Abstract
Despite its frequent use for pain relief, no experimental pain research has tested the analgesic effects of cannabidiol (CBD) in humans. The goal of this study was to experimentally test the effects of CBD and expectancies for receiving CBD on human pain reactivity. Using a crossover, 2 × 2 factorial balanced placebo design, drug administration (given inactive substance or given active CBD) and verbal instruction sets (told inactive substance or told active CBD) were experimentally manipulated. Fifteen healthy adults each completed 4 separate experimental sessions. Participants were randomly assigned to different counterbalanced manipulation conditions at each session: control (told inactive – given inactive); expectancy (told active CBD – given inactive); drug (told inactive – given active CBD); and expectancy+drug (told active CBD – given active CBD). Primary outcomes were pain threshold, tolerance, intensity, unpleasantness, conditioned pain modulation (CPM), and offset analgesia (OA). There was a significant main effect of instructions on OA, such that the OA response was significantly larger when participants were told that they received CBD, regardless of drug content. Pain unpleasantness was significantly reduced in the drug, expectancy, and expectancy+drug conditions, relative to the control condition. The drug and expectancy conditions separately improved CPM, whereas the expectancy+drug and control conditions produced the lowest CPM change scores. We did not detect significant effects for pain threshold, tolerance, or intensity. Our results indicated that separate pain outcomes can be differentially affected by CBD and/or expectancies for receiving CBD. Future investigations of the psychological and pharmacological mechanisms underlying CBD analgesia are warranted.
Keywords: cannabidiol, CBD, expectancy effects, experimental pain reactivity
Cannabidiol (CBD) has attracted widespread interest as a nonintoxicating alternative to traditional cannabinoid-based analgesics (Russo, 2017). CBD has shown a good safety profile and low abuse potential in humans (Corroon & Phillips, 2018; Iffland & Grotenhermen, 2017; Pisanti et al., 2017; World Health Organization Expert Committee on Drug Dependence, 2018). Cross-sectional research has implicated pain as the most common medical condition for which CBD is used (Corroon & Phillips, 2018). Among those using CBD to treat pain, the majority reported believing that CBD works “very well” to “moderately well” for relieving their pain (Corroon & Phillips, 2018). Despite its frequent use for pain relief, no experimental pain trials have tested the analgesic effects of pure CBD in humans.
A recent meta-analytic review concluded that cannabinoid drugs may not reduce the intensity of experimental pain, but instead make pain feel less unpleasant, suggesting a notable influence on affective processes (De Vita et al., 2018). What remains unclear is whether cannabinoid analgesia is attributable to intoxication, analgesic expectancies, and/or pharmacological action. Experimental pain studies have exclusively administered cannabinoids (e.g., Δ9-THC) with intoxicating properties (De Vita et al., 2018). Blinding procedures in placebo-controlled cannabinoid trials often fail due to these effects (Casarett, 2018), which may interact with widely held expectancies (e.g., cannabis reduces pain) to produce placebo effects. Expectations for receiving pain-relieving substances can induce robust placebo analgesia (Flaten & al’Absi, 2012; Forsberg et al., 2017). Despite receiving little empirical attention, expectancies likely play an important role in cannabinoid analgesia.
One promising approach to address these questions involves testing the effects of CBD and analgesic expectancies on human pain reactivity using a balanced placebo design. This experimental design crosses instructions about the substance to be administered with the substance that actually is administered (Gunn et al., 2017; Metrik et al., 2012; Metrik et al., 2009). Experimental pain assessment methods can yield insights into multiple aspects of cannabinoid analgesia (De Vita et al., 2018). Laboratory pain assessments avoid confounds present in clinical data by studying how controlled cannabinoid doses affect quantifiable responses to painful stimuli in healthy humans (Cooper et al., 2013). To date, no experimental pain study has examined cannabinoid analgesia using a balanced placebo design. Given its purported analgesic effects, as well as widely held beliefs that CBD is an effective pain reliever, CBD is an ideal candidate for balanced placebo design trials.
Using a balanced placebo design, we tested the effects of CBD and expectancies for receiving CBD on experimental pain reactivity (i.e., pain threshold, tolerance, intensity, unpleasantness, conditioned pain modulation, offset analgesia) by manipulating both drug administration (given inactive substance or given CBD) and instructions (told inactive substance or told CBD) in healthy humans. We hypothesized a main effect of instructions, such that telling participants they received CBD (versus inactive substance) would produce significant analgesic effects, independent of the substance that participants actually received. We hypothesized that we would not detect administration or interaction effects.
Method
Participants
Fifteen healthy adults were recruited from the Syracuse, NY community between 08/29/2019 and 02/18/2020. Respondents were screened by phone for the following inclusion criteria: (a) between 18–30 years of age and (b) ability to speak and read English. Previous research has suggested that older adults may have altered pain reactivity/sensitivity (Lautenbacher et al., 2005; Lautenbacher et al., 2017). Given our aim to examine relations between CBD, analgesic expectancies, and pain reactivity in healthy adults, the inclusion age range was limited to enhance internal validity. Respondents were excluded if they endorsed: (a) any current pain that was either chronic (i.e., lasting longer than 3 months) or acute (i.e., resulting from a recent injury or pain that is not fleeting); (b) current use of any pain medications; (c) any current or previous diagnosis of chronic medical or psychiatric conditions; (d) a history or diagnosis of any substance use disorder, or a score of 8 or more on either the AUDIT or CUDIT-R; (e) current use of any prescription medication for any physical or mental health condition (except oral contraceptive pills); (f) Current or former tobacco/nicotine use; (g) coconut allergies (contraindicated for CBD isolate oil solution), (h) being pregnant, breast feeding, or planning to become pregnant in the next 4–6 months; and (i) negative expectancies for cannabinoid analgesia. After telephone screening, initial sessions were scheduled. Participants were instructed to refrain from all non-prescription medications or other substances (including alcohol, tobacco, cannabinoids, CBD, and pain medications) for the 24 hours preceding their appointments. Research staff verified abstinence via self-report (i.e., Timeline Followback) and BAC bioverification at each session. After verifying eligibility in-person at the first session, participants provided written informed consent and were randomized to a counterbalanced manipulation order. Recruitment continued until 15 participants completed the entire study. This study was approved by the Syracuse University IRB (protocol #19–059) and preregistered on the Open Science Framework (doi: 10.17605/OSF.IO/5UN2D; De Vita, 2019).
Study Design
Our study employed a crossover, balanced placebo 2 × 2 factorial design. Participants completed 4 experimental sessions and were randomly assigned to different manipulation conditions at each session: control (told inactive – given inactive); expectancy (told active – given inactive); drug (told inactive – given active); and expectancy+drug (told active – given active). Counterbalanced condition order was randomized using a Latin-square approach. A 1-week minimum washout period was used between sessions to prevent carryover effects.
Power Analyses
To determine the target sample size, we examined effect sizes reported in the literature from studies that tested the effect of expectancy manipulations on experimental pain in healthy humans. In a recent meta-analysis of 58 studies (3557 healthy adults), Forsberg and colleagues (2017) found that expectancy manipulations (i.e., describing placebo treatment as a painkiller) produced large (f = .62) analgesic effects on experimentally-induced pain in healthy adults. Considering these data and the tests proposed herein, we selected a more conservative medium-sized estimate (f = .25) for our power analyses. Thus, a sample of 15 was estimated to afford a power of .82 to detect medium-sized main effects using repeated measures ANOVA with 4 within-subjects conditions.
