT delta was ≥5 ng/L during CRT. Moreover, elevated hs-cTnT before and during treatment also predicted poor OS, with respective HRs of 1.95 (P=0.002) and 1.99 (P=0.013) (Table 3).
Table 3.
Multivariate Cox Proportional Hazard Regression Analysis for Association of High-Sensitivity Cardiac Troponin T and Grade ≥3 Cardiac Adverse Events and Overall Survival
| Variables | Grade ≥3 Cardiac Adverse Events |
Overall Survival | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| HR | 95% CI | P | HR | 95% CI | P | |
| hs-cTnT | ||||||
| Baseline >10 ng/L | 4.06 | 1.52–10.88 | 0.005 | 1.95 | 1.28–2.96 | 0.002 |
| delta >5 ng/L | 3.57 | 1.37–9.29 | 0.009 | 1.99 | 1.16–3.42 | 0.013 |
| Tumor location | ||||||
| Left/Mediastinum | Ref | |||||
| Right | 1.72 | 1.06–2.78 | 0.028 | |||
| Other | 1.63 | 0.47–5.63 | 0.442 | |||
| Stage | ||||||
| II-IIIA | Ref | |||||
| IIIB | 0.22 | 0.06–0.78 | 0.019 | |||
| IV, recurrent | 0.45 | 0.06–3.55 | 0.446 | |||
| Total dose, Gy | 0.95 | 0.91–1.00 | 0.033 | |||
| Radiation technique | ||||||
| Photon vs Proton | 0.48 | 0.31–0.76 | 0.002 | |||
Abbreviations: HR, hazard ratio; CI, confidence interval; hs-cTnT, high-sensitivity cardiac troponin T.
Note: Clinical covariates evaluated in multivariate models include age, sex, race, disease stage, tumor histology, gross tumor volume, tumor location, Karnofsky performance status score, smoking status, pre-existing heart disease, induction and adjuvant chemotherapy, treatment modality, total delivered dose, mean heart dose, and mean lung dose, with an inclusion criteria threshold of 0.1.
The cumulative incidence of grade ≥3 CAEs and Kaplan-Meier estimates of OS stratified by cutoffs of hs-cTnT baseline and delta are plotted in Figure 2. Risks of CAEs and mortality were further stratified by combining the two high-risk factors: hs-cTnT baseline >10 ng/L and delta ≥5 ng/L during CRT (Fig. 3). Patients with neither of these high-risk factors (i.e., 0 risk factors) had the lowest risk of grade ≥3 CAEs (13%) or death (median OS time 39 months) compared with patients with 1 or 2 risk factors. Among patients with both high-risk factors, 45% developed grade ≥3 CAEs and had a median OS time of 14 months. Patients with 1 risk factor fell between these two groups, with 13% grade ≥2 CAEs and median OS time of 21 months, even though the risk of death was not significantly different for patients with 1 vs. 2 risk factors (P=0.129).
Fig. 2.
