Abstract
Background
Health-related quality of life (HRQoL) impairment is often reported among COVID-19 ICU survivors, and little is known about their long-term outcomes.
We evaluated the HRQoL trajectories between 3 months and 1 year after ICU discharge, the factors influencing these trajectories and the presence of clusters of HRQoL profiles in a population of COVID-19 patients who underwent invasive mechanical ventilation (IMV). Moreover, pathophysiological correlations of residual dyspnea were tested.
Methods
We followed up 178 survivors from 16 Italian ICUs up to one year after ICU discharge. HRQoL was investigated through the 15D instrument. Available pulmonary function tests (PFTs) and chest CT scans at 1 year were also collected. A linear mixed-effects model was adopted to identify factors associated with different HRQoL trajectories and a two-step cluster analysis was performed to identify HRQoL clusters.
Results
We found that HRQoL increased during the study period, especially for the significant increase of the physical dimensions, while the mental dimensions and dyspnea remained substantially unchanged. Four main 15D profiles were identified: full recovery (47.2%), bad recovery (5.1%) and two partial recovery clusters with mostly physical (9.6%) or mental (38.2%) dimensions affected. Gender, duration of IMV and number of comorbidities significantly influenced HRQoL trajectories. Persistent dyspnea was reported in 58.4% of patients, and weakly, but significantly, correlated with both DLCO and length of IMV.
Conclusions
HRQoL impairment is frequent 1 year after ICU discharge, and the lowest recovery is found in the mental dimensions. Persistent dyspnea is often reported and weakly correlated with PFTs alterations.
Trial registration
Keywords: COVID-19, Acute respiratory distress syndrome, Health-related quality of life, Dyspnea, Respiratory function tests
1. Introduction
As of the end of August 2021, the COVID-19 pandemic resulted in more than 4 million deaths worldwide [1], and it considerably increased the need for healthcare resources dedicated to the acute phase of the syndrome, posing a hard challenge to the national health systems [2].
A series of somatic and psychological consequences are described among survivors, including pulmonary function impairment, such as a reduction in forced vital capacity (FVC) and diffusion capacity of lungs for carbon monoxide (DLCO) [3], neuropsychiatric disorders [4], and cardiac sequelae [5]. The most frequently complained persistent symptoms are dyspnea [6,7] and fatigue, as well as anxiety, depression, and sleep problems, which could significantly undermine the health-related quality of life (HRQoL) of these patients, and together identify the so-called long-COVID syndrome [4,8].
Moreover, patients who underwent ICU admission for COVID-19 related ARDS (C-ARDS) may also experience post-intensive care syndrome (PICS), which eventually overlaps other symptoms related to COVID-19 itself [9].
Little is known about the long-term HRQoL of patients who underwent invasive mechanical ventilation (IMV) for COVID-19 pneumonia, and no studies have yet investigated if the persistence of symptoms is associated with long-term pulmonary function impairment in this particular population.
This study reports the results of the second follow-up (1 year) of a population of C-ARDS survivors who required ICU admission, intubation, and IMV [10], and who were previously evaluated at 3 months after ICU discharge [11]. The primary objective of this study was to describe the HRQoL trajectories between 3 months and 1 year after ICU discharge. Moreover, we defined clusters of patients based on their HRQoL profiles at 1 year and investigated the factors influencing the recovery trajectories of the survivors.
Finally, a subgroup analysis was performed for patients having pulmonary function tests available between 9 and 12 months after ICU discharge to evaluate the relationship between the persistence and entity of dyspnea and pulmonary function.
2. Material and methods
This prospective multicenter observational study involved 16 Italian ICUs. Patients admitted to the participating ICUs from 22nd February through 4th May 2020 (the end of lockdown in Italy), who survived hospital discharge were subsequently followed up until one year after ICU discharge.
The study was approved by the Institutional Review Board (IRB) of the study coordinator centre (Maggiore Hospital, Bologna, Italy) and by each institutional review committee of the participating hospitals. Informed consent was asked at the time of the first follow-up if not obtained during ICU stay according to the approval of the local Ethics committee, and researchers analyzed anonymized data. The study was registered in ClinicalTrials.gov (NCT04411459).
2.1. Data collection
Data were collected in an electronic case report form developed by YGHEA, CRO division of Ecol Studio SPA (Bologna Operational Headquarters), and hosted by Actide Nubilaria (Novara, Italy). Collected data comprised demographic data, comorbidities, and ICU-related variables such as SAPS II and SOFA score at ICU admission and the severity of ARDS following the Berlin criteria [12]. Social related variables recorded were: marital status, occupational status, and instruction degree. Residual symptoms at 1 year evaluated were: dyspnea measured with the modified Medical Research Council (mMRC) scale [13], arthromyalgia, palpitations, cough.
Follow-up pulmonary function tests (PFTs) data were collected for tests performed between 9 and 12 months after ICU discharge and consisted of: forced expiratory volume in the first second (FEV1), FVC, FEV1/FVC ratio, and DLCO. Follow-up CT scan data between 9 and 12 months after ICU discharge were also recorded, in particular concerning the presence of signs of fibrosis, residual ground glass, and atelectasis.
HRQoL was measured at 3 months and 1 year by telephonic interview and administration of the 15D instrument.
2.2. The 15D instrument
The 15D instrument (http://www.15d-instrument.net/15d/) is a generic, 15-dimensional multiutility instrument assessing different aspects of HRQoL (including mobility, vision, hearing, breathing, sleeping, eating, speech, excretion, usual activities, mental function, discomfort and symptoms, depression, distress, vitality, and sexual activity).
The respondents are required to answer about their state of health at the moment of the interview, and each answer is scored on a 5 points scale, with 1 being the best value and 5 being the worst [14]. The valuation system is based on an application of the multiattribute utility theory. The single index score (15D score), representing the overall HRQoL on a 0–1 scale (1 = full health, 0 = being dead) and the dimension level values, reflecting the goodness of the levels relative to no problems on the dimension (=1) and to being dead (=0), are calculated from the health state descriptive system using a set of population-based preference or utility weights. Mean dimension level values are used to draw 15D profiles for groups. The minimum clinically important change or difference in the 15D score has been estimated to be ±0.015 on the basis that people can on average feel such a difference [15].
The 15D scores are shown to be highly reliable, sensitive and responsive to change, there is a considerable degree of agreement between health state valuations from several European countries [11], and these latter are generalizable at least in Western-type societies [14,16].
2.3. Statistical analysis
Continuous variables were reported as mean and standard deviation (SD) or median and interquartile range (IQR) when appropriate, and comparisons were performed with Student's t-test or Mann-Whitney U test when appropriate. One way analysis of variance (ANOVA) and Kruskal-Wallis ANOVA were used to compare means and medians in the multiple HRQoL clusters. Categorical variables were expressed as numbers and percentages and compared using the Chi-square test or Fisher exact test. To evaluate the modifications of the 15D score and its dimensions throughout the study period, a paired-samples t-test was adopted.
Since the 15D value can be calculated only if all the 15 dimensions values are available, patients with three or more missing dimensions were excluded from the analysis while a multiple imputation technique was adopted when information regarding less than three dimensions was missing [14].
Two-Step cluster analysis, an exploratory tool designed to reveal natural groupings (clusters) within a dataset, was adopted to define the presence and number of HRQoL profile clusters one year after ICU discharge. This selection procedure allows both categorical and standardized continuous variables and it is fairly robust even when the assumptions of independence and normality of distribution of the variables are violated [17]. To determine the best number of clusters automatically, the indicators BIC (Schwarz's Bayesian Information Criterion) or AIC (Akaike's Information Criterion) are calculated for each number of clusters from a specified range. Automatic clustering was performed based on the 15 dimensions of the 15D instrument, the distance between clusters was calculated using the log-likelihood distance and both AIC and BIC were used to evaluate the best number of clusters.
