Abstract
Colorectal cancer (CRC) is an aggressive malignancy. Critical mechanisms that support CRC progression include cell migration, invasion, metastasis, and angiogenesis, which is associated with L1 cell adhesion molecule (L1CAM) and nuclear factor-kappa B (NF-κB) signaling pathways. In this study, viability of HT-29 cells and human umbilical vein endothelial cells (HUVECs) was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays, and cell apoptosis was investigated by flow cytometry assays. HT-29 cell migration and invasion were observed by wound healing and Transwell invasion assays, respectively, and tube formation of HUVECs was observed by tubulogenesis assays. L1CAM and NF-κB protein expressions in HT-29 cells treated with onion peel extract were determined by indirect immunofluorescence. Results showed that high dose treatments of onion peel extract inhibited cell viability of both HT-29 cells and HUVECs, induced HT-29 cell apoptosis, and inhibited HT-29 cell migration and invasion. Moreover, onion peel extract decreased total HUVEC tube length and, at a concentration of 10 μg/mL, showed potential to downregulate L1CAM and NF-κB. In conclusion, onion peel extract inhibits HT-29 cell growth, migration, and invasion through suppressing pathways related to angiogenesis downstream of L1CAM-activated NF-κB.
Keywords: angiogenesis, colorectal cancer, L1CAM, NF-κB, onion peel extract
INTRODUCTION
Globally, colorectal cancer (CRC) is the third most-commonly diagnosed malignancy and has one of the highest mortality rates of all cancer types, especially in Western countries. The number of people diagnosed with CRC increases every year (Harada and Morlote, 2020; Wan et al., 2020). CRC is the second most common cancer in women, accounting for 9.2% of cases, and the third most common cancer in men, accounting for approximately 10% of cases (Mármol et al., 2017). The US and Europe have recently observed an increase in the incidence of CRC in individuals under 50 years of age (Seiwert et al., 2020). Both genetic and environmental factors play important roles in the etiology of CRC (Kuipers et al., 2015). The rise of CRC cases in young patients is most often attributed to lifestyle factors, such as alcohol, tobacco, physical inactivity, and bad dietary habits, including regular intake of red meat, and food with low fiber and high fat contents (Granados-Romero et al., 2017; Seiwert et al., 2020). Growth and progression of CRC involve various signaling pathways and oncogenes, with several studies having reported that metastasis is a major cause of patient fatality (Chen and Zhao, 2020). Recent studies have shown that epithelial-mesenchymal transition (EMT) is critical for cancer cell migration, invasion, and metastasis. EMT events occur when cancer epithelial cells lose their epithelial feature and cell-to-cell junctions, leading to the development of mesenchymal cells under physiological or pathological conditions. EMT transformation causes the cancer cells to change their morphology, which facilitate migration, invasion, and metastasis (Versluis et al., 2018; Huang et al., 2020).
L1 cell adhesion molecule (L1CAM) is a transmembrane adhesion molecule essential for nerve cell growth and development. However, L1CAM is also relevant to human cancer progression, with roles in cell proliferation, migration, invasion, and survival (Kiefel et al., 2012a; Chen et al., 2018). Several studies have supported the correlation between L1CAM and EMT (Huszar et al., 2010; Kiefel et al., 2012a; Kiefel et al., 2012b; Doberstein et al., 2015). Huszar et al. (2010) demonstrated that increased expression of L1CAM is associated with reduced expression of the epithelial phenotype. Consequently, L1CAM may be a mesenchymal phenotype marker. Additionally, many studies have shown that L1CAM induces nuclear factor-kappa B (NF-κB) activation, promotes cell proliferation in vitro and metastasis in vivo, and encourages cancer cell migration and invasion, which is related to poor prognosis (Kiefel et al., 2011; Bondong et al., 2012; Kiefel et al., 2012b; Versluis et al., 2018).
NF-κB plays a key role in the initiation of CRC progression, specifically mediating the induction of EMT, to enhance migration and cell invasion (Nomura et al., 2016; Sadremomtaz et al., 2018; Sun et al., 2019). Moreover, the pivotal mechanism associated with cancer growth and progression is angiogenesis. Indeed, new blood vessels sprout from pre-existing vessels to provide efficient nourishment, oxygen, and metabolite drainage, acting as the route for cancer metastasis (Tonini et al., 2003; Eichhorn et al., 2007). Without a blood supply, cancer cells grow to 1∼2 mm3 in diameter; however, when placed in an angiogenic area, the cells are able to grow larger than 2 mm3. In the absence of vascular support, cancer may die by necrosis or even apoptosis (Nishida et al., 2006). Angiogenesis results from an angiogenic switch, characterized by increased stimulators and decreased inhibitors. Vascular endothelial growth factor (VEGF) is an important stimulator for cancer angiogenesis that promotes the growth of new blood vessels (Carmeliet, 2005).
