Abstract
Human immunodeficiency virus (HIV) pre-exposure prophylaxis (PrEP) and buprenorphine decrease HIV acquisition. Between November, 2016 - July, 2017, we surveyed persons (N=200) at a drug detoxification center to assess their interest in PrEP and in buprenorphine, and to examine factors associated with such interests. Over the previous 6 months, 58% (117/200) injected drugs, 87% (173/200) used opioids, 50% (85/171) had condomless sex. Only 22% (26/117) of persons who injected drugs were aware of PrEP, yet 74% (86/116) and 72% (84/116) were interested in oral or injectable PrEP, respectively. Thirty-eight percent (47/125) of persons not receiving buprenorphine or methadone expressed interest in buprenorphine. After multivariable adjustment, Latinx ethnicity was associated with interest in PrEP (aOR: 3.80; 95% CI, 1.37–10.53), while male gender (aOR: 2.76; 95% CI, 1.21–6.34) was associated with interest in buprenorphine. Opportunities exist to implement PrEP and buprenorphine within drug detoxification centers.
Keywords: pre-exposure prophylaxis, persons who inject drugs, human immunodeficiency virus, medication for opioid use disorder, drug detoxification center
Introduction
Human immunodeficiency virus (HIV) pre-exposure prophylaxis (PrEP) and medications for opioid use disorder (MOUD) are evidence-based approaches to improve health outcomes in people who use drugs.1,2 PrEP decreases HIV incidence among persons who inject drugs (PWID)1 and is recommended by the Centers for Disease Control and Prevention (CDC) and the US Preventive Services Task Force (USPSTF) for HIV prevention among PWID.3,4 MOUD such as methadone and buprenorphine decrease morbidity and mortality among individuals with opioid use disorder.5–7 In addition, buprenorphine is associated with decreased drug-related HIV risk.8 As evidence-based tools, PrEP and MOUD should be integral to the ongoing efforts to decrease new HIV infections and overdose deaths among PWID during the opioid epidemic.9,10
Despite robust safety and efficacy data, PrEP and MOUD have not been widely adopted in real-world settings where individuals at high risk of substance use-related complications access care.11–14 Drug detoxification centers serve individuals with active substance use disorders and represent important touchpoints for individuals who may lack access to or feel uncomfortable in traditional clinical settings.15 Although drug detoxification centers are a potential venue to expand PrEP implementation for PWID and buprenorphine, little is known about the knowledge and interest in PrEP and buprenorphine among individuals accessing these facilities during the opioid epidemic.
Given the paucity of information on the feasibility and acceptability of PrEP among PWID, we undertook the current study to survey patients at a drug detoxification center to better understand their knowledge of and interest in HIV PrEP and outpatient medications for opioid use disorder.
Methods
2.1. Participants and setting
We conducted a single site randomized trial comparing the real-world case notification of rapid testing to that of laboratory testing for HIV and HCV at a drug detoxification center. The study was conducted at the Boston Treatment Center (BOSTC) between November, 2016 and July, 2017.16 The primary outcome was receipt of test results within two weeks. The survey for the current sub-analysis of the parent study was included as part of the initial questionnaire that we administered to all 200 patients included in the clinical trial.
BOSTC is the largest, short-term inpatient drug and alcohol detoxification center in the Boston metropolitan area, and its standard opioid withdrawal protocol during the study period was a 6day methadone or buprenorphine taper. BOSTC also had a case management program aimed at linking patients to substance use disorder treatment after discharge. The questionnaire focused on sex and drug use behaviors and overdose history. We also collected information on knowledge of and interest in HIV PrEP and buprenorphine. Eligibility criteria for the trial were as follows: 1) English-speaking; 2) 18 years or older; 3) Admission to BOSTC with a history of self-reported drug use; and 4) Willingness to provide locator information and sign a release of medical records form to enable collection of follow-up visit information from Boston Medical Center, a safety net hospital situated across from BOSTC. Persons reporting known HIV or HCV infection, and patients who had been tested for HIV and HCV within 6 months were excluded. Patients received a $20 gift card for completing the study. The study was approved by the Institutional Review Board of Boston University Medical Campus and a Certificate of Confidentiality was obtained from the National Institutes of Health.
