The GTP hydrolases of the Rho/Rac family participate in the generation of coordinated cellular responses to extracellular stimuli (52, 153). Their actions are essential to promote the formation of cytoskeletal structures that contribute to changes in cell shape and motility, the activation of lipid and protein kinase cascades, and the induction of patterns of gene expression required for both developmental and proliferative decisions (52, 153). The functions of these proteins are also associated with a number of human disorders. Thus, hereditary diseases, such as immunodeficiencies (Wiscott-Aldrich syndrome), certain types of X-linked mental retardation, and developmental abnormalities (Aarskog-Scott syndrome), have been linked to null mutations in either upstream or downstream elements of the Rho/Rac pathways (2, 113, 144). The activities of these proteins have also been associated with cellular transformation and oncogenesis, either by enhancing the metastatic properties of transformed cells or by serving as ancillary factors that contribute to the transforming activities of oncoproteins such as Ras (153, 172). Rho/Rac family members are found amplified in some tumors but, unlike Ras, no evidence has been found for their activation by gain-of-function mutations (40, 143). This indicates that the constitutive activation of their pathways is mediated by the deregulation of upstream signals in most cancer cells. Due to these observations, the manipulation of the activation-deactivation cycles of these proteins has received special attention as a potential point of pharmacological intervention to stop the growth of cancer cells.
The key step in this activation cycle is the differential binding of guanosine nucleotides (7). In quiescent cells, these proteins are locked in an inactive state maintained by the presence of bound GDP molecules. In this state, Rho/Rac proteins bind to negative regulators (Rho GDP dissociation inhibitors) that keep them sequestered in the cytosol and block the intrinsic release of bound GDP (7). After cell simulation, there is an exchange of GDP for GTP molecules, resulting in the release of the inhibitory molecules, the translocation of the GTPases to the plasma membrane, and their interaction with their effector molecules (7). Eventually, the action of the GTPase-activating proteins leads to the hydrolysis of the bound GTP molecules and the reversion of the GTPases to the inactive, GDP-bound state (7). Since the intrinsic exchange rate of these proteins is low under normal physiological conditions, the stimulation of Rho/Rac proteins requires the participation of regulatory molecules known as guanosine nucleotide exchange factors (GEFs) (153). To date, two different families of Rho/Rac GEFs have been characterized. The first group is composed of Rho GDP dissociation stimulators (GDSs), a family of proteins that show distant homology with the Cdc25 domains of Ras-specific GEFs (7). GDS molecules work only at stoichiometric concentrations and display broad enzyme specificity, being active on Rap, Rho/Rac, and K-Ras proteins (7). The second group comprises a large number of enzymes containing Dbl and pleckstrin homology (DH and PH) domains whose catalytic activities are directed exclusively towards Rho/Rac GTPases (16). Since these proteins work catalytically on their substrates, it is believed that they represent the main activators of Rho/Rac proteins during signal transduction processes. The majority of these GEFs are highly transforming when overexpressed either as wild-type or truncated proteins, a property that highlights the importance of the regulation of the GDP-GTP cycle of Rho/Rac proteins for cell growth control.
While the mechanism by which these GEFs trigger the activation of GTPases has been studied in detail biochemically, an important issue that remains to be fully resolved is how the stimulated receptors and upstream oncogenic proteins communicate with Rho/Rac GEFs. In this regard, recent reports have demonstrated the activation of a number of exchange factors by translocation to the plasma membrane (Tiam1), interaction with lipids (Sos), or association with the β and γ subunits of heterotrimeric G proteins (Ras GDP-releasing factor, PDZ-RhoGEF, and p115RhoGEF) (45, 71, 74, 93, 103). However, the Rho/Rac GEF proteins that have been best characterized at the biochemical, signaling, and functional level are the members of the Vav family. Originally discovered due to the transforming activity of the first member of the family (Vav), these GEFs have been now found in many tissues and distributed phylogenetically from nematodes to humans. As will be seen in this review, the most relevant feature of these proteins is the tight regulation of their GDP-GTP exchange activities by direct phosphorylation. In the absence of mitogens or antigens, these proteins remain inactive in the cell due to low levels of tyrosine phosphorylation. In the course of cell stimulation, Vav proteins become rapidly and transiently phosphorylated on tyrosine residues, leading to the activation of their GDP-GTP exchange activity towards Rho/Rac proteins. More recently, it has been discovered that tyrosine phosphorylation events also regulate other functions in these proteins, including the association with tyrosine kinases and adapter molecules, the formation of heteromolecular complexes that modulate the signaling output of these GEFs, and the termination of the activity of Vav proteins at the end of the stimulation cycle. Given this close relationship with phosphorylation events, it is not surprising that these GEFs are the only ones known to combine in the same molecule the canonical DH-plus-PH motifs of Rho/Rac GEFs and the structural hallmark of tyrosine phosphorylation pathways, the Src homology 2 (SH2) domain. In addition to this singular mechanism of activation, the Vav family has also attracted recent attention due to the essential role of the founding member, Vav, in cell signaling. Using gene targeting techniques with mice, it has been demonstrated that the expression of this protein is essential for cytoskeletal, proliferative, and apoptotic pathways that determine the development and the signaling responses of lymphoid cells. Moreover, it has been demonstrated that the subversion of the normal activation-deactivation cycle of some members of the Vav family results in severe alterations in cell behavior, including tumorigenesis, changes in F actin organization, and the acquisition of metastatic properties. All these observations establish the Vav family as an essential and direct link between receptors with intrinsic or associated tyrosine kinase activity and the mitogenic and cytoskeletal pathways regulated by Rho/Rac proteins. In this review, I will summarize the most recent developments pertaining to the regulatory, catalytic, and signaling properties of these proteins. Complementary information can be found in a number of previous publications (9, 38, 117, 126).
MEMBERS AND STRUCTURAL FEATURES OF THE VAV FAMILY
The Vav family has three known members in mammalian cells (Vav, Vav2, and Vav3) and one in nematodes (CelVav). The first member of the family, Vav, was identified by Katzav and colleagues in 1989 during the search for oncogenes present in human tumor DNA (66). Since this new transforming gene was the sixth one isolated in that laboratory, it was designated “vav,” the name of the sixth letter of the Hebrew alphabet. The proto-oncogenic versions of this gene were subsequently isolated in the human and mouse species (24, 65). CelVav was found in 1994 during the characterization of the genome of Caenorhabditis elegans (155). The vav2 gene was identified 1 year later in humans due to its close proximity to the tuberous sclerosis disorder gene and, in rodents, during PCR experiments with degenerated oligonucleotides (56, 134). The isolation of the human vav3 gene was reported recently, after an expressed sequence tag cDNA clone encoding a fragment of a new protein with Vav SH2-SH3-like domains was found (99). No family members have been identified in the genome of Saccharomyces cerevisiae despite the presence of several Rho GTPases and DH family proteins in this species. It seems, therefore, that the necessity for Vav function occurred during the transition from unicellular to multicellular organisms, an evolutionary step that coincided with an increased regulatory role for tyrosine phosphorylation in the modulation of signaling cascades.
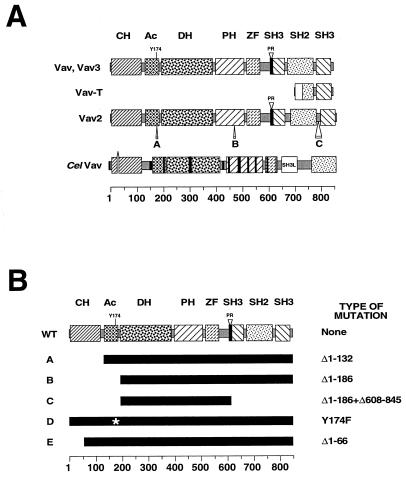
All Vav family members are characterized by similar structures (Fig. 1A). Mammalian Vav proteins contain a calponin homology (CH) domain, an acidic (Ac) region, the DH and PH domains typical of members of the Dbl family of GDP-GTP exchange factors, a zinc finger (ZF) domain similar to those present in c-Raf and atypical members of the protein kinase C (PKC) family, a short proline-rich (PR) region, and two SH3 domains flanking a single SH2 region. The Vav protein of nematodes differs mostly at the C terminus, where the PR and the most distal SH3 of the mammalian Vav proteins are missing (Fig. 1A). In addition, the more proximal SH3 is highly divergent from the canonical sequences of known SH3s, indicating that it is probably not functional. Other major changes are found in the CelVav ZF region, since this domain lacks the cysteine residues involved in the formation of the fifth β sheet and the unique alpha helix typical of ZF domains. Since the integrity of the C-terminal SH3 and the ZF is a prerequisite for the function of the wild-type versions of the mammalian Vav proteins (24, 50, 99), these structural changes suggest that the regulation of CelVav will diverge significantly from that of its mammalian counterparts. In addition to these changes, CelVav contains small deletions and insertions of amino acid residues throughout the molecule when compared with its mammalian counterparts (Fig. 1A). The most conspicuous are a 17- and a 15-amino-acid insert in the DH and PH region, respectively (Fig. 1A). From structural data gathered from other DH and PH regions, it seems that these insertions will not have major effects on the functions of these domains, as they are located in linker regions that do not interfere with the respective substrate binding sites. Indeed, insertions of similar size are found in the DH region of Tiam1 and in the PH domains of the Bcr, Abr, and Cdc24 proteins. Although the structural domains found on Vav proteins are also detected in a plethora of signal transduction molecules, two structural features are unique to the Vav family. One is the DH-PH-ZF cassette present in the central regions of these proteins. The other is the presence in the same molecule of a DH and an SH2 region (Fig. 1A). As will be discussed below, these structural peculiarities translate into regulatory and catalytic properties also unique for this group of GDP-GTP exchange factors. The spatial organization of the C-terminal SH3 and SH2 is also unusual among signaling proteins, although it is seen in other SH2- and SH3-containing proteins, such as Grb2, Gbr3, and Grab/Grap proteins.
FIG. 1.
(A) Structures of Vav family proteins. The tyrosine residue involved in Vav downmodulation (Y174) is shown. The alternative sequences of Vav2 generated by alternative splicing of its hnRNA are indicated by A, B, and C. Deletions or insertions of sequences found in the CelVav protein are illustrated by using empty spaces and solid boxes, respectively. The lack of the two cysteine residues in the C terminus of the CelVav ZF is indicated by a gray box. SH3L is the region with low resemblance to SH3 domains present in CelVav. (B) Oncogenic mutations found in Vav family members. WT, wild type. An asterisk indicates the Y174F mutation. Amino acid numbers are shown at the bottom of each panel.
Despite these structural similarities, the mammalian vav genes differ in chromosomal localization and expression patterns. Thus, the human vav, vav2, and vav3 genes have been assigned to chromosomes 19 (region 19p13.2), 9 (region 3q34.1), and 1, respectively (56, 88; http://www.ncbi.nlm.nih.gov /LocusLink/LocRpt.cgi?l=10451 [the Human Genome Project]). At the expression level, Vav is restricted mostly to hematopoietic cells, being found from the pluripotential stem cells to the most mature stages of the lymphoid and myeloid-erythroid lineages. Vav is also detected in a few nonhematopoietic tissues, such as the pancreas, the tooth enamel, and the trophoblast layer (9). On the other hand, Vav3 shows a much broader expression profile, and Vav2 displays an almost ubiquitous distribution (99, 134). Although all vav family genes are expressed in hematopoietic cells, vav transcripts are present at significantly higher levels than those for vav2 and vav3 (M. Movilla and X. R. Bustelo, unpublished results). Several isoforms have been described recently for Vav and Vav2. Vav-T, a truncated version of Vav, is found in the spermatocytes and spermatids of mice (Fig. 1A) (105). In the case of Vav2, there are at least three differentially spliced forms that differ in the presence of insertions in the Ac domain, the PH region, and the SH2-SH3 linker region (Fig. 1A). There is evidence that some of these isoforms show a tissue-specific pattern of expression (56, 134). It is not yet known whether these isoforms display different regulatory or signaling properties. CelVav is located on chromosome 3 and appears to be expressed preferentially in muscle and neural tissues (http://wormsrv1.sanger.ac.uk/cgi-bin/ace/paper/worm?name=%5Bwcwm98ab48%5D).
REGULATION OF VAV ACTIVITY
The most distinctive functional property of Vav family members is the fact that their enzyme activities are triggered by the tyrosine phosphorylation of the respective molecules. To date, no other GDP-GTP exchange factor of the Ras superfamily shows this type of regulation. Recent results have shown that the levels of Vav enzyme activity are additionally modulated by second messengers derived from the enzyme activity of phosphatidylinositol 3-kinase (PI-3K). This regulatory property is shared with a small number of Ras superfamily GEFs, such as Sos and Arf nucleotide binding-site opener (ARNO) (19, 103). By analyzing the behavior of transforming versions of these proteins, evidence has recently been obtained that other regulatory steps are also in place to control the activities of these proteins in living cells.
The initial link between phosphorylation and Vav activation was obtained by Crespo et al. after setting up an in vitro assay that allowed the side by side comparison of the exchange activity of the phosphorylated and nonphosphorylated forms of Vav (27). Using this system, it was found that Vav had no detectable enzyme activity when tested with different GTP hydrolases of the Ras superfamily. However, the phosphorylation of Vav by treatment with a GST-Lck fusion protein led to a strong activation of its GDP-GTP exchange activity towards Rac1 and, to a lesser extent, RhoA. Vav activity was circumscribed to those two GTPases because no GDP-GTP exchange was observed on Cdc42 or other Ras superfamily proteins, such as Ras and Ran. The fact that this activation could occur in vitro with highly purified preparations of proteins proved that the phosphorylation is the regulatory event triggering Vav activation. Subsequently, it was demonstrated that such activation could be reproduced in vivo by the coexpression of Vav with several tyrosine kinases of the Src and Syk families. Since these early studies, the phosphorylation-dependent activation of Vav has been validated in a number of independent systems both in vitro and in living cells (53, 94, 129, 146). Recently, other studies have shown that Vav2 and Vav3 are also subjected to the same regulation (99, 135). Interestingly, the analysis of the catalytic specificities of these proteins indicates that they target overlapping, but not identical, spectra of Rho/Rac substrates. Thus, Vav works catalytically on Rac1 and RhoG but is significantly less active on RhoA proteins (27, 135). Activation of Rac2 by phosphorylated Vav was also observed recently (135). On the other hand, Vav2 and Vav3 act catalytically on RhoA and RhoG GTPases but are less active on Rac1 (99, 135). There is disagreement about the possible implication of Cdc42 in the Vav pathway. Thus, we and others have shown that none of the known Vav family members is active in vivo or in vitro on Cdc42 under conditions in which this GTPase is activated by either Dbl or Rho GDS (27, 99, 135, 146). In addition, dominant-negative mutants of Cdc42 do not seem to have any effect on several Vav-mediated cellular responses, such as transformation (26), c-Jun N-terminal kinase (JNK) activation (26), and NF-κB and serum-responsive factor (SRF) stimulation (96, 97). In contrast, two groups have shown the activation of Cdc42 by Vav either in vitro (53) or in vivo (106). At this point, the reason for the discrepancy between these two sets of observations is unknown.
