Abstract
CD200-CD200R pathway regulates immune responses and has been implicated in the pathogenesis of a number of cancer types. CD200 blockade is considered a strategy for immunotherapy of CD200-positive cancers such as melanoma. Thus, it is critical to understand the potential impacts of CD200 blockade in a more human relevant tumor model. In this study, we evaluated these issues using the CD200+ Yumm1.7 mouse melanoma model. Yumm1.7 cells bear Braf/Pten mutations resembling human melanoma. We found that Yumm1.7 tumors grow significantly faster in CD200R–/– mice compared to wild type mice. Analysis of tumor immune microenvironment (TIME) revealed that tumors from CD200R–/– or anti-CD200 treated mice had downregulated immune cell contents and reduced TCR clonality compared to tumors from untreated wild type mice. T cells also showed impaired effector functions, as reflected by reduced numbers of IFN-γ+ and TNF-α+ T cells. Mechanistically, we found upregulation of the CCL8 gene in CD200R–/– tumors. In vitro co-culture experiments using Yumm1.7 tumor cells with bone marrow derived macrophages (BMDM) from WT and CD200R–/– mice confirmed upregulation of macrophage CCL8 in the absence of CD200-CD200R interaction. Finally, we found that anti-CD200 therapy failed to show efficacy either alone or in combination with checkpoint inhibitors such as anti-PD-1 or anti-CTLA4 in inhibiting Yumm1.7 tumor growth. Given that CD200R-deficiency or anti-CD200 treatment leads to reduced T cell responses in TME, using blockade of CD200 as an immunotherapy for cancers such as melanoma should be practiced with caution.
Keywords: CD200, CD200R, tumor immune microenvironment, cancer immunotherapy, tumor associated macrophage (TAM)
Introduction
Melanoma accounts for 1% of all skin cancers, but it is the deadliest of all such cancers (Siegel et al., 2018). Melanoma is sensitive to immune modulation, partially due to the presence of tumor-infiltrating lymphocytes (TIL) in its tumor microenvironment (TME), whose presence correlates with increased survival and reduced metastasis (Candido et al., 2014; Lee et al., 2016). For decades, chemotherapy was the dominant and preferred therapeutic option for melanoma patients. However, in the past decade, we have seen major advancements in treatment options. Immune inhibitors to checkpoint molecules such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) (Buchbinder and Hodi, 2016) and programmed cell death (PD-1) (Robert et al., 2019) have shown promise in treating human melanoma. However, the high rate of resistance to therapy has proven challenging. Thus, developing combination immunotherapy strategies or targeting new checkpoint molecules have gained increasing favor in overcoming resistance.
CD200 and CD200R are transmembrane glycoproteins belonging to the immunoglobulin superfamily of proteins (IgSF). CD200 contains a small 19 amino acids (AA) intracellular domain with no known signaling motif (Barclay et al., 1986). It is expressed on the surface of a wide variety of normal cells such as T and B cells, and many types of cancer cells including melanoma (Petermann et al., 2007; Liu et al., 2016; Xin et al., 2020). CD200R, its cognate ligand, is primarily expressed on myeloid cells (Wright et al., 2003). CD200R has a 67 AA cytoplasmic tail with three tyrosine residues. The 3rd tyrosine residue, located within an NPXY motif (Mihrshahi et al., 2009) inhibits phosphorylation of the ERK pathways upon ligation of CD200R to CD200 (Minas and Liversidge, 2006; Mihrshahi and Brown, 2010).
CD200R functions to control or decrease collateral damage by preventing excessive immune cell activation. Mice deficient in CD200R or CD200 are healthy, but more susceptible to induced immune pathology (Rygiel et al., 2009; Simelyte et al., 2010). The role of CD200-CD200R pathway in tumor growth, immunity and immunotherapy in mouse models is, at best, controversial. Some studies (McWhirter et al., 2006; Kretz-Rommel et al., 2007; Rygiel et al., 2012) show that CD200-CD200R interaction inhibits anti-tumor immune responses and CD200-CD200R signaling pathway plays a pro-tumor role. However, opposite results have also been reported. We previously found that B16-CD200 tumors grew significantly faster in CD200R–/– mice (Liu et al., 2016). Similarly, 4THM breast tumors were shown to exhibit accelerated growth and metastasis in CD200R–/– mice when compared to WT mice (Erin et al., 2015). Given that blockade of CD200 was recently used in a clinical trial for Chronic Lymphocytic Leukemia (CLL) and Multiple Myeloma (MM) (Mahadevan et al., 2019), and has been proposed for the treatment of patients with solid tumors such as melanoma, it is necessary to evaluate its potential impacts in more human relevant tumor models.
In this study, we evaluated the impact of blocking CD200-CD200R pathway in a murine model of melanoma (Yumm1.7) that bears Braf/Pten mutations (Meeth et al., 2016) resembling human melanoma. We found that blockade of CD200-CD200R either genetically or using a monoclonal antibody could significantly alter tumor immune microenvironment (TIME). We found that CD200R-deficiency resulted in significantly upregulated production of CCL8, which could partially explain why these tumors grow faster in the absence of CD200-CD200R interaction in TME. Given that CD200R-deficiency or anti-CD200 treatment leads to reduced T cell responses and fails to show benefits either alone or in combination with checkpoint inhibitors, blockade of CD200 should not be considered for immunotherapy of cancers such as melanoma.
Materials and Methods
Mice and Tumor Establishment
C57BL/6 mice were purchased from The Jackson laboratory, and CD200R–/– mice were originally generated via a contract with Taconic Farms and were bred into the C57BL6 background for over 12 generations (Liu et al., 2016). All mice were maintained and cared for in The Ohio State University (OSU) laboratory animal facilities which are fully accredited by OSU Institutional Animal Care and Use Committee (IACUC). To establish subcutaneous (s.c.) tumors in C57BL/6 and CD200R–/–, 5 × 105 Yumm1.7 cells in 100 μl PBS were injected into each mouse s.c. Development of tumors was monitored and tumors were measured for length (a) and width (b) every 2–3 days using a digital caliper, and tumor volumes were calculated as (a∗b2)/2.
