Abstract
One major health issue is the microbial and chemical contamination of natural freshwater, particularly in Latin American countries, such as Ecuador, where it is still lacking wastewater treatment plants. This study analyzed the water quality in twelve rivers of Ecuador (Coastal, Andean, and Amazonian regions). All rivers showed levels of E. coli and total coliforms above the maximum limit according to International and Ecuadorian legislations. The most polluted rivers were Zamora, Esmeraldas and Machángara. Also, E. coli pathotypes were found in six rivers. Several physicochemical and metal parameters were detected in high levels, such as CODTOTAL (in eight rivers), TSS (in six rivers), TS (in two rivers), Al (in nine rivers), Zn (in eight rivers), Pb (in three rivers), Cu (in three rivers), Fe (in two rivers), and Mn (in Machángara River). Our results agree with other studies in Latin America (such as Colombia, Brazil, and Peru) reporting similar contamination in water resources used for agriculture, livestock, and human consumption. Overall, Guayas, Guayllabamba, and Machángara Rivers showed the highest levels of physicochemical parameters (such as CODTOTAL and TSS) and metal concentrations (such as copper, zinc, aluminum, iron, and manganese). Further studies should evaluate contamination sources and public health impact.
Subject terms: Environmental chemistry, Environmental impact, Environmental microbiology, Policy and public health in microbiology
Introduction
The ongoing discharge of untreated wastewater into the environment is a major concern worldwide. Even more so in developing countries, where untreated domestic wastewater is usually discharged into the nearest freshwater system, inducing severe impacts on ecosystems. Pollution in rivers leads to low yields of agricultural and industrial production1. Increased bacterial and chemical contamination contributes to severe problems in the food industry, since its production, processing, and distribution2. The continuous discharge of untreated effluents favors microbial proliferation (either commensal, opportunistic, or even pathogen microorganisms) and chemical contamination of surface water3, which is commonly used in rural areas as a drinking water source and for agriculture and livestock farming. This contamination eventually leads to serious public health risks and costs, such as the augmentation of chronic diseases and persistence of microorganisms with antibiotic resistance4, which is more evident in greater population density areas due to untreated domestic and industrial discharges5.
According to the United Nations Water Division, globally 80% of the domestic streams are discharged directly into rivers, lakes, and coastal zones without treatment, and Ecuador is not an exception6. This scenario represents a serious problem when surface water is used as an alternative to potable water, which currently occurs in numerous locations in Ecuador. Usually, in developed countries, potable water meets drinking water quality standards, being safe to drink or use for food preparation. However, in Ecuador, only 83% of the population has access to potable water, but may not always be drinkable quality water7. In rural regions, the situation is even worse, where only 53.9% of the population has potable water7. Numerous problems of access to drinking water lead part of the population to use river water for various domestic activities, including laundry, personal hygiene, and, on occasion, food preparation8–10. This national context led 28, 787 people to suffer, in 2015, from diarrhea and gastroenteritis due to a presumed infectious origin11. Some studies in Ecuador already postulated the contamination of water sources with potentially pathogenic microorganisms for human health12–14. These authors analyzed water resources through the general indicators of bacteriological quality, such as Escherichia coli and total coliforms15. Additionally, the contamination of surface waters by trace metallic elements due to mining supplies or industrial activities has been reported in several rivers located in the south of Ecuador, specifically in the localities of Nambija, Portovelo-Zaruma, and Ponce Enriquez. Due to the use of cyanide in mineral processing, water pollution was reported in several regions of Ecuador, through poor management of mining waste and conflicts related to regulations and policies16,17. So, the safety of these natural freshwater resources is also affected by various contaminants (trace metals, and major elements). These contaminants cause variations of the physicochemical properties of water resources, which directly influence microbial proliferation, and therefore physicochemical analysis is also an indispensable feature for the water quality assessment. Finally, high levels of heavy metals (such as Pb, Cr, Cu, and Zn) represent a serious public health risk because they are not biodegradable18.
Quito is the capital city of Ecuador with a population of 2,239,191 people based on the last census conducted in 201019. Surprisingly, Quito has a small wastewater treatment plant (WWTP) in the southern part of the city, and, currently, 97% of domestic effluents are still being discharged directly into Machángara and Monjas Rivers without prior treatment20. In 2015, Voloshenko-Rossin et al. studied the water quality and the organic pollutants in the San Pedro–Guayllabamba–Esmeraldas watershed, while Benítez and colleagues characterized domestic wastewater samples from six different discharge points in the southern area of Quito22. In 2020, a study evaluated the quality of eighteen rivers located in Quito23, identifying Machángara and Monjas Rivers as the most contaminated rivers based on the physicochemical and microbiological parameters. However, little is still known about the microbial and chemical contamination in Ecuador’s main rivers, despite some studies recently realized in rivers of certain major cities (Guayaquil, Esmeraldas, and Quito) of Ecuador21,22,24.
Other potentially pathogenic microorganisms to human health and even food production should also be evaluated in the water quality assessment, such as Pseudomonas, Shigella, Salmonella, Legionella, and Campylobacter spp.3. Besides commensal E. coli quantification, as fecal contamination biomarker, the microbial load analysis should include the determination of certain E. coli pathotypes, more exactly, enteroaggregative E. coli (EAEC), enterohemorrhagic E. coli (EHEC), enteropathogenic E. coli (EPEC), and enteroinvasive E. coli (EIEC)3. In developing countries, E. coli pathotypes are responsible for numerous infections among the population, particularly, children under five years old25. Certain E. coli pathotypes are associated with the consumption of contaminated food and water. In Ecuador, the prevalence of EAEC, EHEC, EPEC, and EIEC are reported in single locations26, but few studies are characterizing their prevalence in space. So, by monitoring these E. coli pathotypes in natural freshwater resources, we aim to better understand E. coli transmission among Ecuadorian regions. These E. coli pathotypes contain both extended‐spectrum beta‐lactamase genes and virulence factors for intestinal and extraintestinal infections, which could eventually lead to a trade‐off between resistance and virulence of E. coli or other bacteria27. The dissemination of antibiotic resistance and virulence factors in natural environments is currently not well understood, and therefore needs to be clarified. These virulence factors can affect a wide range of cellular processes, such as cell–cell signaling, ion secretion, protein synthesis, mitosis, cytoskeletal structure, and mitochondrial function27. Presently, the microbial load evaluation in water samples uses classic and molecular methodologies. E. coli and total coliforms counting are typically obtained by classic techniques. Molecular techniques, such as polymerase chain reaction (PCR) or quantitative PCR (qPCR), could be an efficient complementary analysis, allowing a rapid detection and quantification of certain microorganisms in water samples28.
Our study aimed to analyze the physicochemical characteristics (including major and trace metallic elements) and microbiological quality of natural freshwater resources in twelve rivers located in urban areas of eleven provinces of Ecuador (Coastal, Andean, and Amazonian regions). All analyzed samples were collected from areas of high population densities located next to these rivers, allowing us to evaluate the current contamination panorama of the main rivers of Ecuador that could affect human health.
Results
Escherichia coli and total coliform counts
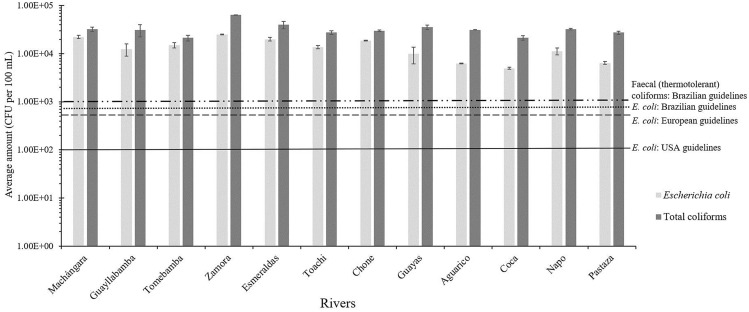
The counts of Escherichia coli and total coliforms were realized in the twelve rivers of the study set (Table 1). As shown in Fig. 1, all rivers showed concentrations of both E. coli and total coliforms above the maximum limits allowed by the United States of America (USA) standard values of the Recreational Water Quality Criteria29, European Union guidelines30, and Brazilian guidelines for bathing waters under Resolution CONAMA no. 274 of 29 November 200031 (see Table S1 for additional information). Although microbial contamination was found in all rivers, the most polluted rivers were Zamora River in Loja at the southern Andean region (E. coli: 2.50 × 104 CFU per 100 mL; and total coliforms: 6.38 × 104 CFU per 100 mL), Esmeraldas River in Esmeraldas at the northeastern Coastal region of the country (E. coli: 2.00 × 104 CFU per 100 mL; and total coliforms: 4.00 × 104 CFU per 100 mL), and Machángara River in Quito at the central Andean region (E. coli: 2.25 × 104 CFU per 100 mL; and total coliforms: 3.25 × 104 CFU per 100 mL). Overall, the rivers of the Amazonian region showed the lower contamination levels of the present study, more exactly, Coca (E. coli: 5.00 × 103 CFU per 100 mL; and total coliforms: 2.13 × 104 CFU per 100 mL), Aguarico (E. coli: 6.25 × 103 CFU per 100 mL; and total coliforms: 3.13 × 104 CFU per 100 mL), and Pastaza (E. coli: 6.42 × 103 CFU per 100 mL; and total coliforms: 2.75 × 104 CFU per 100 mL) Rivers.