Experimental Procedure
At session 1, participants completed baseline self-report measures before undergoing a pre-manipulation experimental pain assessment. Participants then received the instructional set and drug administration combination corresponding to their order assignment. Following a 30-minute absorption period, post-manipulation pain assessments were performed. Afterwards, participants completed manipulation check measures. All sessions followed similar procedures. Participants were compensated $20 per session and $20 for study completion (i.e., $100 total). All participants were fully debriefed after the study. During debriefing, they were informed that the analgesic properties of cannabinoid-related substances remain poorly understood, as there are a notable lack of high quality experimental trials. Participants were also debriefed about the nature and purpose of the instructional set deception.
Manipulations
Instructional Sets
Verbal instructional sets were used to manipulate expectancies for receiving CBD or an inactive substance (coconut oil). In the control (told coconut oil – given coconut oil) and drug (told coconut oil – given CBD) conditions, participants were told, “In today’s session, you will not be receiving the pain-reliever, cannabidiol. Instead, you will be given control dose of coconut oil, which does not have any effects on anything, including pain.” In the expectancy (told CBD – given coconut oil) and expectancy+drug (told CBD – given CBD) conditions, participants were told, “In today’s session, you will be receiving a concentrated dose of cannabidiol, or CBD. This experimental CBD produces strong pain-relieving effects without making you high, or producing any other negative mind altering effects.” Participants never received the same instructions consecutively. The instructions describing CBD analgesia were informed by cross-sectional research reporting that the majority of CBD users believe that cannabidiol works well for relieving pain (Corroon & Phillips, 2018).
Drugs
In active drug conditions, participants received 50mg of hemp-derived CBD isolate in a 0.3mL oil solution (Infinite CBD, LLP, Colorado, USA) administered sublingually via dropper. This dosage approximates widely-available commercial CBD recommendations for pain. Isolate CBD crystals are oil-soluble, and this product uses fractionated coconut oil as a base. No other compounds or flavors are added by the manufacturer. Cannabinoid content and purity (CBD: 99.46 wt %; THC: none detected) were verified by an independent accredited testing facility (ProVerde Laboratories, Inc.; see Appendix) by use of convergence chromatography. Fractionated coconut oil (the CBD oil base) was administered in equal volume in inactive conditions. The CBD isolate and fractionated coconut oil products were clear in color and did not have noticeable flavors or odors. Artificial flavors (Perfumer’s Apprentice, LLC, California, USA) were used to enhance the experimental manipulations. In conditions where participants were told they were receiving coconut oil, an inert ‘coconut’ flavor was added to the substances administered. An inert ‘Mary Jane’ flavor was added to substances administered in conditions where participants were told they were receiving CBD.
Blinding
Two researchers, a dose administrator and a pain assessor, conducted sessions. Substance dosages were prepared by the primary author (M.D.) and coded with letters (A, B, C, D) to maintain blinding among the administrator and assessor. Instructions and coded letter labels corresponding to substances in each condition were sealed into pre-prepared envelopes prior to enrollment. The dose administrator opened envelopes immediately before selecting substances and administering instructions, and were thus blinded to the substance content. The pain assessor was blinded to instruction and substance administration.
Manipulation Checks
A single item was used at the end of each session to assess whether participants believed they had received the active or inactive substance. At the end of the experiment, participants were asked whether they felt deceived about anything during the experiment. If so, they were asked to explain using an open-ended response format. These items were administered privately via an online survey platform (Qualtrics).
Baseline Measures
Problematic alcohol and cannabis use were screened using the AUDIT (Babor et al., 2001; Saunders et al., 1993) and CUDIT-R (Adamson et al., 2010), respectively. To screen for negative expectancies for cannabinoid analgesia, participants were asked whether cannabis-based medicines were helpful for treating 10 different clinical conditions, including pain. Participants self-reported demographic information. The Timeline Followback procedure (TLFB; Babor et al., 1990; Dennis et al., 2004; Sobell & Sobell, 1992) was used to establish a 30-day retrospective substance use baseline, including cannabinoid drugs (e.g., cannabis, CBD). A 7-day TLFB was given at each follow-up session to assess recent substance use. The Marijuana Smoking History Questionnaire assessed cannabis use history (Bonn-Miller & Zvolensky, 2009). Participants were asked whether they had ever used CBD and/or personally knew anyone who has used CBD. Common cannabis-related side effects of drowsiness, euphoria, sedation, nausea, dry mouth, and vertigo were assessed before and after each session using visual analog scales (Kraft et al., 2008).
Experimental Pain Outcomes
Pain Reactivity Assessment
Experimental pain methods systematically apply quantifiable sensory stimuli to evaluate perceptual pain reactivity (Boivie, 2003; Cruz-Almeida & Fillingim, 2014; Hansson et al., 2007; Olesen et al., 2012). Static pain measures evaluate overall sensitivity to painful stimulation at a specific moment in time (Arendt-Nielsen & Yarnitsky, 2009; Mogil & Bailey, 2010; Tesarz et al., 2012; Walk et al., 2009), and include rating the intensity (sensory dimension), unpleasantness (affective dimension), threshold (level at which painful stimuli are first perceived), and tolerance (level at which one can no longer withstand a painful stimulus) of experimental pain (Arendt-Nielsen & Yarnitsky, 2009; Coghill et al., 1999; Schaible, 2006; Walk et al., 2009). Dynamic pain measures activate and evaluate complex pain processing systems, providing advanced assessments of the central inhibitory mechanisms underlying analgesic responses (Arendt-Nielsen & Yarnitsky, 2009; Cruz-Almeida & Fillingim, 2014; Goodin et al., 2013). Standardized contact-heat protocols were administered using a Q-Sense unit (Medoc LTD.) equipped with two 30×30 mm thermodes. Dynamic pain ratings were measured continuously using a computerized visual analog scale (CoVAS) ranging from 0 (no pain) to 100 (pain as intense as possible).
Static Pain Measures
Pain threshold and tolerance were assessed using a ‘method-of-limits’ protocol with a thermode attached to the non-dominant forearm (McMahon et al., 2013). Six pain stimulation trials were administered at 30-second intervals. In each trial, stimulation temperature increased from baseline (32°C) at a rate of 0.3°C/second. For the first 3 trials, participants indicated when they first perceived the stimuli as painful (threshold) using a response device, at which point the temperature was digitally recorded and returned to baseline. During the last 3 trials, participants indicated when they could no longer withstand the stimulus (tolerance). Trial temperatures for each outcome were averaged to generate final threshold and tolerance values, respectively. Suprathreshold values were calculated as the average of threshold and tolerance to individualize destination temperatures for the dynamic pain measures. Pain intensity and unpleasantness were assessed using a visual analogue scale (VAS) ranging from 0 (not at all intense/unpleasant) to 100 (maximum intensity/unpleasantness possible). Participants provided ratings at 30-second intervals throughout the continuous application of 46°C heat pain for 2 minutes. Final ratings were averaged across measurement intervals.
Dynamic Pain Measures
Conditioned pain modulation (CPM) and offset analgesia (OA) are two dynamic pain measures used to evaluate central pain inhibition systems. CPM was measured using a ‘single test-stimulus’ procedure with 2 thermodes (Granovsky et al., 2015). A ‘test-stimulus’ thermode was attached to the non-dominant forearm, whereas a ‘conditioning-stimulus’ thermode was placed on the upper posterior of the opposite arm. During the procedure, the test-stimulus temperature increased from 32°C at a rate of 2°C/s until it reached the participant’s suprathreshold level. Ten seconds later, the conditioning-stimulus temperature began increasing at the same rate until it reached the suprathreshold level. For the following 20 seconds, both thermodes remained at suprathreshold temperature before returning to baseline. Participants continuously rated the test-stimulus using the CoVAS. OA was assessed with a 3-temperature paradigm that utilizes a single thermode attached to the volar surface of the participant’s non-dominant forearm (Grill & Coghill, 2002). The temperature increased from 32°C at a rate of 2°C/s until it reached the participant’s suprathreshold value. This temperature was held constant for 5 seconds, followed by a 1°C increase for 5 seconds, and then a subsequent 1°C decrease for 20 seconds, before returning to baseline. Participants provided continuous CoVAS ratings. For both CPM and OA, pain inhibition was calculated from CoVAS data using the ‘percent decrease’ formula (M. Niesters et al., 2011; Marieke Niesters et al., 2011): .