Nanostring Pan-Cancer Immune Profiling of Tumors
Total RNA was extracted from fresh tumors (n = 3/group) using Trizol® according to the manufacturer’s instructions. Approximately 50 ng of total RNA were hybridized with the mouse PanCancer immune profiling code set containing 770 unique pairs of 35–50 base pair biotin-labeled capture probes and reporter probes with internal reference controls (NanoString nCounter® PanCancer IO360 panel, NanoString Technologies, Inc., Seattle, WA). Hybridization was performed overnight at 65°C. Unbound probes were washed away, the tripartite structure was bound to the streptavidin-coated cartridge by the biotin capture probe, aligned by an electric current, and immobilized. Degradation of fluorophore and photobleaching were prevented by adding SlowFade. Read counts from the raw data output were assessed for differential gene expression and cell type scoring after normalization using Rosalind Software.1 Briefly, Log2 counts were represented as z-scores in heat map to indicate alterations in gene expression and immune cell profile for each sample.
T Cell Receptor-Seq Analysis of Tumor Samples
Genomic DNA was prepared from tumor tissues and submitted for the immunoSEQ assay (Adaptive Biotechnologies, Seattle, WA). The somatically rearranged mouse T cell receptor (TCR) hypervariable complementarity-determining region 3 (CDR3) was amplified from genomic DNA of tumor samples using a two-step, amplification bias-controlled multiplex PCR approach (Robins et al., 2009; Carlson et al., 2013). Specifically, the first PCR consists of forward and reverse amplification primers specific for every V and J gene segment, and amplifies the CDR3 of the TCR locus. The second PCR adds a proprietary barcode sequence and Illumina adapter sequences. CDR3 libraries were sequenced on an Illumina instrument according to the manufacturer’s instructions. Raw Illumina sequence reads were demultiplexed according to Adaptive’s proprietary barcode sequences. Demultiplexed reads were then further processed to: remove adapter and primer sequences; identify and correct for technical errors introduced through PCR and sequencing; and remove primer dimer, germline and other contaminant sequences (Robins et al., 2012). The data is filtered and clustered using both the relative frequency ratio between similar clones and a modified nearest-neighbor algorithm, to merge closely related sequences. The resulting sequences were sufficient to allow annotation of the V(N)D(N)J genes constituting each unique CDR3 and the translation of the encoded CDR3 amino acid sequence. V, D and J gene definitions were based on annotation in accordance with the IMGT database.2 The set of observed TCR CDR3 sequences were normalized to correct for residual multiplex PCR amplification bias and quantified against a set of synthetic TCR CDR3 sequence analogs (Carlson et al., 2013). Data was analyzed using the immunoSEQ Analyzer toolset.
Antibodies and Flow Cytometry
Fluorescence labeled monoclonal antibodies to mouse CD45 (30-F11), CD3 (145-2c11), CD4 (GK1.5), CD8α (53-6.7), NK1.1 (Pk136), CD11b (M1/70), Gr-1 (RB6-8C5), Ly6C (HK1.4), F4/80 (745-2342), Foxp3 (Nrrf-3c), TNF-α (XT22), IFN-γ (XMG1.2), and isotype-matched control antibodies were purchased from Biolegend or BD Biosciences. Mononuclear cells from tumors were prepared as we previously described (Liu et al., 2016). For cell surface staining, cells were incubated with antibodies in 0.1 M PBS (pH7.4) supplemented with 1% FCS and 0.1% sodium azide on ice for 30 min. Cells were then washed three times and fixed in 1% paraformaldehyde followed by flow cytometry analysis. For intracellular staining of TNF-α, IFN-γ or Foxp3, cells were first stimulated with cell stimulation cocktail (Invitrogen) for 4 h in the presence of Gorgistop (BD Biosciences). The cells were first stained for the cell surface markers (CD4/8), followed by a standard intracellular cytokine staining procedure. A Celesta flow cytometer (BD) was used to detect stained cells. Data was analyzed using the flowjo software (Tree Star, Inc., OR).
Generation of Bone Marrow Derived Macrophages and Co-culture With Tumor Cells
Bone marrow (BM) cells from C57BL/6 and CD200R–/– mice were plated in 15cm culture dishes in DMEM medium containing 20% FBS and 30% culture supernatant from L929-G-CSF cells. The cells were allowed to grow in an incubator at 37°C for 3 days. On day 3, fresh culture medium was added to the plate and allowed it to grow for another 2 days. Macrophages (adherent cells) were dissociated on day 5 using 5mM EDTA and re-suspended in DMEM medium containing 10% FBS and 1% L929-G-CSF supernatant. The macrophages (1 × 106 cells/ml) were then co-cultured with Yumm1.7 tumor cells at 1:1 ratio or Yumm1.7 supernatant. Supernatant from another tumor cell line (NB9464D) was used as a negative control. 24 and 48 h later, culture supernatants were collected for ELISA. The detached cells were used for flow cytometry analysis or qPCR for chemokine gene expression analysis.
ELISA
MAX CCL8 (MCP-2) ELISA kit (Biolegend) was used to quantify CCL8 in culture supernatants from BMDM/Yumm1.7 co-cultures according to manufacturer’s instructions.
Real-Time PCR
Quantitative real-time PCR was done using previously determined conditions (33). The following primers were used for amplifying the CCL8 gene: 5′-ACGCTAGCCTCCACTCCAAA-3′ (forward) and 5′-GAGCCTTATCTGGCCCAGTC-3′ (reverse) and HPRT gene (endogenous control): 5′-AGCCTAAGATGA GCGCAAGT-3′ (forward) and 5′-TTACTACGCAGATGGCCA CA-3′ (reverse). Each sample was assayed in triplicate, and the relative gene expression was calculated by plotting the Ct (cycle number) and the average relative expression for each group was determined using the comparative method (2–ΔΔCt).
Treatment of Mice With Established Tumors
We first established Yumm1.7 tumors in male or female C57BL/6 mice by injecting 5 × 105 Yumm1.7 cells in 100 μl PBS. When tumors are palpable (typically 7 days after tumor cell injection), mice were treated with 250 μg/mouse of the following antibodies i.p. alone or in combination: αCD200 (OX-90), αPD1 (RPM1-14) or αCTLA-4 (9H10). Mice were treated every 3 days until the end of the experiments.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 9.1.1 software (GraphPad Software Inc., La Jolla, CA, United States). The differences between the treatments compared to the untreated control were analyzed by multiple t-tests without multiple comparisons correction. The nanostring data were represented as mean of log2 fold change relative to control. All other data were presented as mean ± standard error of the mean (SEM) unless otherwise indicated. The overall P-value for Kaplan-Meier analysis was calculated using the log-rank test. Analysis of differences between two normally distributed test groups was performed using an unpaired t-test assuming unequal variance and multiple t-tests. P < 0.05 was considered to be statistically significant.