Table 1.
Selection of the main Ecuadorian rivers and their collection samples for microbial, physicochemical, and metal analysis in this study.
| Location | River | GPS Coordinates | City (Province) | Region | Collection sampling | Mean annual discharge (m3 s-1) a | Monthly average temperature (°C)b | Annual Precipitation (mm) b | Name of INAMHI Stations | GPS Coordinates of INAMHI Stations | Height of INAMHI Stations (m) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Machángara | 0°14′03.6"S/78°30′53.0"W | Quito (Pichincha) | Andean | April 2016 | 4.2 | N/A | 1381.9 | M0325 Garcia Moreno | 0°14′5"S/78°37′38"W | 1950 |
| 2 | Guayllabamba | 0°4′6,961″S/78° 22′21,87″W | Guayllabamba (Pichincha) | Andean | April 2016 | N/A | N/A | 847 | M0345 Calderon | 0°5′54″S/78°25′15″W | 2645 |
| 3 | Tomebamba | 2°53′44.1″S/78°58′07.5″W | Santa Ana de los Cuatro Ríos de Cuenca, commonly referred as Cuenca (Azuay) | Andean | May 2016 | 21.37 | N/A | 878 | M0426 Ricaurte-Cuenca | 2°51′3″S/78°56′55″W | 2545 |
| 4 | Zamora | 3°58′42.21″S/79°12′10.68″W | Loja (Loja) | Andean | June 2016 | N/A | N/A | 621.3 | M0759 El Tambo-Loja | 4°4′25″S/79°18′0″W | 1580 |
| 5 | Esmeraldas | 0°56′31.3"N/79°38′34.5"W | Esmeraldas (Esmeraldas) | Coastal | July 2016 | 88.25 | N/A | 614.3 | M0441 Sague (San Mateo) | 0°53′13″S/79°37′54″W | 15 |
| 6 | Toachi | 0°14′46.2″S/79°8′02,1″W | Santo Domingo (Santo Domingo de los Tsáchilas) | Coastal | July 2016 | 48.20 | N/A | 2792.4 | M0348 Santa Anita-Km 10 Via Chone | 0°13′50″S/79°14′54″W | 560 |
| 7 | Chone | 0°41′41.6″ S/80°5′15.3″ W | Chone (Manabí) | Coastal | July 2016 | N/A | 25.32 | 1486.4 | M0162 Chone—U. Catolica | 0°39′51″S/80°2′11″W | 13 |
| 8 | Guayas | 2°06′55.5"S/79°52′43.3"W | Guayaquil (Guayas) | Coastal | July 2016 | 1654.50 | 26.2 | 1064.5 | M1096 Guayaquil—U. Estatal | 2°12′0″S/79°53′0″W | 6 |
| 9 | Aguarico | 0°03′36,8"N/76°52′25,0"W | Nueva Loja, also known as Lago Agrio (Sucumbios) | Amazonian | June 2016 | N/A | 23.8 | 4637.8 | M1203 Lumbaqui | 0°2′26″S/77°20′2″W | 580 |
| 10 | Coca | 0°27′24,43″S/76°59′9,143″W | Puerto Francisco de Orellana, also known as El Coca (Orellana) | Amazonian | June 2016 | 32.23 | 25.5 | 3261.4 | M1221 San Jose De Payamino | 0°30′14″S/77°19′3″W | 345 |
| 11 | Napo | 1°02′40.12″S/77°47′37.61″W | Tena (Napo) | Amazonian | June 2016 | 1105 | N/A | 2186.7 | M0490 Sardinas | 0°22′16″S/77°48′6″W | 1615 |
| 12 | Pastaza | 1°27′05.8"S/78°09′18.6"W | Puyo (Pastaza) | Amazonian | June 2016 | N/A | 21.8 | 3557.1 | M0041 Sangay (P. Santa Ana) | 1°41′18″S/77°57′31″W | 880 |
a Data from the National Institute of Meteorology and Hydrology (INAMHI, http://www.serviciometeorologico.gob.ec/); b INAMHI. (2013). Anuario Metereológico. Retrieved from: https://www.serviciometeorologico.gob.ec/docum_institucion/anuarios/meteorologicos/Am_2013.pdf; N/A: Not available.
Figure 1.
Average amount of Escherichia coli and total coliforms quantified in the rivers and their water classification accordingly to bathing-water standards by the USA, European and Brazilian guidelines. Legend: Threshold of faecal (thermotolerant) coliforms by Brazilian guidelines (- -- -); threshold of E. coli by Brazilian guidelines (- - -); threshold of E. coli by European guidelines (-- -- --); threshold of E. coli by USA guidelines (--------).
Prevalence of bacterial genera and Escherichia coli pathotypes in river samples
Other growth media cultures were also assessed to detect several bacterial genera. In MacConkey agar, all water samples showed growth of Gram-negative rods, which can include Escherichia, Salmonella, Shigella (enteric bacteria), and Pseudomonas (non-enteric bacterium) genera. Yet, during the culture on Legionella CYE Agar Base, none of the rivers evidenced the growth of pure colonies, displaying bacterial contamination. No growth was detected on Salmonella-Shigella agar and Campylobacter agar in any water sample. Therefore, the presence or absence of these genera (Legionella, Pseudomonas, Salmonella, Shigella, and Campylobacter spp.) was then evaluated through polymerase chain reaction (PCR) analysis. As suspected by growth media culture, none of the rivers revealed the presence of Salmonella, Shigella, or even Campylobacter spp., but all rivers showed the presence of Pseudomonas and Legionella spp. Finally, PCR analysis also evidenced the presence of EIEC pathotype in the Esmeraldas, Chone, Machángara, Guayllabamba, and Napo Rivers. EPEC pathotype was detected in the Zamora River and EAEC pathotype was also found in the Machángara River. However, the EHEC pathotype was not observed in any river.
Analysis of physicochemical parameters
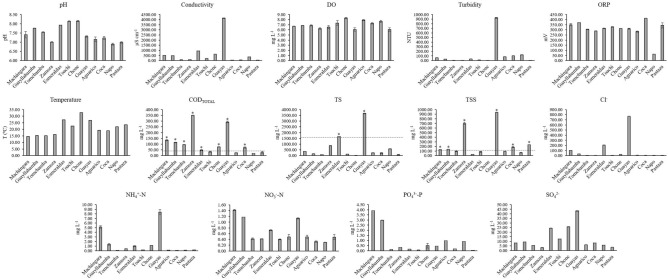
Additionally, we analyzed the physicochemical parameters (Fig. 2). These parameters were compared to the maximum contaminant levels for the preservation of aquatic and wildlife in freshwater established in the Ecuadorian legislation32 (see Table S2 for additional information). The Chone River showed the highest temperature (32.7 ºC) while the Machángara River registered the lowest value (14.5 ºC). This is not surprising, as the Chone River is located in the Coastal region, where high ambient temperatures occur, and the Machángara River is located in the Andean region, at 2800 m.a.s.l. Values for pH (6.89–8.14), DO (6.08–8.30 mg L−1) and NO3−-N (0.30–1.42 mg L−1) were within the recommended ranges and illustrated the intrinsic natural variance due to the high geomorphological diversity between the three main regions of Ecuador (i.e., Coastal, Andean, and Amazonian)32.
Figure 2.
Average and standard deviation values of physicochemical parameters quantified in water samples of the twelve rivers in this study. Legend: Threshold of a certain physicochemical parameter (-—-); * exceedance values according to legislation.
High values of conductivity were found in Guayas (4137.33 µS cm−1), Esmeraldas (938.53 μS cm−1), and Machángara (501.10 µS cm−1) Rivers. The Environmental Protection Agency (EPA) suggests a range of conductivity between 150 and 500 μS cm−1, and therefore the conductivity values found in the river basins are higher than the recommended values18. In addition, the ORP values found in this study were between 62.44 and 412.77 mV, in which Coca River showed the highest value of ORP.
Ammonium levels ranged from 0.08 to 8.38 mg L−1, evidencing the highest value in the Guayas River (8.38 mg L−1), and followed by the Machángara River (5.15 mg L−1).
Regarding the total COD (CODTOTAL) values, it was found that eight rivers exceeded the value recommended by the Ecuadorian legislation (40 mg L−1)32. The Zamora River registered the highest CODTOTAL value (349.73 mg L−1), followed by the Guayas (292.67 mg L−1) and the Machángara Rivers (133.58 mg L−1). The Guayllabamba, Tomebamba, Chone, Coca, and Esmeraldas Rivers exceeded the recommended CODTOTAL value by a factor of 2.9, 2.4, 1.9, 1.7, and 1.2, respectively.
