Abstract
This study aimed to investigate the underlying mechanisms of red ginseng extract (RGE) on regulating hair growth and hair follicle development. Results from in vitro studies showed that RGE treatment simultaneously enhanced viability and inhibited apoptosis in human hair dermal papilla cells. Moreover, RGE administration promoted telogen-to-anagen transition, prolonged anagen in hair follicular cycling, and increased the size of hair follicles and skin thickness in a C57BL/6 mouse model. Furthermore, RGE treatment significantly upregulated the expression of β-catenin, phospho-glycogen synthase kinase 3β, cyclin D1, cyclin E, and Bcl-2, phospho-extracellular signal-regulated protein kinase, and phospho-Akt, which are associated with promoting hair growth. In addition, RGE enhanced skin health by activation of antiox-idant defense systems. Our data demonstrates that hair regenerative mechanisms of RGE may be mediated by stimulating dermal papilla cell proliferation and enhancing skin functions.
Keywords: alopecia, β-catenin, dermal papilla cell, hair growth, red ginseng extract
INTRODUCTION
Hair, a vital skin appendage, stems from hair follicles and has diverse functions, including thermoregulation, protection, sensory, and tactile activities, and social interactions (Schneider et al., 2009). Hair follicles are complex regenerative organs that undergo repetitive cycles, with phases including anagen (growth), catagen (regression), and telogen (rest). Its function and cycling depend on effective interactions between different cell populations. Hair shafts are produced in anagen and their lengths corresponds to the duration of the anagen phase (Cotsarelis and Millar, 2001; Schneider et al., 2009).
Hair loss (alopecia or baldness) is a common condition that affects both men and women. Recently, the incidence of hair loss has dramatically increased in young people. Although alopecia is not life-threatening in humans, it causes psychological stress. Hair loss can be attributed to a wide range of factors, such as genetics, disease, nutritional insufficiency, aging, hormone change, and stress, and results in alterations to hair follicle cycles, including shortening of the anagen phase, augmenting the number of telogenic hair follicles, and changes to hair follicle morphology (Cotsarelis and Millar, 2001). It has been suggested that effective strategies to combat hair loss include extending anagen, stimulating telogen-to-anagen transition of follicles, reversing miniaturization, and enhancing dermal papilla cell viability to stimulate new follicle generation. Minoxidil (MNX) and finasteride are current hair loss treatments for patients with alopecia in many countries. However, their therapeutic benefits are limited due to their transient and adverse effects (Park et al., 2011). Therefore, alternative strategies are necessary to develop new agents capable of preventing and/or treating hair loss.
Red ginseng (Panax ginseng Mayer) has long been used in traditional medicine in Asian countries, including China, Korea, and Japan. Recent studies have reported that red ginseng extract (RGE) contains a wide variety of ginsenosides, such as ginsenosides-Rb1, -Rb2, -Rc, -Rd, -Rg3, -Rg1, and -Ro, which are primarily responsible for its biological activities, including antioxidant, antitumor, antidiabetic, anti-inflammatory, and hepatoprotective effects (Yun, 2001; Lü et al., 2009; Lee and Kim, 2014). Previous reports have indicated that P. ginseng may stimulate hair growth, possibly due to action of its ginsenosides ginsenoside-Rb1 and -Rg3, in cultured mouse vibrissal hair follicles (Matsuda et al., 2003; Shin et al., 2014a), and in cultured human hair follicles (Park et al., 2015). Ginsenoside F2, an intestinal microorganism-mediated metabolite of ginsenoside-Rb1, has been reported to trigger telogen-to-anagen transition of hair follicles and hair regrowth via modulating Wnt/β-catenin signal pathways in mouse (Shin et al., 2014c). Furthermore, ginsenoside F2 has been shown to reduce hair loss in dihydrotestosterone (DHT)-treated in vitro and in vivo models through preventing apoptosis (Shin et al., 2014b). In addition, ginseng rhizome and ginsenoside-Ro have been shown to exhibit inhibitory activity against 5α-reductase and enhance hair regrowth in a mouse model of androgenic alopecia (Murata et al., 2012). Although clinical trials have found that consumption of Korean RGE improves hair density and thickness in patients with alopecia (Kim et al., 2009; Oh and Son, 2012), the molecular mechanisms of red ginseng on anagen induction have not been fully elucidated.
The study aimed to investigate the possible molecular mechanisms of RGE in promoting hair growth in human hair dermal papilla cells and a mouse model.
MATERIALS AND METHODS
Materials
Formaldehyde, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT), hematoxylin, and eosin were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). β-Catenin, vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), cyclin E, β-actin, cyclin D1, p-extracellular signal-related kinase (ERK), p-Akt, p-glycogen synthase kinase 3β (GSK-3β), Bcl-2, Bax, peroxidase-conjugated anti-rabbit, and peroxidase-conjugated anti-goat antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) or Cell Signaling Technology (Boston, MA, USA). MNX (3%) was purchased from Hyundai Pharm. Co., Ltd. (Chungnam, Korea). All other reagents used were of the analytical grade commercially available.
Preparation of RGE
RGE was kindly given by Korea Ginseng Corporation (Daejeon, Korea). Briefly, six-year-old red ginseng powders were extracted with ten volumes of potable water at 98°C for 48 h. After filtering, filtrates were condensed under reduced pressure to obtain darkish brown-red ginseng extracts.
