Abstract
The first genus- and subgenus-specific fluorescent oligonucleotide probes for in situ staining of Acanthamoeba are described. Sequences of these phylogeny-based probes complement the 18S rRNA and the gene encoding it (18S rDNA). The genus-specific probe (GSP) is a fluorescein-labeled 22-mer specific for Acanthamoeba as shown here by its hybridization to growing trophozoites of all 12 known Acanthamoeba 18S rDNA sequence types and by its failure to hybridize with amoebae of two other genera (Hartmannella vermiformis and Balamuthia mandrillaris), two human cell lines, and two bacteria (Pseudomonas aeruginosa and Escherichia coli). The sequence type T4-specific probe (ST4P) is a rhodamine-labeled 30-mer specific for Acanthamoeba 18S rDNA sequence type T4, as shown here in hybridization tests with trophozoites of all 12 sequence types. T4 is the subgenus group associated most closely with Acanthamoeba keratitis (AK). GSP also was tested with corneal scrapings from 17 patients with a high index of clinical suspicion of AK plus 5 patient controls. GSP stained both trophozoites and cysts, although nonspecific cyst wall autofluorescence also was observed. Results could be obtained with GSP in 1 to 2 days, and based on results from cell culture tests, the probe correctly detected the presence or absence of Acanthamoeba in 21 of 24 specimens from the 22 patients. The use of GSP with cultured trophozoites and cysts from corneal scrapings has illustrated the suitability of using fluorescent oligonucleotide probes for identification of the genus Acanthamoeba in both environmental and clinical samples. In addition, the use of ST4P with cultured amoebae has indicated the potential of oligonucleotide probes for use in subgenus classification.
The genus Acanthamoeba consists of small, ubiquitous amoebae that exhibit a biphasic life cycle consisting of a vegetative trophozoite stage and a physiologically static cyst stage. These amoebae have been isolated from a variety of environmental sources (7) and are associated with human infection (17, 24–26, 37, 39). In immunocompromised patients, Acanthamoeba infections include granulomatous amoebic encephalitis (GAE), which is a fatal brain disease, and disseminated infections of various other tissues. In otherwise healthy individuals, the prominent disease is Acanthamoeba keratitis (AK), a potentially sight-threatening eye infection. This disease was discovered in 1973 (19, 27). More than 500 cases of AK had been reported in the United States by 1993 (31), and another 200 cases had been reported by 1997 (25). AK is most prevalent among contact lens (CL) wearers (34). A recent prospective population-based study estimated that the incidence of AK among soft-CL wearers in Scotland was 1 in 6,710 (32). In other countries—for example, India—AK usually is associated with non-CL-associated ocular trauma (33).
Identification of acanthamoebae in ocular and other tissues can be difficult and time-consuming, even for trained microscopists. In histological preparations, these amoebae look very similar to keratoplasts as well as neutrophils and monocytes, and this often leads to false-negative and/or false-positive results. It has been estimated that up to 70% of clinical AK cases are misdiagnosed as viral keratitis (2, 12, 18). Thus, many patients are initially treated with inappropriate drug therapies.
Early detection of Acanthamoeba infection is important because trophozoites, which predominate in the initial stages of infection, are more susceptible to treatment than the subsequent cysts. Calcafluor white (42) and similar fluorescent stains, which often are used to detect Acanthamoeba, stain cyst walls and fail to detect trophozoites. The mean time to diagnosis of AK can average 2.5 weeks longer for non-CL wearers than for CL users (6). This lag time may hamper disease resolution, since several studies have found that diagnosis and treatment within 1 month of onset results in lower morbidity and a better visual outcome (1, 2, 38). Thus, the availability of a rapid, accurate, and relatively simple diagnostic test would significantly enhance the initiation of appropriate chemotherapy.
Several RNA- and/or DNA-based methods are being developed to aid in the detection and accurate identification of Acanthamoeba in clinical and environmental settings. These include PCR with and without DNA sequencing (16, 23, 35, 40), restriction fragment length polymorphism analysis (5, 36), and nucleic acid blotting methods (9). Although these techniques are important diagnostic aids, they involve indirect evaluation of samples. They are unable to distinguish different developmental stages of Acanthamoeba spp. and lack precision in the localization of the organisms in situ. These methods cannot determine whether the majority of amoebae in a sample are cysts or trophozoites; they also cannot discern the density of the infection or the depth of corneal penetration of the amoebae. Traditional histological techniques, in trained hands, often can identify amoebae in situ, but this depends on morphology or immunoreactivity. Furthermore, structural characteristics for identification of acanthamoebae at the subgenus level are unreliable.
The most specific and sensitive technique devised to date for in situ staining is phylogenetic staining, first used by DeLong and coworkers (8) for identification of single cells in samples of mixed species. This technique uses oligonucleotide probes complementary to phylogenetically informative segments of rRNA. Different probes can provide accurate identification of an organism at various levels of taxonomic organization. In addition, specific probes labeled with different chromophores can be used in fluorescent in situ hybridization (FISH) to differentiate the different species or other taxonomic levels in a mixed sample.
The present article reports the development and use of two fluorescent probes. The first identifies all members of the genus Acanthamoeba (genus-specific probe [GSP]); the second is specific for the subgenus group that is most commonly identified in AK infections (sequence type T4-specific probe [ST4P]). We have used these probes for identification of Acanthamoeba in laboratory cultures and in human corneal scrapings from patients presumed to have AK based on traditional methods of microscopy and follow-up cultures.
MATERIALS AND METHODS
Cell cultures and corneal scrapings.
