Abstract
The frequencies of 19 respiratory pathogens other than SARS-CoV-2 were assessed in 6,"?>235 Brazilian individuals tested for COVID-19. Overall, only 83 individuals who tested positive for SARS-CoV-2 had codetection of other pathogens. Individuals infected with Rhinovirus/Enterovirus, Human Coronavirus (HCoV)-HKU1, HCoV-NL63, HPIV-4, Influenza A (-H1N1 and other subtypes), Influenza B, Human Respiratory Syncytial Virus and Human Metapneumovirus were less likely to test positive for SARS-CoV-2. Infection with Streptococcys pyogenes, Chlamydophila pneumoniae, Mycoplasma pneumoniae, and Bordetella pertussis were more frequent in individuals who tested negative for SARS-CoV-2, but without significancy. We found 150 individuals infected with ≥2 pathogens other than SARS-CoV-2, only 3 out of whom tested positive for COVID-19. The codetection frequency was low in individuals diagnosed with COVID-19. Other viral infections may provide a cross-reactive, protective immune response against SARS-CoV-2. Screening for bacterial respiratory infections upon COVID-19 testing is important to drive suitable therapeutic approaches and avoid unnecessary antibiotic prescription.
Keywords: Codetection, COVID-19, Pandemic, SARS-CoV-2
1. Introduction
Screening for different pathogens that can cause symptoms similar to COVID-19 is important for driving appropriate disease-specific measures, and codetection of other viruses such as Rhinoviruses, Influenza viruses, other human Coronaviruses and Adenoviruses has been reported during SARS-CoV-2 testing (Calcagno et al., 2021; Ma et al., 2020; Matos et al., 2020; Zhu et al., 2020). Unlike COVID-19, the diagnosis of several respiratory viral infections is usually based on clinical history and physical examination, with no additional laboratory tests being required, even though it is possible to use reverse transcriptase real-time polymerase chain reaction (RT-PCR) to identify the causative pathogen (Turner, 2015). In addition, bacterial and fungal pathogens have also been codetected during SARS-CoV-2 testing, such as Streptococcus pneumoniae, Klebsiella pneumoniae, Staphylococcus aureus and Aspergillus sp. (Calcagno et al., 2021; Ma et al., 2020; Matos et al., 2020; Zhu et al., 2020). For this reason, we aimed to describe the frequency of micro-organisms other than SARS-CoV-2 detected during COVID-19 testing in a Brazilian cohort.
2. Methods
We used data obtained from the COVID-19 Data Sharing/BR database, an initiative of the São Paulo Research Foundation (FAPESP) (Mello et al., 2020), in conjunction with 5 public and private health services located in the state of São Paulo, Brazil: University of São Paulo's Hospital de Clínicas (HC-USP), Hospital Israelita Albert Einstein, Hospital Sírio-Libanês, Beneficência Portuguesa, and Fleury Institute. This database is available at https://repositoriodatasharingfapesp.uspdigital.usp.br/.
The included individuals were first grouped according to sex and age range. Clinical outcomes, such as hospitalization or death, were described only by Hospital Sírio-Libanês. SARS-CoV-2 detection was done using RT-PCR and the patients were classified as positive and negative for SARS-CoV-2. The different protocols for RT-PCR testing were not detailed in the datasets.
2.1. Screening for viruses, bacteria and detection of yeasts in urine
The following respiratory viruses were screened together with SARS-CoV-2: Influenza A viruses (-H1N1 and other subtyes), Influenza B virus, Human Respiratory Syncycial Virus (HRSV), Human Parainfluenza Viruses Type I (HPIV-1), II (HPIV-2), III (HPIV-3), IV (HPIV-4), Rhinovirus/Enterovirus (RV/EV), Adenoviruses (ADVs), Human Metapneumovirus (hMPV), Human Coronavirus (HCoV)-229E, HCoV-HKU1, HCoV-NL63, and HCoV-OC43. The viruses were detected using specific RT-PCR assays.
Four bacterial species were screened: Streptococcus pyogenes, Bordetella pertussis, Mycoplasma pneumoniae and Chlamydophila pneumoniae. S. pyogenes was detected using a quick monoclonal antibody-based test for detection of bacterial surface antigens. B. pertussis, M. pneumoniae and C. pneumoniae were detected using a molecular panel for detection of respiratory pathogens. Serological tests for detection of specific IgM and IgG were also used to detect M. pneumoniae and C. pneumoniae. The datasets did not describe the reference or brands of the diagnostic kits for bacterial infections.
Detection of yeasts in urine was made using microscopic examination of the first urine sample of the day during routine urinalysis.
2.2. Database management
Databases for the present study were provided by Fleury Institute, Hospital Israelita Albert Einstein (2 MS Excel files each, one with demographic data and one with clinical data and test results). Hospital Sírio-Libanês provided 3 MS Excel sheets, one with demographic information, one with test results and one with the clinical data, including outcomes.