Results
Yumm1.7 Tumors Grow Faster in CD200R–/– Mice
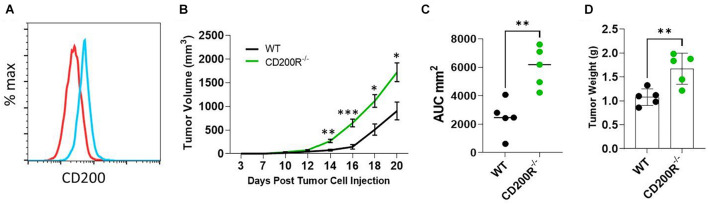
The Yumm1.7 melanoma tumor cell line was derived from a spontaneous tumor originally developed in a mouse bearing Braf/Pten mutations (Meeth et al., 2016). We found that Yumm1.7 cells constitutively expressed CD200 on its cell surface (Figure 1A). To investigate the role of CD200-CD200R signaling pathway in melanoma tumor establishment and progression, C57BL/6 and CD200R–/– C57BL/6 mice were subcutaneously inoculated with CD200+ Yumm1.7 melanoma cells. About 1 week after tumor cell injection, tumors started to show up in all mice regardless of CD200R-deficiency (Figure 1B). However, tumors grew faster in CD200R–/– mice compared to their wild type counterpart as measured by higher tumor growth curve (Figure 1B) and bigger area under the tumor volume curve (AUC) (Duan et al., 2012; Figure 1C). At the end of the experiment (day 20 after tumor cell injection), we sacrificed mice and measured tumor weight. We found that tumors from CD200R–/– mice were significantly heavier than tumors harvested from WT mice (Figure 1D). Overall, our results show that the growth of CD200+ Yumm1.7 melanoma is enhanced when CD200R signaling is absent.
FIGURE 1.
Accelerated growth of Yumm1.7 melanoma tumors in CD200R–/– mice. (A) Flow cytometry analysis of CD200 expression in Yumm1.7 cells. Red line represents isotype antibody control, blue line represents cells stained with CD200 Antibody. (B) Tumor growth kinetics. Yumm1.7 cells were injected into each mouse subcutaneously at a dose of 5 × 105/mouse. Tumor growth was monitored by measuring tumor length and width every 2 days after the appearance of tumors. Growth kinetics (changes of mean tumor volume over time) are shown. ∗∗P = 0.0012; ∗∗∗P = 0.00067; ∗P = 0.0101 on day 18; ∗P = 0.0017 on day 20. (C) Area under the curve (AUC) was calculated based on the tumor volume data shown in B (∗∗P = 0.0079). (D) Tumors isolated from wild type and CD200R–/– mice were weighted at the end of the experiment (∗∗P = 0.0069). Data shown represents three experiments with similar results. Two sided Student’s t-test was used for the statistical analysis.
Diminished Anti-tumor T Cell Responses in Yumm1.7 Tumors From CD200R–/– Mice
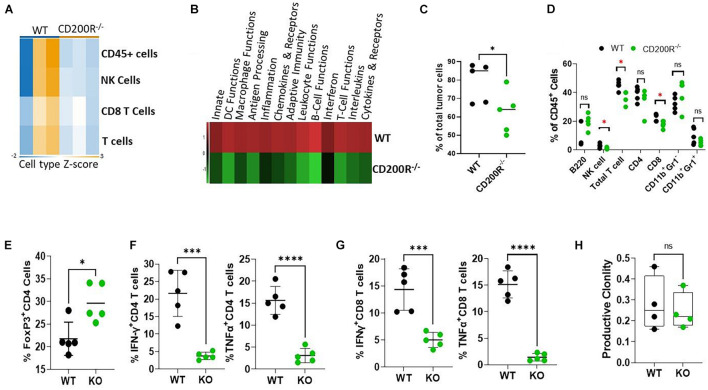
To understand what factors were driving the enhanced Yumm1.7 tumor growth in CD200R–/– mice, RNA samples from tumors were used for Nanostring analysis. We used the pre-formatted Pan-cancer 360 IO panel to characterize and measure the expression of about 750 vital genes in the TME. We first compared Immune cell type abundance within the two TME. As shown in Figure 2A, Nanostring analysis revealed decreased immune cell presence in the CD200R–/– TME. In 3 out of 3 CD200R–/– tumor samples, the abundance scores of immune cells were low, while 2 out of 3 tumor samples from WT mice exhibited higher enrichment of immune cells, including total CD45+ leukocytes, total T cells and CD8+ T cells. Strikingly, pathway analysis revealed that majorities of immune pathways including adaptive immunity/T cell functions were downregulated (Figure 2B). To validate the immune cell type abundance data from Nanostring, we analyzed immune cell subsets using flow cytometry. As seen in Figure 2C, flow cytometry confirmed significant decreases of CD45+ cells in tumors from CD200R–/– mice. Additionally, we observed decreased percentages of NK cells, T cells (CD3+) and CD8+ T cells among total leukocytes (CD45+) in tumors from CD200R–/– mice, while percentages of B cells (B220+) and CD4+ T cells among total leukocytes were not significantly different between tumors from CD200R–/– and WT mice. Similarly, we did not detect differences in CD11b+Gr1– tumor associated macrophage (TAM) population and CD11b+Gr1+ myeloid derived suppressor (MDSC) cells (Figure 2D). CD4+FoxP3+ regulatory T cells (Tregs) are significant players in regulating T cell responses in TME. We therefore quantified Tregs and found that Tregs were significantly increased in the CD200R–/– tumors (Figure 2E). To determine whether the functions of immune cells such as T cells were impacted by the lack of the CD200-CD200R interaction, we quantified IFN-γ and TNF-α levels in CD4+ and CD8+ tumor infiltrating lymphocytes (TIL). We found that both IFN-γ and TNF-α production were significantly suppressed in CD4+ (Figure 2F) and CD8+ T cells (Figure 2G) within the CD200R–/– tumors.
FIGURE 2.
