Abstract
Introduction
To evaluate the radiological sequelae of coronavirus disease (COVID-19) in a mid-term follow-up and investigate their relationship with clinical-radiological findings.
Methods
This prospective study included COVID-19 patients who underwent a CXR three months after discharge. The relationship between CXR score at three months after discharge and clinical findings and previous CXR scores, at admission and before the discharge, were evaluated. Then, based on mid-term follow-up CXR score, patients were divided in Group A (score = 0) and Group B (score≥1), and clinical-radiological findings were compared between two Groups. Finally, we calculated the CXR scores at admission and before the discharge with the highest sensitivity and specificity to predict normal and abnormal CXR score at mid-term follow-up.
Results
The study included 119 patients, mean age 65.9 ± 14.6 years. The oxygen saturation (SaO2) (p = 0.0006), the days of hospitalization (p < 0.0001) and the CXR score before the discharge (p = 0.0091) were independent factors to predict the mid-term follow-up CXR score. The Group A, 59 (49.6%) patients, had CXR scores at admission and before the discharge lower than Group B. The CXR scores at admission and before the discharge with the highest sensitivity and specificity to predict normal and abnormal CXR score at mid-term follow-up were, respectively, 3 and 2 (p < 0.0001).
Conclusions
The radiological abnormalities were present in about half patients three months after discharge, which had higher age, previous CXR scores and longer hospitalization. The SO2, days of hospitalization and previous CXR scores were independent factors for predicting the CXR at three months.
Implications for practice
The radiologist with CXR could play a central role in mid to long-term follow-up of COVID-19, assessing the radiological sequelae of patients and identifying those who might require a closer follow-up.
Keywords: COVID-19, Follow up, Radiological sequelae, Chest X-ray
Introduction
Coronavirus disease 2019 (COVID-19) is caused by a novel coronavirus, known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 , 2 Though most symptomatic patients have mild flu-like symptoms, a significant minority develop acute respiratory distress syndrome, leading to considerable morbidity and mortality.3 Despite chest computed tomography (CT) is the best modality to detect lung abnormalities,4 in case of a high number of hospitalized patients chest X-ray (CXR) is the most common radiological method to monitor the rapid course of COVID-19.5 , 6 Moreover, some CXR scoring systems, including the Brixia Score, were developed to rate pulmonary involvement according to the type and the extension of lung abnormalities.7
Despite several previous studies reporting radiological temporal changes of COVID-19 in-patients until four weeks after the disease onset, most patients who have recovered still have residual abnormalities on CXR: a close follow-up during the hospitalization is essential but may not be enough.8, 9, 10 Indeed, some studies concluded that long-term follow-up is needed to evaluate the development of irreversible fibrosis.11, 12, 13 However, the optimal time for follow-up imaging is unknown; the American Thoracic Society does not recommend routine follow-up imaging for patients recovering satisfactorily from community-acquired pneumonia.14 , 15 Trying to solve this problem, George et al.14 provided a structure for long-term follow-up in COVID-19 patients. However, studies about COVID-19 patients after discharge reported different conclusions. According to Mo et al.,16 the impairment of diffusion capacity is the most common abnormality of lung function in COVID-19 patients and it is associated with the CT pneumonia severity score. Conversely, Frija-Masson et al.17 reported that more than half of patients exhibited abnormal lung function unrelated to CT severity 12 weeks post discharge.
Nevertheless, studies on a mid-term or long radiological follow-up of sequelae in COVID-19 are scarce.
Therefore, the aim of this study is to evaluate the radiological sequelae of COVID-19 patients in a mid-term follow-up (3 months) and to investigate their relationship with clinical and radiological findings.
Materials and methods
Study population
All procedures on studies involving human participants were performed in accordance with the ethical standards of the Institutional and National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The Institutional Review Board of our Hospital approved the study protocol (013087S). Written informed consent was obtained from all the patients.
In this prospective study from May 01 to June 31, 2021 we enrolled 122 consecutive patients with previous COVID-19 who underwent a follow-up with CXR three months after Hospital discharge.