Cell culture
Human hair dermal papilla cells (HHDPC) were purchased from ScienCell Research Laboratories, Inc. (Carlsbad, CA, USA) and maintained in mesenchymal stem cell medium containing 5% fetal bovine serum, 1% mesenchymal stem cell growth supplement, and 1% penicillin/ streptomycin at 37°C in a 5% CO2 incubator. Cell culture medium was replenished every 2 days.
Cell viability assays
HHDPC was seeded onto 96-well microplate (3×104 cells/well) and incubated with different RGE concentrations (100, 200, 300, and 400 µg/mL) or water (control) for 24 h. MTT solution (5 mg/mL) was added to each well and cells were incubated at 37°C for 4 h in the dark. Purple formazan product, a metabolite of MTT, was then solubilized with 100 mL of dimethyl sulfoxide. The absorbance at 570 nm was measured using a microplate reader (BioTek Instruments, Winooski, VT, USA).
Animal experiments
Six-week-old male C57BL/6 mice (18∼20 g) were obtained from Hyochang Science (Deagu, Korea). Mice were maintained under a standard condition, according to Institutional Animal Care and Use Guidelines of Inje University, who approved the mouse experiments with the approval number 2015-05 (Gimhae, Korea). After a week for acclimation, dorsal hairs were removed with hair clippers and hair removal cream from the 7-week-old mice (Veet, Oxy Reckitt Benckiser, Chartres, France). The control group received the vehicle (normal saline) alone, the RGE group received orally administered 50 mg/kg RGE, and the MNX group (positive control) received 3% MNX (topically, 200 µL per mouse) every day for up to 21 days. The dorsal skin of the mice was photographed at 0, 7, 14, 17, and 21 days after treatment. Mice were subsequently sacrificed and skin tissues were harvested and stored at −80°C for further experiments.
Histological analysis
Dorsal skin tissues were collected and subjected to hematoxylin and eosin staining by using standard techniques. The stained sections were observed and analyzed through an optical microscope. Bulb diameter of hair follicles in the deep subcutis were measured at the level of the largest hair diameter of the hair bulbs with clearly visible dermal papilla. Skin thickness was measured from the epidermis to subcutaneous fat. Digital microimages were taken from representative areas using a digital camera (PAXcam, PAXit, Villa Park, IL, USA).
Alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (γ-GT), and antioxidant enzyme activity assays
ALP activity assays were performed using an Alkaline Phosphatase Assay kit, according to manufacturer’s instructions (BioAssay Systems, Wien, Austria). γ-GT activity assays were conducted using an Activity Colorimetric Assay kit (BioVision Inc., Milpitas, CA, USA). Catalase (CAT) activity was determined according to the method of Aebi (1984). One unit of CAT was described as the amount of enzyme required to decompose 1.0 µM of H2O2 in a minute. Glutathione peroxidase (GPx) activity assays were conducted following the method of Bogdanska and colleagues (2003). The amount of enzyme that oxidizes 1 nM of nicotinamide adenine dinucleotide phosphate hydrogen per min was defined as a unit of GPx. Manganese-superoxide dismutase (Mn-SOD) was measured by the method of Oyanagui (1984). One unit of SOD was defined as the mount of enzyme needed to inhibit 50% of the superoxide radicals.
Western blot analysis
Proteins were extracted from skin tissues or cultured (human hair dermal papilla cells) HHDPCs by homogenizing cells with radioimmunoprecipitation assay buffer. The protein content of each sample was measured using a BCA protein assay kits (Pierce Manufacturing, Appleton, WI, USA). To analyze protein expression, an equal amount of proteins from each group were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred onto polyvinylidene fluoride membranes using a semidry transfer system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were blocked with 5% non-fat milk and then hybridized with proper primary antibodies overnight at 4°C. After washing with 0.1% Tween-20 in phosphate-buffered saline, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 3 h at 4°C. Protein bands were visualized using enhanced chemiluminescence Western blotting reagents (Santa Cruz Biotechnology, Inc.).
Statistical analysis
Results are presented as mean±standard deviation (SD). Student’s t-tests were used to analyze significant differences between control and treatment groups. Values of P<0.05 were considered to be statistically significant.
RESULTS
Effect of RGE on viability and apoptosis of HHDPC
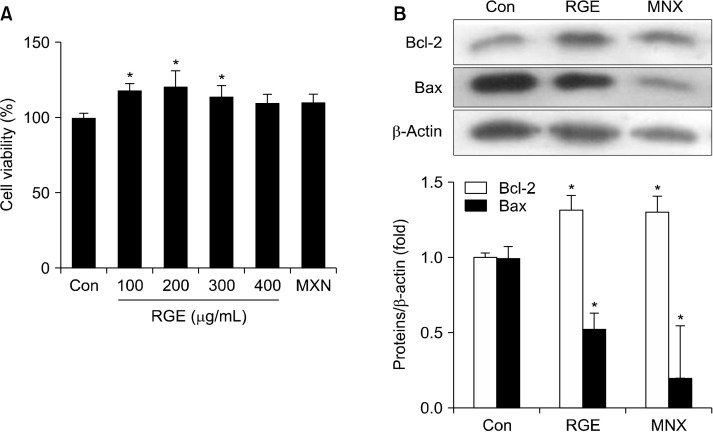
To evaluate the effect of RGE on survival of HHDPCs, cells were incubated with different concentrations of RGE and cell viability was determined using MTT assays. RGE treatment significantly enhanced cell survival compared with the vehicle-treated control cells (Fig. 1A). RGE at 200 mg/mL showed the greatest effect on cell viability, even higher than MNX, a positive control. However, the highest dose of RGE (400 µg/mL) was unlikely to significantly increase the viability of HHDPCs. Apoptosis directly affects cell viability and disturbs hair follicle cycling. RGE treatment upregulated expression of anti-apoptotic Bcl-2 and downregulated expression of apoptotic Bax protein. A similar result was observed for MNX-treated HHDPCs (Fig. 1B).
