Abstract
Background
Immunogenicity and safety of the AZD1222 (ChAdOx1 nCoV-19) vaccine was evaluated in Japanese adults in an ongoing phase 1/2, randomized, double-blind, parallel-group, placebo-controlled, multi-centre trial (NCT04568031).
Methods
Adults (n=256, age ≥18 years) seronegative for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) were stratified by age into 18–55- (n=128), 56–69- (n=86) and ≥70-year-old cohorts (n=42), and randomized 3:1 to receive AZD1222 or placebo (two intramuscular injections 4 weeks apart). Immunogenicity and safety were coprimary endpoints. Data collected up to Day 57 are reported.
Results
Positive seroresponses to SARS-CoV-2 spike and receptor-binding domain antigens were seen in all 174 participants who received two doses of AZD1222. Neutralizing antibody seroresponses were seen in 67.5%, 60.3% and 50.0% of participants receiving AZD1222 aged 18–55, 56–69 and ≥70 years, respectively. Solicited adverse events (AEs) were typically mild/moderate in severity and included pain and tenderness at the injection site, malaise, fatigue, muscle pain and headache. Common unsolicited AEs included pain and tenderness at the injection site, fatigue and elevated body temperature. No vaccine-related serious AEs or deaths were reported.
Conclusions
AZD1222 elicited a strong humoral immune response against SARS-CoV-2, and was well tolerated in Japanese participants, including elderly participants.
Keywords: COVID-19, ChAdOx1 nCoV-19, AZD1222, Japan, Elderly adult, Humoral response
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has resulted in widespread morbidity and mortality, prompting the most extensive and rapid global vaccine development programme in history (World Health Organization, 2020a). Accelerated development and approval has been crucial to providing effective, well-tolerated vaccines against symptomatic SARS-CoV-2 infection (COVID-19). By the end of 2020, several vaccines had reached their phase 3 efficacy milestones and received emergency authorization for use (Polack et al., 2020; Baden et al., 2021; Voysey et al., 2021a, Voysey et al., 2021b).
As of 21 September 2021, 1,679,116 confirmed cases of COVID-19 and 17,233 deaths related to the disease have been reported in Japan, with greater numbers seen in older age groups (World Health Organization, 2020b; National Institute of Population and Social Security Research, 2021). In 2019, people aged ≥65 years accounted for 28.4% of the Japanese population (Statistics Bureau of Japan, 2021). Age is a prominent risk factor for COVID-19 morbidity and mortality, with adults aged ≥70 years at greater risk of severe disease and death than younger age groups (Wu et al., 2020; Chen et al., 2021). Risk of severe disease is also increased by the presence of comorbidities, including hypertension, cardiovascular disease, diabetes and obesity (Wu et al., 2020). The rapid approval of COVID-19 vaccines in Japan is therefore crucial to help protect an aging population.
The AZD1222 (ChAdOx1 nCoV-19) vaccine is a replication-deficient simian adenovirus-vectored vaccine encoding the full-length SARS-CoV-2 spike protein (Folegatti et al., 2020). The immunogenicity, safety and efficacy of AZD1222 is being assessed globally in randomized controlled trials (Folegatti et al., 2020; AstraZeneca, 2021; Barrett et al., 2021; Ewer et al., 2021; Ramasamy et al., 2021; Voysey et al., 2021a, Voysey et al., 2021b). In a pooled analysis of four trials conducted in the UK (phase 1/2 and 2/3), Brazil (phase 3) and South Africa (phase 1/2), AZD1222 exhibited an acceptable safety profile and overall vaccine efficacy of 66.7% [95% confidence interval (CI) 57.4–74.0] against COVID-19 >14 days after the second dose (Voysey et al., 2021a). A single dose of AZD1222 induced spike and receptor-binding domain (RBD) antibodies as well as neutralizing antibody (nAb) titres against SARS-CoV-2, which were increased substantially after a second dose 28 days later (Folegatti et al., 2020; Barrett et al., 2021; Ewer et al., 2021; Ramasamy et al., 2021).
A growing number of studies suggest that seroresponse and presence of nAbs may indicate protection against COVID-19 (Addetia et al., 2020; Folegatti et al., 2020; Robbiani et al., 2020; Wang et al., 2020; Hall et al., 2021; Hansen et al., 2021; Harvey et al., 2021; Krammer, 2021; Pilz et al., 2021). For the development of a vaccine, the presence of an immune response can provide a reasonable indication of efficacy, and immunogenic assessments can be made in a shorter time and with smaller sample sizes compared with efficacy assessments.
This article reports immunogenicity and safety results from a randomized, placebo-controlled, phase 1/2 trial of AZD1222 in Japanese adults. To the authors’ knowledge, this is the first randomized, double-blind, placebo-controlled study to evaluate immunogenicity and safety of a replication-deficient simian adenovirus-vectored vaccine in an East Asian country.
Methods
Study design and participants
This phase 1/2, randomized, double-blind, parallel-group, placebo-controlled trial (ClinicalTrials.gov identifier: NCT04568031) is being conducted at five centres in Japan, with a planned study duration of 1 year following dosing for each participant. Safety and immunogenicity data up to Day 57 for all enrolled participants are reported here.
Ethics and oversight
The study is being conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. The protocol, including amendments and consent form, were approved by an institutional review board prior to study initiation. All participants provided written informed consent before enrolment.