Data Analytic Strategy
Statistical analyses were conducted using SPSS, v23 (IBM, Armonk, New York). Baseline descriptive statistics were calculated to characterize the sample. Pre-post change scores were computed for each pain outcome. Bivariate correlations tested associations between order assignment and pain outcomes. Two-way repeated measures ANOVAs tested for main and interaction effects on QST outcomes and side-effects. Significant interactions were followed up with paired samples t-tests to identify simple main effects between conditions. Significance testing was conducted with alpha .05.
Results
Study Recruitment and Retention
Figure 1 presents a CONSORT diagram. Research staff telephone screened 75 respondents and 25 were deemed eligible. Throughout the study, 21 eligible respondents were scheduled for a baseline session. Five were no-shows for their first appointment and 4 were waitlisted. Sixteen eligible participants were enrolled in the study and randomized. Incomplete data from 1 participant who did not return after the first session were excluded from analyses. Fifteen participants completed the study and were included in analyses.
Figure 1.
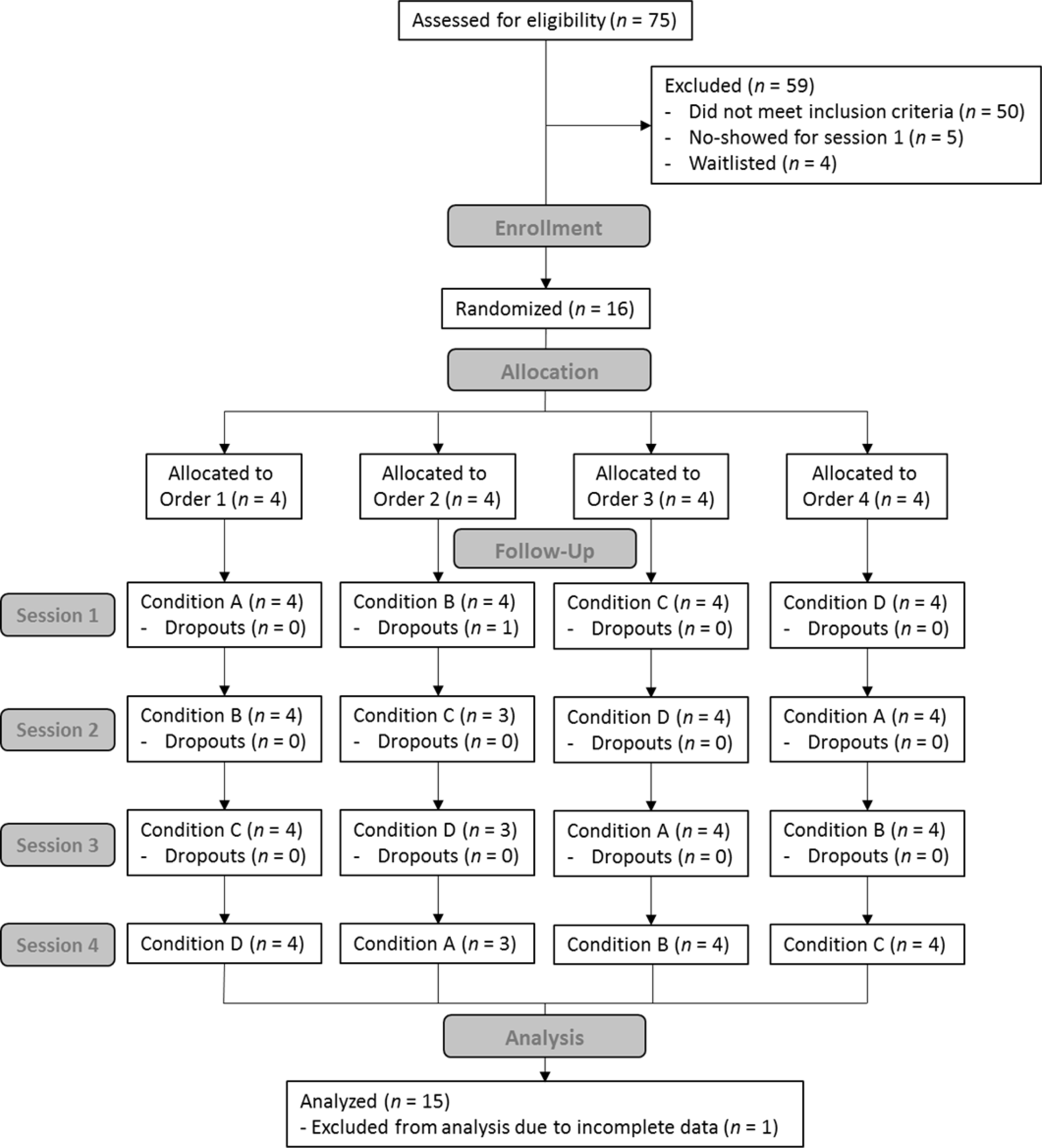
CONSORT diagram illustrating participant enrollment, allocation, follow-up, and analysis.
Sample Characteristics
Table 1 reports participant characteristics. The sample was predominately comprised of young adults (67% Female; Mage = 20.73, SD = 2.60). Approximately 67% of participants endorsed having ever used CBD, whereas 80% reported personally knowing someone who has used CBD. Thirteen (86.7%) participants endorsed current or ever use of cannabis and an average of 3.10 years of cannabis use. At session 1, participants reported using cannabinoid drugs for 10.2% of days in the past 30 days.
Table 1.
Sociodemographic and Substance Use Characteristics
| Participant Characteristics | Sample No. (%) |
|---|---|
| Total Sample Size | 15 |
| Gender | |
| Female | 10 (66.7) |
| Income | |
| <30K | 6 (40.0) |
| 30–50K | 2 (13.3) |
| >50K | 7 (46.7) |
| Education | |
| High school graduate | 2 (13.3) |
| Some college | 9 (60.0) |
| Four-year college degree | 2 (13.3) |
| School beyond 4-year degree | 2 (13.3) |
| Marital Status | |
| Single | 15 (100) |
| Ethnicity | |
| Hispanic/Latino | 4 (26.7) |
| Not Hispanic/Latino | 11 (73.3) |
| Race | |
| Caucasian | 8(53.3) |
| Black/African American | 2 (13.3) |
| Asian | 4 (26.7) |
| Native Hawaiian or Other Pacific Islander | 1 (6.7) |
| Have you ever used Cannabidiol/CBD? | |
| Yes | 10 (66.7) |
| No | 5 (33.3) |
| Do you personally know anyone who has used Cannabidiol/CBD? | |
| Yes | 12 (80.0) |
| No | 3 (20.0) |
|
| |
| M (SD) | |
|
| |
| Age | 20.73 (2.60) |
| AUDIT Total1 | 4.20 (2.54) |
| CUDIT-R Total2 | 3.13 (2.61) |
| PACE Total3 | 69.93 (14.06) |
| 30-Day TLFB4 | |
| % Drinking Days | 17.33 (17.19) |
| % Cannabis-Use Days | 10.22 (24.67) |
| MSHQ5 | |
| Frequency | 2.62 (1.85) |
| Quantity | 2.38 (1.50) |
| Years of Cannabis Use | 3.10 (1.72) |
| Age of Onset | 16.46 (1.66) |
Note.