The Guayas and Esmeraldas Rivers from the Coastal region showed high concentrations of total solids (TS) with values of 3667.50 and 1657.50 mg L−1, respectively. These values are 2.3 and 1.1 times higher than the maximum allowable limit for discharges to water bodies established by Ecuadorian legislation33. Meanwhile, Guayas (939 mg L−1), Zamora (697.50 mg L−1), Coca (182.50 mg L−1), Pastaza (237.50 mg L−1), Machángara (132.50 mg L−1), and Guayllabamba (137.50 mg L−1) Rivers had TSS values above the maximum value (130 mg L−1) specified by Ecuadorian legislation for discharges to freshwater bodies33. Concentrations of sulphates (3.27–43.15 mg L−1), phosphates (0.04–3.91 mg L−1), and chlorides (0.07–769.58 mg L−1) were within the limits established by the Ecuadorian legislation33. However, in the case of chlorides, it is important to note that Machángara, Esmeraldas, and Guayas Rivers registered concentrations between one and two orders of magnitude higher than the remaining rivers.
Statistical analysis was performed between the concentration of E. coli and total coliforms against physicochemical parameters, using linear and multiple logistic regressions. Several correlation analyses were examined and we only found a statistically significant correlation between total coliforms and CODTOTAL (R2 = 0.501, P-value = 0.010; N = 12). However, this correlation did not reveal a good fit (Fig. 3), which could be partially attributable to total coliforms as a variable. The Benjamini–Hochberg method was then used for multiple test corrections. The correlation between total coliforms and CODTOTAL did not show statistical significance in the adjusted P-value (P = 0.089).
Figure 3.
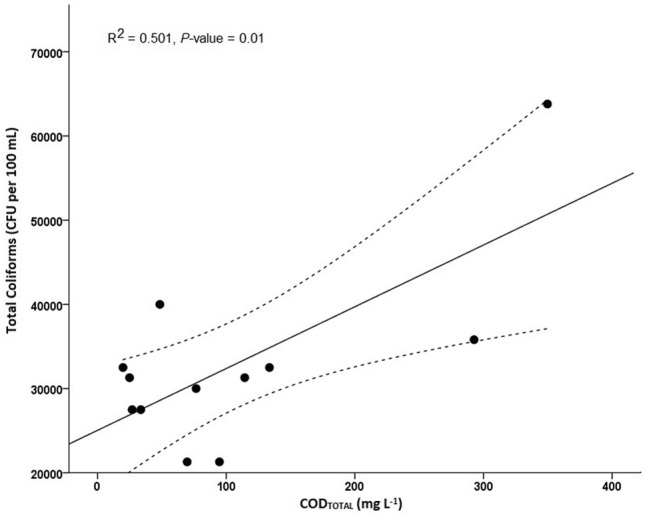
Linear logistic regression between total coliforms and CODTOTAL (R2 = 0.501, P-value = 0.010; N = 12). Legend: Upper and lower 95% Confident Interval (95% CI) limit in the linear logistic regression (-—-).
Analysis of trace metals and major elements
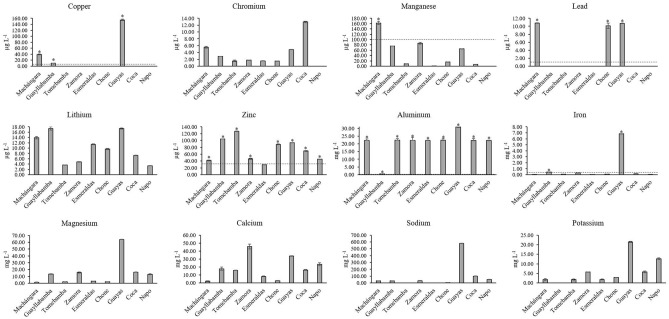
It is worth mentioning that the samples from three rivers (Toachi, Pastaza, and Aguarico) were not analyzed for trace metals and major elements due to the cross-contamination of the samples during transport. Therefore, only nine rivers out of twelve were analyzed, as shown in Fig. 4. The following trace elements were analyzed: copper (Cu), chromium (Cr), manganese (Mn), lead (Pb), lithium (Li), and zinc (Zn); whereas the major elements were: aluminum (Al), iron (Fe), magnesium (Mg), calcium (Ca), sodium (Na), and potassium (K). Chromium (1.52–12.93 µg L−1) and Li (3.35–17.39 µg L−1) were the trace metals that were consistently below the limits (see Table S3 for additional information). Concentration ranges of Pb (10.12–10.82 µg L−1) and Cu (10.17–154.67 µg L−1) were the lowest measured in the surface water samples. The Chone, Machángara, and Guayas Rivers exceeded the maximum value of Pb by a factor of 10; while the Guayllabamba, Machángara, and Guayas Rivers exceeded the established value of Cu by a factor of 2.0, 7.8, and 30.9, respectively. The concentration of Zn (29.50–127.02 µg L−1) exceeded the limits established by Ecuador (30 µg L−1) in almost 100% of analyzed samples except for the Esmeraldas River. In the case of major elements, all rivers analyzed in this study evidenced Al levels exceeding the Ecuadorian threshold, in particular, the Guayas River (30.80 mg L−1), followed by Chone (22.45 mg L−1), Tomebamba (22.44 mg L−1), Esmeraldas (22.26 mg L−1), Zamora (22.25), and Machángara (22.17 mg L−1) Rivers. The concentrations of Fe were 1.5 and 22.8 times higher than the recommended values of Ecuadorian legislation (0.3 mg L−1) in Guayllabamba and Guayas Rivers, respectively. The highest concentrations of Fe and Al were observed in the same river (Guayas River). Mg (1.47–64.18 mg L−1), Ca (2.20–45.69 mg L−1), Na (4.85–578.82 mg L−1), and K (1.73–21.43 mg L−1) were also detected in high concentrations in several rivers. The Guayas River also registered the highest concentrations of Mg, Na, and K, while the highest concentration of Ca was detected in the Zamora River.
Figure 4.
Average and standard deviation values of trace metals and major elements quantified in water samples of the nine rivers in this study. Legend: Threshold of a certain trace metal or major element (-—-); * exceedance values according to legislation.
Finally, the statistical analysis did not find any correlation between metal concentrations and E. coli or total coliforms. Despite these results, we found a significant correlation between Fe and Cu concentrations (R2 = 0.986, P-value < 0.010; data not shown).
Evaluation of the contamination between regions
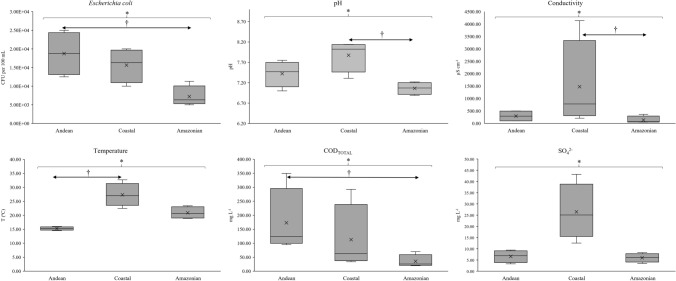
To better evaluate different river systems in the present study, all parameters (microbial, physicochemical, trace metals, and major elements) were further analyzed between different regions of Ecuador (Fig. S1 and Fig. S2 for additional information). So, the Kruskal–Wallis non-parametric one-way analysis of variance was used to compare contamination between regions (Andean, Coastal, and Amazonian), followed by a Mann–Whitney test for paired comparisons. Statistical differences were found on six parameters (P-values < 0.050; Fig. 5), more exactly, E. coli (P = 0.031), pH (P = 0.030), conductivity (P = 0.039), temperature (P = 0.010), CODTOTAL (P = 0.030), and SO42− (P = 0.025). The Mann–Whitney paired comparisons demonstrated the following differences: E. coli concentrations between Andean and Amazonian regions (P = 0.032), showing average concentrations of 1.88 × 104 and 7.24 × 103 CFU per 100 mL, respectively; pH values between Coastal and Amazonian regions (P = 0.024), showing mean values of 7.88 and 7.06, respectively; conductivity values between Coastal and Amazonian regions (P = 0.032), showing mean values of 1476.46 and 137.14 μS cm−1, respectively; river temperatures between Coastal and Andean regions (P = 0.007), showing mean values of 27.33 and 15.28 ºC, respectively; and CODTOTAL values between Andean and Amazonian regions (P = 0.024), showing mean values of 173.10 and 35.26 mg L−1, respectively. The Mann–Whitney paired comparisons did not evidence statistically differences in SO42− concentrations between regions. Although the average concentration of SO42− in the Coastal region (26.45 mg L−1) was higher than Andean and Amazonian regions (6.60 and 5.98 mg L−1, respectively), the adjusted P value was 0.056 against both regions. No statistically significant values were found among trace metals and major elements.
Figure 5.
Statistical differences between regions (Andean, Coastal, and Amazonian) on microbial and physicochemical contamination in water samples of the present study. Legend: Statistical P-value obtained through Kruskal–Wallis non-parametric one-way analysis of variance (P < 0.050); † Statistical P-value obtained through Mann–Whitney test for paired comparisons (P < 0.050).
Discussion
Bacterial contamination in urban areas of the main Ecuadorian rivers
All rivers showed E. coli levels above standard concentrations for bathing-water recommended by the USA, European and Brazilian guidelines (Fig. 1), in concordance with other studies in Latin America, such as Colombia34, Mexico4, and Perú35. Most of the rivers in this study could be treated to produce drinking or bathing water, however, a drastic and expensive treatment would be necessary, being economically challenging in Ecuador.