Participants
Eligibility criteria
Adults aged ≥18 years, seronegative for SARS-CoV-2 at screening, and with a negative reverse-transcriptase polymerase chain reaction test for SARS-CoV-2 were eligible for inclusion. Participants with a history of laboratory-confirmed SARS-CoV-2 infection, pregnant women, new onset of fever, any confirmed or suspected immunodeficient state, receipt of any vaccine within 30 days before or after each study dose, or prior or planned receipt of an investigational or licensed vaccine or product that may impact interpretation of trial data (e.g. adenovirus-vectored vaccines or coronavirus vaccines) were excluded. Participants with severe and/or uncontrolled cardiovascular, respiratory, gastrointestinal, hepatic or renal disease, endocrine disorder or neurologic illness were also excluded. Mild/moderate, well-controlled comorbidities were allowed.
Randomization and masking
Participants were stratified into two age cohorts: 18–55 years and ≥56 years. The ≥56-year-old cohort was further divided into subcohorts aged 56–69 years and ≥70 years. Approximately 30% of participants in the ≥56-year-old cohort were aged ≥70 years. Participants in each cohort were randomized (3:1) to receive either AZD1222 or placebo (saline) using a centralized Interactive Response Technology system.
AZD1222 and placebo were prepared by an unblinded pharmacist, in accordance with local and institutional regulations at each site. Participants, clinical investigators and sponsor staff were blinded to the study vaccination received until completion of safety data lock at Day 57.
Study procedures
Vaccination
AZD1222 was manufactured in accordance with current Good Manufacturing Practice guidelines (AstraZeneca K.K., Osaka, Japan; MedImmune Pharma BV, Nijmegen, The Netherlands). Participants received two doses of AZD1222 (5 × 1010 viral particles/dose) or placebo (saline; 0.9% weight/volume) on Days 1 and 29, administered as intramuscular injections into the deltoid muscle.
Clinical and immunogenicity assessments
Blood samples for immunogenicity assessments were collected before and after each dose (serology: Days 1, 15, 29, 43 and 57; nAb: Days 1, 29 and 57). Humoral responses at baseline and after dosing were assessed using a validated, multiplex, electrochemiluminescent immunoassay against the SARS-CoV-2 spike, RBD and nucleocapsid antigens (PPD Vaccines, Richmond, VA, USA), and a validated HIV-1-based pseudo-virus neutralization assay (Monogram Biosciences, South San Francisco, CA, USA).
Safety assessments
Participants were monitored for 30 min after dosing to assess for adverse events (AEs). Solicited AEs were predefined as local (pain and tenderness at the injection site, redness, swelling and induration) or systemic (fever, chills, muscle pain, fatigue, headache, malaise, nausea and vomiting) and were collected by participants using an electronic diary for 6 days after each dose (Days 1–7 and Days 29–35). Unsolicited AEs were recorded for 28 days following each dose (Days 1–29 and Days 29–57).
Blood samples were collected at Days 1, 8, 29, 36 and 57 for determination of clinical laboratory safety, including assessment of haemoglobin concentration, leukocyte count, platelet count and clinical chemistry.
Severity of safety endpoints was assessed according to toxicity grading scales adapted from Food and Drug Administration (FDA) grading guidance.
Outcomes
The coprimary endpoints were immunogenicity, measured by anti-SARS-CoV-2 spike seroresponse (≥4-fold rise in titre from Day 1 baseline value) (European Medicines Agency, 2018) at Day 57 following vaccination with AZD1222, and safety, measured by occurrence of solicited local and systemic reactogenicity signs/symptoms in the 6 days after each dose; occurrence of unsolicited AEs, serious AEs (SAEs) and AEs of special interest (AESIs) for 28 days after each dose; and change from baseline in safety laboratory measures.
Secondary endpoints included proportion of participants with a seroresponse for the RBD antigen and SARS-CoV-2 nAb 28 days after the second dose of AZD1222 (Day 57); geometric mean titre (GMT) and geometric mean fold rise of immunogenicity against SARS-CoV-2 spike and RBD antigens and SARS-CoV-2 nAb at each time point up to Day 365; and occurrence of SAEs and AESIs up to Day 365.
Statistical analyses
Sample size calculations
A sample size of 128 participants (96 and 32 randomized to AZD1222 and placebo, respectively) in each cohort was determined mainly for the evaluation of safety and based on feasibility. With a sample size of 96 participants in the AZD1222 arm for each cohort, at least one participant with an AE at an incidence of 2.5% could be detected with a probability of approximately 90%.
Analysis of study endpoints
The proportions of participants with a seroresponse for the SARS-CoV-2 spike and RBD antigens, as well as for nAb, were compared between AZD1222 and placebo using Fisher's exact test at the two-sided 5% alpha level for each cohort. Two-sided 95% CIs were calculated using the Clopper–Pearson method for proportions within each cohort/subcohort. Given the exploratory nature of the study, no adjustment for multiple comparisons and multiplicity was performed; only nominal P-values were provided for immunogenicity endpoints. All statistical analyses were conducted using SAS Version 9.4.
Assessments of immunogenicity were performed on the fully vaccinated analysis set (FVS), which included all participants who received two study doses, had no protocol deviations judged to potentially interfere with generation/interpretation of immune responses, and did not exhibit a seroresponse (≥4-fold rise in titre from the Day 1 baseline value) to nucleocapsid antibodies by Meso Scale Discovery serology assay up to Day 57. Safety analyses (unless otherwise stated) were performed on the total vaccinated analysis set (TVS), which included all participants who received at least one dose of study intervention. Data are summarized by descriptive statistics.