The in situ hybridization of the modified universal probe (MUP), GSP, and ST4P described below was tested with cultured trophozoites from 21 strains (Table 1) representing the 12 different Acanthamoeba 18S rDNA sequence types previously described (35). Cultures were maintained at Ohio State University (OSU) as described previously (4, 10). GSP also was tested with 24 human corneal scraping specimens obtained in a study of microbial keratitis in Scotland (32). Acanthamoeba terricola was a gift from Frederick Schuster, Brooklyn College, Brooklyn, N.Y. Human U937 lymphocytes and human HeLa cells were provided by the OSU laboratories of Ing-ming Chiu and Mark Muller, respectively. The amoeba Balamuthia mandrillaris and the Acanthamoeba strains prefixed by CDC in Table 1 were gifts from Govinda S. Visvesvara of the U.S. Public Health Service’s Centers for Disease Control and Prevention, Atlanta, Ga. Hartmannella vermiformis and Acanthamoeba palestinensis, originally from the Cambridge Collection of Algae and Protozoa (see Table 2), were provided by Peter H. H. Weekers, University of Nijmegen, Nijmegen, The Netherlands. The remainder of the Acanthamoeba cultures (see Table 2) were obtained from Thomas Nerad at the American Type Culture Collection, Manassas, Va. Bacterial specimens were from laboratory cultures at OSU.
TABLE 1.
Acanthamoeba strains used in testing the genus- and T4-specific probesa
| Strain | Source | 18S rDNA sequence type |
|---|---|---|
| Acanthamoeba species strain V006a | CDC0981:V006 | T1 |
| A. palestinensis GE 3aa | ATCC 50252 | T2 |
| A. palestinensis 1501/3ca | CCAP 1501/3c | T2 |
| A. griffini S7 | ATCC 30731 | T3 |
| A. griffini Panola Mt.a | ATCC 30487 | T3 |
| A. castellanii Neff | ATCC 50373 | T4 |
| A. castellanii V042 | ATCC 50493 | T4 |
| A. castellanii V014 | ATCC 50492 | T4 |
| A. castellanii Ma | ATCC 50370 | T4 |
| Acanthamoeba species strain Diamonda | CDC Diamond | T4 |
| A. polyphaga V029 | CDC0884:V029 | T4 |
| A. rhysodes Singh | ATCC 30976 | T4 |
| Acanthamoeba species strain 88-2-37 | ATCC 50497 | T4 |
| A. lenticulata PD2S | ATCC 30841 | T5 |
| A. palestinensis 2802 | ATCC 50708 | T6 |
| A. astronyxis Ray & Hayes | ATCC 30137 | T7 |
| A. tubiashi OC-15c | ATCC 30867 | T8 |
| A. terricola FS | F. Schuster | T9 |
| A. culbertsoni Lilly A1 | ATCC 30137 | T10 |
| A. hatchetti BH-2 | T. Sawyer | T11 |
| A. heayli V013 | CDC1283:V013 | T12 |
Species name has been revised according to the nomenclature of Stothard et al. (35).
TABLE 2.
Comparison of bright-field and phase-contrast microscopy, culture growth, and FISH evaluations of corneal scrapings as follow-up to clinical presumption of AK
| Scraping code no.a | Date of scraping (mo/day/yr) | Clinical diagnosisb | Acanthamoebae detected by microscopy of wet filmsc | Acanthamoebae cultured from scrapingsd | Acanthamoebae detected by GSP |
|---|---|---|---|---|---|
| 1ae | 06/06/95 | Typical AK | − | −/+ | − |
| 1be | 06/07/95 | Typical AK | + | +/+ | + |
| 6 | 06/27/95 | Typical AK | + | +/+ | + |
| 4 | 07/06/95 | Typical AK | − | +/+ | + |
| 12 | 07/10/95 | Typical AK | + | +/+ | + |
| 2 | 09/13/95 | Typical AK | − | +/+ | +p |
| 13 | 10/02/95 | Typical AK | + | +/+ | + |
| 9 | 10/17/95 | Typical AK | + | +/+ | + |
| 10 | 02/05/96 | Typical AKf | − | +/+ | +q |
| 19 | ? | Atypical AKg | − | +/+ | + |
| 18 | 12/03/94 | Atypical AKh | − | +/+ | + |
| 8 | 09/18/95 | Atypical AKi | − | −/+ | +p |
| 5 | 10/12/95 | Atypical AK | − | +/+ | + |
| 15a | 08/12/95 | Typical AKj | ND | −/− | − |
| 7 | 09/07/95 | Typical AKk | − | −/− | − |
| 16a | 09/22/95 | Typical AKk | ND | −/− | − |
| 23 | 10/23/95 | Typical AK | − | +/− | − |
| 3akl | 10/25/95 | Typical AK | − | −/− | − |
| 3bkl | 10/25/95 | Typical AK | − | −/− | − |
| 21 | 05/09/95 | Non-AK BKNm | − | −/− | − |
| 22 | 10/03/95 | Non-AK MKn | − | −/− | − |
| 14 | 12/14/95 | Non-AK Ci | − | −/− | − |
| 11 | 01/24/96 | Non-AK MK | ND | −/− | − |
| 17 | 05/24/96 | Non-AK BKSo | + | −/+ | − |
All samples except no. 15 and 16 were fresh corneal scrapings transferred to sterile isotonic saline. The exceptions are scrapings originally spread on non-nutrient agar plates and then recovered from plate washings.
C, conjunctivitis; MK, presumed microbial keratitis of bacterial origin; BKN, bacterial keratitis due to Neisseria meningitidis; BKS, bacterial keratitis due to Staphylococcus aureus.
