Abstract
Background
To explore evolving surgical techniques and outcomes for aortic arch surgery.
Methods
A total of 2435 consecutive patients underwent aortic arch repair with hypothermic circulatory arrest between 2008 and 2018 in 12 institutions across Canada. Trends in patient characteristics, surgical techniques, and in-hospital outcomes, including major morbidity or mortality, were examined.
Results
From 2008 to 2018, the age of patients (62.3 ± 13.2 years) and the proportion of women (30.2%) undergoing arch surgery did not change significantly. Aortic diameters at operation decreased (2008: 58 ± 13 mm; 2018: 53 ± 11 mm; P < 0.01). Surgeons performed more valve-sparing root replacements (2008: 0%; 2018: 15%; P < 0.001) and fewer Bentall procedures (2008: 27%; 2018: 20%; P < 0.01). Total arch replacement rates were similar (P = 0.18); however, elephant trunk (2008: 9.5%; 2018: 19%; P < 0.001) and frozen elephant trunk (2008: 3.1%; 2018: 15%; P < 0.001) repair rates have increased. Over time, higher nadir temperatures (2008: 18 [17-21]°C; 2018: 25 [23-28]°C; P < 0.001), and more frequent antegrade cerebral perfusion (2008: 61%; 2018: 83%; P < 0.001) were used. For elective cases, in-hospital mortality rates declined (2008: 6.8%; 2018: 1.2%; P = < 0.01), as did major morbidity or mortality (2008: 24%; 2018: 13%; P < 0.001) and transfusion rates (2008: 61%; 2018: 41%; P < 0.001), but stroke rates remained constant (2008: 6.8%; 2018: 5.3%; P = 0.12). Outcomes remained the same over time for urgent or emergent cases.
Conclusions
Outcomes have improved over the past decade in Canada for elective aortic arch surgery, in the context of operating on smaller aortas, and more frequent use of moderate hypothermia and antegrade cerebral perfusion. Further research is needed to improve stroke rates and outcomes in the emergency setting.
Résumé
Introduction
Examiner l’évolution des techniques chirurgicales et les résultats de l'intervention chirurgicale de l'arc aortique.
Méthodes
Un total de 2 435 patients consécutifs ont subi une réparation de l'arc aortique en arrêt circulatoire en hypothermie entre 2008 et 2018 dans 12 établissements du Canada. Nous avons examiné les tendances en ce qui concerne les caractéristiques des patients, les techniques chirurgicales et les résultats cliniques intrahospitaliers, y compris les principales causes de morbidité ou de mortalité.
Résultats
De 2008 à 2018, l’âge des patients (62,3 ± 13,2 ans) et la proportion de femmes (30,2 %) subissant l'intervention chirurgicale de l'arc n'a pas montré de changement significatif. Les diamètres aortiques à l'opération ont diminué (2008 : 58 ± 13 mm; 2018 : 53 ± 11 mm; P < 0,01). Les chirurgiens ont réalisé un plus grand nombre de remplacements de la racine aortique sans remplacement de la valve (2008 : 0 %; 2018 : 15 %; P < 0,001) et un moins grand nombre d'opérations de Bentall (2008 : 27 %; 2018 : 20 %; P < 0,01). Les taux totaux de remplacements de l'arc étaient similaires (P = 0,18). Toutefois, les taux de réparation avec la technique de la trompe d’éléphant; (2008 : 9,5 %; 2018 : 19 %; P < 0,001) et de la trompe d’éléphant congelée (2008 : 3,1 %; 2018 : 15 %; P < 0,001) ont augmenté. Avec le temps, des nadirs supérieurs de température (2008 : 18 [17-21]°C; 2018 : 25 [23-28]°C; P < 0,001) et des perfusions cérébrales antérogrades plus fréquentes (2008 : 61 %; 2018 : 83 %; P < 0,001) ont été utilisés. Pour les cas non urgents, les taux de mortalité intrahospitalière (2008 : 6,8 %; 2018 : 1,2 %; P = < 0,01) et les taux de morbidité grave et de mortalité (2008 : 24 %; 2018 : 13 %; P < 0,001) et de transfusion (2008 : 61 %; 2018 : 41 %; P < 0,001) ont décru, mais les taux d'accidents vasculaires cérébraux (2008 : 6,8 %; 2018 : 5,3 %; P = 0,12) sont demeurés constants. Les résultats cliniques sont demeurés identiques au fil du temps pour les cas urgents ou les nouveaux cas.
Conclusions
Au Canada, les résultats de l'intervention chirurgicale non urgente de l'arc aortique se sont améliorés au cours de la dernière décennie dans le contexte de l'opération d'aortes plus petites et de l'utilisation plus fréquente de l'hypothermie modérée et de la perfusion cérébrale antérograde. D'autres recherches sont nécessaires pour améliorer les taux d'accidents vasculaires cérébraux et les résultats cliniques dans le cadre d'interventions urgentes.
Aortic arch surgery has traditionally been associated with high rates of morbidity and mortality, largely due to complications associated with interrupting flow to the cerebral and systemic circulations. Over the past several decades, stepwise advances in circulatory management, surgical technique, anesthetic administration, and perioperative care have made it possible to more safely repair the aortic arch.1, 2, 3, 4 The first successful arch replacement was performed in 1957 by DeBakey, using cardiopulmonary bypass.5 In 1975, Griepp and colleagues introduced the concept of hypothermia to reduce the metabolic demand on the brain.6 Ueda in 1990, based on work by Mills and Ochsner, proposed the use of continuous retrograde cerebral perfusion as an adjunct to hypothermic circulatory arrest (HCA).7 In the 1980s and 1990s, the more physiologic antegrade cerebral perfusion (ACP) with HCA was proposed by Kazui, aimed at extending the safe duration of HCA.8 These advances, along with modifications in cannulation techniques, use of the frozen elephant trunk, and changes in temperature management,9, 10, 11 have contributed to better outcomes, with contemporary studies reporting mortality rates of 2%-5%12, 13, 14 and stroke rates of 2%-7%.12,15, 16, 17, 18
The adoption and impact of these evolving techniques on outcomes of aortic arch surgery are unclear. This study aimed to explore the trends in patient characteristics, surgical techniques, and outcomes of aortic arch surgery over time in Canada.