Results
Participant disposition
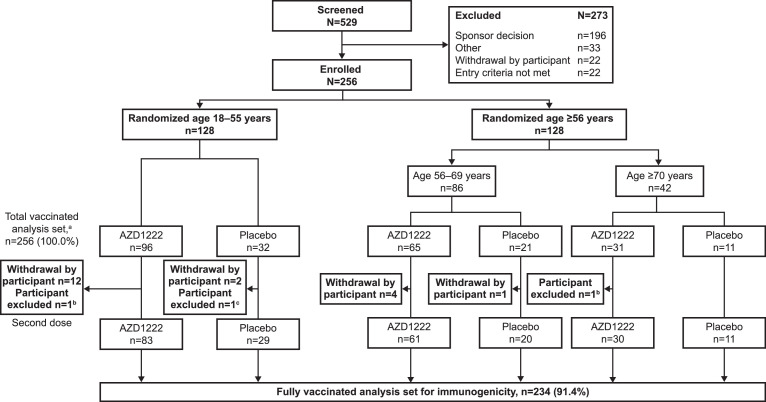
Participants were recruited from August 2020 (final data lock: 24 February 2021), with follow-up scheduled for approximately 1 year after the first dose. This study was temporarily interrupted by the safety data review on the occurrence of SAEs outside Japan. Participant disposition is outlined in Figure 1 . All screen failures that were categorized as ‘Sponsor decision’ were associated with this interruption and a consequent pause in enrolment (Figure 1).
Figure 1.
Participant disposition (CONSORT). aIncluding all participants who received at least one dose of study intervention. bHad seroresponse (≥4-fold rise in titre from Day 1 baseline value) to nucleocapsid antibodies by Meso Scale Discovery serology assay up to Day 57. cHad protocol deviations judged to have potential to interfere with the generation or interpretation of an immune response. The study was interrupted temporarily following the occurrence of serious adverse events in clinical trials outside Japan. All screen failures categorized as ‘Sponsor decision’ were associated with this interruption and a consequent pause in enrolment.
Participant baseline characteristics
Demographic and other participant baseline characteristics were well balanced between AZD1222 and placebo in the TVS (Table 1 ). At baseline, the prevalence of comorbidities associated with higher risk of severe COVID-19 was 27.0%, 4.3%, 1.6%, 1.6% and 0.4% for hypertension, obesity (body mass index ≥30 kg/m2), cardiac disorder, type 2 diabetes and type 1 diabetes, respectively. All participants were Japanese (Asian), and 32.8% (42/128) of participants aged ≥56 years were aged ≥70 years.
Table 1.
Participant baseline characteristics by age group.
| Age 18–55 years |
Age 56–69 years |
Age ≥70 years |
Total |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | AZD1222 (n=96) | Placebo (n=32) | AZD1222 (n=65) | Placebo (n=21) | AZD1222 (n=31) | Placebo (n=11) | AZD1222 (n=192) | Placebo (n=64) |
| Age, years | 45.6 (8.2) | 46.1 (6.7) | 61.0 (3.7) | 62.2 (3.6) | 73.1 (3.0) | 73.5 (4.7) | 55.3 (12.2) | 56.1 (12.1) |
| Females, n (%) | 25 (26.0) | 8 (25.0) | 27 (41.5) | 9 (42.9) | 13 (41.9) | 5 (45.5) | 65 (33.9) | 22 (34.4) |
| Weight, kg | 67.3 (14.1) | 69.4 (10.2) | 63.4 (12.3) | 60.2 (10.2) | 58.6 (9.6) | 61.7 (16.6) | 64.6 (13.2) | 65.1 (12.1) |
| Height, cm | 168.6 (7.3) | 169.1 (5.5) | 163.8 (8.5) | 162.9 (9.3) | 159.5 (7.2) | 161.6 (9.9) | 165.5 (8.4) | 165.8 (8.3) |
| BMI, kg/m2 | 23.6 (4.2) | 24.2 (3.2) | 23.5 (3.4) | 22.6 (2.8) | 22.9 (2.7) | 23.4 (4.4) | 23.4 (3.7) | 23.6 (3.3) |
| BMI group | ||||||||
| <30 kg/m2, n (%) | 90 (93.8) | 30 (93.8) | 63 (96.9) | 21 (100.0) | 31 (100.0) | 10 (90.9) | 184 (95.8) | 61 (95.3) |
| ≥30 kg/m2, n (%) | 6 (6.3) | 2 (6.3) | 2 (3.1) | 0 | 0 | 1 (9.1) | 8 (4.2) | 3 (4.7) |
| Comorbidities | ||||||||
| Hypertension | 17 (17.7) | 6 (18.8) | 19 (29.2) | 8 (38.1) | 14 (45.2) | 5 (45.5) | 50 (26.0) | 19 (29.7) |
| Cardiac disorders | 0 | 0 | 2 (3.1) | 0 | 1 (3.2) | 1 (9.1) | 3 (1.6) | 1 (1.6) |
| Type 2 diabetes | 0 | 0 | 3 (4.6) | 1 (4.8) | 0 | 0 | 3 (1.6) | 1 (1.6) |
| Type 1 diabetes | 0 | 0 | 0 | 0 | 0 | 1 (9.1) | 0 | 1 (1.6) |
BMI, body mass index.
Table parameters presented were predefined. Values are mean (standard deviation) unless otherwise stated.
Immunogenicity endpoints
Coprimary endpoint: antibody responses to AZD1222 spike antigen
Seroresponses to the SARS-CoV-2 spike antigen in the FVS on Day 57 were exhibited by 100% (n=174) of participants who received AZD1222 and 0% (n=60) of participants who received placebo (P<0.001 for AZD1222 vs placebo for both cohorts).