The level of activity of Vav is also dependent on the presence of phosphorylated forms of phosphatidylinositol (PI). Thus, it has been shown that the enzyme activity of phosphorylated Vav increases twofold when Vav is incubated with either PI-3,4-P2 or PIP3, two products of PI-3K (54). Conversely, the GDP-GTP exchange activity of Vav is totally inhibited when the PI-3K substrate PIP2 is included in the reactions (54). These effects are mediated by the binding of those molecules to the Vav PH domain (54). In agreement with these in vitro observations, Vav activity is enhanced in vivo when the protein is coexpressed with PI-3Kγ (83). Likewise, this lipid kinase seems to be upstream of Vav during the signaling of both CD5 and Fcɛ receptor I (FcɛRI) as the PI3-K inhibitor wortmannin can inhibit to some extent Vav-dependent responses in both systems (49, 138). Since phospholipids cannot activate nonphosphorylated Vav (54), their role seems to be the fine modulation of the enzyme activity of phosphorylated Vav rather than to act as an independent mechanism for Vav activation. This alternative regulatory step may therefore be relevant only under stimulation conditions that do not trigger optimal Vav phosphorylation. This interpretation is consistent with recent results showing that inhibitors of PI-3K do not affect the generation of Vav-mediated signals in either anti-CD3-stimulated thymocytes or CHO cells overexpressing Vav and Syk (72, 94). Whether PI phosphates regulate the activities of Vav2 and Vav3 is as yet unknown.
In addition to the physiological stimulation of Vav during signal transduction, the activities of Vav proteins can be upregulated by gain-of-function mutations that subvert the normal regulation of these proteins in vivo. As a consequence, these mutations often correlate with the generation of proteins with high transforming potential. Interestingly, the high biological activities of some of these mutants cannot be attributed to the loss of the known regulatory properties of the Vav family, suggesting that new mechanisms must exist that contribute to the tight control of these proteins inside the cell. The deregulated Vav mutants can be classified in two classes, based on how their activities depend on tyrosine phosphorylation. The first group includes proteins that have lost either the entire CH domain (Δ1–132) or the CH domain plus the Ac region (Δ1–186) (Fig. 1B, forms A to C). These truncated proteins show constitutive, phosphorylation-independent exchange activity both in vitro and in vivo. As a consequence, these mutants can induce biological responses even in the absence of the SH3-SH2-SH3 domain (Fig. 1B, form C), the region involved in the interaction with protein tyrosine kinases (99, 135; M. Lopez-Lago, N. Movilla, and X. R. Bustelo, unpublished observations). Since these proteins are totally unregulated, they show the highest transforming activity of all known Vav gain-of-function mutants (135; Lopez-Lago et al., unpublished). From a regulatory point of view, the members of the second group of mutants are perhaps more interesting, because they include oncogenic versions of Vav proteins that still conserve a phosphorylation-dependent exchange activity. One of the mutants belonging to this group is a recently identified Vav protein containing a tyrosine (Y)-to-phenylalanine (F) substitution in one of the phosphorylation sites of the Vav Ac region (Y174) (Fig. 1B, form D) (82). Interestingly, this mutant is also active in short-term biological responses, such as the induction of F-actin reorganization in fibroblasts (82) and the stimulation of transcriptional responses in T lymphocytes (77, 82). This is the only known case of oncogenic activation of Vav proteins by a single point mutation. Using a phosphospecific antibody for that position, it has been shown that the residue affected by this mutation becomes phosphorylated after receptor stimulation, suggesting that it is probably involved in a feedback mechanism that downmodulates the activity of Vav at the end of the stimulation cycle (82). Since this residue is located in consensus sequences for the binding of SH2 domains, this negative regulation presumably involves the action of a hitherto-unknown SH2-containing protein. The identification of this Vav Y174F mutant is therefore especially valuable, because it indicates that phosphorylation plays both positive and negative roles, depending on the tyrosine residue that is targeted on the Vav molecule. Another protein included in this second class of mutants is the truncated version of Vav (Δ1–66) initially isolated by Katzav and coworkers (Fig. 1B, form E) (66). The mechanism by which this oncogenic version promotes cellular transformation is enigmatic as yet, since its exchange activity is phosphorylation dependent (27, 53, 135). Recent results have shown that wild-type Vav and Vav (Δ1–66) are found in Triton-soluble and -insoluble fractions, respectively, after cell lysis (75). Thus, this mutant may have a different subcellular localization than wild-type Vav, suggesting that it may trigger cell transformation by a closer proximity to protein kinases and/or GTPases. The identification of these two phosphotyrosine-dependent mutants indicates that the regulation of Vav proteins in vivo is more complex than the simple controls by phosphorylation levels and/or phospholipid binding.
The complexity of the regulation of Vav proteins in vivo is further emphasized by recent observations indicating that the biological effects of a particular gain-of-function mutation are highly dependent on the member of the Vav family used and on the cellular context in which the function of a specific Vav protein is tested. For instance, the deletion of the CH region leads to the phosphorylation-independent exchange activity of Vav, Vav2, and Vav3 and to enhanced biological responses when tested in transient-transfection assays (99, 135). Despite this, only the mutant versions of Vav and Vav2 can induce morphological transformation in long-term assays (99, 135). Since Vav2 and Vav3 share the same spectrum of GTPase substrates (99, 135), these results indicate that other factors should control the strength of the signal emanating from each Vav family member in vivo. Moreover, while Vav (Δ1–66) and Vav (Δ1–186) are transforming in rodent fibroblasts, they are not functional in certain hematopoietic specific responses (5, 156). In contrast, Vav (Y174F) is still capable of effective signaling in both hematopoietic and nonhematopoietic cells (77, 82). Thus, it seems that the Vav CH may have negative roles in cis but also cell-type-specific positive regulatory functions in trans. At this moment, it is difficult to predict the function of this domain in mediating such positive responses. Based on the homology of this region with domains involved in F-actin binding, it was initially proposed that the CH domain could regulate the function of Vav by binding to this cytoskeletal protein (15). However, the recent characterization of other CH-containing proteins has indicated that F-actin binding is only possible when two CH domains are present in tandem in the same molecule, suggesting that such a regulatory possibility does not occur in the case of Vav proteins (141). In fact, both Vav and Vav3 seem to associate with cytoskeletal structures only when the CH domain is missing (75, 99). CH regions have also been shown to bind to vesicles composed of phosphatidylserine and phosphatidylinositol, suggesting a potential regulation by lipids (6, 42). The formation of protein-protein interactions cannot be excluded, given the high alpha-helical content of this domain (14). Arguably, more work on this area is required to unveil all the regulatory mechanisms that modulate the activity of these proteins in vivo.
MECHANISM OF VAV EXCHANGE ACTIVITY
The ultimate functional objective of activated Vav proteins is to interact catalytically with the GTPases, leading to the rapid exchange of their bound GDP molecules by GTP. Recent experiments have shed light on several molecular aspects of this catalytic relationship. Thus, it has been shown that activated Vav3 can interact physically with the nucleotide-free GTPases (99). This suggests that Vav proteins, like most GEFs known so far, promote nucleotide exchange by stabilizing this highly unstable transition state of the GTPases. This physical interaction occurs only when Vav3 is tyrosine phosphorylated, indicating that one of the consequences of this posttranslational modification is to increase the binding affinity of Vav3 towards its substrates (99). This effect appears to correlate with the release of an inhibitory conformation induced by the N-terminal CH region, as Vav proteins lacking this domain display phosphorylation-independent GDP-GTP exchange activity (99, 135). It is not yet known whether this inhibitory effect is due to an intramolecular interaction between the CH domain and other structural domains of Vav proteins or to the induction by that region of an unstable conformation in the Vav molecules. Structural studies of the Vav3-substrate interaction have also revealed that the Vav3 DH region is not catalytically autonomous, as it requires the presence of an intact ZF region for both the binding to and activation of the GTPase substrates (99). Accordingly, deletions or point mutations that inactivate the ZF abrogate the enzyme activity of Vav3 in vitro and its biological activity in vivo (99). The contribution of the ZF region to the catalysis of nucleotide exchange appears to be dual, offering points of contact with the substrates and allowing an optimal conformation of the DH-PH-ZF cassette that is compatible with the action of the catalytic DH domain (99). Interestingly, point mutations that inactivate the functions of the Vav and Vav3 PH domains show no major alterations in the exchange activities of these proteins, indicating that this domain does not have an active role in the catalytic reaction of Vav proteins (54, 99). However, since the binding of PIP2 to the Vav PH domain results in the downmodulation of the exchange activity of this protein (54), it is possible that the PIP2-bound PH domain may indirectly affect the catalytic activity of Vav in vivo by blocking the interaction of the substrates with the DH and/or the ZF region. A similar regulatory role has been proposed recently for the modulation of the Rac-1 GDP-GTP exchange activity of the Sos DH region by PI phosphates (103). The dependency of Vav proteins on the ZF region for catalytic activity is, with the activation by tyrosine phosphorylation, a unique functional property of this GEF family.
SIGNALING ELEMENTS OF THE VAV PATHWAY
The elucidation of the biochemical function of Vav proteins has evolved in parallel with the characterization of the signal transduction elements that work coordinately with these proteins in the induction of several biological responses. In addition, synergistic interactions between Vav and other pathways during cell signaling have also been found. Below, we summarize the major signaling elements involved in the Vav pathway.
(i) Upstream elements.
The upstream elements of the Vav pathway include molecules that trigger the activation of Vav during signal transduction. This category includes receptors, protein tyrosine kinases, and adaptor molecules whose function is to favor the interaction of Vav with the upstream kinases. The role of PI3-K as a possible coactivator of Vav has been described in the previous section and will not be included here. Since the initial reports indicating the phosphorylation of Vav during the signaling of the epidermal growth factor (EGF), platelet-derived growth factor (PDGF), B-cell, and T-cell receptors (EGF-R, PDGF-R, BCR, and TCR) and FcɛRI (10, 12, 86), this protein has been found to be involved in the pathways of 35 membrane receptors (Table 1). This number will probably grow larger, as the receptors for several cytokines (i.e., interleukin 2 (IL-2) and IL-15) share the same signaling subunits. Although the study of Vav2 and Vav3 during signal transduction is in its initial stages, there are already data regarding their participation in the pathways regulated by the EGF-R, PDGF-R, and TCR (99, 111; Movilla and Bustelo, unpublished). Phosphorylation of Vav2 by the receptor protein kinases c-Kit and Flk2 was also found recently (R. Rottapel, personal communication). In most cases examined, the activation of those receptors by the respective ligands leads to a rapid and transient phosphorylation of Vav on tyrosine residues, although the kinetics change depending on the receptor involved. However, it is known now that the same receptor can trigger different phosphorylation levels of Vav, depending on the type of ligand bound or the cellular context in which such response is generated. For example, the overall levels of Vav phosphorylation induced by the TCR-αβ of immature T cells depend significantly on whether the receptor binds to agonistic or antagonistic peptides, a property that can affect decisions about whether to maintain or kill the stimulated thymocyte (136). The extent and duration of the phosphorylation-dephosphorylation cycle of Vav can also be modulated either positively or negatively by the costimulation of adjacent surface molecules. Thus, the coligation of the TCR with the CD28 receptor leads to higher and more sustained phosphorylation levels of Vav during T-cell signaling than when each receptor is cross-linked alone (104, 129). In B cells, the phosphorylation levels of Vav are increased when the BCR and CD19 molecules are cross-linked simultaneously (108, 131). On the negative side, the phosphorylation levels of Vav induced by the BCR-CD19 complex are significantly lowered if the CD22 molecule is engaged simultaneously (131). A similar response is observed when CD16-stimulated basophils are treated at the same time with IL-10 (47). These dynamic changes in the levels of Vav phosphorylation indicate that the regulation of the Vav signaling output is probably one of the parameters used by cells for establishing the signaling thresholds that mediate the strength and duration of immune responses.
TABLE 1.
Phosphorylation of Vav mediated by membrane receptors
| Receptora | Referenceb |
|---|---|
| Tyrosine kinase receptors | |
| c-Fms | 163 |
| c-Kit | 1 |
| EGF-R | 12, 86 |
| Flk-2 | 33 |
| IGF-1-R | 152 |
| Insulin-R | 151 |
| PDGF-R | 12 |
| TrkA | 90 |
| Receptors signaling via the Syk/Zap70 family | |
| BCR | 10 |
| CD28 | 3, 104 |
| FcγRI | 69 |
| FcɛRI | 86 |
| FcγRII | 69 |
| FcγRIII | 160 |
| Integrins | 21 |
| NK complexc | 5 |
| TCR | 12, 86 |
| Receptor signaling via the Src family | |
| CD19 | 154 |
| Receptors signaling via the Jak family | |
| c-Mpl | 31, 100, 102, 130 |
| Epo-R | 84 |
| G-CSF-R | 164 |
| GM-CSF-R | 89 |
| IL-2-R | 34 |
| IL-3-R | 89 |
| IL-5-R | 132 |
| IL-6-R | 78 |
| Prolactin R | 76 |
| Type I IFN-R | 119 |
| Receptors with uncharacterized kinases | |
| CD14 | 47 |
| CD2 | 121 |
| CD40 | 110 |
| CD43 | 115 |
| CD5 | 49 |
| G-coupled receptors | |
| PAR1 | 22 |
| Thrombin-R | 21 |
Receptors shown to phosphorylate Vav3 are underlined. IGF, insulin growth factor; IFN, interferon; PAR, protease-activated receptor.
For simplicity, only the first articles reporting the participation of Vav in a particular receptor pathway are included.
The receptor for the cytotoxic response of NK cells has not been identified yet. It is possible that it is not a single molecular entity but rather a set of membrane proteins (79).