Patients and Methods
Study population
The Canadian Thoracic Aortic Collaborative (CTAC) is a national collaborative of cardiac surgeons with expertise in aortic repair who retrospectively compiled a comprehensive national registry of consecutive patients undergoing thoracic aortic surgery with circulatory arrest. All extents of thoracic aortic surgery, including hemiarch replacements, total arch reconstructions, distal arch, and descending thoracic aortic repairs, were included in the registry if circulatory arrest was used during the procedure. Elective and emergent cases were included, as were cases with concomitant surgery. Circulatory arrest cases for thoracoabdominal aortic repair, or those not involving aortic repair (eg, congenital cases, tumor removal, etc.), were excluded.
For the present study, a total of 2435 consecutive patients underwent aortic arch repair with HCA from 2008 to 2018. There were a total of 52 contributing surgeons from 12 centres, with the lowest-volume centre contributing 43 cases, and the highest-volume centre contributing 387 cases. Each centre obtained local ethics approval from their respective institutional review boards, and individual informed consent was waived at all centres.
Trends analysis
Trends in 3 clinical areas were examined: (i) patient characteristics; (ii) surgical techniques; and (iii) in-hospital outcomes. For preoperative baseline characteristics, variables assessed included age, aortic valve disease, aortic diameter, presence of dissection or rupture, urgency, and comorbidities. Surgical techniques evaluated included extent of aortic reconstruction, concomitant surgeries, surgical times, nadir temperatures, circulatory arrest times, and cerebral protection strategies. Outcomes evaluated included in-hospital mortality, in-hospital stroke, transfusion rates, and a modified Society of Thoracic Surgeons–defined composite endpoint for major morbidity and operative mortality.19 This composite endpoint was defined as the occurrence of 1 or more of the following: in-hospital mortality, stroke, dialysis-dependent renal failure, deep sternal wound infection, reoperation, or prolonged ventilation of more than 40 hours.9 A subgroup analysis of elective (n = 1510) and urgent or emergent operations (n = 905) was performed as well, and outcomes were evaluated.
Statistical methods
Trends over time for binary, ordinal, categorical, or continuous variables were assessed using mixed-effect models with logit, cumulative ordinal, multinomial logit, or identity link, respectively, with a random-effect for the centre to account for the effect of the individual centres using PROC GLIMMIX in SAS 9.4 (SAS Institute, Cary, NC). Statistical significance was set at α = 0.05. For variables that were not normally distributed (eg, surgical times), a logarithmic transformation was used in mixed-effect models. Two mixed-effect models were built for every variable. First, a piecewise linear random-effect model was built using date of surgery as a continuous measure with time knots every 2 years (ie, at 2008, 2010, 2012, 2014, and 2016). Second, a simpler model was built without time knots to assess an overall linear trend through the years. For linear trend analyses, values at the beginning and the end of the curve may not exactly correspond to the 2008 or 2018 biyearly raw values or biyearly model estimates. For all models, for binary, categorical, and continuous variables, results are presented with a 95% confidence interval.
Results
Baseline characteristics
A total of 2435 patients undergoing aortic arch surgery, with an average age of 62.3 years ± 13.2 years, were included in this study, of whom 1700 (69.8%) were males. From 2008 to 2018, the age of patients (P = 0.60) and the proportion of women (P = 0.46) remained the same. There has been a decrease in several patient comorbidities over time (Table 1), including preoperative renal failure (2008:13%; 2018: 5.4%; P < 0.01) and chronic obstructive pulmonary disease (2008: 20%; 2018:12%; P < 0.001). The majority of patients had preserved left ventricular ejection fraction (≥60%); this has not changed over time (P = 0.48). Over the study period, the maximum aortic diameter of patients undergoing surgery decreased over time (2008: 58 ± 13 mm; 2018: 53 ± 11 mm; P < 0.01). There was also a significant decline in the proportion of urgent (2008: 20%; 2018: 9.1%; P = 0.04) and emergent (2008: 28%; 2018: 22%; P = 0.04) cases, and a similar increase in the proportion of elective cases (2008: 50%; 2018: 67%; P = 0.04). The rates of aortic stenosis (2008: 19%; 2018: 34%; P < 0.001) and aortic insufficiency (2008: 48%; 2018: 53%; P = 0.02) have increased over time. The proportion of patients with a bicuspid aortic valve has remained stable at about 25% of the population undergoing aortic arch surgery (2008: 24%; 2018: 26%; P = 0.42).