2.2. Measures
A research assistant administered the questionnaire that collected information on knowledge and interest in PrEP and buprenorphine, including long-acting injectable forms of both. The questionnaire also collected demographics, substance use, psychiatric history (defined as having received a prescription for mental health disorder in the past 6 months), past HIV and HCV testing, sex and drug use behaviors, prior substance use treatment and overdose history. In terms of sexual history, we asked participants about the number and type of partners they had over the past 6 months as well as the frequency of condom use during the same time period. We also identified the proportion of patients with unstable housing, defined as living on the street or in an overnight shelter in the past 6 months.
Our primary outcomes of interest for this analysis was interest in PrEP and buprenorphine among BOSTC trial participants. We focused on buprenorphine because it is an MOUD that can be prescribed more easily in the outpatient setting with relatively lower barriers to linkage from drug detoxification centers when sufficient number of waivered prescribers exist in the locale. We also evaluated factors associated with patient interest in PrEP and buprenorphine. This study explores at-risk individuals’ interest in the adoption of these evidence-based tools while seeking care at drug detoxification centers. Nonfatal overdoses are surrogates of substance use disorder severity and are predictive of future fatal overdoses.17 As nonfatal overdoses represent potential windows of opportunity to engage patients in care, we present awareness and interest in PrEP in the subset of patients who had previously experienced a drug overdose.
2.3. Data Analysis
We used descriptive statistics to determine the proportion of individuals interested in PrEP and buprenorphine, separately. Variables significant in bivariate analysis and known potential confounders were included in logistic regression to determine factors associated with interest in PrEP and buprenorphine. We used 95% confidence intervals and all p-value significance levels were two-sided. Statistical significance was set at p < 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
A total of 341 individuals were screened for the parent randomized clinical trial and 200 participated, all of whom were included in this analysis. Patients who did not meet criteria for the randomized clinical trial were excluded for the following reasons: HIV or HCV testing within 6 months, 50% (71/141); refusal to take part in the study, 20% (28/141); unwilling or unable to share contact information, 17% (24/141); unable to sign a release form, 5% (7/141); HIV/HCV co-infection, 4% (5/141); non-English speaking, 3% (5/141); and age younger than 18 years <1% (1/141).
Among participants, mean [SD] age was 39 [10] years; 62% (124/76) were male; 45% (90/200) were White, 23% (46/200) Black, 23% (46/200) Latinx, 7% other (8/200) (Table 1). In addition, 10% (20/200) identified as gay or bisexual; 68% (136/200) had at least a high school level of education; and nearly 27% (55/100) had unstable housing.
Table 1.
Baseline characteristics of participants seen at a drug detoxification center (N=200)
| Characteristic | (N=200) | |
|---|---|---|
|
| ||
| Mean age (SD) --- years | 39 ± 10 | |
| Gender ---no. (%) | Female | 76 (38) |
| Male | 124 (62) | |
| Race/Ethnicity ---no. (%) | Latinx | 47 (23) |
| White | 90 (45) | |
| Black | 46 (23) | |
| Other | 17 (8) | |
| Education level ---no. (%) | < High School | 9 (5) |
| High School | 136 (68) | |
| > High School | 55 (27) | |
| Unstable Housing* ---no. (%) | 55 (27) | |
| Sexual Orientation ---no. (%) | Straight | 180 (90) |
| Gay | 7 (4) | |
| Bisexual | 13 (6) | |
| Substance use ---no. (%) | Heroin | 159 (80) |
| Sedative | 59 (30) | |
| Tranquilizers | 91 (46) | |
| Amphetamines | 57 (29) | |
| Prescription opioids | 80 (40%) | |
| Inhalants | 8 (4%) | |
| Cocaine/crack | 110 (55%) | |
| Hallucinogens | 8 (4%) | |
| Two or more substances | 158 (79%) | |
| Substance use services in past 6 mo. —no. (%) | 92 (46%) | |
| Prescription for mental health disorder in past 6 mo.---no. (%) | 70 (35%) | |
Defined as living on the street or in an overnight shelter in the past 6 months.