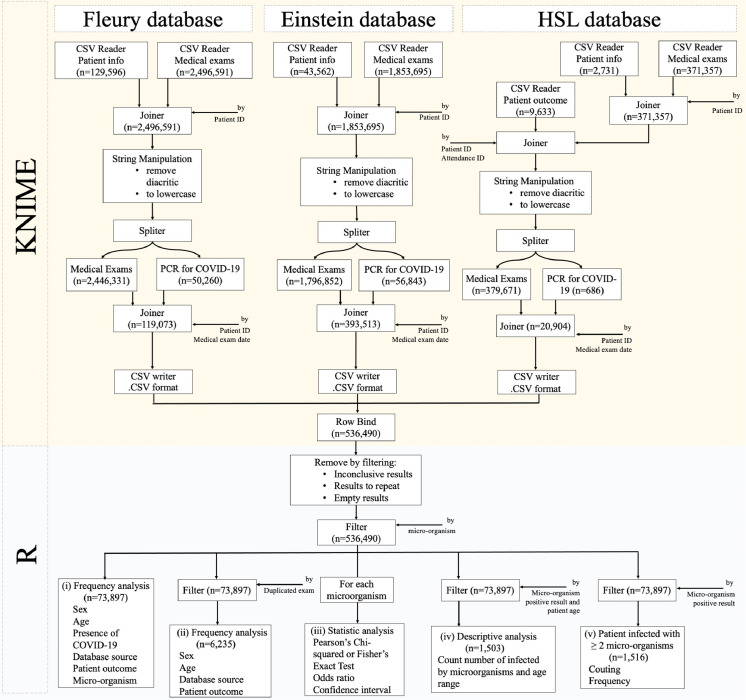
Using the KNIME software (Berthold et al., 2008), patients’ information and medical results datasets were inner-joined by patient ID (Joiner, 2021). Data on patients’ outcomes from Hospital Sírio-Libanês Hospital were later inner-joined by patient and attendance ID. These 3 datasets were processed by a string manipulation module to remove diacritics and transform every word to lowercase (String, 2021). RT-PCR results were split by a row splitter module and inner-joined by patient ID and medical exam date (Joiner, 2021; Row Splitter, 2021). These 3 datasets were then exported as an .CSV file (CSV Writer, 2021). Using the R software (R, 2021), the 3 datasets were read, and row-bound together using the rbind.fill function (Wickham, 2011). Different words associated to the same meaning were normalized along the dataset. Results for repetition, inconclusive results and empty results were removed by the filter. Medical exams, with the exception of testing for micro-organisms, were also removed by the same function (Wickham et al., 2021). Demographic characteristics and data on laboratory exams performed on the day of respiratory sampling for COVID-19 investigation were included in the first screening stage. The second screening stage included cases where at least one respiratory pathogen was investigated in addition to SARS-CoV-2 (Fig. 1 ).
Fig. 1.
Database management and statistical analysis protocol.
2.3. Statistical analysis
The data were described as absolute and relative frequencies when categorizing individuals according to sex, age, presence of COVID-19, database source, patient outcome and presence of other micro-organisms. Difference in the proportions between 2 groups or among more than 2 groups were assessed using the Pearson Chi-square test or Fisher Exact Test. Associations between variables were analyzed using odds ratio (OR) and their correspondent 95% confidence intervals (95% CI) (Aragon et al., 2020; R, 2021; Wickham et al., 2021). For all tests, a P-value ≤ 0.05 indicated statistical significance. The analyses were performed using the R software.
3. Results
3.1. Demographic characteristics
Data of 73,897 individuals were included in the first screening, out of whom 16,850 (22.8%) tested positive to SARS-CoV-2. Among the individuals, 6,"?>235 (8.44%) people were screened for at least one micro-organism in addition to SARS-CoV-2. In the final cohort, the majority of individuals were female (3,"?>049/6,235; 51.10%) and aged between 25 and 61 years old (3,"?>757/6,114; 61.45%). Most individuals were seen in a single hospital, 69 were hospitalized and recovered from COVID-19 and one individual died of COVID-19 (Table 1 ). There were 5,"?>850 tests for Influenza A virus, 5,"?>728 for Influenza B virus, 3,"?>780 for Influenza A-H1N1 virus, 3,"?>778 for M. pneumoniae, 3,"?>775 for HSRV, 3,768 for C. pneumoniae, 3,"?>767 for RV/EV, 3,"?>766 for ADVs, B. pertussis, HCoV-229E, HCoV-HKU1, HCoV-NL63, HCoV-OC43, HPIV-1, -2, -3, -4 and hMPV, 1,"?>304 for yeasts in urine and 935 for S. pyogenes (SM-1 and 2).
Table 1.
Demographic data from the individuals included in the study.