Phosphorylation plays two convergent roles during these early stages of the activation of Vav proteins. It promotes the final activation of the latent enzyme activity of Vav, but in addition, it makes possible such activation by favoring the prior formation of heteromolecular complexes between Vav and its upstream elements. In reductionistic terms, these interactions use as molecular glue the avidity of the Vav SH2 domain towards the consensus sequence Y(PO4)XEP (where X is M, L, or E) (139). However, the type of complexes formed depends on the nature of the receptor involved. In the case of some receptors with intrinsic tyrosine kinase activity (EGF-R and PDGF-R), Vav proteins associate, via their SH2 domains, with the autophosphorylated receptors (Fig. 2A) (12, 86, 99, 111; Movilla and Bustelo, unpublished). This is not always the case, because the Vav SH2 domain cannot bind to the activated Flk2 and c-Kit receptors, suggesting that in this case the interaction occurs indirectly via adapter proteins not yet characterized (1, 33, 127). When the stimulated receptors lack intrinsic tyrosine kinase activity, Vav interacts via its SH2 domain with cytoplasmic kinases of three distinct families. The first group is composed of Syk and Zap70 (20), two kinases that contain two SH2 domains and two regulatory regions (interdomains A and B) (Fig. 3). Syk is expressed in B lymphocytes, basophils, NK cells, and immature T-cell populations. Zap70, on the other hand, is distributed in T lymphocytes (20). Presently, there is extensive genetic evidence indicating that these two kinases are the key mediators of Vav phosphorylation during the signaling of many receptors present in lymphoid and myeloid cells. Thus, the elimination of the syk gene by homologous recombination in chicken DT-40 B cells leads to the blocking of Vav phosphorylation after BCR cross-linking (158). Likewise, Jurkat T cells lacking a functional Zap70 kinase (P116 clone) also fail in phosphorylating Vav during both TCR- and CD28-mediated signaling (129). Similar results were observed in Zap70-deficient T cells obtained from individuals with severe combined immunodeficiency (17). Interestingly, the overexpression of Zap70 in Syk-deficient cells rescues Vav phosphorylation, indicating that the kinases are functionally redundant in this process (158). Since both DT-40B and P116 cells contain functional kinases of the Src and Btk families, these experiments prove indirectly that these other kinase families are not implicated in the tyrosine phosphorylation of Vav induced by the TCR, the BCR, and CD28. Outside the lymphoid lineage, Syk has been linked to the phosphorylation of Vav in other signaling pathways, including those for integrins (94), the FcɛRI receptor (57, 146), and the cytotoxic response of NK cells (46).
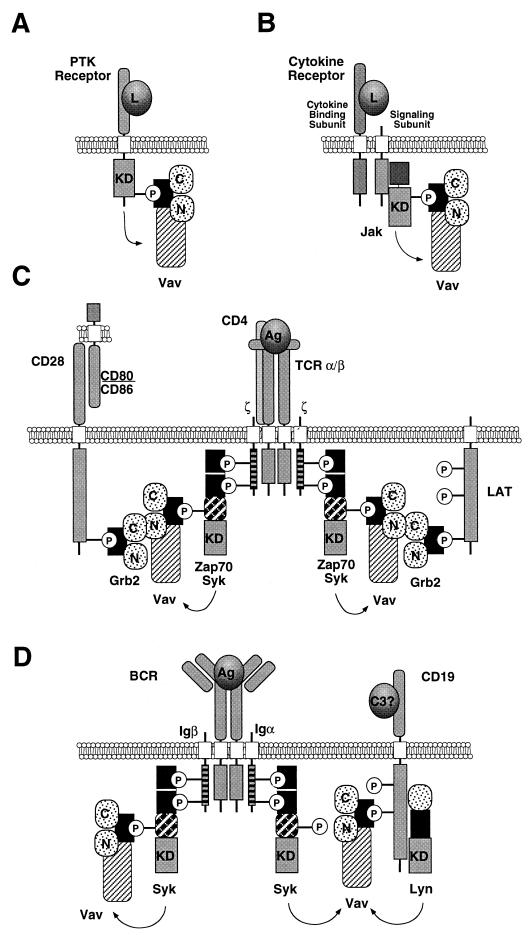
FIG. 2.
Types of interaction between Vav and upstream elements during the signaling of membrane PTKs (A), cytokine receptors (B), the TCRαβ (C), and the BCR (D). PTK, protein tyrosine kinase; L, ligand; Ag, antigen; KD, kinase domain; P, phosphorylated tyrosine residue; N, proximal SH3 domain; C, distal SH3 domain. Transmembrane domains are indicated by open boxes, immunoreceptor tyrosine-based activation motif-containing molecules are shown as boxes with horizontal lines, SH2 domains are shown as solid boxes, SH3 domains are shown as dotted circles, and the rest of the N-terminal domains of Vav are shown as a shaded box. The proteins included are not shown to scale. The two possible transmembrane ligands for CD28 (CD80 and CD86) are indicated. The ligand for CD19 is as yet unknown, although it is believed that it binds to complement (C3) with the help of a second coreceptor, CD21 (43).
FIG. 3.
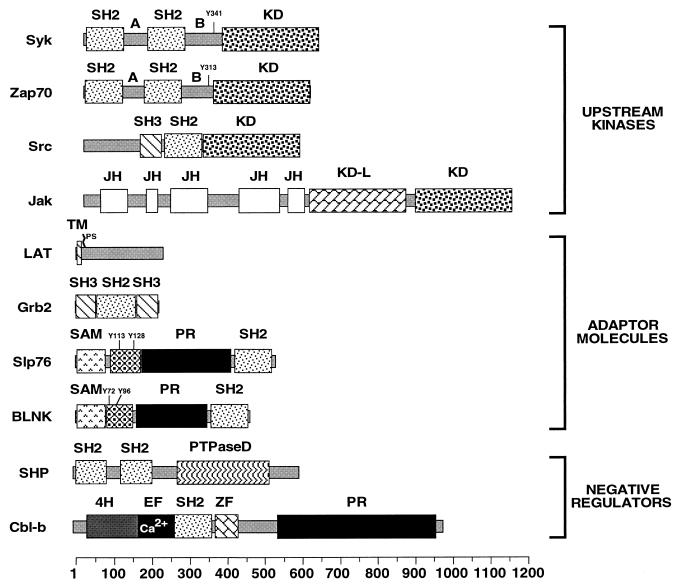
Structures of regulatory elements of the Vav pathway. A and B, interdomains A and B; KD, kinase domain; JH, Jak homology; KD-L, kinase domain-like; TM, transmembrane region; PS, prenylation site; PR, proline-rich region; PTPaseD, phosphatase domain; 4H, four-helix bundle; EF, EF hand. The phosphorylation sites recognized by the Vav SH2 domain in Syk, Zap70, Slp76, and BLNK are indicated (the sites in BLNK have not been formally identified). Amino acid numbers are shown at the bottom of the figure.
The sequence of events that triggers the activation of these kinases and the subsequent tyrosine phosphorylation of Vav phosphorylation is now well established (Fig. 2C and D). The activation of the antigen receptors leads to the activation of Src kinases, resulting in the phosphorylation of the immunoreceptor tyrosine-based activation motifs (defined by the sequence YX2[L/I]X6–8YX2[L/I]) of the signaling subunits of receptors (20). The phosphorylation of two tyrosine residues (boldface) in these motifs leads in turn to the binding of the two SH2 domains of Zap70 (Fig. 2C and D). Finally, the phosphorylation of Zap70 by Src family kinases leads to the activation of its kinase activity and the phosphorylation of downstream signaling elements (20). Syk follows a similar mechanism of activation, although it does not require phosphorylation by Src family kinases for the final activation step (20). The phosphorylation of Vav during this process is linked to the physical interaction with these kinases, an event mediated by the binding of the Vav SH2 region to one phosphorylated residue located in interdomain B of Zap70 (Y313ESP) and Syk (Y341ESP) (Fig. 3 and 2C and D) (28, 67). Mutation of those tyrosine residues to phenylalanine eliminates the binding of Vav to the kinases and its subsequent phosphorylation, indicating that the physical interaction is mandatory for this step of Vav activation (28, 158, 171).
A second group of kinases involved in Vav activation during the signaling of cytokine receptors is the Jak family (Fig. 3). Unlike Syk proteins, these kinases are associated constitutively with the cytoplasmic tails of cytokine receptors, becoming activated by cross phosphorylation after the aggregation of the receptors induced by the binding of the cytokines (60). The Vav-Jak interaction appears to be mediated by the association of the Vav SH2 domain with hitherto-unidentified phosphorylated sequences of the activated kinases (Fig. 2B) (89). The physiological significance of the Vav-Jak interaction is now well established through several studies using mutant versions of the erythropoietin receptor (Epo-R) and the thrombopoietin (c-Mpl) receptor. Thus, mutations in the Epo-R that disrupt the association of Jak2 concomitantly abolish the phosphorylation of Vav by Epo (95). Conversely, the expression of an activated form of the Epo-R mutant leads to the constitutive kinase activity of Jak2 and to Vav phosphorylation in a ligand-independent manner (95). The use of deletion mutants of the c-Mpl receptor has yielded similar results (32, 98), although some other unidentified tyrosine kinases also seem to play secondary roles (32).
Finally, there have been reports indicating the possible involvement of Src family kinases in Vav phosphorylation. Thus, Michel et al. have shown that Vav coimmunoprecipitates with Fyn upon cross-linking of the CD28 receptor in a T-cell hybridoma (92). This binding could be reproduced in vitro by using the isolated Fyn SH2 domain and is blocked by phosphopeptides containing the Y174EDL sequence present in the Vav Ac region (92). This result represents a biological paradox, because the phosphorylation of Vav by Fyn would require the prior phosphorylation of Vav by other kinases. The role of Fyn in this signaling response is unclear because, as stated before, cells lacking Zap70 cannot phosphorylate Vav after CD28 stimulation (129). In the case of B cells, a report has shown that the cross-linking of the CD19 receptor leads to the colocalization of Vav and Lyn in the cytoplasmic tail of this receptor, leading to Vav phosphorylation (Fig. 3D) (44). Interestingly, the ability of Lyn to phosphorylate Vav in vitro is enhanced by the addition of exogenous CD19, indicating that this receptor serves not only as a scaffold but also as a facilitator of the Lyn-dependent phosphorylation of Vav (44). Despite these two examples, the evidence available so far indicates that the implication of Src kinases in the direct phosphorylation of Vav is very limited under physiological conditions. However, it is worth noting that several Src proteins (Lck, Fyn, and Hck) induce the efficient phosphorylation and activation of the GDP-GTP exchange activity of all mammalian Vav members when used in vitro or in overexpression systems (27, 99, 135). Thus, it is possible that these kinases may have some role in the activation of the Vav pathways under abnormal conditions, such as tumors containing alterations in the upstream elements of the Vav pathway.
Despite the formation of complexes with all the kinases described above, recent data indicate that the optimal phosphorylation of Vav in some cell lineages requires the participation of adaptor molecules that facilitate the spatial proximity between Vav and the upstream kinases. These associations also depend on the tyrosine phosphorylation of these adapter proteins and the utilization of either the Vav SH3 or SH2 domains as interacting domains. In T cells, the translocation of Vav is facilitated by the binding of Vav to the adapter protein LAT and, less importantly, to the receptor CD28. LAT is a 36- to 38-kDa palmitoylated protein specifically expressed in T, NK, and mast cells (Fig. 3) (168). This protein resides in rafts of the plasma membrane enriched in cholesterol and glycolipids that are essential for optimal signaling responses of T lymphocytes (159, 170). The function of LAT is to work as an adapter molecule to allow the translocation of several signaling proteins into these membrane rafts (168, 170). In order to perform such a role, activated Syk/Zap70 family proteins phosphorylate LAT on tyrosine residues, leading to the creation of anchoring sites for SH2-containing proteins (168). The important role of LAT in Vav signaling was recently demonstrated genetically using LAT-deficient Jurkat T cells (J.CaM2). In these cells, the phosphorylation of Vav upon TCR stimulation is reduced despite the normal activation of upstream kinases (36). Reintroduction of LAT in J.CaM2 cells restores Vav phosphorylation, but only when versions of LAT capable of localizing in the cholesterol- and glycolipid-rich rafts are utilized (36, 167). In good agreement with such a translocation role, a percentage of Vav distributes into the membrane rafts after TCR cross-linking (170). Interestingly, the Vav SH2 domain cannot bind phosphorylated LAT, indicating that other bridge molecules are required (168). One possible candidate is Grb2, an adapter protein that can bind simultaneously to LAT (via its SH2 domain) and to Vav (via its C-terminal SH3 domain) (Fig. 2C) (122, 162, 168). Grb2 is found constitutively associated with Vav in different hematopoietic cells (9, 126). The use of Grb2 for the interaction of Vav with LAT is probably a molecular stratagem to keep the Vav SH2 domain free to interact with the phosphorylated tyrosine kinases (Fig. 2C).
A second functional alternative for the translocation of Vav to the plasma membrane is the interaction of Vav with CD28, a receptor that is engaged simultaneously with the TCR. As in the case of LAT, this interaction is also indirect, requiring the Grb2-binding site of CD28 (Y191MNM) (Fig. 2C) (70). Mutation of this site leads to a lack of Vav phosphorylation after CD28 cross-linking (70). The functional interaction between Vav and CD28 appears to be limited to the enhancement of the activity of Vav downstream of the TCR, since CD28-specific responses are not affected in vav−/− T cells (116). B cells utilize a different strategy for Vav translocation (Fig. 2D). In this case, Vav binds to the cytoplasmic tail of the CD19 receptor, a step that facilitates the proximity of Vav to the CD19-associated Lyn and the immunoglobulin M-associated Syk (81, 108, 154). The interaction between Vav and CD19 is direct, due to the recognition by the Vav SH2 domain of the Y391EEP sequence present in the cytoplasmic tail of this receptor (108). This site is presumably a target of Syk, as its phosphorylation is dependent on prior BCR stimulation (108). Mutation of this site abrogates Vav binding and CD19-mediated responses, indicating that this interaction mediates both Vav phosphorylation and the activation of downstream signaling events (81, 108). No LAT-like proteins have been found in B cells, suggesting that the interaction between Vav and Syk is either direct or mediated by CD19.