Table 1.
Baseline patient characteristics over the study period, 2008 to 2018
| Year | 2008(n = 148) | 2010(n = 267) | 2012(n = 310) | 2014(n = 584) | 2016(n = 753) | 2018(n = 373) | Modeled linear regression P value* |
|---|---|---|---|---|---|---|---|
| Age, y | 63 ± 13 | 62 ± 13 | 62 ± 13 | 63 ± 13 | 62 ± 14 | 62 ± 13 | 0.60 |
| Female | 41 (32) | 73 (27) | 102 (33) | 180 (31) | 218 (29) | 108 (29) | 0.46 |
| Hypertension | 80 (63) | 188 (70) | 222 (72) | 422 (72) | 506 (67) | 254 (68) | 0.86 |
| Connective tissue disorder | 0.23 | ||||||
| None | 117 (94) | 235 (89) | 279 (91) | 539 (93) | 723 (96) | 350 (94) | |
| Confirmed | 8 (6.4) | 28 (11) | 27 (8.8) | 42 (7.2) | 28 (3.7) | 22 (5.9) | |
| Suspected | 0 (0) | 0 (0) | 2 (0.65) | 1 (0.17) | 1 (0.13) | 0 (0) | |
| Diabetes mellitus | 12 (9.4) | 35 (13) | 38 (12) | 63 (11) | 95 (13) | 45 (12) | 0.67 |
| Dyslipidemia | 45 (36) | 136 (52) | 149 (49) | 270 (46) | 324 (43) | 134 (36) | 0.29 |
| Renal failure | 16 (13) | 28 (10) | 28 (9) | 54 (9.2) | 44 (5.8) | 20 (5.4) | < 0.01 |
| Cerebrovascular disease | 18 (12) | 23 (8.6) | 34 (11) | 61 (10) | 67 (8.9) | 41 (11) | 0.48 |
| Peripheral vascular disease | 20 (14) | 53 (20) | 56 (18) | 84 (14) | 79 (10) | 32 (8.6) | 0.77 |
| Smoker | 63 (43) | 140 (52) | 140 (45) | 253 (43) | 304 (40) | 139 (37) | < 0.001 |
| COPD | 25 (20) | 45 (17) | 40 (13) | 77 (13) | 70 (9.3) | 43 (12) | < 0.001 |
| Previous cardiac surgery | 20 (16) | 47 (18) | 47 (15) | 112 (19) | 117 (16) | 51 (14) | 0.61 |
| Atrial fibrillation | 20 (16) | 31 (12) | 44 (14) | 74 (13) | 89 (12) | 35 (9.4) | 0.26 |
| Coronary artery disease | 19 (15) | 59 (22) | 64 (21) | 144 (25) | 143 (19) | 63 (17) | 0.37 |
| LVEF, % | 0.48 | ||||||
| > 60 | 97 (76) | 197 (74) | 257 (83) | 469 (80) | 571 (76) | 293 (79) | |
| 40–60 | 23 (18) | 54 (20) | 30 (10) | 81 (14) | 125 (17) | 58 (16) | |
| 20–40 | 6 (4.7) | 14 (5.2) | 19 (6.1) | 22 (3.8) | 49 (6.5) | 16 (4.3) | |
| < 20 | 2 (1.6) | 2 (0.75) | 4 (1.3) | 12 (2.1) | 8 (1.1) | 6 (1.6) | |
| Anatomy | |||||||
| Body surface area, m2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.3 | 2.0 ± 0.3 | < 0.001 |
| Maximum aortic diameter, mm | 58 ± 13 | 55 ± 11 | 54 ± 12 | 53 ± 10 | 52 ± 12 | 53 ± 11 | < 0.01 |
| Maximum indexed aortic diameter, mm/m2 |
31 ± 8 | 29 ± 7 | 28 ± 7 | 28 ± 12 | 27 ± 7 | 27 ± 6 | < 0.001 |
| Aortic valve anatomy | 0.42 | ||||||
| Tricuspid valve | 97 (76) | 196 (74) | 208 (68) | 386 (68) | 520 (73) | 268 (72) | |
| Bicuspid valve | 31 (24) | 68 (26) | 95 (31) | 174 (31) | 193 (27) | 97 (26) | |
| Unicuspid valve | 0 (0) | 0 (0) | 2 (0.66) | 5 (0.88) | 3 (0.42) | 5 (1.4) | |
| Aortic stenosis | 24 (19) | 59 (22) | 80 (26) | 160 (27) | 218 (29) | 125 (34) | < 0.001 |
| Aortic insufficiency | 62 (48) | 144 (54) | 166 (54) | 312 (53) | 359 (48) | 198 (53) | 0.02 |
| Presentation | |||||||
| Acute dissection or rupture | 38 (30) | 69 (26) | 65 (21) | 135 (23) | 181 (24) | 81 (22) | 0.03 |
| Dissection | 51 (40) | 110 (41) | 108 (35) | 198 (34) | 243 (32) | 112 (30) | 0.01 |
| Rupture | 8 (6.3) | 16 (6.0) | 16 (5.2) | 31 (5.3) | 37 (4.9) | 15 (4.0) | 0.17 |
| Urgency status | 0.04 | ||||||
| Elective | 64 (50) | 146 (55) | 202 (65) | 384 (66) | 477 (63) | 249 (67) | |
| Urgent | 25 (20) | 44 (16) | 38 (12) | 58 (10) | 80 (11) | 34 (9.1) | |
| Emergent (< 6 h) | 36 (28) | 70 (26) | 61 (20) | 133 (23) | 186 (25) | 81 (22) | |
| Salvage | 3 (2.3) | 7 (2.6) | 9 (2.9) | 9 (1.5) | 10 (1.3) | 9 (2.4) | |
| Emergent or salvage | 39 (30) | 77 (29) | 70 (23) | 142 (24) | 196 (26) | 90 (24) | 0.04 |
Table shows trends in baseline characteristics in 2435 patients who underwent aortic arch surgery with hypothermic circulatory arrest, between 2008 and 2018, in 12 institutions across Canada. Values are n (%) or mean ± standard deviation.
COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction.
Whether these characteristics changed significantly over time was assessed by mixed-effect linear regression models using date of surgery as a continuous variable to assess a linear trend across the years from 2008 to 2018.
Operative characteristics
Over the study period, the types of surgeries have evolved (Table 2). Surgeons performed fewer Bentall procedures (2008: 27%; 2018: 20%; P < 0.01) and more valve-sparing root replacements (2008: 0%; 2018: 15%; P < 0.001), although the overall proportion of patients undergoing concomitant root replacement remained stable (P = 0.29). There was no statistically significant change in the rates of total arch replacement (2008: 18%; 2018: 20%; P = 0.18); however, the elephant trunk (2008: 9.5%; 2018: 19%; P < 0.001) and frozen elephant trunk (2008: 3.1%; 2018: 15%; P < 0.001) repair rates have increased significantly over time.
Table 2.