Diminished anti-tumor T cell responses in Yumm1.7 tumors from CD200R–/– mice. (A,B) Nanostring analysis of tumors (n = 3/group) from WT and CD200R–/– mice was performed using total tumor RNA and the Nanostring Pan cancer IO 360 panel, and results were analyzed using Rosalind software. Heat maps showing cell type abundance (A) in tumors and directed global significance scores (B) of altered pathways were generated based on Nanostring data. (C–G) Flow cytometry analyses of subtypes of tumor infiltrating leukocytes and effector functions of T cells. Percent of positive cells from each group was calculated relative to total tumor cells (C, ∗P = 0.046) or total CD45+ cells (D, ∗P < 0.05). Intracellular staining was performed to quantify Tregs (E, ∗P = 0.013), IFN-γ (∗∗∗P = 0.0003) and TNF-α (****P < 0.0001) producing CD4+ (F) or IFN-γ (∗∗∗P = 0.0009) and TNF-α (****P < 0.0001) producing CD8+ (G) T cells. (H) TCR-seq was performed on genomic DNA samples (n = 4/group) from tumors grown in WT and CD200R–/– mice. Results were analyzed using ImmunoSEQ software. Depicted is productive clonality (higher values in this graph represent more clonal T cell response). Ns: not significant. Two sided Student’s t-test was used for all the statistical analyses.
To assess the impact of CD200-CD200R signaling on the diversity of the immune repertoire of tumor-infiltrating T cells, we prepared genomic DNA from tumors (n = 4/group) and performed TCR-seq analysis (Adaptive Technology, Seattle, Washington). We found that in the absence of CD200R signaling, clonality of tumor infiltrating T cells were similar (Figure 2H), suggesting that lack of CD200R signaling did not significantly affect dominant T cell populations in TME.
Deficiency of CD200R Signaling Alters Tumor Microenvironment and Upregulates CCL8
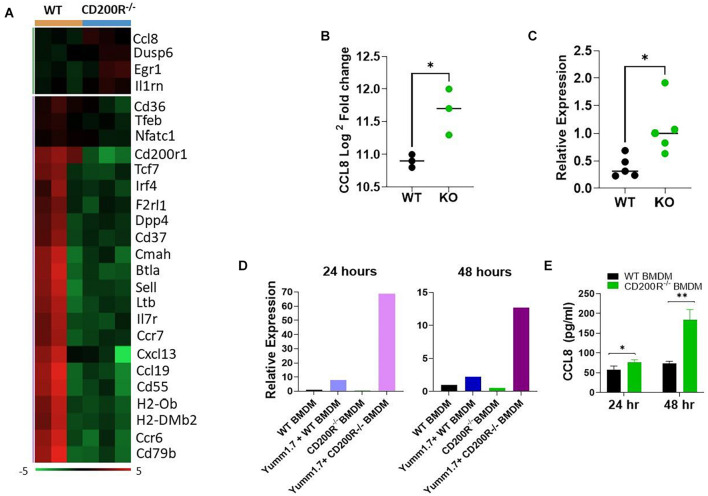
Differential gene expression analysis of the data generated from the Nanaostring assay revealed significant alterations in TME (Figure 3A). Among the 26 differentially expressed genes, 22 were genes downregulated in the CD200R–/– TME. Consistent with the downregulated immune pathways indicated in Figure 2A, the downregulated genes included genes important for T cell trafficking and effector functions. Notably, several chemokine and chemokine receptors (CCR6, CCR7, CCL19, CXCL13) were also downregulated. On the other end of the spectrum, there were 4 upregulated genes in TME in the absence of CD200R. Notable among them was CCL8, a chemokine ligand for murine CCR8 (Figure 3B). Our qPCR analysis verified its upregulation in tumors from CD200R-dificient mice (Figure 3C).
FIGURE 3.
Deficiency of CD200R signaling alters TME and upregulates CCL8. (A) Heat map of all differentially expressed genes based on the Nanostring analysis. (B) CCL8 gene expression was significantly elevated in CD200R–/– vs. wild type tumors based on the Nanostring analysis (∗P = 0.022 by two-sided Student’s t-test). (C) qPCR was used to quantify CCL8 gene expression in tumors (n = 5/group) from wild type vs. CD200R–/– mice. ∗P = 0.042 by two-sided STUDENT’s t-test. (D,E) Bone marrow derive macrophages (BMDM) was generated and co-cultured with YUMM1.7 tumor cells. qPCR was used to quantify CCL8 gene (D) expression in macrophages, and ELISA was used to quantify CCL8 protein (E) production in culture supernatants. CCL8 gene expression or protein production in BMDM alone or YUMM1.7 tumor cell alone were low or undetectable. ∗P = 0.05; ∗∗P = 0.0019 by two-sided Student’s t-test.
To determine if tumor CD200 interacts with CD200R on macrophages to regulate CCL8 production, we generated bone marrow derived macrophages (BMDM) from CD200R–/– and WT mice, co-cultured them with Yumm1.7 tumor cells and measured CCL8 gene expression and protein secretion. As shown in Figure 3D, we found that CCL8 gene expression was significantly higher in CD200R–/– macrophages after a 24 and 48 h co-culture with Yumm1.7 cells. At the protein level, most significant up-regulation of CCL8 was detected 48 h after co-culture (Figure 3E). Overall, these results indicate that tumor CD200 interacts with CD200R and limits the secretion of CCL8.
CD200 Blockade Using a Monoclonal Antibody Alters Tumor Immune Microenvironment Without Inhibiting Tumor Growth
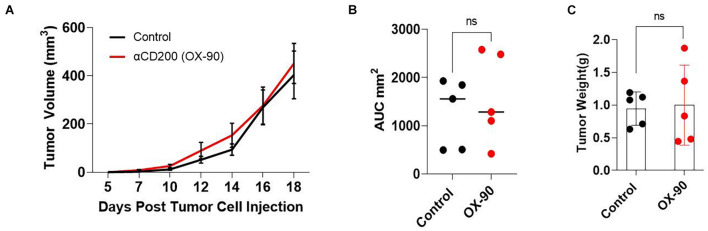
It has been reported (Kretz-Rommel et al., 2007; Choueiry et al., 2020) that CD200 blockade may improve the efficacy of cancer immunotherapy. To determine if CD200 blockade could inhibit the growth of CD200+ melanoma, we tested a monoclonal antibody (OX-90) in the Yumm1.7 tumor model. OX-90 has been shown to block CD200-CD200R interaction (Snelgrove et al., 2008; Choueiry et al., 2020). We inoculated C57BL/6 mice with 0.5 × 106 Yumm1.7 melanoma cells subcutaneously. Once the tumors were established, the mice were injected with the OX-90 antibody (250 μg/mouse) i.p. every 3 day until the end of the experiment. As shown in Figure 4, treatment of mice with OX-90 failed to affect Yumm1.7 tumor growth, as measured by tumor growth kinetics (Figure 4A), mean area under curve (Figure 4B) and tumor weight at the end of the experiment (Figure 4C).