Bright-field or phase-contrast microscopy of original wet films. +, acanthamoebae detected; −, acanthamoebae not detected; ND, not done.
Culture results at TIO/OSU.
Scrapings 1a and b were from the same patient.
Severe AK after 3 weeks on chlorhexidine therapy.
Trachoma and acanthamoebae in a non-CL wearer (30).
Large epithelial defect, sporadic diffuse corneal endotheliitis thought to be due to herpes simplex virus in a non-CL wearer.
CL-associated conjunctival inflammation without keratitis.
Patient was on Brolene and neomycin therapy at time of scraping; acanthamoebae found in CL storage case.
Patient was on topical 0.02% chlorhexidine therapy at time of scraping; acanthamoebae found in CL storage case.
Both eyes of same patient; acanthamoebae found in CL storage case.
Basement membrane dystrophy.
Red eye, pain, and photophobia in a CL wearer.
Peripheral ulcer in a CL wearer due to S. aureus, with transient acanthamoebae in the scraping sample (28).
One cyst seen.
Two cysts seen.
The corneal scrapings examined here were isolated by two of us (J.H. and D.V.S.) during a population-based longitudinal study of microbial keratitis in western Scotland (32). Scrapings were placed in sterile isotonic saline after being obtained from the eye by an ophthalmologist. Unstained aliquots were examined by bright-field or phase-contrast microscopy. This was followed by culture of samples for Acanthamoeba in a medium described previously (15). One to 19 months after the initial specimen collection, portions of the original scraping samples were delivered to OSU, where they were retested for growth in culture and analyzed with the GSP probe described in this paper.
Pretreatment and fixation of amoebae and other cells for FISH.
All solutions, with the exception of culture media, were prepared with diethylpyrocarbonate-treated double-distilled water (DEPC water). Portions of the human corneal scrapings (see Table 2) that had been suspended in sterile saline and stored at 4°C for 1 to 19 months were fixed. One milliliter of a laboratory cell culture or 100 μl of a scraping specimen was spun in a Microcentrifuge (Costar model 10) for 15 s to loosely pellet the cells. The medium was pipetted off, and the cells were resuspended by gentle shaking in 1× phosphate-buffered saline (PBS; 0.13 M NaCl, 7 mM Na2HPO4, and 3 mM NaH2PO4 in DEPC water; pH 7.2). The cells were then loosely pelleted again by centrifugation for 15 s. The wash buffer was pipetted off, and the cells were gently resuspended in 1 ml of cold (4°C) 12% freshly depolymerized paraformaldehyde (PFA). The PFA was made by dissolving 6 g of paraformaldehyde in 40 ml of 1× PBS for 10 min at 60°C with stirring. Then 10 ml of 0.1 M NaOH in DEPC water was added to clear the solution, and the mixture was chilled before being used. The cells were fixed at 4°C for at least 5 h and to a maximum of overnight. After being fixed, the cells were loosely pelleted by a 15-s centrifugation. The PFA was pipetted off, and the cells were washed in 1 ml of DEPC water with gentle shaking. The cells were loosely pelleted again by a 15-s centrifugation. The DEPC water was pipetted off, and the cells from cultures or corneal scrapings were resuspended in ∼100 or ∼30 μl, respectively, of 70% ethanol and stored at 4°C indefinitely.
Probes.
Three oligonucleotide probes containing a fluorochrome at the 5′ end were synthesized by Amitof Biotech, Inc. (Boston, Mass.). The 21-base MUP (5′-rhodamine-GWATTACCGCGGCTGCTGGCA-3′) was complementary to positions 653 to 633 in the 18S rRNA of Acanthamoeba castellanii Neff (13). The 22-base GSP (5-fluorescein-TTCACGGTAAACGATCTGGGCC-3′) was complementary to positions 957 to 936 in the Neff strain rRNA. The 30-base ST4P (5′-rhodamine-GCTGCCAAAACCAACTGAAAATAGGAGGAC-3′) was complementary to positions 1066 to 1037 in the Neff strain rRNA. MUP is based on the universal probe designed by Giovannoni and coworkers (11), with three modifications: base 14 was changed from K to T, a G between bases 16 and 17 was removed, and a GCA triplet was added to the 3′ end. Probes were resuspended in DEPC water to a final concentration of 1 μg/ml and stored at −20°C. Immediately before hybridization, probes were diluted to 30 ng/ml in DEPC water.
Whole-cell hybridization.
Cells fixed in suspension were pipetted onto poly(l-lysine)-coated microscope slides (Sigma, St. Louis, Mo.) in 2- to 5-μl aliquots and air dried. The slides were then incubated in a methanol-formalin mixture (9:1) at room temperature for 20 min. The slides were then rinsed briefly in DEPC water and allowed to air dry. Thirty microliters of a hybridization mixture containing 60 ng of each probe in hybridization buffer (0.9 M NaCl, 20 mM Tris [pH 7.8], 5 mM EDTA, 0.01% sodium dodecyl sulfate) was pipetted onto each dry slide and covered with a 24- by 50-mm coverslip. The slides were immediately transferred to a dark chamber humidified by a sponge soaked in DEPC water and incubated at 50 to 52°C for no more than 2 h of hybridization. Temperatures above 52°C led to a reduction in the fluorescence signal for all probes used.