Secondary immunogenicity endpoints
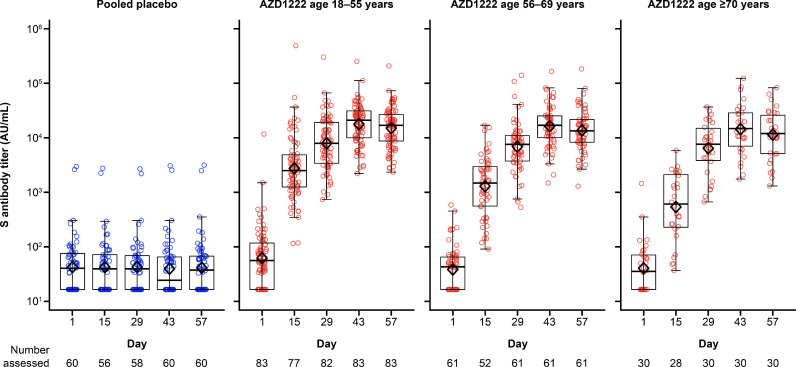
In participants who received AZD1222, antibody titres for SARS-CoV-2 spike antigen increased substantially after the first dose and increased further after the second dose across all age cohorts/subcohorts in the FVS. Titres peaked on Day 43 and remained above pre-dose Day 29 levels up to Day 57 (Figure 2 ).
Figure 2.
Box plots and individual plots of anti-spike antibody titres over time after two doses of AZD1222 or placebo in Japanese participants (fully vaccinated analysis set), determined by Meso Scale Discovery serological assay. The bottom and top edges of the box indicate the first and third quartiles [the difference is the interquartile range (IQR)], the line inside the box is the median, and the diamond mark inside the box is the geometric mean. The whiskers that extend from the box indicate the minimum and maximum within the range of 1.5 × IQR from the box. Box plots are created using log-transformed values. Titre values measured as below the lower limit of quantification (LLoQ) (33) are imputed to a value that is half of the LLoQ. Titre values measured as above the upper limit of quantification (ULoQ) (2,000,000) are imputed at the ULoQ value. AU/mL, arbitrary units per mL; S, SARS-CoV-2 spike antigen.
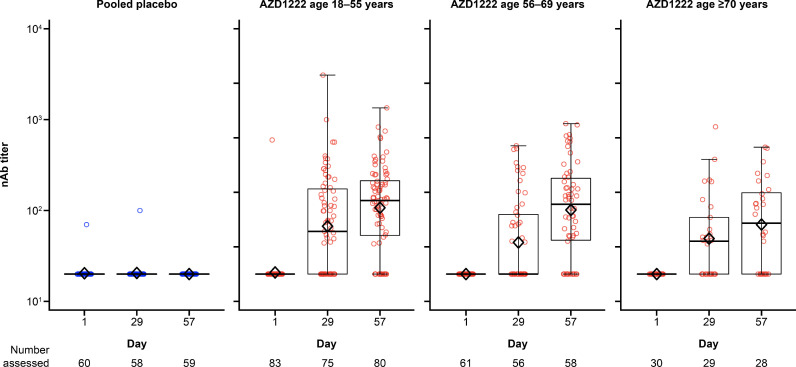
Results for SARS-CoV-2 RBD antigen were similar to those for the spike antigen (Figure 3 ). A seroresponse to the SARS-CoV-2 RBD antigen was observed in 100% (n=174) of participants who received AZD1222 and 0% (n=60) of participants who received placebo in the FVS on Day 57 (P<0.001 for AZD1222 vs placebo for both cohorts). nAb responses against SARS-CoV-2 on Day 57 were observed in 67.5% (54/83), 60.3% (35/61) and 50.0% (14/28) of participants receiving AZD1222 in the FVS among the 18–55, 56–69 and ≥70 years age cohorts, respectively. In contrast, no nAb response was observed in any participant receiving placebo. The difference in nAb responses between AZD1222 and placebo was statistically significant in each age cohort (P<0.001). Titres for nAb increased in a similar manner to the anti-spike and anti-RBD antibodies, remaining above pre-dose Day 29 levels up to Day 57 (Figure 4 ). For participants aged 18–55, 56–69 and ≥70 years, peak nAb GMTs on Day 57 were 107.3 (95% CI 84.2–136.7), 101.5 (95% CI 74.3–138.5) and 70.2 (95% CI 45.6–108.1), respectively. In the placebo groups, there was no change from baseline in antibody titres for the SARS-CoV-2 spike and RBD antigens, and nAb for any age cohort.
Figure 3.
Box plots and individual plots of anti-receptor-binding domain (RBD) antibody titres over time after two doses of AZD1222 or placebo in Japanese participants (fully vaccinated analysis set), determined by Meso Scale Discovery serological assay. The bottom and top edges of the box indicate the first and third quartiles [the difference is the interquartile range (IQR)], the line inside the box is the median, and the diamond mark inside the box is the geometric mean. The whiskers that extend from the box indicate the minimum and maximum within the range of 1.5 × IQR from the box. Box plots are created using log-transformed values. Titre values measured as below the lower limit of quantification (LLoQ) (204) are imputed to a value that is half of the LLoQ. Titre values measured as above the upper limit of quantification (ULoQ) (2,000,000) are imputed at the ULoQ value. AU/mL, arbitrary units per mL.
Figure 4.