The inclusion criteria were: COVID-19 infection at admission and COVID-19 resolution at discharge, confirmed by real-time reverse transcription polymerase chain reaction test using nasal and oropharyngeal swab specimens; the execution of a CXR exam at admission and before discharge. The exclusion criteria were: inaccessible clinical data and CXRs images (n = 3).
The final study population was composed by 119 patients.
For each patient sex, age and medical history (comorbidities and smoking) were collected.
Moreover, we retrospectively collected the clinical (fever, cough, dyspnea, myalgia, diarrhea, ambient air oxygen saturation, type of ventilation support during hospitalization) and laboratory data (value of lactate dehydrogenase, number of lymphocytes) at hospital admission, the number of days between disease onset and hospital admission, and the number of days of hospitalization. The date of disease onset was defined as the day when the first symptoms were noticed.
CXR evaluation
For each patient, two radiologists in consensus evaluated the CXR at admission, at discharge and at mid-term follow-up (3 months), using an 18-points score system.
In each CXR, lungs were divided into three equal parts: upper, middle and lower, for a total of six zones. A score (from 0 to 3) was assigned to each zone based on lung abnormalities detected on a frontal view, as follow:
0 – no abnormalities;
1 – interstitial infiltrates; defined as septal thickenings and focal or extensive opacity, with the evidence of extravascular structure;
2 – interstitial and alveolar infiltrates (interstitial predominance);
3 – interstitial and alveolar infiltrates (alveolar predominance).
The single scores of the six lung zones were added to obtain an overall CXR score ranging from 0 to 18. To minimize bias, two radiologists were blinded to patient histories.
Relationship of mid-term follow-up CXR score
The mid-term follow-up CXR score was correlated with age, ambient air oxygen saturation (SO2), days from disease onset to hospital admission, days of hospitalization, CXR scores at admission and before discharge.
Comparison of long-term follow up CXR score
Based on mid-term follow-up CXR score, the patients were divided in two Groups: Group A, with radiological complete recovery (CXR score = 0) and Group B, with radiological abnormalities (CXR score ≥1). Age, sex, oxygen saturation (SO2), days from disease onset to hospital admission, days of hospitalization, CXR scores at admission and before discharge, were compared between the two Groups.
Then we calculated the cut-off CXR score at admission and before discharge with the highest sensitivity and specificity to distinguish patients with normal (score = 0) and abnormal (score ≥1) CXR at mid-term follow-up.
Statistical analysis
A dedicated statistical software was used (MedCalc v19.1.6, MedCalc Software, Ostend, Belgium). Continuous variables were displayed as mean ± standard deviation and categorical variables were reported as counts and percentages.
CXR score was evaluated at admission, before discharge and in a mid-term follow-up (three months after the discharge). Regression analysis was used to study the independent covariates for the mid-term follow-up CXR score. Regression coefficient (b) and partial regression coefficient (r) were calculated.
Mann–Whitney U test and χ2 test were used to compare, respectively, continuous and categorical variables between the two Groups A and B. A receiving operating characteristic (ROC) curve with area under the curve (AUC) and the Youden's index were used to calculate the cut-off CXR score at admission and before discharge with the highest sensitivity and specificity to distinguish patients with normal (score = 0) and abnormal (score ≥1) CXR at mid-term follow-up.
p < 0.05 was defined as statistically significant.
Results
Study population
Our study population included 119 patients, 72 (60.5%) males and 47 (39.5%) females and mean age was 65.9 ± 14.6 [95% CI: 63.2–68.5]. Fever was present in 103 (86.6%) patients; it was the most frequent onset symptom. Hypertension was the most frequent comorbidity: it was present in 26 (21.8%) patients. Ambient air SO2 was 88.6 ± 12.4 mm Hg [95% CI: 85.7–91.3]. Lactate dehydrogenase (LDH) was 312.5 ± 146.6 U/L [95% CI: 278.7–346.2] (normal value < 214); lymphocytes count was 1.0 ± 0.5 × 109 L [95% CI: 0.9–1.1]. Among the 119 included patients, 11 (9.2%) needed of invasive ventilation during hospitalization.
Time from disease onset to hospital admission was 6.8 ± 3.5 days [95% CI: 6.1–7.4]. The number of days of hospitalization was 17.6 ± 13.1 [95% CI: 14.7–20.4].