FIGURE 4.
CD200 blockade using a monoclonal antibody failed to inhibit tumor growth. (A) Tumor growth kinetics. Yumm1.7 cells were injected into each mouse s.c. at a dose of 5 × 105/mouse. Mice were treated with anti-CD200 antibody (OX-90) at a dose of 250 μg/mouse or vehicle i.p. every 3 days when tumors were palpable. Tumor growth was monitored by measuring tumor length and width every 2 days. Growth kinetics (changes of tumor volume over time) of individual tumors are shown. (B) Area under the curve (AUC) was calculated based on the tumor volume data shown in (A). (C) Tumors isolated from OX-90 and vehicle treated mice were weighted at the end of the experiment. Data shown represents three experiments with similar results. Ns: not significant by two sided Student’s t-test.
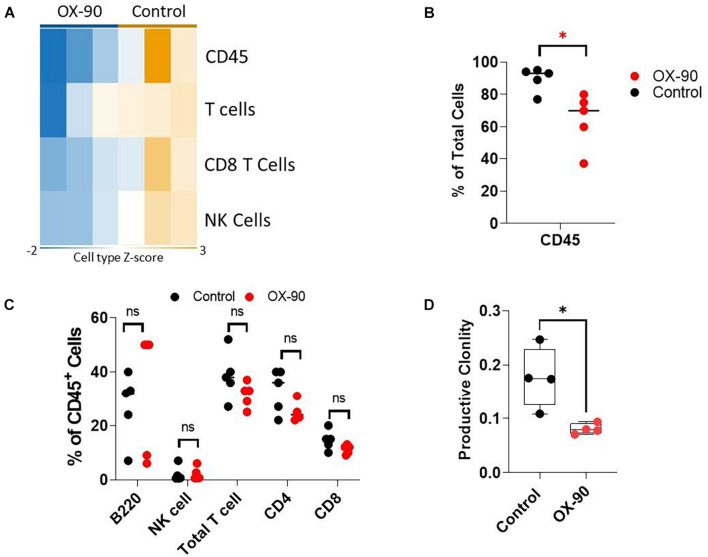
To understand if OX-90 treatment alters TIME, we analyzed the immune cell abundance using Nanostring assay. The OX-90 treated group followed a pattern similar to that seen in the CD200R–/– mice. Specifically, we found reduction of total CD45+ cells, total T cells, CD8+ T cells and NK cells in tumors from OX-90 treated mice (Figure 5A). Consistent with this observation, our flow cytometry analysis verified that total CD45+ and T cells were reduced (Figure 5B), while proportions of other lymphocyte subsets were not significantly altered by OX-90 treatment (Figure 5C). Strikingly, ImmunoSEQ analysis indicated that OX-90 treatment decreased the clonality of tumor infiltrating T cells (Figure 5D), suggesting that OX-90 treatment decreases the quality of T cell responses in TME.
FIGURE 5.
CD200 blockade alters tumor immune microenvironment. (A) Nanostring analysis of tumors (n = 3/group) from OX-90 or vehicle treated mice was performed using total tumor RNA and the Nanostring Pan cancer IO 360 panel, and results were analyzed using Rosalind software. Heat map showing cell type abundance in tumors are presented. (B,C) Flow cytometry analyses of subtypes of tumor infiltrating leukocytes. Percent of positive cells from each group was calculated relative to total tumor cells (B) or total CD45+ cells (C). ∗P = 0.016 by two-sided Student’s t-test. ns: not significant. (D) Productive clonality of tumor infiltrating T cells. TCR-seq was performed on genomic DNA samples (n = 4/group) from tumors grown in OX-90 or vehicle treated mice. Results were analyzed using ImmunoSEQ software. ∗P = 0.0154 by two-sided Student’s t-test.
Anti-CD200 Therapy Failed to Show Synergy With Checkpoint Inhibitors
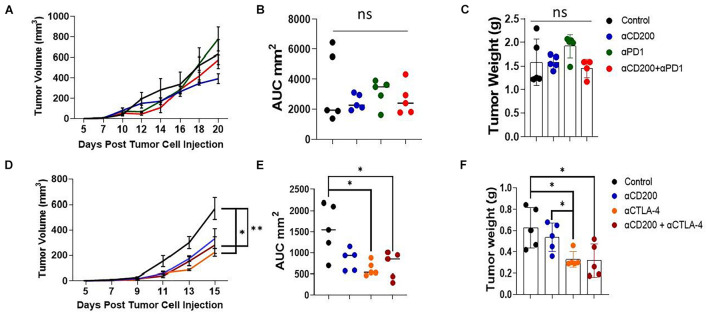
Since anti-CD200 monotherapy failed to show efficacy in inhibiting Yumm1.7 tumor growth, we tested if anti-CD200 in combination with other checkpoint inhibitors will result in tumor growth inhibition. We first tested if anti-CD200 treatment had synergy with anti-PD-1 therapy. As shown in the upper panel of Figure 6, neither anti-CD200 or anti-PD-1 monotherapy, nor their combination showed any efficacy, as measured by tumor growth kinetics (Figure 6A), AUC (Figure 6B) or tumor weight at the end of the experiment (Figure 6C). Since we observed increased Tregs in TME of CD200R-deficient tumors (Figure 2D), we also examined if anti-CD200 could inhibit tumor growth in combination with anti-CTLA4 antibody 9H10. 9H10 has been shown to deplete Tregs and inhibits tumor growth (Simpson et al., 2013). We found that anti-CTLA4 antibody alone significantly reduced tumor growth as measured by tumor growth kinetics (Figure 6D), AUC (Figure 6E) or tumor weight at the end of the experiment (Figure 6F). Although OX-90 + αCTLA4 also showed significant efficacy, this combination did not prove to be more effective than αCTLA4 monotherapy. Thus, anti-CD200 therapy failed to show synergy with checkpoint inhibitors.
FIGURE 6.
