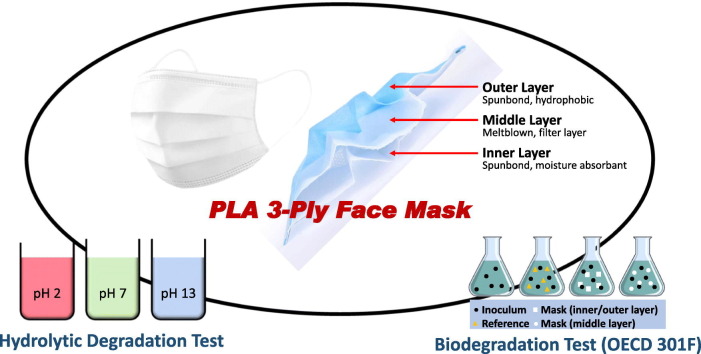
Abstract
The global massive consumption of disposable face masks driven by the ongoing COVID-19 pandemic has emerged as a blooming disaster to both the land and marine environment that might last for generations. Growing public concerns have been raised over the management and control of this new form of plastic pollution, and one of the proposed sustainable solution is to use renewable and/or biodegradable resources to develop mask materials in order to minimize their environmental impacts. As a representative biodegradable polymer, polylactic acid (PLA) has been proposed as a promising candidate to produce non-woven face masks instead of those fossil-based polymers. To further explore the feasibility of this alternative mask material, the present work aims to study both the hydrolytic and bio-degradation behaviors of pure PLA-derived 3-ply disposable face masks at ambient temperature. Hydrolytic degradability was investigated at different pH conditions of 2, 7 and 13 with the whole piece of face mask soaked for regular timed intervals up to 8 weeks. Weight loss study showed neutral and acidic conditions had minimal effect on PLA masks, but rapid degradation occurred under basic conditions in the first week with a sharp 25% decrease in weight that slowly tapered off, coupled with solution pH dropping from 13 to 9.6. This trend was supported by mechanical property, bacterial filtration efficiency (BFE) and particulate filtration efficiency (PFE) studies. Masks soaked in basic conditions had their modulus and tensile strength dropped by more than 50% after 8 weeks where the middle layer reached 68% and 90% respectively just after 48 h, and BFE and PFE decreased by 14% and 43% respectively after 4 weeks, which was much more significant than those in neutral and acidic conditions. Base degradation was also supported by nuclear magnetic resonance (NMR) and fourier transform infrared (FTIR), which disclosed that only the middle layer undergo major degradation with random chain scission and cleavage of enol or enolate chain ends, while outer and inner layers were much less affected. Scanning electron microscopy (SEM) attributed this observation to thinner PLA fibers for the middle layer of 3–7 μm diameter, which on average is 3 times smaller. This degradation was further supported by gel permeation chromatography (GPC) which saw an increase in lower molecular weight fragment Mw ~ 800 Da with soaking duration. The biodegradation behavior was studied under OECD 301F specification in sewage sludge environment. Similarly, degradation to the middle meltblown layer was more extensive, where the average weight loss and carbon loss was 25.8% and 25.7% respectively, double that of outer/inner spunbond layer. The results showed that the face masks did not completely disintegrate after 8 weeks, but small solubilized fragments of PLA formed in the biodegradation process can be completely mineralized into carbon dioxide without generation of secondary microplastic pollution in the environment. PLA masks are therefore a slightly greener option to consider in times of a pandemic that the world was caught unprepared; however future research on masks could be geared towards a higher degradability material that fully breaks down into non-harmful components while maintaining durability, filtration and protection properties for users.
Keywords: Hydrolytic degradation, Biodegradation, Microplastic pollution, Meltblown, Non-woven, PLA
Graphical abstract
1. Introduction
The ongoing COVID-19 pandemic has sparked a massive consumption of personal protective equipment (PPE) worldwide every day (Daniel et al., 2021; Ooi et al., 2021; Suwardi et al., 2021). Wearing a face mask in public places has become the norm in most countries as an efficient and inexpensive way to control the spread of this coronavirus. Recent studies estimated that an astounding 129 billion (roughly 451,500 tons) face masks being used and disposed each month globally, which when placed next to one another, could cover an area roughly three times the size of Singapore (Mungcal et al., 2021; Xu and Ren, 2021). Currently, there is no official guidance on mask recycling. Those discarded masks, most of which were single-use disposable masks, have become an impending disaster to both the land and marine environment (Benson et al., 2021; Dharmaraj et al., 2021; De-la-Torre et al., 2021; Roberts et al., 2020; Selvaranjan et al., 2021; Hasan et al., 2020). Generally, disposable surgical or medical face masks consist of three or four layers of non-woven fabrics produced by spun bonding and melt-blowing manufacturing processes, or by emerging electrospinning or texturized film extrusion technologies (Chellamani et al., 2013; Pan et al., 2020; Chua et al., 2020a). These fabrics, which are prevalently microfibers derived from petroleum-based non-degradable polymers (including polypropylene, polyethylene, polyurethane, polystyrene, polycarbonate, polyacrylonitrile, etc.) (Chua et al., 2020b; Abbasi et al., 2020), can generate a large number of micro-sized particles more easily compared with those bulk plastic wastes, and further fragment into nanoplastics that disperse into ecosystems. It is therefore pertinent to recognize this looming environmental threat and start planning for sustainable approaches so as to reduce the impacts while meeting the huge mask demand during this COVID-19 pandemic.
In addition to waste management for face masks and promoting the use of reusable masks (Torres and De-la-Torre, 2021), one promising solution is to use renewable and/or biodegradable resources to manufacture disposable masks (Morganti and Morganti, 2020; Morganti et al., 2020; Choi et al., 2021; Garcia et al., 2021). In recent years, significant progress has been made in the development of biodegradable plastics (Rai et al., 2021; Ghosh and Jones, 2021; Din et al., 2020; Song et al., 2009), largely from renewable natural resources, to produce degradable materials with similar functionalities to those of fossil-based polymers (Song et al., 2009; Coppola et al., 2021). It has been demonstrated that these bio-based plastics not only expressed superiority in terms of degradation, but also exhibited potential benefits for greenhouse gas balances and reduction of other environmental impacts over the whole life cycles (Song et al., 2009). Polylactic acid (PLA), one of the most representative eco-friendly biodegradables, is considered to be a versatile substitute to those finite resources with cost-effective industrial processes to derive its monomers from vegetable resources (Garlotta, 2001; Balla et al., 2021). PLA is a linear aliphatic thermoplastic polyester synthesized by poly-condensation of naturally produced lactic acid via corn starch fermentation or by ring opening polymerization of lactide (a cyclic dimer of lactic acid) (Mehta et al., 2005; Fan et al., 2017; Fan et al., 2019). The ester linkages in PLA are sensitive to both chemical hydrolysis and enzymatic chain cleavage (Höglund et al., 2012; Huang et al., 2014; Babu Valapa et al., 2016; Elsawy et al., 2017; Yagi et al., 2009; Fukushima et al., 2009; Pinto et al., 2016). Due to its low cost, excellent mechanical property, easy processability, hydrophobility and antimicrobial activity, PLA has been widely explored for biomedical and food packaging applications (Singhvi et al., 2019; Siracusa et al., 2012).
