Graphical abstract
Abbreviations: CAT, Catalase; CD, Crohn’s disease; DAD, Diode array detection; DAI, Disease Activity Index; DSS, Dextran sodium sulfate; ELISA, Enzyme-linked immunosorbent assay; ESI, Electrospray ionization; FID, Flame ionization detector; FRAP, Ferric reducing antioxidant power; GC, Gas chromatograph; GPx, glutathione peroxidase; GR, Glutathione reductase; GSH, Glutathione; HT, Hawthorn tea; IBD, Inflammatory bowel disease; IL-1β, Interleukin-1beta; MDA, Malondialdehyde; MPO, Myeloperoxidase; MS, Mass spectrometry; ORAC, Oxygen-radical absorbing capacity; SCFA, Short-chain fatty acid; SOD, Superoxide dismutase; TFC, Total flavonoids content; TNBS, 2,4,6-trinitrobenzene sulfonic acid; TNF-α, Tumor necrosis factor-alpha; TPC, Total polyphenols content; TPOC, Total proanthocyanidin oligomers content; UC, Ulcerative colitis; UHPLC, Ultra-high-performance liquid chromatography
Keywords: Crataegus oxyacantha, Polyphenol, Vitexin-2-O-rhamnoside, Inflammatory bowel diseases, Colon
Highlights
-
•
A tea from the leaves and flowers of hawthorn is rich in flavonoids, especially vitexin-2-O-rhamnoside.
-
•
Mesalamine and hawthorn tea have positive healing effects in rats with colitis.
-
•
Hawthorn tea reduces the length and area of the brownish necrotic lesions.
-
•
Hawthorn tea diminishes the levels of the inflammatory markers MPO and IL-1β.
-
•
Hawthorn tea regulates the activity of the oxidative stress enzymes CAT and GR.
Abstract
The purpose of the study was to determine the effects of a tea from the leaves and flowers of Crataegus oxyacantha in rats with colitis. Colitis was induced by administration of 2,4,6-trinitrobenzene sulfonic acid. Hawthorn tea (HT) (100 mg/kg) was given via gavage for 21 days and the mesalamine drug (100 mg/kg) was administrated during the period of disease onset. HT was rich in total phenolic compounds (16.5%), flavonoids (1.8%), and proanthocyanidins (1.5%); vitexin-2-O-rhamnoside was the main compound detected. Mesalamine and the HT diminished the length of the lesions formed in the colon, in addition to reducing the levels of myeloperoxidase and interleukin-1β. Mesalamine was able to significantly reverse the body weight loss, while HT improved the activity of glutathione reductase and catalase. Histological scoring was not changed by the interventions, but it was highly correlated with the necrotic area. HT given at 100 mg/kg can be effective against colitis.
1. Introduction
In the last 20 years, there has been increasing in vitro and in vivo evidence of the beneficial health effects of functional foods, teas, and extracts from plants. Such alternative or complementary medicine has been suggested as a new tool against non-communicable diseases, which eventually may impact in the future for changes in prevention guidance and the treatment of debilitating chronic illness, such as cardiovascular diseases, inflammatory bowel diseases (IBDs), and cancer (Campos, 2019). However, more studies are needed in order to understand and deepen the effective dosages and mechanisms behind the biological action of natural bioactive compounds. Notably, the research on the utilization of the residues of plants, usually commercially discarded, such as stems, leaves, and flowers, is probably one of the strands that should be more investigated within the field of medicinal plants and herbs, given their great chemical and nutritional composition (Simpson et al., 2019).
Europe has a wide range of native plants spread through its territory, and one of the most common ones is hawthorn (Crataegus spp.), a tree belonging to the Rosaceae family (Chang et al., 2002). Hawthorn is commonly used as a hedging species in England and Denmark (Billing et al., 1974, Weber, 2010), and as a herbal remedy throughout the continent (Dahmer & Scott, 2010). Especially, the hawthorn extracts or teas from the leaves and flowers of Crataegus oxyacantha and Crataegus monogyna have been officially approved in Germany to treat patients with mild to moderate chronic congestive heart failure (Nathan, 1999) due to its high content in flavonoids and proanthocyanidins (Ngoc et al., 2019). It is known that flavonoids and proanthocyanidins can 1. decrease the inflammatory processes, 2. act by increasing the activity of enzymes that control the oxidation of the cell membrane (Panche et al., 2016, Rauf et al., 2019), and 3. possibly modulate the microbiome environment by being fermented by the microbiota (Cardona et al., 2013). Despite that, hawthorn has been mostly associated with beneficial effects in cardiovascular complications and hypertension (Dalli et al., 2011, Walker et al., 2006), while research on their effects in intestinal health and against gut diseases, like IBDs, for example, are lacking.