Intraoperative characteristics over the study period, 2008 to 2018
| Year | 2008(n = 148) | 2010(n = 267) | 2012(n = 310) | 2014(n = 584) | 2016(n = 753) | 2018(n = 373) | Modeled linear regression P value* |
|---|---|---|---|---|---|---|---|
| Aortic replacement | |||||||
| Ascending aorta | 111 (87) | 231 (87) | 274 (88) | 492 (84) | 648 (86) | 315 (84) | 0.92 |
| Arch replacement | |||||||
| Hemiarch replacement | 85 (66) | 177 (66) | 216 (70) | 446 (76) | 600 (80) | 290 (78) | 0.30 |
| Total arch replacement | 23 (18) | 44 (16) | 43 (14) | 76 (13) | 126 (17) | 75 (20) | 0.18 |
| Elephant trunk repair | 14 (9.5) | 24 (9.0) | 14 (4.5) | 41 (7.0) | 93 (12) | 70 (19) | < 0.001 |
| Frozen elephant trunk repair | 4 (3.1) | 9 (3.4) | 4 (1.3) | 11 (1.9) | 46 (6.1) | 56 (15) | < 0.001 |
| Aortic valve or root surgery | |||||||
| Aortic valve replacement | 17 (13) | 36 (13) | 47 (15) | 104 (18) | 128 (17) | 81 (22) | 0.08 |
| Bentall procedure | 35 (27) | 80 (30) | 90 (29) | 194 (33) | 208 (28) | 73 (20) | < 0.01 |
| Ross procedure | 0 (0) | 1 (0.37) | 5 (1.6) | 8 (1.4) | 16 (2.1) | 7 (1.9) | 0.03 |
| Valve-sparing root replacement | 0 (0) | 16 (6.0) | 24 (7.7) | 44 (7.5) | 72 (10) | 55 (15) | < 0.001 |
| Aortic valve repair | 27 (21) | 51 (19) | 61 (20) | 80 (14) | 113 (15) | 81 (22) | 0.88 |
| Concomitant surgery | |||||||
| Any concomitant surgery | 49 (38) | 92 (34) | 110 (35) | 207 (35) | 224 (30) | 125 (34) | 0.72 |
| Mitral valve replacement | 1 (0.78) | 7 (2.6) | 11 (3.5) | 11 (1.9) | 19 (2.5) | 3 (0.8) | 0.71 |
| Mitral valve repair | 2 (1.6) | 1 (0.37) | 3 (1.0) | 12 (2.1) | 12 (1.6) | 5 (1.3) | 0.25 |
| Coronary artery bypass grafting | 17 (13) | 52 (19) | 54 (17) | 120 (21) | 114 (15) | 64 (17) | 0.58 |
| ASD or VSD closure | 0 (0) | 1 (0.37) | 8 (2.6) | 14 (2.4) | 10 (1.3) | 4 (1.1) | 0.50 |
| Head or neck vessel surgery | 22 (17) | 42 (16) | 31 (10) | 65 (11) | 88 (12) | 53 (14) | 0.43 |
| Other | 16 (13) | 21 (7.9) | 29 (9.4) | 52 (8.9) | 62 (8.2) | 34 (9.1) | 0.28 |
| Perfusion, min | |||||||
| Cardiopulmonay bypass time | 173 [143, 221] | 192 [142, 245] | 187 [141, 237] | 180 [142, 230] | 178 [135, 230] | 176 [125, 224] | < 0.001 |
| Cross-clamp time | 92 [64, 129] | 107 [65, 144] | 107 [67, 154] | 120 [77, 161] | 114 [74, 165] | 111 [72, 165] | 0.36 |
| Hypothermic circulatory arrest time | 23 [15, 33] | 22 [16, 36] | 20 [15, 31] | 20 [14, 30] | 21 [14, 32] | 20 [16, 30] | < 0.001 |
| Hypothermic circulatory arrest time categories, min | < 0.001 | ||||||
| ≤ 30 | 90 (70) | 182 (68) | 227 (73) | 440 (75) | 552 (73) | 283 (76) | |
| 31–59 | 30 (23) | 54 (20) | 59 (19) | 104 (18) | 148 (20) | 65 (17) | |
| ≥ 60 | 8 (6.3) | 31 (12) | 24 (7.7) | 40 (6.8) | 53 (7.0) | 25 (6.7) | |
| Lowest temperature,°C | 18 [17, 21] | 19 [18, 23] | 22 [18, 25] | 24 [20, 26] | 25 [21, 26] | 25 [23, 28] | < 0.001 |
| Lowest temperature, ≥ 24°C | 14 (12) | 36 (16) | 110 (42) | 290 (55) | 447 (62) | 274 (74) | < 0.001 |
| Cerebral perfusion strategy | |||||||
| None | 49 (38) | 86 (32) | 80 (26) | 115 (20) | 96 (13) | 42 (11) | < 0.001 |
| Antegrade | 78 (61) | 181 (68) | 222 (72) | 431 (74) | 618 (82) | 309 (83) | < 0.001 |
| Retrograde | 1 (0.78) | 0 (0) | 8 (2.6) | 38 (6.5) | 39 (5.2) | 22 (5.9) | 0.70 |
| Cerebral perfusion time, min | 11 [0, 25] | 15 [0, 26] | 16 [0, 26] | 17 [9, 26] | 19 [11, 28] | 19 [13, 27] | < 0.01 |
| Cerebral ischemia time, min | 2 [0, 20] | 0 [0, 18] | 0 [0, 12] | 0 [0, 9] | 0 [0, 4] | 0 [0, 4] | < 0.001 |
| Cerebral ischemia time, ≥ 30 mins | 15 (12) | 29 (11) | 22 (7.1) | 21 (3.6) | 27 (3.6) | 9 (2.4) | < 0.001 |
| Transfusion | |||||||
| Any | 92 (72) | 194 (73) | 220 (71) | 365 (63) | 471 (63) | 204 (55) | < 0.001 |
| Any pRBC used | 67 (52) | 133 (50) | 153 (49) | 248 (42) | 340 (45) | 147 (39) | < 0.001 |
| Any FFP used | 74 (58) | 156 (58) | 178 (57) | 279 (48) | 362 (48) | 159 (43) | < 0.001 |
| Any platelet used | 76 (59) | 163 (61) | 180 (58) | 285 (49) | 358 (48) | 173 (46) | < 0.001 |
| Any factor VII used | 19 (15) | 36 (14) | 34 (11) | 38 (6.8) | 39 (5.2) | 18 (4.8) | < 0.001 |
Table shows trends in intraoperative characteristics and surgical techniques in 2435 patients who underwent aortic arch surgery with hypothermic circulatory arrest, between 2008 and 2018, in 12 institutions across Canada. Values are n (%) or median [interquartile range].
ASD, atrial septal defect; FFP, fresh frozen plasma; pRBC, packed red blood cell; VSD, ventricular septal defect.
Trend in time for binary, ordinal, categorical, or continuous variables was assessed using mixed-effect regression models using date of surgery as a continuous variable to assess a linear trend across the years from 2008 to 2018.
Techniques surrounding HCA have also changed over time. HCA was performed at higher median [IQR] nadir temperatures (2008: 18 [17, 21]°C; 2018: 25 [23, 28]°C; P < 0.001), with more frequent use of ACP (2008: 61%; 2018: 83%; P < 0.001). Accordingly, the use of “no cerebral perfusion” was decreased (2008: 38%; 2018: 11%; P < 0.001), and the median [IQR] cerebral perfusion time was increased (2008: 11 [0, 25] minutes; 2018: 19 [13, 27] minutes; P < 0.01) as the cerebral ischemia time decreased (2008: 2 [0, 20] minutes; 2018: 0 [0, 4] minutes; P < 0.001).