The study population's characteristics are summarized in Table 1 .
Table 1.
Study population characteristics.
| Parameters | Value |
|---|---|
| Epidemiological Data | |
| Sex – M/F | 72 (60.5%)/47 (39.5%) |
| Age (years) | 65.9 ± 14.6 [95% CI: 63.2–68.5] |
| Clinical Data | |
| Fever (n, %) | 103 (86.6%) |
| Cough (n, %) | 64 (53.8%) |
| Dyspnea (n, %) | 29 (24.4%) |
| Diarrhea (n, %) | 6 (5.0%) |
| Ambient air SO2 (mm Hg) | 88.6 ± 12.4 [95% CI: 85.7–91.3] |
| Ventilation support during hospitalization | |
| Face mask NIV | 86 (72.3%) |
| Helmet NIV | 22 (18.5%) |
| IV | 11 (9.2%) |
| Laboratory Data | |
| LDH (U/L) | 312.5 ± 146.6 [95% CI: 278.7–346.2] |
| Lymphocytes (L) | 1.0 ± 0.5 × 109 [95% CI: 0.9–1.1] |
| Comorbidities | |
| Hypertension (n, %) | 26 (21.8%) |
| Diabetes (n, %) | 8 (6.7%) |
| Neoplasia (n, %) | 7 (5.9%) |
| Smoking (n, %) | 17 (14.2%) |
Abbreviations – SO2, oxygen saturation; NIV, non-invasive ventilation; IV, invasive ventilation; CI: confidence interval.
CXR evaluation
A total of 357 CXRs, three CXRs per patient (at admission, before discharge and at mid-term follow-up) were evaluated and scored.
CXR score at admission was 6.1 ± 3.5 (95% CI: 5.2–6.8).
CXR score before the discharge was 5.4 ± 3.1 (95% CI: 5.0–5.8).
CXR score at mid-term follow-up was 1.8 ± 1.5 (95% CI: 1.1–2.0), and 59/119 patients (49.6%) had a CXR score of 0.
CXR scores at admission and before discharge were not statistically different (p = 0.2682).
CXR scores at admission and at mid-term follow up were statistically different (p < 0.0001).
CXR scores at discharge and at mid-term follow up were statistically different (p < 0.0001).
Relationship of mid-term follow-up CXR score
Mid-term follow-up CXR score had a negative linear relationship with SO2 (r = −0.6186, p = 0.0006) and a positive linear relationship with the number of days of hospitalization (r = 0.6351, p > 0.0001) and with CXR score before discharge (r = 0.5242, p = 0.0091). The multiple correlation coefficient was 0.8419; it was statistically significant (p < 0.0001). Relationship between mid-term follow-up CXR score and clinical and radiological findings is shown in Table 2 .
Table 2.
Relationship between mid-term follow-up CXR score and clinical and radiological findings.
| Clinical and radiological findings | Regression coefficients (b) | Partial correlation coefficient (r) | P |
|---|---|---|---|
| Age | 0.2240 | 0.2126 | 0.1786 |
| Ambient air SO2 | −0.0572 | −0.6186 | 0.0006 |
| Days before admission | −0.0912 | −0.2182 | 0.2589 |
| Days of hospitalization | 0.0864 | 0.6351 | <0.0001 |
| CXR at admission | −0.0051 | −0.0116 | 0.8942 |
| CXR at discharge | 0.2242 | 0.5242 | 0.0091 |
Abbreviations – SO2, oxygen saturation; CXR, chest X-ray.
Comparison of mid-term follow up CXR score
In Group A (CXR score = 0 at three months), composed by 59 (49.6%), age, days of hospitalization and CXR scores at admission and before the discharge were statistically lower than in Group B, composed by 60 (50.4%), with a CXR score of 3.0 ± 2.6 (95% CI: 2.4–3.7) at three months. No statistical difference was observed in sex distribution, ambient air SO2, LDH, lymphocytes count and days between disease onset to hospital admission. In addition, the mean CXR scores at admission and before the discharge were lower in Group A than in Group B, respectively, 4.2 ± 3.6 vs 7.1 ± 2.3 (p = 0.0003) and 3.9 ± 3.1 vs 6.9 ± 3.1 (p < 0.0001). Comparison between the two Groups is illustrated in Table 3 .