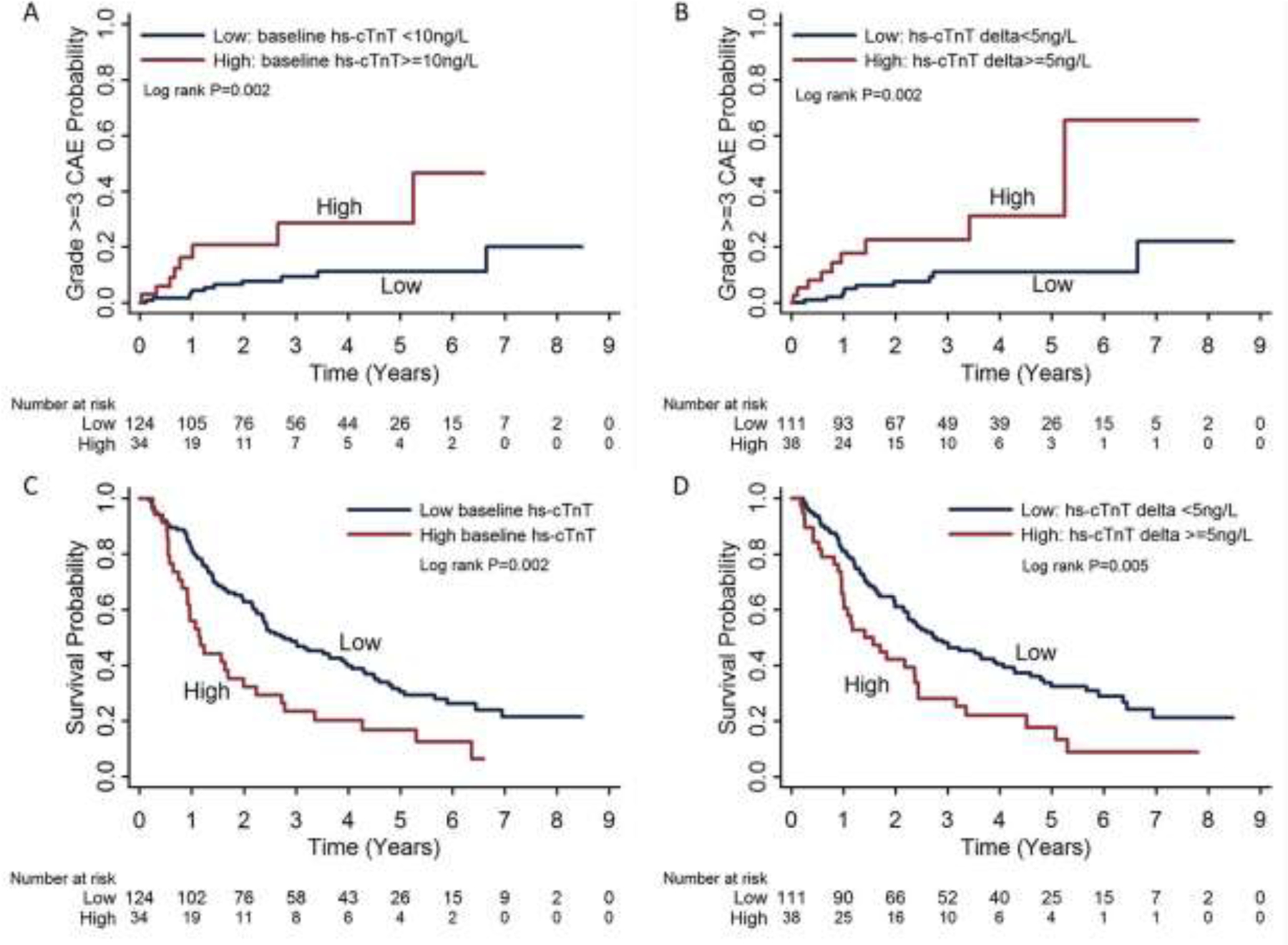
Risks of grade ≥3 cardiac adverse events (A and B) and overall survival (C and D) stratified by serum high-sensitivity cardiac troponin T (hs-cTnT) levels: baseline hs-cTnT levels <10 ng/L vs. ≥10 ng/L and hs-cTnT delta (peak minus baseline) during chemoradiation therapy <5 ng/L vs. ≥5 ng/L.
Fig. 3.