In-hospital outcomes
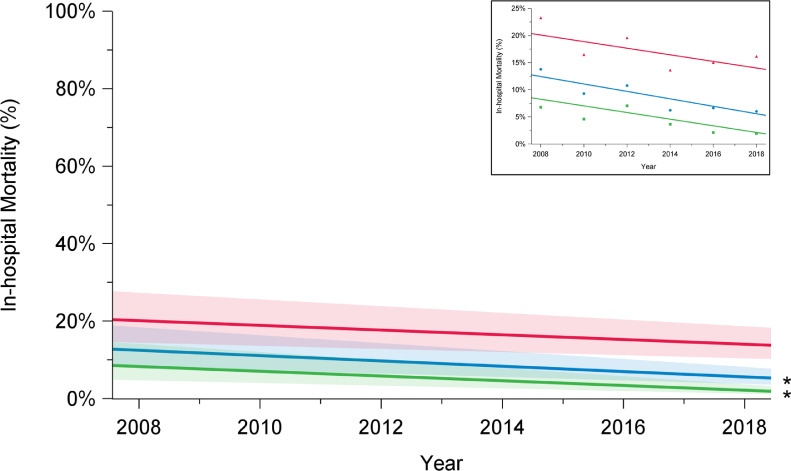
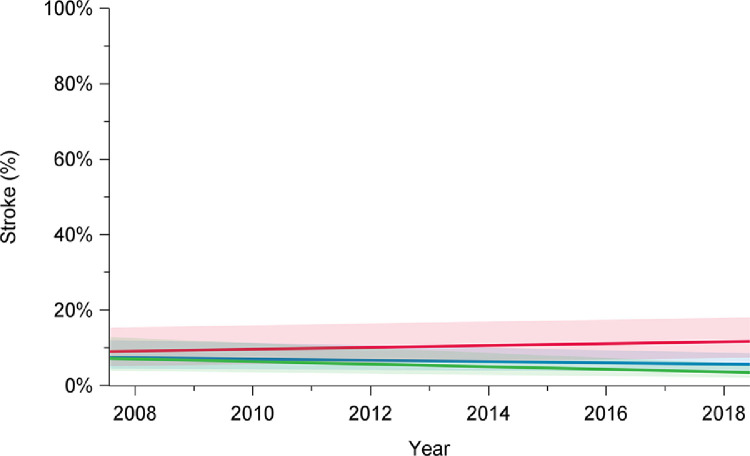
In-hospital mortality following arch repair declined from 16% in 2008 to 6.2% in 2018 (P = 0.001) (Fig. 1; Supplemental Table S1). Among elective cases, in-hospital mortality rates declined from 6.8% in 2008 to 1.2% in 2018 (P < 0.01). However, for urgent and emergent cases, in-hospital mortality remained higher and constant over the study period (2008: 23%; 2018: 16%; P = 0.15). Unlike the improvements observed for in-hospital mortality, in-hospital stroke rates remained constant over time for the overall group (2008: 10%; 2018: 8.9%; P = 0.36), patients undergoing elective surgery (2008: 6.8%; 2018: 5.3%; P = 0.12), and those undergoing urgent or emergent repair (2008: 15%; 2018: 16%; P = 0.49; Fig. 2; Supplemental Table S1).
Figure 1.
Trends in in-hospital mortality among elective (green), urgent (red), and overall (blue) cases, in patients who underwent aortic arch surgery with hypothermic circulatory arrest between 2008 and 2018, in 12 institutions across Canada. There has been a decline in in-hospital mortality rates over time among overall cases (P = 0.001) and elective cases (P = 0.0001), but not among urgent cases (P = 0.17). The significance of trends was assessed using mixed-effect regression models with random-effect for the centre to account for the effect of individual centres. The percentages presented are derived from a model and do not translate to a specific number of cases, and they are presented with their 95% confidence intervals. P value was obtained from a linear regression model using date of surgery as a continuous variable to assess a linear trend across the years from 2008 to 2018. *P < 0.05.
Figure 2.
Trends in stroke rates among elective (green), urgent (red), and overall (blue) cases, in patients who underwent aortic arch surgery with hypothermic circulatory arrest between 2008 and 2018, in 12 institutions across Canada. Stroke rates did not significantly change over time for overall cases (P = 0.52), elective cases (P = 0.13), or urgent cases (P = 0.34). Trend in time was assessed using mixed-effect regression models with random-effect for the centre to account for the effect of individual centres. The percentages presented are derived from a model and do not translate to a specific number of cases, and they are presented with their 95% confidence intervals. P value was obtained from a linear regression model using date of surgery as a continuous variable to assess a linear trend across the years from 2008 to 2018.