Recently, PLA and its polymer blends have been reported to be promising candidates for fabricating non-woven mask materials instead of the conventional fabrics made from fossil-based polymers. He and co-workers developed a transparent, nanoporous PLA mask filter with hierarchical structure via electrospinning and three-dimensional (3D) printing techniques (He et al., 2020). The electrospun PLA nanofibrous webs combined with the 3D printed PLA structural supports can achieve similar particle filtration performance as KN95/N95 (≥95 wt%) masks. Müller et al. presented a high throughput production system by combining solution-based electrospinning with centrifugal spinning to generate interconnected ultrathin nanofiber filters with diameters in the tens of nanometer regime using PLA and polyethylene glycol (Müller et al., 2020). Palmieri et al. reported a hybrid electrospun PLA-cyclodextrins composite for joint filtration of particulate matter and enhanced adsorption of volatile organic compounds (VOCs) (Palmieri et al., 2020). In early 2021, Ahlstrom-Munksjö, the world's leading player in sustainable and innovative fiber-based products, has launched a novel biodegradable and compostable TenderGuard™ biobased fabric, which is mainly comprised of PLA and can be used as an inner or outer coverstock layer of civil use face masks. Currently, research and development activities are still ongoing to explore the feasibility and efficiency of bio-based mask materials. However, to the best of our knowledge, the degradation behaviors of those face masks made from PLA have yet to be investigated. In the present work, we report the degradation behavior studies of PLA-derived disposable 3-ply face masks under both hydrolytic and bio-degradation processes. Are these biodegradable face masks truly the solution to reducing the plastic waste brought about by the huge consumption of disposal face masks? This study focuses on the degradability evaluation, degradation mechanism as well as the changes associated with the degradation process using various analytical techniques.
2. Material and methods
2.1. Materials
Disposable 3-ply nonwoven PLA face masks (17.5 × 9.5 cm) containing three layers; the hydrophobic spun-bond PLA fabric (outer layer), the melt-blown PLA fabric (middle layer) and the hydrophilic spun-bond PLA fabric (inner layer) were used for the analysis. Analytical grade sodium hydroxide and hydrochloric acid were used for preparation of soaking solutions for hydrolytic degradation. The activated sludge inoculum freshly collected from the aeration basin of a Singapore sewage treatment plant a month prior to the biodegradation tests was used for the biodegradation studies.
2.2. Hydrolytic degradation test
Soaking solutions of pH 2 and pH 13 were prepared by diluting 37% HCl and NaOH pellets respectively and adjusted by an Eutech pH 700 m. A pH 7 soaking solution was prepared by using de-ionized water. To each soaking solution, pre-weighed biodegradable masks with mass M0 were submerged in the solution and left to soak for the respective duration. When soaking duration was reached, the pH of the soaking solution was measured, and the masks were removed from the soaking solution and rinsed with de-ionized water, then hung to air dry for 2 days at room temperature. The weight loss of each sample was referred to the difference between the initial weight (M0) and final weight. The percentage of weight loss was calculated as the weight loss divided by the initial weight. In total, 6 mask samples were measured for each soaking solution at each interval. After different periods of hydrolytic degradation, individual mask layers were separated and subjected to further characterizations.
2.3. Biodegradation test
The spunbond and meltblown layers of the PLA masks were subjected to biodegradation test according to OECD 301F solutions aerobic biodegradation test method in the presence of sewage sludge. Coarse suspended particles of the freshly activated sludge (obtained 1 mth prior to biodegradation studies) were filtered out by a fine sieve (<1 mm), and the remaining sludge with a total solids content of 16.5 g/L was stored in a glass bottle at 4 °C until usage. The sludge was then pre-conditioned by aeration at room temperature for 1 day before inoculation. The tests were conducted in 500 mL incubation bottles under aerobic conditions. The mask layers were cut into small pieces and pulverized in an Ultra Centrifugal Mill (Retsch ZM200) after frozen in liquid nitrogen. The total organic carbon (TOC) contents of the samples were measured using a TOC analyzer. In each test, 25 mg of the sample (12.5 mg TOC) was added to the mineral medium (per OECD 301F) and activated sludge inoculum to achieve a final liquid volume of 250 mL. Cellulose powder was used as a control material and the mixture of mineral medium and activated sludge was used as blank. All the tests were conducted in duplicates and the initial pH was adjusted to 7.4. The bottles were sealed from atmosphere, stirred continuously, and maintained at 22 ± 2 °C for 28 days. After 28 days, the liquid in each bottle was filtered by a nylon filter and the retained solids were dried and weighed. The weight loss of each sample due to biodegradation was calculated as the difference between the initial weight and final weight. The percentage of weight loss was calculated as the weight loss divided by the initial weight as shown in the supporting information.
2.4. Characterization
2.4.1. Chemistry analysis
The different mask layers and the basic soaking solutions at different time intervals were subjected to nuclear magnetic resonance (NMR) analysis. For the mask layers analysis, a small segment of 0.5 cm by 0.5 cm of each treated mask layer was cut out and dissolved in 0.75 mL deuterated chloroform solution. The solution was then subjected to NMR analysis on a Jeol 500 MHz spectrometer at room temperature. One sample of each layer in the respective soaking solution at each soaking duration interval was tested for NMR.
50 mL of the basic soaking solutions were withdrawn at the respective duration and subjected to acid work-up with 70 mL of chloroform twice, and 70 mL of ethyl acetate twice. The organic phase was combined and dried over magnesium sulfate, and the solvent was removed under vacuum via the rotary evaporator. The residue was then dissolved in 0.75 mL deuterated chloroform solution and subjected to NMR analysis at room temperature. Only 1 sample from the respective basic soaking solution at each time interval was withdrawn for the NMR test.
The respective dried mask layers before and after the respective soaking duration intervals were cut into small segments of 3 cm by 2 cm, and then clipped onto the sample holder and subjected to fourier transform infrared spectrometer (FTIR) analysis on a PerkinElmer Spectrum 2000 spectrometer. One sample of each layer in the respective soaking solution at each soaking duration interval was tested for FTIR. The peak height ratio can be calculated based on the Eq. (1):
| (1) |
where H x is the height of the respective characteristic bands, and H Ref is the height of the reference peak at 1455 cm−1. These peak height (H) can be calculated by Eq. (2):
| (2) |
where B is the height of the baseline and A is the height of the absorption peak at the respective wavenumber (Oliveira et al., 2016; Sabnis and Block, 1997).
2.4.2. Morphology observation
The morphologies of different layers of PLA masks before and after degradation were monitored by scanning electron microscopy (SEM) using a JEOL JSM-6700F field-emission scanning electron microscope at 5 kV. All specimens were sputter coated with gold prior to analysis. One sample of each layer in the respective soaking solution at each soaking duration interval was tested for SEM.
2.4.3. Mechanical testing
The tensile performance of the different layers of PLA face masks before and after hydrolytic degradation was tested using Instron 5569 Table Universal testing machine with a 10 N load cell capacity at a rate of 10 mm/min and a gauge length of 20 mm. The various layers of the PLA fabrics were cut into a rectangular shape with dimensions of 30 mm × 5 mm and a thickness of about 150–200 μm. At least five samples were prepared and tested for each layer and condition. Mechanical properties such as Young's modulus and tensile strength at maximum were measured.