Phytochemicals and natural products have been reported as alternative treatments for various types of cancer. Quercetin is a major flavonol found in several plant-based foods such as onions, apples, tea, broccoli, red grapes, and several types of berries. Onion peel contains two main bioactive compounds: alk(en)yl cysteine sulfoxides and flavonoids (Celano et al., 2021). Quercetin is the most abundant flavonol in onion peel extract from Allium cepa L. (Fredotović et al., 2021). The potential effects of quercetin have been attributed to a variety of mechanisms, including its anti-oxidative, anti-inflammatory, and anti-cancer properties, as well as its capacity to prevent various diseases, such as neurological, cardiovascular, and hepatoprotective effects (Carmeliet, 2005; Maalik et al., 2014).
The aim of this study was to investigate the effect of onion peel extract on CRC cell growth, migration, and invasion through the inhibition of L1CAM and NF-κB signaling, a transduction pathway related to angiogenesis.
MATERIALS AND METHODS
Onion peel extraction
Crude extracts of onion peel were obtained from the co-operation of Detox (Thailand) Co., Ltd. (Chiang Mai, Thailand). Briefly, onion peel (Allium cepa L.) was obtained from Chiang Mai at an altitude 1,200 m. Onion peel was homogenized, extracted with ethanol, and the extract was freeze-dried in a vacuum chamber under pressure for 24 h. The powder was then dissolved with dimethyl sulfoxide (DMSO), filtered with a 0.22 µm pore filter and stored at −20°C until use.
Cell culture
HT-29 cells were cultured in Dulbecco’s modified Eagle medium-F12 (Sigma-Aldrich Co., St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS), 1% L-glutamine, 1% non-essential amino acid, and 1% penicillin-streptomycin. HUVECs were cultured in endothelial cell growth media supplemented with 2% FBS and growth factor supplements included in the Endothelial Cell Growth Medium 2 kit (PromoCell, Heidelberg, Germany). Cells were maintained in a humidified incubator at 37°C with 5% CO2.
MTT assays
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays were used to evaluate cell viability. First, 1.5×104 of HT-29 cells or 8.0×103 of HUVECs were seeded into a 96-well plates and maintained at 37°C with 5% CO2. After 24 h, the media was replaced by onion peel extract at various concentration in serum media for 24 h. Then, media was removed and 200 µL of serum-free media containing 20 µL of 5 mg/mL MTT stock solution was added and cells were incubated for 2 h. DMSO was then added, and the absorbance was detected by a microplate reader (VICTOR2 1420, Wallac Oy, Shelton, CT, USA) at 570 nm.
Flow cytometry assays
Flow cytometry was used to determine the apoptotic cell population by use of Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining. First, 1.0×105 HT-29 cells were plated into 24-well plates and incubated at 37°C with 5% CO2 for 48 h. Media was then removed and replaced with serum-free media containing onion peel extract. After 24 h, cells were trypsinized, rinsed with phosphate-buffered saline (PBS), and centrifuged. Pellets were then resuspended in binding buffer and FITC-conjugated Annexin V and phycoerythrin-conjugated PI were added. The cells were washed with PBS, fixed with 1% paraformaldehyde, and then incubated at room temperature in the dark for 15 min. The labeled cells were analyzed by using FACScan (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Wound healing assays
Cell migration was observed by wound healing assays. First, 1.0×106 HT-29 cells were seeded into 6-well plates and maintained at 37°C with 5% CO2 for 20 h. Next, cells were treated with mitomycin C (Naprod Life Sciences Pvt. Ltd., Maharashtra, India) for 2 h, before a straight scratch was created by a scratcher (SPL Life Sciences Co., Ltd., Pocheon, Korea). Cells were washed with PBS, followed by addition of onion peel extract, and incubated for 48 h. Results were analyzed by ImageJ software (National Institutes of Health, Bethesda, MD, USA) and expressed as a percentage of the cell migration area (Glenn et al., 2016).
Transwell invasion assays
Transwell invasion assays were used to access the ability of cells to invade the surrounding tissue. 24-well cell culture insert plates with an 8.0-µm membrane pore size were used in this study, with 30 µL extracellular matrix (ECM, Sigma-Aldrich Co.) added on the top of the Transwell membranes. First, 5.0×105 HT-29 cells were seeded on top of the ECM and treated with serum-free media containing onion peel extract. In the lower part, media contained 20% FBS were added. Cells were incubated at 37°C with 5% CO2 for 48 h, before the invaded cells were stained with hematoxylin and eosin (H&E) and observed under a light microscope.
Tubulogenesis assays
Tubulogenesis assays were used to study the endothelial lumen formation in HUVECs. First, 50 µL of Matrigel layer (Corning, Corning, NY, USA) was added to 96-well plates and plates were incubated at room temperature for 30 min. Then, 8.0×103 HUVECs were resuspended in 20 ng/mL media supplemented with VEGF-A as a positive control, and HUVECs were treated with conditioned media of HT-29 cells, as previously described (Uttarawichien et al., 2021). The HUVECs resuspended in conditioned media of HT-29 cells treated with onion peel extract at concentrations of 5 and 10 mg/mL were seeded onto a Matrigel layer and incubated for 6 h. The tubular structures on the Matrigel layer were photographed at 3 random fields. The total lengths of the tubes per area were measured and analyzed using ImageJ software (National Institutes of Health) with an angiogenic analyzer program.