Fig. 1.
Effect of RGE on HHDPC proliferation and apoptosis. HHDPCs were treated with various concentrations of RGE or MNX (100 μM) for 24 h. (A) Cell viability was measured using MTT assays, added as a percentage of the vehicle-treated control. (B) Cells were treated with RGE (100 μg/mL) or MNX (100 μM) for 24 h and protein expressions of Bcl-2 and Bax were analyzed by Western blotting. Data show the mean±SD of at least three independent experiments. *P<0.05 values are considered significantly different. HHDPC, human hair dermal papilla cells; MNX, minoxidil; MTT, 3-[4,5-dimeth-ylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; RGE, red ginseng extract.
Effect of RGE on the Wnt/β-catenin pathway in HHDPCs
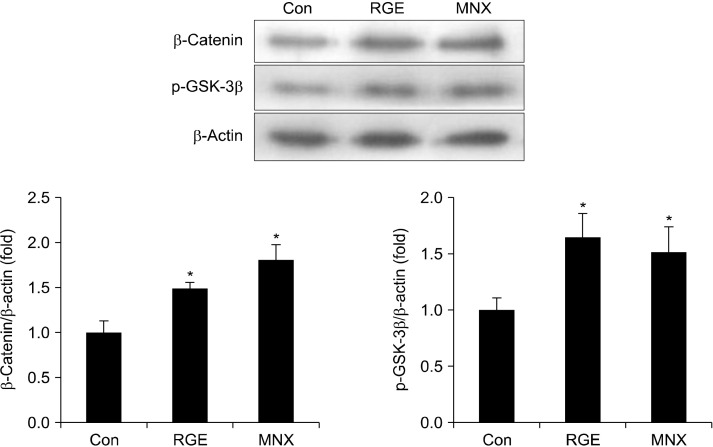
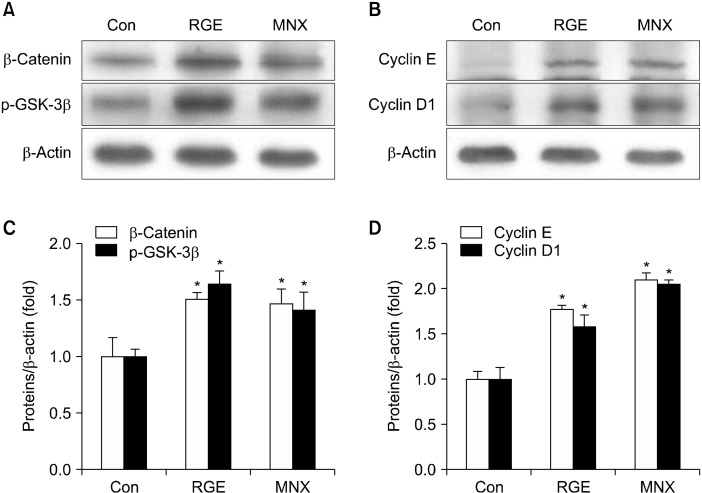
We next examined the role of RGE in regulating the Wnt/β-catenin pathway, which plays a central roles in hair development. Treatment with RGE and MNX significantly upregulated expression of β-catenin in HHDPCs compared with vehicle-treated control cells (Fig. 2). Moreover, the amount of p-GSK-3β was enhanced by treatment with RGE and MNX. These data suggest that phosphorylation-induced inactivation of GSK-3β by RGE results in an increase of β-catenin proteins in HHDPC.
Fig. 2.
Effect of RGE on the Wnt/β-catenin pathway in HHDPCs. HHDPCs were incubated with RGE or MNX (100 μM) for 24 h. Expressions of β-catenin and p-GSK-3β were detected using Western blotting. Data show the mean±SD of at least three independent experiments. *P<0.05 values are considered significantly different. HHDPC, human hair dermal papilla cells; MNX, minoxidil; p-GSK-3β, phospho-glycogen synthase kinase 3 beta; RGE, red ginseng extract.