Alcohol Use Disorders Identification Test
Cannabis Use Disorders Identification Test – Revised
Pain and Cannabinoid Expectancies
Timeline-Followback
Marijuana Smoking History Questionnaire.
Manipulation Checks and Side Effects
All participants reported receiving the substance consistent with their instructional set for each condition. No participants endorsed feeling deceived about drug content. Order assignment was not significantly correlated with any QST outcome (ps > .05). No cannabis-related side-effects were detected or observed (ps > .05).
Experimental Pain Outcomes
Table 2 presents results for the two-way repeated measures ANOVAs for experimental pain outcomes. Figure 2 presents the mean change scores in each condition for all experimental outcomes.
Table 2.
Two-Way Repeated Measures ANOVA Results for Pain Outcomes
| Outcome | MS | F(1, 14) | p | η2p |
|---|---|---|---|---|
| Pain Threshold | ||||
| Instruction | .52 | .36 | .558 | .025 |
| Administration | 18.29 | 4.23 | .059 | .232 |
| Interaction | .16 | .06 | .807 | .004 |
| Pain Tolerance | ||||
| Instruction | .34 | .68 | .425 | .046 |
| Administration | .16 | .55 | .469 | .038 |
| Interaction | .04 | .16 | .696 | .011 |
| Pain Intensity | ||||
| Instruction | 256.27 | 3.65 | .077 | .207 |
| Administration | 8.56 | .22 | .650 | .015 |
| Interaction | 125.19 | 3.10 | .100 | .181 |
| Pain Unpleasantness | ||||
| Instruction | 180.27 | 2.50 | .136 | .152 |
| Administration | 84.81 | 1.66 | .219 | .106 |
| Interaction | 548.03 | 8.45 | .011* | .376 |
| Conditioned Pain Modulation | ||||
| Instruction | 32.12 | .09 | .774 | .006 |
| Administration | 326.28 | .45 | .513 | .031 |
| Interaction | 1942.75 | 7.07 | .019* | .336 |
| Offset Analgesia | ||||
| Instruction | 4072.76 | 7.58 | .016* | .351 |
| Administration | 304.91 | .47 | .506 | .032 |
| Interaction | 110.35 | .13 | .724 | .009 |
Note. N = 15. MS = Mean Square; η2p = Partial Eta Squared (effect size).
p < .05
p < .01
p < .001
Figure 2.
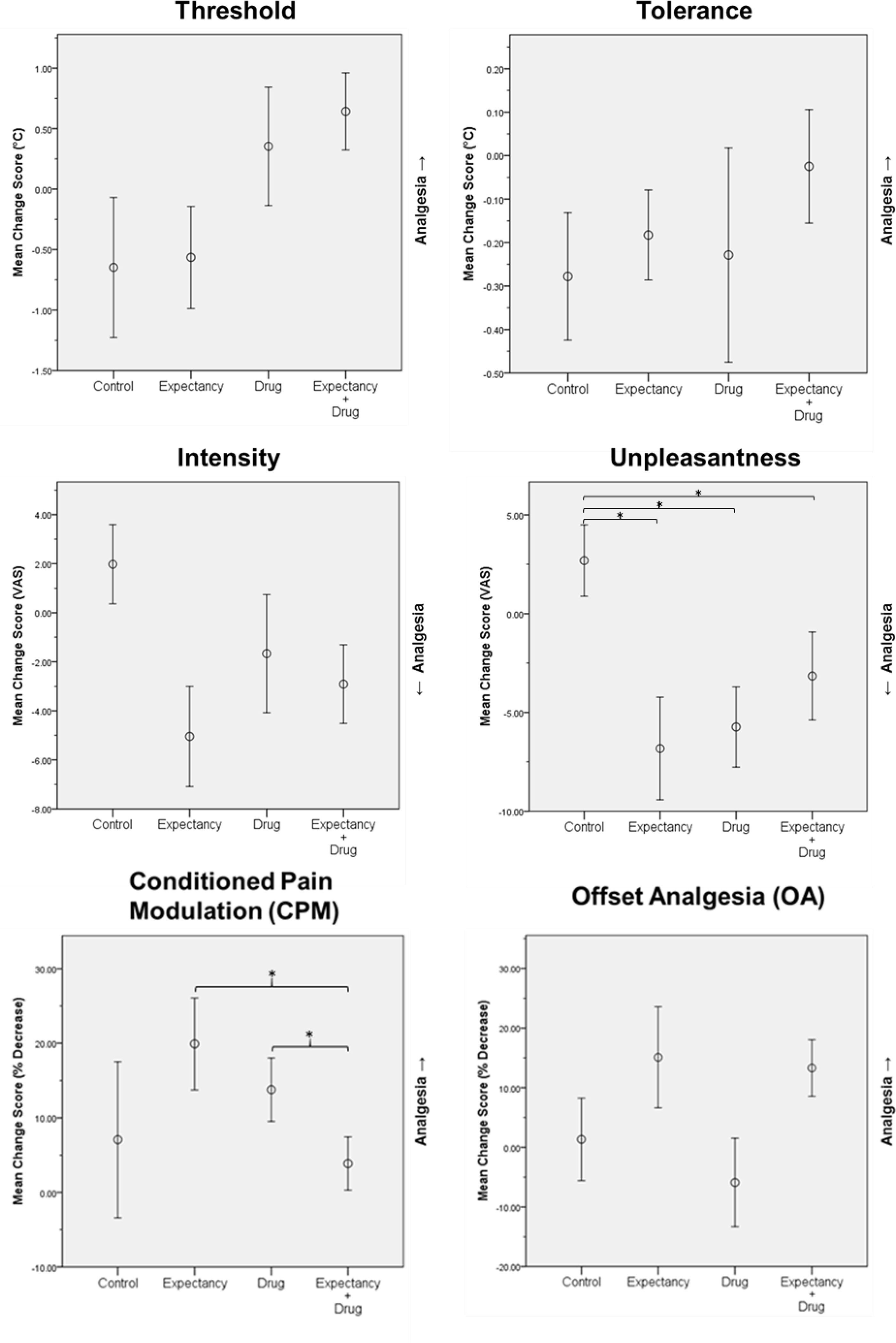
Mean change scores (post minus pre) for experimental pain variables in each condition. Error bars represent standard error. For pain unpleasantness and CPM, significant simple main effects are displayed. *p < .05
Static QST Measures
We detected no main or interaction effects for pain threshold, tolerance, or intensity. There were no main effects for pain unpleasantness, but results revealed a significant interaction (F[1, 14] = 8.45, p = .011, η2p = .376). In follow-up comparisons, paired-samples t-tests revealed significantly greater pain unpleasantness reductions in the expectancy (t[14] = 3.26, p = .006, d = .860), drug (t[14] = 3.17, p = .007, d = .820), and expectancy+drug conditions (t[14] = 2.36, p = .033, d = .616) when compared to the control condition.
Dynamic QST Measures
There were no significant main effects for CPM, but results revealed a significant interaction (F[1, 14] = 7.07, p = .019, η2p = .336). Follow-up comparisons revealed significantly greater CPM responses (i.e., more pain inhibition) in the expectancy (t[14] = −2.75, p = .016, d = −.76) and drug (t[14] = −3.20, p = .006, d = −0.84) conditions when compared to expectancy+drug condition. There was a significant instruction effect (F[1, 14] = 7.58, p = .016, η2p = .351) on OA, but no administration effect or interaction.