Immunofluorescence assay
Immunofluorescence assays were used to investigate L1CAM (Abcam, Waltham, MA, USA) and NF-κB (BioLegend, Inc., San Diego, CA, USA) expression. First, 1.0×105 HT-29 cells were seeded onto coverslips placed in 6-well plates and incubated at 37°C with 5% CO2 for 48 h. Then, media was removed with serum-free media containing onion peel extract for 48 h. Next, cells were fixed with cold absolute methanol and washed with PBS. Primary antibodies against L1CAM and NF-κB were added for 1.5 h followed by FITC-conjugated secondary antibodies for 30 min. Then, HT-29 cells were counterstained with Hoechst-33342 for 15 min, and imaged using a fluorescent microscope (Model BX53, Olympus, Tokyo, Japan). The fluorescence intensities of 3 random fields were analyzed in triplicate using ImageJ software (National Institutes of Health).
Statistical analysis
Results were analyzed by using one-way analysis of variance (ANOVA) in GraphPad Prism version 9 (GraphPad, San Diego, CA, USA). Data were presented as mean±standard deviation (SD) of three independent experiments (n=3). P-values <0.05 were considered significantly different.
RESULTS
Effect of onion peel extract on cell growth of HT-29 cells and HUVECs
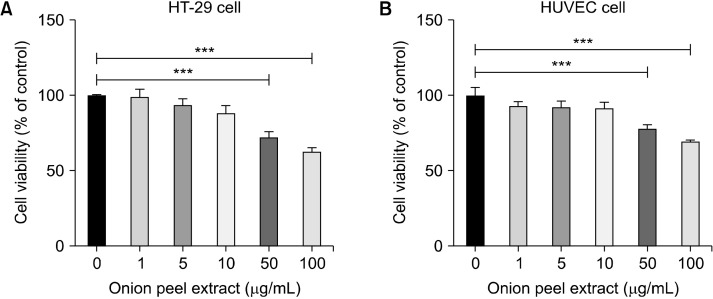
The effects of various concentrations of onion peel extract (1, 5, 10, 50, and 100 µg/mL) on HT-29 cell and HUVEC growth were determined using MTT assays. Treatment onion peel extract at concentrations of 50 and 100 µg/mL inhibited both HT-29 cell and HUVEC growth in a dose-dependent manner. HT-29 cells treated with 1, 5, and 10 µg/mL of onion peel extract were not significantly different from untreated cells (99.34±4.85%, 93.83±4.01%, and 88.32±4.87%, respectively, of untreated cells). These results indicate that onion peel extract is not toxic at concentrations of 1, 5, and 10 µg/mL. However, the survival rate of HT-29 cells treated with 50 and 100 µg/mL onion peel extract decreased to 72.53±3.69% and 62.93±2.51%, respectively, of that of untreated cells (P<0.001) (Fig. 1A).
Fig. 1.
Effect of onion peel extract on growth of HT-29 cells and HUVECs. The viability of HT-29 cells and HUVECs after treatment with onion peel extract for 24 h at various concentrations. Viability of HT-29 cells treated with onion peel extract at 1, 5, 10, 50, and 100 mg/mL (A) and HUVECs (B). Data show mean±SD. ***P<0.001.
HUVECs were treated with various concentrations of onion peel extract (1, 5, 10, 50, and 100 µg/mL) for 24 h. Results showed that HUVEC survival of after they were treated with onion peel extract at concentrations of 1, 5, and 10 µg/mL did not change significantly vs. untreated cells (93.03±2.96%, 92.62±3.73%, and 91.69±3.94%, respectively, of untreated cells). This indicates that onion peel extract at concentrations of 1, 5, and 10 µg/mL had a non-anti-proliferative effect on the HUVECs. However, after HUVECs were treated with 50 and 100 µg/mL onion peel extract, their survival rate decreased to 78.33±2.30% and 69.97±0.66% compared with that of untreated cells (P<0.001) (Fig. 1B). Thus, the maximum safe dose of onion peel extract was 10 µg/mL. Therefore, 5 µg/mL and 10 µg/mL concentrations of onion peel extract were used in further experiments.
Onion peel extract induces apoptosis of HT-29 cells
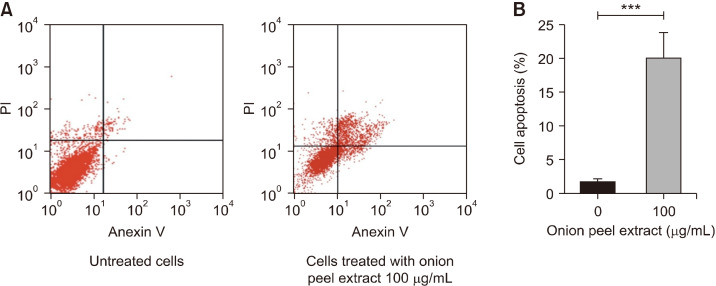
At high doses, treatment with onion peel extract reduced viability of HT-29 cells. Therefore, we investigated the ability of onion peel extract to induce HT-29 cell apoptosis. Flow cytometry was used to investigate the apoptotic population of HT-29 cells (Fig. 2A). After treatment with onion peel extract at a concentration of 100 µg/mL for 24 h, the HT-29 cells showed higher summation of early and late apoptotic cells compared with untreated cells (20.07±3.69% vs. 1.84±0.38%, P<0.001). This indicated that a high dose (100 µg/mL) of onion peel extract induced apoptosis in HT-29 cells (Fig. 2B).