IBDs, mainly ulcerative colitis (UC) and Crohn’s disease (CD), largely affect the gastrointestinal tract, producing a high-grade immune-associated inflammatory and oxidative process (Ungaro et al., 2017). Such diseases possess a high prevalence in Europe and are increasing at a high incidence rate since the ’90s in newly industrialized countries, such as Brazil and Taiwan (Ng et al., 2017). Treatment options for IBDs are commonly not well accepted or tolerable for a great part of patients (Horne et al., 2009, Lasa et al., 2020, Pieper et al., 2009), suggesting the need for studies capable of proving new effective options. From our knowledge, four studies to date have attested the positive effects of hawthorn in IBDs; however, these investigations used the fruits, not the leaves or flowers, and distinct hawthorn species (Fujisawa et al., 2005, Guo et al., 2021, Liu et al., 2020, Malekinejad et al., 2013). Fujisawa et al. (2005) reported that the freeze-dried aqueous extract from the fruit of Crataegi fructus given in the drinking water prevented the inflammatory outcome and high mortality of chemically-induced models of colitis (Fujisawa et al., 2005). Liu et al. (2020) described that the freeze-dried hydroalcoholic extract from the fruit of Crataegus pinnatifida, rich in flavonoids, inhibited the secretion of proinflammatory cytokines and alleviated the increase of paracellular permeability in Caco-2 cells, therefore, indicating its protective effects against epithelial barrier dysfunction (Liu et al., 2020). Such initial studies indicate the potential of the hawthorn plant against IBDs.
No publication to date investigated the preventive effects of Crataegus oxyacantha or the tea from its flowering tops (leaves and flower buds) in broader aspects of IBDs, such as inflammation, oxidative stress, and microbiome health. Therefore, in order to understand if the effects of the residual parts of this plant are extended further than its most-known cardioprotective role, this study aimed to investigate the preventive implications of the administration of a polyphenol-rich dry tea from the Crataegus oxyacantha flowering tops in a model of IBD-like colitis induced in rats by 2,4,6-trinitrobenzene sulfonic acid (TNBS).
2. Material and methods
2.1. Chemicals
Aluminum chloride hexahydrate, n-butanol, Folin-Ciocalteu reagent, gallic acid (GA), quercetin (Q), and cyanidin chloride (CY) were purchased from Merck (Saint-Quentin Fallavier, France). Crataegus spp. extract standard (R1) was acquired from HWI Group (Rülzheim, Germany) and standardized at 29 mg of vitexin 2-O-rhamnoside per g of extract. Procyanidin A2, B2 and C1, epicatechin, cinnamtannin A2, and isoquercetin were purchased from Phytolab (Vestenbergsgreuth, Germany). 2,2′-Azobis(2-amidinopropane) dihydrochloride was purchased from Cayman Chemical (Ann Arbor, MI, USA), fluorescein was purchased from Synth (Diadema, SP, Brazil) and metaphosphoric acid was purchased from Vetec Quimica Fina Ltda. (Rio de Janeiro, RJ, Brazil). Protein Assay Dye Reagent Concentrate was acquired from Bio-Rad Laboratories (Hercules, CA, USA). Thiobarbituric acid was obtained from Merck KGaA (Darmstadt, Germany). Enzyme-linked immunosorbent assay (ELISA) kits were purchased from Peprotech (Rocky Hill, NJ, United States). 1,1,3,3-tetraethoxypropane, 2,4,6-Tripyridyl-S-triazine, 5,5′-dithiobis-(2-nitrobenzoic acid), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), bovine serum albumin, catalase (CAT), glutathione (GSH), myeloperoxidase (MPO), nitro blue tetrazolium chloride, oxidized glutathione, reduced nicotinamide-adenine dinucleotide phosphate, standards of short-chain fatty acids (SCFAs) (2-ethylbutyric acid, acetic acid, butyric acid, and propionic acid), sodium dodecyl sulfate and TNBS were all obtained from Sigma Aldrich (St. Louis, MO, USA).