Table 3.
Comparison between Groups with different mid-term follow-up CXR score.
| Findings | Group A (n = 59) | Group B (n = 60) | P |
|---|---|---|---|
| Male/Female | 36/23 | 38/22 | 0.8511 |
| Age (years) | 61.8 ± 16.7 (95% CI: 57.5–66.2) | 69.8 ± 10.1 (95% CI: 67.0–72.6) | 0.0026 |
| SO2 (mm Hg) | 89.8 ± 11.0 (95% CI: 86.4–93.2) | 87.3 ± 13.6 (95% CI: 82.9–91.0) | 0.3913 |
| Lymphocytes (L) | 1.1 ± 0.5 × 109 (95% CI: 0.9–1.2) | 1.0 ± 0.4 × 109 (95% CI: 0.9–1.1) | 0.1947 |
| LDH (U/L) | 282.9 ± 134.0 (95% CI: 236.7–329.1) | 338.4 ± 153.4 (95% CI: 289.3–387.4) | 0.1023 |
| Days before admission | 6.5 ± 3.5 (95% CI: 5.6–7.5) | 7.0 ± 3.5 (95% CI: 6.0–7.0) | 0.5404 |
| Days of hospitalization | 18.1 ± 12.3 (95% CI: 13.1–23.2) | 26.2 ± 17.1 (95% CI: 21.1–31.2) | 0.0409 |
| CXR at admission | 4.2 ± 3.6 (95% CI: 2.8–5.9) | 7.1 ± 2.3 (95% CI: 6.2–7.9) | 0.0003 |
| CXR before discharge | 3.9 ± 3.1 (95% CI: 3.1–4.7) | 6.9 ± 3.1 (95% CI: 6.1–7.7) | <0.0001 |
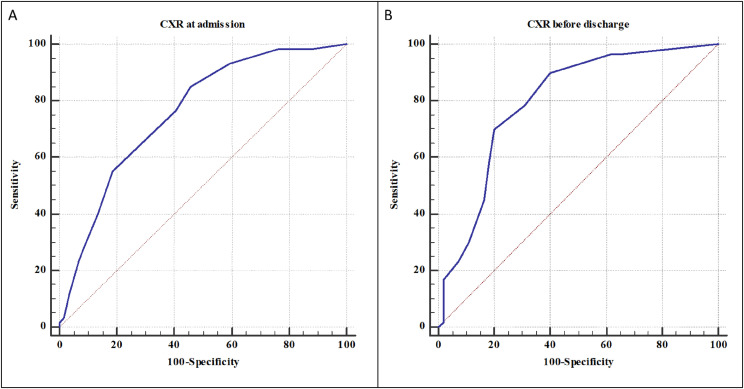
The cut-off CXR score at admission with the highest sensitivity (85.0%) and specificity (64.0%) to distinguish normal (score = 0) and abnormal (score≥1) CXR at mid-term follow-up was 3 with AUC of 0.757 ± 0.041 (95% CI: 0.670–0.830, p < 0.0001).
The cut-off CXR score before discharge with the highest sensitivity (92.1%) and specificity (61.3%) to distinguish normal (score = 0) and abnormal (score≥1) CXR at mid-term follow-up was 2 with AUC of 0.798 ± 0.043 (95% CI: 0.713–0.867, p < 0.0001).
The ROC curves and the Youden's index are illustrated in Fig. 1 .
Figure 1.
The cut-off CXR score at admission with the highest sensitivity and specificity to distinguish normal and abnormal CXR at mid-term follow-up was 3 (A). The cut-off CXR score before discharge with the highest sensitivity and specificity to distinguish normal and abnormal CXR at mid-term follow-up was 2 (B).