2.4.4. Molecular weight analysis
The number (Mn) and weight average (Mw) molar mass and polydispersity index (PDI) were measured by gel permeation chromatography (GPC) using chloroform as the solvent. A Waters Alliance e2695 HPLC system with Waters 2414 refractive index detector, equipped with 2 columns of Phenomenex linear (7.8 × 300 mm) together with guard column were used for the analysis. Chloroform was used as eluent with a flow rate of 0.8 mL/min and both the detector and column temperatures are 40 °C. One sample of each layer in the respective soaking solution at each soaking duration interval, and 1 sample of the respective worked-up basic soaking solution at each time interval was tested for GPC.
2.4.5. Filtration efficiency tests
The 3-ply PLA masks before and after degradation under acid, neutral and basic conditions for 4 weeks were dried and subjected to filtration efficiency tests, where 3 sample under each conditions were tested. The bacterial filtration efficiency (BFE) was performed at testing organisation TUV-SUD using an aerosol of Staphylococcus aureus according to the ASTM F2101-19 standard test method. The inside of the mask was challenged with the bacteria, and the mean particle size of the challenge aerosol was 3 μm ± 0.3 μm. Statistical testing was performed using ANOVA and Bonferroni test. The particle filtration efficiency (PFE) test of mask samples were conducted in accordance to ASTM F2299 by penetration of 0.1 μm polystyrene latex spheres. The effective sample size was about 45.6 cm2 and the flow rate was controlled around 28.3 L/min. All samples were tested under fixed conditions with 1 min sampling time. Five upstream and downstream aerosol counts were obtained and averaged per sample. PFE can be calculated with the following equation:
3. Results and discussion
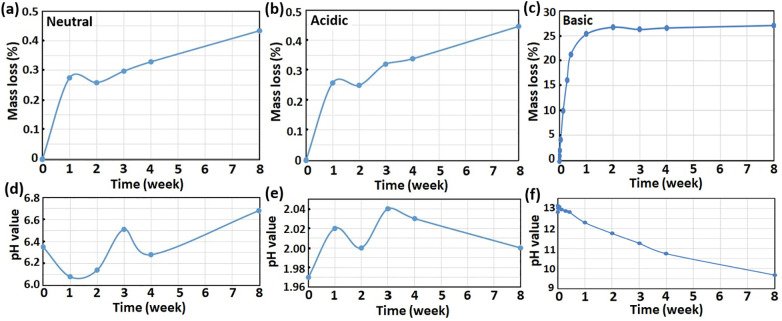
The 3-ply PLA face mask evaluated in this study was fabricated in a spunbond-meltblown-spunbond (SMS) PLA fabric layered design, as illustrated in Fig. S1. The outer and inner layers (0.168–0.184 mm in thickness) were made of random spunbond PLA fibers with diameters between 14 and 17 μm, and the middle layer (0.137 to 0.149 mm in thickness) was made of meltblown PLA non-woven fabric with smaller fiber diameters of 3 to 7 μm. The 3-ply masks were soaked in neutral (pH ~ 7), acidic (pH ~ 2) and basic (pH ~ 13) solutions at room temperature, and the mask's weight loss and change in pH value of the soaking solutions were monitored at specific time intervals for up to 8 weeks. As shown in Fig. 1a–c, it was apparent that the hydrolytic degradation rate in basic condition was faster, with a sharp weight loss of 25% in the first week that tapers off for the rest of the test duration. The pH dropped gradually from 13 to 9.6 during the degradation period of 8 weeks (Fig. 1f). In comparison, negligible differences were observed for the weight loss anf pH change in both neutral and acidic pH conditions. Similar pH-dependent degradation behavior for PLA was also reported by Łysik et al. (2019) and Xu et al. (2011). It has been proposed that the hydrolytic degradation of PLA occurred mainly by cleavage of ester linkage in the presence of water (Huang et al., 2014; Oliveira et al., 2016), generating more hydroxyl and carboxyl end groups that can improve the hydrophilicity of the PLA surface and promote further degradation. Also due to the increased amount of end carboxyl groups, the pH value of the basic soaking solution decreased and the degradation rate slowed down.
Fig. 1.
Changes of the mask mass loss (a, b, c) and pH value of the hydrolytic soaking solutions (d, e, f) with different degradation periods under neutral (pH = 7), acid (pH = 2) and basic (pH = 13) conditions, respectively.
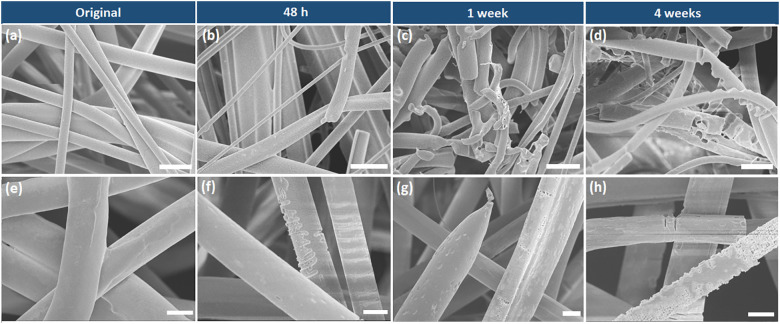
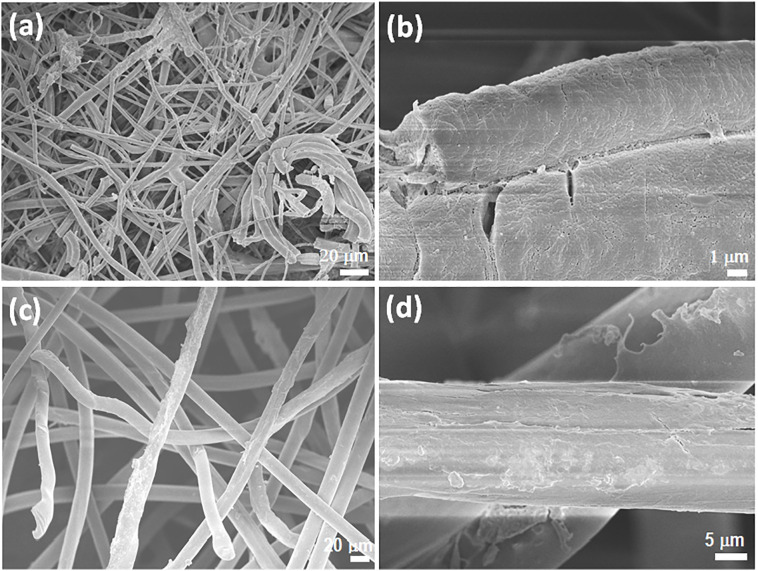
The morphology of the spunbond and meltblown mask layers throughout the degradation process under basic condition was monitored by SEM imaging. As depicted in Fig. 2a and e, both the spunbond and meltblown fibers exhibited smooth surface before the hydrolytic degradation process. Once the samples were subjected to alkaline hydrolysis, erosions can be seen distinctly on the surface of both the fibers after 48 h (Fig. 2b and f). Apart from surface erosion, cavities were also found on the spunbond fibers after one week (Fig. 2g and h). More severe corrosion and breakage was observed with the meltblown fibers with prolonged degradation time (Fig. 2c and d), due to the smaller diameter and larger specific surface area in contact with the degradation soaking solution.