| Marker | n/N (%) |
|---|---|
| Sex | |
| Female | 3186/6,235 (51.10%) |
| Male | 3049/6,235 (48.9%) |
| Age (y) | |
| ≤ 25 y old | 1373/6,114 (22.46%) |
| 25 to < 61 y old | 3757/6,114 (61.45%) |
| ≥ 61 y old | 984/6,114 (16.09%) |
| Health units | |
| Einstein (hospital) | 6106/6,235 (97.93%) |
| Sírio-Libanês (hospital) | 71/6,235 (1.14%) |
| Fleury laboratory | 58/6,235 (0.93%) |
3.2. Viral codetection
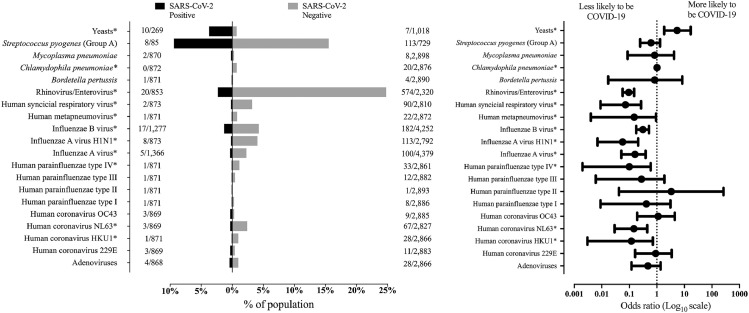
RV/EV was the most frequently detected viral group, being identified in 594 individuals, followed by Influenza B virus (n = 199), Influenza A-H1N1 virus (n = 115), other Influenza A subtypes (n = 105) (Fig. 2 ). All viruses detected in 3 or more individuals were mostly detected in individuals who tested negative for COVID-19. Statistical significance was observed for RV/EV, HCoV-HKU1, HCoV-NL63, HPIV-4, Influenza A-H1N1, other Influenza A subtypes, Influenza B virus, Hmpv, and HRSV (SM-1).
Fig. 2.
Prevalence of different micro-organisms in individuals included in our cohort according to the COVID-19 status. In the graphic on the left, each bar represents the frequency of individuals who tested positive for the corresponding micro-organism. In the graphic on the right, the dots represent the odds ratio (OR) values for testing positive for COVID-19, and the extremities of each line represent the upper and lower limits of the 95% confidence interval.
*, represents the pathogens with significative difference between the patients distributed by COVID-19 status – P-value <0.05.
3.3. Bacterial codetection
S. pyogenes was the most frequently detected bacteria, being identified in 121 individuals, out of whom 8 tested positive for COVID-19. Infection with C. pneumoniae was detected in 20 individuals, all of whom tested negative for COVID-19. Infection with M. pneumoniae was detected in 10 individuals, 2 of whom tested positive for COVID-19. B. pertussis infection was detected in 4 individuals, one of whom tested positive for COVID-19 (SM-2 and Fig. 2).
3.4. Yeasts in urine
Yeasts were identified in urine samples of 17 individuals, and the presence of yeasts in urine was more frequent in individuals who tested positive for SARS-CoV-2 [OR = 5.397 (95%CI = 1.835−16.870)] (SM-2 and Fig. 2).
3.5. Frequency of micro-organisms according to the COVID-19 infection in each age group
Overall, 1,"?>419 out 1,503 (94.4%) individuals with a negative COVID-19 test tested positive for at least one micro-organism, 755 of whom were 25 to 60 years old, 401 were 1 to 12 years old, 155 were 13 to 24 years old, 66 were 61 to 72 years old, 32 were 73 to 85 years old, 7 were <1 years old and 3 were more than 85 years old. Among individuals with a positive COVID-19 test, 84 out of 1,"?>503 (5.6%) tested positive for at least one additional micro-organism, 38 of whom were 25 to 60 years old, 7 were 1 to 12 years old, 9 were 13 to 24 years old, 26 were 61 to 72 years old, 3 were 73 to 85 years old and one was older than 85 years old (Table 2 ).
Table 2.
Prevalence of micro-organism of individuals with COVID-19 symptoms regarding the SARS-CoV-2 infection and distributed by age group.
| Micro-organism | Individuals with SARS-CoV-2 infection (y old) |
Individuals without SARS-CoV-2 infection (y old) |
Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1-12 | 13-24 | 25-60 | 61-72 | 73-85 | >85 | <1 | 1-12 | 13-24 | 25-60 | 61-72 | 73-85 | >85 | ||
| Bordetella pertussis | 1 | 1 | 2 | 1 | 5 | ||||||||||
| Chlamydophila pneumoniae | 6 | 1 | 13 | 20 | |||||||||||
| Mycoplasma pneumoniae | 1 | 1 | 5 | 3 | 10 | ||||||||||
| Streptococcus pyogenes (Group A) | 2 | 1 | 4 | 1 | 31 | 35 | 43 | 2 | 2 | 121 | |||||
| Yeastsa | 2 | 2 | 1 | 3 | 2 | 1 | 1 | 12 | |||||||
| Adenoviruses | 1 | 2 | 1 | 24 | 3 | 1 | 32 | ||||||||
| Human coronavirus 229E | 1 | 2 | 1 | 10 | 14 | ||||||||||
| Human coronavirus HKU1 | 1 | 2 | 1 | 23 | 2 | 29 | |||||||||
| Human coronavirus NL63 | 2 | 1 | 10 | 4 | 43 | 9 | 1 | 70 | |||||||
| Human coronavirus OC43 | 1 | 2 | 1 | 8 | 12 | ||||||||||
| Human Parainfluenza virus type I | 1 | 4 | 4 | 9 | |||||||||||
| Human Parainfluenza virus type II | 1 | 1 | 2 | ||||||||||||
| Human Parainfluenza virus type IIIa | 1 | 2 | 8 | 1 | 12 | ||||||||||
| Human Parainfluenza virus type IV | 1 | 1 | 14 | 2 | 15 | 1 | 34 | ||||||||
| Influenza A virus | 2 | 2 | 1 | 30 | 12 | 50 | 6 | 2 | 105 | ||||||
| Influenza A virus subtype H1N1 | 2 | 26 | 11 | 72 | 2 | 2 | 115 | ||||||||
| Influenza B virusa | 2 | 1 | 11 | 3 | 50 | 20 | 102 | 8 | 1 | 198 | |||||
| Human Metapneumovirus | 1 | 1 | 7 | 1 | 11 | 2 | 23 | ||||||||
| Human Respiratory Syncytial virus | 2 | 3 | 66 | 2 | 17 | 1 | 1 | 92 | |||||||
| Rhinovirus|Enterovirusa | 1 | 3 | 11 | 4 | 1 | 2 | 122 | 62 | 327 | 31 | 22 | 2 | 588 | ||
| Total | 7 | 9 | 38 | 26 | 3 | 1 | 7 | 401 | 155 | 755 | 66 | 32 | 3 | 1,503 | |
A total of 13 positive exams were screened in individuals without the description for age being 2 (2 cases of yeasts) in individuals with SARS-CoV-2 infection and 11 (6 cases of Rhinovirus/Enterovirus, 3 cases of yeasts, one case of Influenza B virus and one case of Human Parainfluenzae virus type III) in individuals without SARS-CoV-2. The data is associated with the number of exams performed in our casuistic.