The physical interaction of Vav with the prolactin receptor (23), the Epo-R (76), the insulin receptor β subunit (151), gp130 (78), CD5 (49), and the FcɛRIII γ subunit (137) have also been reported, although the structural bases of those interactions have not been studied in detail. Thus, the use of adapters or receptors for the membrane translocation of Vav seems to be a conserved mechanism in most signaling systems. In addition to promoting Vav protein phosphorylation, it is likely that such translocation events will favor the signaling output of Vav proteins by bringing these molecules in close proximity to their GTPase substrates or to other regulatory molecules that impinge on Vav function, such as PI3-K.
(ii) Negative regulators.
Much less is known about the regulatory molecules that trigger the downmodulation of Vav signals at the end of the stimulation cycle. Vav was shown to coimmunoprecipitate in a stimulation-dependent manner with the tyrosine phosphate SHP (also known as PTP1C, HCP, and SHPTP1), a cytoplasmic, SH2-containing protein encoded by the motheaten locus (Fig. 3) (73). This interaction requires the SH2 region of Vav, although the C-terminal Vav SH3 seems to play a cooperative role (73, 112). The complex between Vav and SHP can probably also occur indirectly, via the association of SHP with receptors such as CD22 (30). This may explain the negative effect of this receptor on the phosphorylation of Vav induced by the BCR-CD19 complex (131). In addition to SHP, other phosphatases are likely to be implicated in Vav inactivation, since the phosphorylation levels of Vav do not change in SHP-deficient cells (112). A second potential negative regulator of Vav is Cbl-b, a protein initially isolated in two hybrid-system experiments aimed at identifying binding partners of the Vav SH3-SH2-SH3 domain (11). Cbl-b is a ubiquitously expressed protein with a multidomain structure that includes a four-helix bundle, an EF-hand calcium-binding domain, a cryptic SH2 region, a RING finger domain, and a C-terminal proline-rich region (91, 147) (Fig. 3). This protein belongs to a well-known family of signal transduction proteins (c-Cbl, Cbl3, Sli-1, and Drosophila Cbl) that inhibit transduction cascades either by downmodulating the kinase activity of cytoplasmic tyrosine kinases or by promoting the ubiquitination of membrane tyrosine kinase receptors (62, 80, 109, 147). The interaction of Cbl-b with Vav requires a PR domain present in the central region of Cbl-b and the intact Vav SH3-SH2-SH3 domain (11). The possible implication of Cbl-b in the Vav pathway is demonstrated by two independent observations. First, the overexpression of Cbl-b in COS-1 cells leads to the inhibition of the activation of JNK mediated by Vav (11). This effect requires the integrity of both the SH2 and the C-terminal regions of Cbl-b (11). Moreover, recent experiments using gene-targeting techniques have shown that the deletion of the cbl-b gene removes the necessity for the TCR-CD28 coengagement in order to obtain optimal phosphorylation levels of Vav during antigen-mediated T-cell signaling (J. Penninger and H. Gu, personal communication). Despite these functional observations, nothing is yet known about the mechanism by which Cbl-b downmodulates the Vav pathway. There is also evidence that other proteins in addition to SHP and Cbl-b can contribute to the downmodulation of the function of Vav during signal transduction, as demonstrated by the oncogenic activation of Vav by the Y174F mutation (Fig. 1B) (82). Although many candidates for such a role have been isolated using two-hybrid or coimmunoprecipitation experiments (c-Cbl, Socs, and hSiah2) (29, 48, 85), their precise functional roles in the Vav pathways remain to be fully addressed.
(iii) Downstream elements.
The signaling pathway used by Vav proteins was ill defined until the discovery of its biochemical activity. At that point, the Vav field converged with parallel studies of Rho/Rac proteins that had found an extensive number of effector molecules and biological responses under the control of these GTPases. Since then, a number of Rho/Rac effectors have been demonstrated for Vav, further confirming the functional relationship between this GEF and Rho/Rac proteins.
The biological responses induced by Vav can be placed in three different, although interdependent, groups: stimulation of known Rho/Rac effectors, activation of transcriptional factors, and induction of cytoskeleton-related responses. In the first category, several reports have shown the activation by Vav of JNK (26, 27, 48, 94, 138, 146), p21-activated kinase (PAK) (8), and PI-4-P5-kinase (PIP5-K) (108). JNK is a serine-threonine kinase involved in the phosphorylation of several transcriptional factors, such as c-Jun and ATF2 (153). PAKs are also serine-threonine kinases apparently linked to cytoskeletal responses and transcriptional activation (153). In addition, these kinases contribute to the activation of the Ras pathway via the stimulation of either Raf (in the case of PAK3) or MAPK/ERK kinase (MEK; in the case of PAK1) (133). PIP5-K is a lipid kinase involved in the phosphorylation of PI-4-P to produce PIP2. One of the functions of this kinase is to keep the intracellular pools of PIP2 constant during cell stimulation. Since PIP2 is the substrate of phospholipase C-γ1 (PLC-γ1), it is believed that the action of PIP5-K is crucial for maintaining Ca2+ fluxes during cell stimulation. PIP5-K has also been linked to cytoskeletal effects by facilitating the uncapping of F-actin ends (124). The activation of JNK and PIP5-K by Vav is mediated by Rac1 activation (26, 108). At the level of transcriptional regulation, Vav has been linked to the activation of the nuclear factor of activated T cells (NF-AT), SRF, and NF-κB (59, 96, 97, 156). NF-AT proteins are involved in a number of transcriptional responses in lymphocytes, including the transcription of the il-2 gene during T-cell stimulation (123). Incidentally, this is the only downstream element that was characterized prior to the identification of the biochemical function of Vav proteins. SRF and NF-κB both have roles in hematopoietic and nonhematopoietic cell signaling, linking extracellular stimuli to patterns of gene expression important for both cell growth and immune-specific responses (63, 123, 148). In agreement with the induction of these transcriptional responses, it has been shown that Vav stimulates the transcription of the il-2 and il-6 genes in T cells and mast cells (59, 138, 156). This action seems to be highly specific, since no effects have been observed in the transcription of other genes involved in hematopoietic regulation, such as those encoding IL-3, tumor necrosis factor alpha, transforming growth factor β, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (138).
While the above-mentioned signaling cascades have been characterized only for Vav, the morphological changes associated with F-actin reorganization have been characterized for all of the mammalian members of the family by using transient-transfection assays. For example, Vav proteins induce typical Rac1- and RhoG-like cytoskeletal changes in NIH 3T3 cells, including cell spreading, membrane ruffling, the formation of extensive lamellipodia, and contraction of the actomyosin ring. These changes are the consequence of the extensive reorganization of F-actin induced by Vav proteins (99, 135). In addition, Vav2 and Vav3 also induce the roundup of cells, a phenotype observed upon the transient expression of GTPases of the RhoA subfamily (99, 135). The intimate relationship between Vav proteins and the cytoskeleton is further demonstrated by recent results showing the partial colocalization of Vav3 with F-actin in several cytoskeletal structures (99). Interestingly, recent observations have established a direct link between changes in the actin dynamics and the induction of specific transcriptional responses (140). It is possible, therefore, that Vav-mediated changes will have an impact not only on the migration and morphology of cells but also in patterns of gene expression. F-actin polymerization was also found in the case of Vav by using Jurkat cells (8).
The coincidence between the biological responses induced by Vav and Rho family proteins suggests that the function of these exchange factors is primarily dependent on the activation of Rho/Rac pathways. Accordingly, mutations that affect the catalytic regions of Vav proteins destroy most of their known biological activities, including cellular transformation (26, 58, 99, 138; Lopez-Lago et al., unpublished). The importance of this pathway is also underlined by recent genetic experiments that have linked the phenotypes of vav-deficient mice to the disruption of many of the biological responses described above (see below). As an exception to this rule, recent observations from Weiss' laboratory suggest that the activation of NF-AT by Vav in T cells is, at least in part, independent of the catalytic activity of this exchange factor (77). It is likely, therefore, that other structural domains in addition to the DH region will contribute to the effector functions of the Vav proteins in hematopoietic cells. One obvious candidate for such a role is the N-terminal CH domain, since the deletion of this region totally eliminates the ability of Vav to induce NF-AT responses (5, 82, 156).
(iv) Synergy with other signaling pathways.
Recent advances in the signal transduction field indicate that the cellular responses triggered by extracellular stimuli are not the result of the additive effect of all the activated pathways but rather the multiplicative effect of cross talks that are established among them. The objective of those cross talks is to generate coherent signals by inducing synergistic interactions among pathways with complementary functions and, at the same time, by inhibiting routes with antagonistic effects in the biological response being generated by the cell (114). In the case of Vav, there are at least two important routes that synergize with this protein to induce more robust signaling responses: the Ras and the Slp76 pathways.
The connection of the Vav and Ras pathways was initially detected in focus formation assays, when it was observed that the cotransfection of vav and ras at suboptimal concentrations led to higher levels of transformation than when each oncogene was transfected alone (13). Moreover, it has been shown that the activation of complex biological responses by Vav (transformation and NF-AT stimulation) is dependent on the integrity of both Ras and Raf pathways (13, 68, 156). This is not a linear pathway, because other Rac1 responses, such as morphological change or JNK activation, are not affected by the inhibition of the Ras pathway (26; Movilla and Bustelo, unpublished). Although a detailed dissection of the Vav-Ras interaction remains to be done, several points of cross talk between them can be envisioned from the recent characterization of their signaling pathways. Thus, Vav could contribute to enhanced Ras signals via the activation of Raf and MEK by PAK3 and PAK1, respectively (Fig. 4B) (133). Some of these responses seem to be mediated by cell-type-specific signaling elements, because Vav stimulates extracellular signal-regulated kinase (ERK) activity in some cell types (NIH 3T3 and CHO) (68, 94) but not in others (COS1 and mast cells) (26, 106, 138). Conversely, Ras could enhance Vav-mediated signals via PI-3K, a known Ras effector (Fig. 4B) (87). However, Ras mutants that cannot interact with PI-3K can still synergize with Vav in cellular transformation, indicating that other, PI-3K-independent pathways are in place (Movilla and Bustelo, unpublished). This cross talk is conserved in other Vav family proteins, as Vav2 also synergizes with Ras in cellular transformation (135).
FIG. 4.
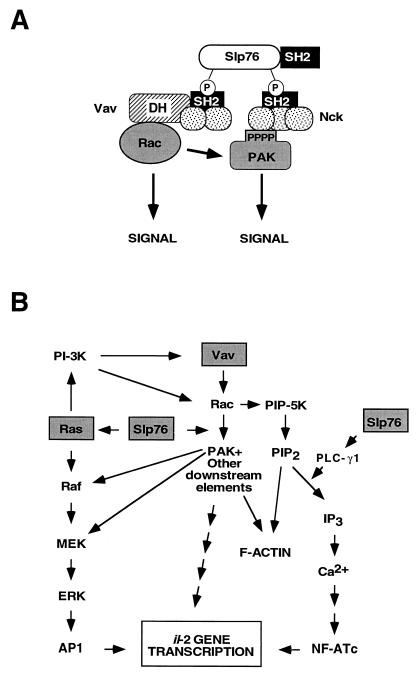
(A) Model for the synergy observed between Vav and Slp76. SH2 domains are indicated by closed boxes, SH3 domains are shown as dotted circles, the Vav DH domain is depicted as a shaded box, and the N-terminal domains of Slp76 are shown as an open box. PPPP, proline-rich region; P, phosphorylated tyrosine residues. (B) Possible points of functional interaction among the Vav, Ras, and Slp76 pathways. For simplicity, only one of the effector molecules of Rac (PAK) has been represented. The exact downstream elements of Vav leading to NF-AT activation have not been identified. The same applies to the pathways of Slp76 leading to ERK, PLC-γ1, and NF-AT activation.
Slp76 is a signaling protein expressed in T cells and myeloid cells that is composed of an N-terminal sterile alpha motif (SAM), an Ac region that contains several phosphorylation sites, a PR region, and a C-terminal SH2 domain (Fig. 3). Despite the absence of catalytic domains, Slp76 plays essential roles in T-cell signaling, coupling the activation of the TCR with increases in Ca2+, the stimulation of the Ras–mitogen-activated protein kinase pathway, and the activation of il-2 gene transcription (128). Coexpression of Vav with Slp76 induces a synergistic response, leading to enhanced activation of the NF-AT transcriptional activator (157). Interestingly, Vav and Slp76 form a very stable complex upon TCR cross-linking, an interaction mediated by the recognition of two phosphorylated residues located in the acidic domain of Slp76 (Y113ESP and, with less affinity, Y128ESP) by the Vav SH2 (Fig. 3 and 4A) (107, 120, 149, 157). However, this interaction seems to be optional rather than compulsory for NF-AT activation, because the synergistic response is still observed with mutants of Slp76 that cannot bind to Vav (35). Recent data indicate that these proteins talk to each other at different levels. On one hand, it has been shown that Slp76 serves as a scaffold protein to bring together the Vav-Rac complex and the Nck/PAK complex (Fig. 4A) (8). Nck is an adaptor protein containing three SH3 domains and one C-terminal SH2 that shows transforming potential. Nck binds to PAK via an SH3-PR region interaction and, at the same time, to the phosphorylated sequence Y145EPP of Slp76 via its SH2 domain (Fig. 4A) (8). By allowing the close proximity of all these proteins, Slp76 facilitates the rapid transmission of signals from Vav-activated Rac1 to PAK, leading to enhanced downstream signals (Fig. 4A) (8). In agreement with this model, it has been shown that Vav and Nck interact in a Slp76-dependent manner, leading to both enhanced PAK activation and actin polymerization after TCR cross-linking (8). Moreover, dominant-negative mutants of Vav and Nck abrogate both responses (8). Despite these observations, the scaffold model cannot be the determinant of the Vav-Slp76 synergy because this response is also observed in the absence of physical interaction between these two proteins (35, 120). There are at least three other routes for the possible cross talk between Vav and Slp76 (Fig. 4B). Thus, it is believed that Slp76 participates in the activation of Ca2+ fluxes during T-cell stimulation by facilitating the phosphorylation of PLC-γ1 (161). Vav can impinge on this route via the stimulation of PIP5-K (108), a Rac1 downstream element specialized in the conversion of PI-4-P into the PLC-γ1 substrate PIP2 (124). Slp76 has also been shown to be necessary for the activation of the Ras-ERK route, thus giving a new point of interaction with the Vav pathway (101, 161). At more distal locations in the signaling response, the NF-AT activation mediated by Vav can be enhanced by the stimulation of NF-AT cofactors (such as c-Jun and c-Fos) via Slp76 (101). The detailed understanding of the Vav-Slp76 synergy will have to wait for the characterization of the Slp76 binding proteins that mediate each of the biological responses attributed to this adapter protein. In addition to this functional cooperativity with Vav, it is likely that Slp76 also has functions of its own, as the phenotype of slp76-deficient mice is more severe than that observed in vav−/− mice (118). A similar pathway is likely to exist in B lymphocytes, as these cells express another adaptor protein (B-cell linker protein [BLNK]; also called Slp65) with structural and functional characteristics similar to those of Slp76 (41, 61).