Fig. 2.
Scanning electron microscopy (SEM) images of the meltblown layers (a–d) before and after degradation under basic conditions for 48 h, 1 week and 4 weeks (scale bar: 10 μm). SEM images of the spunbond layers (e–h) before and after degradation under basic conditions for 48 h, 1 week and 4 weeks (scale bar: 10 μm).
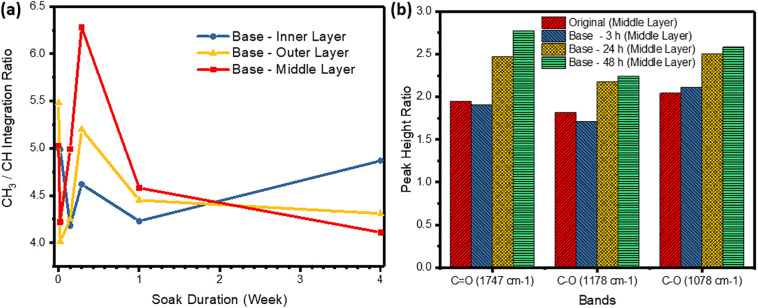
For better understanding of the degradation mechanism, 1H NMR analysis was performed for the different mask layers soaked in the respective pH solutions, and the chemical shifts (δ) and integration data can be found in Table S1. The chemical shift values were consistent with that of PLA pellets (Oliveira et al., 2016; Carrasco et al., 2010; Liu et al., 2006), where the values for the –CH3 and –CH protons were between the range of 1.56–1.59 ppm and 5.14–5.18 ppm respectively. No change in chemical shift values was observed for the mask layers at different soak duration and soaking pH. However, integration ratio of CH3/CH proton area changed with soak duration due to degradation of the PLA polymer chain. The theoretical CH3/CH integration ratio for PLA is 3, however the different layers of an original PLA mask has a ratio of more than 5. This could be due to pyrolytic elimination taking place in PLA during the melt blowing manufacturing process, where -CH-CH3 degrades to -CH=CH2 (Scheme S1a) (Carrasco et al., 2010) at high temperatures of 200 °C (Vadas et al., 2018). Also with different processing methods of face masks (Liao et al., 2021) to meet safety standards or confer different properties (Patil et al., 2021; Hiragond et al., 2018), lactic acid within the polymer chain may exist in the enol or enolate form (Scheme S1b) which reduces the amount of CH present in the structure, causing the CH3/CH integration ratio to be greater than 3. For the middle layers soaked in basic condition (pH ~ 13), a clear trend was observed for the CH3/CH integration ratio where there was an initial decrease from original value, followed by a rapid increase for the first 48 h, and a slow decrease over the course of 4 week Fig. 3 (a). The initial decrease could be attributed to rapid hydrolysis of fragments containing enol or enolate form near the ends of the PLA chains (Scheme S1c). This decreases the amount of CH3 group in the main PLA chain, causing the integration ratio to decrease. As time progresses, random chain scission (Oliveira et al., 2016; Carrasco et al., 2010) may start to dominate and cause an increase in integration ratio in 48 h. But with prolonged soaking in the basic solution for up to 4 weeks, the PLA fibers become thinner (Fig. 2d), thus exposing more enol/enolate fragments to hydrolysis which cause the integration to fall gradually with time. Degradation of the outer and inner mask layers appear to be unaffected by soak duration with no observable trend in the CH3/CH integration ratio Table S1. This could be due to thicker spunbond PLA fibers in these layers coupled with non-uniform thickness distributed across the surface, resulting in uneven degradation. For mask layers soaked in acid and neutral conditions, gradual hydrolysis of enol/enolate fragments resulted in an overall decrease in integration ratio Table S2.
Fig. 3.
(a) Integration ratio (CH3/CH) with respect to soak duration for polylactic acid (PLA) mask layer samples. (b) Fourier transform infrared spectroscopy (FTIR) peak height ratio for the respective characteristic stretching vibration bands of PLA in the middle layer.
The degradation due to structural changes in PLA by oxidation was further analyzed by FTIR. The characteristic FTIR stretching peaks of PLA are C O stretching (around 1747 cm−1) and C—O stretching (around 1178 cm−1 and 1078 cm−1) bands of its ester group (Fig. S2). To compare the layers at different soak duration, peak height ratio of the three bands can be calculated against at reference peak at 1455 cm−1 (Oliveira et al., 2016; Sabnis and Block, 1997), which corresponds to the CH3 bending. As seen in Fig. 3b, peak height ratios for the bands involving with the ester groups increased in the first 48 h, indicating formation of new carbonyl compounds. This would probably be due to hydrolysis and oxidation, coupled with other degradation processes such as random chain scission and β elimination. Nevertheless, since there was no significant increase in the –OH band (Fig. S2), degradation of the PLA mask would be mainly due to random chain scission.
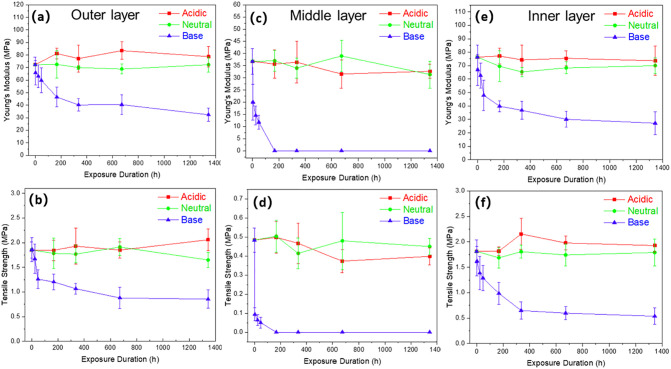
The influence of various PH conditions with different exposure durations to the mechanical performance of the three mask layers were evaluated and the results are summarized in Table S3. As shown in Fig. 4 , The mechanical properties of the outer and inner layers (spun-bonded) show higher modulus and tensile strength than the middle layer (melt-blown), attributed to the more robust and thicker fibers produced by the spun-bonded process (Dharmalingam et al., 2015; Yeo et al., 2020). As observed in Fig. 4a and b, all the three layers within the mask exhibited high resistance to degradation under neutral conditions (pH = 7), evinced by the unchanged mechanical performance over eight weeks. A similar trend was observed with the mask under acidic conditions (pH = 2) for over eight weeks, with unaffected mechanical performance as shown in Fig. 4c and d. It was reported that the degradation rates of solid polylactide samples were similar between pH 0 to pH 7. They were ineffectual since both the H+ and OH− ions from the testing medium have very low solubility in polymers (Lyu and Untereker, 2009). On the contrary, the mask under basic condition displayed a massive deterioration in mechanical performance over eight weeks, as illustrated in Fig. 4e and f. The modulus and tensile strength of both the outer and inner layer reduce by 8.9% and 12.4%, respectively, after 3 h of exposure. It further decreased by 55.2% and 64.3%, respectively, after eight weeks, attributed to the hydrolytic degradation. In comparison, the middle layer showed a more significant decline in mechanical performance. In addition, the thinner and shorter fibrous network allowed ions to diffuse readily into the structure, which further exacerbates and accelerates the degradation process (Elsawy et al., 2017). The modulus and tensile strength were reduced by 68% and 89.5% respectively, after 48 h of exposure. Subsequent exposure caused the layer to disintegrate physically, and it could not be evaluated mechanically. It was worth noting that the degradation in basic conditions could mimic the ocean, which is slightly alkaline. This could be a potential solution to adverse marine pollution caused by inconsiderate disposal of the existing non-biodegradable mask that frequently ends up in the ocean (Manfra et al., 2021).