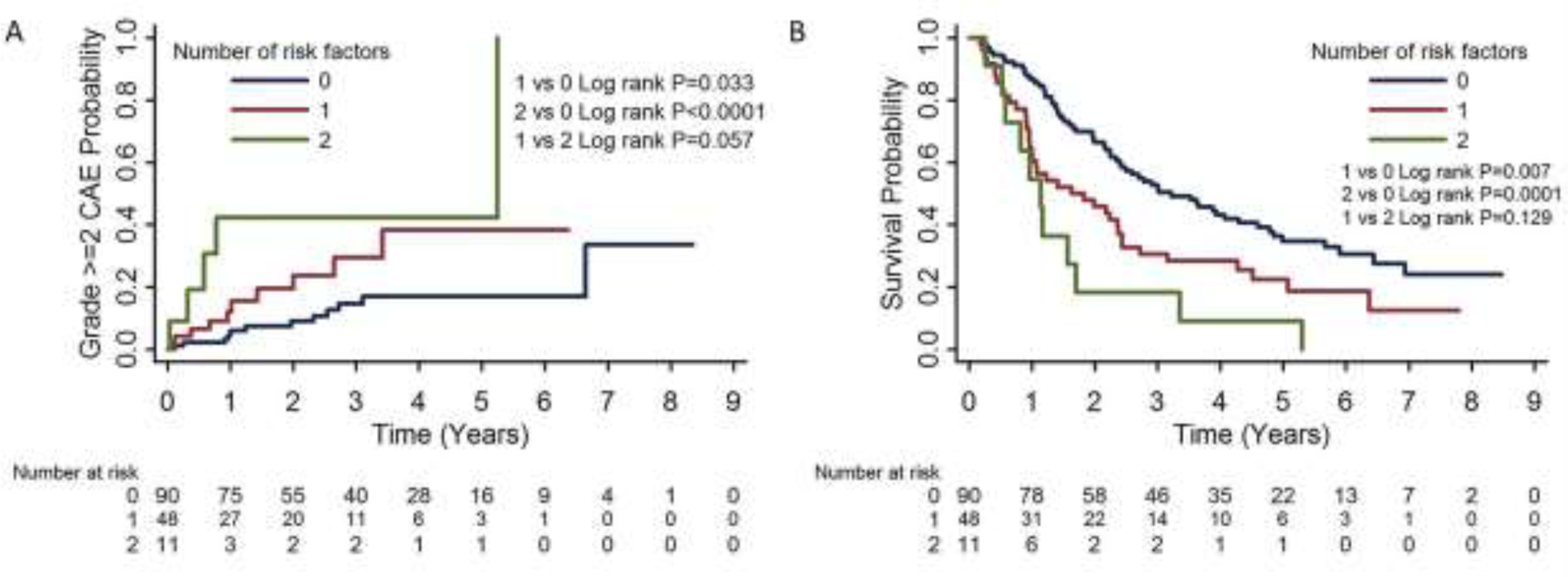
Risk stratification of grade ≥2 cardiac adverse events (CAEs) (A) and survival (B) by number of risk factors. High-risk factors were baseline hs-cTnT levels ≥10 ng/L and hs-cTnT delta during chemoradiation therapy ≥5 ng/L.
Discussion
Our investigation of cardiotoxicity and cardiac troponin levels in NSCLC patients revealed several significant findings. First, the incidence of CAEs was quite high, and most occurred within 24 months after the start of CRT, suggesting that cardiotoxicity is an early complication aafter treatment for NSCLC (73% of the cumulative incidence of grade ≥3 events occurred at a median 9 months after the start of CRT). Second, we found that serum hs-cTnT levels at any time [before CRT (10 ng/L), during CRT (peak 16 ng/L or increase of 5 ng/L), or after CRT (12 ng/L)] were associated with CAE and OS and therefore might be helpful to stratify patients for risk of CAEs. hs-cTnT levels at baseline and the extent of elevation during CRT were two independent risk factors for CAEs and mortality; specifically, patients who had high baseline and large increases in hs-cTnT during CRT were at the highest risk for CAEs. Third, we found links between radiation dose-volume metrics and change in hs-cTnT levels during CRT, with mean heart dose showing the strongest correlation. We did not find any difference in incidences of CAEs between IMRT vs. PSPT, a finding that may be explained by larger volumes in the high dose region (V40–V70 Gy) even though the mean heart dose was lower with PSPT (P >0.05, not shown).