3.1. HIV risk behavior, knowledge and interest in HIV preexposure prophylaxis including injectable formulation
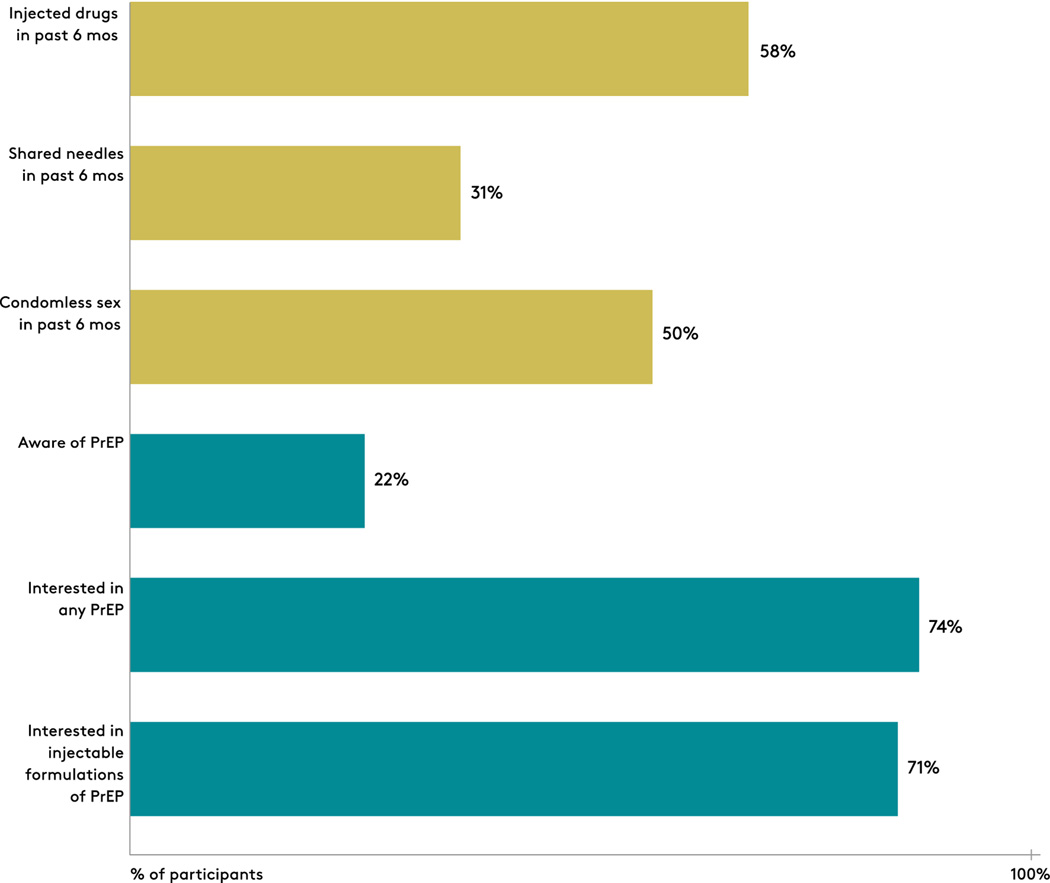
The cohort reported multiple risk factors for HIV acquisition (Figure 1). Over the past 6 months, 58% (117/200) injected drugs and 31% (63/200) shared needles. Of individuals who reported being sexually active over the past 6 months (n=171), 50% (85/171) had condomless sex. In addition, of individuals who reported injecting drugs in the past 6 months (n=117), only 22% (26/117) were aware of PrEP and only 15% (18/117) knew that PrEP was recommended for people who inject drugs. When provided information about PrEP as an HIV prevention method (n=116), 74% (86/116) provided an answer and expressed interest in receiving a drug to prevent HIV, and 72% (84/116) specifically indicated an interest in an injectable form of HIV PrEP. We determined awareness and interest in PrEP in the subgroup of patients who had experienced a nonfatal drug overdose and found that similar to the larger cohort, 24% (24/98) had heard of PrEP and 73% (71/97) were interested in PrEP.
Figure 1:
Human immunodeficiency virus (HIV) risk and interest in pre-exposure prophylaxis (PrEP) among participants recruited at a drug detoxification center.