Some studies in the USA reported lower levels of E. coli and total coliforms contamination than those reported in Latin America36,37. In particular, the study of Bower and colleagues37 demonstrated that 28 of the 74 analyzed samples did not exceed 235 CFU per 100 ml of E. coli showing a drastically lower level of contamination when compared to this study. In addition, other studies reported different levels of E. coli ranging from 3.1 × 105 to 6.4 × 105 CFU per 100 mL in Asia (India, Nepal and Iran), and 4.2 × 104 to 5.4 × 104 CFU per 100 mL in Spain5,38,39. Therefore, the contamination levels were higher than the results obtained in our study (5.00 × 103 to 2.50 × 104 CFU per 100 mL).
The selection of the sampling locations was an important step for the analysis of the water quality. In our study, all sampling locations were selected from dense urban areas and downstream of the most contaminated zones (Table 1). It is important to mention that the levels of total coliforms and E. coli were obtained at a similar order of magnitude, suggesting that most of the total coliforms were constituted by typical E. coli from animals and humans’ enteric origin. Most likely, these results evidenced environmental contamination of the rivers set by urban sewages, as previously reported40. Although all water samples were collected from areas of high population density, the contamination in our study was most probably due to the lack of wastewater treatment plants. Untreated sewage, combined with the geographical locations and the ambient temperatures, could contribute to the bacteria proliferation in surface waters6.
Next, we reported the presence of three Escherichia coli pathotypes (EAEC, EPEC, and EIEC). EHEC was not detected in any samples from our study. Although EHEC is one of the most prevalent E. coli pathotypes among environmental samples, Stanford et al. demonstrated seasonal variations in the prevalence of E. coli pathotypes41. The lack of positive EHEC results could be due to the cross-sectional study realized during a single season, showing one of the limitations of the present study. The EIEC was the most prevalent E. coli pathotype and it was found in five rivers. On the other hand, the EPEC and EAEC pathotypes were detected only once. More exactly, the EPEC was found in Zamora River while the EAEC was observed in Machángara River. These E. coli strains are more commonly found in rivers from developing countries, even in surface water resources42. E. coli pathotypes even on samples with low concentrations of total coliforms and E. coli constitute a greater threat to public health. All E. coli pathotypes are potentially dangerous to the population (particularly, in children). E. coli pathotypes may cause urinary tract infections, bacteremia, and bacterium-related diarrhea, being also the main cause of neonatal meningitis in humans and animals25. These findings represent a possible public health problem taking into account the type of distribution of the untreated water to the surrounding population, where the river water is usually used for numerous local practices (domestic, agricultural, live stocking, and even recreational activities). Currently, public health officials rely on infection reports by certain communities (such as indigenous, and rural communities) or public health outbreaks for assessing pathogen and/or chemical levels in water resources. So, future monitoring should be simultaneously realized in untreated wastewaters and natural freshwater resources. Finally, besides the standard quantification of E. coli and total coliforms, the detection of E. coli pathotypes could be useful as an additional indicator in water analysis to prevent waterborne disease outbreaks.
Physicochemical parameters of surface waters
The majority of values found in the rivers were below the maximum limits established by the local legislation. However, certain parameters, such as TSS (132.5 to 939 mg L−1 > 130 mg L−1), CODTOTAL (48.37 to 349.73 mg L−1 > 40 mg L−1), and TS (1657.50 to 3667.50 mg L−1 > 1600 mg L−1), were above Maximum Contaminant Levels (MCL; Fig. 2). In Ecuador, few studies assessed these chemical parameters in rivers 21,24. Voloshenko-Rossin and colleagues evaluated some physicochemical parameters in the San Pedro, Guayllabamba and Esmeraldas Rivers21, obtaining similar values of pH, conductivity, dissolved oxygen (DO), and turbidity when compared to our study. In Guayas, Damanik-Ambarita and colleagues studied the water quality of the Guayas River basin, evidencing also analogous values of pH, temperature, and DO. However, other physicochemical parameters were reported in lower levels when compared to our results, such as conductivity, turbidity, CODTOTAL, and TSS24. Other studies in Latin American countries also analyzed these basic parameters reporting similar levels of temperature, pH, and turbidity, such as Brazil42.
The high conductivity values were found in Guayas (4137.33 µS cm−1) and Esmeraldas (938.53 µS cm−1) Rivers. However, samples from the Guayas and Esmeraldas Rivers were collected in the urban area located near the Pacific Ocean, and so their high conductivity values could be associated with the presence of high concentrations of certain salts (such as Na and Mg) due to the entrance of sea waters. When measuring mixed water or saline water, conductivity values can easily achieve values greater than 5000 µS cm−1, in which case these rivers demonstrated normal conductivity values32. It is important to mention that samples from the Guayas River could have also shown a higher conductivity due to geological factors of the studied area, where it possesses clay soil. Therefore, it was expected to find high indices of conductivity among Guayas and Esmeraldas Rivers in opposite to rivers with granite associated soils (such as Toachi, Tomebamba, and Zamora Rivers), where this type of soil does not ionize and usually shows low conductivity values. In addition, brackish water samples with high conductivity values generally show higher values of TS and TSS, as previously detected in Guayas and Esmeraldas Rivers. Therefore, their higher TS and TSS values were considered normal among brackish systems32. On the other hand, DO values were quantified between 6.08 and 8.30 mg L−1, being slightly above the minimum value allowed by the Ecuadorian Legislation (at least 6 mg L−1 or 80% saturation). It is important to mention that DO values could vary with temperature12,18, where higher temperatures usually diminished dissolved oxygen levels in the water. The dissolved O2 range measured in the rivers of this study was found to be suitable for natural waters depending on turbulence, temperature, salinity, and altitude43.
Trace metals in surface waters
The majority of the elements were below the permitted limit for water aimed at agricultural use or for the preservation of aquatic and wildlife (Fig. 4)32,44. However, some levels of Cu, Pb, and Fe, and most levels of Zn and Al were the exceptions, showing high concentrations above the MCL at several sampling points. Although the maximum values recommended by the WHO are usually lower than the Ecuadorian legislation, it is important to mention that most of the elements were below both limits.
Nevertheless, Guayas and Machángara Rivers, indicators of the surface water quality of the two most populated cities of Ecuador (Guayaquil and Quito, respectively), and Chone River registered concentrations of Pb ten times higher than the maximum contaminant level (1 µg L−1). Lead is considered an important toxic heavy element in the environment, affecting almost every function in humans45. Even though lead is naturally present in the environment, anthropogenic activities (fossil fuels burning, mining, and manufacturing) contribute to its increase45. The Pb levels found in these three rivers were similar to the contamination levels reported by Cui et al. in urban zones of rivers in Northeast China (Harbin City)46. It is important to mention that the values of lead contamination in our study were very close to the limit of quantification (LOQ; 10.12 µg L−1). Therefore, it is plausible that these concentrations could not be accurately distinguished in these rivers. However, Machángara River already showed superior lead contamination (59.7 µg L−1) in a previous study23.
Cu was detected in the Guayas, Machángara, and Guayllabamba Rivers at concentrations exceeding the Ecuadorian guidelines. Similar contamination values of Cu were already reported in other countries, such as Bangladesh (50–100 µg L−1)47 and Canada (1–110 µg L−1)48. Some sources mentioned that these levels of Cu could be associated with the contamination from water pipes from households or industries49. However, other countries, such as Chile (170–630 µg L−1)50 and the USA (10–570 µg L−1)51, reported higher values of Cu on rivers. These higher concentrations could be explained by mining industries or activities near the water sources. Excess copper induces oxidative stress, DNA damage, and reduced cell proliferation leading to copperiedus52.
Although Zn is an essential element for all organisms, an excess of zinc plays a significant role in cytotoxic events in the cells. This element is involved in cell death of the brain, and its cytotoxicity induces ischemia or trauma53. In our study, eight rivers revealed Zn levels above the quality criteria32, ranging from 1.5 until 4.2 times higher than the MCL (30 µg L−1). These levels were still found below contamination levels from other studies realized in China46 and Brazil54. However, our levels of Zn are superior to the levels reported in Argentina55. These authors analyzed water samples from La Plata basin, showing levels of Zn between 0.2 and 11.9 µg L−1. Although their levels of Zn were below our results, these authors suggested that people would eventually experience high health risks through continuous consumption. So, these health risks are also plausible to the Ecuadorian population exposed to the rivers in our study.