The incidence of CAEs reported here is similar to that of a multicenter prospective study reported by Dess et al,32 which showed a 24-month cumulative incidence of grade ≥3 cardiac events of 11%, with the events occurring at a median 11 months after treatment. However, a pooled analysis reported by Wang et al9,33 demonstrated an even higher cumulative incidence of symptomatic (grade ≥2) cardiac events of 23% at a median time of 26 months. Differences in radiation dose, treatment modalities, and length of follow-up may underlie these differences in rates of cardiotoxicity events. Specifically, the radiation dose ranges in our study (60‒74 Gy in 2-Gy fractions) were well within the dose range used in the multicenter trial (47–96 Gy) and generally below that in the pooled analysis (70‒90 Gy); the treatment modalities were either IMRT or PSPT in the current study, but were 3D conformal radiation therapy (3DRT) or IMRT in the multicenter trial, and 3DRT only in the pooled analysis. The median follow-up times were 26 months in our study, 23 months in the multicenter study, and 8.8 years in the pooled analysis. Recently, Atkins et al7 reported a cumulative incidence of grade ≥3 cardiac events at 2 years of 23%, slightly higher than that in our study. The difference might be due to fact that most patients in the Atkins et al. study were treated with 3DRT (78%), and cardiac dose constraints were not adopted before 2008 (36%).
Several cardiac biomarkers have been tested in clinical settings. Biomarkers of cardiac injury, including hs-cTnT, brain natriuretic peptide (BNP), and C-reactive protein (CRP), also predict a higher likelihood of myocardial ischemia. Several studies have shown that hs-cTnT assays enable the early detection and prediction of chemo- or radiotherapy-induced cardiac toxicity.34–37 Among cancer patients receiving radiotherapy, a meta-analysis suggested that BNP could be useful as a biomarker of cardiac damage.38,39 Another marker of cardiac damage from inflammation and ischemia is CRP, which has been shown to effectively monitor trastuzumab-induced cardiac toxicity in early-stage breast cancer40 and is associated with severity of thoracic radiotherapy-induced cardiomyopathy.41 Two other putative biomarkers, placental growth factor (PIGF) and growth differentiation factor (GDF)-15 were not tested in the current study because serum sample size was limited.
Many groups have explored and been unable to define the predictive value of using cardiac biomarkers (e.g., cardiac troponin, CRP, NT-proBNP) for early detection of cancer therapy–induced cardiac toxicity.19,42–45 These studies usually involved small numbers of patients with quite heterogenous thoracic disease19,42,43 as well as lower prescribed radiation doses; for example, in the Kuo study, only 13 of 30 patients had NSCLC, and the radiation dose was only 50.4 Gy. Other studies involved patients with breast cancer, for whom cardiac structures can be avoided in radiation treatment planning. Reported cardiac exposures associated with treatment of breast cancer are usually quite low, and the total dose is lower than that typically used for definitive concurrent chemoradiation for NSCLC.43–45 Interestingly, both De Sanctis43 and Palumbo45 reported finding no elevation in longitudinal measurements of NT-proBNP levels or any association with cardiac toxicity in prospective studies of patients with early breast cancer; Palumbo reported finding a strong correlation between the normalized NT-proBNP and cardiac dosimetry. In another study, cardiac radiation dosimetric parameters correlated strongly with DGF-15 and PIGF levels during treatment for patients with lung cancer.46
hs-cTnT has been well established as a quantitative marker of myocardial injury.18,19,47–49 Cardiac troponin elevation may indicate the use of enalapril for cardioprotection in anthracycline-induced cardiotoxicity according to one study50 and has shown high diagnostic accuracy for major CAEs after immune checkpoint-inhibitor therapy at discharge.51 Currently, the American Society of Clinical Oncology recommends screening for cardiotoxicity by 2D echocardiography if the prescribed radiation dose is ≥30 Gy when the heart is in the treatment field.52 Our study suggests that hs-cTnT may be used as a biomarker in addition to imaging for predicting CAEs associated with radiotherapy.