Box plots and individual plots of neutralizing antibody (nAb) titres over time after two doses of AZD1222 or placebo in Japanese participants (fully vaccinated analysis set), determined by pseudo-virus neutralization assay. The bottom and top edges of the box indicate the first and third quartiles [the difference is the interquartile range (IQR)], the line inside the box is the median, and the diamond mark inside the box is the geometric mean. The whiskers that extend from the box indicate the minimum and maximum within the range of 1.5 × IQR from the box. Box plots are created using log-transformed values. Titre values measured as below the lower limit of quantification (LLoQ) (40) are imputed to a value that is half of the LLoQ. Titre values measured as above the upper limit of quantification (ULoQ) (787,339) are imputed at the ULoQ value.
Coprimary endpoint: safety and reactogenicity
Solicited AEs
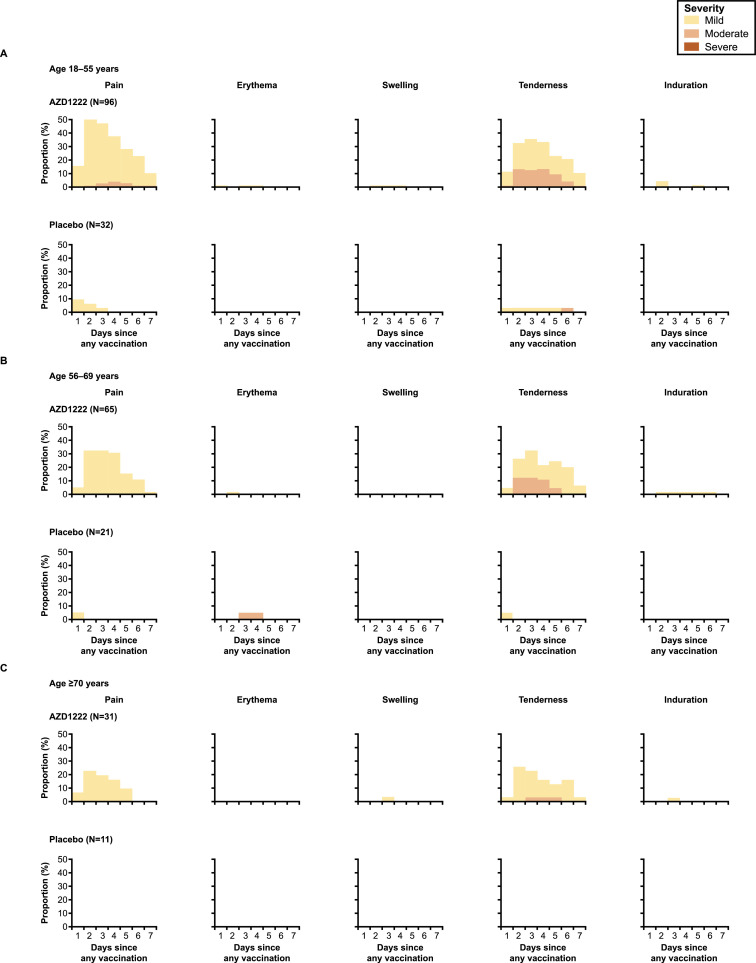
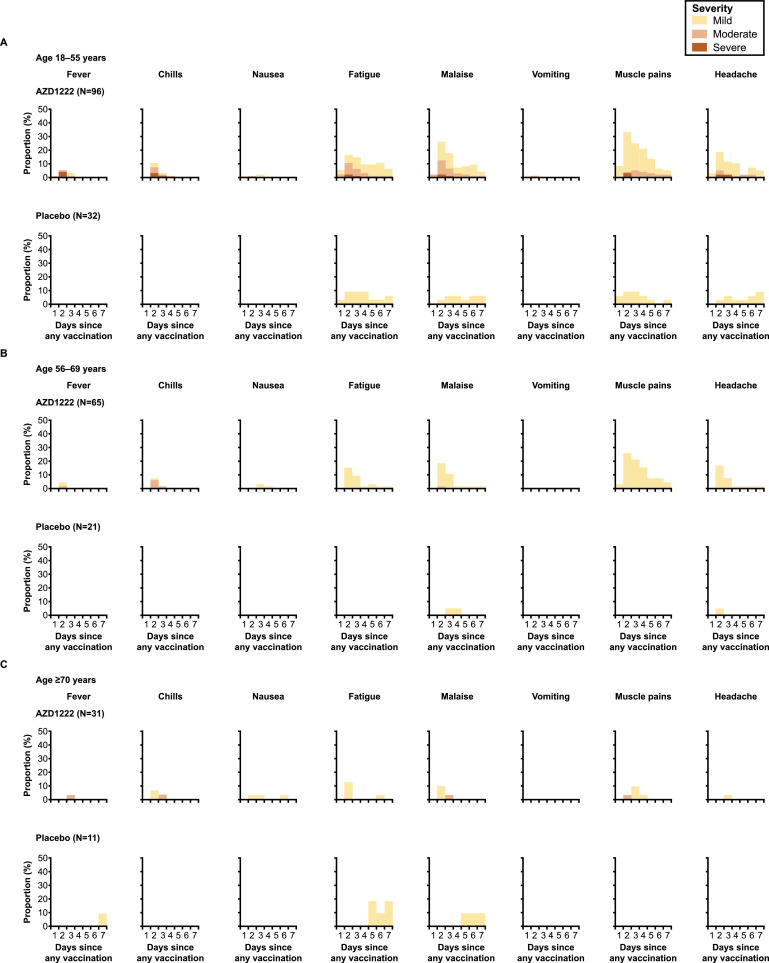
Incidences of solicited local and systemic AEs in the first 6 days are reported in Figure 5, Figure 6 , respectively. Solicited AEs (within 6 days of any dose) were reported by 85.4% (82/96) and 37.5% (12/32) of participants in the AZD1222 and placebo groups in the 18–55-year-old cohort, 72.3% (47/65) and 19.0% (4/21) in the 56–69-year-old subcohort, and 51.6% (16/31) and 27.3% (3/11) in the ≥70-year-old subcohort, respectively. Across all ages, the most frequently reported solicited AEs within 6 days of any dose of AZD1222 were pain and tenderness at the injection site (local), and malaise, muscle pain, fatigue and headache (systemic). The majority were of mild or moderate severity. Severe solicited AEs occurred in 9.4% (9/96) of participants aged 18–55 years receiving AZD1222, and were not observed in the other age groups. No potentially life-threatening solicited AEs were reported. Solicited local and systemic AEs were most frequently reported in the first 2–4 days following any injection (Figures 5 and 6). Solicited AEs were more frequently reported after the first dose than the second (Table S1, see online supplementary material). In participants who received AZD1222, solicited AEs were milder and reported less frequently in those aged ≥70 years compared with those aged 18–55 years and 56–69 years.