Furthermore, Al and Fe were detected in values higher than those established by WHO (2011), by Ecuadorian legislation, or even in surface waters used for human consumption in the country56. As previously described, Al comes mainly from natural sources being one of the main constituents of the silicates that make up the mineral clay57. More exactly, Al concentrations were quantified between 0.49 and 30.80 mg L−1. Interestingly, seven rivers showed similar elevated Al concentrations (around 22 mg L−1). However, a previous study in Ecuador already reported analogous Al concentrations (17.30–18.25 mg L−1) in seven of eighteen rivers of Pichincha province23. These similar levels can probably be attributed to a strong build-up of Al from natural resources rather than directly from wastewater discharges due to anthropogenic activities. It is important to mention that Ecuador is a country famous for its large number of volcanoes contributing to the Al accumulation in soil and natural water resources58. Therefore, it is plausible that the high levels of Al in surface water on these locations did not differ significantly between them even with the anthropogenic activities in the urban areas of the rivers. Even though anthropogenic activities, such as discharge of industrial and domestic effluents, use of agricultural chemicals, land use, and cover changes, are typically the major factors that influence surface water quality. Accumulative exposure to this metal in low concentrations does not cause any harm to humans or animals. However, high concentrations of metals (such as Al) can trigger complications in the kidney due to metal accumulation and also induce cases of infertility in animals58 but its bioavailability depends on its species. Dissolved Al in water may induce risk for human health when reaching values for the internal aluminum load above 15 µg L−1 in urine or 5 µg L−1 in serum59. In Ecuador, the MCL of Al for the preservation of aquatic and wildlife in fresh and marine water is 0.1 mg L−1. Therefore, most rivers surpassed this legal value by more than 200 times, excepting the Guayllabamba River (approximately 5 times more than the MCL). The accumulative exposure of Al in these rivers could be potentially dangerous for aquatic life and even for human through regular water consumption. On the other hand, high concentrations of Fe were only detected in the Guayas (6.84 mg L−1) and Guayllabamba (0.46 mg L−1) Rivers, showing approximately a Fe concentration in the Guayas River of 10 times higher than the MCL (0.7 mg L−1) recommended by the World Health Organization44. Although this Fe concentration is not an immediate danger to public health, cumulative Fe contamination could cause hemorrhagic necrosis and disorders in the stomach mucosa60. So, further studies should monitor Fe variations in these rivers.
Previous studies18,60 reported similar metal analysis, showing also elevated concentrations of dissolved Fe, Mn, Al, Pb, and Zn. These large metal concentrations are usually associated with high soil erosion and discharges of contaminated water from different anthropogenic activities (such as industrial, oil, and agricultural), and followed by several public health issues in the surrounding communities, such as neurological problems, skin irritation, hormonal imbalances, atopic dermatitis, and thyroid problems2.
In Latin America, in the last decades, high concentrations of metals have been found in several rivers2,42,60. In Colombia, Cd and Pb were the highest metal values found nearby crops of vegetables and legumes2. These studies reported the contamination by several metals in water resources and warned for the use of these waters in the food industry (livestock and agriculture). Likewise, studies in the United States realized similar metal analysis in water supplies, showing significantly lower metal concentrations51,61. These low levels of metals in surface water could be due to the strict national regulations that control the heavy metal levels of effluents from large-scale industries61.
In summary, the main rivers of Ecuador showed unacceptable microbial, physicochemical, and metal levels for the preservation of aquatic and wildlife in freshwater, nor human consumption or bathing waters, and agriculture activities. To the best of our knowledge, this is the first study in Ecuador that simultaneously analyzed the microbial and physicochemical parameters in the three main natural regions (Coastal, Andean, and Amazonian), demonstrating statistically significant differences between these regions. However, this statistical analysis should be validated in future studies with a greater number of samples. Also, it is important to mention that there are some major limitations of the present study: (1) it is a cross-sectional study, and therefore unable to evaluate seasonal variations of microbial and physicochemical levels, (2) all physicochemical analyses were realized using water samples taken once in each river, (3) the sampling points were selected close to the main cities or even in urban areas, and it would be useful to extend this monitoring downstream in order to evaluate the extension of the observed contamination, and (4) this study only evaluated the presence or absence of several bacterial genera through PCR analysis without sequencing analysis.
Despite the increasing legislation in Ecuador, there is still an exceedance of the established standards, which suggests that practical control on effluent levels is underdeveloped. Finally, it is essential to evaluate a future scenario of reversing these high rates of microbial and chemical contamination by installing efficient wastewater treatment plants.
Material and methods
Sample site and collection
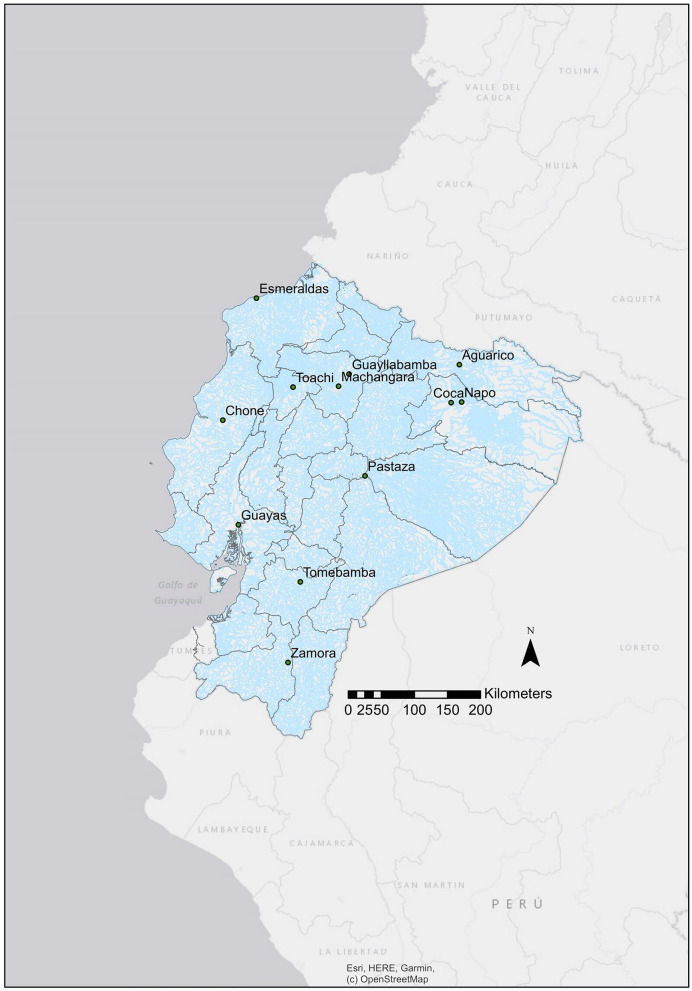
Surface water samples were taken from rivers located along with eleven provinces of Ecuador (Fig. 6), twelve rivers were selected due to their proximity to high-populated cities and their location in the three geomorphologic regions (Coastal, Andean, and Amazonian). All samples were collected from urban sites, where the population lived close to the rivers. Water samples for the microbial analysis were taken on three different dates of collection during a month in each river (Table 1), while water samples for the physicochemical and metal analysis were collected only once on the last collection date. All microbial and analytical methods described below are similar to our previous publication realized by Borja et al.23 and reference publications cited in each subsection. For microbial analysis, samples were taken in previously sterilized glass containers by autoclaving at 121 ºC for 15 min. A total volume of 800 mL was collected from each river. Additionally, for the physicochemical analysis, water samples were taken once in each river, between April and July 2016, during the high-water stage for Amazonian Rivers, and the values of each parameter were obtained by triplicate measurements of each analyzed river sample. For chemical analysis and trace metallic elements, surface water samples were collected in amber glass bottles cleaned in a muffle oven at 550 ºC and in acid clean 1 L Teflon bottles previously washed with 10% hydrochloric acid and later rinsed with distilled water, respectively. Dissolved and suspended phases were separated immediately after collection by vacuum filtration using a 0.45 µm cellulose pre-cleaned filter. For metal analysis, the filtrate was transferred to acid cleaned high-density polyethylene Nalgene bottles and preserved with high purity concentrated nitric acid (HNO3; Merck, Massachusetts, USA) to obtain a final concentration of 2% v/v at the Laboratory of Environmental Engineering at Universidad San Francisco de Quito USFQ (LIA-USFQ).
Figure 6.
Illustration of the collection sampling points selected in this study for the microbial and chemical evaluation of the main Ecuadorian rivers. Legend: The map of Ecuador with the collection sampling points was created through ArcGIS Desktop software (version 10.8, available online: https://desktop.arcgis.com/es/).
Sample preparation
Surface water samples (800 mL) were filtered through a nitrocellulose membrane 0.45 μm (Millipore) into a vacuum pump under aseptic conditions (Chemical Duty Pump, Millipore Inc.). Then, the following procedure was adapted from a previous study realized by Dobrowsky and colleagues with slight modifications3. Briefly, the membrane was removed and placed in a sterile falcon tube with 20 mL of distilled water. The tube was vortexed over 15 min to suspend the particles and microorganisms. The membrane was removed, and the tubes were centrifuged at 5000 rpm for 15 min to precipitate the sediments. The obtained pellet was suspended in 500 μL of sterile distilled water. Subsequently, this sample was then divided for bacterial DNA extraction through Power Soil Extraction Kit (Mo Bio Laboratories Inc.) and for bacterial growth cultures.
Cultivation, quantification, and isolation of dominant bacteria from river samples
Different media cultures were employed to isolate or count the most diverse microorganisms in the samples. More precisely, a volume of 50 μL of the previous aliquot (pellet sample suspended in sterile distilled water) was incubated on MacConkey agar (Difco Laboratories Inc.) at 37 ºC for 18 to 24 h for the recovery of the genus Escherichia; on Salmonella-Shigella agar (Difco Laboratories Inc.) for the cultivation of Salmonella and Shigella genera at same conditions; on Legionella CYE agar (Difco Laboratories Inc.) at 35 ºC for 48 h to isolate Legionella spp.; and on Campylobacter agar for the isolation of Campylobacter spp. at 37 ºC for 18 to 24 h. Finally, for the quantification of Escherichia coli and total coliforms, successive dilutions of the initial aliquot were cultured in Chromocult agar medium (Biolab Laboratories, Merck Inc.) through classic dilution method62, and the results were obtained after 24–48 h of culture.