Our finding of a heart radiation dose–dependent increase in this biomarker is consistent with a report by Darby et al,8 who found that rates of major coronary events increased linearly with the mean dose to the heart by 7.4% per Gy (95% confidence interval, 2.9 to 14.5; P<0.001), with no apparent threshold. In that study, only major coronary events (i.e., myocardial infarction, coronary revascularization, or death from ischemic heart disease) were included. With our current results, we are working to develop a predictive model to clarify the relationship of heart radiation dose and hs-cTnT as well as various cardiac events. A prospective study monitoring the continuum of hs-cTnT levels through the course of treatment and follow-up, coupled with functional assessment of the heart, will be crucial to verify the predictive power of hs-cTnT for radiation-induced cardiac injury and to screen patients for high risk of CAEs. This would be a first step toward development of individualized strategies to modify cancer therapy by reducing the radiation dose to the heart and developing medical intervention for mitigation and prevention of CAEs.
The strengths of our study are that the clinical outcome and biomarker data were prospectively collected, and it is the first to report radiation dose–dependent dynamics of serum hs-cTnT during radiation in patients with lung cancer; this is also the first study to evaluate the predictive role of hs-cTnT for radiation-induced CAEs and mortality in patients with lung cancer. Among the limitations of our study are that measuring hs-cTnT only 2 or 3 times during CRT may not reveal the true peak in hs-cTnT. Also, we did not obtain blood samples at the time at which CAEs occurred. Third, patients had cardiac imaging or functional examination only when medically indicated, and the lack of prospective systematic cardiac testing (e.g., electrocardiography) for all study subjects may have led to under-detection of subclinical, asymptomatic events. Therefore, our findings should be considered hypothesis-generating and must be validated in a larger prospective study with weekly hs-cTnT measurements.
In conclusion, we found that most clinically significant CAEs among patients given definitive CRT for locally advanced NSCLC developed within 24 months after treatment; that the extent of change in hs-cTnT levels during CRT depended on heart dose; and that hs-cTnT was a sensitive quantitative biomarker associated with radiation-induced symptomatic CAEs and OS. Because hs-cTnT is a well-established cardiac-specific biomarker in clinical practice, its use could be expanded to radiation oncology as a routine test to monitor cardiac toxicity, especially for patients receiving thoracic radiation when heart exposure cannot be avoided. A prospective trial is currently being developed to confirm our findings.
Supplementary Material
Acknowledgements
The high-sensitivity cardiac troponin (hs-cTnT) reagents were provided by Roche Diagnostics, Indianapolis, IN, USA as part of a research agreement. The funding agencies and Roche Diagnostics played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr. Zhongxing Liao and Dr. Ting Xu had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors are extremely grateful for the expert editorial work performed by Christine Wogan, MS, ELS, of MD Anderson’s Division of Radiation Oncology.
Conflict of Interest Statement for All Authors
Zhongxing Liao received support from the US National Cancer Institute (grants 1R21 CA222749–01A1, 5 P01 CA021239, 5 U19 CA021239, P30 CA016672), had a research agreement with Roche Diagnostics during the conduct of the submitted work, and received personal fees from Varian Speaking Bureau outside the submitted work; Radhe Mohan received grants support from US National Cancer Institute (5 P01 CA021239, 5 U19 CA021239) during the conduct of the submitted work; Steven H. Lin received grants and other from Beyond Spring Pharmaceuticals, grants from Hitachi Chemical Diagnostics, other from STCube Pharmaceuticals, and other from AstraZeneca, outside the submitted work.