2.2. Hawthorn tea production and chemical characterization
Dry hawthorn flowering tops (Crataegus oxyacantha) (leaves and flower buds) were purchased from France Herboristerie (Lot number 55849, Noidans-Lès-Vesoul, France). The plants were previously grounded using an Ika grinder (Ika-Werke GmbH, Model MF10 basic, Staufen, Germany) and a “1 mm” grid. The material size distribution was determined by dry laser granulometry.
The tea was produced according to Ngoc et al. (Ngoc et al., 2019). A sample of 50 g was infused in 1 L of boiling water using a ‘French press’ Bodum® under 500 rpm magnetic stirring. After 30 min, the herbal tea solution was filtered first with the Bodum® cover to remove the largest particles, then with a Whatman filter paper placed in a Büchner funnel using a vacuum pump to remove any residue of the solid plant. Finally, the herbal tea solution was concentrated using a rotary evaporator and later freeze-dried (Cryotec Model CRIOS-80, Saint-Gély-du-Fesc, France) to obtain the dry hawthorn tea (HT). The same experiment was repeated three times to get a total of 30 g. Lyophilized tea was stored at 4 °C (Ngoc et al., 2019).
Total polyphenols (TPC) (mg of gallic acid equivalent/g dry weight), total flavonoids (TFC) (mg of quercetin equivalent/g dry weight), and total proanthocyanidin oligomers (TPOC) (mg of cyanidin equivalent/g dry weight) contents were determined according to the Folin-Ciocalteu method (Singleton & Rossi, 1965), the aluminum chloride method (Lamaison & Carnat, 1990) and the HCl/n-butanol assay (Porter et al., 1985), respectively. Analyses were performed in triplicate.
For the quantification and identification of specific phenolic compounds, an ultra-high-performance liquid chromatography associated with diode array detection (UHPLC-DAD) and coupled with electrospray ionization and mass spectrometry (UHPLC-ESI-MS), respectively, were utilized. First, 20 mg of the dry HT was dissolved in 1 mL of water and vortexed for 2 min. The resulting solution was diluted five times with water and vortexed for another 2 min prior to the analysis.
The UHPLC-DAD system consisted of a Thermo Scientific™ Dionex™ UltiMate™ 3000 BioRS equipped with a WPS-3000TBRS autosampler and a TCC-3000RS column compartment set at 35 °C (Thermofisher Scientific, Waltham MA, USA). The system was operated using Chromeleon 7 software. A Luna® Omega polar C18 column (1.6 μm, 100 × 2.1 mm) combined with a security guard ultra-cartridge was used (Phenomenex Inc., Torrance CA, USA). A binary solvent system was utilized, consisting of water/formic acid (1%, v/v) as solvent A and acetonitrile/formic acid (1%, v/v) as solvent B. The gradient program started with 5% B, then B was increased to 100% in 30 min with a convex increase (curve 5 in Chromeleon 7). The flow rate of the mobile phase was 0.4 mL/min, and the injection volume was 4 μL. The peaks were monitored at 273 nm. The UV–Vis spectra of the different compounds were recorded between 200 and 550 nm using the diode array detector.
The UHPLC-ESI-MS analysis was performed using a Synapt G2-S (Waters Corp., Milford MA, USA) equipped with an ESI. The column, injection volume, flow rate, and gradient program were the same as for UHPLC-DAD. A positive mode was used. The capillary voltage was set to 3 kV, the cone voltage was set to 30 V, and the extract or voltage was set to 3 V. The source temperature was 100 °C, and the desolvation temperature was 450 °C. MS spectra were obtained by scanning ions between 100 and 1500 m/z. The system was operated using MassLynx 4.1 software.
The quantification of vitexin-2-O-rhamnoside, a common and abundant flavonoid found in hawthorn species (Alirezalu et al., 2018, Kumar et al., 2012), was determined by external calibration, using a commercially available standard. All other components identified in the HT were expressed as peak area ratio from the chromatogram, since the standards were not available.