Discussion
Using a balanced placebo design, we tested the effects of CBD and analgesic expectancies on human pain reactivity (i.e., pain threshold, tolerance, intensity, unpleasantness, conditioned pain modulation, offset analgesia) by manipulating both drug administration (given coconut oil or given CBD) and instructions (told coconut oil or told CBD). We hypothesized finding significant analgesic effects of the instructional set (expectancy) manipulation, but null effects for drug administration and interactions. As hypothesized, there was a significant main effect of instructions on OA, such that the OA response was significantly larger (e.g., greater pain inhibition) when participants were told that they received CBD, regardless of drug content. Our hypotheses were only partially supported for the pain unpleasantness and CPM results. Pain unpleasantness was significantly reduced in the drug, expectancy, and expectancy+drug conditions, relative to the control condition. Whereas the drug and expectancy conditions separately improved CPM, the expectancy+drug condition produced the lowest CPM change scores, closely resembling the control condition. Several factors may explain this observation. When combined, CBD and participant expectancies may have interacted in such a way that an effect cancellation occurred. Additionally, CPM estimates may have had limited precision given our modest sample size. CPM measures are typically more variable than static pain outcomes (Marcuzzi et al., 2017). Larger trials are needed to test the validity of these possible explanations and would yield more precise estimates of the true mean. We did not detect significant effects for pain threshold, tolerance, or intensity, indicating that our hypotheses were not supported for these outcomes. Overall, our results indicated that separate pain outcomes can be differentially affected by CBD and/or expectancies for receiving CBD. These findings suggest that the analgesic profile for CBD is complex and multifaceted.
The effects of CBD and analgesic expectancies on pain unpleasantness were robust. Our results were consistent with previous meta-analytic findings that cannabinoid administration primarily decreases the unpleasantness, but not intensity of experimental pain (De Vita et al., 2018). Until now, cannabinoid analgesia trials have been unable to determine whether decreases in unpleasantness were attributable to intoxication, expectancies, and/or pharmacological action. Our study was designed to address these questions. Specifically, we found that cannabinoid-induced reductions in pain unpleasantness were caused by both psychological expectancies for receiving a CBD analgesic and pharmacological administration of CBD. We did not detect main or interaction effects on self-report measures (VAS) of common cannabis-related side effects. Intoxicating cannabinoids may not always suffice when treatment aims to relieve pain without impairing function. CBD may relieve pain unpleasantness while potentially mitigating these concerns. However, comprehensive side-effect assessments that include measures of cognitive and psychomotor functioning would be needed to confirm this hypothesis in future research. Given its low abuse liability and good safety profile (Babalonis et al., 2017), further research investigating CBD as a therapeutic alternative to traditional cannabinoid-based analgesics is warranted.
Mechanistic Considerations
Mechanistic insights may be drawn from the results obtained in this study. Notably, our results suggested that expectancies for receiving CBD and administration of CBD enhance central nervous system processes that inhibit pain (i.e., descending inhibition). Conditioned pain modulation (CPM) and offset analgesia (OA) evaluate endogenous systems that promote descending pain inhibition via spatial and temporal processing, respectively (Grill & Coghill, 2002; Pud et al., 2009). We observed an amplified CPM response in both the drug and expectancy conditions, indicating that CBD and expectancies for receiving CBD can independently enhance endogenous inhibition mediated by spatial pain processing. OA was exclusively enhanced by telling participants they received CBD, regardless of what was actually administered. Thus, expectancies for receiving CBD enhanced endogenous pain inhibition mediated by temporal processing. Importantly, expectancies played a large role in amplifying both CPM and OA. Numerous chronic pain conditions (e.g., fibromyalgia, chronic tension headache, complex regional pain syndrome) are characterized by deficiencies in endogenous pain inhibition (King et al., 2009; Lautenbacher & Rollman, 1997; Olesen et al., 2010; Seifert et al., 2009). Our results suggest that CBD may improve such deficiencies, particularly when analgesic expectancies are leveraged to optimize central effects. Our findings are consistent with those from neuroimaging studies that implicate top-down inhibiting mechanisms in expectancy-induced analgesia (Eippert et al., 2009). Expectancy-induced analgesia reduces nociceptive processing in the dorsal spinal cord, implicating processing between cortical regions and descending pain inhibitory systems (Eippert et al., 2009; Medoff & Colloca, 2015). Our results support this observation, as expectancies for receiving a CBD analgesic enhanced spatial and temporal measures of central descending inhibition.
Our finding that CBD administration reduced pain unpleasantness is similar to results obtained in other cannabinoid administration studies (De Vita et al., 2018). Pre-clinical research has described how cannabinoids may influence mechanisms underlying affective pain responses (Lötsch et al., 2017). Rodent models suggest that cannabinoids reduce the stress response by acting on CB1 receptors, which may alter affective responses to pain and the ability to cope with pain-induced stress (Busquets-Garcia et al., 2016; Rácz et al., 2015). Cannabinoids have been shown to activate CB2 receptors as well, resulting in the release of endogenous opioids that reduce nociception (Ibrahim et al., 2005; Ibrahim et al., 2006). Yet, unlike many of the cannabinoids examined in pre-clinical research (e.g., Δ9-THC), CBD has poor binding affinity for CB1/2 receptors (Thomas et al., 1998). CBD may act as a negative allosteric modulator at CB1 receptors and a partial agonist at CB2 receptors (Tham et al., 2019). On the other hand, CBD may have some indirect agonist activity via fatty-acid-binding-protein (FABP) inhibition and increased endocannabinoid tone (do Nascimento et al., 2020). Although not fully elucidated, some of the mechanisms of action underlying CBD analgesia have been increasingly characterized as disparate from the endocannabinoid system (Mlost et al., 2020). Other molecular targets involved in CBD analgesia may include 5HT1a, TRPV1, TRPA1, and adenosine A1 receptors. However, pharmacological interactions with these targets vary in the literature, leading some researchers to hypothesize state-dependent CBD effects (Mlost et al., 2020). Given that CBD pharmacodynamics are still not completely understood, additional research is needed to explicate specific mechanisms underlying CBD analgesia.
Implications
The finding that expectancies play a large role in CBD analgesia has several clinical implications. Indeed, there is an emerging clinical consensus that expectancy-induced analgesia can be leveraged to enhance pain interventions (Bingel, 2013; Castelnuovo et al., 2018; Colloca, 2019; Evers et al., 2018; Klinger et al., 2014; Klinger et al., 2018; Peerdeman, Van Laarhoven, Peters, et al., 2016; Sanders et al., 2020). Integrative models of pain and expectancies describe several processes (e.g., instructional learning, conditioning, social observations) that can influence placebo analgesia (Bajcar & Bąbel, 2018; Colloca, 2019; Peerdeman, Van Laarhoven, Peters, et al., 2016). Brief psychological interventions may promote analgesic expectancies and optimize pain treatments. Verbal instructions emphasizing analgesic treatment outcomes are particularly effective in reducing clinical pain (Peerdeman, van Laarhoven, Keij, et al., 2016). Verbally emphasizing the positive and realistic effects of CBD on pain, without overemphasizing negative side-effects, may optimize analgesic responses. Conditioning processes may involve reinforcing successful features of treatment experiences while altering less desirable aspects (Peerdeman, Van Laarhoven, Peters, et al., 2016). For instance, switching from cannabis to CBD might maintain positive expectancies about cannabinoid analgesia while reducing negative expectancies for intoxicating side-effects. Social learning may be facilitated by interacting with others who hold positive expectancies, or by exposure to media (e.g., online video) that promotes analgesic expectancies (Bajcar & Bąbel, 2018; Hunter et al., 2014; Peerdeman, Van Laarhoven, Peters, et al., 2016). Although expectancy interventions are promising for optimizing analgesic treatments, several considerations are worth noting. Deception should not be considered a necessary treatment component (Evers et al., 2018), as expectancy-induced analgesia can occur even when individuals are aware that they are using placebo (Carvalho et al., 2016; Locher et al., 2017). Instead, explaining expectancy effects and their clinical use has been encouraged (Klinger et al., 2014; Klinger et al., 2018). Overstatements about CBD analgesia should be avoided, as this can lead to negative treatment outcomes when exaggerated expectancies are not fulfilled (Klinger et al., 2014). Importantly, expectancy interventions may complement, but not replace, first-line evidence based pain treatments (Forsberg et al., 2017; Sanders et al., 2020). A systematic approach to expectancy assessment and intervention may help capitalize on the clinical utility of CBD analgesia. Nonetheless, further explicating the individual and combined effects of CBD and expectancies on pain is needed.