Funding Statement: Supported in part by the National Cancer Institute at the National Institutes of Health through grants 1 R21 CA222749–01A1, 5 P01 CA021239, 5 U19 CA021239, and Cancer Center Support (Core) Grant P30 CA016672 to The University of Texas MD Anderson Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement for this Work
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol 2010;28:1308–15. [DOI] [PubMed] [Google Scholar]
- 2.Tapio S. Pathology and biology of radiation-induced cardiac disease. J Radiat Res 2016;57:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087–106. [DOI] [PubMed] [Google Scholar]
- 4.McGale P, Darby SC, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol 2011;100:167–75. [DOI] [PubMed] [Google Scholar]
- 5.Haque W, Verma V, Fakhreddine M, Butler EB, Teh BS, Simone CB 2nd. Trends in Cardiac Mortality in Patients With Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2018;100:470–7. [DOI] [PubMed] [Google Scholar]
- 6.Yegya-Raman N, Wang K, Kim S, et al. Dosimetric Predictors of Symptomatic Cardiac Events After Conventional-Dose Chemoradiation Therapy for Inoperable NSCLC. J Thorac Oncol 2018;13:1508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkins KM, Rawal B, Chaunzwa TL, et al. Cardiac Radiation Dose, Cardiac Disease, and Mortality in Patients With Lung Cancer. J Am Coll Cardiol 2019;73:2976–87. [DOI] [PubMed] [Google Scholar]
- 8.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98. [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Eblan MJ, Deal AM, et al. Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol 2017;35:1387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speirs CK, DeWees TA, Rehman S, et al. Heart Dose Is an Independent Dosimetrie Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:293–301. [DOI] [PubMed] [Google Scholar]
- 11.Niska JR, Thorpe CS, Allen SM, et al. Radiation and the heart: systematic review of dosimetry and cardiac endpoints. Expert Rev Cardiovasc Ther 2018;16:931–50. [DOI] [PubMed] [Google Scholar]
- 12.Demirci S, Nam J, Hubbs JL, Nguyen T, Marks LB. Radiation-induced cardiac toxicity after therapy for breast cancer: interaction between treatment era and follow-up duration. Int J Radiat Oncol Biol Phys 2009;73:980–7. [DOI] [PubMed] [Google Scholar]
- 13.Adams MJ, Lipshultz SE, Schwartz C, Fajardo LF, Coen V, Constine LS. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol 2003;13:346–56. [DOI] [PubMed] [Google Scholar]
- 14.Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys 2010;76:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 16.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- 17.Gray JE, Villegas A, Daniel D, et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J Thorac Oncol 2020;15:288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg P, Morris P, Fazlanie AL, et al. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med 2017;12:147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozak KR, Hong TS, Sluss PM, et al. Cardiac blood biomarkers in patients receiving thoracic (chemo)radiation. Lung Cancer 2008;62:351–5. [DOI] [PubMed] [Google Scholar]
- 20.Apple FS, Jaffe AS, Collinson P, et al. IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clin Biochem 2015;48:201–3. [DOI] [PubMed] [Google Scholar]
- 21.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Glob Heart 2018;13:305–38. [DOI] [PubMed] [Google Scholar]
- 22.Cao L, Zhu W, Wagar EA, Meng QH. Biomarkers for monitoring chemotherapy-induced cardiotoxicity. Crit Rev Clin Lab Sci 2017;54:87–101. [DOI] [PubMed] [Google Scholar]
- 23.Goodman SG, Steg PG, Eagle KA, et al. The diagnostic and prognostic impact of the redefinition of acute myocardial infarction: Lessons from the Global Registry of Acute Coronary Events (GRACE). Am Heart J 2006;151:654–60. [DOI] [PubMed] [Google Scholar]
- 24.Thygesen K, Mair J, Katus H, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care(dagger). Eur Heart J 2010;31:2197–204b. [DOI] [PubMed] [Google Scholar]
- 25.Katrukha IA. Human cardiac troponin complex. Structure and functions. Biochemistry (Mosc) 2013;78:1447–65. [DOI] [PubMed] [Google Scholar]
- 26.Parmacek MS, Solaro RJ. Biology of the troponin complex in cardiac myocytes. Prog Cardiovasc Dis 2004;47:159–76. [DOI] [PubMed] [Google Scholar]