Figure 5.
Solicited local adverse events in the first 7 days after any dose of AZD1222 or placebo for participants aged (A) 18–55 years, (B) 56–69 years and (C) ≥70 years (total vaccinated analysis set). Day 1 is the day of vaccination. Participants with more than one event within one interval and category are counted once for maximum severity.
Figure 6.
Solicited systemic adverse events in the first 7 days after any dose of AZD1222 or placebo for participants aged (A) 18–55 years, (B) 56–69 years and (C) ≥70 years (total vaccinated analysis set). Day 1 is the day of injection. Participants with more than one event within one interval and category are counted once for maximum severity.
Unsolicited AEs
Unsolicited AEs (28 days following each dose) for AZD1222 and placebo, respectively, were reported by 28.1% (27/96) and 9.4% (3/32) of participants aged 18–55 years, 21.5 % (14/65) and 28.6% (6/21) in those aged 56–69 years, and 22.6% (7/31) and 27.3% (3/11) in those aged ≥70 years. The majority were of mild or moderate severity. Severe unsolicited AEs occurred in 4.2% (4/96) of participants aged 18–55 years receiving AZD1222, and 3.1% (2/65) and 9.5% (2/21) of participants aged 56–69 years receiving AZD1222 or placebo, respectively. The most common unsolicited AEs were consistent with AEs commonly observed following vaccination, including pain and tenderness at the injection site, fatigue and elevated body temperature (Table S2, see online supplementary material). There were no notable imbalances between AZD1222 and placebo for AEs that were not commonly associated with vaccination. In participants who received AZD1222, unsolicited AEs were milder and reported less frequently in those aged ≥56 years compared with those aged 18–55 years.
Deaths, SAEs and AESIs
No deaths, SAEs or AESIs were reported among participants receiving AZD1222. Among participants receiving placebo, one SAE of cervical intra-epithelial neoplasia (grade 3) occurred in a 69-year-old female after the first dose. This SAE was considered to be unrelated to placebo and the participant received her second dose as planned.
Clinical laboratory parameters
No clinically significant differences in haematologic and clinical chemistry parameters were observed in the AZD1222 and placebo groups over time. Isolated increases from baseline in FDA toxicity grades were observed for haematology/chemistry parameters in a minority of participants, but this was not considered clinically significant. A temporary decrease in neutrophils in one male participant was reported in the AZD1222 group as an unsolicited AE (grade 3), and recovered to the normal range within 3 weeks after each vaccination. There was no clinically relevant change in average platelet count in the AZD1222 group compared with the placebo group (Tables S3 and S4, see online supplementary material).
Discussion
The coprimary objectives of this randomized, placebo-controlled trial were to assess immunogenicity and safety of AZD1222 in a Japanese population.
All measures of immunogenicity indicated a strong humoral response to AZD1222 on Day 57, irrespective of participant age. All participants who received both doses of AZD1222 had seroresponses against the SARS-CoV-2 spike and RBD antigens on Day 57. nAb response rates were 62.0% among participants who received two doses of AZD1222, ranging from 50.0% in those aged ≥70 years to 67.5% in those aged 18–55 years. Titre values of anti-spike antibodies, anti-RBD antibodies and nAb increased substantially after the first dose of AZD1222 (Day 29), and increased further after the second dose (Day 57).
Seroresponse rates against SARS-CoV-2 spike and RBD antigens were almost identical to those reported in a pooled analysis of trials conducted in the UK, Brazil and South Africa, in which the majority of participants were white (seroresponse rates ∼100%) (Voysey et al., 2021a), although the respective titre values in this study were less than reported previously (Voysey et al., 2021a). nAb response rates and titres were also numerically lower than observed in the pooled analysis, although this is likely to be due to differences in intervals between first and second doses (4 weeks ±2 days in this study compared with 3–26 weeks for the pooled analysis) (Voysey et al., 2021a). However, nAb titres observed in this study (overall GMT 97.96) were comparable with those in clinical trials conducted in the UK (GMT 97.43) and Brazil (GMT 110.96), which employed similar dosing intervals (4–8 weeks) to this study (Pharmaceuticals and Medical Devices, 2021). Furthermore, the GMTs observed for anti-spike antibodies, anti-RBD antibodies and nAb after the first dose of AZD1222 were comparable between Japanese and non-Japanese participants (Voysey et al., 2021a).
In this study, there was a decreasing trend in anti-spike antibodies, anti-RBD antibodies and nAb titres with increasing age, although large variability was observed among individual titres with wide, overlapping CIs between age groups. These data align with the reduced humoral responses observed in older adults in the previous pooled analysis (Voysey et al., 2021a). However, given the robust vaccine efficacy of AZD1222 observed previously in adults aged >65 years (Voysey et al., 2021a), these numerically reduced responses may not be clinically meaningful.