DNA extraction
DNA from the microbial community in water samples was extracted following the instructions of the commercial PowerSoil DNA Isolation Kit (Mo Bio Laboratories Inc.). Briefly, 250 µL of the pellet obtained from the river water filtration was placed in the PowerBead tubes. The PowerBead tubes contained a buffer that allowed to disperse the soil particles and to dissolve humic acids while protecting nucleic acids from degradation. Later, solution C1 was placed, which contained sodium dodecyl sulfate (SDS) and other disruption agents required for complete cell lysis. Then, a step of 20 min vortexing was performed for complete homogenization and cell lysis in the samples. Subsequently, the tubes were centrifuged at 10,000 × g for 30 s at room temperature. A total volume of 500 μL of the supernatant was taken and placed in a 2 mL Collection Tube, afterward 250 μL of solution C2 was added, and the total volume in the tubes was incubated at 4 ºC for 5 min. Solution C2 contained a flocculant mixture (a combination of ammonium acetate, magnesium chloride (MgCl), ferric chloride (Fe(Cl), a salt of iron, a salt of aluminum, calcium chloride (CaCl), polyacrylamide, aluminum ammonium sulphate, and derivates) to precipitate non-DNA organic and inorganic material including humic substances, cell debris, and proteins. The tubes were centrifuged at 10,000 × g for 30 s at room temperature. 600 mL of supernatant from each tube was transferred to a new 2 mL Collection tube with 200 μL of solution C3. Solution C3 allowed to precipitate additional non-DNA organic and inorganic material. The tubes were centrifuged at 10,000 × g for 30 s at room temperature and 750 μL of the supernatant was mixed with 1.2 mL of Solution C4 (a high concentration salt solution). Half volume was placed inside Spin Filter and centrifuged at 10,000 × g for one minute at room temperature. Afterward, the liquid was discarded and the previous step was repeated twice with the remaining volume. In the next step, 500 μL of the C5 solution was added inside the Spin Filter, centrifuged at 10,000 × g for 30 s at room temperature, and discarded the liquid in each tube. The tubes were again centrifuged at 10,000 × g for 1 min at room temperature, removing the residual solution C5. Carefully the Spin Filter was placed on a new 2 mL Collection Tube. Finally, 100 μL of solution C6 sterile elution buffer was added to the center of the filter membrane. Then the tubes were centrifuged for 30 s at 10,000 × g and the Spin Filter was discarded. The DNA solution of each tube was stored at − 20 °C for further PCR analysis.
Molecular identification of bacterial genera
Once the genomic DNA had been extracted from the different samples, specific primer pairs from previous studies were employed to identify several bacterial genera by polymerase chain reaction (PCR; see Table S4 for additional information). The PCR mixtures consisted of a final volume of 20 μL, containing 4 μL of 5X Green GoTaq Flexi buffer (1X final concentration; Promega, Madison, USA), 1.6 μL of MgCl2 (2.0 mM final concentration; Promega, Madison, USA), 0.2 μL of dNTP Mix (0.1 mM final concentration; Promega, Madison, USA), 1.0 μL of each PCR primer (0.5 μM final concentration; Table S4), 0.3 μL of GoTaq Flexi DNA polymerase (1.5 U final concentration; Promega, Madison, USA), 2 μL of template DNA, and the remaining volume of DNA-free water. For Shigella and Salmonella spp., the same PCR mix was used, with the exception that 0.2 μL of each primer (0.1 μM) was added. For Pseudomonas, Legionella, and Campylobacter spp., the same reaction mixture was used, with the exception that 0.8 μL, 1.0 μL, and 0.6 μL of each primer (0.3 μM) was added, respectively. The PCR analysis was performed in a thermocycler (Bio-Rad Laboratories Inc.) with the standard procedure illustrated in Table S4. The respective use of negative (without DNA sample and samples with other DNA-related bacteria) and positive (collection of identified strains of each genus or species through DNA sequencing) controls were used in each PCR assay. These positive controls were provided by the Microbiology Institute at Universidad San Francisco de Quito (MI-USFQ). All samples were randomly performed in triplicate with different negative and positive controls.
Molecular identification of Escherichia coli pathotypes
For the molecular identification of E. coli pathotypes, the PCR mixtures consisted of a final volume of 20 μL, containing 4 μL of 5X Green GoTaq Flexi buffer (1X final concentration; Promega, Madison, USA), 2 μL of MgCl2 (2.5 mM final concentration; Promega, Madison, USA), 0.4 μL of dNTP Mix (0.2 mM final concentration; Promega, Madison, USA), 0.5 μL of GoTaq Flexi DNA polymerase (2.5 U final concentration; Promega, Madison, USA), 2 μL of template DNA, and the remaining volume of each PCR primer and DNA-free water. Volumes of 0.6 μL for EAEC, 1 μL for EHEC, 0.5 μL for EPEC, and 0.8 μL for EIEC were added of each PCR primer set (0.5 μM final concentration; see Table S5 for additional information). The positive control pathotypes (previously sequenced E. coli isolates, such as EHEC O157:H7 and EAEC 3591–87) were provided by the MI-USFQ from the microbial collection. All samples were randomly performed in triplicate with different negative and positive controls.
PCR product analysis
The PCR products were visualized using electrophoresis in 2% agarose gels and staining with ethidium bromide 0.1%, with the respective use of negative and positive controls.
Analytical methods
Physicochemical characterization of water samples was conducted, as described previously by Benitez et al. (2018) and Grube et al. (2020) according to US Standard Methods from the American Public Health Association14,22. Dissolved oxygen (DO), temperature (SM 4500-O A), conductivity (SM 2510), and pH (SM 4500 H+) were measured in situ with a portable multiparameter and corresponding probes (Thermo Fisher Scientific Model A329, Waltham, USA). Turbidity (EPA 180.1 Rev 2.0) was measured using a portable turbidimeter (Thermo Fisher Scientific AQUAFast AQ4500, Waltham, USA). Ammonium (NH4+; SM 4500-NH3), nitrate (NO3−; SM 4500-NO3−D), and chlorides (Cl−; SM 4500 Cl−D) were measured using an ion-selective electrode (Thermo Specific Ion Selective Electrode, ISE Orion). A calibration curve between potential (mV) and concentrations (R2 = 0.99) was constructed for every test. Chemical oxygen demand (CODTOTAL; SM 5520) and phosphates (PO43−; SM 4500-P B) were measured by a colorimetric method, using a Spectronic 20D + spectrophotometer (Thermo Fisher Scientific, Waltham, USA). Sulphates (SO42−; SM 426 C) were measured following filtrations, using Whatman glass microfiber filters (Grade 934-AH). Total solids (TS) (SM 2540 B) and total suspended solids (TSS) were measured using 0.45 µm cellulose filters, and dried in a 40 GC Lab Oven. Metal analysis on filtered and acidified water samples was conducted with a ThermoScientific iCAP 7400 ICP-OES at the LIA–USFQ. Standard solutions were prepared in dilute nitric acid from commercial standards (Sigma Aldrich, Trace–CERT multielement standard solution 6, USA). The detection and quantification limits were calculated by analyzing blank samples with at least 8 replicates, adding the average of the blank values with 3 and tenfold the standard deviation to obtain the limit of detection (LOD) and the limit of quantification (LOQ), respectively.
Quality assurance/quality control
Quality control in metals analysis was conducted employing CRM 1640a–Trace elements in natural waters (National Institute of Standards and Technology, Gaithersburg, USA), which was measured every 10 samples (see Table S6 for additional information). Recovery percentages were calculated to determine matrix effects and measurements accurateness, and all concentrations were corrected based on the percentage recoveries. The recoveries varied between 89.43 and 105.42% for nickel and calcium, respectively.
Statistical analysis
The data obtained from the microbial and physicochemical analysis of the water samples was analyzed by using the statistical software SPSS version 23.0 package. Linear and multiple logistic regressions were performed between the concentration of E. coli and coliforms, physicochemical parameters, and metal concentrations. To evaluate the relevance of the correlations, P-values were then adjusted for multiple testing using the Benjamini–Hochberg (BH) method63 implemented in RStudio software (version 1.3.1073; https://rstudio.com/). Adjusted P-values by the BH method were obtained using the option method = ~ BH ~ of the p.adjust function from the stats base R package (Package stats version 4.1.0). In all hypothesis tests, a significant level of 5% was used as the standard. Also, differences in contamination between Andean, Coastal, and Amazonian regions were evaluated using Kruskal–Wallis non-parametric one-way analysis of variance with Mann–Whitney test for paired comparisons. In all tests, a P-value below 0.05 was considered a statistically significant value.
Ethical approval and consent to participate
The study did not require approval from the research ethics committee as it did not involve human subjects or records.
Consent to publish
The authors declare that they consented to publish the present study.