Fig. 4.
Young's modulus and tensile strength were obtained from the tensile test of (a, b) outer layer, (c, d) middle layer, and (e, f) inner layer of the polylactic acid (PLA) facial mask.
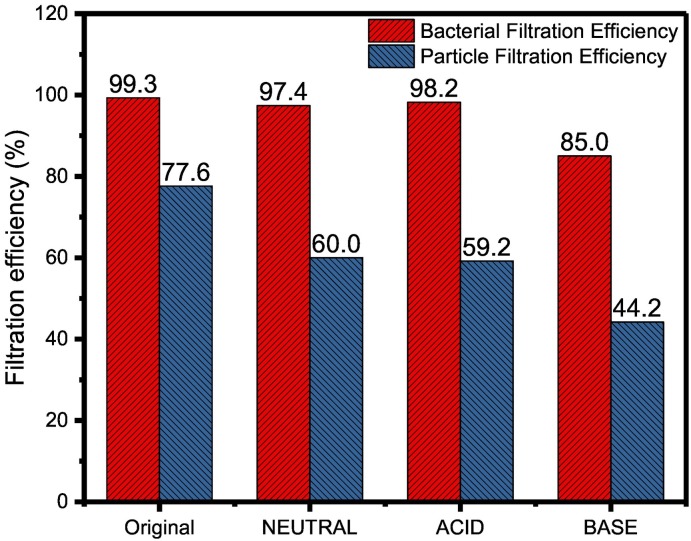
The bacterial filtration efficiency (BFE) and particulate filtration efficiency (PFE) of these PLA masks before and after degradation under different pH conditions over a period of 4 weeks were also evaluated using the standard methods. The BFE test validates how well the masks were able to block the release of aerosols and therefore is important in the regulation of medical masks (Pourchez et al., 2021). As shown in Fig. 5 , before degradation, these masks exhibited a high BFE value (> 99%) and moderate PFE. After hydrolytic degradation in neutral and acidic conditions for 4 weeks, BFE decreased by an insignificant amount of 1–2% for masks. However, a substantial drop in BFE by about 14% was observed for masks under basic conditions. The masks were challenged with bacteria droplets which are approximately 3 μm in size, therefore in addition to mask protection effect, it also gives an idea of the porosity of the mask. While the original mask and masks at pH 2 and pH 7 mostly retained a dense network structure and completely blocked micron sized particles, the mask at pH 13 showed degradation and became partially porous to 3 μm particles.
Fig. 5.
Filtration efficiency of the polylactic acid (PLA) masks before and after degradation under different pH conditions for 4 weeks.
In the case of PFE, where masks were bombarded with particles of even smaller size of 0.1 μm (polystyrene latex spheres), the original face masks exhibit only a moderate PFE of 77.6%. After 4 weeks of hydrolytic degradation, PFE decreased significantly by an average of 23% for masks under neutral and acidic conditions, and the drop almost doubled for masks under alkaline condition to a PFE of 44%. The degradation of mask layers greatly affected its filtration efficiency for small sized particle.
PLA has been reported to be fully biodegradable when composted in a large-scale operation with temperatures of 60 °C and above. In the biodegradation process, PLA was first hydrolyzed into water-soluble compounds and lactic acid, and the hydrolyzed products can undergo metabolisation into carbon dioxide, water and biomass by a variety of microorganisms (Yagi et al., 2009; Fukushima et al., 2009; Pinto et al., 2016; Napper and Thompson, 2019). The biodegradation behavior of these PLA masks was also investigated in the presence of sewage sludge under ambient temperature using cellulose as controls. The biodegradation process can be monitored by SEM imaging and measurements of weight loss and carbon loss. The morphology of the spunbond and meltblown mask layers after biodegradation for 4 weeks was observed by SEM analysis. As can be seen in Fig. 6c–d, the spunbond fiber surface became very rough after 4 weeks. In comparison, numerous cracks and corrosion holes were observed on the meltblown fiber surface (Fig. 6a–b), apparently showing that degradation of PLA. As shown in Table S4, the average weight loss and carbon loss of the cellulose controls are 100% and 79.4%, respectively. After immersed in sewage sludge for 4 weeks, the spunbond layers demonstrated an average weight loss and carbon loss of 12.6% and 12.5%, respectively. Higher degree of degradation was observed for the meltblown layers, with an enhanced average weight loss and carbon loss of 25.8% and 25.7%, respectively. This means that over 70% of the materials within the face mask remained. However, the part that was measured by the weight loss value indicates the amount of sample that is hydrolyzed and solubilized by the microorganisms, whereas the carbon loss value indicates the amount of sample that was bio-mineralized into carbon dioxide. It is worth mentioning that the biodegradation testing results suggest that although small fractions of the mask materials were solubilized after four weeks, but such fragments were completely bio-mineralized. Therefore, the materials do not result in secondary formation of microplastics and generation of non-biodegradable soluble compounds in the environment.
Fig. 6.
Scanning electron microscopy (SEM) images of the meltblown (a and b) and spunbond (c and d) mask layer after biodegradation in the presence of sewage sludge under room temperature for 4 weeks.
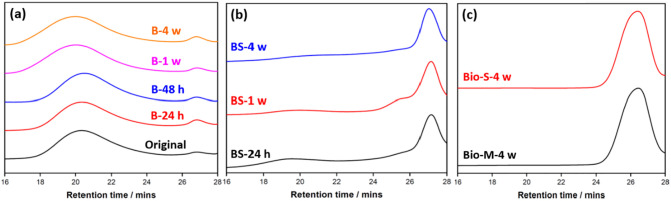
To further demonstrate the degradation process, GPC analysis was employed to determine the changes in molecular weight of the PLA mask layers and the results are illustrated in Table 1 . The solid meltblown layers before and after different hydrolytic degradation periods (0 h, 24 h, 48 h, 1 week and 4 weeks) in basic conditions were collected and dissolved in chloroform for GPC analysis. Although the mass loss of the meltblown layer increased with the extension of degradation time, the molecular weights of the solid residues were almost unchanged, which were all in 35,000–40,000 Da range. However, it was worth noting that after 24 h, the amount of PLA oligomer (Mw ~ 800 Da) in all samples increased obviously (Fig. 7a), which clearly demonstrated the mask degradation. The basic solution residue samples after different degradation periods have also been tested to determine the molecular weights of the dissolved PLA oligomers (Fig. 7b). Compared to the results of solid residues, the molecular weights of solution residues dropped dramatically with the increase of the degradation time. After 4 weeks, only low molecular weight PLA oligomers with Mw~ 500 Da were detected, which indicated the complete degradation of the dissolved meltblown mask layers. These GPC results illustrated that undissolved mask residue still retained the large molecular weights, while the dissolved oligomers could fully degrade to small fragments in a short span of period. The spunbond and meltblown mask layers were individually subjected to biodegradation process, and the soaking medium samples were also collected after 4 weeks and measured by GPC respectively (Fig. 7c). Both samples showed low molecular weights of ~1300 Da after 4 weeks, which demonstrated the successful degradation of both mask layers to small oligomer fragments, which will not cause the secondary contamination.