Strengths, Limitations, and Future Directions
The current study has notable strengths. It is the first balanced placebo design experiment to examine both drug and expectancy effects in cannabinoid analgesia. This pilot study is also the first experimental pain study to test the analgesic effects of pure CBD. Our novel approach enhanced the feasibility of experimental manipulations while overcoming blinding failures that confound placebo-controlled cannabinoid trials. Additionally, we assessed analgesic responses using advanced static and dynamic experimental pain methods.
Several limitations should be considered. Our pilot study employed experimental pain measures, which approximate clinical pain features (McMahon et al., 2013). Research must be conducted in clinical samples to support the generalizability of our findings. This study was limited to testing acute analgesic effects of a single sublingual 50mg CBD isolate dose. Future work should examine CBD analgesia using varying doses, preparations, and measurement periods. The age range in our study was limited to young adults between 18 and 27 years old. The effects observed in this study may vary as a function of age for various reasons. Different age groups may hold distinct expectancies for cannabinoids due to their lifetime experiences. For cannabinoid drugs, especially CBD, there has been a dramatic shift in societal attitudes/beliefs, public policy, and empirical knowledge within recent years. Motives for CBD use may also differ between age groups. Whereas younger adults may use CBD recreationally or as part of a health/lifestyle regimen, older adults may be motivated to use CBD to treat conditions that commonly co-occur with aging, such as chronic pain. More research is needed to characterize attitudes, beliefs, expectancies, and motives regarding CBD use in different age groups. Experimental work that tests the analgesic effects of CBD and analgesic expectancies in different age groups is also warranted. The current study tested the analgesic effects of expectancies for receiving a CBD analgesic, which differ from expectancies about the strength of CBD-induced pain relief. Future research should test whether expectancy strength moderates analgesic effect sizes. Another related, yet distinct empirical question is how CBD may affect pain with and without manipulating expectancies (given instruction sets vs. given no instruction sets). Our design was limited in this regard, as all conditions received instruction sets to manipulate expectancies (i.e., told active CBD vs. told inactive coconut oil). Future research may benefit from including expectancy ‘neutral’ control conditions wherein no instructions are given. Another limitation was that drug screening at each study session was limited to breath analysis for alcohol and the Timeline Followback procedure (TLFB; Babor et al., 1990; Dennis et al., 2004; Sobell & Sobell, 1992) for all other substances. Most commercially available drug screens do not assess CBD specifically. The TLFB was selected for this preliminary study, as extensive research has shown it to be a reliable and valid measure of substance use (i.e., type, quantity, frequency) with a high degree of agreement (87% for cannabis) with biological measures (Hjorthoj et al., 2012). Nevertheless, administering both self-report and bioverification measures in future trials would provide more comprehensive substance use assessments. This would enable future studies to reduce threats to internal validity and/or examine associations between cannabinoid analgesia and relevant substance use factors (e.g., polysubstance use, recreational vs. medicinal use, substance use history). Lastly, although the statistical power of this study was enhanced by a within-subjects design, larger sample sizes in future research would likely yield more stable effect size estimates. Unfortunately, there is a notable lack of experimental trials examining CBD analgesia. Experimental pain research examining expectancy-induced analgesia is far more prevalent. Using the available data, we primarily designed our study to be powered to detect main effects of instructional sets (i.e., expectancy manipulation). In doing so, we were able to detect interactions and simple main effects in the drug, expectancy, and expectancy+drug conditions in this preliminary trial. Indeed, future trials with larger samples would afford greater power to detect main and interaction effects with greater precision.
Conclusions
Our findings from this pilot study indicated that CBD and analgesic expectancies differentially affect several dimensions of pain reactivity. CBD and analgesic expectancies reduced pain unpleasantness separately and when combined. Although CPM was separately amplified in the drug and expectancy conditions, the expectancy+drug condition produced the lowest CPM values. OA was primarily enhanced by expectancies for receiving CBD. Future investigations of the psychological and pharmacological mechanisms underlying CBD analgesia are warranted.
Supplementary Material
Public Health Significance.
Despite its frequent use for pain relief, no experimental pain research has tested the analgesic effects of cannabidiol (CBD) in humans. We experimentally tested the effects of CBD and expectancies for receiving CBD on human pain reactivity. This study found that CBD analgesia was driven by both psychological expectancies and pharmacological action.
Disclosures and Acknowledgements
Martin J. De Vita’s work on this project was supported by the Syracuse University Dissertation Fellowship. Stephen A. Maisto’s work on this manuscript was supported in part by grant 2KO5 AA16928 from the National Institute on Alcohol Abuse and Alcoholism. The funding sources had no role in the design and conduct of the study; collection management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, the Department of the Air Force and Department of Defense or the U.S. Government.
All authors made substantial contributions, which are as follows: Martin J. De Vita (primary author) and Stephen A. Maisto (senior author) served as leads for conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, as well as writing, revising, reviewing, and editing the manuscript. Christina E. Gilmour served in a supporting role for data curation, formal analysis, investigation, project administration, supervision, validation, and writing, revising, reviewing and editing the manuscript. Lauren McGuire and Elizabeth Tarvin served in a supporting role data curation, investigation and writing, revising, reviewing, and editing the manuscript. Dezarie Moskal served in a supporting role for investigation, project administration, and writing, revising, reviewing, and editing the manuscript. All authors have read and approved the final manuscript for submission.
The authors thank Drs. Sarah Woolf-King, Kevin Antshel, Kyle Possemato, Dessa Bergen-Cico, Lael Schooler and Collin Mullins for providing insightful feedback on this project. Additional thanks are given to Shockey Sanders for reviewing data input consistency.
Footnotes
The authors declare that they have no conflict of interests.