- 27.Liao Z, Lee JJ, Komaki R, et al. Bayesian Adaptive Randomization Trial of Passive Scattering Proton Therapy and Intensity-Modulated Photon Radiotherapy for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:1813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng M, Moran JM, Koelling T, et al. Development and Validation of a Heart Atlas to Study Cardiac Exposure to Radiation Following Treatment for Breast Cancer. Int J Radiat Oncol 2011;79:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 30.Cox DR. Regression Models and Life-Tables. J R Stat Soc B 1972;34:187-+. [Google Scholar]
- 31.Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data An 1999;30:253–70. [Google Scholar]
- 32.Dess RT, Sun Y, Matuszak MM, et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- 34.Cardinale D, Sandri MT. Role of biomarkers in chemotherapy-induced cardiotoxicity. Prog Cardiovasc Dis 2010;53:121–9. [DOI] [PubMed] [Google Scholar]
- 35.Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011;107:1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tzolos E, Adamson PD, Hall PS, et al. Dynamic Changes in High-Sensitivity Cardiac Troponin I in Response to Anthracycline-Based Chemotherapy. Clin Oncol (R Coll Radiol) 2020;32:292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pudil R, Mueller C, Čelutkienė J, et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail 2020;22:1966–83. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C, Shi D, Yang P. BNP as a potential biomarker for cardiac damage of breast cancer after radiotherapy: a meta-analysis. Medicine (Baltimore) 2019;98:e16507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michel L, Mincu RI, Mahabadi AA, et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail 2020;22:350–61. [DOI] [PubMed] [Google Scholar]
- 40.Onitilo AA, Engel JM, Stankowski RV, Liang H, Berg RL, Doi SA. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: a pilot study. Breast Cancer Res Treat 2012;134:291–8. [DOI] [PubMed] [Google Scholar]
- 41.Canada JM, Thomas GK, Trankle CR, et al. Increased C-reactive protein is associated with the severity of thoracic radiotherapy-induced cardiomyopathy. Cardiooncology 2020;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuo AH, Ancukiewicz M, Kozak KR, Yock TI, Padera TP. Cardiac and inflammatory biomarkers do not correlate with volume of heart or lung receiving radiation. Radiat Oncol 2015;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Sanctis V, Alfò M, Vitiello C, et al. Markers of Cardiotoxicity in Early Breast Cancer Patients Treated With a Hypofractionated Schedule: A Prospective Study. Clin Breast Cancer 2020. [DOI] [PubMed] [Google Scholar]
- 44.Serrano NA, Mikkelsen R, Canada J, Mezzaroma E, Weiss E, Abbate A. Biomarkers of cardiac injury in patients undergoing thoracic radiation therapy. Int J Cardiol 2016;223:507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palumbo I, Palumbo B, Fravolini ML, et al. Brain natriuretic peptide as a cardiac marker of transient radiotherapy-related damage in left-sided breast cancer patients: A prospective study. Breast 2016;25:45–50. [DOI] [PubMed] [Google Scholar]
- 46.Demissei BG, Freedman G, Feigenberg SJ, et al. Early Changes in Cardiovascular Biomarkers with Contemporary Thoracic Radiation Therapy for Breast Cancer, Lung Cancer, and Lymphoma. Int J Radiat Oncol Biol Phys 2019;103:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlton EW, Cullen L, Than M, Gamble J, Khattab A, Greaves K. A novel diagnostic protocol to identify patients suitable for discharge after a single high-sensitivity troponin. Heart 2015;101:1041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller C. Biomarkers and acute coronary syndromes: an update. Eur Heart J 2014;35:552–6. [DOI] [PubMed] [Google Scholar]
- 49.Tan LL, Lyon AR. Role of Biomarkers in Prediction of Cardiotoxicity During Cancer Treatment. Curr Treat Options Cardiovasc Med 2018;20:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cardinale D, Ciceri F, Latini R, et al. Anthracycline-induced cardiotoxicity: A multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The International CardioOncology Society-one trial. Eur J Cancer 2018;94:126–37. [DOI] [PubMed] [Google Scholar]
- 51.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol 2018;71:1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armenian SH, Lacchetti C, Barac A, et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology 2017;35:893–911.27918725 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