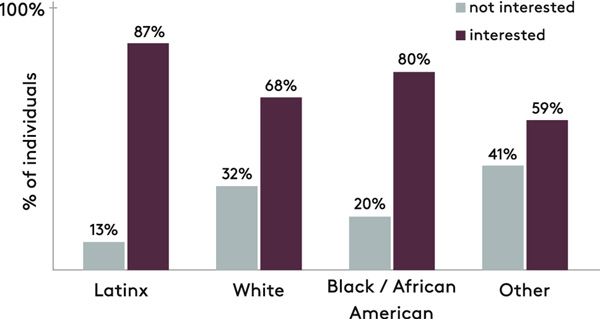
Given both sexual and injection risk behaviors in the cohort, we determined the factors associated with interest in PrEP in the full sample (n=200). In multivariable modeling, identifying as Latinx (OR: 3.80; 95% CI, 1.37–10.53) was independently associated with interest in PrEP after controlling for age, gender, race/ethnicity, risk behavior, unstable housing, HCV/HIV testing history, and prescription for mental health disorder. Eighty seven percent (40/46) of individuals identifying as Latinx were interested in PrEP compared to 80% (37/46) of Black/African American and 68% (62/90) of White participants (Figure 2).
Figure 2:
Interest in human immunodeficiency virus (HIV) pre-exposure prophylaxis (PrEP) stratified by race/ethnicity of participants recruited at a drug detoxification center.
3.2. Substance use history and interest in buprenorphine including long-acting injectable formulation
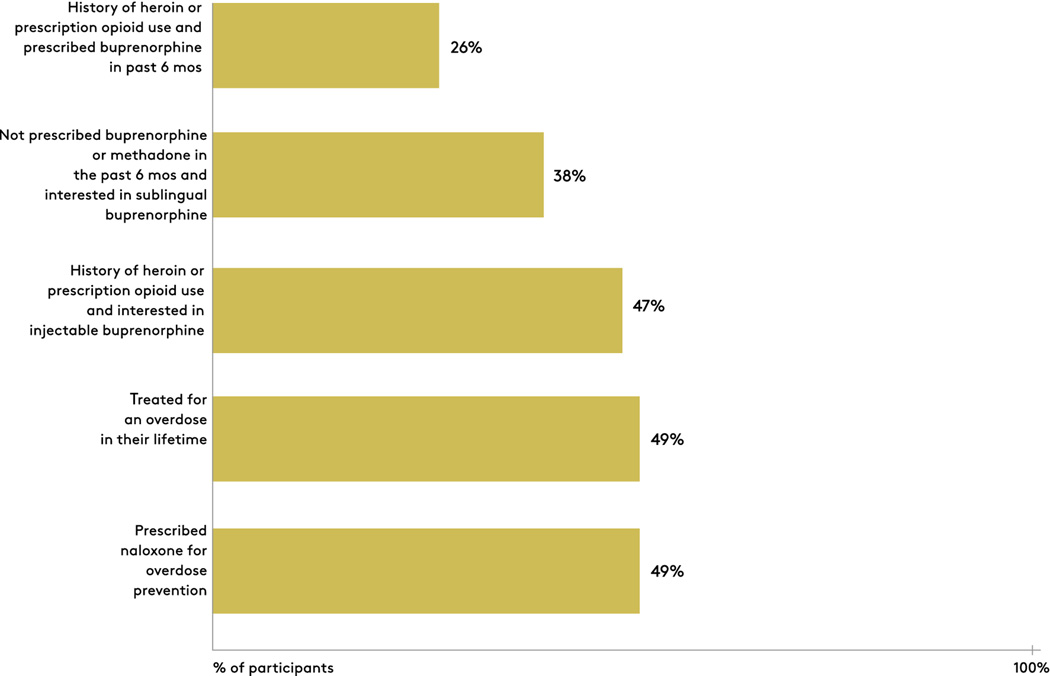
In terms of drug use history, heroin was the most common substance with 80% (159/200) reporting use in the past 12 months, followed by cocaine at 55% (110/200) and prescription opioids at 40% (80/200) (Table 1). Seventy nine percent (158/200) reported two or more substances. Forty nine percent (98/200) had a lifetime history of opioid overdose, and the same percentage had been prescribed naloxone for overdose prevention (Figure 3). Forty seven percent (94/200) reported ever using a drug to reverse an overdose. Over the past 6 months, 46% (92/200) had accessed substance use services in the form of drug detoxification programs, halfway house or residential facilities, day treatment programs for alcohol or drugs, and methadone treatment programs. Approximately one-half had received services at a drug detoxification center prior to the current admission. Of the participants who reported either heroin or prescription opioid use in the past 6 months (n=173), 26% (45/173) had been prescribed buprenorphine within the 6-month time frame (Figure 3). Of participants who had not been on prescribed buprenorphine or methadone in the past 6 months (n=125), 38% (47/125) were interested in being prescribed sublingual buprenorphine. Among individuals with either heroin or prescription opioid use (n=173), 47% (82/173) were interested in injectable buprenorphine. In multivariable modeling, male gender (OR: 2.76; 95% CI, 1.21–6.34) was independently associated with interest in buprenorphine after controlling for age, gender, race/ethnicity, unstable housing, injection drug use, past HIV/HCV testing, and prescription for mental health disorder.