Fig. 2.
Effect of onion peel extract on apoptosis of HT-29 cells. Flow cytometry analysis showed four quadrants, including lower-left, upper-left, lower-right, and upper-right, that show viable cells, necrotic cells, and early and late apo-ptotic cells (A). The bar graph shows the percentage of early and late ap-optotic cells (B). Data show mean± SD . ***P<0.001.
Onion peel extract inhibits HT-29 cell migration
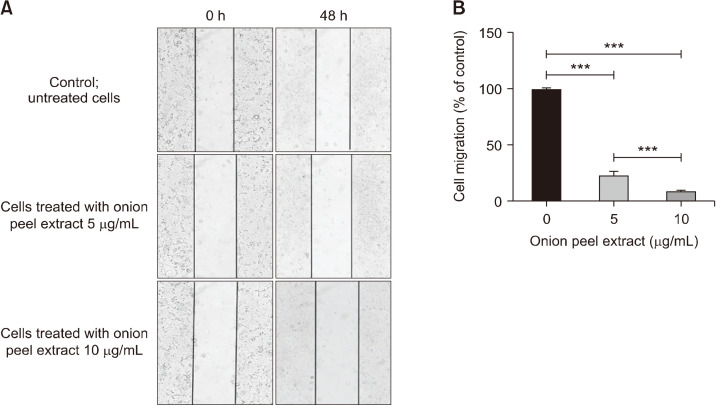
HT-29 cell migration was investigated by wound healing assays. HT-29 cells were treated with onion peel extract at concentrations of 5 and 10 µg/mL for 48 h. At 5 and 10 µg/mL, onion peel extract significantly decreased HT-29 cell migration in a dose-dependent manner [by 23.48 ±3.50% and 9.37±0.74%, respectively, compared with untreated cells (100%), P<0.001]. In addition, results for extracts at 5 and 10 µg/mL significantly differed (P< 0.001) (Fig. 3). These results indicate that onion peel extract inhibits cancer cell migration of HT-29 cells.
Fig. 3.
Effect of onion peel extract on inhibition of HT-29 cell migration. HT-29 cell migration was observed in wound healing assays using an inverted microscope with 100× magnification (A). Data show mean±SD compared with untreated cells (100%) (B). ***P<0.001.
Onion peel extract inhibits HT-29 cell invasion
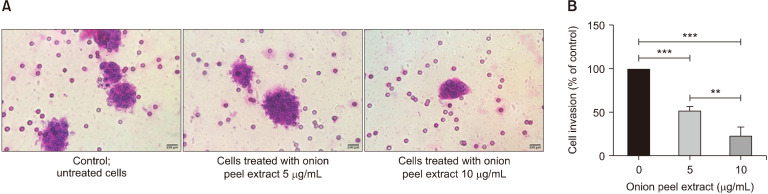
Transwell invasion assay was used to assess the effect of onion peel extract on HT-29 cell invasion. HT-29 cells were treated with onion peel extract at concentrations of 5 and 10 µg/mL for 48 h. Invading cells were then stained with H&E and imaged under a light microscope. Onion peel extract at concentrations of 5 and 10 µg/mL for 48 h significantly attenuated HT-29 cell invasion [by 52.13± 4.65% and 23.48±9.97%, respectively, vs. untreated cells (100%), P<0.001]. In addition, results for extracts at 5 and 10 µg/mL significantly differed (P<0.01) (Fig. 4). These results indicate that onion peel extract inhibits HT-29 cells invasion.
Fig. 4.
Effect of onion peel extract on inhibition of HT-29 cell invasion. HT-29 cells were treated with onion peel extract at 5 and 10 mg/mL for 48 h in Transwell invasion assays. Invading cells were stained with H&E and observed under a light microscope at 400× magnification (A). Data show mean±SD compared with untreated cells (100%) (B). **P<0.01 and ***P<0.001.