2.3. Animal experimentation
2.3.1. Ethics, diet, and conditions
The study is in accordance with the ARRIVE guidelines and followed the guide for the care and use of laboratory animals of the National Institutes of Health (NIH Publications No. 8023, revised 1978), and the Brazilian National Council for Animal Experiments Control - CONCEA. The Ethics Committee on the Use of Animals of the University of Campinas approved the experimental protocol (number 5042–1/2018, UNICAMP).
Male Wistar rats (n = 29) were obtained from the Multidisciplinary Center for Biological Research on Laboratory Animal Science at UNICAMP and allocated in an experimentation room with the temperature at controlled levels (20–22 °C). Rats were submitted to a standard daily 12 h:12 h light–dark cycle. During the acclimatization and experimental periods, rats received a commercial pelleted diet (Nuvilab CR-1, Nuvital) and water ad libitum. Animals entered the experimentation room at four weeks of age and were submitted to the procedures starting at seven weeks of age.
2.3.2. Intervention, colitis induction, and clinical evaluation
A total of 29 Wistar rats were divided in five experimental groups: control-C (n = 5), control colitis-CC (n = 6), hawthorn tea-H (n = 5), hawthorn tea colitis-HC (n = 6) and mesalamine colitis-MC (n = 7). Groups with colitis had more animals since death may happen in the TNBS model (Antoniou et al., 2016). Dry HT was diluted in distilled water and given preventively for 14 days at a dosage of 100 mg/kg of body weight via gavage. The dosage chosen was based on the majority of studies found in the literature (Elango et al., 2009, Elango and Devaraj, 2010, Hatipoʇlu et al., 2015). The intervention with HT (groups H and HC) continued until the end of the experiment, including the period after colitis induction (when applicable). Other groups (C, CC, MC) received distilled water via gavage instead.
Acute colitis was induced on the 14th day using TNBS (10 mg) dissolved in 250 μL of 50% ethanol (v/v) (da Silva-Maia et al., 2019) and administrated via rectal in rats previously sedated intraperitoneally with ketamine (75 mg/kg of body weight) and xylazine (10 mg/kg of body weight). Groups without colitis induction (C, H) received saline solution (0.9%) instead of TNBS.
Mesalamine, a drug commonly used to treat IBDs (Lacucci et al., 2010), was diluted in distilled water and given via gavage at a dosage of 100 mg/kg/day (da Silva-Maia et al., 2019). Mesalamine was administrated for seven days, from the day of the induction until the end of the experiment.
The dietary intake (g) and body weight (g) were measured during the whole experiment. Additionally, for the period of colitis, the Disease Activity Index (DAI) was assessed (days 14, 15, 17, 19, and 20), according to described by Gommeaux et al. (2007). The DAI evaluates the body weight loss, anal bleeding, and stool consistency (Gommeaux et al., 2007). Photographs of the rat’s stool and anus were taken to analyze anal bleeding and stool consistency. A blinded researcher to animals' identification and with experience in IBDs, did the evaluations. A photographic representation of the scores of bleeding and stool consistency from this study was created similarly to Nascimento, Lima et al. (2020) to showcase the differences between the score’s degrees. A Nikon® SLR D3100 digital camera with 14.2-megapixel resolution and an 18–55 mm lens was utilized.
2.3.3. Euthanasia and tissue measurements
Euthanasia was performed on day 21 by exsanguination through cardiac puncture. Intraperitoneal administration of ketamine (300 mg/kg of body weight) and xylazine (30 mg/kg of body weight) were used to anesthetize the animals.
Colon was dissected, cleaned with saline solution, weighted (g), and measured with a ruler (cm). The colon weight/length (g/cm) ratio was analyzed. Brownish necrotic lesions are common features of TNBS-induced colitis (De Almeida et al., 2015, Paiotti et al., 2013); therefore, their width (cm), length (cm), and estimated area (width × length, cm2) were measured for this study. Additionally, the relation between the larger necrotic lesion length (cm) and the colon length (cm) was evaluated as an indicator of the colon proportion (%) affected by the disease. A small portion of the distal colon was separated for histology. The remaining, composed of proximal, middle, and distal colon, and rectum, were mixed and preserved at −80 °C for further analyses (inflammation and oxidative stress).
Blood was centrifuged, and the serum was collected and stored at −80 °C for posterior antioxidant analyses. Feces from the cecum and colon were collected and kept at −80 °C for the analysis of the concentration of SCFAs. The liver, spleen, and kidney were weighted (g). The carcasses of the animals and unused tissues or organs were discarded as biological materials.