Figure 3:
Experience and interest in buprenorphine, and history of nonfatal drug overdose among participants recruited at a drug detoxification center.
Discussion
We investigated interest in both HIV pre-exposure prophylaxis (PrEP) and buprenorphine among individuals accessing acute drug detoxification services. In the midst of the opioid crisis, which has seen high rates of drug overdose, as well as several outbreaks of HIV infection among persons who inject drugs10, interest has grown in developing models of care to deliver accessible substance use disorder treatment and HIV prevention services outside of traditional medical settings.18,19 Experts have also called for reevaluating the role of drug detoxification centers, potentially restructuring these facilities into venues for initiating medication for opioid use disorder (MOUD) and addressing co-occurring conditions such as HIV.20 Our work provides several key messages that can inform such efforts.
First, our findings underscore potential opportunities for implementing HIV PrEP in drug detoxification centers. The need for education and outreach is clear. We found that very little awareness of HIV PrEP existed among patients accessing drug detoxification centers, despite high self-reported risk for HIV acquisition through injection and sexual risk behaviors. Of note, high interest in PrEP was found once participants were informed of this biomedical prevention method. Also, participants expressed notable enthusiasm for the use of injectable forms of HIV PrEP. Interest in injectable PrEP is particularly timely given the recent release of HIV Prevention Trials Network (HPTN) 0083 findings, which demonstrated the effectiveness of injectable HIV PrEP in preventing HIV transmission.21 This interest in PrEP was similar to prior studies showing that PWID are open to using PrEP once they learn about this effective intervention.22 Although these data cannot speak to the proper model for PrEP implementation in drug detoxification centers, they suggest that interest in PrEP is present among PWID seen in these locations. Although information on the acceptability of long-acting injectable formulation of PrEP is limited, a qualitative study found that longer delivery methods would reduce barriers to daily oral PrEP adherence.23 Our study adds to the current literature by suggesting a potential role for long-acting PrEP initiation at drug detoxification centers. These centers could be novel platforms for PrEP implementation in the midst of HIV outbreaks and in the context of the U.S. effort aimed at Ending the HIV Epidemic.10,24 Future work should explore models for PrEP implementation in this setting.
Of interest, we found that participants who identified as Latinx were more likely to be interested in PrEP. Reasons for this finding are unclear. A recent study demonstrated gaps in knowledge about PrEP among Latinx men who have sex with men and Latinx transgender women, but this study did not delve into interest in PrEP.25 There are however data showing that when compared to heterosexual White women, African American women were more likely to report potential use of PrEP.26 The higher proportion of interest in PrEP among Latinx and Black/African-American patients is important as recent data show that these racial/ethnic groups are at higher risk for acquiring HIV, but are less likely to have access to PrEP. 27,28 Our findings support additional studies to increase uptake among these groups.