Hair growth-promoting activity of RGE in C57BL/6 mice
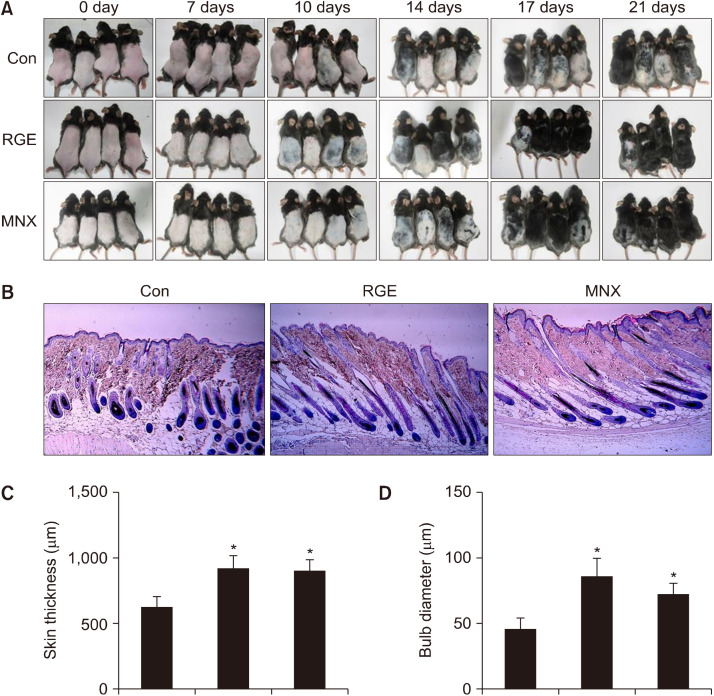
Hair regenerative activity of RGE was further examined in an in vivo model. Mice were orally administered RGE or MNX (positive control) once daily for 21 days. Changes in back skin color, which is bright pink in telogen and turns gray/black in anagen, were used to evaluate hair growth. RGE administration significantly stimulated hair growth and induced premature progression of hair follicles in the anagen phase of the hair cycle compared with vehicle-treated mice (Fig. 3A). Mice treated with both RGE and MNX exhibited gray skin by day 10 of treatment and dark skin with visible hair shafts by day 14, whereas large areas of dorsal skins remained without pigmentation in the control group. Dorsal skins of mice in both the RGE and MNX groups were covered with long hair shafts at day 17. Moreover, hair growth-promoting efficacy of RGE was comparable with that of MNX after 21 days of treatment.
Fig. 3.
Hair growth-promoting activity of RGE in C57BL/6 mice. After shaving, mice were treated daily with RGE (50 mg/kg) or 3% MNX for 21 consecutive days. (A) Dorsal skins were photographed at 0, 7, 14, 17, and 21 days. After treatment, dorsal skins were collected and subjected to H&E staining. Representative micro-images of (B) skin sections, (C) skin thickness and (D) hair follicle bulb diameters. Data show the mean±SD (n=4). *P<0.05 values are considered significantly different. H&E, hematoxylin and eosin; MNX, minoxidil; RGE, red ginseng extract.
Effects of RGE on development and structure of hair follicles
Histological analysis showed premature telogen-to-anagen conversion of hair follicles in both the RGE and MNX groups compared with the control group. Almost all hair follicles in the RGE and MNX groups appeared at anagen V/VI with hair shaft erupting out of the epidermis. However, some of hair follicles in the control group entered anagen IV/V of the hair cycle (Fig. 3B). In addition, RGE administration significantly increased skin thickness compared with administration of the vehicle (Fig. 3C). Furthermore, the sizes of the hair bulbs of mice in the RGE-treated group were greater than those of both the vehicle-treated and MNX-treated groups (Fig. 3D). These data suggest that RGE may induce early telogen-to-anagen transition of hairs and promote development of hair follicles, thereby stimulating hair regrowth.
Effect of RGE on levels of growth factors and hair growth indicators
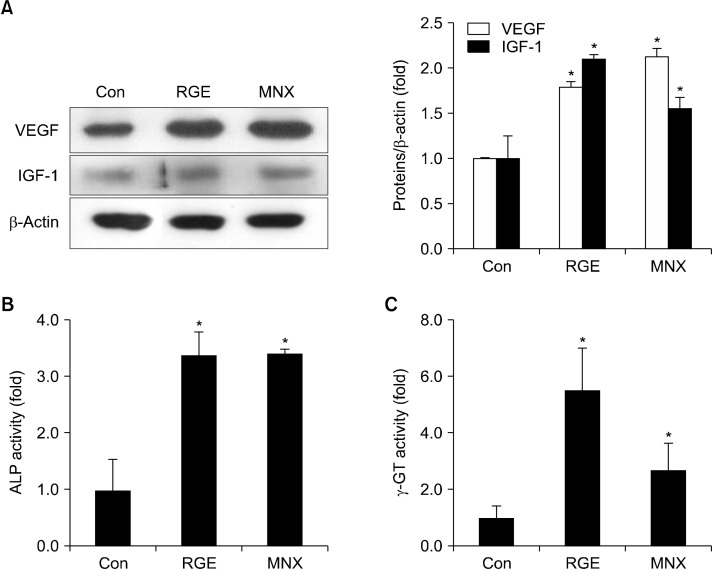
Growth factors are involved in regulation of hair development and differentiation. After 21 days of treatment, RGE significantly increased VEGF levels in dorsal skin tissues compared with the vehicle. Likewise, IGF-1 protein expression was upregulated by RGE administration (Fig. 4A), and ALP and γ-GT, typical biomedical markers of anagen in growing hair, were significantly elevated in mice treated with RGE (Fig. 4B and 4C). Furthermore, γ-GT activity was greater in the RGE-treated group than in the MNX-treated group (Fig. 4C).
Fig. 4.
Effect of RGE on levels of growth factors and hair growth indicators in C57BL/6 mice. (A) Expression of IGF-1 and VEGF in skin tissues, detected by Western blotting. Levels of ALP (B) and γ-GT (C) in skin tissues determined using commercial kits. Data show the mean±SD (n=3). *P<0.05 values are considered significantly different. ALP, alkaline phosphates; γ-GT, gamma-glutamyl transpeptidase; IGF-1, insulin-like growth factor; RGE, red ginseng extract; VEGF, vascular endothelial growth factor.