A linear mixed-effects model was used to determine which factors predicted changes in HRQoL between 3 months and 1 year after ICU discharge using different demographic, ICU-related, and social variables as fixed effects and subject-level random effects while 15D score measured at 3 months and 1 year was the dependent variable. Fixed effects associated with 15D score change with a p < 0.2 in the univariate analyses were introduced in the final multivariate model.
Finally, to assess eventual correlations between the 15D breathing score and mMRC scale with pulmonary function tests and ICU stay-related variables, bivariate Spearman correlation tests were performed.
P-values < 0.05 were considered statistically significant, and all tests were two-sided. All data were analyzed using SPSS Statistics 26 (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.) and RStudio (RStudio Team 2020. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA).
3. Results
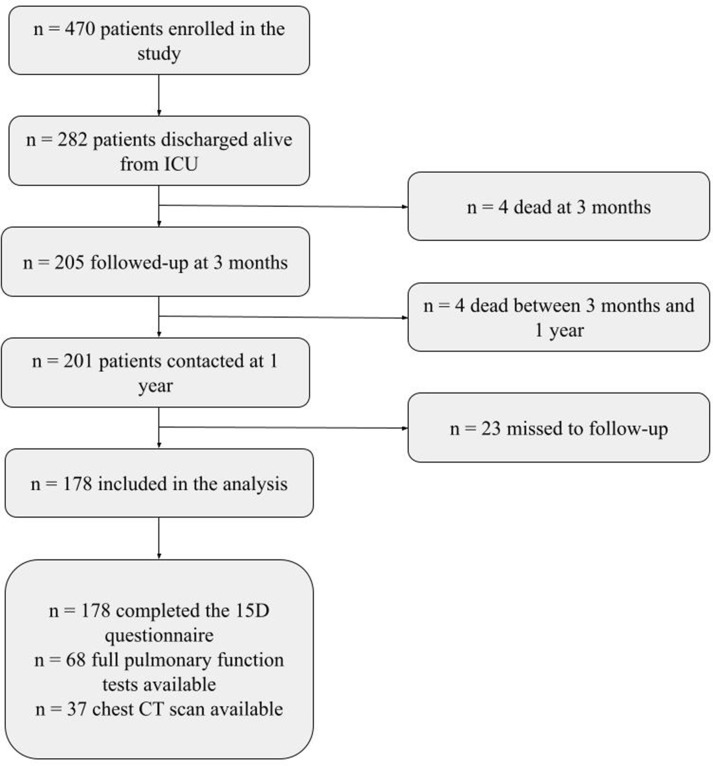
Of the 470 patients initially enrolled in the study, 282 (60%) were discharged alive from the ICU and 205 (43.6%) were followed up at 3 months after ICU discharge.
Four (2%) out of the 205 patients who were initially interviewed, died between three months and 1 year after ICU discharge, while another 23 (11.2%) were lost to follow-up, therefore, the final follow-up cohort was represented by 178 patients (86.8%). The main characteristics of the study population are described in Table 1 , while supplement Fig. 1 shows the flow of patients throughout the study.
Table 1.
General characteristics of the study population and the recovery clusters based on the 15D dimensions.
| Total population n = 178 | Full recovery n = 84 | Partial recovery Mental n = 68 |
Partial recovery Physical n = 17 |
Bad recovery n = 9 | p | |
|---|---|---|---|---|---|---|
| Age - years (IQR) | 64 (55–70) | 60 (52–68) | 66 (57–71) | 69 (55–75) | 66 (60–74) | 0.011 |
| Sex - male - no (%) | 129 (72.5%) | 67 (79.8%) | 46 (67.6%) | 11 (64.7%) | 5 (55.6%) | 0.184 |
| BMI - kg/m2 (IQR) | 28 (26–31) | 28 (26–31) | 28 (26–31) | 27 (25–29) | 32 (27–35) | 0.116 |
| Comorbidities | ||||||
| Hypertension - no (%) | 88 (49.4%) | 36 (42.9%) | 36 (52.9%) | 9 (52.9%) | 7 (77.8%) | 0.190 |
| Chronic ischemic heart disease - no (%) | 13 (7.3%) | 3 (3.6%) | 7 (10.3%) | 2 (11.8%) | 1 (11.1%) | 0.345 |
| Chronic kidney disease - no (%) | 6 (3.4%) | 2 (2.4%) | 2 (2.9%) | 0 (0%) | 2 (22.2%) | 0.013 |
| COPD - no (%) | 13 (7.3%) | 4 (4.8%) | 7 (10.3%) | 0 (0%) | 2 (22.2%) | 0.112 |
| Diabetes - no (%) | 28 (15.7%) | 9 (10.7%) | 11 (16.2%) | 2 (11.8%) | 6 (66.7%) | < 0.001 |
| Number of comorbidities - (IQR) | 1 (0–1) | 0 (0–1) | 1 (0–1) | 1 (0–2) | 2 (1–3) | 0.001 |
| Intensive care and hospital stay | ||||||
| ARDS class - no (%) | 0.745* | |||||
| mild (PaO2/FiO2 200–300) | 7 (3.9%) | 4 (4.8%) | 3 (4.4%) | 0 (0%) | 0 (0%) | |
| moderate (PaO2/FiO2 100–200) | 93 (52.2%) | 47 (56.0%) | 35 (51.5%) | 7 (41.2%) | 4 (44.4%) | |
| severe (PaO2/FiO2 < 100) | 78 (43.8%) | 33 (39.3%) | 30 (44.1%) | 10 (58.8%) | 5 (55.6%) | |
| SAPS II score (IQR) | 35 (29–42) | 34 (28–40) | 38 (29–42) | 38 (32–42) | 38 (31–49) | 0.431 |
| SOFA score at ICU admission (IQR) | 4 (3–6) | 4 (3–6) | 4 (3–6) | 5 (4–7) | 3 (3–5) | 0.182 |
| Tracheostomy - no (%) | 110 (61.8%) | 53 (63.1%) | 43 (63.2%) | 10 (58.8%) | 4 (44.4%) | 0.722 |
| Length of invasive mechanical ventilation - d (IQR) | 16 (10–27) | 14 (9–26) | 18 (13–26) | 18 (9–32) | 20 (11–33) | 0.499 |
| Length of ICU stay - d (IQR) | 23 (15–35) | 19 (14–35) | 24 (17–36) | 25 (13–37) | 29 (13–55) | 0.671 |
| Socioeconomic variables | ||||||
| Marital status - married/cohabitee - no (%) | 135 (75.8%) | 62 (73.8%) | 52 (75.6%) | 14 (82.4%) | 7 (77.8%) | 0.893 |
| Instruction degree - high school or higher - no (%) | 113 (63.5%) | 58 (69.0%) | 39 (57.4%) | 11 (64.7%) | 5 (55.6%) | 0.479 |
| Actual occupational status | 0.594* | |||||
| Employed - no (%) | 87 (48.9%) | 47 (56.0%) | 28 (41.2%) | 7 (41.2%) | 5 (55.6%) | |
| Unemployed - no (%) | 10 (5.6%) | 4 (4.8%) | 4 (5.9%) | 1 (5.9%) | 1 (11.1%) | |
| Retiree - no (%) | 81 (45.5%) | 33 (39.3%) | 36 (52.9%) | 9 (52.9%) | 3 (33.3%) | |
Abbreviations: IQR – interquartile range; BMI – body mass index; COPD – chronic obstructive pulmonary disease; ARDS – acute respiratory distress syndrome; SAPS – simplified acute physiology score; SOFA – sequential organ failure assessment; ICU – intensive care unit. Notes: significant p values for differences among the recovery clusters are evidenced in bold. * p-value referred to the Chi-square test for the whole contingency table.
Globally, patients were mostly males (n = 129, 72.5%), and had a median age of 64 years (IQR 55–70), the median number of comorbidities was one (IQR 0–1) and the most frequent were hypertension (n = 88, 49.4%), and diabetes (n = 28, 15.7%). The median length of invasive mechanical ventilation was 16 (IQR 10–27) days, and the median ICU stay was 23 (IQR 15–35) days.