Second, the current study highlights important areas of future research that will be foundational to any successful effort to utilize drug detoxification centers as venues for initiating buprenorphine, including the need for qualitative research exploring reasons why many patients might not be interested in this medication. It is essential to understand why some patients who access drug detoxification centers are not interested in starting buprenorphine treatment. Potential reasons might include a preference to treat opioid withdrawal with methadone given that this medication is a full opioid agonist (i.e. it can be initiated soon after heroin use without precipitating opioid withdrawal) and competing priorities such as housing, barriers to outpatient care including transportation, or a lack of readiness to change substance use. Our survey did not probe deeper to understand the motivators and barriers to initiating buprenorphine in detoxification facilities. What is clear, however, is that simply offering buprenorphine to patients entering detoxification centers might not result in a majority of patients initiating and continuing this medication. Nevertheless, the fact that nearly 4 in 10 patients not on MOUD in the past 6 months were interested in initiating buprenorphine suggests an important opportunity in drug detoxification centers. Buprenorphine initiation coupled with direct linkage to low-barrier bridge clinic programs or Office-Based Addiction treatment (OBAT) programs 29 has the potential to enhance the care continuum for individuals with OUD and reduce loss to follow-up between acute treatment and outpatient care settings.
Third, future research investigating interest in MOUD in drug detoxification centers should explore the potential to deliver injectable long-acting formulations of buprenorphine in this setting. A monthly injectable form of buprenorphine was FDA-approved for the treatment of opioid use disorder in November, 2017 but was not routinely available clinically at nearby institutions until late 2018, after the study period. Long-acting formulations have the potential to improve retention on medications, but the real-world effectiveness of these MOUD formulations when initiated in acute treatment settings is still unknown. Injectable buprenorphine has the potential to address structural barriers to sublingual buprenorphine adherence faced by some patients with unstable housing, including medication loss, theft, pressure to sell or trade medication, and ability to obtain regular refills.30 The real-world effectiveness of monthly injectable buprenorphine initiated in detoxification settings merits future study. Simply initiating long-acting medications may not lead to retention on MOUD without additional support. For example, a prior study demonstrated that approximately half of patients on injectable extended-release naltrexone discontinued this medication after the first injection.31 Buprenorphine and extended-release naltrexone have different mechanisms of action, and it is not known how retention on long-acting buprenorphine will compare to extended-release naltrexone. Patients initiating long-acting injectable buprenorphine may require case management to ensure linkage to a long-term care setting equipped to continue this buprenorphine formulation. Ongoing studies might provide some additional information on the best approach to integrate injectable formulations within existing care models with the goal of improving retention on MOUD and patient outcomes.32
Our findings confirm that the potential for drug detoxification centers to reach patients not previously treated with buprenorphine for opioid use disorder is high. Consistent with prior work, just one-third of participants with a history of heroin or prescription opioid use had been prescribed buprenorphine in the past 6 months.12 Approximately 40% of individuals who had not been prescribed buprenorphine and were not on methadone reported interest in buprenorphine initiation, and a similar proportion were open to an injectable buprenorphine formulation. Results suggest substantial opportunity to increase buprenorphine initiation in drug detoxification facilities; they also highlight the challenges of meeting the needs of all patients with opioid use disorder with a single MOUD approach, as a little over one-half of participants were not interested in buprenorphine.
Limitations to the current study include the cross-sectional study design and the use of a single, urban site located in close proximity to significant MOUD infrastructure. Although these factors might influence generalizability to rural or lower-resource urban settings, we provide novel information on the opportunity to expand PrEP and buprenorphine initiation, including the potential role for long-acting injectable formulations, in non-traditional settings such as acute detoxification centers. In addition, we did not ask participants about fentanyl use as it was much less prevalent in our location at the time of the study; however, we obtained some detailed information about other substances used including heroin, amphetamines, crack/cocaine and noted the use of multiple substances.
Conclusion
We documented limited awareness of HIV PrEP among people entering a substance use treatment center, but observed enthusiasm for this biomedical prevention method once patients were made aware. We also identified an opportunity to expand MOUD initiation with buprenorphine during a stay at substance use treatment centers. Recent and pioneering studies have shown that starting MOUD is feasible and acceptable in various settings such as jails or prisons, HIV and HCV specialty clinics, and inpatient settings during reachable moments.33–36 The current study provides additional data on potentially initiation these treatments in substance use treatment centers.
The potential for drug detoxification centers to become venues for addressing the syndemics of opioid use disorder and HIV is real, and the current findings frame some of the significant challenges and opportunities inherent in this approach. Future studies should further explore how to increase interest in buprenorphine among drug detoxification patients during the opioid overdose epidemic and evaluate implementation strategies for PrEP and MOUD and in these facilities.