After hybridization, the coverslips were floated off in DEPC water at room temperature and the slides were washed in wash buffer (30 mM NaCl, 4 mM Tris [pH 7.8], 1 mM EDTA) at 50°C for 20 min in the dark. The slides were rinsed quickly in DEPC water at room temperature and then counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 1 μg/ml) for 10 min at room temperature in the dark. The slides were next washed briefly in DEPC water and allowed to air dry in the dark. Coverslips were mounted on dry slides by using 1 drop of Anti-fade Light mounting medium (Molecular Probes, Eugene, Oreg.) and permanently sealed with nail polish. The slides were stored indefinitely in the dark at 4°C. The preparations were examined with an inverted microscope (Zeiss Axioscope, Oberkochen, Germany) fitted for epifluorescence detection with a high-pressure mercury bulb and filter sets CZ902 (for DAPI), CZ 915 (for rhodamine), and CZ 909 (for fluorescein).
RESULTS
Use of MUP with trophozoites.
MUP was used here as a control that would stain all organisms tested in the FISH analyses. It was derived from the universal probe, designed by Giovannoni et al. (11), which originally was made to hybridize to all three domains of life: archaebacteria, eubacteria, and eukaryotes. In the present study, however, the probe of Giovannoni and coworkers did not provide a satisfactory signal due to the higher-stringency conditions used. Modification of two sites to be more consistent with the eubacteria and eukaryotes used in the present study and addition of 3 bases at the 3′ end led to a 100% match between MUP and targets and an unambiguously positive red fluorescent signal from all cells tested. For example, hybridization of MUP with B. mandrillaris and A. castellanii is illustrated by the red stain in Fig. 1.
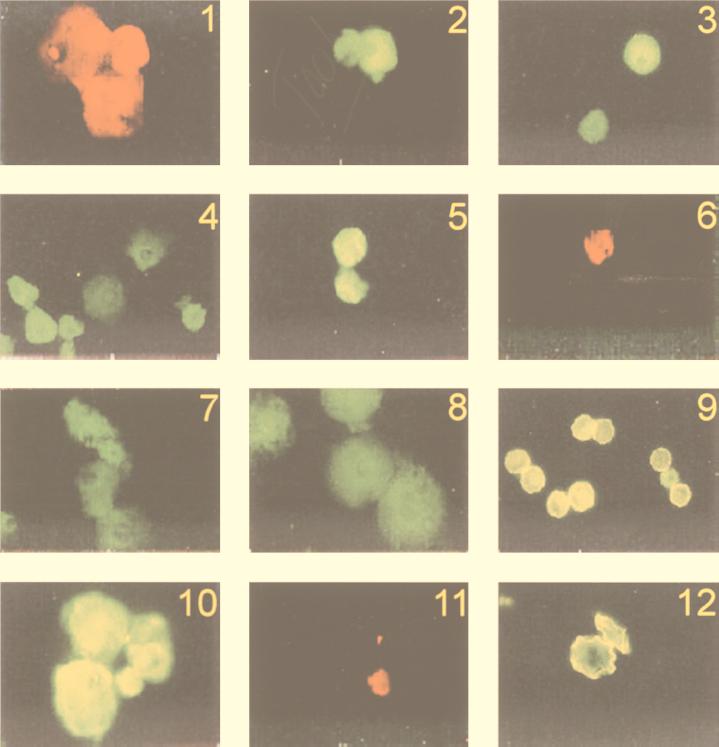
FIG. 1.
–12. In situ hybridization of MUP, GSP, and ST4P with seven sequence types of Acanthamoeba and with its closest relative, the genus Balamuthia.
Use of GSP with trophozoites.
Trophozoites double stained with MUP and GSP were used in tests for determining the specificity of GSP. Representatives from all 12 previously identified sequence types of Acanthamoeba (35) (Table 1), two amoebae relatively closely related to the genus Acanthamoeba (H. vermiformis [41] and B. mandrillaris), bacteria (Pseudomonas aeruginosa and Escherichia coli), and human cells (U937 and HeLa) were tested for hybridization with these two probes. As indicated above, MUP hybridized with Acanthamoeba and all control cells. GSP hybridized with all acanthamoebae tested but with no control organisms. The differential staining of MUP and GSP is illustrated in Fig. 1 and 2, which include a mixture of one relatively small acanthamoeba of sequence type T4 and two larger Balamuthia amoebae. With the rhodamine filter (Fig. 1), all three amoebae are red, indicating hybridization with MUP. With the fluorescein filter (Fig. 2), only the smaller amoeba is green, indicating that GSP hybridized specifically to A. castellanii. Evidence that GSP hybridizes with divergent sequence types of Acanthamoeba is illustrated by the green staining of amoebae of seven sequence types that had been hybridized with this probe, as shown in Fig. 3 (T1), 4 (T2), 5 (T3 and T4), 7 (T5), 8 (T8), and 10 (T9 and T4). See below for identification of T4 amoebae in Fig. 6 and 11.
Use of ST4P with trophozoites.
The specificity of ST4P was tested by using cells doubly stained with GSP and ST4P. Amoebae from each of the 12 described 18S rRNA gene (rDNA) sequence types of Acanthamoeba (Table 1) plus the bacteria, human cells, and other amoebae described above were used. ST4P successfully hybridized with all acanthamoebae of the T4 sequence type, but not with acanthamoebae of any of the other sequence types or with control cells. The degree of specificity is best observed in Fig. 5 and 6, which illustrate differential staining of the two most closely related sequence types, T3 (A. griffini Panola Mt.) and T4 (A. castellanii V014). In Fig. 5, amoebae of both sequence types appear green with the fluorescein filter, indicating that both types have hybridized with GSP. In Fig. 6, however, only the amoeba of sequence type T4 appears red with the rhodamine filter, indicating that it alone has hybridized with ST4P. The specificity of ST4P also is seen in Fig. 10 and 11, in which staining with this probe and with GSP is compared for T4 (A. castellanii V014) and a representative of a more distantly related sequence type, T9 (A. terricola FS). Both amoebae are green in Fig. 10, indicating that both hybridized with GSP, but only the smaller A. castellanii is red in Fig. 11 and, thus, was able to hybridize with ST4P.