2.4. Histopathology
A sample of the distal colon was disposed in a small piece of paper and preserved in formaldehyde 4% (v/v) until histological processing. Briefly, samples were dehydrated with increasing ethanol concentrations, included in paraffin blocks, and subjected to sectioning (4 μm) follow by insertion in slides. Slides were standardly stained with hematoxylin and eosin and analyzed by a specialist, blind to the identification of the groups, according to a score modified from Dieleman et al. (1998) (Table 1). Photos were taken at 10x/0.25 magnification, 6.31x/0.25 AxioCam ICc5 (Zeiss, Germany).
Table 1.
Histological grading of colitis.
| Score | Leukocyte infiltration | Inflammation extent | Crypt damage |
|---|---|---|---|
| 0 | None | None | None |
| 1 | Discrete | Mucosa | 1/3 damaged (basal) |
| 2 | Moderate | Mucosa and submucosa | 2/3 damaged (basal) |
| 3 | Severe | Transmural | Only the surface of the epithelium is intact |
| 4 | – | – | Loss of crypt and epithelium |
| Area involved in percentage (score): 1–25 (1), 26–50 (2), 51–75 (3), 76–100 (4) | |||
For each category of the score (leukocyte infiltration, inflammation extent, and crypt damage), points were multiplied by a factor of involvement of the colonic tissue. The sum of the three categories adds up to the total score of each section (Dieleman et al., 1998).
2.5. Antioxidant capacity of the serum
The total antioxidant capacity of the serum was evaluated by two methods, the ferric-reducing antioxidant power (FRAP) and the oxygen-radical absorbing capacity (ORAC). Trolox was utilized as a standard for both analyses. Before the tests, samples were submitted to treatment with metaphosphoric acid in order to precipitate proteins and extract the antioxidant substances (Leite et al., 2011).
For the FRAP analysis, samples were added with a solution composed of 2,4,6-tripyridyl-S-triazine, ferric chloride, and acetate buffer at proportions of 1:1:10, respectively. Samples were included in a microplate and incubated for 30 min at 37 °C. The absorbance was read at 595 nm using an Epoch™ spectrophotometer (BioTek Instruments Inc., Winooski, VT, USA), which was utilized for all the applicable analyses. The results were expressed in µmol of Trolox equivalent/mL of serum (Benzie & Strain, 1996).
For the ORAC analysis, in a microplate, samples were added with fluorescein and incubated for 10 min at 37 °C. After that, 2,2′-azobis(2-amidinopropane) dihydrochloride was added, and the fluorescence was read for 80 min with filters set at 520 nm for emission and 485 nm for excitation. The area under the curve was calculated, and the results were expressed in µmol of Trolox equivalent/mL of serum (Ou et al., 2013).
2.6. Inflammation and oxidative stress analyses
Colonic samples were homogenized in a potassium phosphate buffer (75 mM, pH 7.4) and centrifuged at 10,000 rpm for 30 min. The supernatant was collected and subjected to protein quantification (mg of bovine serum albumin/mL) by the Bradford assay (Bradford, 1976). Supernatants were utilized for the determination of MPO and cytokines concentration, and the oxidative stress analyses.
The concentration of MPO (U/g protein), tumor necrosis factor-alpha (TNF-α), and interleukin-1beta (IL-1β) (ng/g of protein or mL of serum) were determined as indicators of inflammation. MPO was determined by mixing the diluted homogenates (1:4) or a standard with a cocktail of O-dianisidine dihydrochloride and hydrogen peroxide. MPO was utilized as the standard. Absorbance was read every min for 10 min at 460 nm, and the area under the curve was calculated (Winterbourn et al., 1975). The concentrations of the proinflammatory cytokines TNF-α and IL-1β in colon homogenates and serum were determined by ELISA, following the protocols of commercial kits.
The activity or concentration of CAT (U/g of protein), glutathione reductase (GR) (consumed NADPH/min/g of protein), GSH (nmol of GSH/mg of protein), malondialdehyde (MDA) (nmol of MDA/g of protein), and superoxide dismutase (SOD) (SOD/g of protein) were determined as indicators more closely related to oxidative stress.