3.6. Codetection of more than one micro-organism other than SARS-CoV-2
We identified 150 cases of codetection of more than one micro-organism other than SARS-CoV-2 and grouped them into 55 microbiological profiles. Three cases were individuals who tested positive for SARS-CoV-2, one of whom tested positive for 16 microrganims. Of note, we are not able to state whether this was a database error, or if all micro-organisms were true pathogens, and this finding should therefore be looked at with caution. One individual tested positive for 3 micro-organisms and had yeasts in the urine, and one tested positive for 3 micro-organisms without having yeasts in the urine (Table 3 ).
Table 3.
Micro-organism profile for the codetection (more than 2 without taking into account the SARS-CoV-2) in individuals with COVID-19 symptoms regarding the SARS-CoV-2 infection.
| Micro-organisms | Number of | |
|---|---|---|
| Individuals who tested positive for SARS-CoV-2 | micro-organism | cases |
| Bordetella pertussis + Mycoplasma pneumoniae + Adenoviruses + Human coronavirus 229E (HCoV-229E) + Human coronavirus HKU1 (HCoV-HKU1) + Human coronavirus NL63 (HCoV-NL63) + Human coronavirus OC43 (HCoV-OC43) + Human Parainfluenza virus types I (HPIV-1), II (HPIV-2), III (HPIV-3) and IV (HPIV-4) + Influenza A virus + Influenza A virus subtype H1N1 + Influenza B virus + Human Metapneumovirus (hMPV) + Rhinovirus|Enterovirus | 16a | 1 |
| M. pneumoniae + Adenoviruses + Rhinovirus|Enterovirus | 3 | 1 |
| Yeasts + Influenza A virus + Influenza B virus + Rhinovirus|Enterovirus | 3 | 1 |
| Individuals who tested negative for SARS-CoV-2 | ||
| Chlamydophila pneumoniae + Adenoviruses + Human Respiratory Syncytial virus + Rhinovirus|Enterovirus | 4 | 1 |
| Adenoviruses + HCoV-NL63 + Rhinovirus|Enterovirus | 3 | 2 |
| Adenoviruses + Influenza B virus + Rhinovirus|Enterovirus | 3 | 1 |
| Adenoviruses + Human Respiratory Syncytial virus + Rhinovirus|Enterovirus | 3 | 3 |
| C. pneumoniae + Influenza A virus subtype H1N1+ Rhinovirus|Enterovirus | 3 | 1 |
| HCoV-HKU1 + HPIV-4 + Rhinovirus|Enterovirus | 3 | 1 |
| HCoV-NL63 + Human Respiratory Syncytial virus + Rhinovirus|Enterovirus | 3 | 1 |
| Influenza A virus + Influenza A virus subtype H1N1 + Rhinovirus|Enterovirus | 3 | 2 |
| Influenza A virus subtype H1N1 + Influenza B virus + Rhinovirus|Enterovirus | 3 | 1 |
| Streptococcus pyogenes (Group A) + Influenza A virus + Influenza B virus | 3 | 1 |
| HPIV-4 + Human Respiratory Syncytial virus + Rhinovirus|Enterovirus | 3 | 1 |
| C. pneumoniae + Adenoviruses | 2 | 1 |
| Adenoviruses + Influenza A virus subtype H1N1 | 2 | 1 |
| Adenoviruses + Influenza B virus | 2 | 1 |
| Adenoviruses + HPIV-4 | 2 | 1 |
| Adenoviruses + Rhinovirus|Enterovirus | 2 | 4 |
| Adenoviruses + Human Respiratory Syncytial virus | 2 | 2 |
| C. pneumoniae + Influenza B virus | 2 | 1 |
| C. pneumoniae + HPIV-4 | 2 | 1 |
| C. pneumoniae + Rhinovirus|Enterovirus | 2 | 2 |
| HCoV-229E + HPIV-4 | 2 | 1 |
| HCoV-229E + Rhinovirus|Enterovirus | 2 | 1 |
| HCoV-HKU1 + HCoV-OC43 | 2 | 1 |
| HCoV-HKU1 + Influenza A virus subtype H1N1 | 2 | 1 |
| HCoV-HKU1 + HPIV-4 | 2 | 1 |
| HCoV-HKU1 + Rhinovirus|Enterovirus | 2 | 1 |
| HCoV-HKU1 + Human Respiratory Syncytial virus | 2 | 1 |
| HCoV-NL63 + Influenza A virus subtype H1N1 | 2 | 1 |
| HCoV-NL63 + hMPV | 2 | 1 |
| HCoV-NL63 + HPIV-3 | 2 | 1 |
| HCoV-NL63 + Rhinovirus|Enterovirus | 2 | 10 |
| S. pyogenes (Group A) + HCoV-NL63 | 2 | 1 |
| HCoV-NL63 + Influenza B virus | 2 | 1 |
| HCoV-NL63 + Rhinovirus|Enterovirus | 2 | 2 |
| Influenza A virus + Influenza A virus subtype H1N1 | 2 | 28 |
| Influenza A virus subtype H1N1 + Influenza B virus | 2 | 1 |
| Influenza A virus subtype H1N1 + Rhinovirus|Enterovirus | 2 | 4 |
| Influenza A virus + Influenza B virus | 2 | 2 |
| HPIV-4 + Influenza A virus | 2 | 1 |
| S. pyogenes (Group A) + Influenza A virus | 2 | 1 |
| HPIV-1 + Influenza B virus | 2 | 1 |
| Influenza B virus + Rhinovirus|Enterovirus | 2 | 15 |
| S. pyogenes (Group A) + Influenza B virus | 2 | 1 |
| Yeasts + Rhinovirus|Enterovirus | 2 | 1 |
| hMPV + Rhinovirus|Enterovirus | 2 | 2 |
| HPIV-1 + Rhinovirus|Enterovirus | 2 | 2 |
| HPIV-1 + Human Respiratory Syncytial virus | 2 | 1 |