Use of GSP with cysts from corneal scrapings.
Hybridization of GSP with intact cysts from a corneal scraping is illustrated by the green stain in Fig. 9. In addition, Acanthamoeba cyst walls autofluoresced either in the presence or in the absence of hybridization conditions. Attempts to quench the fluorescence with a number of different agents failed. Nevertheless, the fluorescein filter set did distinguish between the yellow autofluorescence of the cyst walls and the green fluorescence attributable to hybridization of the fluorescein-labeled GSP to the rRNA and rDNA of the encysted amoebae. This distinction is best seen in sectioned cysts from the human cornea (Fig. 12).
To test the usefulness of GSP with corneal scrapings, we employed 24 specimens obtained from 22 patients, 17 with either confirmed AK or a high index of suspicion of AK and 5 with non-Acanthamoeba keratitis or conjunctivitis (Table 2). Bright-field and phase-contrast microscopy were used with wet films to evaluate the specimens. Fifteen scrapings produced positive Acanthamoeba cultures at Tennent Institute of Ophthalmology (TIO), OSU, or both institutions. It was observed, however, that wet-film microscopy detected acanthamoebae in only 6 (40%) of the 15 culture-positive specimens.
Microscopy using FISH with GSP was then used to evaluate the corneal scraping specimens. Acanthamoebae were detected in 12 (80%) of the 15 culture-positive samples. Overall, FISH results were consistent with the positive or negative culture results for 21 (88%) of the 24 specimens. The only discrepancies were three FISH-negative specimens (no. 1a, 17, and 23) that were considered culture positive even though positive results were obtained at only one of the two institutions (see Discussion).
DISCUSSION
The major advantage of using fluorescent oligonucleotide probes and FISH for identification of Acanthamoeba is that such probes have the potential to simultaneously detect and classify amoebae in situ. FISH provides a means of rapid, unequivocal identification of Acanthamoeba in cases of AK for which clinical diagnosis is putative and the appropriate expertise for identification by bright-field and/or phase-contrast microscopy is unavailable. As we have seen here, even when that expertise is available, the number of false negatives can be relatively high in the absence of FISH unless the observations are backed up by tests for culture growth. However, culture may take a week or more to yield satisfactory division of amoeba, especially if the cornea has been exposed to a range of antimicrobial or antiviral drugs prior to the procurement of the scraping specimen.
Although the examples of GSP and ST4P staining described in this paper have used amoebae growing in culture or in corneal scrapings, we also have been able to detect Acanthamoeba in sectioned human cornea (Fig. 12) (20).
In these studies, successful culture of a specimen at both TIO and OSU provided the highest level of confidence that viable acanthamoebae were present in an original scraping. However, successful culture at either one of the two institutions also was considered strong evidence that amoebae were originally present in the scraping. This is because the amoebae in the scrapings we examined were encysted. In our experience, negative culture results due to failure of excystment are relatively common whereas positive culture results due to accidental contamination with acanthamoebae are relatively rare. Thus, in the present study, the observance of an occasional failure of culture growth for scrapings that included cysts was not surprising. However, obtaining positive cultures from scrapings that did not originally include amoebae was highly unlikely. Therefore, in our analysis, all specimens giving rise to cultures at TIO, OSU, or both institutions were considered to have included acanthamoebae in the original scrapings. In some cases, positive cultures might have been obtained from samples with very few amoebae. Because FISH was performed on the original samples, small numbers of cells could have resulted in negative FISH results. On this basis, FISH with GSP detected Acanthamoeba in 80% of the culture-positive samples, and FISH and culture results were consistent for 88% of all samples. The comparable values are slightly higher if the FISH results, which were all obtained at OSU, are separately compared with culture results from each of the two laboratories. In this case, FISH detected amoebae in 86% (12 of 14) of culture-positive samples, and FISH and culture results were consistent for 92% (22 of 24) of all samples tested at OSU. At TIO, the values were 92% (11 of 12) and 92% (22 of 24), respectively. If comparisons of FISH and cell culture results are limited to the 20 specimens for which positive or negative culture results were identical at TIO and OSU, there was 100% agreement. Thus, FISH results were most consistent with culture results when there was no ambiguity in the latter.
The data indicate that FISH with GSP clearly improves the accuracy of microscopy for detection of Acanthamoeba. This method produced no false positives and only three false-negative results among the 24 scrapings. The most likely explanation for the false negatives is that viable amoebae, although present, were too sparse to be picked up by FISH. This problem probably could have been overcome by the use of a more effective method of cell concentration. The FISH result for specimen 1a (Table 2) appears to be a false negative since amoebae were successfully cultured at OSU and because specimen 1b, obtained from the same eye on the next day, was culture positive by all methods used, including FISH.
The FISH results for scraping specimens 17 and 23 also appear to be false negatives because amoebae were cultured successfully at either TIO or OSU and because PCR results to be discussed elsewhere (31a) indicated the presence of acanthamoebae in both scrapings. Specimen 17 is a special case. The clinical diagnosis was bacterial keratitis, and the acanthamoebae detected by wet-film microcopy and cell culture were identified as transients present in the scraping. This result emphasizes that detection of Acanthamoeba should not be the only criterion used for a diagnosis of AK. Finally, the FISH result for scraping specimen 8 appears to be a true positive because the presence of acanthamoebae also is supported by positive cell culture and PCR results at OSU.