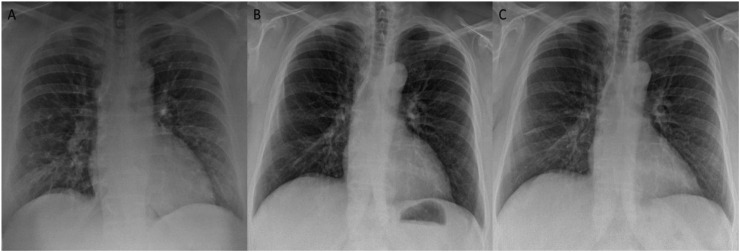
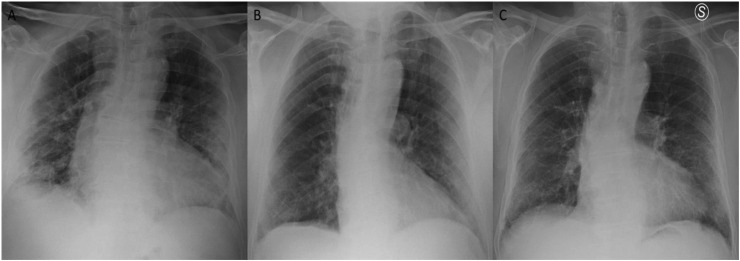
The Figure 2, Figure 3 show the CXRs images of two patients of this study.
Figure 2.
The figure shows the examples of patient with a CXR score of 3 at admission and CXR scores of 0 before the discharge and at three months follow-up.
Figure 3.
The figure shows the examples of patient with a CXR score of 6 at admission and CXR scores of 4 before the discharge and at three months follow-up.
Discussion
COVID-19 has spread around the world, causing hundreds of thousands of deaths.20 During the acute phase of the pandemic, many patients with severe COVID-19 were hospitalized and radiological temporal changes were evaluated with a close follow-up; it was necessary, but not sufficient because most patients who have recovered still had residual radiological abnormalities.8, 9, 10 Therefore, a mid-term follow-up is needed to show eventual clinical-radiological COVID-19 sequelae, such as irreversible fibrosis, and to understand if further diagnostic insights and therapeutic actions are needed.11, 12, 13 Therefore, we aimed to evaluate the radiological sequelae of COVID-19 patients in a mid-term follow-up and to investigate their relationship with clinical and radiological findings.
Our study enrolled 119 patients who underwent a CXR three months after hospital discharge. The multivariate regression analysis revealed that ambient air SO2 at admission (p = 0.0006), the days of hospitalization (p < 0.0001) and the CXR score before discharge (p = 0.0091) are independent factors for predicting the mid-term follow-up CXR score at three months. In particular, ambient air SO2 has a negative linear relationship, probably because its reduction reflects a higher radiological disease severity, as indicated in previous works.18, 19, 20, 21 On the contrary, the number of days of hospitalization and CXR score before discharge have a positive linear relationship, probably because they reflect a higher radiological disease severity, which requests a longer recovery time.
Out of the 119 patients included, 59 (49.6%) had a CXR score of 0 (Group A) which indicated a complete radiological recovery 3 months post discharge; the remaining 60 (50.4%) had radiological abnormalities with a CXR score of 3.0 ± 2.6 (Group B). In Group A, age, days of hospitalization, CXR scores at admission and before discharge were statistically lower than in Group B (patients with radiological abnormalities). These results could have different possible explanations. First, we hypothesize that in severe COVID-19 healing takes more time and the probability of a complete recovery is lower; consequently, CXR score at three months is higher. Indeed, older age and a long hospitalization could represent indirect factors of severe COVID-19 infection. In fact, in a recent systematic review and meta-analysis, Del Sole et al.22 showed that patients with severe/complicated SARS-CoV-2 infection had a mean older age (7 years) compared to those with non-severe disease. Moreover, as suggested by previous authors, higher CXR scores at admission and before discharge can represent direct elements of severe COVID-19 disease.23 Second, we suppose that older age and longer hospitalization could reduce the physiological lung reserve and postpone the rehabilitative intervention,24 increasing healing times and, consequently, mid-term follow-up CXR score at three months.
Interestingly, CXR score of 3 at admission and CXR score of 2 before discharge were the values with the highest sensitivity and specificity to distinguish the patients with normal (score = 0) and abnormal (score ≥1) CXR at mid-term follow-up. These score could be used, as cut-off values, to decide if the mid-term follow-up is necessary, avoiding, in this way, an excessive number of CXRs and giving priority to patients with more severe clinical and radiological findings.