Acknowledgement:
The authors would like to thank study participants and our research team for their contributions to this work.
Funding:
This work was supported by the National Institute of Allergy and Infectious Diseases [P30AI042853 Providence/Boston Center for AIDS Research collaborative developmental grant to S.A.A]; and the National Institute of Drug Abuse [R25DA035163 pilot award to S.A.A, K23DA044085 to S.A.A, R01DA046527 to B.P.L, and P30DA040500 to B.P.L]. The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institutes of Health.
Findings were presented as a poster at the College on Problems of Drug Dependence (CPDD), San Diego, California, June 11th, 2018.
Abbreviations:
- MA
Massachusetts
- RI
Rhode Island
- USA
United States of America
Footnotes
Clinical trial registration: NCT02869776
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. Jun 15 2013;381(9883):2083–2090. [DOI] [PubMed] [Google Scholar]
- 2.Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. The New England journal of medicine. Sep 4 2003;349(10):949–958. [DOI] [PubMed] [Google Scholar]
- 3.USPSTF, Owens DK, Davidson KW, et al. Preexposure Prophylaxis for the Prevention of HIV Infection: US Preventive Services Task Force Recommendation Statement. JAMA : the journal of the American Medical Association. June 11 2019;321(22):2203–2213. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention: US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 Update: a clinical practice guideline. March 2018. Available at https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed on June 12, 2020.
- 5.Schwartz RP, Gryczynski J, O’Grady KE, et al. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995–2009. American journal of public health. May 2013;103(5):917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. Apr 16 2008(2):CD002207. [DOI] [PubMed] [Google Scholar]
- 7.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. Jul 08 2009(3):CD002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan LE, Moore BA, Chawarski MC, et al. Buprenorphine/naloxone treatment in primary care is associated with decreased human immunodeficiency virus risk behaviors. Journal of substance abuse treatment. Jul 2008;35(1):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad C, Bradley HM, Broz D, et al. Community Outbreak of HIV Infection Linked to Injection Drug Use of Oxymorphone--Indiana, 2015. MMWR. Morbidity and mortality weekly report. May 1 2015;64(16):443–444. [PMC free article] [PubMed] [Google Scholar]
- 10.Alpren C, Dawson EL, John B, et al. Opioid Use Fueling HIV Transmission in an Urban Setting: An Outbreak of HIV Infection Among People Who Inject Drugs-Massachusetts, 2015–2018. American journal of public health. Jan 2020;110(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazzi AR, Drainoni ML, Biancarelli DL, et al. Systematic review of HIV treatment adherence research among people who inject drugs in the United States and Canada: evidence to inform pre-exposure prophylaxis (PrEP) adherence interventions. BMC public health. Jan 8 2019;19(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. March 2011;5(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo I, Olsen H, Patrick R, et al. Willingness to use HIV pre-exposure prophylaxis among community-recruited, older people who inject drugs in Washington, DC. Drug and alcohol dependence. Jul 1 2016;164:8–13. [DOI] [PubMed] [Google Scholar]
- 14.Walters Suzan M., Kral Alex H., Simpson Kelsey A., Wenger Lynn & Bluthenthal Ricky N. (2020) HIV Pre-Exposure Prophylaxis Prevention Awareness, Willingness, and Perceived Barriers among People Who Inject Drugs in Los Angeles and San Francisco, CA, 2016–2018, Substance Use & Misuse, 55:14, 2409–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biancarelli DL, Biello KB, Childs E, et al. Strategies used by people who inject drugs to avoid stigma in healthcare settings. Drug and alcohol dependence. May 1 2019;198:8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assoumou SA, Paniagua SM, Linas BP, et al. Rapid Versus Laboratory-Based Testing for HIV and Hepatitis C at a Drug Detoxification Treatment Center: A Randomized Trial. The Journal of infectious diseases. Sep 2 2020;222(Supplement_5):S376–S383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caudarella A, Dong H, Milloy MJ, Kerr T, Wood E, Hayashi K. Non-fatal overdose as a risk factor for subsequent fatal overdose among people who inject drugs. Drug and alcohol dependence. May 1 2016;162:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snow RL, Simon RE, Jack HE, Oller D, Kehoe L, Wakeman SE. Patient experiences with a transitional, low-threshold clinic for the treatment of substance use disorder: A qualitative study of a bridge clinic. Journal of substance abuse treatment. Dec 2019;107:1–7. [DOI] [PubMed] [Google Scholar]
- 19.Springer SA, Del Rio C. Addressing the Intersection of Infectious Disease Epidemics and Opioid and Substance Use Epidemics. Infect Dis Clin North Am. Sep 2020;34(3):xiii–xiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedmann PD, Suzuki J. More beds are not the answer: transforming detoxification units into medication induction centers to address the opioid epidemic. Addict Sci Clin Pract. November 15 2017;12(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HIV Prevention Trials Network. “HPTN 083 Study Demonstrates Superiority of Cabotegravir for the Prevention of HIV”. Available at https://www.hptn.org/news-and-events/press-releases/hptn-083-study-demonstrates-superiority-cabotegravir-prevention-hiv. Accessed on August 10th, 2020.