Two important advantages of FISH are the unambiguous identification of the organisms and the relative rapidity with which results can be obtained. Identification with FISH can be accomplished in 1 to 2 days, whereas cell culture time is variable and can take up to several weeks. It is recommended, however, that all scrapings, especially those that give negative FISH results, be cultured. Any amoebae that grow can then be tested by FISH with GSP to determine whether they are acanthamoebae.
The availability of a large number of DNA sequences for the nuclear small-subunit rRNA gene (35) has made it possible to design cytological stains that are specific for the genus Acanthamoeba and for separate lineages within this genus. We plan to develop a set of probes that could be used to identify the amoebae at the subgenus level. ST4P, which identifies 18S rDNA sequence type T4, is the first of these probes to become available. The specificity of this probe has been demonstrated for trophozoites in this study but remains to be demonstrated for cysts. We focused on this sequence type for the design of a probe mostly because of its apparent importance in AK. Our previous sequence studies (35) plus 18S rDNA sequences obtained for corneal scrapings used in the present study (31a) indicate that 29 of the 30 AK isolates we have examined to date have T4 sequences. The only exception is a T3 isolate (22), and this sequence type is very closely related to T4. Another reason for concentrating on the T4 probe is because we have 18S rDNA sequence information for many more T4 strains than for any other sequence type. The greater amount of information available about sequence variation for this lineage permitted the design of a more robust probe. ST4P hybridizes to a region of the 18S rRNA gene that differs among the sequence types. Thus, this region may be a good target for other probes designed to be specific for other sequence types. However, more information on sequence variation in most of these other types is required before a complete set of robust sequence type-specific probes can be developed.
The FISH protocol was designed to be both specific and sensitive. The cells tested included axenic cultures of trophozoites and corneal scraping specimens that mostly included cysts. GSP worked very well with either developmental stage, but ST4P was used only with multiplying amoebae. Amoebae actively growing in cultures gave the brightest signal, most likely because of a high rRNA content in the cytoplasm. However, amoebae that have been stored in isotonic saline for 1 to 19 months, as was the case for some of the corneal scraping specimens, also were detectable. The fact that this technique works on stored samples is important because there can often be some delay between the collection of a scraping and its analysis in a specialized laboratory. The success of FISH with older stored samples is not surprising because the amoebae were present as cysts. It previously has been shown that encysted acanthamoebae can remain viable in CL saline for 14 to 90 days (3). In the present study, however, cysts remained viable during refrigeration in isotonic saline for at least 19 months, as shown, for example, by scraping sample 18 (Table 2), which was collected 3 December 1994 and still produced a culture at OSU in early July 1996.
The probes were designed to hybridize with 18S rRNA in addition to the large number of rDNA copies in the nucleolus in order to maximize the number of intracellular targets. Probe specificity at the level of sequence type was tested only with trophozoites because interpretation of results from cysts might be complicated by the autofluorescence of the cyst walls. It should be possible to alleviate this problem by using colorimetric detection of a nonfluorescent tag such as digoxigenin, which has been used with probes for Acanthamoeba 26S rRNA (21).
The probes have a number of potential applications in addition to those tested in the present study. For example, they can detect amoebae in deparaffinized tissue sections (Fig. 12). Also, Paillasson and coworkers (29) have recently developed a procedure for in situ hybridization of FISH probes to RNA in living human cells. The cells were permeabilized with streptolysin O and probed without fixation. They then were detected with a fluorescence-activated cell sorter. We recently proved a linkage between a patient with AK, the CL storage case contents, and the patient’s home water supply (22). Thus, it might be possible to use FISH probes in a fluorescence-activated cell sorter format for prophylactic studies of water from treatment plants, home water supplies, or other sources.
Although this report has focused on AK, Acanthamoeba also is responsible for GAE. Two of the specimens tested in this study, Acanthamoeba healyi V013 and A. castellanii V006, are isolates from patients with GAE. This disease has been increasing in incidence due to its association with AIDS (14). Hawley et al. (14) recently reported the ability to identify Acanthamoeba in cerebrospinal fluid via culturing. Nearly all reported cases of GAE have been fatal, but early diagnosis, possibly using FISH on cerebrospinal fluid, might improve the odds of successful treatment.
The level of specificity required in probes will depend on their use. In some cases, it may only be necessary to distinguish between organisms that are known to cause a particular infection and those that do not. For studies of biodiversity, a more varied set of probes might be needed. The large collection of 18S rDNA sequences now available at GenBank plus the sequence alignments available on the Internet (3a) should be consulted by those wishing to design other probes. In addition, since cysts can be the major component of clinical samples, methods of suppressing the autofluorescence of cyst walls or the use of nonfluorescent markers for the genus- and sequence type-specific probes will be explored.
ACKNOWLEDGMENTS
The molecular biology studies described in this report were funded by Public Health Service grant EY09073 from the National Eye Institute.
We thank our colleague Gregory Booton for helpful discussions and assistance with the figures.
REFERENCES
- 1.Bacon A S, Dart J K, Ficker L A, Matheson M M, Wright P. Acanthamoeba keratitis. The value of early diagnosis. Ophthalmology. 1993;100:1238–1243. doi: 10.1016/s0161-6420(93)31499-5. [DOI] [PubMed] [Google Scholar]
- 2.Bacon A S, Frazer D G, Dart J K, Matheson M, Ficker L A, Wright P A. A review of 72 consecutive cases of Acanthamoeba keratitis, 1984–1992. Eye. 1993;7:719–725. doi: 10.1038/eye.1993.168. [DOI] [PubMed] [Google Scholar]
- 3.Brandt F H, Ware D A, Visvesvara G S. Viability of Acanthamoeba cysts in ophthalmic solutions. Appl Environ Microbiol. 1989;55:1144–1146. doi: 10.1128/aem.55.5.1144-1146.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Byers, T. J. 1 April 1998, posting date. 18S rDNA sequence alignments. [Online.] http://www.biosci.ohio-state.edu/∼tbyers/byers.htm.