Table 1.
Gel permeation chromatography (GPC) testing results.
| Sample name | Mn (Da) |
Mw (Da) |
PDI |
|||
|---|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 1 | Peak 2 | Peak 1 | Peak 2 | |
| Original | 39,000 | 810 | 74,000 | 870 | 1.9 | 1.1 |
| B-24 h | 40,000 | 800 | 74,000 | 850 | 1.9 | 1.1 |
| B-48 h | 35,000 | 840 | 67,000 | 930 | 1.9 | 1.1 |
| B-1w | 44,000 | 820 | 106,000 | 910 | 2.4 | 1.1 |
| B-4w | 44,000 | 850 | 111,000 | 950 | 2.5 | 1.1 |
| BS-24 h | 85,000 | 820 | 119,000 | 970 | 1.4 | 1.2 |
| BS-1w | 74,000 | 1100 | 102,000 | 1600 | 1.4 | 1.5 |
| BS-4 w | − | 470 | − | 560 | − | 1.2 |
| Bio-S-4w | 1300 | 1700 | 1.3 | |||
| Bio-M-4w | 1300 | 1700 | 1.3 | |||
Fig. 7.
Gel permeation chromatography (GPC) profiles of (a) the meltblown layer before and after hydrolytic degradation in basic condition for different periods; (b) the basic solution residues (BS) associated with the meltblown layer after different degradation periods; (c) the biodegradation soaking mediums of the spunbond (Bio—S) and meltblown (Bio-M) layers after 4 weeks.
4. Conclusions and outlook
The degradability of PLA-derived 3-ply face masks was investigated for hydrolytic and bio-degradation, and the results showed that it was mainly the middle meltblown layer that degrades, and most significantly under basic conditions. Smaller meltblown fiber diameters gives the middle layer a larger specific surface area in contact with the degradation soaking solution compared to thicker spunbond fibers. Hence with intrinsic hydrolytic property of the ester group in the PLA, both hydrolysis of enol or enolate chain ends and random chain scission in basic conditions occurs rapidly within the first week of exposure, and tapers off thereafter. Mechanical strength of the PLA masks also dropped by more than 50% at the end of 8 weeks, and more significantly so for the middle meltblown layer, but remains generally unaffected for mask under neutral and acidic conditions. Therefore structure of PLA masks can be deemed as severely weakened under alkaline environments, however this degradation only reduced the mass of the mask by a stagnating of about 27% at the end of 8 weeks, and is insufficient to consider as a full degradation of the PLA mask. Hydrolytic degradation therefore aids in the process for PLA degradation, and would require other factors to increase its degradability. An interesting point to note was that the protective and filtration effectiveness of PLA masks under such environment was greatly reduced, with BFE and PFE dropping by 14% and 43% respectively after 4 weeks. Hence care must be taken to keep PLA masks out of alkaline atmospheres to prevent reduced efficiency of new masks. Biodegradation test under OECD 301F specification showed only a small fraction of mask materials solubilized, with meltblown layer degradation double that of spunbond layer, having an average weight loss and carbon loss of 25.8% and 25.7% respectively. Though such levels of biodegradability is not ideal to solve the pollution problem, and it does not reduce the carbon dioxide emissions into the atmosphere, it is comforting to know that the solubilized fragments completely bio-mineralized without formation of secondary microplastic pollution.
In light of the above studies, PLA-derived face masks displayed limited potential to salvage the problem of plastic pollution that emerges during this COVID pandemic. The dissolution of the polymers in basic environment render the hydrolytic conditions to be very specific. The use of biological methods to degrade these materials appear to be slow and will eventually result in the release of carbon dioxide. The ability of PLA masks to degrade under basic conditions and in the presence of micro-organisms without causing secondary contamination provides a possible temporary solution to the growing demand for face masks for prevention and protection. Nevertheless future research could gear towards enhancing the rate of degradation or have more conditions that allow for disintegration of the mask material. The current research work will open a new gate to resolve the severe challenge of face mask pollution during the COVID-19 pandemic. However, much work remains to be done.
CRediT authorship contribution statement
Xiang Yun Debbie Soo: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft. Suxi Wang: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft. Chee Chuan Jayven Yeo: Methodology, Writing – review & editing. Jiuwei Li: Methodology, Writing – review & editing. Xi Ping Ni: Methodology, Writing – review & editing. Lu Jiang: Methodology, Writing – review & editing. Kun Xue: Writing – review & editing, Supervision, Project administration, Funding acquisition. Zibiao Li: Writing – review & editing, Supervision, Project administration, Funding acquisition. Xunchang Fei: Writing – review & editing, Supervision, Project administration, Funding acquisition. Qiang Zhu: Writing – review & editing, Supervision, Project administration, Funding acquisition. Xian Jun Loh: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the financial support from the Agency for Science, Technology and Research (A*STAR), Science and Engineering Research Council for this work (Grant No.: GAP/2019/00314).
Editor: Damià Barceló
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.151084.
Appendix A. Supplementary data
Supplementary material contains schematic of possible mask degradation mechanisms, characterization of masks after degradation and biodegradation test set up information.