The multiple imputation technique was adopted only for replacing missing values in the sleeping (n = 2, 1.1%) and sexual activity (n = 37, 20.8%) dimensions.
Table 2 shows the mean values for each of the 15D dimensions at 1 year. Globally, the 15D score significantly increased during the study period, with mean values increasing from 0.857 to 0.880 (p = 0.006). The change was clinically important since it is above the threshold of ±0.015 [15].
Table 2.
Quality of life and reported symptoms details of the study population and the recovery clusters based on the 15D dimensions.
| Health related Quality of Life | Respondents (n = 178) | Full recovery n = 84 | Partial recovery Mental n = 68 |
Partial recovery Physical n = 17 |
Bad recovery n = 9 | p |
|---|---|---|---|---|---|---|
| 15D score 3 months - mean ± SD | 0.857 ± 0.133 | 0.927 ± 0.061 | 0.800 ± 0.135 | 0.853 ± 0.114 | 0.637 ± 0.204 | < 0.001 |
| 15D score 1 year - mean ± SD | 0.880 ± 0.115 | 0.964 ± 0.033 | 0.820 ± 0.068 | 0.866 ± 0.088 | 0.572 ± 0.112 | < 0.001 |
| Mobility - mean ± SD | 0.876 ± 0.207 | 0.963 ± 0.104 | 0.828 ± 0.191 | 0.901 ± 0.166 | 0.375 ± 0.298 | < 0.001 |
| Vision - mean ± SD | 0.953 ± 0.119 | 0.992 ± 0.040 | 0.942 ± 0.108 | 0.949 ± 0.094 | 0.681 ± 0.280 | < 0.001 |
| Hearing - mean ± SD | 0.968 ± 0.098 | 1.000 ± 0.000 | 1.000 ± 0.000 | 0.745 ± 0.135 | 0.857 ± 0.192 | < 0.001 |
| Breathing - mean ± SD | 0.746 ± 0.238 | 0.879 ± 0.154 | 0.620 ± 0.227 | 0.753 ± 0.223 | 0.438 ± 0.238 | < 0.001 |
| Sleeping - mean ± SD | 0.838 ± 0.238 | 0.940 ± 0.135 | 0.716 ± 0.274 | 0.929 ± 0.142 | 0.632 ± 0.312 | < 0.001 |
| Eating - mean ± SD | 0.979 ± 0.102 | 1.000 ± 0.000 | 1 .000 ± 0.000 | 1.000 ± 0.000 | 0.587 ± 0.221 | < 0.001 |
| Speech - mean ± SD | 0.980 ± 0.090 | 0.996 ± 0.032 | 0.996 ± 0.036 | 0.948 ± 0.117 | 0.777 ± 0.276 | < 0.001 |
| Excretion - mean ± SD | 0.974 ± 0.110 | 1.000 ± 0.000 | 1.000 ± 0.000 | 0.872 ± 0.191 | 0.720 ± 0.292 | < 0.001 |
| Usual activities - mean ± SD | 0.845 ± 0.234 | 0.977 ± 0.078 | 0.768 ± 0.211 | 0.831 ± 0.231 | 0.224 ± 0.085 | < 0.001 |
| Mental function - mean ± SD | 0.889 ± 0.174 | 0.962 ± 0.111 | 0.844 ± 0.185 | 0.811 ± 0.183 | 0.903 ± 0.248 | < 0.001 |
| Discomfort - mean ± SD | 0.853 ± 0.206 | 0.979 ± 0.077 | 0.726 ± 0.214 | 0.859 ± 0.216 | 0.633 ± 0.202 | < 0.001 |
| Depression - mean ± SD | 0.853 ± 0.203 | 0.966 ± 0.091 | 0.719 ± 0.220 | 0.901 ± 0.172 | 0.706 ± 0.203 | < 0.001 |
| Distress - mean ± SD | 0.815 ± 0.210 | 0.949 ± 0.128 | 0.683 ± 0.184 | 0.825 ± 0.186 | 0.549 ± 0.201 | < 0.001 |
| Vitality - mean ± SD | 0.816 ± 0.196 | 0.931 ± 0.125 | 0.718 ± 0.160 | 0.806 ± 0.227 | 0.499 ± 0.185 | < 0.001 |
| Sexual activity - mean ± SD | 0.820 ± 0.235 | 0.966 ± 0.103 | 0.723 ± 0.215 | 0.749 ± 0.219 | 0.317 ± 0.185 | < 0.001 |
| Reported symptoms at 1 year | ||||||
| Cough - n (%) | 18 (10.1%) | 2 (2.4%) | 13 (19.1%) | 1 (5.9%) | 2 (22.2%) | 0.004 |
| Arthromialgia - n (%) | 62 (34.8%) | 15 (17.9%) | 33 (48.5%) | 7 (41.7%) | 7 (77.8%) | < 0.001 |
| Palpitations - n (%) | 12 (6.7%) | 1 (1.2%) | 8 (11.2%) | 2 (11.8%) | 1 (11.1%) | 0.050 |
| Dyspnoea (mMRC ≥1) - n (%) | 104 (58.4%) | 32 (38.1%) | 51 (75.0%) | 13 (76.5%) | 8 (88.9%) | < 0.001 |
| Dyspnoea mMRC scale - (IQR) | 0 (0–2) | 0 (0–0) | 1 (0–2) | 1 (0–2) | 3 (2–4) | < 0.001 |
Abbreviations: mMRC - modified Medical Research Council dyspnoea scale.
Notes: significant p values for differences among the recovery clusters are evidenced in bold.
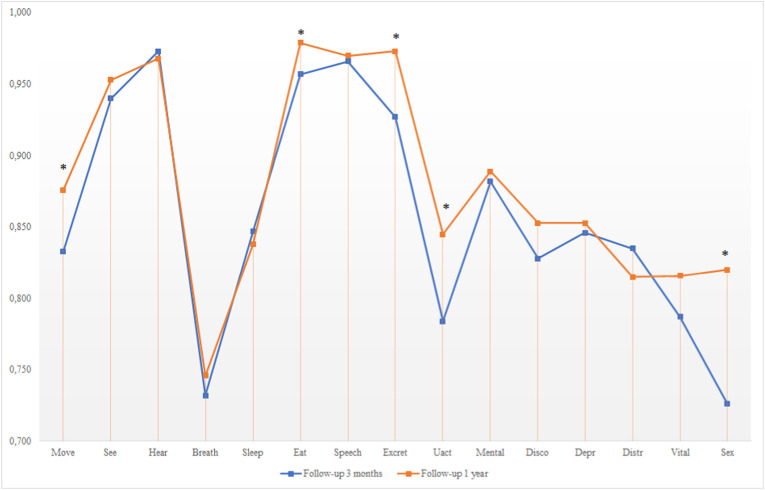
Most of the increase in HRQoL between 3 months and 1 year was related to physical dimensions (mobility, eating, excretion, usual activities, and sexual activity), while the psychological and breathing dimensions were substantially unchanged (Fig. 1).
Fig. 1.
Health-related quality of life profiles at 3 months and 1 year after ICU discharge. Notes: asterisks highlight the dimensions significantly different between 3 months and 1 year. Abbreviations: Move – mobility; See – vision; Hear – hearing; Breath – breathing; Sleep – sleeping; Eat – eating; Speech – speech; Excret – excretion; Uact – usual activities; Mental – mental function; Disco – discomfort; Depr – depression; Distr – distress; Vital – Vitality; Sex – sexual activity.
The two-step cluster analysis identified three different clusters based on BIC and four different clusters based on AIC (see supplement Table 1, Table 2). Based on the clinical relevance of the clustering process, AIC-based clustering was chosen. Fig. 2 shows the 15D dimensions values among the four different clusters, 84 patients (47.2%) were classified in the “full recovery” group (15D score = 0.964 ± 0.033), and 9 patients (5.1%) were classified in the “bad recovery” group (15D score = 0.572 ± 0.112).