- 4.Byers T J, Akins R A, Maynard B J, Lefken R A, Martin S M. Rapid growth of Acanthamoeba in defined media; induction of encystment by glucose-acetate starvation. J Protozool. 1980;27:216–219. doi: 10.1111/j.1550-7408.1980.tb04684.x. [DOI] [PubMed] [Google Scholar]
- 5.Chung D I, Yu H-S, Hwang M-Y, Kim T-H, Kim T-O, Yun H-C, Kong H-H. Subgenus classification of Acanthamoeba by riboprinting. Korean J Parasitol. 1998;36:69–80. doi: 10.3347/kjp.1998.36.2.69. [DOI] [PubMed] [Google Scholar]
- 6.Chynn E W, Lopez M A, Pavan-Langston D, Talamo J H. Acanthamoeba keratitis. Contact lens and noncontact lens characteristics. Ophthalmology. 1995;102:1369–1373. doi: 10.1016/s0161-6420(95)30862-7. [DOI] [PubMed] [Google Scholar]
- 7.De Jonckheere J F. Ecology of Acanthamoeba. Rev Infect Dis. 1991;13(Suppl. 5):S385–S387. doi: 10.1093/clind/13.supplement_5.s385. [DOI] [PubMed] [Google Scholar]
- 8.DeLong E F, Wickham G S, Pace N R. Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science. 1989;243:1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- 9.Gast R J, Byers T J. Genus- and subgenus-specific oligonucleotide probes for Acanthamoeba. Mol Biochem Parasitol. 1995;71:255–260. doi: 10.1016/0166-6851(94)00049-s. [DOI] [PubMed] [Google Scholar]
- 10.Gast R J, Ledee D R, Fuerst P A, Byers T J. Subgenus systematics of Acanthamoeba: four nuclear 18S rDNA sequence types. J Eukaryot Microbiol. 1996;43:498–504. doi: 10.1111/j.1550-7408.1996.tb04510.x. [DOI] [PubMed] [Google Scholar]
- 11.Giovannoni S J, DeLong E F, Olsen G J, Pace N R. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J Bacteriol. 1988;170:720–726. doi: 10.1128/jb.170.2.720-726.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodall K, Brahma A, Ridgeway A. Acanthamoeba keratitis: masquerading as adenovirus keratitis. Eye. 1996;10:643–644. doi: 10.1038/eye.1996.148. [DOI] [PubMed] [Google Scholar]
- 13.Gunderson J H, Sogin M L. Length variation in eukaryotic rRNAs: small-subunit rRNAs from the protists Acanthamoeba castellanii and Euglena gracilis. Gene. 1986;44:63–70. doi: 10.1016/0378-1119(86)90043-0. [DOI] [PubMed] [Google Scholar]
- 14.Hawley H B, Czachor J S, Malhotra V, Funkhouser J W, Visvesvara G S. Acanthamoeba encephalitis in patients with AIDS. AIDS Reader. 1997;7:137–144. [Google Scholar]
- 15.Hay J, Kirkness C M, Seal D V, Wright P. Drug resistance and Acanthamoeba keratitis: the quest for alternative antiprotozoal chemotherapy. Eye. 1994;8:555–563. doi: 10.1038/eye.1994.137. [DOI] [PubMed] [Google Scholar]
- 16.Howe D K, Vodkin M H, Novak R J, Visvesvara G, McLaughlin G L. Identification of two genetic markers that distinguish pathogenic and nonpathogenic strains of Acanthamoeba. Parasitol Res. 1997;83:345–348. doi: 10.1007/s004360050259. [DOI] [PubMed] [Google Scholar]
- 17.John D T. Opportunistically pathogenic free-living ameba. In: Baker J R, Kreier J P, editors. Parasitic protozoa. 2nd ed. New York, N.Y: Academic Press; 1993. pp. 143–246. [Google Scholar]
- 18.Johns K J, O’Day D M, Head W S, Neff R J, Elliott J H. Herpes simplex masquerade syndrome: Acanthamoeba keratitis. Curr Eye Res. 1987;6:207–212. doi: 10.3109/02713688709020092. [DOI] [PubMed] [Google Scholar]
- 19.Jones D B, Visvesvara G S, Robinson N M. Acanthamoeba polyphaga keratitis and Acanthamoeba uveitis associated with fatal meningoencephalitis. Trans Ophthalmol Soc UK. 1975;95:221–232. [PubMed] [Google Scholar]
- 20.Kinnear, F., J. Hay, J. M. Schroeder-Diedrich, and T. J. Byers. Unpublished data.