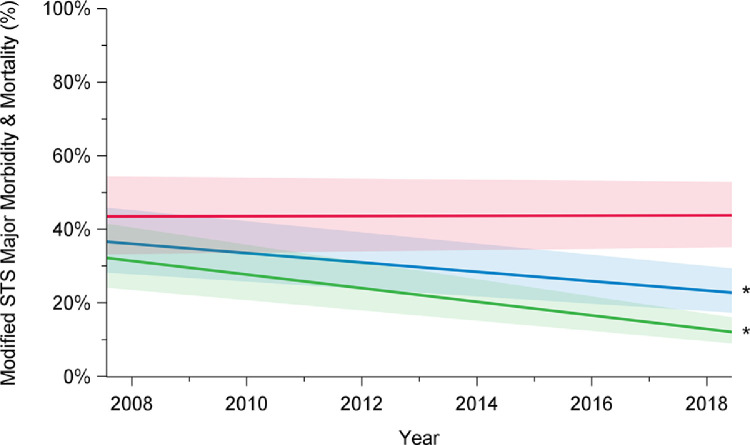
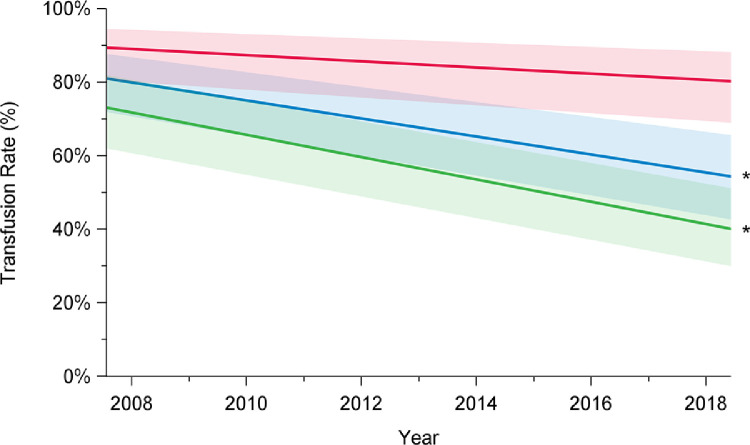
Rates of composite outcome of interest major morbidity and operative mortality decreased over time, from 41% in 2008 to 27% in 2018 (P < 0.001; Fig. 3; Supplemental Table S1). This improvement was limited to patients undergoing elective surgery (2008: 24%; 2018: 13%; P < 0.001) and was not observed after urgent or emergent repair (2008: 55%; 2018: 54%; P = 0.96). Overall total transfusion rates, including transfusion of the products packed red blood cells, fresh frozen plasma, platelets, and factor VII, significantly decreased over time (2008: 72%; 2018: 55%; P < 0.001; Fig. 4; Supplemental Table S1). Transfusion rates declined for elective cases (2008: 61%; 2018: 41%; P < 0.001), but not for patients undergoing urgent or emergent surgery (2008: 85%; 2018: 83%; P = 0.06).
Figure 3.
Trends in major morbidity and mortality among elective (green), urgent (red), and overall (blue) cases, in patients who underwent aortic arch surgery with hypothermic circulatory arrest between 2008 and 2018, in 12 institutions across Canada. There has been a decline in major morbidity and mortality rates over time among overall cases (P < 0.001) and elective cases (P < 0.001), but not among urgent cases (P = 0.98). The significance of the trends was assessed using mixed-effect regression models with random-effect for the centre to account for the effect of individual centres. The percentages presented are derived from a model and do not translate to a specific number of cases, and they are presented with their 95% confidence intervals. P value was obtained from a linear regression model using date of surgery as a continuous variable to assess a linear trend across the years from 2008 to 2018. STS, Society of Thoracic Surgeons. *P < 0.05.
Figure 4.
Trends in transfusion rates among elective (green), urgent (red), and overall (blue) cases, in patients who underwent aortic arch surgery with hypothermic circulatory arrest between 2008 and 2018, in 12 institutions across Canada. Transfusion rates included packed red blood cells, fresh frozen plasma, platelets, and factor VII. There has been a decline in total transfusion rates over time among overall cases (P < 0.001) and elective cases (P < 0.001), but not among urgent cases (P = 0.06). Trend in time was assessed using mixed-effect regression models with random-effect for the centre to account for the effect of individual centres. The percentages presented are derived from a model and do not translate to a specific number of cases, and they are presented with their 95% confidence intervals. P value was obtained from a linear regression model using date of surgery as a continuous variable to assess a linear trend across the years from 2008 to 2018. *P < 0.05.
Discussion
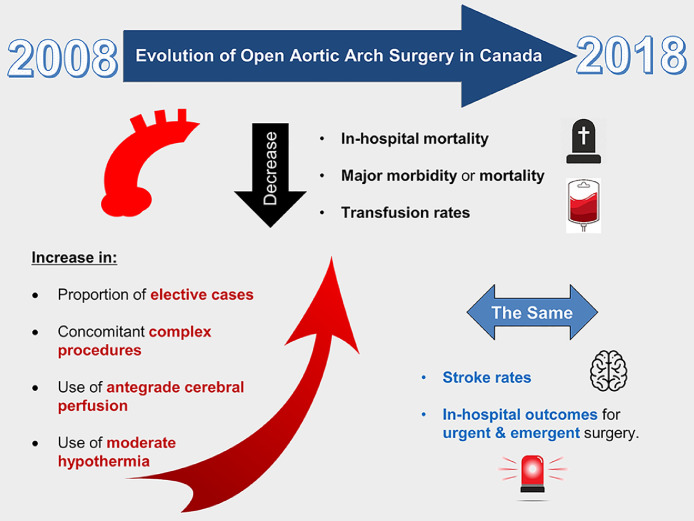
Through our study of a nationwide registry, we documented the evolution of open aortic arch surgery in Canada from 2008 to 2018. We observed a significant decline in in-hospital mortality, major complications, and transfusion rates over the past 10 years, primarily driven by improved outcomes after elective repair of the aortic arch. The main findings of our study are summarized in Figure 5.
Figure 5.
Evolving trends for open aortic arch surgery between 2008 and 2018 in Canada. Outcomes have improved over the past decade for elective aortic arch surgery, in the context of operating on smaller aortas, and more frequent use of moderate hypothermia and antegrade cerebral perfusion. Further research is needed to improve stroke rates and outcomes in the emergency setting.
A number of trends were observed in patient characteristics that may be linked to these improvements in in-hospital mortality and other complications. The size of the aorta at the time of intervention has decreased, along with the rates of baseline comorbidities such as renal failure and chronic obstructive pulmonary disease, and the proportion of elective cases has increased. These changes suggest that surgeons are intervening on healthier patients earlier in their aortic disease process. This difference may account for the improved outcomes, despite increases in surgical complexity, as exemplified by the higher use rates of valve-sparing root procedures and elephant trunk technique in recent years.
Recent advances in surgical technique were also associated with these improved outcomes. During HCA, there was increased use of ACP and higher nadir temperatures over the past decade. Retrograde cerebral perfusion has not been popular in Canada and has been used in only a minority of cases, ranging from 0% to 5.9% over the study period. By 2008, the start of the study period, ACP was already used in the majority of cases (61%), and by 2018, ACP was the standard cerebral perfusion strategy during HCA in Canada (used 83% of the time). ACP may be instituted through the innominate or right axillary artery for cardiopulmonary bypass and ACP inflow, providing constant perfusion to the brain during arch surgery, and reducing the brain ischemic time to zero.13,14,17,20,21
The nadir temperature during HCA has increased to 25°C, from 18°C a decade earlier. This change was likely driven by the increased use of ACP, limiting brain ischemic time. Compared with the use of deep hypothermia, the use of moderate hypothermia has been associated with shorter cardiopulmonary bypass time, shorter length of hospital stay, and lower early mortality.22, 23, 24 The Canadian Thoracic Aortic Collaborative recently performed an analysis of 647 propensity score–matched pairs of patients undergoing aortic arch surgery, and compared HCA with a nadir temperature < 24°C to a nadir temperature ≥ 24°C. Use of a nadir temperature ≥ 24°C was a predictor of improved survival and neurologic outcomes.25 An additional benefit of avoiding deep HCA is reduced coagulopathy, which helps to decrease transfusion requirements and prevent the development of acute lung injury and other transfusion-related sequelae.26 This benefit was corroborated by the current study, in which a significant decline in intraoperative use of blood products over the decade was observed.