Anti-CD200 therapy failed to show synergy with checkpoint inhibitors. Yumm1.7 cells were injected into each mouse s.c. at a dose of 5 × 105/mouse. When tumors were palpable, mice (n = 5/group) were treated with 250 μg/mouse of anti-CD200 (OX-90), anti-PD-1 (RPM1-14) or αCTLA-4 (9H10) or vehicle i.p. every 3 days. Tumor growth was monitored by measuring tumor length and width every 2 days. (A–C) Anti-CD200 therapy failed to show synergy with anti-PD-1 therapy. Growth kinetics (A), AUC (B) and tumor weight (C) are shown. Ns: not significant by one way ANOVA. (D–F) Anti-CD200 therapy failed to show synergy with anti-CTLA4 therapy. Growth kinetics (D, ∗P = 0.02; ∗∗P = 0.005), AUC (E, ∗P = 0.011, control vs. aCTLA4; ∗P = 0.026, control vs. aCD200/CTLA4) and tumor weight (F, ∗P = 0.0115, control vs. aCTLA4; ∗P = 0.023, control vs. aCD200/CTLA4; ∗P = 0.015, aCD200 vs. aCTLA4) are shown. Data shown represents two to three experiments with similar results. One way ANOVA was used for the comparison.
Discussion
In this study, we used the human-relevant, CD200 expressing Yumm1.7 tumor model to evaluate the role of CD200-CD200R pathway in melanoma tumor growth, immunity and immunotherapy. We found that in CD200R–/– mice, Yumm1.7 tumors grew significantly faster than in wild type mice. Consistent with this observation, nanostring analysis revealed that tumors from CD200R–/– mice had downregulated immune cell contents. Flow cytometry analysis further suggested that Yumm1.7 tumors from CD200R–/– mice had reduced infiltration of CD8+ T cells but increased Tregs. T cells also showed impaired effector functions, as reflected by reduced numbers of IFN-γ+ and TNF-α+ T cells. These observations are consistent with our previous observations in B16 melanoma model where CD200 was artificially expressed in B16.F10 cells (Liu et al., 2016). Similarly, 4THM breast tumors were shown to exhibit accelerated growth and metastasis in CD200R–/– mice compared to WT mice (Erin et al., 2015). On the opposite end of the spectrum, it was observed that CD200–/– mice had reduced carcinogen-induced tumor development (Rygiel et al., 2012). In CD200R-deficient mice, decreased growth and metastasis of CD200-positive EMT6 tumors were observed (Podnos et al., 2012). We recently provided a model (Liu et al., 2020) to explain the differential tumor growth in CD200/CD200R-deficient mice among different tumor models. Expansion of M2 macrophages and MDSCs will enhance tumor-associated inflammation/angiogenesis, activating tumor invasion/metastasis, and regulating tumor-specific T cell responses, therefore enhancing tumor growth. In contrast, expansion of M1 macrophages will lead to tumor growth inhibition due to direct anti-tumor effects imposed by M1 macrophages and induction of tumor-specific T cell responses. In this work, we found that M2 associated genes such as Il1rn (Sun et al., 2015) are significantly upregulated in TME of CD200R–/– mice, suggesting more M2 conversion in the absence of CD200R signaling.
CD200-CD200R interaction may potentially affect production of inflammatory mediators by tumor-associated macrophages. In this work, we observed upregulation of CCL8 gene in CD200R–/– tumors. In vitro co-culture experiments using Yumm1.7 tumor cells with BMDM from WT and CD200R–/– mice confirmed upregulation of CCL8 in macrophages in the absence of CD200-CD200R interaction (Figure 3). CCL8 also known as MCP-2, was originally described as an antimicrobial protein involved in the inflammatory process (Asano et al., 2015). CCL8 has since been known to contribute to immune response by attracting monocytes, T lymphocytes, natural killer cells (NK), basophils, mast cells, and eosinophils (Farmaki et al., 2017). In tumor models, tumor CCL8 production has been shown to promote a pro-metastatic environment of cervical cancer (Chen et al., 2019). In TME, TAMs are major sources of CCL8 that support tumor cell survival (Cassetta et al., 2019) and increase invasion and stem-like characteristics of GBM cells (Zhang et al., 2020). CCL8 promotes the migration and invasion of esophageal squamous cell carcinoma as well (Zhou et al., 2018). High levels of CCL8 has been indicated in recruiting Tregs to the TME through CCR5 (Halvorsen et al., 2016). CCL8 is also involved in promoting metastasis in mice inoculated with human melanoma cell lines (Barbai et al., 2015). Several studies have implicated elevated CCL8 in breast cancer progression, metastasis and relapse free survival (RFS) (Farmaki et al., 2016; Farmaki et al., 2020; Tang et al., 2020). In light of these studies, we assume that upregulation of CCL8 in the absence of CD200R signaling plays a role in promoting tumor growth, which can partially explain the accelerated Yumm1.7 tumor growth observed in CD200R–/– mice.
Mahadevan et al. (2019) published the results of their phase I clinical trial where they administered a novel recombinant humanized monoclonal antibody called Samalizumab that blocks CD200 in chronic lymphocytic leukemia (CLL) and multiple myeloma (MM) patients. In their study, Samalizumab was associated with reduced tumor burden in most advanced CLL patients and suggested further development of Samalizumab as an immune checkpoint inhibitor. However, Samalizumab did not prove effective in MM patients. In this work, we investigated whether CD200 blockade could be used as a potential therapy in melanoma. We found that anti-CD200 monotherapy failed to show efficacy in inhibiting Yumm1.7 tumor growth. Strikingly, we found that tumors from anti-CD200-treated mice also had downregulated abundance of immune cell contents and TCR clonality. Since activated immune cells upregulate CD200 expression, and in human trials anti-CD200 has been shown to deplete activated CD4 T cells (Mahadevan et al., 2019), we examined whether CD200 blockade resulted in depletion of CD200 expressing CD4 T cells. However, we did not observe any depletion of B cells or T cells (Figure 5C). Thus, our data suggests that anti-CD200 therapy alters tumor microenvironment through similar mechanisms observed in CD200R–/– mice. Previous studies have suggested (Coles et al., 2015) that CD200 and PD-L1 should be targeted in combination to improve immunotherapy outcomes. However, in this work, we found that anti-CD200 Ab failed to show synergy with anti-PD-1 or anti-CTLA4 therapy. Thus, CD200 blockade failed to show efficacy in inhibiting Yumm1.7 tumor growth either alone or in combination with checkpoint inhibitors.