RGE modulates the Wnt/β-catenin pathway in mice
To explore possible hair regenerative mechanisms of RGE, we analyzed expression of hair induction-associated genes in skin tissues of the mice. The Wnt/β-catenin pathway is involved in initiating and maintaining the anagen phase of the hair cycle (Enshell-Seijffers et al., 2010). Therefore, we surmised that RGE may stimulate hair regrowth by triggering the Wnt/β-catenin pathway. RGE-treated mice exhibited a significant increase in the level of β-catenin compared with vehicle-treated mice. GSK-3β, a β-catenin cytosolic suppressor, was inactivated by enhanced phosphorylation in mice treated with RGE (Fig. 5A). Similar results were observed for mice treated with MNX. In addition, Wnt target genes, including cyclin D1 and cyclin E, were upregulated by both RGE and MNX (Fig. 5B).
Fig. 5.
Effect of RGE on the Wnt/β-catenin pathway in C57BL/6 mice. Expression of (A) p-GSK-3β and β-catenin, and (B) cyclin D1 and cyclin E in skin tissues were measured by Western blotting. Data show the mean±SD (n=3). *P<0.05 values are considered significantly different. p-GSK-3β, phospho-glycogen synthase kinase 3 beta; RGE, red ginseng ex-tract.
Effect of RGE on antioxidant status and apoptosis in mice
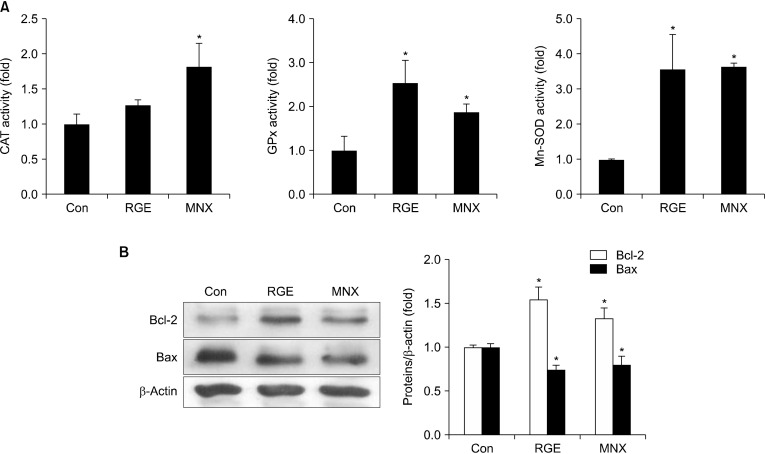
Oxidative stress may inhibit hair growth, cause skin and hair aging, and delay onset of the anagen phase of the hair cycle (Prie et al., 2015). Enhanced antioxidant defense systems in skin helps inhibit hair aging and promotes hair regeneration. We explored the effect of RGE on the antioxidant status of skin tissues by evaluating activity of antioxidant enzymes, such as CAT, GPx, and SOD-2. Activities of GPx and SOD-2 in RGE-treated mice were markedly increased. Although not significant, CAT activity was also slightly enhanced by RGE (Fig. 6A). Therefore, RGE treatment may enhance antioxidant defense system in mouse skin by increasing endogenous antioxidant enzyme activity. Moreover, apoptosis that suppresses hair growth can be regulated by the ratio of Bcl-2 and Bax during the apoptosis-driven involution phase of the hair cycle. To examine whether RGE blocks apoptosis in hair follicles, we elucidated expression of two key proteins, Bcl-2 and Bax, in skin tissues. Results indicated that treatment with RGE or MNX increased anti-apoptotic Bcl-2, but decreased pro-apoptotic Bax in skin tissue (Fig. 6B). These findings suggest that enhanced skin health induced by increased antioxidant status and regulation of apoptosis may, in part, contribute to the hair growth-promoting ability of RGE.
Fig. 6.
Effect of RGE on antioxidant status of and apoptosis in C57BL/6 mice. (A) Activities of CAT, GPx, and Mn-SOD enzymes in skin tissues were measured as mentioned in the Materials and Methods. (B) Protein expressions of Bcl-2 and Bax in C57BL/6 mouse skin tissue were analyzed by Western blotting. Data show the mean±SD (n=3). *P<0.05 values are considered significantly different. CAT, catalase; GPx, glutathione peroxidase; Mn-SOD, manganese-superoxide dismutase; RGE, red ginseng extract.
Effect of RGE on kinase signaling pathways in mice
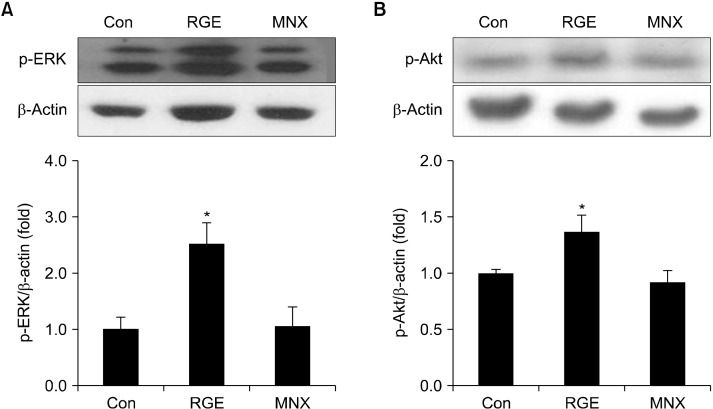
Mitogen-activated protein kinase/ERK and phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways are involved in the regulation of dermal papilla cell proliferation in hair follicles (Yun et al., 2005; Zhang et al., 2013). To investigate whether RGE activates intracellular signaling pathways, phosphorylated levels of ERK and Akt were determined in skin tissues. Results indicated that RGE significantly increased phosphorylation of ERK compared with the vehicle (Fig. 7A). Likewise, Akt phosphorylation was elevated by treatment with RGE (Fig. 7B).