Fig. 2.
Comparison of the 1-year health-related quality of life profiles clusters. Notes: the blue dashed lines highlight the significant differences between the partial recovery-mental and the partial recovery-physical groups. Abbreviations: Move – mobility; See – vision; Hear – hearing; Breath – breathing; Sleep – sleeping; Eat – eating; Speech – speech; Excret – excretion; Uact – usual activities; Mental – mental function; Disco – discomfort; Depr – depression; Distr – distress; Vital – Vitality; Sex – sexual activity. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Finally, two different clusters of intermediate recovery were distinguished: 68 patients (38.2%) were classified in a cluster of “partial recovery with mental dimensions most affected” while 17 patients (9.6%) were grouped in a cluster of “partial recovery with physical dimensions most affected”. These two intermediate clusters did not significantly differ in terms of absolute 15D score, but only in terms of 15D profiles. The partial recovery-physical cluster indeed demonstrated significantly lower mean values in the hearing and excretion dimensions but higher values for sleep, discomfort, depression, and distress values. Patients grouped into the four clusters demonstrated different characteristics concerning age, the median number of comorbidities and prevalence of hypertension, and diabetes (Table 2), with those in the partial and bad recovery clusters being older and affected by more comorbidities.
Most of the patients reported persistent symptoms 1 year after ICU discharge, of which dyspnea (n = 104, 58.4%) and arthromyalgia (n = 62, 34.8%) were the most frequent. The prevalence of symptoms and the severity of dyspnea measured with the mMRC scale demonstrated significant differences in distribution across the four clusters, with the bad recovery and partial recovery demonstrating a significantly higher prevalence of persistent symptoms and more severe dyspnea.
Univariate linear mixed-effects model analyses selected sex, age, BMI, number of comorbidities, duration of IMV, and tracheostomy for introduction in the multivariate model. The final multivariate model demonstrated that the male gender was associated with a higher increase in HRQoL from 3 to 12 months after ICU discharge while the increase in the duration of IMV and number of comorbidities were negatively associated with HRQoL change (Table 3 ).
Table 3.
Univariate and multivariate analysis for the general mixed model.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | β | 95% CI | p | β | 95% CI | p |
| Gender (male) | 0.031 | −0.005 : 0.068 | 0.092 | 0.038 | 0.004 : 0.073 | 0.030 |
| Age (years) | −0.001 | −0.003 : 0.001 | 0.097 | - 0.001 | −0.002 : 0.001 | 0.606 |
| BMI | −0.003 | −0.006 : 0.001 | 0.098 | −0.003 | −0.006 : 0.001 | 0.118 |
| Number of comorbidities | −0.036 | −0.053: −0.019 | <0.001 | −0.033 | −0.051: −0.015 | < 0.001 |
| ARDS class (per class increase)٭ | 0.011 | −0.018 : 0.040 | 0.467 | |||
| SAPS II score | −0.001 | −0.002 : 0.001 | 0.315 | |||
| SOFA score at ICU admission | 0.002 | −0.005 : 0.009 | 0.597 | |||
| Duration of invasive mechanical ventilation | −0.002 | −0.003: −0.001 | 0.001 | −0.002 | −0.003: −0.001 | 0.004 |
| Tracheostomy | −0.026 | −0.060 : 0.007 | 0.129 | 0.003 | −0.032 : 0.039 | 0.857 |
| Marital status - married/cohabitee | −0.004 | −0.042 : 0.035 | 0.840 | |||
| Instruction degree - high school or higher | 0.005 | −0.029 : 0.039 | 0.767 | |||
| Occupational status - unemployed* | 0.015 | −0.058 : 0.088 | 0.680 | |||
| Occupational status - retiree* | 0.004 | −0.030 : 0.037 | 0.837 | |||
Abbreviations: BMI – body mass index; ARDS – acute respiratory distress syndrome; SAPS – simplified acute physiology score; SOFA – sequential organ failure assessment; ICU – intensive care unit.
Notes: significant p values are evidenced in bold; ٭reference class: severe ARDS; * reference class: employed.
Pulmonary function tests at 1 year were available for 68 patients (Table 4 ), 35 of these (51.5%) had a reduction in DLCO values (DLCO lower than 80% of the predicted), while 12 out of 68 (17.6%) showed a restrictive ventilatory defect (FVC lower than 80% of the predicted). Only 2 patients (0.5%) showed an obstructive ventilatory impairment (FEV1/FVC <0.7).
Table 4.
Pulmonary function tests and health-related quality of life correlations.
| Total population n = 68 | DLCO <80% n = 35 | DLCO >80% n = 33 | p | FVC <80% n = 12 | FVC >80% n = 56 | p | |
|---|---|---|---|---|---|---|---|
| Age - years (IQR) | 62 (54–71) | 65 (57–71) | 59 (52–68) | 0.077 | 60 (57–66) | 64 (54–71) | 0.705 |
| Sex - male - no (%) | 6 (9%) | 22 (62.9%) | 29 (87.9%) | 0.017 | 11 (91.7%) | 40 (71.4%) | 0.269 |
| BMI - kg/m2 (IQR) | 28 (25–30) | 28 (25–31) | 28 (25–29) | 0.598 | 28 (26–37) | 28 (25–30) | 0.292 |
| COPD - no (%) | 6 (8.8%) | 2 (5.7%) | 4 (12.1%) | 0.667 | 0 (0%) | 6 (10.7%) | 0.581 |
| Number of comorbidities - (IQR) | 1 (0–1) | 1 (0–1) | 0 (0–1) | 0.203 | 0 (0–1) | 1 (0–1) | 0.505 |
| Intensive Care Unit stay related variables | |||||||
| SAPS II score (IQR) | 35 (27–43) | 38 (29–49) | 34 (27–42) | 0.161 | 35 (27–40) | 35 (29–44) | 0.384 |
| SOFA score at ICU admission (IQR) | 4 (3–7) | 6 (3–7) | 4 (3–6) | 0.053 | 4 (3–5) | 5 (3–7) | 0.128 |
| Worst PaO2/FiO2 5d - (IQR) | 105 (80–149) | 118 (82–146) | 90 (80–150) | 0.165 | 81 (69–108) | 112 (83–150) | 0.020 |
| Worst respiratory system compliance 5d - IQR | 40 (32–49) | 37 (31–46) | 40 (33–50) | 0.299 | 34 (30–49) | 40 (33–49) | 0.380 |
| Length of invasive mechanical ventilation - d (IQR) | 14 (8–21) | 15 (9–28) | 14 (8–18) | 0.140 | 16 (7–23) | 14 (9–21) | 0.942 |
| Length of ICU stay - d (IQR) | 19 (14–33) | 22 (15–42) | 19 (13–29) | 0.100 | 32 (15–42) | 19 (14–29) | 0.102 |
| Pulmonary function tests at 1 year after ICU discharge | |||||||
| FEV 1 - % of predicted - mean ± SD | 99.7 ± 17.4 | 85.5 ± 14.5 | 86.7 ± 14.5 | 0.195 | 77.2 ± 11.0 | 104.6 ± 14.6 | < 0.001 |
| FVC - % of predicted - mean ± SD | 97.3 ± 18.5 | 95.0 ± 22.6 | 99.7 ± 12.7 | 0.293 | 70.3 ± 8.7 | 103.0 ± 14.5 | < 0.001 |
| FEV/FVC - % of predicted - mean ± SD | 86.1 ± 14.6 | 85.5 ± 14.9 | 86.7 ± 14.5 | 0.729 | 95.4 ± 21.8 | 84.1 ± 11.9 | 0.105 |
| DLCO - % of predicted - mean ± SD | 77.6 ± 21.6 | 63.6 ± 11.9 | 92.6 ± 19.5 | < 0.001 | 68.2 ± 17.6 | 79.7 ± 22.0 | 0.096 |
| Health-related quality of life and dyspnoea | |||||||
| Outcome cluster | 0.039• | 0.122• | |||||
| Bad recovery – n (%) | 1 (1.5%) | 1 (100%) | 0 (0%) | 0 (0%) | 1 (100%) | ||
| Partial recovery physical – n (%) | 9 (13.2%) | 4 (44.4%) | 5 (55.6%) | 0 (0%) | 9 (100%) | ||
| Partial recovery mental – n (%) | 20 (29.4%) | 15 (75%) | 5 (25.0%) | 6 (30%) | 14 (70%) | ||
| Good recovery – n (%) | 38 (55.9%) | 15 (39.5%) | 23 (60.5%) | 6 (15.8%) | 32 (84.2%) | ||
| 15D score 1 year - mean ± SD | 0.906 ± 0.095 | 0.881 ± 0.105 | 0.933 ± 0.075 | 0.022 | 0.886 ± 0.078 | 0.911 ± 0.098 | 0.413 |
| 15D - Breathing - mean ± SD | 0.768 ± 0.215 | 0.731 ± 0.189 | 0.807 ± 0.236 | 0.150 | 0.711 ± 0.159 | 0.780 ± 0.225 | 0.223 |
| mMRC grade of dyspnea - (IQR) | 0 (0–1) | 1 (0–1) | 0 (0–1) | 0.061 | 1 (0–1) | 0 (0–1) | 0.251 |
| Bivariate correlations* | |||||||
| PaO2/FiO2 | CRS | DLCO | FVC | FEV1 | FEV/FVC | MV length | |
| 15D - breathing (ρ) | - 0.029 | - 0.071 | 0.244 | 0.109 | 0.159 | 0.133 | - 0.162 |
| mMRC grade of dyspnea (ρ) | - 0.105 | 0.066 | - 0.196 | - 0.068 | - 0.191 | - 0.137 | 0.204 |
Abbreviations: IQR – interquartile range; COPD – chronic obstructive pulmonary disease; ARDS – acute respiratory distress syndrome; SAPS – simplified acute physiology score; SOFA – sequential organ failure assessment; ICU – intensive care unit; mMRC modified Medical Research Council; CRS Respiratory system compliance; DLCO – diffusion capacity of lungs for carbon monoxide, FVC - forced vital capacity; FEV1 – forced expiratory volume in the first second; MV - mechanical ventilation.