| HPIV-3 + Rhinovirus|Enterovirus | 2 | 2 |
| HPIV-4 + Rhinovirus|Enterovirus | 2 | 9 |
| S. pyogenes (Group A) + Rhinovirus|Enterovirus | 2 | 6 |
| Human Respiratory Syncytial virus + Rhinovirus|Enterovirus | 2 | 17 |
Of note, we are not able to state whether this was a database error, or if all micro-organisms were true pathogens, and this finding should therefore be looked at with caution.
Among individuals not diagnosed with COVID-19, one tested positive for 4 micro-organisms, 14 tested positive for 3 micro-organisms and 132 tested positive for 2 micro-organisms (Table 3). Overall, we found 95 cases of codetection with RV/EV, 43 with Influenza A-H1N1 virus, 28 with Influenza B virus, 27 with HRSV and 21 with HCoV-NL63 (SM-3).
4. Discussion
Notably, most individuals in our cohort who were infected with other respiratory viruses tested negative for SARS-CoV-2, and, in a cross-sectional analysis, 9 viruses had their presence associated with a lower risk of testing positive for SARS-CoV-2, among which HCoVs -NL63 and -HKU1, 2 coronaviruses that cause the common cold, are worth highlighting. In a cohort with more than 800,000 individuals in Israel, individuals aged more than 18 years old who had common cold symptoms in the year before the COVID-19 pandemic were shown to have a lower risk of having COVID-19 (Aran et al., 2020), which was suggested to be due to past coronavirus infections and is corroborated by studies showing that serum and plasma samples from individuals without COVID-19 reacted against proteins of SARS-CoV-2 and other human coronaviruses in immunofluorescence and flow cytometry assays (Ng et al., 2020; Tso et al., 2021). Immunity induced by infections with HCoVs -NL63 and -HKU1 may have provided individuals in our cohort with a protective, cross-reactive response against SARS-CoV-2. This association was not significant for HCoVs -229E and -OC43, but most individuals infected with these coronaviruses also tested negative for COVID-19. As there were fewer of these individuals, one can speculate that the impact of HCoV -229E and -OC43 infections has been underestimated.
Most of the codetected pathogens in individuals who tested positive for SARS-CoV-2 reported here were viruses. Overall, 1,334 (88.88%) individuals tested positive for other viruses, with RV/EV being the most common one (44.1%), which is in accordance with the literature (Kim et al., 2020). Testing positive for HPIV-4, Influenza A, Influenza A-H1N1, Influenza B viruses, hMPV, HSRv, and RV/EV were associated with a lower risk of testing positive for SARS-CoV-2. Coronaviruses have molecular structures that are similar to Influenza viruses (Abdella et al., 2020; Aragon et al., 2020; Aran et al., 2020; CSV Writer, 2021; Kim et al., 2020; Maltezou et al., 2020; Ng et al., 2020; R, 2021; Row Splitter, 2021; Tso et al., 2021; Wickham, 2011; Wickham et al., 2021; Zeng et al., 2008), possibly leading to cross-reactivity between the 2 viral groups (Zheng and Perlman, 2018). RV/EV and HRSV also have some degree of similarity with SARS-CoV-2, but they have been shown to be poor sources of cross-reactivity (Reche, 2020). Thus, a different mechanism may be associated with the low SARS-CoV-2 positive testing rate among patients infected with these viruses. In a study performed at the Icahn School of Medicine (New York, USA), with 8,990 individuals tested for SARS-CoV-2, 1,204 individuals tested positive for other respiratory viruses, and codetection was found in only 36 (<3%) individuals. On the other hand, codetection with at least one respiratory viral pathogen other than SARS-CoV-2 was found in 13.1% of individuals who tested negative for SARS-CoV-2. Additionally, in patients who tested negative for SARS-CoV-2, the most common respiratory viral codetections were community-acquired (Nowak et al., 2020). Worse outcomes have been reported, especially when codetection happened with Influenza viruses (Hashemi et al., 2020; Ma et al., 2020; Maltezou et al., 2020), which resulted in a more detrimental immune response (Chen et al., 2020; Tay et al., 2020). That seems not to be the case in our cohort, as none of the individuals with severe or fatal outcomes were infected with pathogens other than SARS-CoV-2.