Fig. 7.
Effect of RGE on kinase signaling pathways in C57BL/6 mice. Phosphorylation levels of ERK (A) and Akt (B) were detected by Western blotting. Data show the mean±SD (n=3). *P<0.05 values are considered significantly different. ERK, extracellular signal-related kinase; RGE, red ginseng extract.
DISCUSSION
Hair follicles are constituted by multiple layers of epithelial cells and a mass of dermal papilla cells. Hair dermal papilla cells that inhabit the dermal papilla anchored at the bottom of hair roots are necessary for hair formation, growth, and cycling (Yang and Cotsarelis, 2010). Dermal papilla cells are responsible for induction and maintenance of epithelial cell growth and differentiation by instructing surrounding epithelial cells to proliferate and differentiate into the multiple layers of the outgrowing hair shaft and the channel surrounding the hair shaft (Millar, 2002; Driskell et al., 2011). Moreover, the quantity of dermal papilla cells in the follicles correlates with the size and shape of the hair, with degradation of the dermal papilla population causing several hair thinning and loss conditions (Chi et al., 2013). Therefore, stimulation of dermal papilla cell proliferation helps promote hair growth. Our study indicated that RGE may enhance proliferation of HHDPCs.
In addition, apoptosis affects both hair cell survival and hair growth. Activation of signaling pathways that trigger apoptosis in hair cells occurs throughout hair follicle regression. Bcl-2 family proteins, such as anti-apoptotic Bcl-2 and pro-apoptotic Bax, play roles as master regulators of apoptotic process in almost all cell types (Lindner et al., 1997). Treatment of HHDPCs with cisplatin induces production of reactive oxygen species and decreases of Bcl-2/Bax ratios, leading to HHDPC apoptosis and massive hair loss (Luanpitpong et al., 2011). Tea polyphenol epigallocatechin-3-gallate (Kwon et al., 2007) and MNX (Han et al., 2004) enhanced proliferation and inhibited apoptosis (by increasing Bcl-2/Bax ratios) of dermal papilla cells, thereby stimulating hair growth. In mice, 6-gingerol in ginger suppresses cultured human hair elongation and delays telogen-to-anagen transition of hair follicles by inhibitory and pro-apoptotic activities on dermal hair cells (Miao et al., 2013). Furthermore, DHT-mediated hair loss may arise from triggering of apoptotic cell death in hair follicles (Kwack et al., 2008). In our study, we demonstrated that RGE simultaneously upregulated Bcl-2 and downregulated Bax in both HHDPCs and in mouse skin, indicating enhanced survival of dermal cells in hair follicles.
Telogen-to-anagen transition of hair follicle is mediated by effective interactions among various signaling molecules and pathways (Rishikaysh et al., 2014). The Wnt/β-catenin pathway is one of the most important signaling pathways producing a signal for hair follicle induction (Millar et al., 1999; Kishimoto et al., 2000). Usually, transcription factor β-catenin is anchored in the cytoplasm by phosphorylation of GSK-3β, subsequently leading to proteasomal degradation of β-catenin (Ikeda et al., 2000). However, activation of upstream signal transduction cascades induces release of β-catenin from this inhibitory complex. Excessive β-catenin is translocated into the nucleus, where it cooperates with other transcription factors to activate target genes contributing to initiation of the transition from the late G1 to S phases of the cell cycle, including cyclin D1, cyclin E, or hair growth-promoting ALP (Behrens et al., 1996; Tetsu and McCormick, 1999). Recent reports have demonstrated that deletion of β-catenin abolishes direct Wnt/β-catenin-mediated target gene cyclin D1 and decreased hair regeneration, and eventually causes hair loss (Huelsken et al., 2001; Choi et al., 2013). In contrast, activation of β-catenin in the epidermis promotes expansion of hair follicles during development (Zhang et al., 2008). In addition, inactivation of GSK-3β by phosphorylation leads to liberation of β-catenin from inhibitory complexes, which enhances expression of β-catenin-mediated target genes crucial for dermal papilla cells growth and proliferation and promotes hair follicle regeneration and hair growth (Yamauchi and Kurosaka, 2009; Soma et al., 2012). In addition to induction of the telogen-to-anagen transition, Wnt/β-catenin signaling helps prolong and maintain anagen (Kishimoto et al., 2000; Shimizu and Morgan, 2004). Blockage of the β-catenin gene within the dermal papilla of mature hair follicles attenuated proliferation of progenitors and their immediate progeny that produce the hair shaft, and prematurely induced the destructive phase (catagen) of the hair cycle (Enshell-Seijffers et al., 2010). Our findings show that RGE treatment upregulates β-catenin, p-GSK-3β, cyclin D1, and cyclin E in HHDPCs and mouse skin, suggesting that RGE may stimulate transition of telogenic hair follicles to the anagen phase through activating β-catenin signaling pathway, thereby promoting hair regrowth.