References
- Abbasi S.A., Khalil A.B., Arslan M. Extensive use of face masks during COVID-19 pandemic:(micro-) plastic pollution and potential health concerns in the arabian peninsula. Saudi J. Biol. Sci. 2020;27(12):3181–3186. doi: 10.1016/j.sjbs.2020.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu Valapa R., Pugazhenthi G., Katiyar V. Hydrolytic degradation behaviour of sucrose palmitate reinforced poly (lactic acid) nanocomposites. Int. J. Biol. Macromol. 2016;89:70–80. doi: 10.1016/j.ijbiomac.2016.04.040. [DOI] [PubMed] [Google Scholar]
- Balla E., Daniilidis V., Karlioti G., Kalamas T., Stefanidou M., Bikiaris N.D., Vlachopoulos A., Koumentakou I., Bikiaris D.N. Poly (lactic acid): a versatile biobased polymer for the future with multifunctional properties—from monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers. 2021;13(11):1822. doi: 10.3390/polym13111822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson N.U., Fred-Ahmadu O.H., Bassey D.E., Atayero A.A. COVID-19 pandemic and emerging plastic-based personal protective equipment waste pollution and management in Africa. J. Environ. Chem. Eng. 2021;9(3) doi: 10.1016/j.jece.2021.105222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco F., Pagès P., Gámez-Pérez J., Santana O., Maspoch M.L. Processing of poly (lactic acid): characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010;95(2):116–125. [Google Scholar]
- Chellamani K., Veerasubramanian D., Balaji R.V. Surgical face masks: manufacturing methods and classification. 2013;2(6):320–324. [Google Scholar]
- Choi S., Jeon H., Jang M., Kim H., Shin G., Koo J.M., Lee M., Sung H.K., Eom Y., Yang H.S. Biodegradable, efficient, and breathable multi-use face mask filter. Adv. Sci. 2021;8(6):2003155. doi: 10.1002/advs.202003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua M.H., Cheng W.R., Goh S.S., Kong J.H., Li B., Lim J.Y.C., Mao L., Wang S.X., Xue K., Yang L., Ye E.Y., Zhang K.Y., Cheong W.C.D., Tan B.H., Li Z.B., Tan B.H., Loh X.J. Face masks in the new COVID-19 normal: materials. 2020;2020:7286735. doi: 10.34133/2020/7286735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua M.H., Cheng W., Goh S.S., Kong J., Li B., Lim J.Y., Mao L., Wang S., Xue K., Yang L. Face masks in the new COVID-19 normal: materials, testing, and perspectives. Research. 2020;2020 doi: 10.34133/2020/7286735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G., Gaudio M.T., Lopresto C.G., Calabro V., Curcio S., Chakraborty S. Bioplastic from renewable biomass: a facile solution for a greener environment. Earth Syst. Environ. 2021:1–21. [Google Scholar]
- Daniel D., Lin M., Luhung I., Lui T., Sadovoy A., Koh X., Sng A., Tran T., Schuster S.C., Jun Loh X., Thet O.S., Tan C.K. Effective design of barrier enclosure to contain aerosol emissions from COVID-19 patients. Indoor Air. 2021;31(5):1639–1644. doi: 10.1111/ina.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-la-Torre G.E., Rakib M.R.J., Pizarro-Ortega C.I., Dioses-Salinas D.C. Occurrence of personal protective equipment (PPE) associated with the COVID-19 pandemic along the coast of Lima,Peru. 2021;774 doi: 10.1016/j.scitotenv.2021.145774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmalingam S., Hayes D.G., Wadsworth L.C., Dunlap R.N., DeBruyn J.M., Lee J., Wszelaki A.L. Soil degradation of polylactic acid/polyhydroxyalkanoate-based nonwoven mulches. J. Polym. Environ. 2015;23(3):302–315. [Google Scholar]
- Dharmaraj S., Ashokkumar V., Hariharan S., Manibharathi A., Show P.L., Chong C.T., Ngamcharussrivichai C. The COVID-19 pandemic face mask waste: a blooming threat to the marine environment. Chemosphere. 2021;272 doi: 10.1016/j.chemosphere.2021.129601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din M.I., Ghaffar T., Najeeb J., Hussain Z., Khalid R., Zahid H. Potential perspectives of biodegradable plastics for food packaging application-review of properties and recent developments. 2020;37(4):665–680. doi: 10.1080/19440049.2020.1718219. [DOI] [PubMed] [Google Scholar]
- Elsawy M.A., Kim K.-H., Park J.-W., Deep A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sust. Energ. Rev. 2017;79:1346–1352. [Google Scholar]
- Fan X.S., Tan B.H., Li Z.B., Loh X.J. Control of PLA stereoisomers-based polyurethane elastomers as highly efficient shape memory materials. ACS Sustain. Chem. Eng. 2017;5(1):1217–1227. [Google Scholar]
- Fan X.S., Win K.Y., Hu Z.G., Loh X.J., Li Z.B. Precise synthesis of PS-PLA Janus star-like copolymer. Macromol. Rapid Commun. 2019;40(5) doi: 10.1002/marc.201800217. [DOI] [PubMed] [Google Scholar]
- Fukushima K., Abbate C., Tabuani D., Gennari M., Camino G. Biodegradation of poly (lactic acid) and its nanocomposites. Polym. Degrad. Stab. 2009;94(10):1646–1655. [Google Scholar]
- Garcia R.A., Stevanovic T., Berthier J., Njamen G., Tolnai B., Achim A. Cellulose, nanocellulose, and antimicrobial materials for the manufacture of disposable face masks: a review. Bioresources. 2021;16(2) [Google Scholar]
- Garlotta D. A literature review of poly (lactic acid) J. Polym. Environ. 2001;9(2):63–84. [Google Scholar]
- Ghosh K., Jones B.H. Roadmap to biodegradable plastics—current state and research needs. ACS Sustain. Chem. Eng. 2021;9(18):6170–6187. [Google Scholar]
- Hasan N.A., Bashar A., Heal R.D., Haque M.M. Available at SSRN 3722737. 2020. Face masks-protecting the wearer but neglecting the aquatic environment? [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Gao M., Illés B., Molnar K. 3D printed and electrospun, transparent, hierarchical polylactic acid mask nanoporous filter. Int. J. Bioprinting. 2020;6(4) doi: 10.18063/ijb.v6i4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiragond C.B., Kshirsagar A.S., Dhapte V.V., Khanna T., Joshi P., More P.V. Enhanced anti-microbial response of commercial face mask using colloidal silver nanoparticles. Vacuum. 2018;156:475–482. [Google Scholar]
- Höglund A., Odelius K., Albertsson A.-C. Crucial differences in the hydrolytic degradation between industrial polylactide and laboratory-scale poly (L-lactide) ACS Appl. Mater. Interfaces. 2012;4(5):2788–2793. doi: 10.1021/am300438k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Ge F., Zhou Y., Jiang L., Dan Y. Hydrolytic behavior of poly (lactic acid) films with different architecture modified by poly (dodecafluorheptyl methylacrylate) Euro. Polym. J. 2014;59:189–199. [Google Scholar]
- Liao M., Liu H., Wang X., Hu X., Huang Y., Liu X., Brenan K., Mecha J., Nirmalan M., Lu J.R. A technical review of face mask wearing in preventing respiratory COVID-19 transmission. Curr. Opin. Colloid Interface Sci. 2021;101417 doi: 10.1016/j.cocis.2021.101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zou Y., Li W., Cao G., Chen W. Kinetics of thermo-oxidative and thermal degradation of poly (D, L-lactide)(PDLLA) at processing temperature. Polym. Degrad. Stab. 2006;91(12):3259–3265. [Google Scholar]