The CAT assay was realized by mixing first the diluted homogenates (1:2) with hydrogen peroxide and incubating for two min at 37 °C. After that, ammonium metavanadate was included, and the absorbance was read after 10 min at 452 nm. CAT was utilized as a standard. CAT activity was determined by a logarithmic formula, adapted from a recent methodological approach (Hadwan & Ali, 2018).
For the GR analysis, homogenates (diluted at 1:1 or not diluted, depending on the sample) were added with a cocktail containing reduced nicotinamide-adenine dinucleotide phosphate, oxidized glutathione, and ethylenediaminetetraacetic acid. The reading was realized at 340 nm per min for 10 min and the area under the curve was calculated (Carlberg & Mannervik, 1985).
GSH was determined by first mixing a tris reagent plus ethylenediaminetetraacetic acid buffer with diluted homogenates (1:4) or a standard and read at 421 nm. This step was followed by the inclusion of 5,5′-dithiobis-(2-nitrobenzoic acid) and a 15 min incubation period. The second reading was also done at 421 nm. GSH was utilized as the standard. The calculation was realized by subtracting the first reading data from the second one, followed by linear regression (Yoshikawa et al., 1993).
The concentration of MDA was determined by the thiobarbituric acid reactive substances (TBARS) method. Briefly, samples or a standard were mixed with sodium dodecyl sulfate and a solution containing thiobarbituric acid, sodium hydroxide, and acetic acid. This mixture was left for 60 min in a boiling water bath and then cooled for 10 min. Samples were centrifuged at 10,000 rpm for 10 min at low temperature (4 °C). The absorbance of the supernatant was read at 532 nm. A standard curve was made using 1,1,3,3-tetraethoxypropane, and the results were expressed as nmol MDA/g protein (Ohkawa et al., 1979).
SOD was quantified by mixing the diluted homogenates (1:4) with a cocktail composed of hypoxanthine, xanthine oxidase, and nitro blue tetrazolium chloride. Absorbance was read every min for 10 min at 560 nm, and the area under the curve was calculated (Winterbourn et al., 1975).
2.7. Short-chain fatty acids concentration
Feces sample preparation followed the protocol described by Zhao et al. (2006), with modifications. Approximately 300 mg of stool was diluted in distilled water (1:6) and mixed with HCl for adjustment in pH 2. Afterward, the samples were centrifuged at 3000 rpm for 60 min. The supernatant was collected and mixed with the internal standard 2-ethyl-butyric acid. Once prepared, the samples were injected into a gas chromatograph coupled to a flame ionization detector. The samples were injected with an auto-injector brand Shimadzu, model Ai20, in a GC-FID Shimadzu, model GC-2010 plus, equipped with a capillary column of fused silica Nukol (30 m × 0.25 mm, inside diameter × 0.25 μm). The chromatographic conditions were as follow: injector at 200 °C operating in split mode 1:5 for 1.0 min; helium carrier gas at 1 mL/min; oven temperature ramp starting at 100 °C, with an increase of 8 °C/min to 190 °C, remaining at this temperature for 3.25 min; and detector at 200 °C. The co-injection of authentic standards identified the analytes. All chromatographic analyzes were performed in triplicate (Zhao et al., 2006).
2.8. Statistical analysis and Pearson’s correlation
Results are presented as mean ± standard deviation (SD) for data from the chemical characterization and as mean ± standard error of the mean (SEM) for data from the biological analyses.
For data from the biological analysis, first, outliers were discarded after realizing the Grubbs test (5%). Then, One-way ANOVA followed by Tukey (parametric data) was utilized when making comparisons among all experimental groups. Two-way ANOVA followed by Tukey (parametric data) was utilized for analyses over time (body weight, DAI). Data between groups were considered statistically different when p < 0.05.
Data were submitted to Pearson’s correlation and included in a correlation matrix. The correlations were only considered parameters previously attested to be significantly altered by the induction of colitis or the intervention with the HT (p < 0.05). Groups without colitis induction (C, H) were not included, as they could bias the results. Pearson’s r placed between 0.70 and 1 (positive) or −0.70 and −1 (negative) were classified as highly correlated (Hinkle et al., 2003). A heatmap was created to represent the correlation matrix.
Analyses were performed using GraphPad Prism.