Hair regeneration is a complex process that involves growth factors, cytokines, and hormones (Peus and Pit-telkow, 1996; Chi et al., 2013). Induction of angiogenesis is essential to satisfy the increased nutritional need of hair follicles during the anagen phase of rapid cell division. VEGF has been identified to induce hair follicle progression into anagen phase of the hair cycle and to enhance hair shaft elongation through stimulating vasculogenesis and angiogenesis (Yano et al., 2001). IGF-1 is an important growth factor that regulates cellular proliferation and differentiation, tissue remodeling and the hair cycle during development of hair follicles (Weger and Schlake, 2005). Loss of IGF-1 induces resting in the telogen phase and retards the onset of a second anagen phase. In the present study, we observed significant increases in VEGF and IGF-1 levels in RGE-treated mouse skins, indicating that RGE may promote hair regrowth, to some extent by inducing growth factors such as VEGF and IGF-1 in skin tissues. Moreover, stimulation of ERK and PI3K/Akt signaling pathways was also observed in mice treated with RGE. These pathways regulate cell survival, cell proliferation, transcription, and cell migration (Hayashi et al., 2013). A previous study showed that RGE and its ginsenoside Rb1 enhances proliferation of human dermal papilla cells through activating ERK and Akt signaling pathways (Park et al., 2015). VEGF and IGF-1 have shown to signal through cooperation with several different signaling transduction pathways, such as ERK and Akt (Zachary, 2003; Hayashi et al., 2013). Furthermore, some studies have demonstrated that ERK and Akt pathways contribute to activation of transcription factors including β-catenin, resulting in enhanced hair follicular cell survival and proliferation, thereby promoting hair growth (Yun et al., 2005; Zhang et al., 2013).
Overall, this study demonstrates the role of RGE in promoting hair growth and suggests plausible underlying mechanisms for RGE-mediated activity. Our findings show that RGE induces early progression of hair follicles into the anagen phase and stimulates hair regrowth through enhancing proliferation of dermal papilla cells, activating Wnt/β-catenin pathway, and inducing growth factors. Therefore, RGE could be a potent therapeutic agent for prevention and/or treatment of hair loss.
Footnotes
FUNDING
We are grateful to the Korea Ginseng Corporation for the preparation of RGE. This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIT) (NRF-2014R1A2A1A11050006, 2020R1F1A1073595, and 2021R1A2C2006745).
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
AUTHORS’ CONTRIBUTION
Van-Long Truong: Methodology; Formal analysis; Writing-original draft. Woo-Sik Jeong: Project administration; Supervision.
REFERENCES
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, et al. Functional interaction of β-catenin with the transcrip-tion factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bogdanska JJ, Korneti P, Todorova B. Erythrocyte superoxide dismutase, glutathione peroxidase and catalase activities in healthy male subjects in Republic of Macedonia. Bratisl Lek Listy. 2003;104:108–114. [PubMed] [Google Scholar]
- Chi W, Wu E, Morgan BA. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development. 2013;140:1676–1683. doi: 10.1242/dev.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Zhang Y, Xu M, Yang Y, Ito M, Peng T, et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 2013;13:720–733. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Millar SE. Towards a molecular understanding of hair loss and its treatment. Trends Mol Med. 2001;7:293–301. doi: 10.1016/S1471-4914(01)02027-5. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011;124:1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. β-Catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Kwon OS, Chung JH, Cho KH, Eun HC, Kim KH. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J Dermatol Sci. 2004;34:91–98. doi: 10.1016/j.jdermsci.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Yamamoto N, Nakagawa T, Ito J. Insulin-like growth factor 1 inhibits hair cell apoptosis and promotes the cell cycle of supporting cells by activating different downstream cas-cades after pharmacological hair cell injury in neonatal mice. Mol Cell Neurosci. 2013;56:29–38. doi: 10.1016/j.mcn.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/S0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A. GSK-3β-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by β-catenin and protein phosphatase 2A complexed with Axin. Oncogene. 2000;19:537–545. doi: 10.1038/sj.onc.1203359. [DOI] [PubMed] [Google Scholar]
- Kim JH, Yi SM, Choi JE, Son SW. Study of the efficacy of Korean red ginseng in the treatment of androgenic alopecia. J Ginseng Res. 2009;33:223–228. doi: 10.5142/JGR.2009.33.3.223. [DOI] [Google Scholar]
- Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- Kwack MH, Sung YK, Chung EJ, Im SU, Ahn JS, Kim MK, et al. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol. 2008;128:262–269. doi: 10.1038/sj.jid.5700999. [DOI] [PubMed] [Google Scholar]
- Kwon OS, Han JH, Yoo HG, Chung JH, Cho KH, Eun HC, et al. Human hair growth enhancement in vitro by green tea epigal-locatechin-3-gallate (EGCG) Phytomedicine. 2007;14:551–555. doi: 10.1016/j.phymed.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Lee CH, Kim JH. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R. Analysis of apoptosis during hair follicle regression (catagen) Am J Pathol. 1997;151:1601–1617. [PMC free article] [PubMed] [Google Scholar]