Notes: significant p values are evidenced in bold, as well as the rho (ρ) values of significant bivariate Spearman correlations. • p values referred to the likelihood ratio of the Chi-square test. * bivariate correlations are calculated with a pairwise exclusion of missing data.
Median PaO2/FiO2 ratio nadir during the first five days of IMV was significantly lower in patients showing restrictive ventilatory abnormality (81, IQR 69–108 vs 112 IQR 83–150, p = 0.020), while this difference was not observed about DLCO. On the other hand, DLCO impairment was more frequent in the subgroup with partial or bad recovery compared with the subgroup reporting good recovery (Table 4), and the mean 15D score was significantly lower among those patients showing DLCO impairment (0.881 ± 0.105 vs 0.933 ± 0.075, p = 0.022).
Finally, bivariate Spearman correlations showed that the 15D breathing dimension at one year after ICU discharge was significantly correlated with both DLCO and duration of IMV, even if the strength of these associations was low (ρ = 0.244 and −0.162, respectively), while the degree of dyspnea measured with mMRC scale was significantly correlated only with the duration of IMV (ρ = 0.204).
Only 37 out of 178 patients had a chest CT scan performed within 9 months and 1 year after ICU discharge, therefore data are only reported in supplement Table 3, pulmonary fibrosis signs were observed in 26 (70.3%) of the available CT scans, while non-fibrosing signs such as persistent ground-glass opacities or consolidations in 15 (40.5%) patients.
4. Discussion
Approximately half of the mechanically ventilated ICU patients subsequently develop PICS [18], a multidimensional syndrome that concerns both the physical and psychological traits of the survivors. Signs of persistent impairment in these traits could be found up to two years after ICU discharge among ARDS patients [19,20]. The COVID-19 pandemic produced waves of ICU survivors at risk of developing both PICS and long-COVID syndrome, moreover, these patients experienced ICU stay in a healthcare system sustaining profound structural and organizational changes in response to the pandemic.
In this cohort of COVID-19 survivors who underwent ICU admission and IMV, we found that: a) HRQoL significantly increased from 3 months to 1 year after ICU discharge; b) this increase was mainly due to an increase in physical dimensions scores, while mental and breathing dimensions scores remained substantially unchanged; c) four main clusters of HRQoL profiles could be identified, two groups at the extremities of the sample showing good and bad recovery and two distinct groups with partial recovery, a larger one showing more severe alterations in mental dimensions, and a smaller group with alterations in physical dimensions; d) factors influencing HRQoL trajectories between 3 months and 1 year were: sex, duration of IMV and number of comorbidities; e) dyspnea remains the most reported symptom in ICU survivors and it is only partially explained by pulmonary function tests.
4.1. Health-related quality of life trajectories
Due to the lack of long-term information about COVID-19 sequelae, there is currently no consensus about follow-up measures after hospital discharge for COVID-19 patients [21]. Moreover, the follow-up of these patients could be furtherly complicated by the health systems overload due to the repeated pandemic waves and by the need to recover ordinary activity for non-COVID diseases [22]. A recent online survey evidenced both physical and mental sequelae one year after COVID-19 syndrome for both survivors and their relatives, with age, sex, distance from COVID-19 diagnosis, and length of hospital stay being significant predictors of HRQoL impairment [23].
This is the first literature report specifically focusing on ICU survivors who required IMV and our results strengthen the information that more than half of C-ARDS survivors report significant impairment in HRQoL and persistent symptoms, in particular dyspnea, at 1 year after ICU discharge. In fact, despite a significant increase in HRQoL from 3 months to 1 year after ICU discharge being evidenced, only 47.2% of the patients showed a complete recovery at 1 year after ICU discharge with 15D values comparable to those of the general population [11,24].
We have to underline that only physical functioning dimensions showed a significant improvement in this time frame, while mental dimensions remained substantially unchanged. This aspect is confirmed by previous literature about ARDS survivors demonstrating a slower recovery in psychological dimensions [25,26]. Therefore, a significant proportion of patients (38.2%) with partial recovery showed a long-lasting impairment in mental dimensions. Specific COVID-19 ICU policies about isolation and visits from relatives, in particular for patients admitted during the first wave, could have had a role in worsening this aspect, and this should probably demand different visiting policies and psychological support during hospital stay [27].
Several factors influenced the slope of HRQoL trajectories. In our previous study at 3 months after ICU discharge [11], we found that increasing age resulted negatively associated with HRQoL scores. Indeed, in the current follow-up, the “bad recovery” cluster showed a higher median age (Table 1). Older age, however, did not significantly influence the entity of HRQoL change towards the study period, which was instead significantly affected by the number of comorbidities and duration of IMV, like in available follow-up literature for other diseases [28]. Male sex was associated with a greater increase in HRQoL over time, this is possibly related to gender-based different shapes of the HRQoL trajectories already reported in the literature [29].
The absence of association between young age and HRQoL trajectories is another clinically relevant aspect because it underlines that even young patients, often missed at follow-up, could have significantly hampered recovery trajectories and could benefit from a longer follow-up and specific interventions. Finally, the impairment in mental dimensions has been demonstrated to be significant and long-lasting [30], therefore, psychological advice may be considered when planning the follow-up. Moreover, a not negligible fraction of patients reported dyspnea and a variable degree of impairment in pulmonary function tests that should be furtherly discussed.
4.2. Persistent dyspnea and pulmonary function tests
We reported a very high prevalence (58.4%) of persistent dyspnea at 1 year after ICU discharge in comparison to that reported from papers, this phenomenon could be explained by the fact that our population was made by critically ill patients (ICU survivors) and not by a mixed population of critical and non-critical COVID-19 patients [3]. Analogously, the 15-D breathing dimension score significantly contributed to the reduction in HRQoL at 3 months, and it did not significantly improve during the study period (see Fig. 1 and Table 2).