3. Results and discussion
3.1. Tea characterization and dosage of compounds administrated to rats
The extraction yield was about 20% in mass, meaning that 200 mg of HT was obtained from 1 g of the dry ground plant. The contents of TPC, TFC and TPOC obtained from HT were 165.55 ± 2.20 mg of gallic acid equivalent/g of dry tea, 18.8 ± 0.70 mg of quercetin equivalent/g of dry tea, and 15.35 ± 0.60 mg of cyanidin equivalent/g of dry tea, respectively. The levels found from the three analyses are in accordance with recent studies using similar plant species and portions (Issaadi et al., 2020, Ngoc et al., 2019). By utilizing only water for the extraction of the ground plant, the results of TPC (33.11 mg/g of dry plant) shown in the present study are comparable or even higher than less green extracts utilized by the literature (Edwards et al., 2012). For example, in a study by Alirezalu et al. (2018), the TPC of different Crataegus species (flowers and leaves), extracted with methanol/water (80%, v/v), ranged between 20 and 50 mg/g. Also, in a study by Keser et al. (2014), the polyphenol content of the aqueous extract of Crataegus monogyna leaves and flowers was found to be higher than the extract with 100% ethanol, while also possessing a high antioxidant activity (Keser et al., 2014). Such findings suggest that the use of water for the extraction of hawthorn can be equally important to other solvents, as far as the dry plant is ground (<1 mm).
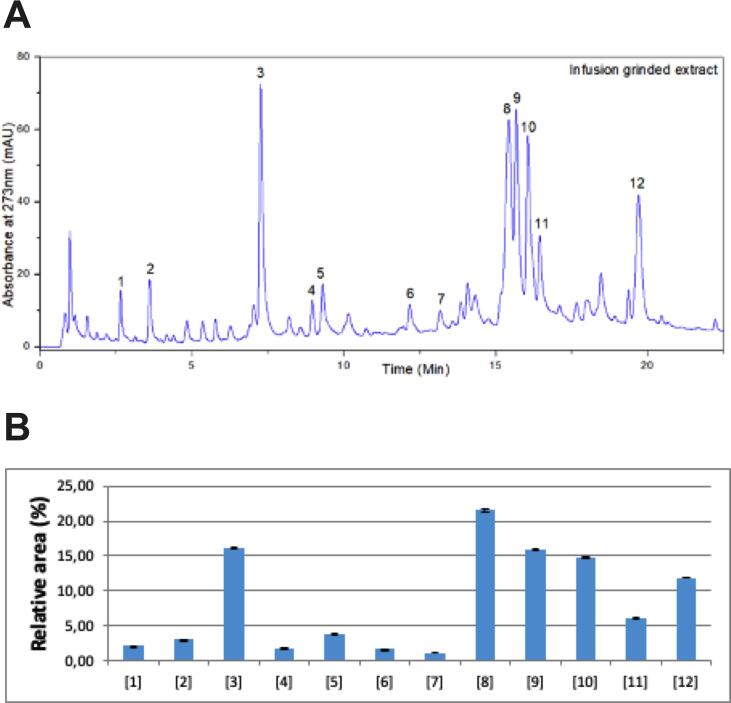
The main compounds found in the HT were flavonoids, like chlorogenic acid, vitexin 2-O-rhamnoside, pinnatifinoside A, hyperoside, and apigenin-C-hexoside, as detected by the UHPLC retention time (Table 2) and peak (Fig. 1A) profiles. Especially, vitexin 2-O-rhamnoside presented the highest relative peak area (Fig. 1B), as later quantified. The content of this flavonoid by UHPLC was determined to be 3.53 ± 0.036 mg/g of dry plant or 17.66 ± 0.18 mg/g of dry HT. Vitexin 2-O-rhamnoside is the main flavonoid found in hawthorn species, with content reaching up to 6.6 mg/g of dry plant (Martino et al., 2008) or 26 mg/g of the tea (i.e., 5.2 mg/g of dry tea) (Ngoc et al., 2019). Studies have shown the capacity of vitexin 2-O-rhamnoside against induced-oxidative stress damage (Wei et al., 2014) and endothelial injury (Zhu et al., 2006), making it also a probable candidate for inflammatory intestinal conditions. However, such flavonoid has poor oral bioavailability in rats (<5%) (Gao et al., 2016), which could indicate its biotransformation by the microbiota in similarity to other prebiotic-like flavonoids (e.g., anthocyanins) (Kawabata et al., 2019), but studies are needed to investigate such assumption.