GENETIC ANALYSIS OF THE VAV SIGNALING PATHWAY
Recently, several groups have developed mouse models in which the vav gene has been inactivated by homologous-recombination techniques. The phenotypic analysis of these mice confirmed the important role of Vav in the regulation of biological responses mediated by Rho/Rac proteins. Moreover, they have indicated that this role is highly specific, as only a few types of hematopoietic cells and, within them, only specific signaling pathways are affected. Perhaps more interestingly, the analysis of vav-deficient cells has revealed the implication of Vav in cellular responses not anticipated previously.
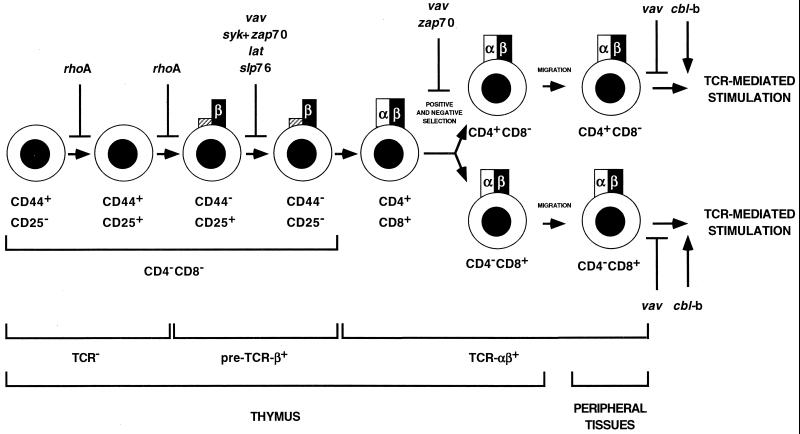
vav-deficient mice develop normally and are fully viable (37, 150). In addition, they show no developmental or functional abnormalities in most hematopoietic lineages, such as red cells, leukocytes, and platelets (37, 150, 166). The major defects are concentrated in the lymphoid compartment, as these animals lack B-1a B cells, have reduced numbers of T cells, and suffer from mature T and B lymphocytes incapable of mounting immune responses to antigens (37, 39, 145, 150, 165). The T lymphocytopenia found in vav-deficient mice derives from signaling defects of the pre-TCR and the TCR-αβ (Fig. 5). The inefficient signaling of the pre-TCR leads to a defective, although not totally impaired, transition from the CD44− CD25+ to the CD44− CD25− stage (37, 150). Later on, the reduced signaling output of the newly expressed TCR-αβ complex in the CD4+ CD8+ population results in impaired positive and negative selection and, as a consequence, in a significant reduction in the total number of mature cytotoxic and helper T cells (72, 150). The signaling defect of the TCR-αβ is maintained in the reduced populations of mature T cells, resulting in a lack of proliferative responses after TCR cross-linking (37, 39, 145, 150, 165). The main culprit in this proliferative arrest is the lack of IL-2 production, as addition of exogenous IL-2 to the stimulated cultures of vav−/− cells restores full cell growth (37, 145, 165). The absence of IL-2 production is due to a lack of transcriptional activation of the il-2 gene (25). These results agree with previous studies of Jurkat cells linking Vav to the activation of NF-AT and the IL-2 promoter (59, 156). IL-2 receptor (IL-2-R) and CD28-mediated responses are not affected in vav−/− T cells (37, 116, 145, 165), indicating that the function of Vav is a prerequisite for the function of a restricted set of receptors.
FIG. 5.
Schematic representation of thymocyte development. The processes affected by the vav gene knockout are indicated. Defects observed in other signaling proteins involved in the Vav pathways have been included for comparison. Inhibition or upregulation of a given T-cell response is indicated by blunt or sharp arrows, respectively. The α and β subunits of the TCR are indicated as open and solid boxes, respectively. The surrogate α chain of the pre-TCR is indicated as a hatched box.
The effect of the vav gene knockout in the B-cell compartment depends primarily on the type of B-cell lineage. Conventional B cells develop in normal numbers but fail to mount responses to stimuli specific for the BCR and CD19 receptors (37, 108, 145, 150, 165). In addition to these cell-autonomous defects, some deficient B-cell responses found in vivo were attributed to a lack of IL-4 secretion by helper T cells (51). These dysfunctions are also highly specific, because vav−/− B cells can proliferate normally in response to lipopolysaccharide or CD40 ligand plus IL-4 (145, 165). While vav gene expression is inconsequential for the development of normal B cells, it is a prerequisite for the generation of the B-1a (immunoglobulin M+ CD5+) subset, a specialized B-cell subpopulation localized in the peritoneum (37, 145, 165). Since these cells are only produced by the embryonic stem cells and rely on periodic BCR stimulation for their perpetuation in the adult (64), their dependency on Vav function is probably a reflection of the inefficacy of their BCR and CD19 receptors. Consistent with this, cd19−/− mice also lack this B-cell subpopulation (43).
The phenotype of vav-null animals is similar to, albeit milder than, those found for other proteins previously inserted in the Vav pathway (Fig. 5). Thus, lat, slp76, and syk-zap70 double-knockout animals show a complete arrest in the CD44− CD25+→CD44− CD25− transition (20, 118, 169). Zap70-deficient CD4+ CD8+ thymocytes show a total lack of positive and negative selection (20). Likewise, syk−/− B cells cannot proliferate after antigen stimulation (20). However, unlike vav−/− animals, syk-deficient animals also display abnormalities in B-cell development and platelet function (20). Abnormalities in platelet function are also observed in slp76-deficient animals (118). The more severe phenotype of those mutations is consistent with the idea that, in addition to Vav, these proteins mediate the activation of multiple pathways during receptor stimulation. In good agreement with the putative inhibitory role of Cbl-b in the Vav pathway, the phenotype of cbl-b−/− mice is the reverse of that found in vav−/− animals, including hyperproliferation of B and T cells upon antigen receptor stimulation and overproduction of IL-2. Interestingly, pathways not affected by the Vav deficiency (IL-4, CD40, and lipopolysaccharide) are not affected in cbl-b−/− mice either. The signaling dysfunctions of cbl-b−/− animals result in an autoimmune disease characterized by autoantibody production and infiltration of activated T and B lymphocytes into multiple organs. These studies have also indicated that the function of Cbl-b appears to be concentrated in mature cells, since no obvious abnormalities of T-cell development have been observed so far (J. Penninger and H. Gu, personal communication).
On the downstream side, Henning et al. have shown that the simultaneous inactivation of several Rho (RhoA to -C and Rac2) GTPases via the expression of the C3 transferase in thymocytes does not affect the positive and negative selection of thymocytes or the proliferation of mature T cells (55). Instead, these proteins seem to be required for the proliferation of CD4− CD8− thymocytes before the CD44− CD25+ stage (Fig. 5) (55). Likewise, the recent knockout of the rac2 locus showed that this GTPase has essential roles in neutrophils but not in lymphoid cells (125). Thus, it seems that most of the effects of the vav knockout may derive from the defective activation of Rac1, Rac3, or RhoG. The elucidation of the role of Rac1 in T-cell development will have to wait for the generation of rac1−/− rag−/− chimeric mice, as the deletion of the rac1 gene results in early-embryonic lethality (142).
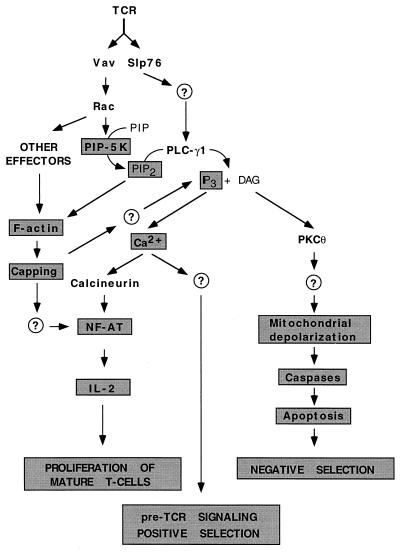
The signaling defect of vav-deficient cells seems concentrated in a very narrow window between the early and the late signaling responses triggered by antigen receptors (Fig. 6). For example, stimulated vav−/− T cells can trigger normal levels of Zap70 activation and CD3ζ phosphorylation. Likewise, the pattern of tyrosine-phosphorylated proteins is not altered, including the usual suspects, LAT, Slp76, and PLC-γ1 (25, 37, 39, 58). These results agree with the previous signaling experiments, indicating that Vav is downstream (CD3ζ, Zap70, and LAT), or in parallel position (Slp76 and PLC-γ1), to those molecules. Other distal signaling elements are fully functional, as demonstrated by the effective proliferation of vav−/− T cells after the addition of exogenous IL-2 or after treatment with phorbol myristate acetate plus ionophore (37, 145, 165). Despite this apparently normal sequence of events, vav−/− T cells cannot induce F-actin polymerization upon stimulation, and as a result, they show no aggregation of receptors after activation (Fig. 6) (37, 58). In addition, vav−/− cells cannot generate IP3 (25), leading to reduced levels of intracellular Ca2+ after TCR cross-linking (25, 37, 39, 58, 150). In B cells, this defect has been attributed to the lack of stimulation of PIP5-K (Fig. 6) (108). Interestingly, the treatment of wild-type T cells with an F-actin inhibitor induces vav−/−-like effects while the addition of a calcium ionophore partially restores the signaling properties of mature vav−/− T cells (25, 37, 58). These results indicate that these two defective signaling events are probably the crucial ones for the proliferative defects observed in mature T cells containing a null vav gene. While the lack of Ca2+ signaling is found in all knockouts reported, there exist considerable discrepancies regarding the signaling defects that follow. For instance, one group has reported that other expected downstream elements of the Vav pathway, such as NF-AT and NF-κB, are impaired in vav−/− T cells (25). In addition, the lack of JNK and PIP5-K activation was not observed after stimulation of vav−/− B cells via the CD19 receptor (108). In contrast, other groups have found no major alterations in JNK, NF-AT, or NF-κB in lymphocytes (37, 58). At this moment, it is unclear whether these discrepancies are due to the stimulation conditions used, the genetic background of the mice, or the nature of the knockout construct used. Thus, the nature of the defective signaling cascade that ultimately leads to a lack of proliferation of B and T lymphocytes remains to be fully explored.
FIG. 6.
Integrated view of the signaling defects observed in vav-deficient T cells. The biological process or signaling steps whose disruptions have been demonstrated experimentally are boxed. The role of PIP5-K in T cells is inferred from parallel studies with the B-cell CD19 receptor, but its role in T cells remains to be demonstrated experimentally. Possible new pathways predicted from the analysis of vav−/− lymphocytes are indicated by question marks. See the text for details.
Interestingly, the analysis of the role of Vav during negative selection has revealed new routes and signaling elements that are connected to the Vav pathway. Thus, the lack of negative selection in vav-deficient thymocytes has been attributed to a lack of activation of the apoptotic cascade (Fig. 6) (72). This is again a defect specific for signals derived from the TCR-αβ, since vav−/− thymocytes undergo normal apoptosis after treatment with dexamethasone or stimulation with the CD95 receptor (72). Interestingly, this abnormal response is also a reflection of inefficiencies in the pathways leading to F-actin polymerization and PLC-γ1 activity (72). However, in this case, the causative agent is not the absence of Ca2+ fluxes but the other product of PLC-γ1 hydrolytic activity, the second messenger diacylglycerol (Fig. 6) (72). Thus, the treatment of vav−/− thymocytes with phorbol myristate acetate restores the apoptotic defect of these cells, apparently by activating PKCθ (72), a Ca2+-independent PKC preferentially expressed in T cells (Fig. 6). Activation of PKCs was previously correlated with the function of the GTPases of the Rho/Rac family (4, 18), thus offering a new pathway for connecting the enzyme activity of Vav to its final biological effects.
In summary, the phenotype of vav-deficient mice can be attributed to problems in two possibly intertwined Rho/Rac-dependent signaling responses: abnormal F-actin organization and low performance of the PLC-γ1 pathway. However, more work will be needed in the future to fill the gaps between these two events and the final proliferative and apoptotic defects seen in vav−/− lymphocytes. In addition, it will be important to know the level of functional redundancy of Vav family members and how the catalytic and tissue expression specificities of each family member translate into different phenotypes.
CONCLUDING REMARKS
A decade has passed since the initial isolation of vav as an oncogene with an obscure function and an interesting pattern of expression. Work since then has revealed that the protein encoded by this transforming gene is a member of a highly conserved protein family, identified the biochemical function of the members of the family in vertebrates, and illuminated different aspects of the regulation of the activities of Vav proteins during signal transduction and oncogenesis. Despite this progress, much work remains to be done. Biochemical and structural studies have to be performed in order to identify the phosphorylation site(s) that triggers the activation of Vav proteins, to uncover the intramolecular changes that take place during the activation-deactivation cycle of these GEFs, and to understand the complex regulatory properties of the N-terminal CH region. Likewise, a careful dissection of the structural determinants that mediate the Vav-GTPase interaction is still missing. Currently, little information is available on the negative regulation of Vav via the formation of heteromolecular complexes, including the function of the negative regulatory site Y174. The interconnections between the Vav, Ras, and Slp76 pathways remain, for the most part, enigmatic. Finally, significant advances have to be made to understand all the signaling defects observed in vav−/− cells and to establish the role of each Vav family member, and of the family as a whole, in the development and physiology of mice. Most of these studies are approachable now thanks to the information gathered on Vav proteins during the last 10 years. In addition, the study of the pathway of Vav family proteins will benefit from related areas, such as the functional characterization of Slp76 and Rho/Rac proteins. The finding that Vav proteins are conserved in all multicellular organisms will allow us to approach, for the first time, the characterization of this family using genetic models, such as Drosophila melanogaster and C. elegans. Arguably, these multifaceted studies will define with more precision the pathways already discovered and unveil new ones not predicted yet. These future advances will, in turn, allow us to see with new eyes the mechanisms by which membrane receptors convert the incoming extracellular signals into the intracellular responses required for proliferation, differentiation, and apoptosis.