Most of the survivors showed some kind of impairment in both DLCO (51.5%) and FVC (17.6%) values, in line with previous literature about ARDS survivors patients [31]. In particular, in our study, reduction of FVC was significantly more frequent in patients experiencing more severe forms of ARDS (Table 4). According to actual literature [3,30], respiratory functional impairment after COVID-19 pneumonia is associated with persistent pulmonary radiological abnormalities so that PFTs may therefore represent a marker of radiological sequelae of severe COVID-19.
On the other hand, in our study population DLCO impairment degree, which is the most reported respiratory functional impairment in post-COVID syndrome, was not associated with ARDS severity. Even if potentially controversial, this result confirms that COVID-19 patients requiring intensive care and IMV can fully recover [32,33].
Interestingly, the presence and severity of dyspnea, measured by the mMRC scale, was correlated with IMV length and not with DLCO and FVC values, while the 15D breathing dimension was correlated to both IMV length and DLCO (Table 4).
According to our results, pulmonary function tests alterations do not completely explain the persistence of dyspnea, so that the perceived breathing impairment complained by patients after COVID-19 may not be fully related to pulmonary function impairment [34,35]. Other clinical and neuro-psychological aspects such as experiences of anxiety and pain, the sedative regimens, and mechanical ventilation settings during ICU stay [36,37], could play a role in determining the presence and entity of dyspnea among survivors.
Dyspnea is defined as a subjective perception of uncomfortable breathing and could be determined by complex and multiple mechanisms including social, psychological, and physical conditions [38], and it is one of the major components of the post-COVID syndrome [39]. This study evaluated a population made of critically ill patients, which could be also affected by the so-called post-intensive care syndrome (PICS), a multisystemic syndrome characterized by new or worsening physical, mental and neurocognitive disorders [40,41], that could overlap post-COVID syndrome manifestations.
It is impossible to precisely estimate the proportion of mental and physical components potentially responsible for dyspnea as well as the relative roles of PICS and post-COVID syndrome from the available data in our population. However, the very high prevalence of psychological consequences, together with the only partial concordance between dyspnea and pulmonary functional impairment (especially DLCO), may suggest a cardinal role of neuropsychological mechanisms at the root of this symptom.
Exploring this topic in larger studies could be helpful to better understand an important aspect of both PICS and post-COVID syndrome, and to foster their management in a multidisciplinary approach.
4.3. Limitations
Several limitations should be discussed: first, we adopted the telephonic interview rather than follow-up visits, this was chosen to reach the maximum number of patients taking into account the travel restrictions due to the second and third waves of the COVID-19 pandemic. Other limitations are also represented by the small proportion of patients with available PFTs at 1-year, so that the prevalence of functional impairment could be biased. Moreover, the lack of association with lung radiological data at 1-year due to data unavailability and the absence of baseline PFTs information prevented us from deriving clinical-radiological correlations and carrying out pre-post analyses.
Missing data for the sexual activity dimension exceeded 20%, despite this aspect is in line with our previous follow-up [11] and data substitution was obtained with the multiple imputation technique, cautious interpretation of these data should be warranted.
Finally, among ICU survivors significant modifications in HRQoL dimensions were reported up to ten years after discharge [42], therefore, longer follow-up times could help in better defining the HRQoL trajectories and the effects of eventual interventions [43].
5. Conclusions
COVID-19 survivors who needed IMV reported a significant HRQoL impairment at 1 year after ICU discharge in most of the cases. Despite a trend towards an increase in HRQoL being detected during the first year after ICU discharge, mental dimensions did not significantly improve and dyspnea proved to be the most frequent symptom reported 1 year after ICU discharge. Pulmonary function tests alterations, in particular concerning DLCO, only partially explained the entity of this symptom and other physiological and psychological causes should be investigated.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board (IRB) of the study coordinator centre (Maggiore Hospital, Bologna, Italy, approval number: 273/2020/OSS/AUSLBO) and by each institutional review committee of the participating hospitals. Informed consent was waived for unconscious patients while it was acquired for conscious patients or after liberation from mechanical ventilation or at the time of follow-up. The researchers analyzed anonymized individual data.
Consent for publication
Not applicable.
Availability of data and materials
The datasets related to the Italian population used and/or analyzed during the current study are available at https://doi.org/10.17632/krvnn6dzjx.1.
Funding
None declared.
CRediT authorship contribution statement
Lorenzo Gamberini: Conceptualization, Writing – original draft, Formal analysis, Conceptualized and designed the work, drafted the article, performed the statistical analysis. Carlo Alberto Mazzoli: Conceptualization, Writing – original draft, Conceptualized and designed the work, drafted the article. Irene Prediletto: Writing – original draft, drafted the article. Harri Sintonen: substantively revised the article. . Gaetano Scaramuzzo: substantively revised the article. Davide Allegri: Formal analysis, performed the statistical analysis. Davide Colombo: acquired and interpreted the data. Tommaso Tonetti: acquired and interpreted the data. Gianluca Zani: Conceptualization, Conceptualized and designed the work. Chiara Capozzi: Conceptualization, Conceptualized and designed the work, EG: Conceptualized and designed the work. Vanni Agnoletti: acquired and interpreted the data. FB: acquired and interpreted the data. Iacopo Cappellini: acquired and interpreted the data. Gabriele Melegari: acquired and interpreted the data. Federica Damiani: acquired and interpreted the data. Maurizio Fusari: acquired and interpreted the data. Giovanni Gordini: acquired and interpreted the data. Cristiana Laici: acquired and interpreted the data. Maria Concetta Lanza: acquired and interpreted the data. Mirco Leo: acquired and interpreted the data. Andrea Marudi: acquired and interpreted the data. Raffaella Papa: acquired and interpreted the data. Antonella Potalivo: acquired and interpreted the data. Jonathan Montomoli: acquired and interpreted the data. Stefania Taddei: acquired and interpreted the data. Massimiliano Mazzolini: acquired and interpreted the data. Anna Filomena Ferravante: acquired and interpreted the data. Roberta Nicali: acquired and interpreted the data. Vito Marco Ranieri: acquired and interpreted the data. Emanuele Russo: acquired and interpreted the data.. Carlo Alberto Volta: substantively revised the article. Savino Spadaro: Conceptualization, Conceptualized and designed the work.
Declaration of competing interest
Harri Sintonen is the developer of the 15D and obtains royalties from its electronic versions. The other Authors have nothing to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2021.106665.
Contributor Information
the ICU-RER COVID-19 Collaboration:
Marco Tartaglione, Valentina Chiarini, Virginia Buldini, Carlo Coniglio, Federico Moro, Silvia Orlando, Daniele Fecarotti, Nicola Cilloni, Lorenzo Giuntoli, Angela Bellocchio, Emanuele Matteo, Giacinto Pizzilli, Antonio Siniscalchi, Chiara Tartivita, Irene Cavalli, Andrea Castelli, Annalisa Marchio, Igor Bacchilega, Laura Bernabé, Francesca Facondini, Luca Morini, Luca Bissoni, Lorenzo Viola, Tommaso Meconi, Vittorio Pavoni, Angelica Venni, Aline Pagni, Patrizia Pompa Cleta, Marco Cavagnino, Alessia Guzzo, Anna Malfatto, Angelina Adduci, Silvia Pareschi, Elisabetta Bertellini, Jessica Maccieri, Elisa Marinangeli, Fabrizio Racca, Marco Verri, Giulia Falò, Elisabetta Marangoni, Irene Ottaviani, Francesco Boni, Giulia Felloni, Federico Domenico Baccarini, Marina Terzitta, Stefano Maitan, Lorenzo Tutino, Angelo Senzi, Guglielmo Consales, and Filippo Becherucci
Radiology Collaborators (to be indexed and searchable into PubMed):
Michele Imbriani, Paolo Orlandi, Silvia Candini, Rita Golfieri, Federica Ciccarese, Antonio Poerio, Francesco Muratore, Fabio Ferrari, Martina Mughetti, Emanuela Giampalma, Loredana Franchini, Ersenad Neziri, Marco Miceli, Maria Teresa Minguzzi, Lorenzo Mellini, and Sara Piciucchi
Pneumology Collaborators (to be indexed and searchable into PubMed):
Matteo Monari, Michele Valli, Federico Daniele, Martina Ferioli, Stefano Nava, Luigi Arcangelo Lazzari Agli, Ilaria Valentini, Eva Bernardi, Bruno Balbi, Marco Contoli, Marianna Padovani, Stefano Oldani, Claudia Ravaglia, and Patrizio Goti
Appendix A. Supplementary data
The following are the Supplementary data to this article:
supplement Fig. 1.