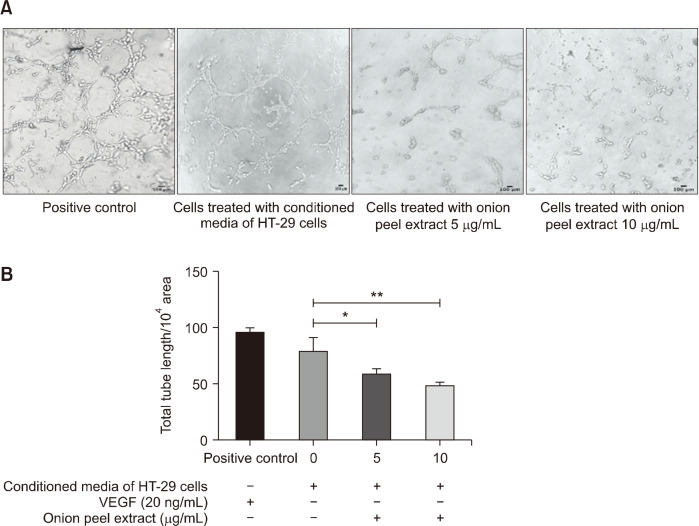
Onion peel extract significantly inhibits tube formation in HUVECs
Tube formation assays were used to determine the effect of onion peel extract on lumen formation in HUVECs, using 20 ng/mL of VEGF-A as a positive control. HUVECs were treated with either conditioned media of HT-29 cells or onion peel extract at concentrations of 5 and 10 µg/mL for 6 h. Then, tube formation was observed and captured under an inverted microscope, as shown in Fig. 5A. We calculated total tube length per 104 pixel area using the ImageJ program with an angiogenic analyzer. The total tube length in the HT-29 conditioned media-induced HUVECs was 79.56±11.78%, which indicates that the HT-29 conditioned media induced tube formation in the HUVECs. Moreover, 5 and 10 µg/mL of onion peel extract significantly decreased total tube length to 60.02±3.76% and 49.73±2.23%, respectively, compared with HT-29 conditioned media treated HUVECs (P<0.05 and P<0.01, respectively) (Fig. 5B). These results indicate that onion peel extract inhibits tube formation in HUVECs in a dose-dependent manner.
Fig. 5.
Effect of onion peel extract on inhibition of tube formation in HUVECs. HUVECs were seeded onto Matrigel layers and treated with 20 ng/mL VEGF-A (positive control), or conditioned media of HT-29 cells with or without onion peel extract at 5 and 10 μg/mL for 6 h. Cells were fixed and tubular structures were photographed (A). Data show total tube length per 104 pixels area, expressed as mean±SD (B). *P<0.05 and **P<0.01.
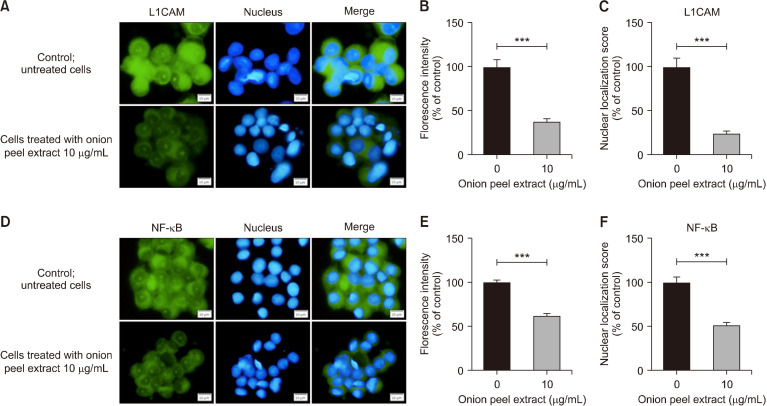
Onion peel extract downregulates L1CAM and NF-κB expression in HT-29 cells
We determined the potential mechanism of onion peel extract on L1CAM and NF-κB protein expression and their localization by indirect immunofluorescence assays. To evaluate the expression of L1CAM, HT-29 cells were treated with 10 µg/mL onion peel extract for 48 h. Treatment with 10 µg/mL of onion peel extract significantly decreased the fluorescence intensity of the L1CAM to 38.00±3.03% compared with untreated cells (100%; P<0.001). In addition, 10 µg/mL of onion peel extract significantly decreased nuclear localization of L1CAM to 24.00±3.17% compared with untreated cells (100%; P<0.001) (Fig. 6A∼6C).
Fig. 6.
Effect of onion peel extract on expression of L1CAM and NF-κB in HT-29 cells after treatment with onion peel extract at 10 mg/mL for 48 h. Immunofluorescence staining of L1CAM (A) and NF-κB (D) (green) and Hoechst-33342 for nuclear staining (blue). L1CAM intensity and nuclear localization were determined relative to untreated cells (100%) (B and C). NF-κB intensity and its nuclear localization were determined relative to untreated cells (100%) (E and F). Data show mean±SD. ***P<0.001.
To assess expression of NF-κB, HT-29 cells were treated with a 10 µg/mL onion peel extract for 48 h. Treatment significantly decreased the fluorescence intensity of NF-κB to 62.32±2.42% compared with untreated cells (100%; P<0.001). Nuclear localization after treatment with 10 µg/mL onion peel extract was significantly lower than untreated cells (51.63±3.28% vs. 100%; P<0.001) (Fig. 6D∼6F).
These findings demonstrate that onion peel extract can suppress the metastasis-related proteins of L1CAM and NF-κB in HT-29 cells. In addition, onion peel extract effectively suppressed expression and inhibited the nuclear translocation of L1CAM and NF-κB in HT-29 cells.
DISCUSSION
Several studies have investigated the molecular factors that encourage CRC progression and metastasis. Li et al. (2020) and Jia et al. (2020) both demonstrated that EMT is associated with metastatic ability in CRC. To date, the most effective therapy against CRC is chemotherapy combined with targeted therapy such as anti-VEGF, which inhibits pro-angiogenic factors to kill both cancer cells and their blood supplies. Nevertheless, this approach may also damage non-cancerous cells, resulting in side effects such as fatigue, nausea, hair loss, vomiting, or even death in severe cases (Aslam et al., 2014). Consequently, the development of therapies to improve outcomes of CRC treatment is necessary.