An observational study of Zhao et al.18 evaluated the radiological sequelae of COVID-19 patients three month after discharge. They reported radiological abnormalities in 74.6% of patients and this value was higher than in our work (52.0%); however, they used a chest-CT to assess radiological sequelae and they had a smaller study population than ours (55 vs 119). Finally, similar to our work, they concluded that patients with abnormal radiological findings were generally older than patients with normal radiological images. Moreover, we carried out a literature review about radiological sequelae in similar pandemics induced by SARS-CoV-1 and Middle East Respiratory Syndrome Coronavirus (MERS-CoV). In a study about SARS-CoV-1,25 36% of 110 patients had residual CXR abnormalities three months after discharge and similar results were obtained in MERS survivors.26 , 27 These results indicate that after three months approximately a half - two third of patients have a full CXR resolution.
George et al.15 proposed a structured long-term follow-up after discharge of patients with previous COVID-19 pneumonia. They suggested use of CXR as the first imaging modality to evaluate discharged COVID-19 patients and, only in the case of radiological abnormalities, they proposed to proceed with clinical assessment and CT evaluation, paying particular attention to patients with previous complicated COVID-19. Based on our results, we agree with this follow-up structure. CXR represents a rapid, widespread and low-dose method allowing evaluation of possible sequelae in COVID-19; it can be a useful and objective first step modality to decide whether to go further with more in-depth diagnostic and expensive exams, such as chest-CT.
Evaluation of clinical data, such as SO2 and, above all, CXR scores at admission and before discharge, we might predict the CXR score at three months and, taking into consideration the patient's age, we could suggest a closer follow-up and a targeted management.
Until an effective vaccine for SARS-Cov-2 is developed, long-term follow-up should be performed to identify and proactively manage sequelae from infection and support patients through pulmonary rehabilitation with the goal of complete recovery.
This study has some limitations. First, it is a mono-centric study; further multi-centric works are needed to confirm our results. Second, CXRs were reviewed in consensus and the inter-observer agreement was not calculated. Third, patients included in the study have predominantly a mild to moderate disease.
Conclusions
Radiological abnormalities persist three months after discharge in a high proportion of COVID-19 patients. SO2 at admission, days of hospitalization and CXR score before discharge are independent factors for predicting the CXR score at three months. Moreover, age, days of hospitalization, CXR scores at admission and before discharge were statistically higher in the Group with radiological abnormalities than in the Group with radiological complete recovery, three months after discharge. The radiologist has an essential role in the detection of the pathology but he also can play a central role in mid to long-term follow-up of COVID-19, assessing the radiological sequelae of patients and identifying those who might require a closer follow-up.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data statement
Data to support the findings of this study are not publicly available, however, data is available upon reasonable request from the corresponding author.
Conflict of interest statement
The authors declared no potential conflicts of interests associated with this study.
Acknowledgments
None
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . 7 September 2020. Weekly epidemiological update. Coronavirus disease 2019 (COVID-19) [Google Scholar]
- 3.Mohanty S.K., Satapathy A., Naidu M.M., Mukhopadhyay S., Sharma S., Barton L.M., et al. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19) - anatomic pathology perspective on current knowledge. Diagn Pathol. 2020;15(1):103. doi: 10.1186/s13000-020-01017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhan J., Li H., Yu H., Liu X., Zeng X., Peng D., et al. 2019 novel coronavirus (COVID-19) pneumonia: CT manifestations and pattern of evolution in 110 patients in Jiangxi, China. Eur Radiol. 2021;31(2):1059–1068. doi: 10.1007/s00330-020-07201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong H.Y.F., Lam H.Y.S., Fong A.H., Leung S.T., Chin T.W., Lo C.S.Y., et al. Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology. 2020;296(2):E72–E78. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasilewski P.G., Mruk B., Mazur S., Półtorak-Szymczak G., Sklinda K., Walecki J. COVID-19 severity scoring systems in radiological imaging - a review. Pol J Radiol. 