- Lü JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luanpitpong S, Nimmannit U, Chanvorachote P, Leonard SS, Pongrakhananon V, Wang L, et al. Hydroxyl radical mediates cisplatin-induced apoptosis in human hair follicle dermal papilla cells and keratinocytes through Bcl-2-dependent mechanism. Apoptosis. 2011;16:769–782. doi: 10.1007/s10495-011-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Yamazaki M, Asanuma Y, Kubo M. Promotion of hair growth by ginseng radix on cultured mouse vibrissal hair follicles. Phytother Res. 2003;17:797–800. doi: 10.1002/ptr.1241. [DOI] [PubMed] [Google Scholar]
- Miao Y, Sun Y, Wang W, Du B, Xiao SE, Hu Y, et al. 6-Gingerol inhibits hair shaft growth in cultured human hair follicles and modulates hair growth in mice. PLoS One. 2013;8:e57226. doi: 10.1371/journal.pone.0057226. https://doi.org/10.1371/journal.pone.0057226 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SE, Willert K, Salinas PC, Roelink H, Nusse R, Sussman DJ, et al. WNT signaling in the control of hair growth and structure. Dev Biol. 1999;207:133–149. doi: 10.1006/dbio.1998.9140. [DOI] [PubMed] [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Murata K, Takeshita F, Samukawa K, Tani T, Matsuda H. Effects of ginseng rhizome and ginsenoside Ro on testosterone 5α-reductase and hair re-growth in testosterone-treated mice. Phytother Res. 2012;26:48–53. doi: 10.1002/ptr.3511. [DOI] [PubMed] [Google Scholar]
- Oh GN, Son SW. Efficacy of Korean red ginseng in the treatment of alopecia areata. J Ginseng Res. 2012;36:391–395. doi: 10.5142/jgr.2012.36.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyanagui Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem. 1984;142:290–296. doi: 10.1016/0003-2697(84)90467-6. [DOI] [PubMed] [Google Scholar]
- Park GH, Park KY, Cho HI, Lee SM, Han JS, Won CH, et al. Red ginseng extract promotes the hair growth in cultured human hair follicles. J Med Food. 2015;18:354–362. doi: 10.1089/jmf.2013.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Shin WS, Ho J. Fructus panax ginseng extract promotes hair regeneration in C57BL/6 mice. J Ethnopharmacol. 2011;138:340–344. doi: 10.1016/j.jep.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Peus D, Pittelkow MR. Growth factors in hair organ development and the hair growth cycle. Dermatol Clin. 1996;14:559–572. doi: 10.1016/S0733-8635(05)70384-3. [DOI] [PubMed] [Google Scholar]
- Prie BE, Voiculescu VM, Ionescu-Bozdog OB, Petrutescu B, Iosif L, Gaman LE, et al. Oxidative stress and alopecia areata. J Med Life. 2015;8:43–46. [PMC free article] [PubMed] [Google Scholar]
- Rishikaysh P, Dev K, Diaz D, Qureshi WM, Filip S, Mokry J. Signaling involved in hair follicle morphogenesis and development. Int J Mol Sci. 2014;15:1647–1670. doi: 10.3390/ijms15011647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Morgan BA. Wnt signaling through the beta-catenin pathway is sufficient to maintain, but not restore, anagen-phase characteristics of dermal papilla cells. J Invest Dermatol. 2004;122:239–245. doi: 10.1046/j.0022-202X.2004.22224.x. [DOI] [PubMed] [Google Scholar]
- Shin DH, Cha YJ, Yang KE, Jang IS, Son CG, Kim BH, et al. Ginsenoside Rg3 up-regulates the expression of vascular endothe-lial growth factor in human dermal papilla cells and mouse hair follicles. Phytother Res. 2014a;28:1088–1095. doi: 10.1002/ptr.5101. [DOI] [PubMed] [Google Scholar]
- Shin HS, Park SY, Hwang ES, Lee DG, Mavlonov GT, Yi TH. Ginsenoside F2 reduces hair loss by controlling apoptosis through the sterol regulatory element-binding protein cleavage activating protein and transforming growth factor-β pathways in a dihydrotestosterone-induced mouse model. Biol Pharm Bull. 2014b;37:755–763. doi: 10.1248/bpb.b13-00771. [DOI] [PubMed] [Google Scholar]
- Shin HS, Park SY, Hwang ES, Lee DG, Song HG, Mavlonov GT, et al. The inductive effect of ginsenoside F2 on hair growth by altering the WNT signal pathway in telogen mouse skin. Eur J Pharmacol. 2014c;730:82–89. doi: 10.1016/j.ejphar.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Soma T, Fujiwara S, Shirakata Y, Hashimoto K, Kishimoto J. Hair-inducing ability of human dermal papilla cells cultured under Wnt/β-catenin signalling activation. Exp Dermatol. 2012;21:307–309. doi: 10.1111/j.1600-0625.2012.01458.x. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Weger N, Schlake T. IGF-I signalling controls the hair growth cycle and the differentiation of hair shafts. J Invest Dermatol. 2005;125:873–882. doi: 10.1111/j.0022-202X.2005.23946.x. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Kurosaka A. Inhibition of glycogen synthase kinase-3 enhances the expression of alkaline phosphatase and insulin-like growth factor-1 in human primary dermal papilla cell cul-ture and maintains mouse hair bulbs in organ culture. Arch Dermatol Res. 2009;301:357–365. doi: 10.1007/s00403-009-0929-7. [DOI] [PubMed] [Google Scholar]
- Yang CC, Cotsarelis G. Review of hair follicle dermal cells. J Der-matol Sci. 2010;57:2–11. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409–417. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun MS, Kim SE, Jeon SH, Lee JS, Choi KY. Both ERK and Wnt/β-catenin pathways are involved in Wnt3a-induced proliferation. J Cell Sci. 2005;118:313–322. doi: 10.1242/jcs.01601. [DOI] [PubMed] [Google Scholar]
- Yun TK. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16:S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–1177. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, et al. Activa-tion of β-catenin signaling programs embryonic epidermis to hair follicle fate. Development. 2008;135:2161–2172. doi: 10.1242/dev.017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yu J, Shi C, Wang Y, Yang J, Yang T. Regulatory effect of β-catenin on proliferation of hair follicle stem cells involves PI3K/Akt pathway. J Appl Biomed. 2013;11:131–141. doi: 10.2478/v10136-012-0019-6. [DOI] [Google Scholar]