Supplementary Information
Acknowledgements
We thank the volunteers that made possible this study, also the people who work at the Institute of Microbiology of USFQ, in particular Pamela Borja, Jose Carrera, and Juan Zurita, who helped in the development of analysis in the laboratory. We also thank Pamela Borja and Izan Chalen for creating the map of this study. We thank Christian Gallardo and Nicolas Saud for participating in the sampling campaigns. Finally, we would like to thank the Laboratory of Environmental Engineering at USFQ (LIA-USFQ) for metal analysis (ThermoScientific iCAP 7400 ICP-OES) and the Research Office of Universidad San Francisco de Quito for financial support.
Author contributions
Molecular analysis: Dayana Vinueza (DV). DNA extraction: DV. Microbiological analysis: DV and Lorena Mejía (LM). Physicochemical analysis: Esteban Tamayo (ET) and Laurence Maurice (LMA). Study design, project coordination, and help with the manuscript draft: António Machado (AM) and Valeria Ochoa-Herrera (VOH). Data analysis: DV, ET, LM, Eduardo Tejera (ETE), VOH, LMA, and AM. Original Draft Preparation: DV and AM. Review and Editing: VOH, LMA, ETE, and AM.
Funding
This work was supported by Chancellor Grant 2015 and COCIBA research budget from Universidad San Francisco de Quito, under the Project ID: 1106 entitled ''Characterization and quantification of microbial pathogens in the natural water resources of Ecuador'' and POLITECNICO research grant from Universidad San Francisco de Quito under the Project ID: 16914 entitled ¨Heavy metal assessment in freshwater and sediments in Ecuador¨. This work was supported by Chancellor Grants from Universidad San Francisco de Quito under jurisdiction of the Contrato Marco de Acceso a los Recursos Genéticos No. MAE-DNB-CM-2016-0046. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Data availability
The data that support the findings of this study are available on request from the corresponding author and Supplementary Information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-96926-z.
References
- 1.Ferronato C, et al. Chemical and microbiological parameters in fresh water and sediments to evaluate the pollution risk in the Reno River Watershed (North Italy) J. Water Resour. Prot. 2013;5:458–468. doi: 10.4236/jwarp.2013.54045. [DOI] [Google Scholar]
- 2.Reyes J, Vergara I, Torres O, Díaz M, González E. Contaminación por metales pesados: implicaciones en salud, ambiente y seguridad alimentaria. Rev. Ing. Investig. y Desarro. 2016;16:66–77. [Google Scholar]
- 3.Dobrowsky PH, De Kwaadsteniet M, Cloete TE, Khan W. Distribution of indigenous bacterial pathogens and potential pathogens associated with roof-harvested rainwater. Appl. Environ. Microbiol. 2014;80:2307–2316. doi: 10.1128/AEM.04130-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramírez Castillo FY, et al. Presence of multi-drug resistant pathogenic Escherichia coli in the San Pedro River located in the State of Aguascalientes, Mexico. Front. Microbiol. 2013;4:1–16. doi: 10.3389/fmicb.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almeida SFP, et al. Water quality assessment of rivers using diatom metrics across Mediterranean Europe: A methods intercalibration exercise. Sci. Total Environ. 2014;476–477:768–776. doi: 10.1016/j.scitotenv.2013.11.144. [DOI] [PubMed] [Google Scholar]
- 6.United Nations Water. Environmental Indicators. 1–31 https://www.unwater.org/publications/un-water-annual-report-2011/ (2011).
- 7.National Institute of Statistics and Censuses. Measurement of the SDG indicators of Water, Sanitation and Hygiene (ASH) in Ecuador. https://www.ecuadorencifras.gob.ec/documentos/web-inec/EMPLEO/2017/Indicadores ODS Agua, Saneamiento e Higiene/Presentacion_Agua_2017_05.pdf (2017).
- 8.Ortiz, D. The paradox of the Amazon River and drinking water. El Comercio (2016).
- 9.Sorgato, V. Water samples from the country’s rivers alerted population about pollution. El Comercio (2017).
- 10.News Unit of El Comercio. The Vindobona project starts for the decontamination of the rivers of Quito. El Comercio (2019).
- 11.Ministry of Public Health of Ecuador. https://www.salud.gob.ec/ (2015).
- 12.Gerhard WA, Choi WS, Houck KM, Stewart JR. Water quality at points-of-use in the Galapagos Islands. Int. J. Hyg. Environ. Health. 2017;220:485–493. doi: 10.1016/j.ijheh.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Levy K, Nelson KL, Hubbard A, Eisenberg JNS. Rethinking indicators of microbial drinking water quality for health studies in tropical developing countries: Case study in northern coastal Ecuador. Am. J. Trop. Med. Hyg. 2012;86:499–507. doi: 10.4269/ajtmh.2012.11-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grube AM, Stewart JR, Ochoa-Herrera V. The challenge of achieving safely managed drinking water supply on San Cristobal Island, Galápagos. Int. J. Hyg. Environ. Health. 2020;228:113547. doi: 10.1016/j.ijheh.2020.113547. [DOI] [PubMed] [Google Scholar]
- 15.Liang X, et al. E. coli surface properties differ between stream water and sediment environments. Front. Microbiol. 2016;7:1–10. doi: 10.3389/fmicb.2016.01732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler Miserendino R, et al. Challenges to measuring, monitoring, and addressing the cumulative impacts of artisanal and small-scale gold mining in Ecuador. Resour. Policy. 2013;38:713–722. doi: 10.1016/j.resourpol.2013.03.007. [DOI] [Google Scholar]
- 17.Carling GT, et al. Particulate and dissolved trace element concentrations in three Southern Ecuador rivers impacted by artisanal gold mining. Water Air Soil Pollut. 2013;224:1415. doi: 10.1007/s11270-012-1415-y. [DOI] [Google Scholar]
- 18.PerezNaranjo C, et al. Determinación de elementos mayores en sedimentos provenientes de zonas afectadas por actividades petroleras en Ecuador. Av. Cienc. Ing. 2015;7:271. [Google Scholar]
- 19.National Institute of Statistics and Censuses. Statistical Compendium 2013. INEC vol. 01 1–92 https://www.ecuadorencifras.gob.ec/documentos/web-inec/Bibliotecas/Compendio/Compendio-2013/compendio_estadistico_2013.pdf (2013).
- 20.Metropolitan Public Drinking Water and Sanitation Company. Wastewater Treatment Plants in Quito. https://www.aguaquito.gob.ec/.
- 21.Voloshenko-Rossin A, et al. Emerging pollutants in the Esmeraldas watershed in Ecuador: discharge and attenuation of emerging organic pollutants along the San Pedro–Guayllabamba–Esmeraldas rivers. Environ. Sci. Process. Impacts. 2015;17:41–53. doi: 10.1039/C4EM00394B. [DOI] [PubMed] [Google Scholar]
- 22.Benítez MB, Champagne P, Ramos A, Torres AF, Ochoa-Herrera V. Wastewater treatment for nutrient removal with Ecuadorian native microalgae. Environ. Technol. (United Kingdom) 2018;3330:1–9. doi: 10.1080/09593330.2018.1459874. [DOI] [PubMed] [Google Scholar]
- 23.Borja-Serrano P, et al. Determination of the microbial and chemical loads in rivers from the Quito capital province of Ecuador (Pichincha)—A preliminary analysis of microbial and chemical quality of the main rivers. Int. J. Environ. Res. Public Health. 2020;17:1–26. doi: 10.3390/ijerph17145048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damanik-Ambarita MN, et al. Impact assessment of local land use on ecological water quality of the Guayas river basin (Ecuador) Ecol. Inform. 2018;48:226–237. doi: 10.1016/j.ecoinf.2018.08.009. [DOI] [Google Scholar]
- 25.Vasco G, et al. Identifying etiological agents causing diarrhea in low income Ecuadorian communities. Am. J. Trop. Med. Hyg. 2014;91:563–569. doi: 10.4269/ajtmh.13-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhavnani D, et al. Distribution of enteroinvasive and enterotoxigenic Escherichia coli across space and time in northwestern Ecuador. Am. J. Trop. Med. Hyg. 2016;94:276–284. doi: 10.4269/ajtmh.14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 28.Law JWF, Mutalib NSA, Chan KG, Lee LH. Rapid methods for the detection of foodborne bacterial pathogens: Principles, applications, advantages and limitations. Front. Microbiol. 2014;5:1–19. doi: 10.3389/fmicb.2014.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.EPA. Recreational Water Quality Criteria. U. S. Environ. Prot. Agency 1–69. https://www.epa.gov/sites/default/files/2015-10/documents/rwqc2012.pdf (2012).
- 30.Council of the European Union. Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality and repealing Directive 76/160/EEC. http://data.europa.eu/eli/dir/2006/7/2014-01-01 (2006).
- 31.Ambiente MDM. Brazilian guidelines for bathing waters were established by Resolution CONAMA no. 274, of 29 November 2000. Dou. 2001;18:70–71. [Google Scholar]
- 32.Ministry of Environment of Ecuador (MAE). Quality criteria for the preservation of aquatic and wildlife in fresh and marine water, and estuary. in Decret 097A.Texto Unificado de Legislación Ambiental Secundaria TULSMA (2015) Book VI. Table 2 (2015).
- 33.Ministry of Environment of Ecuador (MAE). Discharge limits to a fresh water body. In Decret 097A.Texto Unificado de Legislación Ambiental Secundaria TULSMA Table 9 (2015).