References
- 1.Who Coronavirus (Covid-19) Dashboard WHO coronavirus (COVID-19) dashboard with vaccination data. https://covid19.who.int/ n.d. accessed.
- 2.J.W. März, S. Holm, M. Schlander, Resource allocation in the Covid-19 health crisis: are Covid-19 preventive measures consistent with the Rule of Rescue?, (n.d.). 10.1007/s11019-021-10045-0. [DOI] [PMC free article] [PubMed]
- 3.Wu X., Liu X., Zhou Y., Yu H., Li R., Zhan Q., Ni F., Fang S., Lu Y., Ding X., Liu H., Ewing R.M., Jones M.G., Hu Y., Nie H., Wang Y. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir. Med. 2021;9:747–754. doi: 10.1016/s2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236379survivors of COVID -19:a retrospective cohort study using electronic health records. The Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramadan M.S., Bertolino L., Zampino R., Durante-Mangoni E., Iossa D., Ursi M.P., D'Amico F., Karruli A., Ramadan M., Andini R., Bernardo M., Ruocco G., Dialetto G., Covino F.E., Manduca S., Della Corte A., De Feo M., De Vivo S., De Rimini M.L., Galdieri N. Cardiac sequelae after coronavirus disease 2019 recovery: a systematic review. Clin. Microbiol. Infect. 2021;2 doi: 10.1016/j.cmi.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandal S., Barnett J., Brill S.E., Brown J.S., Denneny E.K., Hare S.S., Heightman M., Hillman T.E., Jacob J., Jarvis H.C., Lipman M.C.I., Naidu S.B., Nair A., Porter J.C., Tomlinson G.S., Hurst J.R., Long-Covid’ A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76:396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Augustin M., Schommers P., Stecher M., Dewald F., Gieselmann L., Gruell H., Horn C., Vanshylla K., Di Cristanziano V., Osebold L., Roventa M., Riaz T., Tschernoster N., Altmueller J., Rose L., Salomon S., Priesner V., Luers J.C., Albus C., Rosenkranz S., Gathof B., Fätkenheuer G., Hallek M., Klein F., Suárez I., Lehmann C. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg. Heal. - Eur. 2021;6:100122. doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rousseau A.-F., Minguet P., Colson C., Kellens I., Chaabane S., Delanaye P., Cavalier E., Chase J.G., Lambermont B., Misset B. Post-intensive care syndrome after a critical COVID-19: cohort study from a Belgian follow-up clinic. Ann. Intensive Care. 2021;11:118. doi: 10.1186/s13613-021-00910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamberini L., Tonetti T., Spadaro S., Zani G., Mazzoli C.A., Capozzi C., Giampalma E., Letizia M., Reggiani B., Bertellini E., Castelli A., Cavalli I., Colombo D., Crimaldi F., Damiani F., Fogagnolo A., Fusari M., Gamberini E., Gordini G., Laici C., Lanza M.C., Leo M., Marudi A., Nardi G., Ottaviani I., Papa R., Potalivo A., Russo E., Taddei S. Factors influencing liberation from mechanical ventilation in coronavirus disease 2019 : multicenter observational study in fifteen. Italian ICUs. 2020;4:1–12. doi: 10.1186/s40560-020-00499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gamberini L., Mazzoli C.A., Sintonen H., Colombo D., Scaramuzzo G., Allegri D., Tonetti T., Zani G., Capozzi C., Giampalma E., Agnoletti V., Becherucci F., Bertellini E., Castelli A., Cappellini I., Cavalli I., Crimaldi F., Damiani F., Fusari M., Gordini G., Laici C., Lanza M.C., Leo M., Marudi A., Nardi G., Ottaviani I., Papa R., Potalivo A., Ranieri V.M., Russo E., Taddei S., Volta C.A., Spadaro S., Tartaglione M., Chiarini V., Buldini V., Coniglio C., Moro F., Barbalace C., Citino M., Cilloni N., Giuntoli L., Bellocchio A., Matteo E., Pizzilli G., Siniscalchi A., Tartivita C., Matteo F., Marchio A., Bacchilega I., Bernabé L., Guarino S., Mosconi E., Bissoni L., Viola L., Gamberini E., Meconi T., Pavoni V., Pagni A., Cleta P.P., Cavagnino M., Malfatto A., Adduci A., Pareschi S., Melegari G., Maccieri J., Marinangeli E., Racca F., Verri M., Falò G., Marangoni E., Boni F., Felloni G., Baccarini F.D., Terzitta M., Maitan S., Parise M., Bugiani B., Masoni F. Quality of life of COVID-19 critically ill survivors after ICU discharge: 90 days follow-up. Qual. Life Res. 2021 doi: 10.1007/s11136-021-02865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson N.D., Fan E., Camporota L., Antonelli M., Anzueto A., Beale R., Brochard L., Brower R., Esteban A., Gattinoni L., Rhodes A., Slutsky A.S., Vincent J.L., Rubenfeld G.D., Taylor Thompson B., Marco Ranieri V. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 13.Da M., Ck W. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/CHEST.93.3.580. [DOI] [PubMed] [Google Scholar]
- 14.Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann. Med. 2001;33:328–336. doi: 10.3109/07853890109002086. [DOI] [PubMed] [Google Scholar]
- 15.Alanne S., Roine R.P., Räsänen P., Vainiola T., Sintonen H. Estimating the minimum important change in the 15D scores. Qual. Life Res. 2015;24:599–606. doi: 10.1007/s11136-014-0787-4. [DOI] [PubMed] [Google Scholar]
- 16.Sintonen H., Johansson S., Ohinmaa A., Apajasalo M., Kainulainen P., Heikkinen J. Measuring health-related quality of life in women on hormone replacement therapy. Expert Rev. Pharmacoecon. Outcomes Res. 2003;3:351–361. doi: 10.1586/14737167.3.3.351. [DOI] [PubMed] [Google Scholar]
- 17.Benassi M., Garofalo S., Ambrosini F., Sant'Angelo R.P., Raggini R., De Paoli G., Ravani C., Giovagnoli S., Orsoni M., Piraccini G., M B., S G.G., F A., Rp S., R R., G D.P., C R., S G.G., M O., G P. Using two-step cluster Analysis and latent class cluster Analysis to classify the cognitive heterogeneity of cross-diagnostic psychiatric inpatients. Front. Psychol. 2020;11:1–11. doi: 10.3389/fpsyg.2020.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaffri A., Jaffri U.A. Post-Intensive care syndrome and COVID-19: crisis after a crisis? Heart Lung. 2020;49:883. doi: 10.1016/J.HRTLNG.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins R.O., Weaver L.K., Collingridge D., Parkinson R.B., Chan K.J., Orme J.F. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 20.Fan E., Dowdy D.W., Colantuoni E., Mendez-Tellez P.A., Sevransky J.E., Shanholtz C., Himmelfarb C.R.D., Desai S.V., Ciesla N., Herridge M.S., Pronovost P.J., Needham D.M. Physical complications in acute lung injury survivors: a 2-year longitudinal prospective study. Crit. Care Med. 2014;42:849. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee L., Iyer S., Jose R.J., Manuel A. COVID-19 follow-up planning: what will we be missing? ERJ Open Res. 2020;6 doi: 10.1183/23120541.00198-2020. 00198–02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.L. Rosenbaum, The Untold Toll — The Pandemic's Effects on Patients without Covid-19, 10.1056/nejmms2009984.%20382%20(2020)%202368%5f2371. . [DOI] [PubMed]