Author note: OSF Registration DOI: 10.17605/OSF.IO/5UN2D and link: https://osf.io/5un2d
References
- Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, & Sellman JD (2010). An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend, 110(1–2), 137–143. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, & Yarnitsky D (2009). Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain, 10(6), 556–572. 10.1016/j.jpain.2009.02.002 [DOI] [PubMed] [Google Scholar]
- Babalonis S, Haney M, Malcolm RJ, Lofwall MR, Votaw VR, Sparenborg S, & Walsh SL (2017). Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug Alcohol Depend, 172, 9–13. 10.1016/j.drugalcdep.2016.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Brown J, & Del Boca FK (1990). Validity of self-reports in applied research on addictive behaviors: fact or fiction? Behavioral Assessment. [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, & Monteiro MG (2001). Audit. The Alcohol Use Disorders Identification Test (AUDIT): Guidelines for use in primary care. [Google Scholar]
- Bajcar EA, & Bąbel P (2018). How Does Observational Learning Produce Placebo Effects? A Model Integrating Research Findings [Review]. Frontiers in Psychology, 9(2041). 10.3389/fpsyg.2018.02041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U (2013). The relevance of placebo and nocebo mechanisms for analgesic treatments. In Placebo and Pain (pp. 127–136). Elsevier. [Google Scholar]
- Boivie J (2003). Central pain and the role of quantitative sensory testing (QST) in research and diagnosis. Eur J Pain, 7(4), 339–343. 10.1016/S1090-3801(03)00046-6 [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, & Zvolensky MJ (2009). An Evaluation of the Nature of Marijuana Use and Its Motives among Young Adult Active Users. American Journal on Addictions, 18(5), 409–416. 10.3109/10550490903077705 [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, Gomis-González M, Srivastava RK, Cutando L, Ortega-Alvaro A, Ruehle S, Remmers F, Bindila L, Bellocchio L, & Marsicano G (2016). Peripheral and central CB1 cannabinoid receptors control stress-induced impairment of memory consolidation. Proceedings of the National Academy of Sciences, 113(35), 9904–9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho C, Caetano JM, Cunha L, Rebouta P, Kaptchuk TJ, & Kirsch I (2016). Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain, 157(12), 2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarett D (2018). The Achilles Heel of Medical Cannabis Research-Inadequate Blinding of Placebo-Controlled Trials. JAMA Intern Med, 178(1), 9–10. 10.1001/jamainternmed.2017.5308 [DOI] [PubMed] [Google Scholar]
- Castelnuovo G, Giusti EM, Manzoni GM, Saviola D, Gabrielli S, Lacerenza M, Pietrabissa G, Cattivelli R, Spatola CAM, Rossi A, Varallo G, Novelli M, Villa V, Luzzati F, Cottini A, Lai C, Volpato E, Cavalera C, Pagnini F, Tesio V, Castelli L, Tavola M, Torta R, Arreghini M, Zanini L, Brunani A, Seitanidis I, Ventura G, Capodaglio P, D’Aniello GE, Scarpina F, Brioschi A, Bigoni M, Priano L, Mauro A, Riva G, Di Lernia D, Repetto C, Regalia C, Molinari E, Notaro P, Paolucci S, Sandrini G, Simpson S, Wiederhold BK, Gaudio S, Jackson JB, Tamburin S, & Benedetti F (2018). What Is the Role of the Placebo Effect for Pain Relief in Neurorehabilitation? Clinical Implications From the Italian Consensus Conference on Pain in Neurorehabilitation [Review]. Frontiers in Neurology, 9(310). 10.3389/fneur.2018.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, & Iadarola MJ (1999). Pain intensity processing within the human brain: a bilateral, distributed mechanism. Journal of neurophysiology, 82(4), 1934–1943. [DOI] [PubMed] [Google Scholar]
- Colloca L (2019). The placebo effect in pain therapies. Annual review of pharmacology and toxicology, 59, 191–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Comer SD, & Haney M (2013). Comparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokers. Neuropsychopharmacology, 38(10), 1984–1992. 10.1038/npp.2013.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corroon J, & Phillips JA (2018). A Cross-Sectional Study of Cannabidiol Users. Cannabis and Cannabinoid Research, 3(1), 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Almeida Y, & Fillingim RB (2014). Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med, 15(1), 61–72. 10.1111/pme.12230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita MJ (2019). The analgesic effects of cannabidiol and expectancies on human pain reactivity: A pilot trial. 10.17605/OSF.IO/5UN2D [DOI]
- De Vita MJ, Moskal D, Maisto SA, & Ansell EB (2018). Association of cannabinoid administration with experimental pain in healthy adults: A systematic review and meta-analysis. JAMA Psychiatry, 75(11), 1118–1127. 10.1001/jamapsychiatry.2018.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ML, Funk R, Godley SH, Godley MD, & Waldron H (2004). Cross‐validation of the alcohol and cannabis use measures in the Global Appraisal of Individual Needs (GAIN) and Timeline Followback (TLFB; Form 90) among adolescents in substance abuse treatment. Addiction, 99, 120–128. [DOI] [PubMed] [Google Scholar]
- do Nascimento GC, Ferrari DP, Guimaraes FS, Del Bel EA, Bortolanza M, & Ferreira-Junior NC (2020). Cannabidiol increases the nociceptive threshold in a preclinical model of Parkinson’s disease. Neuropharmacology, 163, 107808. [DOI] [PubMed] [Google Scholar]
- Eippert F, Finsterbusch J, Bingel U, & Büchel C (2009). Direct evidence for spinal cord involvement in placebo analgesia. Science, 326(5951), 404–404. [DOI] [PubMed] [Google Scholar]
- Evers AW, Colloca L, Blease C, Annoni M, Atlas LY, Benedetti F, Bingel U, Büchel C, Carvalho C, & Colagiuri B (2018). Implications of placebo and nocebo effects for clinical practice: expert consensus. Psychotherapy and psychosomatics, 87(4), 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaten MA, & al’Absi M (2012). The placebo effect. In Gellman M & Turner JR (Eds.), Encyclopedia of Behavioral Medicine (pp. 1497–1499). Springer. [Google Scholar]
- Forsberg JT, Martinussen M, & Flaten MA (2017). The Placebo Analgesic Effect in Healthy Individuals and Patients: A Meta-Analysis. Psychosom Med, 79(4), 388–394. 10.1097/psy.0000000000000432 [DOI] [PubMed] [Google Scholar]
- Goodin BR, Glover TL, Sotolongo A, King CD, Sibille KT, Herbert MS, Cruz-Almeida Y, Sanden SH, Staud R, Redden DT, Bradley LA, & Fillingim RB (2013). The association of greater dispositional optimism with less endogenous pain facilitation is indirectly transmitted through lower levels of pain catastrophizing. J Pain, 14(2), 126–135. 10.1016/j.jpain.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granovsky Y, Miller‐Barmak A, Goldstein O, Sprecher E, & Yarnitsky D (2015). CPM Test–Retest Reliability:“Standard” vs “Single Test‐Stimulus” Protocols. Pain Medicine. [DOI] [PubMed] [Google Scholar]
- Grill JD, & Coghill RC (2002). Transient analgesia evoked by noxious stimulus offset. J Neurophysiol, 87(4), 2205–2208. 10.1152/jn.00730.2001 [DOI] [PubMed] [Google Scholar]
- Gunn RL, Skalski L, & Metrik J (2017). Expectancy of impairment attenuates marijuana-induced risk taking. Drug Alcohol Depend, 178, 39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson P, Backonja M, & Bouhassira D (2007). Usefulness and limitations of quantitative sensory testing: clinical and research application in neuropathic pain states. Pain, 129(3), 256–259. 10.1016/j.pain.2007.03.030 [DOI] [PubMed] [Google Scholar]
- Hjorthoj CR, Hjorthoj AR, & Nordentoft M (2012). Validity of Timeline Follow-Back for self-reported use of cannabis and other illicit substances--systematic review and meta-analysis. Addict Behav, 37(3), 225–233. 10.1016/j.addbeh.2011.11.025 [DOI] [PubMed] [Google Scholar]
- Hunter T, Siess F, & Colloca L (2014). Socially induced placebo analgesia: a comparison of a pre-recorded versus live face-to-face observation. Eur J Pain, 18(7), 914–922. 10.1002/j.1532-2149.2013.00436.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, & Mata HP (2005). CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proceedings of the National Academy of Sciences of the United States of America, 102(8), 3093–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, Porreca F, Buckley NE, Makriyannis A, & Malan TP (2006). CB 2 cannabinoid receptor mediation of antinociception. Pain, 122(1), 36–42. [DOI] [PubMed] [Google Scholar]