ACKNOWLEDGMENTS
I thank M. Dosil and J. Benach for their advice and suggestions during the elaboration of this work and A. Weiss, R. Rottapel, H. Gu, and J. Penninger for communicating results prior to publication.
X.R.B. is a Sinsheimer Scholar for Cancer Research whose own work is supported by the National Cancer Institute (CA7373501), the Baldwin Foundation for Breast Cancer Research, and the Association for International Cancer Research.
REFERENCES
- 1.Alai M, Mui A L, Cutler R L, Bustelo X R, Barbacid M, Krystal G. Steel factor stimulates the tyrosine phosphorylation of the proto-oncogene product, p95vav, in human hematopoietic cells. J Biol Chem. 1992;267:18021–18025. [PubMed] [Google Scholar]
- 2.Allen K M, Gleeson J G, Bagrodia S, Partington M W, MacMillan J C, Cerione R A, Mulley J C, Walsh C A. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998;20:25–30. doi: 10.1038/1675. [DOI] [PubMed] [Google Scholar]
- 3.August A, Gibson S, Kawakami Y, Kawakami T, Mills G B, Dupont B. CD28 is associated with and induces the immediate tyrosine phosphorylation and activation of the Tec family kinase ITK/EMT in the human Jurkat leukemic T-cell line. Proc Natl Acad Sci USA. 1994;91:9347–9351. doi: 10.1073/pnas.91.20.9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avraham A, Jung S, Samuels Y, Seger R, Ben-Neriah Y. Costimulation-dependent activation of a JNK-kinase in T lymphocytes. Eur J Immunol. 1998;28:2320–2330. doi: 10.1002/(SICI)1521-4141(199808)28:08<2320::AID-IMMU2320>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 5.Billadeau D D, Brumbaugh K M, Dick C J, Schoon R A, Bustelo X R, Leibson P J. The Vav-Rac1 pathway in cytotoxic lymphocytes regulates the generation of cell-mediated killing. J Exp Med. 1998;188:549–559. doi: 10.1084/jem.188.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogatcheva N V, Gusev N B. Interaction of smooth muscle calponin with phospholipids. FEBS Lett. 1995;371:123–126. doi: 10.1016/0014-5793(95)00868-a. [DOI] [PubMed] [Google Scholar]
- 7.Boguski M S, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 8.Bubeck Wardenburg J, Pappu R, Bu J Y, Mayer B, Chernoff J, Straus D, Chan A C. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity. 1998;9:607–616. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- 9.Bustelo X R. The VAV family of signal transduction molecules. Crit Rev Oncog. 1996;7:65–88. doi: 10.1615/critrevoncog.v7.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 10.Bustelo X R, Barbacid M. Tyrosine phosphorylation of the vav proto-oncogene product in activated B cells. Science. 1992;256:1196–1199. doi: 10.1126/science.256.5060.1196. [DOI] [PubMed] [Google Scholar]
- 11.Bustelo X R, Crespo P, Lopez-Barahona M, Gutkind J S, Barbacid M. Cbl-b, a member of the Sli-1/c-Cbl protein family, inhibits Vav-mediated c-Jun N-terminal kinase activation. Oncogene. 1997;15:2511–2520. doi: 10.1038/sj.onc.1201430. [DOI] [PubMed] [Google Scholar]
- 12.Bustelo X R, Ledbetter J A, Barbacid M. Product of vav proto-oncogene defines a new class of tyrosine protein kinase substrates. Nature. 1992;356:68–71. doi: 10.1038/356068a0. [DOI] [PubMed] [Google Scholar]
- 13.Bustelo X R, Suen K L, Leftheris K, Meyers C A, Barbacid M. Vav cooperates with Ras to transform rodent fibroblasts but is not a Ras GDP/GTP exchange factor. Oncogene. 1994;9:2405–2413. [PubMed] [Google Scholar]
- 14.Carugo K D, Banuelos S, Saraste M. Crystal structure of a calponin homology domain. Nat Struct Biol. 1997;4:175–179. doi: 10.1038/nsb0397-175. [DOI] [PubMed] [Google Scholar]
- 15.Castresana J, Saraste M. Does Vav bind to F-actin through a CH domain? FEBS Lett. 1995;374:149–151. doi: 10.1016/0014-5793(95)01098-y. [DOI] [PubMed] [Google Scholar]
- 16.Cerione R A, Zheng Y. The Dbl family of oncogenes. Curr Opin Cell Biol. 1996;8:216–222. doi: 10.1016/s0955-0674(96)80068-8. [DOI] [PubMed] [Google Scholar]
- 17.Chan A C, Kadlecek T A, Elder M E, Filipovich A H, Kuo W L, Iwashima M, Parslow T G, Weiss A. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–1601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 18.Chang J H, Pratt J C, Sawasdikosol S, Kapeller R, Burakoff S J. The small GTP-binding protein Rho potentiates AP-1 transcription in T cells. Mol Cell Biol. 1998;18:4986–4993. doi: 10.1128/mcb.18.9.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chardin P, Paris S, Antonny B, Robineau S, Beraud-Dufour S, Jackson C L, Chabre M. A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature. 1996;384:481–484. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- 20.Chu D H, Morita C T, Weiss A. The Syk family of protein tyrosine kinases in T-cell activation and development. Immunol Rev. 1998;165:167–180. doi: 10.1111/j.1600-065x.1998.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 21.Cichowski K, Brugge J S, Brass L F. Thrombin receptor activation and integrin engagement stimulate tyrosine phosphorylation of the proto-oncogene product, p95vav, in platelets. J Biol Chem. 1996;271:7544–7550. doi: 10.1074/jbc.271.13.7544. [DOI] [PubMed] [Google Scholar]
- 22.Cichowski K, Orsini M J, Brass L F. PAR1 activation initiates integrin engagement and outside-in signalling in megakaryoblastic CHRF-288 cells. Biochim Biophys Acta. 1999;1450:265–276. doi: 10.1016/s0167-4889(99)00065-8. [DOI] [PubMed] [Google Scholar]
- 23.Clevenger C V, Ngo W, Sokol D L, Luger S M, Gewirtz A M. Vav is necessary for prolactin-stimulated proliferation and is translocated into the nucleus of a T-cell line. J Biol Chem. 1995;270:13246–13253. doi: 10.1074/jbc.270.22.13246. [DOI] [PubMed] [Google Scholar]
- 24.Coppola J, Bryant S, Koda T, Conway D, Barbacid M. Mechanism of activation of the vav protooncogene. Cell Growth Differ. 1991;2:95–105. [PubMed] [Google Scholar]
- 25.Costello P S, Walters A E, Mee P J, Turner M, Reynolds L F, Prisco A, Sarner N, Zamoyska R, Tybulewicz V L. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-κB pathways. Proc Natl Acad Sci USA. 1999;96:3035–3040. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crespo P, Bustelo X R, Aaronson D S, Coso O A, Lopez-Barahona M, Barbacid M, Gutkind J S. Rac-1 dependent stimulation of the JNK/SAPK signaling pathway by Vav. Oncogene. 1996;13:455–460. [PubMed] [Google Scholar]
- 27.Crespo P, Schuebel K E, Ostrom A A, Gutkind J S, Bustelo X R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 28.Deckert M, Tartare-Deckert S, Couture C, Mustelin T, Altman A. Functional and physical interactions of Syk family kinases with the vav proto-oncogene product. Immunity. 1996;5:591–604. doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- 29.De Sepulveda P, Okkenhaug K, Rose J L, Hawley R G, Dubreuil P, Rottapel R. Socs1 binds to multiple signalling proteins and suppresses steel factor-dependent proliferation. EMBO J. 1999;18:904–915. doi: 10.1093/emboj/18.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doody G M, Justement L B, Delibrias C C, Matthews R J, Lin J, Thomas M L, Fearon D T. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242–244. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 31.Dorsch M, Fan P D, Bogenberger J, Goff S P. TPO and IL-3 induce overlapping but distinct protein tyrosine phosphorylation in a myeloid precursor cell line. Biochem Biophys Res Commun. 1995;214:424–431. doi: 10.1006/bbrc.1995.2304. [DOI] [PubMed] [Google Scholar]
- 32.Dorsch M, Fan P D, Danial N N, Rothman P B, Goff S P. The thrombopoietin receptor can mediate proliferation without activation of the Jak-STAT pathway. J Exp Med. 1997;186:1947–1955. doi: 10.1084/jem.186.12.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dosil M, Wang S, Lemischka I R. Mitogenic signalling and substrate specificity of the Flk2/Flt3 receptor tyrosine kinase in fibroblasts and interleukin 3-dependent hematopoietic cells. Mol Cell Biol. 1993;13:6572–6585. doi: 10.1128/mcb.13.10.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans G A, Howard O M, Erwin R, Farrar W L. Interleukin-2 induces tyrosine phosphorylation of the vav proto-oncogene product in human T cells: lack of requirement for the tyrosine kinase lck. Biochem J. 1993;294:339–342. doi: 10.1042/bj2940339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang N, Koretzky G A. SLP-76 and Vav function in separate, but overlapping pathways to augment interleukin-2 promoter activity. J Biol Chem. 1999;274:16206–16212. doi: 10.1074/jbc.274.23.16206. [DOI] [PubMed] [Google Scholar]
- 36.Finco T S, Kadlecek T, Zhang W, Samelson L E, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 37.Fischer K D, Kong Y Y, Nishina H, Tedford K, Marengere L E, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, Nghiem M P, Bouchard D, Barbacid M, Bernstein A, Penninger J M. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 38.Fischer K D, Tedford K, Penninger J M. Vav links antigen-receptor signaling to the actin cytoskeleton. Semin Immunol. 1998;10:317–327. doi: 10.1006/smim.1998.0124. [DOI] [PubMed] [Google Scholar]
- 39.Fischer K D, Zmuldzinas A, Gardner S, Barbacid M, Bernstein A, Guidos C. Defective T-cell receptor signalling and positive selection of Vav-deficient CD4+ CD8+ thymocytes. Nature. 1995;374:474–477. doi: 10.1038/374474a0. [DOI] [PubMed] [Google Scholar]
- 40.Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81:682–687. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 41.Fu C, Turck C W, Kurosaki T, Chan A C. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 42.Fujii T, Yamana K, Ogoma Y, Kondo Y. Interaction of calponin with phospholipids. J Biochem (Tokyo) 1995;117:999–1003. doi: 10.1093/oxfordjournals.jbchem.a124833. [DOI] [PubMed] [Google Scholar]
- 43.Fujimoto M, Poe J C, Inaoki M, Tedder T F. CD19 regulates B lymphocyte responses to transmembrane signals. Semin Immunol. 1998;10:267–277. doi: 10.1006/smim.1998.9999. [DOI] [PubMed] [Google Scholar]
- 44.Fujimoto M, Poe J C, Jansen P J, Sato S, Tedder T F. CD19 amplifies B lymphocyte signal transduction by regulating Src-family protein tyrosine kinase activation. J Immunol. 1999;162:7088–7094. [PubMed] [Google Scholar]
- 45.Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind J S. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J Biol Chem. 1999;274:5868–5879. doi: 10.1074/jbc.274.9.5868. [DOI] [PubMed] [Google Scholar]
- 46.Galandrini R, Palmieri G, Piccoli M, Frati L, Santoni A. Role for the Rac1 exchange factor Vav in the signaling pathways leading to NK cell cytotoxicity. J Immunol. 1999;162:3148–3152. [PubMed] [Google Scholar]
- 47.Geng Y, Gulbins E, Altman A, Lotz M. Monocyte deactivation by interleukin 10 via inhibition of tyrosine kinase activity and the Ras signaling pathway. Proc Natl Acad Sci USA. 1994;91:8602–8606. doi: 10.1073/pnas.91.18.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Germani A, Romero F, Houlard M, Camonis J, Gisselbrecht S, Fischer S, Varin-Blank N. hSiah2 is a new Vav binding protein which inhibits Vav-mediated signaling pathways. Mol Cell Biol. 1999;19:3798–3807. doi: 10.1128/mcb.19.5.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gringhuis S I, de Leij L F, Coffer P J, Vellenga E. Signaling through CD5 activates a pathway involving phosphatidylinositol 3-kinase, Vav, and Rac1 in human mature T lymphocytes. Mol Cell Biol. 1998;18:1725–1735. doi: 10.1128/mcb.18.3.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groysman M, Nagano M, Shaanan B, Katzav S. Mutagenic analysis of Vav reveals that an intact SH3 domain is required for transformation. Oncogene. 1998;17:1597–1606. doi: 10.1038/sj.onc.1202074. [DOI] [PubMed] [Google Scholar]
- 51.Gulbranson-Judge A, Tybulewicz V L, Walters A E, Toellner K M, MacLennan I C, Turner M. Defective immunoglobulin class switching in Vav-deficient mice is attributable to compromised T cell help. Eur J Immunol. 1999;29:477–487. doi: 10.1002/(SICI)1521-4141(199902)29:02<477::AID-IMMU477>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 52.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 53.Han J, Das B, Wei W, Van Aelst L, Mosteller R D, Khosravi-Far R, Westwick J K, Der C J, Broek D. Lck regulates Vav activation of members of the Rho family of GTPases. Mol Cell Biol. 1997;17:1346–1353. doi: 10.1128/mcb.17.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller R D, Krishna U M, Falck J R, White M A, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 55.Henning S W, Galandrini R, Hall A, Cantrell D A. The GTPase Rho has a critical regulatory role in thymus development. EMBO J. 1997;16:2397–2407. doi: 10.1093/emboj/16.9.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henske E P, Short M P, Jozwiak S, Bovey C M, Ramlakhan S, Haines J L, Kwiatkowski D J. Identification of VAV2 on 9q34 and its exclusion as the tuberous sclerosis gene TSC1. Ann Hum Genet. 1995;59:25–37. doi: 10.1111/j.1469-1809.1995.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 57.Hirasawa N, Scharenberg A, Yamamura H, Beaven M A, Kinet J P. A requirement for Syk in the activation of the microtubule-associated protein kinase/phospholipase A2 pathway by Fc epsilon R1 is not shared by a G protein-coupled receptor. J Biol Chem. 1995;270:10960–10967. doi: 10.1074/jbc.270.18.10960. [DOI] [PubMed] [Google Scholar]