Taken together, we have found that blockade of CD200-CD200R either genetically or using a monoclonal antibody could significantly alter TIME. We found that CD200R-deficiency resulted in significantly upregulated production of CCL8, which could partially explain why tumor grows faster in the absence of CD200-CD200R interaction in TME. Given that CD200R-deficiency or anti-CD200 treatment leads to reduced T cell responses and fails to show benefits either alone or in combination with checkpoint inhibitors, blockade of CD200 should not be considered for immunotherapy of cancers such as melanoma.
Data Availability Statement
The data has been deposited and can be accessed by using either the URL https://clients.adaptivebiotech.com/pub/talebian-2021-fcdb or 10.21417/FT2021FCDB.
Ethics Statement
All mice works involved in this study were approved by Institutional Animal Care and Use Committee (IACUC) of the Ohio State University.
Author Contributions
FT performed most of the experiments. JY helped and performed some animal work and flow cytometry analysis. KL and J-QL helped with mouse work and reagents. WC helped with experimental design and provided resources for the experiments. X-FB generated funding support, designed experiments, analyzed data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
Funding
This work was supported by a T32 grant (T32CA090223) and an R01 grant (R012CA211014) from the National Institutes of Health.
References
- Asano K., Takahashi N., Ushiki M., Monya M., Aihara F., Kuboki E., et al. (2015). Intestinal CD169(+) macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat. Commun. 6:7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbai T., Fejõs Z., Puskas L. G., Tímár J., Rásó E. (2015). The importance of microenvironment: the role of CCL8 in metastasis formation of melanoma. Oncotarget 6 29111–29128. 10.18632/oncotarget.5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay A. N., Clark M. J., McCaughan G. W. (1986). Neuronal/lymphoid membrane glycoprotein MRC OX-2 is a member of the immunoglobulin superfamily with a light-chain-like structure. Biochem. Soc. Symp. 51 149–157. [PubMed] [Google Scholar]
- Buchbinder E. I., Hodi F. S. (2016). Melanoma in 2015: immune-checkpoint blockade - durable cancer control. Nat. Rev. Clin. Oncol. 13 77–78. 10.1038/nrclinonc.2015.237 [DOI] [PubMed] [Google Scholar]
- Candido S., Rapisarda V., Marconi A., Malaponte G., Bevelacqua V., Gangemi P., et al. (2014). Analysis of the B-RafV600E mutation in cutaneous melanoma patients with occupational sun exposure. Oncol. Rep. 31 1079–1082. 10.3892/or.2014.2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C. S., Emerson R. O., Sherwood A. M., Desmarais C., Chung M. W., Parsons J. M., et al. (2013). Using synthetic templates to design an unbiased multiplex PCR assay. Nat. Commun. 4:2680. [DOI] [PubMed] [Google Scholar]
- Cassetta L., Fragkogianni S., Sims A. H., Swierczak A., Forrester L. M., Zhang H., et al. (2019). Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell 35 588–602.e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. J., Deng Y. R., Wang Z. C., Wei W. F., Zhou C. F., Zhang Y. M., et al. (2019). Hypoxia-induced ZEB1 promotes cervical cancer progression via CCL8-dependent tumour-associated macrophage recruitment. Cell Death Dis. 10:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiry F., Torok M., Shakya R., Agrawal K., Deems A., Benner B., et al. (2020). CD200 promotes immunosuppression in the pancreatic tumor microenvironment. J. Immunother. Cancer 8:e000189. 10.1136/jitc-2019-000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles S. J., Gilmour M. N., Reid R., Knapper S., Burnett A. K., Man S., et al. (2015). The immunosuppressive ligands PD-L1 and CD200 are linked in AML T-cell immunosuppression: identification of a new immunotherapeutic synapse. Leukemia 29 1952–1954. 10.1038/leu.2015.62 [DOI] [PubMed] [Google Scholar]
- Duan F., Simeone S., Wu R., Grady J., Mandoiu I., Srivastava P. K. (2012). Area under the curve as a tool to measure kinetics of tumor growth in experimental animals. J. Immunol. Methods 382 224–228. 10.1016/j.jim.2012.06.005 [DOI] [PubMed] [Google Scholar]
- Erin N., Podnos A., Tanriover G., Duymus O., Cote E., Khatri I., et al. (2015). Bidirectional effect of CD200 on breast cancer development and metastasis, with ultimate outcome determined by tumor aggressiveness and a cancer-induced inflammatory response. Oncogene 34 3860–3870. 10.1038/onc.2014.317 [DOI] [PubMed] [Google Scholar]
- Farmaki E., Chatzistamou I., Kaza V., Kiaris H. (2016). A CCL8 gradient drives breast cancer cell dissemination. Oncogene 35 6309–6318. 10.1038/onc.2016.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmaki E., Kaza V., Chatzistamou I., Kiaris H. (2020). CCL8 promotes postpartum breast cancer by recruiting M2 macrophages. iScience 23:101217. 10.1016/j.isci.2020.101217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmaki E., Kaza V., Papavassiliou A. G., Chatzistamou I., Kiaris H. (2017). Induction of the MCP chemokine cluster cascade in the periphery by cancer cell-derived Ccl3. Cancer Lett. 389 49–58. 10.1016/j.canlet.2016.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen E. C., Hamilton M. J., Young A., Wadsworth B. J., LePard N. E., Lee H. N., et al. (2016). Maraviroc decreases CCL8-mediated migration of CCR5(+) regulatory T cells and reduces metastatic tumor growth in the lungs. Oncoimmunology 5:e1150398. 10.1080/2162402x.2016.1150398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz-Rommel A., Qin F., Dakappagari N., Ravey E. P., McWhirter J., Oltean D., et al. (2007). CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J. Immunol. 178 5595–5605. 10.4049/jimmunol.178.9.5595 [DOI] [PubMed] [Google Scholar]
- Lee N., Zakka L. R., Mihm M. C., Schatton T. (2016). Tumour-infiltrating lymphocytes in melanoma prognosis and cancer immunotherapy. Pathology 48 177–187. 10.1016/j.pathol.2015.12.006 [DOI] [PubMed] [Google Scholar]