In the present study, we examined the potential of onion peel extract to exhibit anti-cancer activity correlated to angiogenesis. Quercetin is the most abundant flavonol in onion peel extract from Allium cepa L. (Fredotović et al., 2021). Previous studies have shown that quercetin glycosides are the predominant flavonols in all onion cultivars, and account for approximately 10% of the total flavonoid content (Slimestad et al., 2007; Tedesco et al., 2015). Previous publications have reported that quercetin acts as an inhibitor, combatting lung, breast, prostate, and ovarian cancers (Dong et al., 2020; Lu et al., 2020; Mansourizadeh et al., 2020; Vafadar et al., 2020). Quercetin has been shown to induce Kirsten rat sarcoma viral oncogene homolog mutant CRC cell apoptosis by activating the c-Jun N-terminal kinase (JNK) pathway (Yang et al., 2019). Moreover, recent studies have shown anti-inflammatory effects of quercetin in restoring leukocyte counts and reducing oxidative stress markers in azoxymethane/dextran sulfate sodium-induced colon cancer mice models (Lin et al., 2020).
The results of this present study revealed that onion peel extract can inhibit CRC cell proliferation, migration, and invasion, in addition to inducing cancer cell apoptosis through attenuating the expression of the L1CAM molecules and subsequently in the NF-κB signaling pathways. In addition, onion peel extract significantly inhibited angiogenesis in HUVECs, which may be a crucial mechanism to target in CRC treatment. It is well known that tumor growth and metastasis depend on angiogenesis. Cancer development, progression, and metastasis result from efficient vascular responses, which promote cancer cells to grow and disseminate beyond their primary sites (Nishida et al., 2006; Loizzi et al., 2017).
In the present study, the cytotoxicity of onion peel extract on HT-29 cells and HUVECs was accessed. Onion peel extract induced HT-29 cell apoptosis, leading to cell death. Moreover, onion peel extract inhibited HT-29 cell migration and invasion, resulting in decreased metastatic ability. Interestingly, onion peel extract inhibited tube formation in HUVECs, which is the pivotal step in angiogenesis for supplying blood to cancer cells to allow growth and disease progression. Our results revealed a possible pathway, as onion peel extract downregulates protein expression of L1CAM and NF-κB, indicating that these two protein molecules are relevant to cancer cell invasion and metastasis, as well as angiogenesis. Therefore, onion peel extract is effective in inhibiting HT-29 cell migration and invasion through attenuating L1CAM expression, decreasing expression of NF-κB, and inhibiting angiogenesis in HUVECs. These results support the hypothesis that onion peel extract can inhibit CRC growth and progression through inhibition of the L1CAM and NF-κB signaling pathway related to angiogenesis.
However, angiogenesis has multiple steps and involves many molecules, such as angiogenic activators. Inhibitors bind their respective receptors to activate the diverse pathways. Thus, the additional molecules involved in angiogenic processes require further study.
In conclusion, onion peel extract inhibited HT-29 growth, migration, and invasion, while also inducing apoptosis in a dose-dependent manner via downregulating L1CAM and NF-κB expression in vitro. Furthermore, onion peel extract inhibited angiogenesis in HUVECs. Therefore, onion peel extract may be an effective anti-CRC therapy based on its ability to inhibit CRC growth and progression through its anti-angiogenic effects.
ACKNOWLEDGEMENTS
This study was supported by grants from the Faculty of Science, Mahidol University. The crude extract of onion peel was obtained from Detox Thailand company. Fluorescence microscope (Olympus Model BX53, Japan) was kindly supported from SPACEMED CO., LTD., Thailand and Center of nanoimaging, Faculty of Science, Mahidol University.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Aslam MS, Naveed S, Ahmed A, Abbas Z, Gull I, Athar MA. Side effects of chemotherapy in cancer patients and evaluation of patients opinion about starvation based differential chemotherapy. J Cancer Ther. 2014;5:817–822. doi: 10.4236/jct.2014.58089. [DOI] [Google Scholar]