Correlates of protection have not yet been established for SARS-CoV-2 in humans, although nAb titres have been correlated with protection in non-human primates (Deng et al., 2020; van Doremalen et al., 2020a, van Doremalen et al., 2020b; Yu et al., 2020; McMahan et al., 2021). In addition, several studies have suggested that the presence of nAb is associated with protection in humans (Addetia et al., 2020; Robbiani et al., 2020; Wang et al., 2020; Hall et al., 2021; Hansen et al., 2021; Harvey et al., 2021; Krammer, 2021; Pilz et al., 2021). In this study, AZD1222 elicited a strong humoral immune response against SARS-CoV-2 in Japanese adults, irrespective of age. This suggests that AZD1222 may provide effective protection against COVID-19 in this population, extrapolated from immunogenicity and efficacy results observed in the previous pooled analysis (Voysey et al., 2021a, Voysey et al., 2021b).
AZD1222 was well tolerated in Japanese adults across all age groups, and the safety profile was similar to that observed in previous clinical trials (Folegatti et al., 2020; Ramasamy et al., 2021). The most frequently occurring AEs were consistent with previous studies of AZD1222, and included pain and tenderness at the injection site, malaise, fatigue, muscle pain and headache. Up to Day 57, no vaccine-related SAEs were reported, and no deaths have been reported in the trial to date.
Extremely rare cases of thrombosis with thrombocytopenia syndrome (TTS) have been observed following vaccination with AZD1222 during postauthorization use. While no cases of TTS were observed in this study, given the rarity of TTS, no events would be expected in a clinical trial setting such as this; in other studies of the large AZD1222 clinical trial programme, no cases were observed among approximately 30,000 participants who received AZD1222 (Falsey, 2021; Voysey et al., 2021a). Independent safety reviews by regulatory authorities have concluded that the overall protective benefits of AZD1222 against COVID-19, including severe disease, hospitalization and death, outweigh the risks of TTS (European Medicines Agency, 2021a; Medicines and Healthcare Products Regulatory Agency, 2021a; World Health Organization 2021). In individuals with a history of heparin-induced thrombocytopenia and thrombosis or cerebral venous sinus thrombosis, AZD1222 should only be considered where the benefits outweigh any potential risks (European Medicines Agency, 2021b; Medicines and Healthcare Products Regulatory Agency, 2021b). Careful monitoring of safety, including anaphylactic reactions, immune-mediated neurologic disorders and TTS, continues in postmarketing surveillance.
Limitations of this study include the small sample size and short duration of follow-up reported (∼8 weeks after first dose). Pregnant women, individuals with immunodeficiency, and those with severe or uncontrolled underlying disease were excluded from this study, and the results may not be generalized to these populations. Long-term follow-up of this study (Day 365 after first dose), other ongoing clinical studies and emerging real-world evidence will provide further data to characterize the safety, immunogenicity and efficacy of AZD1222. Although correlates of protection against SARS-CoV-2 remain undefined, it is thought that both humoral and cellular immune responses play a protective role (Ewer et al., 2021). Cellular immunity was not investigated in this study, which focused on the humoral immune response. However, data from a previous phase 1/2 trial in UK adults (18–55 years) demonstrated that AZD1222 induced a T-helper-1-biased CD4+ effector response and cytotoxic CD8+ T-cell response (Ewer et al., 2021). Finally, this study was not designed to evaluate the efficacy of AZD1222, and it cannot be concluded definitively that the immune responses observed correlate with protection.
Conclusions
AZD1222 elicited a strong humoral immune response against SARS-CoV-2 regardless of age, and was well tolerated with an acceptable safety profile in Japanese adult participants, including the elderly and those with controlled underlying diseases. Results were comparable with data from clinical trials conducted in the UK and Brazil that employed a similar dosing interval to this study, suggesting that AZD1222 can confer effective protection against COVID-19 in the Japanese population.
Acknowledgments
Acknowledgements
The authors wish to thank the participants, their families and all investigators (Akiyoshi Uchiyama, Tokyo Asbo Clinic, formerly Shinagawa East One Medical Clinic, Tokyo, Japan; Atsuko Abe, Seikoukai New Medical Research System Clinic, Hachioji City, Tokyo, Japan; Takuma Yonemura, Souseikai Sumida Hospital, Sumida City, Tokyo, Japan; Kenjiro Nakamura, Tenjin Sogo Clinic, Fukuoka City, Fukuoka, Japan; Akira Numata, Ikebukuro Metropolitan Clinic, Toshima City, Tokyo, Japan), Tatsuya Nakamura (study leader, AstraZeneca K.K.), clinical site staff and all relevant persons involved in this study. The authors also wish to thank Maria-Claudia Nascimento, MD, PhD, study physician, for her contributions in ensuring data quality and scientific integrity of the study. Medical writing support, including assistance with the development of the outline and initial draft and incorporation of comments, under author direction, was provided by Alex Gavin, PhD, and editorial support was provided by Rachael Cazaly, BSc, both of Core Medica, London, UK, supported by AstraZeneca according to Good Publication Practice guidelines. The sponsor was involved in the study design, collection, analysis and interpretation of data, as well as data checking of information provided in the manuscript.
Conflict of interest statement
JV was an employee and stockholder of AstraZeneca at the time of the study. All other authors are employees of, and hold or may hold stock in, AstraZeneca.