2020;85:e361–e368. doi: 10.5114/pjr.2020.98009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghesi A., Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med. 2020;125(5):509–513. doi: 10.1007/s11547-020-01200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan Y., Guan H., Zhou S., Wang Y., Li Q., Zhu T., et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020 Jun;30(6):3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu N., He G., Yang X., Chen J., Wu J., Ma M., et al. Dynamic changes of Chest CT follow-up in Coronavirus disease-19 (COVID-19) pneumonia: relationship to clinical typing. BMC Med Imag. 2020 Aug 5;20(1):92. doi: 10.1186/s12880-020-00491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Dong C., Hu Y., Li C., Ren Q., Zhang X., et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: sEPHa longitudinal study. Radiology. 2020;296(2):E55–E64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Q., Guan H., Sun Z., Huang L., Chen C., Ai T., et al. Early CT features and temporal lung changes in COVID-19 pneumonia in Wuhan, China. Eur J Radiol. 2020 Jul;128:109017. doi: 10.1016/j.ejrad.2020.109017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu M., Liu Y., Xu D., Zhang R., Lan L., Xu H., et al. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol. 2020 Jun;21(6):746–755. doi: 10.3348/kjr.2020.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei J., Lei P., Yang H., Fan B., Qiu Y., Zeng B., et al. Analysis of thin-section CT in patients with coronavirus disease (COVID-19) after hospital discharge. Clin Imag. 2020;28(3):383–389. doi: 10.3233/XST-200685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George P.M., Barratt S.L., Condliffe R., Desai S., Devaray A., Forrest I., et al. Thorax Published Online First; 24 August 2020. Respiratory follow-up of patients with COVID-19 pneumonia. [DOI] [PubMed] [Google Scholar]
- 15.Metlay J.P., Waterer G.W., Long A.C., Brozek J., Crothers K., Cooley L., et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mo X., Jian W., Su Z., Peng H., Peng P., Mu C., et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frija-Masson J., Debray M.-P., Gilbert M., Gilbert M., Lescure M., Travert F., et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020;56:2001754. doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Yu-miao, Shang Yao-min, Song Wen-bin, Li Qing-quan, Xie Hua, Xu Qin-fu, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinical Medicine. 2020 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zha L., Shen Y., Pan L., Han M., Yang G., Teng X., et al. Follow-up study on pulmonary function and radiological changes in critically ill patients with COVID-19. J Infect. 2021;82(1):159–198. doi: 10.1016/j.jinf.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadeghi Dousari A., Taati Moghadam M., Satarzadeh N. COVID-19 (coronavirus disease 2019): a new coronavirus disease. Infect Drug Resist. 2020 Aug 12;13:2819–2828. doi: 10.2147/IDR.S259279. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K., Kang S., Tian R., Zhang X., Zhang X., Wang Y. Imaging manifestations and diagnostic value of chest CT of coronavirus disease 2019 (COVID-19) in the Xiaogan area. Clin Radiol. 2020 May;75(5):341–347. doi: 10.1016/j.crad.2020.03.004. Epub 2020 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Sole F., Farcomeni A., Loffredo L., Carnevale R., Menichelli D., Vicario T., et al. Features of severe COVID-19: a systematic review and meta-analysis. Eur J Clin Invest. 2020 Aug 9 doi: 10.1111/eci.13378. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borghesi A., Zigliani A., Golemi S., Carapella N., Maculotti P., Farina D., et al. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: a study of 302 patients from Italy. Int J Infect Dis. 2020 Jul;96:291–293. doi: 10.1016/j.ijid.2020.05.021. Epub 2020 May 8. PMID: 32437939 Free PMC article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belli S., Balbi B., Prince I., Cattaneo D., Masocco F., Zaccaria S., et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived the hospitalisation. Eur Respir J. 2020 Aug 6:2002096. doi: 10.1183/13993003.02096-2020. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hui D.S., Joynt G.M., Wong K.T., Gomersall C.D., Li T.S., Antonio G., et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60:401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das K.M., Lee E.Y., Singh R., Enani M.A., Dosari K.A., Gorkom K.V., et al. Follow-Up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imag. 2017;27:342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., et al. Angiotensin-ConvertingEnzyme2: sARS-CoV-2 receptor and regulator of the renin-AngiotensinSystem: celebrating the 20th Anniversary of the discovery of ACE2. Cir Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]