- 58.Holsinger L J, Graef I A, Swat W, Chi T, Bautista D M, Davidson L, Lewis R S, Alt F W, Crabtree G R. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- 59.Holsinger L J, Spencer D M, Austin D J, Schreiber S L, Crabtree G R. Signal transduction in T lymphocytes using a conditional allele of Sos. Proc Natl Acad Sci USA. 1995;92:9810–9814. doi: 10.1073/pnas.92.21.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ihle J N. The Janus protein tyrosine kinases in hematopoietic cytokine signaling. Semin Immunol. 1995;7:247–254. doi: 10.1006/smim.1995.0029. [DOI] [PubMed] [Google Scholar]
- 61.Ishiai M, Kurosaki M, Pappu R, Okawa K, Ronko I, Fu C, Shibata M, Iwamatsu A, Chan A C, Kurosaki T. BLNK required for coupling Syk to PLC gamma 2 and Rac1-JNK in B cells. Immunity. 1999;10:117–125. doi: 10.1016/s1074-7613(00)80012-6. [DOI] [PubMed] [Google Scholar]
- 62.Joazeiro C A, Wing S S, Huang H, Leverson J D, Hunter T, Liu Y C. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 63.Johansen F E, Prywes R. Serum response factor: transcriptional regulation of genes induced by growth factors and differentiation. Biochim Biophys Acta. 1995;1242:1–10. doi: 10.1016/0304-419x(94)00014-s. [DOI] [PubMed] [Google Scholar]
- 64.Kantor A B, Stall A M, Adams S, Herzenberg L A. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci USA. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katzav S, Cleveland J L, Heslop H E, Pulido D. Loss of the amino-terminal helix-loop-helix domain of the vav proto-oncogene activates its transforming potential. Mol Cell Biol. 1991;11:1912–1920. doi: 10.1128/mcb.11.4.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katzav S, Martin-Zanca D, Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989;8:2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katzav S, Sutherland M, Packham G, Yi T, Weiss A. The protein tyrosine kinase ZAP-70 can associate with the SH2 domain of proto-Vav. J Biol Chem. 1994;269:32579–32585. [PubMed] [Google Scholar]
- 68.Khosravi-Far R, Chrzanowska-Wodnicka M, Solski P A, Eva A, Burridge K, Der C J. Dbl and Vav mediate transformation via mitogen-activated protein kinase pathways that are distinct from those activated by oncogenic Ras. Mol Cell Biol. 1994;14:6848–6857. doi: 10.1128/mcb.14.10.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kiener P A, Rankin B M, Burkhardt A L, Schieven G L, Gilliland L K, Rowley R B, Bolen J B, Ledbetter J A. Cross-linking of Fc gamma receptor I (Fc gamma RI) and receptor II (Fc gamma RII) on monocytic cells activates a signal transduction pathway common to both Fc receptors that involves the stimulation of p72 Syk protein tyrosine kinase. J Biol Chem. 1993;268:24442–24448. [PubMed] [Google Scholar]
- 70.Kim H H, Tharayil M, Rudd C E. Growth factor receptor-bound protein 2 SH2/SH3 domain binding to CD28 and its role in co-signaling. J Biol Chem. 1998;273:296–301. doi: 10.1074/jbc.273.1.296. [DOI] [PubMed] [Google Scholar]
- 71.Kiyono M, Satoh T, Kaziro Y. G protein βγ subunit-dependent Rac-guanine nucleotide exchange activity of Ras-GRF1/CDC25Mm. Proc Natl Acad Sci USA. 1999;96:4826–4831. doi: 10.1073/pnas.96.9.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong Y Y, Fischer K D, Bachmann M F, Mariathasan S, Kozieradzki I, Nghiem M P, Bouchard D, Bernstein A, Ohashi P S, Penninger J M. Vav regulates peptide-specific apoptosis in thymocytes. J Exp Med. 1998;188:2099–2111. doi: 10.1084/jem.188.11.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kon-Kozlowski M, Pani G, Pawson T, Siminovitch K A. The tyrosine phosphatase PTP1C associates with Vav, Grb2, and mSos1 in hematopoietic cells. J Biol Chem. 1996;271:3856–3862. doi: 10.1074/jbc.271.7.3856. [DOI] [PubMed] [Google Scholar]
- 74.Kozasa T, Jiang X, Hart M J, Sternweis P M, Singer W D, Gilman A G, Bollag G, Sternweis P C. p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 75.Kranewitter W J, Gimona M. N-terminally truncated Vav induces the formation of depolymerization-resistant actin filaments in NIH 3T3 cells. FEBS Lett. 1999;455:123–129. doi: 10.1016/s0014-5793(99)00857-1. [DOI] [PubMed] [Google Scholar]
- 76.Krumenacker J S, Montgomery D W, Buckley D J, Gout P W, Buckley A R. Prolactin receptor signaling: shared components with the T-cell antigen receptor in Nb2 lymphoma cells. Endocrine. 1998;9:313–320. doi: 10.1385/ENDO:9:3:313. [DOI] [PubMed] [Google Scholar]
- 77.Kuhne, M. R., G. K. Ku, and A. Weiss. A guanine nucleotide exchange factor-independent function of Vav1 in transcriptional activation. J. Biol. Chem., in press. [DOI] [PubMed]
- 78.Lee I S, Liu Y, Narazaki M, Hibi M, Kishimoto T, Taga T. Vav is associated with signal transducing molecules gp130, Grb2 and Erk2, and is tyrosine phosphorylated in response to interleukin-6. FEBS Lett. 1997;401:133–137. doi: 10.1016/s0014-5793(96)01456-1. [DOI] [PubMed] [Google Scholar]
- 79.Leibson P J. Signal transduction during natural killer cell activation: inside the mind of a killer. Immunity. 1997;6:655–661. doi: 10.1016/s1074-7613(00)80441-0. [DOI] [PubMed] [Google Scholar]
- 80.Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon W Y, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li X, Sandoval D, Freeberg L, Carter R H. Role of CD19 tyrosine 391 in synergistic activation of B lymphocytes by coligation of CD19 and membrane Ig. J Immunol. 1997;158:5649–5657. [PubMed] [Google Scholar]
- 82.Lopez-Lago M, Lee H, Cruz C, Movilla N, Bustelo X R. Tyrosine phosphorylation mediates both activation and downmodulation of the biological activity of Vav. Mol Cell Biol. 2000;20:1678–1691. doi: 10.1128/mcb.20.5.1678-1691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma A D, Metjian A, Bagrodia S, Taylor S, Abrams C S. Cytoskeletal reorganization by G protein-coupled receptors is dependent on phosphoinositide 3-kinase gamma, a Rac guanosine exchange factor, and Rac. Mol Cell Biol. 1998;18:4744–4751. doi: 10.1128/mcb.18.8.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Machide M, Mano H, Todokoro K. Interleukin 3 and erythropoietin induce association of Vav with Tec kinase through Tec homology domain. Oncogene. 1995;11:619–625. [PubMed] [Google Scholar]
- 85.Marengere L E, Mirtsos C, Kozieradzki I, Veillette A, Mak T W, Penninger J M. Proto-oncoprotein Vav interacts with c-Cbl in activated thymocytes and peripheral T cells. J Immunol. 1997;159:70–76. [PubMed] [Google Scholar]
- 86.Margolis B, Hu P, Katzav S, Li W, Oliver J M, Ullrich A, Weiss A, Schlessinger J. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 1992;356:71–74. doi: 10.1038/356071a0. [DOI] [PubMed] [Google Scholar]
- 87.Marshall C J. Ras effectors. Curr Opin Cell Biol. 1996;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- 88.Martinerie C, Cannizzaro L A, Croce C M, Huebner K, Katzav S, Barbacid M. The human VAV proto-oncogene maps to chromosome region 19p12-19p13.2. Hum Genet. 1990;86:65–68. doi: 10.1007/BF00205175. [DOI] [PubMed] [Google Scholar]
- 89.Matsuguchi T, Inhorn R C, Carlesso N, Xu G, Druker B, Griffin J D. Tyrosine phosphorylation of p95Vav in myeloid cells is regulated by GM-CSF, IL-3 and steel factor and is constitutively increased by p210BCR/ABL. EMBO J. 1995;14:257–265. doi: 10.1002/j.1460-2075.1995.tb06999.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 90.Melamed I, Patel H, Brodie C, Gelfand E W. Activation of Vav and Ras through the nerve growth factor and B cell receptors by different kinases. Cell Immunol. 1999;191:83–89. doi: 10.1006/cimm.1998.1402. [DOI] [PubMed] [Google Scholar]
- 91.Meng W, Sawasdikosol S, Burakoff S J, Eck M J. Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature. 1999;398:84–90. doi: 10.1038/18050. [DOI] [PubMed] [Google Scholar]
- 92.Michel F, Grimaud L, Tuosto L, Acuto O. Fyn and ZAP-70 are required for Vav phosphorylation in T cells stimulated by antigen-presenting cells. J Biol Chem. 1998;273:31932–31938. doi: 10.1074/jbc.273.48.31932. [DOI] [PubMed] [Google Scholar]
- 93.Michiels F, Stam J C, Hordijk P L, van der Kammen R A, Ruuls-Van Stalle L, Feltkamp C A, Collard J G. Regulated membrane localization of Tiam1, mediated by the NH2-terminal pleckstrin homology domain, is required for Rac-dependent membrane ruffling and c-Jun NH2-terminal kinase activation. J Cell Biol. 1997;137:387–398. doi: 10.1083/jcb.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miranti C K, Leng L, Maschberger P, Brugge J S, Shattil S J. Identification of a novel integrin signaling pathway involving the kinase Syk and the guanine nucleotide exchange factor Vav1. Curr Biol. 1998;8:1289–1299. doi: 10.1016/s0960-9822(07)00559-3. [DOI] [PubMed] [Google Scholar]
- 95.Miura O, Miura Y, Nakamura N, Quelle F W, Witthuhn B A, Ihle J N, Aoki N. Induction of tyrosine phosphorylation of Vav and expression of Pim-1 correlates with Jak2-mediated growth signaling from the erythropoietin receptor. Blood. 1994;84:4135–4141. [PubMed] [Google Scholar]
- 96.Montaner S, Perona R, Saniger L, Lacal J C. Activation of serum response factor by RhoA is mediated by the nuclear factor-kappaB and C/EBP transcription factors. J Biol Chem. 1999;274:8506–8515. doi: 10.1074/jbc.274.13.8506. [DOI] [PubMed] [Google Scholar]
- 97.Montaner S, Perona R, Saniger L, Lacal J C. Multiple signalling pathways lead to the activation of the nuclear factor kappaB by the Rho family of GTPases. J Biol Chem. 1998;273:12779–12785. doi: 10.1074/jbc.273.21.12779. [DOI] [PubMed] [Google Scholar]
- 98.Morita H, Tahara T, Matsumoto A, Kato T, Miyazaki H, Ohashi H. Functional analysis of the cytoplasmic domain of the human Mpl receptor for tyrosine-phosphorylation of the signaling molecules, proliferation and differentiation. FEBS Lett. 1996;395:228–234. doi: 10.1016/0014-5793(96)01047-2. [DOI] [PubMed] [Google Scholar]
- 99.Movilla N, Bustelo X R. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol Cell Biol. 1999;19:7870–7885. doi: 10.1128/mcb.19.11.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mu S X, Xia M, Elliott G, Bogenberger J, Swift S, Bennett L, Lappinga D L, Hecht R, Lee R, Saris C J. Megakaryocyte growth and development factor and interleukin-3 induce patterns of protein-tyrosine phosphorylation that correlate with dominant differentiation over proliferation of mpl-transfected 32D cells. Blood. 1995;86:4532–4543. [PubMed] [Google Scholar]
- 101.Musci M A, Motto D G, Ross S E, Fang N, Koretzky G A. Three domains of SLP-76 are required for its optimal function in a T cell line. J Immunol. 1997;159:1639–1647. [PubMed] [Google Scholar]
- 102.Nagata Y, Todokoro K. Thrombopoietin induces activation of at least two distinct signaling pathways. FEBS Lett. 1995;377:497–501. doi: 10.1016/0014-5793(95)01386-5. [DOI] [PubMed] [Google Scholar]
- 103.Nimnual A S, Yatsula B A, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 104.Nunes J A, Collette Y, Truneh A, Olive D, Cantrell D A. The role of p21ras in CD28 signal transduction: triggering of CD28 with antibodies, but not the ligand B7-1, activates p21ras. J Exp Med. 1994;180:1067–1076. doi: 10.1084/jem.180.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Okumura K, Kaneko Y, Nonoguchi K, Nishiyama H, Yokoi H, Higuchi T, Itoh K, Yoshida O, Miki T, Fujita J. Expression of a novel isoform of Vav, Vav-T, containing a single Src homology 3 domain in murine testicular germ cells. Oncogene. 1997;14:713–720. doi: 10.1038/sj.onc.1200878. [DOI] [PubMed] [Google Scholar]
- 106.Olson M F, Pasteris N G, Gorski J L, Hall A. Faciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr Biol. 1996;6:1628–1633. doi: 10.1016/s0960-9822(02)70786-0. [DOI] [PubMed] [Google Scholar]
- 107.Onodera H, Motto D G, Koretzky G A, Rothstein D M. Differential regulation of activation-induced tyrosine phosphorylation and recruitment of SLP-76 to Vav by distinct isoforms of the CD45 protein-tyrosine phosphatase. J Biol Chem. 1996;271:22225–22230. doi: 10.1074/jbc.271.36.22225. [DOI] [PubMed] [Google Scholar]
- 108.O'Rourke L M, Tooze R, Turner M, Sandoval D M, Carter R H, Tybulewicz V L, Fearon D T. CD19 as a membrane-anchored adaptor protein of B lymphocytes: costimulation of lipid and protein kinases by recruitment of Vav. Immunity. 1998;8:635–645. doi: 10.1016/s1074-7613(00)80568-3. [DOI] [PubMed] [Google Scholar]
- 109.Ota Y, Samelson L E. The product of the proto-oncogene c-cbl: a negative regulator of the Syk tyrosine kinase. Science. 1997;276:418–420. doi: 10.1126/science.276.5311.418. [DOI] [PubMed] [Google Scholar]
- 110.Padmore L, An S, Gunby R H, Kelly K, Radda G K, Knox K A. CD40-triggered protein tyrosine phosphorylation on Vav and on phosphatidylinositol 3-kinase correlates with survival of the Ramos-Burkitt lymphoma B cell line. Cell Immunol. 1997;177:119–128. doi: 10.1006/cimm.1997.1102. [DOI] [PubMed] [Google Scholar]