Despite improvement in outcomes of aortic arch surgery over the past decade, stroke rates remain concerning and have not changed significantly. Identification of the risk factors and understanding of the pathogenesis of intraoperative stroke are necessary to decrease its occurrence. Most previous reports regarding stroke risk during aortic arch surgery have shown several preoperative factors to be predictors of stroke, including age, history of diabetes mellitus, renal insufficiency, coronary artery disease, and clinical presentation.27, 28, 29 Moreover, the amount of transfused blood products, which may be a surrogate indicator of coagulopathy or intraoperative bleeding, has been shown to be predictive of stroke.28 Additional important predictors of stroke are burden of atheroma in the ascending aorta, and prolonged brain ischemia time, as reported by Okada et al. in their retrospective study of 190 consecutive patients who underwent aortic arch surgery.30 Although much improvement has been accomplished in surgical techniques for cerebral protection and the reduction of intraoperative bleeding over the past decade, it appears to not be enough to offset baseline patient risk factors for stroke. Rates of diabetes mellitus, coronary artery disease, and history of cerebrovascular disease, all of which are important predictors for stroke, have not changed significantly over the study period. Moreover, other important predictors for stroke, such as atherosclerotic burden in the aorta are not accounted for in our study and may have contributed to the lack of decline in stroke rates. Stroke remains one of the most devastating complications in aortic arch surgery, and more research is needed to decrease its occurrence. Development of a more personalized approach to the application of adjunctive techniques, such as temperature management and perfusion strategies adapted to patient characteristics and anatomy, may be a worthy subject for future research.
In the subgroup analysis of elective vs urgent or emergent cases, the improvements in outcomes over the study period were noted to be driven by the elective cases. The incremental benefits of the surgical techniques discussed above likely are more pronounced in the elective setting, whereas the hemodynamic and physiological compromise associated with urgent arch surgery may be a more dominating factor in early outcomes. In addition, elective aortic surgery is more likely to be performed by surgeons with subspecialty training in aortic surgery. Hence; the growing experience of the cohort of surgeons may have contributed to the improvements in outcomes over the study period.31 Whether outcomes following urgent aortic surgery would be improved if they were performed by only specialized aortic surgeons is unproven.
Limitations
The main limitation of our study lies in its observational and retrospective nature. The improvement in outcomes was associated with trends in patient characteristics and surgical techniques but cannot be directly attributed to them. We were unable to capture other changes in practice over the same time period that also may have had a significant impact on outcome, including changes in anesthetic practices and perioperative medical management. Nevertheless, this study provides valuable data outlining the current state of aortic arch surgery in Canada in the context of the past decade and crystallizes knowledge gaps in need of further study. Finally, the use of a large national registry does not allow close examination of highly specific patient-level data. Missing details include cannulation strategy for ACP, unilateral vs bilateral cerebral perfusion, head vessel reconstruction technique, and the sequence of events during arch reconstruction, all of which may have an effect on stroke and other complications.
Conclusion
Outcomes have improved over the past decade in Canada for elective aortic arch surgery, in the context of operating on smaller aortas, and more frequent use of moderate hypothermia and ACP. Further research is needed to improve the rate of stroke and clinical outcomes in the emergency setting.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: Each centre obtained local ethics approval from their respective institutional review boards, and individual informed consent was waived at all centres.
See page 1124 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at doi:10.1016/j.cjco.2021.05.001.
Appendix. Supplementary materials
References
- 1.Coselli JS, Green SY. Evolution of aortic arch repair. Tex Heart Inst J. 2009;36:435–437. [PMC free article] [PubMed] [Google Scholar]
- 2.de la Cruz KI, Coselli JS, LeMaire SA. Open aortic arch replacement: a technical odyssey. J Extra Corpor Technol. 2012;44 P42-7. [PMC free article] [PubMed] [Google Scholar]
- 3.LeMaire SA, Price MD, Parenti JL. Early outcomes after aortic arch replacement by using the Y-graft technique. Ann Thorac Surg. 2011;91:700–707. doi: 10.1016/j.athoracsur.2010.11.008. [discussion 7-8] [DOI] [PubMed] [Google Scholar]
- 4.Wong DR, Coselli JS, Palmero L. Axillary artery cannulation in surgery for acute or subacute ascending aortic dissections. Ann Thorac Surg. 2010;90:731–737. doi: 10.1016/j.athoracsur.2010.04.059. [DOI] [PubMed] [Google Scholar]
- 5.De Bakey ME, Crawford ES, Cooley DA, Morris GC., Jr. Successful resection of fusiform aneurysm of aortic arch with replacement by homograft. Surg Gynecol Obstet. 1957;105:657–664. [PubMed] [Google Scholar]
- 6.Griepp RB, Stinson EB, Hollingsworth JF, Buehler D. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg. 1975;70:1051–1063. [PubMed] [Google Scholar]
- 7.Ueda Y, Miki S, Kusuhara K. Surgical treatment of aneurysm or dissection involving the ascending aorta and aortic arch, utilizing circulatory arrest and retrograde cerebral perfusion. J Cardiovasc Surg (Torino) 1990;31:553–558. [PubMed] [Google Scholar]