- Liu J. Q., Hu A., Zhu J., Yu J., Talebian F., Bai X. F. (2020). CD200-CD200R pathway in the regulation of tumor immune microenvironment and immunotherapy. Adv. Exp. Med. Biol. 1223 155–165. 10.1007/978-3-030-35582-1_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Q., Talebian F., Wu L., Liu Z., Li M. S., Wu L., et al. (2016). A critical role for CD200R signaling in limiting the growth and metastasis of CD200+ melanoma. J. Immunol. 197 1489–1497. 10.4049/jimmunol.1600052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan D., Lanasa M. C., Farber C., Pandey M., Whelden M., Faas S. J., et al. (2019). Phase I study of samalizumab in chronic lymphocytic leukemia and multiple myeloma: blockade of the immune checkpoint CD200. J. Immunother. Cancer 7:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter J. R., Kretz-Rommel A., Saven A., Maruyama T., Potter K. N., Mockridge C. I., et al. (2006). Antibodies selected from combinatorial libraries block a tumor antigen that plays a key role in immunomodulation. Proc. Natl. Acad. Sci. U.S.A. 103 1041–1046. 10.1073/pnas.0510081103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeth K., Wang J. X., Micevic G., Damsky W., Bosenberg M. W. (2016). The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res. 29 590–597. 10.1111/pcmr.12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihrshahi R., Barclay A. N., Brown M. H. (2009). Essential roles for Dok2 and RasGAP in CD200 receptor-mediated regulation of human myeloid cells. J. Immunol. 183 4879–4886. 10.4049/jimmunol.0901531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihrshahi R., Brown M. H. (2010). Downstream of tyrosine kinase 1 and 2 play opposing roles in CD200 receptor signaling. J. Immunol. 185 7216–7222. 10.4049/jimmunol.1002858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minas K., Liversidge J. (2006). Is the CD200/CD200 receptor interaction more than just a myeloid cell inhibitory signal? Crit. Rev. Immunol. 26 213–230. 10.1615/critrevimmunol.v26.i3.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann K. B., Rozenberg G. I., Zedek D., Groben P., McKinnon K., Buehler C., et al. (2007). CD200 is induced by ERK and is a potential therapeutic target in melanoma. J. Clin. Invest. 117 3922–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podnos A., Clark D. A., Erin N., Yu K., Gorczynski R. M. (2012). Further evidence for a role of tumor CD200 expression in breast cancer metastasis: decreased metastasis in CD200R1KO mice or using CD200-silenced EMT6. Breast Cancer Res. Treat 136 117–127. 10.1007/s10549-012-2258-3 [DOI] [PubMed] [Google Scholar]
- Robert C., Ribas A., Schachter J., Arance A., Grob J. J., Mortier L., et al. (2019). Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 20 1239–1251. 10.1016/s1470-2045(19)30388-2 [DOI] [PubMed] [Google Scholar]
- Robins H., Desmarais C., Matthis J., Livingston R., Andriesen J., Reijonen H., et al. (2012). Ultra-sensitive detection of rare T cell clones. J. Immunol. Methods 375 14–19. 10.1016/j.jim.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins H. S., Campregher P. V., Srivastava S. K., Wacher A., Turtle C. J., Kahsai O., et al. (2009). Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood 114 4099–4107. 10.1182/blood-2009-04-217604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygiel T. P., Karnam G., Goverse G., van der Marel A. P., Greuter M. J., van Schaarenburg R. A., et al. (2012). CD200-CD200R signaling suppresses anti-tumor responses independently of CD200 expression on the tumor. Oncogene 31 2979–2988. 10.1038/onc.2011.477 [DOI] [PubMed] [Google Scholar]
- Rygiel T. P., Rijkers E. S., de Ruiter T., Stolte E. H., van der Valk M., Rimmelzwaan G. F., et al. (2009). Lack of CD200 enhances pathological T cell responses during influenza infection. J. Immunol. 183 1990–1996. 10.4049/jimmunol.0900252 [DOI] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2018). Cancer statistics, 2018. CA Cancer J. Clin. 68 7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- Simelyte E., Alzabin S., Boudakov I., Williams R. (2010). CD200R1 regulates the severity of arthritis but has minimal impact on the adaptive immune response. Clin. Exp. Immunol. 162 163–168. 10.1111/j.1365-2249.2010.04227.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson T. R., Li F., Montalvo-Ortiz W., Sepulveda M. A., Bergerhoff K., Arce F., et al. (2013). Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 210 1695–1710. 10.1084/jem.20130579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelgrove R. J., Goulding J., Didierlaurent A. M., Lyonga D., Vekaria S., Edwards L., et al. (2008). A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat. Immunol. 9 1074–1083. 10.1038/ni.1637 [DOI] [PubMed] [Google Scholar]
- Sun L., Zhou H., Zhu Z., Yan Q., Wang L., Liang Q., et al. (2015). Ex vivo and in vitro effect of serum amyloid a in the induction of macrophage M2 markers and efferocytosis of apoptotic neutrophils. J. Immunol. 194 4891–4900. 10.4049/jimmunol.1402164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Yang Q., Cui Q., Zhang D., Kong D., Liao X., et al. (2020). Weighted gene correlation network analysis identifies RSAD2, HERC5, and CCL8 as prognostic candidates for breast cancer. J. Cell Physiol. 235 394–407. 10.1002/jcp.28980 [DOI] [PubMed] [Google Scholar]
- Wright G. J., Cherwinski H., Foster-Cuevas M., Brooke G., Puklavec M. J., Bigler M., et al. (2003). Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol. 171 3034–3046. 10.4049/jimmunol.171.6.3034 [DOI] [PubMed] [Google Scholar]
- Xin C., Zhu J., Gu S., Yin M., Ma J., Pan C., et al. (2020). CD200 is overexpressed in neuroblastoma and regulates tumor immune microenvironment. Cancer Immunol. Immunother. 69 2333–2343. 10.1007/s00262-020-02589-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Chen L., Dang W. Q., Cao M. F., Xiao J. F., Lv S. Q., et al. (2020). CCL8 secreted by tumor-associated macrophages promotes invasion and stemness of glioblastoma cells via ERK1/2 signaling. Lab. Invest. 100 619–629. 10.1038/s41374-019-0345-3 [DOI] [PubMed] [Google Scholar]
- Zhou J., Zheng S., Liu T., Liu Q., Chen Y., Tan D., et al. (2018). MCP2 activates NF-κB signaling pathway promoting the migration and invasion of ESCC cells. Cell Biol. Int. 42 365–372. 10.1002/cbin.10909 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data has been deposited and can be accessed by using either the URL https://clients.adaptivebiotech.com/pub/talebian-2021-fcdb or 10.21417/FT2021FCDB.