- 111.Pandey, A., A. V. Podtelejnikov, B. Blagoev, X. R. Bustelo, M. Mann, and H. F. Lodish. Analysis of signaling complexes by mass spectrometry: identification of Vav-2 as a novel substrate of the epidermal growth factor receptor. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 112.Pani G, Fischer K D, Mlinaric-Rascan I, Siminovitch K A. Signaling capacity of the T cell antigen receptor is negatively regulated by the PTP1C tyrosine phosphatase. J Exp Med. 1996;184:839–852. doi: 10.1084/jem.184.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pasteris N G, Cadle A, Logie L J, Porteous M E, Schwartz C E, Stevenson R E, Glover T W, Wilroy R S, Gorski J L. Isolation and characterization of the faciogenital dysplasia (Aarskog-Scott syndrome) gene: a putative Rho/Rac guanine nucleotide exchange factor. Cell. 1994;79:669–678. doi: 10.1016/0092-8674(94)90552-5. [DOI] [PubMed] [Google Scholar]
- 114.Pawson T, Saxton T M. Signaling networks—do all roads lead to the same genes? Cell. 1999;97:675–678. doi: 10.1016/s0092-8674(00)80779-5. [DOI] [PubMed] [Google Scholar]
- 115.Pedraza-Alva G, Merida L B, Burakoff S J, Rosenstein Y. T cell activation through the CD43 molecule leads to Vav tyrosine phosphorylation and mitogen-activated protein kinase pathway activation. J Biol Chem. 1998;273:14218–14224. doi: 10.1074/jbc.273.23.14218. [DOI] [PubMed] [Google Scholar]
- 116.Penninger J M, Fischer K D, Sasaki T, Kozieradzki I, Le J, Tedford K, Bachmaier K, Ohashi P S, Bachmann M F. The oncogene product Vav is a crucial regulator of primary cytotoxic T cell responses but has no apparent role in CD28-mediated co-stimulation. Eur J Immunol. 1999;29:1709–1718. doi: 10.1002/(SICI)1521-4141(199905)29:05<1709::AID-IMMU1709>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 117.Penninger J M, Mak T W. Thymocyte selection in Vav and IRF-1 gene-deficient mice. Immunol Rev. 1998;165:149–166. doi: 10.1111/j.1600-065x.1998.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 118.Pivniouk V, Tsitsikov E, Swinton P, Rathbun G, Alt F W, Geha R S. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 119.Platanias L C, Sweet M E. Interferon alpha induces rapid tyrosine phosphorylation of the vav proto-oncogene product in hematopoietic cells. J Biol Chem. 1994;269:3143–3146. [PubMed] [Google Scholar]
- 120.Raab M, da Silva A J, Findell P R, Rudd C E. Regulation of Vav-SLP-76 binding by ZAP-70 and its relevance to TCR zeta/CD3 induction of interleukin-2. Immunity. 1997;6:155–164. doi: 10.1016/s1074-7613(00)80422-7. [DOI] [PubMed] [Google Scholar]
- 121.Ramos-Morales F, Druker B J, Fischer S. Vav binds to several SH2/SH3 containing proteins in activated lymphocytes. Oncogene. 1994;9:1917–1923. [PubMed] [Google Scholar]
- 122.Ramos-Morales F, Romero F, Schweighoffer F, Bismuth G, Camonis J, Tortolero M, Fischer S. The proline-rich region of Vav binds to Grb2 and Grb3-3. Oncogene. 1995;11:1665–1669. [PubMed] [Google Scholar]
- 123.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 124.Ren X D, Schwartz M A. Regulation of inositol lipid kinases by Rho and Rac. Curr Opin Genet Dev. 1998;8:63–67. doi: 10.1016/s0959-437x(98)80063-4. [DOI] [PubMed] [Google Scholar]
- 125.Roberts A W, Kim C, Zhen L, Lowe J B, Kapur R, Petryniak B, Spaetti A, Pollock J D, Borneo J B, Bradford G B, Atkinson S J, Dinauer M C, Williams D A. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 126.Romero F, Fischer S. Structure and function of vav. Cell Signal. 1996;8:545–553. doi: 10.1016/s0898-6568(96)00118-0. [DOI] [PubMed] [Google Scholar]
- 127.Rottapel R, Turck C W, Casteran N, Liu X, Birnbaum D, Pawson T, Dubreuil P. Substrate specificities and identification of a putative binding site for PI3K in the carboxy tail of the murine Flt3 receptor tyrosine kinase. Oncogene. 1994;9:1755–1765. [PubMed] [Google Scholar]
- 128.Rudd C E. Adaptors and molecular scaffolds in immune cell signaling. Cell. 1999;96:5–8. doi: 10.1016/s0092-8674(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 129.Salojin K V, Zhang J, Delovitch T L. TCR and CD28 are coupled via ZAP-70 to the activation of the Vav/Rac-1-/PAK-1/p38 MAPK signaling pathway. J Immunol. 1999;163:844–853. [PubMed] [Google Scholar]
- 130.Sasaki K, Odai H, Hanazono Y, Ueno H, Ogawa S, Langdon W Y, Tanaka T, Miyagawa K, Mitani K, Yazaki Y, et al. TPO/c-mpl ligand induces tyrosine phosphorylation of multiple cellular proteins including proto-oncogene products, Vav and c-Cbl, and Ras signaling molecules. Biochem Biophys Res Commun. 1995;216:338–347. doi: 10.1006/bbrc.1995.2629. [DOI] [PubMed] [Google Scholar]
- 131.Sato S, Jansen P J, Tedder T F. CD19 and CD22 expression reciprocally regulates tyrosine phosphorylation of Vav protein during B lymphocyte signaling. Proc Natl Acad Sci USA. 1997;94:13158–13162. doi: 10.1073/pnas.94.24.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sato S, Katagiri T, Takaki S, Kikuchi Y, Hitoshi Y, Yonehara S, Tsukada S, Kitamura D, Watanabe T, Witte O, et al. IL-5 receptor-mediated tyrosine phosphorylation of SH2/SH3-containing proteins and activation of Bruton's tyrosine and Janus 2 kinases. J Exp Med. 1994;180:2101–2111. doi: 10.1084/jem.180.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schaeffer H J, Weber M J. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schuebel K E, Bustelo X R, Nielsen D A, Song B J, Barbacid M, Goldman D, Lee I J. Isolation and characterization of murine vav2, a member of the vav family of proto-oncogenes. Oncogene. 1996;13:363–371. [PubMed] [Google Scholar]
- 135.Schuebel K E, Movilla N, Rosa J L, Bustelo X R. Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 1998;17:6608–6621. doi: 10.1093/emboj/17.22.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smyth L A, Williams O, Huby R D, Norton T, Acuto O, Ley S C, Kioussis D. Altered peptide ligands induce quantitatively but not qualitatively different intracellular signals in primary thymocytes. Proc Natl Acad Sci USA. 1998;95:8193–8198. doi: 10.1073/pnas.95.14.8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Song J S, Gomez J, Stancato L F, Rivera J. Association of a p95 Vav-containing signaling complex with the FcepsilonRI gamma chain in the RBL-2H3 mast cell line. Evidence for a constitutive in vivo association of Vav with Grb2, Raf-1, and ERK2 in an active complex. J Biol Chem. 1996;271:26962–26970. doi: 10.1074/jbc.271.43.26962. [DOI] [PubMed] [Google Scholar]
- 138.Song J S, Haleem-Smith H, Arudchandran R, Gomez J, Scott P M, Mill J F, Tan T H, Rivera J. Tyrosine phosphorylation of Vav stimulates IL-6 production in mast cells by a Rac/c-Jun N-terminal kinase-dependent pathway. J Immunol. 1999;163:802–810. [PubMed] [Google Scholar]
- 139.Songyang Z, Shoelson S E, McGlade J, Olivier P, Pawson T, Bustelo X R, Barbacid M, Sabe H, Hanafusa H, Yi T, et al. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994;14:2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 141.Stradal T, Kranewitter W, Winder S J, Gimona M. CH domains revisited. FEBS Lett. 1998;431:134–137. doi: 10.1016/s0014-5793(98)00751-0. [DOI] [PubMed] [Google Scholar]
- 142.Sugihara K, Nakatsuji N, Nakamura K, Nakao K, Hashimoto R, Otani H, Sakagami H, Kondo H, Nozawa S, Aiba A, Katsuki M. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene. 1998;17:3427–3433. doi: 10.1038/sj.onc.1202595. [DOI] [PubMed] [Google Scholar]
- 143.Suwa H, Ohshio G, Imamura T, Watanabe G, Arii S, Imamura M, Narumiya S, Hiai H, Fukumoto M. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer. 1998;77:147–152. doi: 10.1038/bjc.1998.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Symons M, Derry J M, Karlak B, Jiang S, Lemahieu V, McCormick F, Francke U, Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- 145.Tarakhovsky A, Turner M, Schaal S, Mee P J, Duddy L P, Rajewsky K, Tybulewicz V L. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature. 1995;374:467–470. doi: 10.1038/374467a0. [DOI] [PubMed] [Google Scholar]
- 146.Teramoto H, Salem P, Robbins K C, Bustelo X R, Gutkind J S. Tyrosine phosphorylation of the vav proto-oncogene product links FcɛRI to the Rac1-JNK pathway. J Biol Chem. 1997;272:10751–10755. doi: 10.1074/jbc.272.16.10751. [DOI] [PubMed] [Google Scholar]
- 147.Thien C B, Langdon W Y. c-Cbl: a regulator of T cell receptor-mediated signalling. Immunol Cell Biol. 1998;76:473–482. doi: 10.1046/j.1440-1711.1998.00768.x. [DOI] [PubMed] [Google Scholar]
- 148.Treisman R. Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev. 1994;4:96–101. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 149.Tuosto L, Michel F, Acuto O. p95vav associates with tyrosine-phosphorylated SLP-76 in antigen-stimulated T cells. J Exp Med. 1996;184:1161–1166. doi: 10.1084/jem.184.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Turner M, Mee P J, Walters A E, Quinn M E, Mellor A L, Zamoyska R, Tybulewicz V L. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 1997;7:451–460. doi: 10.1016/s1074-7613(00)80367-2. [DOI] [PubMed] [Google Scholar]
- 151.Uddin S, Katzav S, White M F, Platanias L C. Insulin-dependent tyrosine phosphorylation of the vav protooncogene product in cells of hematopoietic origin. J Biol Chem. 1995;270:7712–7716. doi: 10.1074/jbc.270.13.7712. [DOI] [PubMed] [Google Scholar]
- 152.Uddin S, Yetter A, Katzav S, Hofmann C, White M F, Platanias L C. Insulin-like growth factor-1 induces rapid tyrosine phosphorylation of the vav proto-oncogene product. Exp Hematol. 1996;24:622–627. [PubMed] [Google Scholar]
- 153.Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 154.Weng W K, Jarvis L, LeBien T W. Signaling through CD19 activates Vav/mitogen-activated protein kinase pathway and induces formation of a CD19/Vav/phosphatidylinositol 3-kinase complex in human B cell precursors. J Biol Chem. 1994;269:32514–32521. [PubMed] [Google Scholar]
- 155.Wilson R, Ainscough R, Anderson K, Baynes C, Berks M, Bonfield J, Burton J, Connell M, Copsey T, Cooper J, et al. 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. Nature. 1994;368:32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]
- 156.Wu J, Katzav S, Weiss A. A functional T-cell receptor signaling pathway is required for p95vav activity. Mol Cell Biol. 1995;15:4337–4346. doi: 10.1128/mcb.15.8.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu J, Motto D G, Koretzky G A, Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4:593–602. doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- 158.Wu J, Zhao Q, Kurosaki T, Weiss A. The Vav binding site (Y315) in ZAP-70 is critical for antigen receptor-mediated signal transduction. J Exp Med. 1997;185:1877–1882. doi: 10.1084/jem.185.10.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 160.Xu X, Chong A S. Vav in natural killer cells is tyrosine phosphorylated upon cross-linking of Fc gamma RIIIA and is constitutively associated with a serine/threonine kinase. Biochem J. 1996;318:527–532. doi: 10.1042/bj3180527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yablonski D, Kuhne M R, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 162.Ye Z S, Baltimore D. Binding of Vav to Grb2 through dimerization of Src homology 3 domains. Proc Natl Acad Sci USA. 1994;91:12629–12633. doi: 10.1073/pnas.91.26.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yeung Y G, Wang Y, Einstein D B, Lee P S, Stanley E R. Colony-stimulating factor-1 stimulates the formation of multimeric cytosolic complexes of signaling proteins and cytoskeletal components in macrophages. J Biol Chem. 1998;273:17128–17137. doi: 10.1074/jbc.273.27.17128. [DOI] [PubMed] [Google Scholar]
- 164.Yuo A, Kitagawa S, Iki S, Yagisawa M, Inuo E K, Mimura T, Minoda S, Hanazono Y, Hirai H, Urabe A, et al. Tyrosine phosphorylation of vav protooncogene product in primary human myelogenous leukemic cells stimulated by granulocyte colony-stimulating factor. Biochem Biophys Res Commun. 1995;211:677–685. doi: 10.1006/bbrc.1995.1865. [DOI] [PubMed] [Google Scholar]
- 165.Zhang R, Alt F W, Davidson L, Orkin S H, Swat W. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature. 1995;374:470–473. doi: 10.1038/374470a0. [DOI] [PubMed] [Google Scholar]
- 166.Zhang R, Tsai F Y, Orkin S H. Hematopoietic development of vav−/− mouse embryonic stem cells. Proc Natl Acad Sci USA. 1994;91:12755–12759. doi: 10.1073/pnas.91.26.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang W, Irvin B J, Trible R P, Abraham R T, Samelson L E. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol. 1999;11:943–950. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- 168.Zhang W, Sloan-Lancaster J, Kitchen J, Trible R P, Samelson L E. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 169.Zhang W, Sommers C L, Burshtyn D N, Stebbins C C, DeJarnette J B, Trible R P, Grinberg A, Tsay H C, Jacobs H M, Kessler C M, Long E O, Love P E, Samelson L E. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 170.Zhang W, Trible R P, Samelson L E. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 171.Zhao Q, Williams B L, Abraham R T, Weiss A. Interdomain B in ZAP-70 regulates but is not required for ZAP-70 signaling function in lymphocytes. Mol Cell Biol. 1999;19:948–956. doi: 10.1128/mcb.19.1.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zohn I M, Campbell S L, Khosravi-Far R, Rossman K L, Der C J. Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene. 1998;17:1415–1438. doi: 10.1038/sj.onc.1202181. [DOI] [PubMed] [Google Scholar]