- Bondong S, Kiefel H, Hielscher T, Zeimet AG, Zeillinger R, Pils D, et al. Prognostic significance of L1CAM in ovarian cancer and its role in constitutive NF-κB activation. Ann Oncol. 2012;23:1795–1802. doi: 10.1093/annonc/mdr568. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69:4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- Celano R, Docimo T, Piccinelli AL, Gazzerro P, Tucci M, Di Sanzo R, et al. Onion peel: turning a food waste into a resource. Antioxidants. 2021;10:304. doi: 10.3390/antiox10020304. https://doi.org/10.3390/antiox1002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Gao F, Liu N. L1CAM promotes epithelial to mesenchymal transition and formation of cancer initiating cells in human endometrial cancer. Exp Ther Med. 2018;15:2792–2797. doi: 10.3892/etm.2018.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhao Y. Block of proliferation 1 promotes cell migration and invasion in human colorectal cancer cells via the JNK pathway. J Clin Lab Anal. 2020;34:e23283. doi: 10.1002/jcla.23283. https://doi.org/10.1002/jcla.23283 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doberstein K, Harter PN, Haberkorn U, Bretz NP, Arnold B, Carretero R, et al. Antibody therapy to human L1CAM in a transgenic mouse model blocks local tumor growth but induces EMT. Int J Cancer. 2015;136:E326–E339. doi: 10.1002/ijc.29222. [DOI] [PubMed] [Google Scholar]
- Dong Y, Yang J, Yang L, Li P. Quercetin inhibits the proliferation and metastasis of human non-small cell lung cancer cell line: the key role of Src-mediated fibroblast growth factor-inducible 14 (Fn14)/nuclear factor kappa B (NF-κB) pathway. Med Sci Monit. 2020;26:e920537. doi: 10.12659/MSM.920537. https://doi.org/10.12659/MSM.920537 . [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Eichhorn ME, Kleespies A, Angele MK, Jauch KW, Bruns CJ. Angiogenesis in cancer: molecular mechanisms, clinical impact. Langenbecks Arch Surg. 2007;392:371–379. doi: 10.1007/s00423-007-0150-0. [DOI] [PubMed] [Google Scholar]
- Fredotović Ž, Puizina J, Nazlić M, Maravić A, Ljubenkov I, Soldo B, et al. Phytochemical characterization and screening of antioxidant, antimicrobial and antiproliferative properties of Allium ×cornutum Clementi and two varieties of Allium cepa L. peel extracts. Plants. 2021;10:832. doi: 10.3390/plants10050832. https://doi.org/10.3390/plants10050832 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn H, Messner J, Meldrum D. A simple non-perturbing cell migration assay insensitive to proliferation effects. Sci Rep. 2016;6:31694. doi: 10.1038/srep31694. https://doi.org/10.1038/srep31694 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Romero JJ, Valderrama-Treviño AI, Contreras-Flores EH, Barrera-Mera B, Enríquez MH, Uriarte-Ruíz K, et al. Colorectal cancer: a review. Int J Res Med Sci. 2017;5:4667–4676. doi: 10.18203/2320-6012.ijrms20174914. [DOI] [Google Scholar]
- Harada S, Morlote D. Molecular pathology of colorectal cancer. Adv Anat Pathol. 2020;27:20–26. doi: 10.1097/PAP.0000000000000247. [DOI] [PubMed] [Google Scholar]
- Huang K, Gao N, Bian D, Zhai Q, Yang P, Li M, et al. Correlation between FAK and EGF-induced EMT in colorectal cancer cells. 2020. 2020:5428920. doi: 10.1155/2020/5428920. https://doi.org/10.1155/2020/5428920 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar M, Pfeifer M, Schirmer U, Kiefel H, Konecny GE, Ben-Arie A, et al. Up-regulation of L1CAM is linked to loss of hormone receptors and E-cadherin in aggressive subtypes of endometrial carcinomas. J Pathol. 2010;220:551–561. doi: 10.1002/path.2673. [DOI] [PubMed] [Google Scholar]
- Jia X, Wang H, Li Z, Yan J, Guo Y, Zhao W, et al. HER4 promotes the progression of colorectal cancer by promoting epithelial-mesenchymal transition. Mol Med Rep. 2020;21:1779–1788. doi: 10.3892/mmr.2020.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefel H, Bondong S, Hazin J, Ridinger J, Schirmer U, Riedle S, et al. L1CAM: a major driver for tumor cell invasion and motility. Cell Adh Migr. 2012a;6:374–384. doi: 10.4161/cam.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefel H, Bondong S, Pfeifer M, Schirmer U, Erbe-Hoffmann N, Schäfer H, et al. EMT-associated up-regulation of L1CAM provides insights into L1CAM-mediated integrin signalling and NF-κB activation. Carcinogenesis. 2012b;33:1919–1929. doi: 10.1093/carcin/bgs220. [DOI] [PubMed] [Google Scholar]
- Kiefel H, Pfeifer M, Bondong S, Hazin J, Altevogt P. Linking L1CAM-mediated signaling to NF-κB activation. Trends Mol Med. 2011;17:178–187. doi: 10.1016/j.molmed.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. https://doi.org/10.1038/nrdp.2015.65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, He C, Gao A, Yan X, Xia X, Zhou J, et al. RAD18 promotes colorectal cancer metastasis by activating the epithelial-mesenchymal transition pathway. Oncol Rep. 2020;44:213–223. doi: 10.3892/or.2020.