- Łysik D., Mystkowska J., Markiewicz G., Deptuła P., Bucki R. The influence of mucin-based artificial saliva on properties of polycaprolactone and polylactide. Polymers. 2019;11(11):1880. doi: 10.3390/polym11111880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu S., Untereker D. Degradability of polymers for implantable biomedical devices. Int. J. Mol. Sci. 2009;10(9):4033–4065. doi: 10.3390/ijms10094033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfra L., Marengo V., Libralato G., Costantini M., De Falco F., Cocca M. Biodegradable polymers: A real opportunity to solve marine plastic pollution? J. Hazard. Mater. 2021;416 doi: 10.1016/j.jhazmat.2021.125763. [DOI] [PubMed] [Google Scholar]
- Mehta R., Kumar V., Bhunia H., Upadhyay S. J. Macromol. Science, Part CPolym. Rev. 2005;45(4):325–349. [Google Scholar]
- Morganti P., Morganti G. Post-COVID-19: an opportunity to produce biodegradable goods & surgical masks to save the environment. J. Health Care Res. 2020;1(3):157. [Google Scholar]
- Morganti P., Yudin V.E., Morganti G., Coltelli M.-B. Trends in surgical and beauty masks for a cleaner environment. Cosmetics. 2020;7(3):68. [Google Scholar]
- Müller F., Jokisch S., Bargel H., Scheibel T. Centrifugal electrospinning enables the production of meshes of ultrathin polymer fibers. ACS Appl. Polym. Mater. 2020;2(11):4360–4367. [Google Scholar]
- Mungcal A.K., Tampus C.S., Tan C., Fogarty D., Pearlman J., Yulisman L., Ng H., Dancel R., Mohan R., Chen S., Adeline S. The Straits Times. Singapore Press Holdings; Singapore: 2021. Three million masks every minute: how Covid-19 is choking the planet. [Google Scholar]
- Napper I.E., Thompson R.C. Environmental deterioration of biodegradable, oxo-biodegradable, compostable, and conventional plastic carrier bags in the sea, soil, and open-air over a 3-year period. Environ. Sci. Technol. 2019;53(9):4775–4783. doi: 10.1021/acs.est.8b06984. [DOI] [PubMed] [Google Scholar]
- Oliveira M., Santos E., Araújo A., Fechine G.J., Machado A.V., Botelho G. The role of shear and stabilizer on PLA degradation. Polym. Test. 2016;51:109–116. [Google Scholar]
- Ooi C.C., Suwardi A., Yang Z.L.O., Xu G., Tan C.K.I., Daniel D., Li H., Ge Z., Leong F.Y., Marimuthu K., Ng O.T., Lim S.B., Lim P., Mak W.S., Cheong W.C.D., Loh X.J., Kang C.W., Lim K.H. Risk assessment of airborne COVID-19 exposure in social settings. Phys. Fluids. 2021;33(8) doi: 10.1063/5.0055547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri S., Pierpaoli M., Riderelli L., Qi S., Ruello M.L. Preparation and characterization of an electrospun PLA-cyclodextrins composite for simultaneous high-efficiency PM and VOC removal. J. Compos. Sci. 2020;4(2):79. [Google Scholar]
- Pan L., Wang C., Jin H.R., Li J., Yang L., Zheng Y.J., Wen Y.G., Tan B.O., Loh X.J., Chen X.D. Lab-on-mask for remote respiratory monitoring. ACS Mater. Lett. 2020;2(9):1178–1181. doi: 10.1021/acsmaterialslett.0c00299. [DOI] [PubMed] [Google Scholar]
- Patil N.A., Gore P.M., Prakash N.J., Govindaraj P., Yadav R., Verma V., Shanmugarajan D., Patil S., Kore A., Kandasubramanian B. Needleless electrospun phytochemicals encapsulated nanofibre based 3-ply biodegradable mask for combating COVID-19 pandemic. Chem. Eng. J. 2021;416 doi: 10.1016/j.cej.2021.129152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A.M., Gonçalves C., Gonçalves I.C., Magalhães F.D. Effect of biodegradation on thermo-mechanical properties and biocompatibility of poly (lactic acid)/graphene nanoplatelets composites. Eur. Polym. J. 2016;85:431–444. [Google Scholar]
- Pourchez J., Peyron A., Montigaud Y., Laurent C., Audoux E., Leclerc L., Verhoeven P.O. New insights into the standard method of assessing bacterial filtration efficiency of medical face masks. Sci. Rep. 2021;11(1):1–11. doi: 10.1038/s41598-021-85327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai P., Mehrotra S., Priya S., Gnansounou E., Sharma S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021;124739 doi: 10.1016/j.biortech.2021.124739. [DOI] [PubMed] [Google Scholar]
- Roberts K.P., Bowyer C., Kolstoe S., Fletcher S. Coronavirus face masks: an environmental disaster that might last generations, The Conversation. 2020. https://theconversation.com/coronavirus-face-masks-an-environmental-disaster-that-might-last-generations-144328
- Sabnis S., Block L.H. Improved infrared spectroscopic method for the analysis of degree of N-deacetylation of chitosan. Polym. Bull. 1997;39(1):67–71. [Google Scholar]
- Selvaranjan K., Navaratnam S., Rajeev P., Ravintherakumaran N. Environmental challenges induced by extensive use of face masks during COVID-19: a review and potential solutions. Environ. Chang. 2021;3 doi: 10.1016/j.envc.2021.100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi M., Zinjarde S., Gokhale D. Polylactic acid: synthesis and biomedical applications. J. Appl. Microbiol. 2019;127(6):1612–1626. doi: 10.1111/jam.14290. [DOI] [PubMed] [Google Scholar]
- Siracusa V., Blanco I., Romani S., Tylewicz U., Rocculi P., Rosa M.D. Poly (lactic acid)-modified films for food packaging application: physical, mechanical, and barrier behavior. J. Appl. Polym. Sci. 2012;125(S2):E390–E401. [Google Scholar]
- Song J., Murphy R., Narayan R., Davies G. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B. 2009;364(1526):2127–2139. doi: 10.1098/rstb.2008.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwardi A., Ooi C.C., Daniel D., Tan C.K., Li H.Y., Liang O.Y.Z., Tang Y.K., Chee J.Y., Sadovoy A., Jiang S.Y., Ramachandran S., Ye E.Y., Kang C.W., Cheong W.C.D., Lim K.H., Loh X.J. The efficacy of plant-based ionizers in removing aerosol for COVID-19 mitigation. Research. 2021;2021:2173642. doi: 10.34133/2021/2173642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres F.G., De-la-Torre G.E. Face mask waste generation and management during the COVID-19 pandemic: an overview and the peruvian case. Sci. Total Environ. 2021;147628 [Google Scholar]
- Vadas D., Kmetykó D., Marosi G., Bocz K. Application of melt-blown Poly (lactic acid) fibres in self-reinforced composites. Polymers. 2018;10(7):766. doi: 10.3390/polym10070766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E.G., Ren Z.J. Preventing masks from becoming the next plastic problem. Front. Environ. Sci. Eng. 2021;15(6):125. doi: 10.1007/s11783-021-1413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Crawford K., Gorman C.B. Effects of temperature and pH on the degradation of poly (lactic acid) brushes. Macromolecules. 2011;44(12):4777–4782. [Google Scholar]
- Yagi H., Ninomiya F., Funabashi M., Kunioka M. Anaerobic biodegradation tests of poly (lactic acid) under mesophilic and thermophilic conditions using a new evaluation system for methane fermentation in anaerobic sludge. Int. J. Mol. Sci. 2009;10(9):3824–3835. doi: 10.3390/ijms10093824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo J.C.C., Kai D., Teng C.P., Lin E.M.J.R., Tan B.H., Li Z., He C. Highly washable and reusable green nanofibrous sorbent with superoleophilicity, biodegradability, and mechanical robustness. ACS Appl. Polym. Mater. 2020;2(11):4825–4835. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material contains schematic of possible mask degradation mechanisms, characterization of masks after degradation and biodegradation test set up information.