Funding
This study was sponsored by AstraZeneca K.K.
Author contributions
Michiko Asano contributed to concept/design and data interpretation.
Hiroshi Okada contributed to concept/design and data interpretation.
Yohji Itoh contributed to concept/design, data analysis and data interpretation.
Hajime Hirata contributed to concept/design and data interpretation.
Kensuke Ishikawa contributed to concept/design and data interpretation.
Erika Yoshida contributed to concept/design, data acquisition and data interpretation.
Akiko Matsui contributed to data interpretation.
Elizabeth J. Kelly contributed to concept/design and data interpretation.
Kathryn Shoemaker contributed to data analysis and data interpretation.
Urban Olsson contributed to concept/design and data interpretation.
Johan Vekemans contributed to concept/design, data analysis and data interpretation.
All authors provided critical review of drafts and approved the final manuscript.
Data sharing
Data underlying the findings described in this manuscript may be requested in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.10.030.
Appendix. Supplementary materials
References
- Addetia A, Crawford KHD, Dingens A, Zhu H, Roychoudhury P, Huang ML, et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58:e02107–20. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AstraZeneca. AZD1222 US Phase III primary analysis confirms safety and efficacy. AstraZeneca; 2021. Available at: https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2021/azd1222-us-phase-iii-primary-analysis-confirms-safety-and-efficacy.html (accessed 9 July 2021).
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JR, Belij-Rammerstorfer S, Dold C, Ewer KJ, Folegatti PM, Gilbride C, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27:279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, Osevala NM, et al. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65 doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Bao L, Liu J, Xiao C, Liu J, Xue J, et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. 2020;369:818–823. doi: 10.1126/science.abc5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency. Draft guideline on clinical evaluation of vaccines – Revision 1. EMA; 2018. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-clinical-evaluation-vaccines-revision-1_en.pdf (accessed 28 October 2021).
- European Medicines Agency. AstraZeneca's COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. EMA; 2021a. Available at: https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood (accessed 21 May 2021).
- European Medicines Agency. Vaxzevria (previously covid-19 vaccine AstraZeneca). EMA; 2021b. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca (accessed 25 May 2021).
- Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27:270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. 2021 doi: 10.1056/NEJMoa2105290. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJM, Simmons R, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RA, Rassen JA, Kabelac CA, Turenne W, Leonard S, Klesh R, et al. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern Med. 2021;181:672–679. doi: 10.1001/jamainternmed.2021.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F. Correlates of protection from SARS-CoV-2 infection. Lancet. 2021;397:1421–1423. doi: 10.1016/S0140-6736(21)00782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicines and Healthcare Products Regulatory Agency. Statement on AstraZeneca COVID-19 vaccine following JCVI update. MHRA; 2021a. Available at: https://www.gov.uk/government/news/statement-on-astrazeneca-covid-19-vaccine-following-jcvi-update (accessed 3 June 2021).
- Medicines and Healthcare Products Regulatory Agency. Summary of Product Characteristics for Vaxzevria. MHRA 2021b https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca/information-for-healthcare-professionals-on-covid-19-vaccine-astrazeneca (accessed 25 November, 2021).
- National Institute of Population and Social Security Research. Trend of COVID-19 cases and deaths in Japan (as of 10 May 21). National Institute of Population and Social Security Research; 2021. Available at: http://www.ipss.go.jp/projects/j/choju/covid19/index-en.asp (accessed 13 May 2021).
- Pilz S, Chakeri A, Ioannidis JP, Richter L, Theiler-Schwetz V, Trummer C, et al. SARS-CoV-2 re-infection risk in Austria. Eur J Clin Invest. 2021;51:e13520. doi: 10.1111/eci.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharmaceuticals and Medical Devices Agency. Vaxzevria review report (English). PMDA; 2021. Available at: https://www.pmda.go.jp/files/000242500.pdf (accessed 22 June 2021).
- Statistics Bureau of Japan. Statistical handbook of Japan 2020. Chapter 2: Population. Statistics Bureau of Japan; 2021. Available at: https://www.stat.go.jp/english/data/handbook/pdf/2020all.pdf#page=23 (accessed 1 April 2021).
- van Doremalen N, Haddock E, Feldmann F, Meade-White K, Bushmaker T, Fischer RJ, et al. A single dose of ChAdOx1 MERS provides protective immunity in rhesus macaques. Sci Adv. 2020;6:eaba8399. doi: 10.1126/sciadv.aba8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Guo X, Xin Q, Pan Y, Hu Y, Li J, et al. Neutralizing antibodies responses to SARS-CoV-2 in COVID-19 inpatients and convalescent patients. Clin Infect Dis. 2020:ciaa721. doi: 10.1093/cid/ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2021. Statement of the WHO Global Advisory Committee on Vaccine Safety (GACVS) COVID-19 subcommittee on safety signals related to the AstraZeneca COVID-19 vaccine. [Google Scholar]; Available at: https://www.who.int/news/item/19-03-2021-statement-of-the-who-global-advisory-committee-on-vaccine-safety-(gacvs)-covid-19-subcommittee-on-safety-signals-related-to-the-astrazeneca-covid-19-vaccine. (accessed 6 June 2021).
- World Health Organization . WHO; Geneva: 2020. The COVID-19 candidate vaccine landscape.https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines . Available at: (accessed 31 March 2021) [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. WHO coronavirus disease (COVID-19) dashboard.https://covid19.who.int/ . Available at: accessed 31 March 2021. [Google Scholar]
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yu J, Tostanoski LH, Peter L, Mercado NB, McMahan K, Mahrokhian SH, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.