- 8.Kazui T, Kimura N, Yamada O, Komatsu S. Surgical outcome of aortic arch aneurysms using selective cerebral perfusion. Ann Thorac Surg. 1994;57:904–911. doi: 10.1016/0003-4975(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 9.Evangelista A, Isselbacher EM, Bossone E. Insights from the International Registry of Acute Aortic Dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137:1846–1860. doi: 10.1161/CIRCULATIONAHA.117.031264. [DOI] [PubMed] [Google Scholar]
- 10.El-Hamamsy I, Ouzounian M, Demers P. State-of-the-art surgical management of acute type A aortic dissection. Can J Cardiol. 2016;32:100–109. doi: 10.1016/j.cjca.2015.07.736. [DOI] [PubMed] [Google Scholar]
- 11.Hanif H, Dubois L, Ouzounian M. Aortic arch reconstructive surgery with conventional techniques vs frozen elephant trunk: a systematic review and meta-analysis. Can J Cardiol. 2018;34:262–273. doi: 10.1016/j.cjca.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Thomas M, Li Z, Cook DJ, Greason KL, Sundt TM. Contemporary results of open aortic arch surgery. J Thorac Cardiovasc Surg. 2012;144:838–844. doi: 10.1016/j.jtcvs.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 13.Halkos ME, Kerendi F, Myung R. Selective antegrade cerebral perfusion via right axillary artery cannulation reduces morbidity and mortality after proximal aortic surgery. J Thorac Cardiovasc Surg. 2009;138:1081–1089. doi: 10.1016/j.jtcvs.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 14.Kazui T, Washiyama N, Muhammad BA. Total arch replacement using aortic arch branched grafts with the aid of antegrade selective cerebral perfusion. Ann Thorac Surg. 2000;70:3–8. doi: 10.1016/s0003-4975(00)01535-6. [discussion 9] [DOI] [PubMed] [Google Scholar]
- 15.Svensson LG. Antegrade perfusion during suspended animation? J Thorac Cardiovasc Surg. 2002;124:1068–1070. doi: 10.1067/mtc.2002.127786. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura K, Nakamura E, Yano M. Factors influencing permanent neurologic dysfunction and mortality after total arch replacement with separate arch vessel grafting using selective cerebral perfusion. Ann Thorac Cardiovasc Surg. 2011;17:39–44. doi: 10.5761/atcs.oa.09.01514. [DOI] [PubMed] [Google Scholar]
- 17.Okita Y, Okada K, Omura A. Total arch replacement using antegrade cerebral perfusion. J Thorac Cardiovasc Surg. 2013;145:S63–S71. doi: 10.1016/j.jtcvs.2012.11.070. [DOI] [PubMed] [Google Scholar]
- 18.Iba Y, Minatoya K, Matsuda H. Contemporary open aortic arch repair with selective cerebral perfusion in the era of endovascular aortic repair. J Thorac Cardiovasc Surg. 2013;145:S72–S77. doi: 10.1016/j.jtcvs.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien SM, Feng L, He X. The society of thoracic surgeons 2018 adult cardiac surgery risk models: Part 2-statistical methods and results. Ann Thorac Surg. 2018;105:1419–1428. doi: 10.1016/j.athoracsur.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Leshnower BG, Myung RJ, Kilgo PD. Moderate hypothermia and unilateral selective antegrade cerebral perfusion: a contemporary cerebral protection strategy for aortic arch surgery. Ann Thorac Surg. 2010;90:547–554. doi: 10.1016/j.athoracsur.2010.03.118. [DOI] [PubMed] [Google Scholar]
- 21.Misfeld M, Mohr FW, Etz CD. Best strategy for cerebral protection in arch surgery—antegrade selective cerebral perfusion and adequate hypothermia. Ann Cardiothorac Surg. 2013;2:331–338. doi: 10.3978/j.issn.2225-319X.2013.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leshnower BG, Thourani VH, Halkos ME. Moderate versus deep hypothermia with unilateral selective antegrade cerebral perfusion for acute type A dissection. Ann Thorac Surg. 2015;100:1563–1568. doi: 10.1016/j.athoracsur.2015.05.032. [discussion 8-9] [DOI] [PubMed] [Google Scholar]
- 23.Kornilov IA, Sinelnikov YS, Soinov IA. Outcomes after aortic arch reconstruction for infants: deep hypothermic circulatory arrest versus moderate hypothermia with selective antegrade cerebral perfusion. Eur J Cardiothorac Surg. 2015;48 doi: 10.1093/ejcts/ezv235. e45-50. [DOI] [PubMed] [Google Scholar]
- 24.Vallabhajosyula P, Jassar AS, Menon RS. Moderate versus deep hypothermic circulatory arrest for elective aortic transverse hemiarch reconstruction. Ann Thorac Surg. 2015;99:1511–1517. doi: 10.1016/j.athoracsur.2014.12.067. [DOI] [PubMed] [Google Scholar]
- 25.Hage A, Stevens L-M, Ouzounian M. Impact of brain protection strategies on mortality and stroke in patients undergoing aortic arch repair with hypothermic circulatory arrest: evidence from the Canadian Thoracic Aortic Collaborative. Eur J Cardio-Thorac Surg. 2020;58:95–103. doi: 10.1093/ejcts/ezaa023. [DOI] [PubMed] [Google Scholar]
- 26.Matalanis G, Koirala RS, Shi WY, Hayward PA, McCall PR. Branch-first aortic arch replacement with no circulatory arrest or deep hypothermia. J Thorac Cardiovasc Surg. 2011;142:809–815. doi: 10.1016/j.jtcvs.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Svensson LG, Crawford ES, Hess KR. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg. 1993;106:19–28. [discussion 31] [PubMed] [Google Scholar]
- 28.Goldstein LJ, Davies RR, Rizzo JA. Stroke in surgery of the thoracic aorta: incidence, impact, etiology, and prevention. J Thorac Cardiovasc Surg. 2001;122:935–945. doi: 10.1067/mtc.2001.117276. [DOI] [PubMed] [Google Scholar]
- 29.Khaladj N, Shrestha M, Meck S. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending aortic and aortic arch surgery: a risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg. 2008;135:908–914. doi: 10.1016/j.jtcvs.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 30.Okada T, Shimamoto M, Yamazaki F. Insights of stroke in aortic arch surgery: identification of significant risk factors and surgical implication. Gen Thorac Cardiovasc Surg. 2012;60:268–274. doi: 10.1007/s11748-011-0884-z. [DOI] [PubMed] [Google Scholar]
- 31.Mazine A, Stevens LM, Ghoneim A. Developing skills for thoracic aortic surgery with hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2019;157:1360–1368. doi: 10.1016/j.jtcvs.2018.11.110. .e8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.