- 23.Shah R., Ali F.M., Nixon S.J., Ingram J.R., Salek S.M., Finlay A.Y. Measuring the impact of COVID-19 on the quality of life of the survivors, partners and family members: a cross-sectional international online survey. BMJ Open. 2021;11:47680. doi: 10.1136/bmjopen-2020-047680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koskinen S., Lundqvist A., N.R.-N.I. for H and Welfare, U . 2012. Health, Functional Capacity and Welfare in Finland in 2011.https://www.julkari.fi/bitstream/handle/10024/90832/Rap068_2012_netti.pdf?sequence=1&isAllowed=y n.d.) [Google Scholar]
- 25.Wilcox M.E., Herridge M.S. vol. 40. Press; Medicale: 2011. pp. e595–e603. (Lung Function and Quality of Life in Survivors of the Acute Respiratory Distress Syndrome (ARDS)). [DOI] [PubMed] [Google Scholar]
- 26.Langerud A.K., Rustøen T., Småstuen M.C., Kongsgaard U., Stubhaug A. 2018. Health-related Quality of Life in Intensive Care Survivors: Associations with Social Support, Comorbidity, and Pain Interference. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Ruiz E., Campelo-Izquierdo M., Estany-Gestal A., Rodríguez-Núñez A., Latour J.M. Impact of different visiting policies on family satisfaction in two Spanish ICUs before and during COVID-19. Intensive Care Med. 2021;2021:1–2. doi: 10.1007/S00134-021-06485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unruh M.L., Newman A.B., Larive B., Dew M.A., Miskulin D.C., Greene T., Beddhu S., Rocco M.V., Kusek J.W., Meyer K.B. Hemodialysis Study Group, the influence of age on changes in health-related quality of life over three years in a cohort undergoing hemodialysis. J. Am. Geriatr. Soc. 2008;56:1608–1617. doi: 10.1111/j.1532-5415.2008.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J., Jang S.N., Il Cho S. Gender differences in the trajectories and the risk factors of depressive symptoms in later life. Int. Psychogeriatr. 2017;29:1495–1505. doi: 10.1017/S1041610217000709. [DOI] [PubMed] [Google Scholar]
- 30.Bellan M., Soddu D., Balbo P.E., Baricich A., Zeppegno P., Avanzi G.C., Baldon G., Bartolomei G., Battaglia M., Battistini S., Binda V., Borg M., Cantaluppi V., Castello L.M., Clivati E., Cisari C., Costanzo M., Croce A., Cuneo D., De Benedittis C., De Vecchi S., Feggi A., Gai M., Gambaro E., Gattoni E., Gramaglia C., Grisafi L., Guerriero C., Hayden E., Jona A., Invernizzi M., Lorenzini L., Loreti L., Martelli M., Marzullo P., Matino E., Panero A., Parachini E., Patrucco F., Patti G., Pirovano A., Prosperini P., Quaglino R., Rigamonti C., Sainaghi P.P., Vecchi C., Zecca E., Pirisi M. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw. Open. 2021;4 doi: 10.1001/JAMANETWORKOPEN.2020.36142. e2036142–e2036142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herridge M.S., Cheung A.M., Tansey C.M., Matte-Martyn A., Diaz-Granados N., Al-Saidi F., Cooper A.B., Guest C.B., Mazer C.D., Mehta S., Stewart T.E., Barr A., Cook D., Slutsky A.S. One-year outcomes in survivors of the acute respiratory distress syndrome. N. Engl. J. Med. 2003;348:683–693. doi: 10.1056/nejmoa022450. [DOI] [PubMed] [Google Scholar]
- 32.Daher A., Cornelissen C., Hartmann N.U., Balfanz P., Müller A., Bergs I., Aetou M., Marx N., Marx G., Simon T.P., Müller-Wieland D., Hartmann B., Kersten A., Müller T., Dreher M. Six months follow-up of patients with invasive mechanical ventilation due to covid-19 related ards. Int. J. Environ. Res. Publ. Health. 2021;18 doi: 10.3390/ijerph18115861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carenzo L., Protti A., Dalla Corte F., Aceto R., Iapichino G., Milani A., Santini A., Chiurazzi C., Ferrari M., Heffler E., Angelini C., Aghemo A., Ciccarelli M., Chiti A., Iwashyna T.J., Herridge M.S., Cecconi M. Short-term health-related quality of life, physical function and psychological consequences of severe COVID-19. Ann. Intensive Care. 2021;11:91. doi: 10.1186/s13613-021-00881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Townsend L., Dowds J., O'Brien K., Sheill G., Dyer A.H., O'Kelly B., Hynes J.P., Mooney A., Dunne J., Cheallaigh C.N., O'Farrelly C., Bourke N.M., Conlon N., Martin-Loeches I., Bergin C., Nadarajan P., Bannan C. Persistent poor health after covid-19 is not associated with respiratory complications or initial disease severity. Ann. Am. Thorac. Soc. 2021;18:997–1003. doi: 10.1513/AnnalsATS.202009-1175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Froidure A., Mahsouli A., Liistro G., De Greef J., Belkhir L., Gérard L., Bertrand A., Koenig S., Pothen L., Yildiz H., Mwenge B., Aboubakar F., Gohy S., Pilette C., Reychler G., Coche E., Yombi J.C., Ghaye B. Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir. Med. 2021;181 doi: 10.1016/j.rmed.2021.106383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt M., Demoule A., Polito A., Porchet R., Aboab J., Siami S., Morelot-Panzini C., Similowski T., Sharshar T. Dyspnea in mechanically ventilated critically ill patients. Crit. Care Med. 2011;39:2059–2065. doi: 10.1097/CCM.0B013E31821E8779. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt M., Banzett R.B., Raux M., Morélot-Panzini C., Dangers L., Similowski T., Demoule A. Unrecognized suffering in the ICU: addressing dyspnea in mechanically ventilated patients. Intensive Care Med. 2014;40:1. doi: 10.1007/S00134-013-3117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mechanisms Dyspnea. assessment, and management: a consensus statement. American Thoracic Society, Am. J. Respir. Crit. Care Med. 1999;159:321–340. doi: 10.1164/AJRCCM.159.1.ATS898. [DOI] [PubMed] [Google Scholar]
- 39.Naeije R., Caravita S. Phenotyping long COVID. Eur. Respir. J. 2021;58 doi: 10.1183/13993003.01763-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvey M.A., Davidson J.E. Postintensive care syndrome: right care, right Now…and later. Crit. Care Med. 2016 Feb;44(2):381–385. doi: 10.1097/CCM.0000000000001531. [DOI] [PubMed] [Google Scholar]
- 41.Stam H.J., Stucki G., Bickenbach J., European Academy of Rehabilitation Medicine Covid-19 and post intensive care syndrome: a call for action. J. Rehabil. Med. 2020 Apr 15;52(4) doi: 10.2340/16501977-2677. [DOI] [PubMed] [Google Scholar]
- 42.Hofhuis J.G.M., Schrijvers A.J.P., Schermer T., Spronk P.E. Health-related quality of life in ICU survivors—10 years later. Sci. Rep. 2021;11:1–10. doi: 10.1038/s41598-021-94637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spadaro S., Capuzzo M., Valpiani G., Bertacchini S., Ragazzi R., Dalla Corte F., Terranova S., Marangoni E., Volta C.A. Fatigue in intensive care survivors one year after discharge. Health Qual. Life Outcome. 2016;14 doi: 10.1186/s12955-016-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets related to the Italian population used and/or analyzed during the current study are available at https://doi.org/10.17632/krvnn6dzjx.1.