- Iffland K, & Grotenhermen F (2017). An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis and Cannabinoid Research, 2(1), 139–154. 10.1089/can.2016.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, & Riley JL 3rd. (2009). Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain, 143(3), 172–178. 10.1016/j.pain.2008.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger R, Colloca L, Bingel U, & Flor H (2014). Placebo analgesia: clinical applications. Pain, 155(6), 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger R, Stuhlreyer J, Schwartz M, Schmitz J, & Colloca L (2018). Clinical Use of Placebo Effects in Patients With Pain Disorders. Int Rev Neurobiol, 139, 107–128. 10.1016/bs.irn.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft B, Frickey NA, Kaufmann RM, Reif M, Frey R, Gustorff B, & Kress HG (2008). Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology, 109(1), 101–110. 10.1097/ALN.0b013e31817881e1 [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Kunz M, Strate P, Nielsen J, & Arendt-Nielsen L (2005). Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain, 115(3), 410–418. 10.1016/j.pain.2005.03.025 [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Peters JH, Heesen M, Scheel J, & Kunz M (2017). Age changes in pain perception: A systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neuroscience & Biobehavioral Reviews, 75, 104–113. 10.1016/j.neubiorev.2017.01.039 [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, & Rollman G (1997). Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain, 13(3), 189–196. [DOI] [PubMed] [Google Scholar]
- Locher C, Nascimento AF, Kirsch I, Kossowsky J, Meyer A, & Gaab J (2017). Is the rationale more important than deception? A randomized controlled trial of open-label placebo analgesia. Pain, 158(12), 2320–2328. [DOI] [PubMed] [Google Scholar]
- Lötsch J, Weyer‐Menkhoff I, & Tegeder I (2017). Current evidence of cannabinoid‐based analgesia obtained in preclinical and human experimental settings. European Journal of Pain. [DOI] [PubMed] [Google Scholar]
- Marcuzzi A, Wrigley PJ, Dean CM, Adams R, & Hush JM (2017). The long-term reliability of static and dynamic quantitative sensory testing in healthy individuals. Pain, 158(7), 1217–1223. [DOI] [PubMed] [Google Scholar]
- McMahon S, Koltzenburg M, Tracey I, & Turk D (2013). Wall & Melzack’s Textbook of Pain,Expert Consult - Online and Print,6: Wall & Melzack’s Textbook of Pain. Elsevier/Saunders. https://books.google.com/books?id=bNlvQJSqrB8C [Google Scholar]
- Medoff ZM, & Colloca L (2015). Placebo analgesia: understanding the mechanisms. Pain management, 5(2), 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metrik J, Kahler CW, Reynolds B, McGeary JE, Monti PM, Haney M, de Wit H, & Rohsenow DJ (2012). Balanced placebo design with marijuana: pharmacological and expectancy effects on impulsivity and risk taking. Psychopharmacology, 223(4), 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metrik J, Rohsenow DJ, Monti PM, McGeary J, Cook TAR, de Wit H, Haney M, & Kahler CW (2009). Effectiveness of a Marijuana Expectancy Manipulation: Piloting the Balanced-Placebo Design for Marijuana. Exp Clin Psychopharmacol, 17(4), 217–225. 10.1037/a0016502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlost J, Bryk M, & Starowicz K (2020). Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. International journal of molecular sciences, 21(22), 8870. https://www.mdpi.com/1422-0067/21/22/8870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, & Bailey AL (2010). Sex and gender differences in pain and analgesia. Prog Brain Res, 186, 141–157. 10.1016/B978-0-444-53630-3.00009-9 [DOI] [PubMed] [Google Scholar]
- Niesters M, Dahan A, Swartjes M, Noppers I, Fillingim RB, Aarts L, & Sarton EY (2011). Effect of ketamine on endogenous pain modulation in healthy volunteers. Pain, 152(3), 656–663. 10.1016/j.pain.2010.12.015 [DOI] [PubMed] [Google Scholar]
- Niesters M, Hoitsma E, Sarton E, Aarts L, & Dahan A (2011). Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. The Journal of the American Society of Anesthesiologists, 115(5), 1063–1071. [DOI] [PubMed] [Google Scholar]
- Olesen SS, Brock C, Krarup AL, Funch–Jensen P, Arendt–Nielsen L, Wilder–Smith OH, & Drewes AM (2010). Descending inhibitory pain modulation is impaired in patients with chronic pancreatitis. Clinical Gastroenterology and Hepatology, 8(8), 724–730. [DOI] [PubMed] [Google Scholar]
- Olesen SS, van Goor H, Bouwense SA, Wilder-Smith OH, & Drewes AM (2012). Reliability of static and dynamic quantitative sensory testing in patients with painful chronic pancreatitis. Reg Anesth Pain Med, 37(5), 530–536. 10.1097/AAP.0b013e3182632c40 [DOI] [PubMed] [Google Scholar]
- Peerdeman KJ, van Laarhoven AI, Keij SM, Vase L, Rovers MM, Peters ML, & Evers AW (2016). Relieving patients’ pain with expectation interventions: a meta-analysis. Pain, 157(6), 1179–1191. 10.1097/j.pain.0000000000000540 [DOI] [PubMed] [Google Scholar]
- Peerdeman KJ, Van Laarhoven AIM, Peters ML, & Evers AWM (2016). An integrative review of the influence of expectancies on pain. Frontiers in Psychology, 7, 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, Abate M, Faggiana G, Proto MC, Fiore D, Laezza C, & Bifulco M (2017). Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol Ther, 175, 133–150. 10.1016/j.pharmthera.2017.02.041 [DOI] [PubMed] [Google Scholar]
- Pud D, Granovsky Y, & Yarnitsky D (2009). The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain, 144(1–2), 16–19. 10.1016/j.pain.2009.02.015 [DOI] [PubMed] [Google Scholar]
- Rácz I, Nent E, Erxlebe E, & Zimmer A (2015). CB1 receptors modulate affective behaviour induced by neuropathic pain. Brain research bulletin, 114, 42–48. [DOI] [PubMed] [Google Scholar]
- Russo EB (2017). Cannabidiol Claims and Misconceptions. Trends in pharmacological sciences, 38(3), 198–201. 10.1016/j.tips.2016.12.004 [DOI] [PubMed] [Google Scholar]
- Sanders D, Colloca L, & Finniss DG (2020). Influence of placebo analgesia in pharmacological treatment of pain. Future Drug Discovery, 2(2), FDD34. 10.4155/fdd-2019-0028 [DOI] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De la Fuente JR, & Grant M (1993). Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption‐II. Addiction, 88(6), 791–804. [DOI] [PubMed] [Google Scholar]
- Schaible H-G (2006). Peripheral and central mechanisms of pain generation. In Analgesia (pp. 3–28). Springer. [DOI] [PubMed] [Google Scholar]
- Seifert F, Kiefer G, DeCol R, Schmelz M, & Maihöfner C (2009). Differential endogenous pain modulation in complex-regional pain syndrome. Brain, 132(3), 788–800. [DOI] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back. In Measuring alcohol consumption (pp. 41–72). Springer. [Google Scholar]
- Tesarz J, Schuster AK, Hartmann M, Gerhardt A, & Eich W (2012). Pain perception in athletes compared to normally active controls: a systematic review with meta-analysis. Pain, 153(6), 1253–1262. 10.1016/j.pain.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Tham M, Yilmaz O, Alaverdashvili M, Kelly ME, Denovan‐Wright EM, & Laprairie RB (2019). Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. British journal of pharmacology, 176(10), 1455–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BF, Gilliam AF, Burch DF, Roche MJ, & Seltzman HH (1998). Comparative receptor binding analyses of cannabinoid agonists and antagonists. Journal of Pharmacology and Experimental Therapeutics, 285(1), 285–292. [PubMed] [Google Scholar]
- Walk D, Sehgal N, Moeller-Bertram T, Edwards RR, Wasan A, Wallace M, Irving G, Argoff C, & Backonja MM (2009). Quantitative sensory testing and mapping: a review of nonautomated quantitative methods for examination of the patient with neuropathic pain. Clin J Pain, 25(7), 632–640. 10.1097/AJP.0b013e3181a68c64 [DOI] [PubMed] [Google Scholar]
- World Health Organization Expert Committee on Drug Dependence. (2018). Cannabidiol (CBD) - Critical Review Report.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