- 22.Sherman SG, Schneider KE, Park JN, et al. PrEP awareness, eligibility, and interest among people who inject drugs in Baltimore, Maryland. Drug and alcohol dependence. Feb 1 2019;195:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biello KB, Edeza A, Salhaney P, et al. A missing perspective: injectable pre-exposure prophylaxis for people who inject drugs. AIDS Care. Oct 2019;31(10):1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV Epidemic: A Plan for the United States. JAMA : the journal of the American Medical Association. March 5 2019;321(9):844–845. [DOI] [PubMed] [Google Scholar]
- 25.Barreras JL, Linnemayr SL, MacCarthy S. “We have a stronger survival mode”: exploring knowledge gaps and culturally sensitive messaging of PrEP among Latino men who have sex with men and Latina transgender women in Los Angeles, CA. AIDS Care. Oct 2019;31(10):1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wingood GM, Dunkle K, Camp C, et al. Racial differences and correlates of potential adoption of preexposure prophylaxis: results of a national survey. Journal of acquired immune deficiency syndromes (1999). Jun 1 2013;63 Suppl 1:S95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanny D, Jeffries WLt, Chapin-Bardales J, et al. Racial/Ethnic Disparities in HIV Preexposure Prophylaxis Among Men Who Have Sex with Men - 23 Urban Areas, 2017. MMWR. Morbidity and mortality weekly report. September 20 2019;68(37):801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hess KL, Hu X, Lansky A, Mermin J, Hall HI. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. April 2017;27(4):238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alford DP, LaBelle CT, Kretsch N, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Archives of internal medicine. March 14 2011;171(5):425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godersky ME, Saxon AJ, Merrill JO, Samet JH, Simoni JM, Tsui JI. Provider and patient perspectives on barriers to buprenorphine adherence and the acceptability of video directly observed therapy to enhance adherence. Addict Sci Clin Pract. March 13 2019;14(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. Journal of substance abuse treatment. Jul 03 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Library of Medicine (U.S.). (2021, May- ) A Study Comparing Oral Buprenorphine and Injectable Buprenorphine for the Treatment of Opioid Use Disorder (VA-BRAVE). Identifier NCT04375033https://clinicaltrials.gov/ct2/show/NCT04375033?term=injection+buprenorphine&recrs=ab&draw=2&rank=2.
- 33.Norton BL, Akiyama MJ, Zamor PJ, Litwin AH. Treatment of Chronic Hepatitis C in Patients Receiving Opioid Agonist Therapy: A Review of Best Practice. Infect Dis Clin North Am. Jun 2018;32(2):347–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green TC, Clarke J, Brinkley-Rubinstein L, et al. Postincarceration Fatal Overdoses After Implementing Medications for Addiction Treatment in a Statewide Correctional System. JAMA Psychiatry. Apr 1 2018;75(4):405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fanucchi L, Springer SA, Korthuis PT. Medications for Treatment of Opioid Use Disorder among Persons Living with HIV. Current HIV/AIDS reports. Feb 2019;16(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. Journal of general internal medicine. Aug 2010;25(8):803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]