Even though most studies evaluating bacterial codetection used RT-PCR for bacterial detection (Contou et al., 2020; Langford et al., 2020; Liu et al., 2020; Pongpirul et al., 2020), some reports used serological tests to detect the presence of bacterial antigens (Ma et al., 2020; Zhang et al., 2020), as was the case with individuals of our cohort. Most individuals who had bacterial respiratory infection did not test positive for COVID-19, which has been shown by other studies (Calcagno et al., 2021; Kim et al., 2020). This raises concern whether many bacterial respiratory infections are neglected in the diagnostic routine and underlines the need for more accurate diagnostic tools targeting these infections, especially in the current pandemic setting, as they also cause symptoms that resemble COVID-19. Overall, the impact of bacterial codetection in COVID-19 is not fully clear. In a study, performed at a hospital in the Jiangsu Province (China), with 257 individuals with COVID-19, bacterial codetections were dominant in all COVID-19 cases, with S. pneumoniae being the most common pathogen, followed by K. pneumoniae and H. influenzae. Moreover, patients with viral codetections and bacterial-fungal codetections were shown to be the most severe COVID-19 cases (Zhu et al., 2020). Some studies have shown that bacterial and fungal codetection may be associated with more severe lung injury (Chen et al., 2020; Tan et al., 2020), a 2.5-fold increased risk of death and can impair the diagnosis, treatment, and prognosis of the disease (Chen et al., 2020; Martins-Filho et al., 2020). In our study, like previously described (Calcagno et al., 2021; Hazra et al., 2020), the frequency of bacterial codetection was low and not shown to be associated with worse COVID-19-related outcomes.
It is worth mentioning that irrational antibiotic prescribing has become a major problem when managing COVID-19. A recent metanalysis assessing data of more than 30,000 patients with COVID-19 all over the world found that nearly 75% of them received antibiotics, which was significantly higher than their 8.6% estimated rate of bacterial infection. Although there is no information on treatment in the COVID-19 Data Sharing/BR database, our finding that only a low number of patients had bacterial infection highlights that the empirical use of antibiotics in patients with COVID-19 should not be recommended, as also observed by previous reports (Langford et al., 2021; Oldenburg et al., 2021; PRINCIPLE Trial Collaborative Group, 2021).
It is noticeable that the presence of yeasts in urine was more frequent in individuals in our cohort who tested positive for COVID-19. Previous studies have reported fungemia in patients with COVID-19 who were hospitalized (Bishburg et al., 2021; Salehi et al., 2020), which may corroborate our findings. Lymphopenia is present in most patients with COVID-19 (Pourbagheri-Sigaroodi et al., 2020) and can play a role, as the lymphocyte response is essential against fungal infections (Shoham and Levitz, 2005). Unfortunately, there were no data on fungemia in the COVID-19 Data Sharing/BR database, so we were not able to conduct a deeper investigation into this matter.
A thorough screening for COVID-19 is not only important to avoid overload in public health units but can also be used for screening for other community-acquired respiratory infections. It is concerning that only 37% of the individuals that were screened from the COVID-19 Data Sharing/BR database have been tested for respiratory pathogens other than SARS-CoV-2. Besides, since identification of respiratory pathogens other than SARS-CoV-2 is not routinely performed, this number is likely to be underestimated. Importantly, these individuals were seen in high-standard Brazilian health care institutions, which, unlike most public health services in Brazil, have the facilities and financial resources needed to perform these kinds of tests. This underlines the inequality in the access to tests that could detect varied respiratory infections, especially the bacterial ones, which, in turn, could help to drive better treatment strategies.
5. Limitations
Our study has several limitations, including its retrospective design, with most data being restricted to a single hospital. A longitudinal monitoring of the individuals in the cohort was not possible. Although the first screening stage had a high number of individuals, this number decreased after they were divided into subgroups, limiting our statistical power. Clinical data indicating the need for a SARS-CoV-2 RT-PCR tests were not available and the data on outcomes were informed in only one database with a low number of individuals. The lack of such clinical information, especially regarding clinical severity and therapeutic interventions could have provided us with important insights. Also, due to the diversity of data and low number of patients with detected coinfection, the OR values should be assessed only from an exploratory point of view. The lack of homogeneity in the screening can also be a cause of selection bias. Finally, there were no data on the history of viral respiratory infections of individuals in our cohort in the pre-pandemic period.