- 21.Lai S, Asgari M, Henney H R., Jr Non-radioactive DNA probe and polymerase chain reaction procedures for the specific detection of Acanthamoeba. Mol Cell Probes. 1994;8:81–89. doi: 10.1006/mcpr.1994.1012. [DOI] [PubMed] [Google Scholar]
- 22.Ledee D R, Hay J, Byers T J, Seal D V, Kirkness C M. Acanthamoeba griffini. Molecular characterization of a new corneal pathogen. Invest Ophthalmol Vis Sci. 1996;37:544–550. [PubMed] [Google Scholar]
- 23.Lehmann M O, Green S M, Morlet N, Keys M F, Matheson M M, Dart J K G, McGill J I, Watt P J. Polymerase chain reaction analysis of corneal epithelial and tear samples in the diagnosis of Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. 1998;39:1261–1265. [PubMed] [Google Scholar]
- 24.Ma P, Visvesvara G S, Martinez A J, Theodore F H, Daggett P-M, Sawyer T K. Naegleria and Acanthamoeba infections: a review. Rev Infect Dis. 1990;12:490–513. doi: 10.1093/clinids/12.3.490. [DOI] [PubMed] [Google Scholar]
- 25.Martinez A J, Visvesvara G S. Free-living amphizoic and opportunistic amebas. Brain Pathol. 1997;7:583–598. doi: 10.1111/j.1750-3639.1997.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakawa G J, McCalmont T, Altman J, Telang G H, Hoffman M D, Kanter G R, Berger T G. Disseminated acanthamebiasis in patients with AIDS. A report of five cases and a review of the literature. Arch Dermatol. 1995;131:1291–1296. [PubMed] [Google Scholar]
- 27.Nagington J, Watson P G, Playfair T J. Amoebic infection of the eye. Lancet. 1974;ii:1547–1550. doi: 10.1016/s0140-6736(74)90285-2. [DOI] [PubMed] [Google Scholar]
- 28.Newman W, Hay J, Brown B, Seal D V. Acanthamoeba as a “transient” in the corneal scrape of a poorly compliant soft contact lens wearer with peripheral keratitis. Eye. 1997;11:937–939. doi: 10.1038/eye.1997.233. [DOI] [PubMed] [Google Scholar]
- 29.Paillasson S, Van de Corput M, Dirks R W, Tanke H J, Robert-Nicoud M, Ronot X. In situ hybridization in living cells: detection of RNA molecules. Exp Cell Res. 1997;231:226–233. doi: 10.1006/excr.1996.3464. [DOI] [PubMed] [Google Scholar]
- 30.Pyott A, Hay J, Seal D V. Acanthamoeba keratitis: first recorded case from a Palestinian patient with trachoma. Br J Ophthalmol. 1996;80:849. doi: 10.1136/bjo.80.9.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivasi F, Longanesi L, Casolari C, Croppo G P, Pierini G, Zunarelli E, Visvesvara G S. Cytologic diagnosis of Acanthamoeba keratitis. Report of a case with correlative study with indirect immunofluorescence and scanning electron microscopy. Acta Cytol. 1995;39:821–826. [PubMed] [Google Scholar]
- 31a.Schroeder-Diedrich, J. M. Unpublished data.
- 32.Seal, D. V., C. M. Kirkness, H. G. B. Bennett, M. Peterson, et al. Population-based cohort study of microbial keratitis in Scotland: incidence and features. Submitted for publication. [DOI] [PubMed]
- 33.Sharma S, Srinivassan M, George C. Acanthamoeba keratitis in non-contact lens wearers. Arch Ophthalmol. 1990;108:676–678. doi: 10.1001/archopht.1990.01070070062035. [DOI] [PubMed] [Google Scholar]
- 34.Stehr-Green J K, Bailey T M, Visvesvara G S. The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol. 1989;107:331–336. doi: 10.1016/0002-9394(89)90654-5. [DOI] [PubMed] [Google Scholar]
- 35.Stothard D R, Schroeder-Diedrich J M, Awwad M H, Gast R J, Ledee D R, Rodriguez-Zaragoza S, Dean C L, Fuerst P A, Byers T J. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18s rRNA gene sequence types. J Eukaryot Microbiol. 1998;45:45–54. doi: 10.1111/j.1550-7408.1998.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szénasí Z, Endo T, Yagita K, Nagy E. Isolation, identification and increasing importance of “free-living” amoebae causing human disease. J Med Microbiol. 1998;47:5–16. doi: 10.1099/00222615-47-1-5. [DOI] [PubMed] [Google Scholar]
- 37.Tan B, Weldon-Linne C M, Rhone D P, Penning C L, Visvesvara G S. Acanthamoeba infection presenting as skin lesions in patients with the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1993;117:1043–1046. [PubMed] [Google Scholar]
- 38.Tay-Kearney M L, McGhee C N, Crawford G J, Trown K. Acanthamoeba keratitis: a masquerade of presentation in six cases. Aust N Z J Ophthalmol. 1993;21:237–245. doi: 10.1111/j.1442-9071.1993.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 39.Visvesvara G S, Stehr-Green J K. Epidemiology of free-living ameba infections. J Protozool. 1990;37:25S–33S. doi: 10.1111/j.1550-7408.1990.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 40.Vodkin M H, Howe D K, Visvesvara G S, McLaughlin G L. Identification of Acanthamoeba at the generic and specific levels using the polymerase chain reaction. J Protozool. 1992;39:378–385. doi: 10.1111/j.1550-7408.1992.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 41.Weekers P H H, Gast R J, Fuerst P A, Byers T J. Sequence variation in small-subunit ribosomal RNAs of Hartmannella vermiformis and the phylogenetic implications. Mol Biol Evol. 1994;11:684–690. doi: 10.1093/oxfordjournals.molbev.a040147. [DOI] [PubMed] [Google Scholar]
- 42.Wilhelmus, K. R., M. S. Osato, R. I. Font, N. M. Robinson, and D. M. Jones. Rapid diagnosis of Acanthamoeba using calcafluor white. Arch. Ophthalmol. 104:1309–1312. [DOI] [PubMed]