- 34.Ávila de Navia, S. L. & Estupiñán Torres, S. M. Bacteriological quality of the water for human consumption in urban and rural areas of the municipality of Guatavita, Cundinamarca, Colombia. Revista Cubana de Higiene y Epidemiología. 50, 163–168 (2012).
- 35.Rodríguez C, Leiva-Aravena E, Serrano J, Leiva E. Occurrence and removal of copper and aluminum in a stream confluence affected by acid mine drainage. Water. 2018;10:1–13. doi: 10.3390/w10020001. [DOI] [Google Scholar]
- 36.Mason OU, Canter EJ, Gillies LE, Paisie TK, Roberts BJ. Mississippi river plume enriches microbial diversity in the northern gulf of Mexico. Front. Microbiol. 2016;7:01048. doi: 10.3389/fmicb.2016.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bower PA, Scopel CO, Jensen ET, Depas MM, Mclellan SL. Detection of genetic markers of fecal indicator bacteria in Lake Michigan and determination of their relationship to Escherichia coli densities using standard microbiological methods detection of genetic markers of fecal indicator bacteria in Lake Michiga. Society. 2005;71:8305–8313. doi: 10.1128/AEM.71.12.8305-8313.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Blasi JIP, et al. Analysis and detection of outliers in water quality parameters from different automated monitoring stations in the Miño river basin (NW Spain) Ecol. Eng. 2013;60:60–66. doi: 10.1016/j.ecoleng.2013.07.054. [DOI] [Google Scholar]
- 39.Kittinger C, et al. Water quality assessment of a Central European River—Does the Directive 2000/60/EC cover all the needs for a comprehensive classification? Sci. Total Environ. 2013;447:424–429. doi: 10.1016/j.scitotenv.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Silva S, Rodrigues CF, Araújo D, Rodrigues ME, Henriques M. Candida species biofilms’ antifungal resistance. J. Fungi. 2017;3:8. doi: 10.3390/jof3010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanford K, Johnson RP, Alexander TW, McAllister TA, Reuter T. Influence of season and feedlot location on prevalence and virulence factors of seven serogroups of Escherichia coli in feces of western-Canadian slaughter cattle. PLoS ONE. 2016;11:e0159866. doi: 10.1371/journal.pone.0159866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carvalho KQ, et al. Influence of urban area on the water quality of the Campo River basin, Paraná State, Brazil. Braz. J. Biol. 2015;75:S96–S106. doi: 10.1590/1519-6984.00413suppl. [DOI] [PubMed] [Google Scholar]
- 43.US Environmental Protection. EPA’s Report of the Environment. https://cfpub.epa.gov/roe/documents/EPAROE_FINAL_2008.PDF (2008).
- 44.Gorchev HG, Ozolins G. WHO guidelines for drinking-water quality. WHO Chron. 2011;38:104–108. [PubMed] [Google Scholar]
- 45.Wani AL, Ara A, Usmani JA. Lead toxicity: A review. Interdiscip. Toxicol. 2015;8:55–64. doi: 10.1515/intox-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui S, et al. Heavy metals in sediment from the urban and rural rivers in Harbin City, Northeast China. Int. J. Environ. Res. Public Health. 2019;16:1–15. doi: 10.3390/ijerph16224313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Islam MS, Tusher TR, Mustafa M, Mahmud S. Effects of solid waste and industrial effluents on water quality of Turag River at Konabari Industrial Area, Gazipur, Bangladesh. J. Environ. Sci. Nat. Resour. 2013;5:213–218. [Google Scholar]
- 48.Khan F, Husain T, Lumb A. Water quality evaluation and trend analysis in selected watersheds of the Atlantic region of Canada. Environ. Monit. Assess. 2003;88:221–242. doi: 10.1023/A:1025573108513. [DOI] [PubMed] [Google Scholar]
- 49.Kumpel E, Nelson KL. Mechanisms affecting water quality in an intermittent piped water supply. Environ. Sci. Technol. 2014;48:2766–2775. doi: 10.1021/es405054u. [DOI] [PubMed] [Google Scholar]
- 50.Rivera NR, Encina F, La Mejias AMP. Calidad de las Aguas en los Ríos Cautín e Imperial, IX Región- Chile Water Quality in the Cautín and Imperial Rivers, IX Region-Chile. Inf. Tecnológica. 2004;15:89–101. [Google Scholar]
- 51.Staley C, et al. Bacterial community structure is indicative of chemical inputs in the Upper Mississippi River. Front. Microbiol. 2014;5:1–13. doi: 10.3389/fmicb.2014.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oe S, Miyagawa K, Honma Y, Harada M. Copper induces hepatocyte injury due to the endoplasmic reticulum stress in cultured cells and patients with Wilson disease. Exp. Cell Res. 2016;347:192–200. doi: 10.1016/j.yexcr.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Plum LM, Rink L, Hajo H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reis MM, Tuffi Santos LD, da Silva AJ, de Pinho GP, Montes WG. Metal contamination of water and sediments of the Vieira River, Montes Claros, Brazil. Arch. Environ. Contam. Toxicol. 2019;77:527–536. doi: 10.1007/s00244-019-00666-1. [DOI] [PubMed] [Google Scholar]
- 55.Avigliano E, et al. Distribution and bioaccumulation of 12 trace elements in water, sediment and tissues of the main fishery from different environments of the La Plata basin (South America): Risk assessment for human consumption. Chemosphere. 2019;236:124394. doi: 10.1016/j.chemosphere.2019.124394. [DOI] [PubMed] [Google Scholar]
- 56.Maurice L, et al. Drinking water quality in areas impacted by oil activities in Ecuador: Associated health risks and social perception of human exposure. Sci. Total Environ. 2019;690:1203–1217. doi: 10.1016/j.scitotenv.2019.07.089. [DOI] [PubMed] [Google Scholar]
- 57.Barraza F, et al. Distribution, contents and health risk assessment of metal(loid)s in small-scale farms in the Ecuadorian Amazon: An insight into impacts of oil activities. Sci. Total Environ. 2018;622–623:106–120. doi: 10.1016/j.scitotenv.2017.11.246. [DOI] [PubMed] [Google Scholar]
- 58.Pourgheysari, H., Hajizadeh, Y., Tarrahi, M. J. & Ebrahimi, A. Association between aluminum and silicon concentrations in Isfahan drinking water and their health risk assessments. Int. J. Prev. Med.6, 111 (2015). [DOI] [PMC free article] [PubMed]
- 59.Klotz K, et al. The health effects of aluminum exposure. Dtsch. Arztebl. Int. 2017;114:653–659. doi: 10.3238/arztebl.2017.0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huaranga Moreno F, Méndez García E, Quilcat León V, Huaranga Arévalo F. Pollution by heavy metals in the Moche River Basin, 1980–2010, La Libertad-Peru. Sci. Agropecu. 2012;3:235–247. doi: 10.17268/sci.agropecu.2012.03.05. [DOI] [Google Scholar]
- 61.Smith DL, Cooper MJ, Kosiara JM, Lamberti GA. Body burdens of heavy metals in Lake Michigan wetland turtles. Environ. Monit. Assess. 2016;188:128. doi: 10.1007/s10661-016-5118-5. [DOI] [PubMed] [Google Scholar]
- 62.Pitkänen T, et al. Comparison of media for enumeration of coliform bacteria and Escherichia coli in non-disinfected water. J. Microbiol. Methods. 2007;68:522–529. doi: 10.1016/j.mimet.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- 64.Rawlings DE, Tributsch H, Hansford GS. Reasons why ’Leptospirillum’-like species rather than Thiobacillus ferrooxidans are the dominant iron-oxidizing bacteria in many commercial processes for the biooxidation of pyrite and related ores. Microbiology. 1999;145:5–13. doi: 10.1099/13500872-145-1-5. [DOI] [PubMed] [Google Scholar]
- 65.Kong RYC, Lee SKY, Law TWF, Law SHW, Wu RSS. Rapid detection of six types of bacterial pathogens in marine waters by multiplex PCR. Water Res. 2002;36:2802–2812. doi: 10.1016/S0043-1354(01)00503-6. [DOI] [PubMed] [Google Scholar]
- 66.Jonas D, Rosenbaum A, Weyrich S, Bhakdi S. Enzyme-linked immunoassay for detection of PCR-amplified DNA of legionellae in bronchoalveolar fluid. J. Clin. Microbiol. 1995;33:1247–1252. doi: 10.1128/jcm.33.5.1247-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spilker T, Coenye T, Vandamme P, LiPuma JJ. PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J. Clin. Microbiol. 2004;42:2074–2079. doi: 10.1128/JCM.42.5.2074-2079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan IUH, Edge TA. Development of a novel triplex PCR assay for the detection and differentiation of thermophilic species of Campylobacter using 16S–23S rDNA internal transcribed spacer (ITS) region. J. Appl. Microbiol. 2007;103:2561–2569. doi: 10.1111/j.1365-2672.2007.03511.x. [DOI] [PubMed] [Google Scholar]
- 69.Toma C, et al. Multiplex PCR assay for identification of human diarrheagenic Escherichia coli. J. Clin. Microbiol. 2003;41:2669–2671. doi: 10.1128/JCM.41.6.2669-2671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author and Supplementary Information.