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Piao M, Song Y, Liu C. Quercetin suppresses AOM/DSS-induced colon carcinogenesis through its anti-inflammation effects in mice. J Immunol Res. 2020;2020:9242601. doi: 10.1155/2020/9242601. https://doi.org/10.1155/2020/9242601 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizzi V, Del Vecchio V, Gargano G, De Liso M, Kardashi A, Naglieri E, et al. Biological pathways involved in tumor angiogenesis and bevacizumab based anti-angiogenic therapy with special references to ovarian cancer. Int J Mol Sci. 2017;18:1967. doi: 10.3390/ijms18091967. https://doi.org/10.3390/ijms18091967 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Chen D, Yang F, Xing N. Quercetin inhibits epithelial-to-mesenchymal transition (EMT) process and promotes apoptosis in prostate cancer via downregulating lncRNA MALAT1. Cancer Manag Res. 2020;12:1741–1750. doi: 10.2147/CMAR.S241093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalik A, Khan FA, Mumtaz A, Mehmood A, Azhar S, Atif M, et al. Pharmacological applications of quercetin and its derivatives: a short review. Trop J Pharm Res. 2014;13:1561–1566. doi: 10.4314/tjpr.v13i9.26. [DOI] [Google Scholar]
- Mansourizadeh F, Alberti D, Bitonto V, Tripepi M, Sepehri H, Khoee S, et al. Efficient synergistic combination effect of quercetin with curcumin on breast cancer cell apoptosis through their loading into Apo ferritin cavity. Colloids Surf B Biointerfaces. 2020;191:110982. doi: 10.1016/j.colsurfb.2020.110982. doi : 10.1016/j.colsurfb.2020.110982. [DOI] [PubMed] [Google Scholar]
- Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci. 2017;18:197. doi: 10.3390/ijms18010197. https://doi.org/10.3390/ijms18010197 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2:213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura A, Majumder K, Giri B, Dauer P, Dudeja V, Roy S, et al. Inhibition of NF-kappa B pathway leads to deregulation of epithelial-mesenchymal transition and neural invasion in pancreatic cancer. Lab Invest. 2016;96:1268–1278. doi: 10.1038/labinvest.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadremomtaz A, Kobarfard F, Mansouri K, Mirzanejad L, Asghari SM. Suppression of migratory and metastatic pathways via blocking VEGFR1 and VEGFR2. J Recept Signal Transduct Res. 2018;38:432–441. doi: 10.1080/10799893.2019.1567785. [DOI] [PubMed] [Google Scholar]
- Seiwert N, Heylmann D, Hasselwander S, Fahrer J. Mechanism of colorectal carcinogenesis triggered by heme iron from red meat. Biochim Biophys Acta Rev Cancer. 2020;1873:188334. doi: 10.1016/j.bbcan.2019.188334. https://doi.org/10.1016/j.bbcan.2019.188334 . [DOI] [PubMed] [Google Scholar]
- Slimestad R, Fossen T, Vågen IM. Onions: a source of unique dietary flavonoids. J Agric Food Chem. 2007;55:10067–10080. doi: 10.1021/jf0712503. [DOI] [PubMed] [Google Scholar]
- Sun XH, Chang X, Wang Y, Xu B, Cao X. Oroxylin A suppresses the cell proliferation, migration, and EMT via NF-κB signaling pathway in human breast cancer cells. BioMed Res Int. 2019;2019:9241769. doi: 10.1155/2019/9241769. https://doi.org/10.1155/2019/9241769 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco I, Carbone V, Spagnuolo C, Minasi P, Russo GL. Identification and quantification of flavonoids from two southern Italian cultivars of Allium cepa L., Tropea (red onion) and Montoro (copper onion), and their capacity to protect human erythrocytes from oxidative stress. J Agric Food Chem. 2015;63:5229–5238. doi: 10.1021/acs.jafc.5b01206. [DOI] [PubMed] [Google Scholar]
- Tonini T, Rossi F, Claudio PP. Molecular basis of angiogenesis and cancer. Oncogene. 2003;22:6549–6556. doi: 10.1038/sj.onc.1206816. [DOI] [PubMed] [Google Scholar]
- Uttarawichien T, Kamnerdnond C, Inwisai T, Suwannalert P, Sibmooh N, Payuhakrit W. Quercetin inhibits colorectal cancer cells induced-angiogenesis in both colorectal cancer cell and endothelial cell through downregulation of VEGF-A/VEGFR2. Sci Pharm. 2021;89:23. doi: 10.3390/scipharm89020023. https://doi.org/10.3390/scipharm89020023 . [DOI] [Google Scholar]
- Vafadar A, Shabaninejad Z, Movahedpour A, Fallahi F, Taghavipour M, Ghasemi Y, et al. Quercetin and cancer: new insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020;10:32. doi: 10.1186/s13578-020-00397-0. https://doi.org/10.1186/s13578-020-00397-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versluis M, Plat A, de Bruyn M, Matias-Guiu X, Trovic J, Krakstad C, et al. L1CAM expression in uterine carcinosarcoma is limited to the epithelial component and may be involved in epithelial-mesenchymal transition. Virchows Arch. 2018;473:591–598. doi: 10.1007/s00428-018-2444-8. [DOI] [PubMed] [Google Scholar]
- Wan ML, Wang Y, Zeng Z, Deng B, Zhu BS, Cao T, et al. Colorectal cancer (CRC) as a multifactorial disease and its causal correlations with multiple signaling pathways. Biosci Rep. 2020;40:BSR20200265. doi: 10.1042/BSR20200265. https://doi.org/10.1042/BSR20200265 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang T, Chen D, Ma Q, Zheng Y, Liao S, et al. Quercetin preferentially induces apoptosis in KRAS-mutant colorectal cancer cells via JNK signaling pathways. Cell Biol Int. 2019;43:117–124. doi: 10.1002/cbin.11055. [DOI] [PubMed] [Google Scholar]