6. Conclusions
Codetection was uncommon in patients who tested positive for SARS-CoV-2. HCoV-HKU1, HCoV-NL63, HPIV-4, Influenza A virus, Influenza A-H1N1 virus, Influenza B virus, hMPV, HSRV, and RV/EV were associated with a lower risk of testing positive for SARS-CoV-2. Even though the screening for COVID-19 is beneficial, it is unfeasible for the Brazilian public health system to afford a thorough screening for other respiratory pathogens, making clinical history and physical examination the first-choice methods. All individuals who had a negative test for SARS-CoV-2 went to the hospital to do the test, stressing the need for a protocol for requirement of SARS-CoV-2 RT-PCR to prevent unnecessary exposure to the virus upon spontaneous appearances in the testing center.
Ethical aspects
FAPESP and the participating institutions followed the recommendations of the Research Data Alliance on sharing the data presented in this study (https://doi.org/10.15497/RDA00049). The provided data are pseudonymized by the participating institutions before being launched in the database. Approval by an ethics committee was not required for the present study.
Consent for publication
None.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article.
Authors' contributions
All authors have approved the manuscript and agreed with its submission to the journal. FALM and AD were responsible for the statistical analysis. MNB, CVCP, AEA, and RMM drafted the first version of the manuscript. All authors revised and wrote the final version of the manuscript.
Declaration of competing interest
The authors report no conflicts of interest relevant to this article.
Acknowledgments
Acknowledgments
The present study used data obtained from the COVID-19 Data Sharing/BR database (FAPESP, 2020). The participating institutions do not take responsibility for misuse of the data by any individual or group.
Funding
MNB was granted a fellowship by the São Paulo Research Foundation (FAPESP, grant no. 2021/05810-7).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.diagmicrobio.2021.115576.
Appendix. Supplementary materials
References
- Abdella R, Aggarwal M, Okura T, Lamb RA, He Y. Structure of a paramyxovirus polymerase complex reveals a unique methyltransferase-CTD conformation. Proc Natl Acad Sci U S A. 2020;117:4931–4941. doi: 10.1073/pnas.1919837117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon TJ, Fay MP, Wollschlaeger D, Omidpanah A. epitools: epidemiology Tools. (2020).
- Aran D, Beachler DC, Lanes S, Overhage JM. Prior presumed coronavirus infection reduces COVID-19 risk: a cohort study. J Infect. 2020;81:923–930. doi: 10.1016/j.jinf.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold MR, Cebron N, Dill F, Gabriel TR, Kotter T, Meinl T, et al. In: Data analysis, machine learning and applications. Preisach C, Burkhardt H, Schmidt-Thieme L, Decker R, editors. Springer; 2008. KNIME: The Konstanz Information Miner; pp. 319–326. [DOI] [Google Scholar]
- Bishburg E, Okoh A, Nagarakanti SR, Lindner M, Migliore C, Patel P. Fungemia in COVID-19 ICU patients, a single medical center experience. J Med Virol. 2021;93:2810–2814. doi: 10.1002/jmv.26633. [DOI] [PubMed] [Google Scholar]
- Calcagno A, Ghisetti V, Burdino E, Trunfio M, Allice T, Boglione L, et al. Co-infection with other respiratory pathogens in COVID-19 patients. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27:297–298. doi: 10.1016/j.cmi.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liao B, Cheng L, Peng X, Xu X, Li Y, et al. The microbial coinfection in COVID-19. Appl Microbiol Biotechnol. 2020;104:7777–7785. doi: 10.1007/s00253-020-10814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contou D, Claudinon A, Pajot O, Micaëlo M, Longuet Flandre P, Dubert M, et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care. 2020;10:119. doi: 10.1186/s13613-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSV Writer. KNIME Hub. 2021. Available at: https://hub.knime.com/knime/extensions/org.knime.features.base/latest/org.knime.base.node.io.filehandling.csv.writer.CSVWriter2NodeFactory. Accessed October 7, 2021.
- Hashemi SA, Safamanesh S, Ghafouri M, Taghavi MR, Mohajer Zadeh Heydari MS, Namdar Ahmadabad H, et al. Co-infection with COVID-19 and influenza A virus in two died patients with acute respiratory syndrome, Bojnurd, Iran. J Med Virol. 2020;92:2319–2321. doi: 10.1002/jmv.26014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra A, Collison M, Pisano J, Kumar M, Oehler C, Ridgway JP. Coinfections with SARS-CoV-2 and other respiratory pathogens. Infect Control Hosp Epidemiol. 2020;41:1228–1229. doi: 10.1017/ice.2020.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner (deprecated). KNIME Hub. 2021. Available at: https://hub.knime.com/knime/extensions/org.knime.features.base/latest/org.knime.base.node.preproc.joiner.Joiner2NodeFactory. Accessed October 7, 2021.
- Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323:2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford BJ, So M, Raybardhan S, Leung V, Soucy JR, Westwood D, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27:520–521. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Tao Z-W, Wang L, Yuan M-L, Liu K, Zhou L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Lai X, Chen Z, Tu S, Qin K. Clinical characteristics of critically ill patients co-infected with SARS-CoV-2 and the influenza virus in Wuhan, China. Int J Infect Dis. 2020;96:683–687. doi: 10.1016/j.ijid.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Wang W, Le Grange JM, Wang X, Du S, Li C, et al. Coinfection of SARS-CoV-2 and other respiratory pathogens. Infect Drug Resist. 2020;13:3045–3053. doi: 10.2147/IDR.S267238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltezou HC, Theodoridou K, Poland G. Influenza immunization and COVID-19. Vaccine. 2020;38:6078–6079. doi: 10.1016/j.vaccine.2020.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Filho PR, Tavares CSS, Santos VS. Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data. Eur J Intern Med. 2020;76:97–99. doi: 10.1016/j.ejim.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos A da R, Motta FC, Caetano BC, Ogrzewalska M, Garcia CC, Lopes JCO, et al. Identification of SARS-CoV-2 and additional respiratory pathogens cases under the investigation of COVID-19 initial phase in a Brazilian reference laboratory. Mem Inst Oswaldo Cruz. 2020;115 doi: 10.1590/0074-02760200232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello LE, Suman A, Medeiros CB, Prado CA, Rizzatti EG, Nunes FLS, et al. Opening Brazilian COVID-19 patient data to support world research on pandemics. 2020 Jul 30; Available at: https://zenodo.org/record/3966427. Accessed October 7, 2021.
- Ng KW, Faulkner N, Cornish GH, Rosa A, Harvey R, Hussain S, et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370:1339–1343. doi: 10.1126/science.abe1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MD, Sordillo EM, Gitman MR, Paniz Mondolfi AE. Coinfection in SARS-CoV-2 infected patients: where are influenza virus and rhinovirus/enterovirus? J Med Virol. 2020;92:1699–1700. doi: 10.1002/jmv.25953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg CE, Pinsky BA, Brogdon J, Chen C, Ruder K, Zhong L, et al. Effect of oral azithromycin vs placebo on COVID-19 symptoms in outpatients with SARS-CoV-2 infection: a randomized clinical trial. JAMA. 2021 doi: 10.1001/jama.2021.11517. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongpirul WA, Mott JA, Woodring JV, Uyeki TM, MacArthur JR, Vachiraphan A, et al. Clinical characteristics of patients hospitalized with coronavirus disease, Thailand. Emerg Infect Dis. 2020;26:1580–1585. doi: 10.3201/eid2607.200598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourbagheri-Sigaroodi A, Bashash D, Fateh F, Abolghasemi H. Laboratory findings in COVID-19 diagnosis and prognosis. Clin Chim Acta. 2020;510:475–482. doi: 10.1016/j.cca.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINCIPLE Trial Collaborative Group Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet Lond Engl. 2021;397:1063–1074. doi: 10.1016/S0140-6736(21)00461-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reche PA. Potential cross-reactive immunity to SARS-CoV-2 from common human pathogens and vaccines. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.586984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R: The R project for statistical computing. 2021. Available at: https://www.r-project.org/. Accessed October 7, 2021.
- Row Splitter. KNIME Hub. 2021. Available at: https://hub.knime.com/knime/extensions/org.knime.features.base/latest/org.knime.base.node.preproc.filter.row.RowFilter2PortNodeFactory. Accessed October 7, 2021.
- Salehi M, Ahmadikia K, Mahmoudi S, Kalantari S, Jamalimoghadamsiahkali S, Izadi A, et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: species identification and antifungal susceptibility pattern. Mycoses. 2020;63:771–778. doi: 10.1111/myc.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham S, Levitz SM. The immune response to fungal infections. Br J Haematol. 2005;129:569–582. doi: 10.1111/j.1365-2141.2005.05397.x. [DOI] [PubMed] [Google Scholar]
- String Manipulation. KNIME Hub. 2021. Available at: https://hub.knime.com/knime/extensions/org.knime.features.javasnippet/latest/org.knime.base.node.preproc.stringmanipulation.StringManipulationNodeFactory. Accessed October 7, 2021.
- Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang Y-Q, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso FY, Lidenge SJ, Peña PB, Clegg AA, Ngowi JR, Mwaiselage J, et al. High prevalence of pre-existing serological cross-reactivity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in sub-Saharan Africa. Int J Infect Dis. 2021;102:577–583. doi: 10.1016/j.ijid.2020.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RB. The common cold. Mand Douglas Bennetts Princ Pract Infect Dis. 2015;1:748–752. e2. [Google Scholar]
- Wickham H. The split-apply-combine strategy for data analysis. J Stat Softw. 2011;40:1–29. [Google Scholar]
- Wickham H, François R, Henry L, Müller K. RStudio. dplyr: a grammar of data manipulation. (2021).
- Zeng Q, Langereis MA, van Vliet ALW, Huizinga EG, de Groot RJ. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc Natl Acad Sci U S A. 2008;105:9065–9069. doi: 10.1073/pnas.0800502105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Dong X, Cao Y, Yuan Y, Yang Y, Yan Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Zheng J, Perlman S. Immune responses in influenza A virus and human coronavirus infections: an ongoing battle between the virus and host. Curr Opin Virol. 2018;28:43–52. doi: 10.1016/j.coviro.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ge Y, Wu T, Zhao K, Chen Y, Wu B, et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.