Table 2.
Peak identification of the main compounds detected by UHPLC-ESI-MS.
| Peak | Retention time (min) | λmax (nm) | [M+H]+ | Other ions in the spectrum | Identified compound |
|---|---|---|---|---|---|
| 1 | 2.71 | 204, 218, 260 | 288 | Cyanidin | |
| 2 | 3.73 | 218, 236, 324 | 355 | 377, 711 | 5-O-Caffeoylquinic acid |
| 3 | 7.49 | 219, 238, 325 | 355 | 377, 711 | Chlorogenic acid (3-O-caffeoyquinic acid) |
| 4 | 9.1 | 227, 280 | 579 | 427, 289 | Procyanidin B2 |
| 5 | 9.45 | 224, 279 | 291 | 147, 139, 123 | Epicatechin |
| 6 | 12.24 | 280 | 867 | 579 | Procyanidin C1 |
| 7 | 13.37 | 219, 280 | 1155 | 287, 413, 575 | Cinnamtannin A2 |
| 8 | 15.42 | 216, 269, 338 | 579 | 433, 313 | Vitexin 2-O-rhamnoside |
| 9 | 15.68 | 206, 262, 348 | 415 | 397, 367, 283 | Pinnatifinoside A |
| 10 | 16.13 | 220, 256, 353 | 465 | 303 | Hyperoside |
| 11 | 16.52 | 202, 257, 353 | 303 | 621 | Isoquercetin |
| 12 | 19.85 | 268, 337 | 433 | 621 | Apigenin-C-hexoside |
λmax is the local maximum absorbance in the UV spectrum. [M + H]+ provided the m/z value of the precursor ion. Other ions provided the m/z value from fragments detected in the mass spectrometry.
Fig. 1.
UHPLC profile of the main identified polyphenols in the hawthorn tea Peak or relative area (%) identification: 1: Cyanidin, 2: 5-O-caffeoylquinic acid, 3: chlorogenic acid, 4: procyanidin B2, 5: epicatechin, 6: procyanidin C1, 7: cinnamtannin A2, 8: vitexin-2-O-rhamnoside, 9: pinnatifinose A, 10: hyperoside, 11: isoquercetin, 12: apigenin C-hexoside. The analysis was performed in triplicate. A. Peak profile of compounds from hawthorn tea by UV monitoring at 273 nm. B. Relative peak area (%) of compounds peaks from hawthorn tea. The relative area was calculated by dividing the peak area of each component by the sum of the peak area of the 12 identified components.
Considering the results from the chemical characterization of the hawthorn plant, rats received 16.5 mg of total phenolic compounds/kg of body weight and 1.76 mg of vitexin 2-O-rhamnoside/kg of body weight daily during 14 days for prevention and the following 7 days while with colitis.
3.2. Macroscopic and microscopic parameters
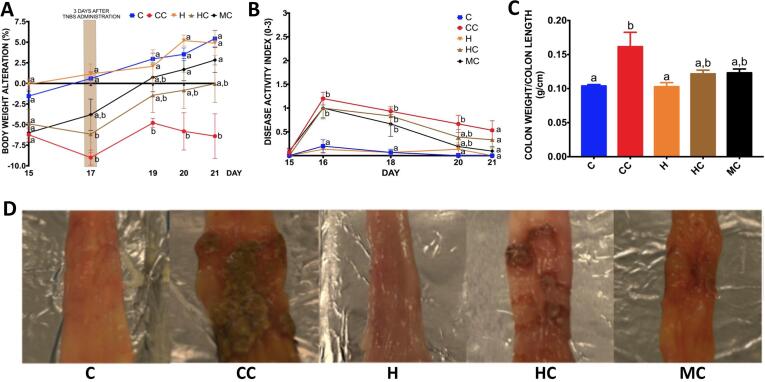
Colitis was successfully induced in rats, as seen by an increased body weight loss (Fig. 2A) and high DAI after one day from the colitis induction (p < 0.01) (Fig. 2B). In general, animals with colitis presented softening of the stools and slight bleeding, as shown by the photographic representation of their respective scores in Fig. 3, Fig. 4, respectively. As expected, the treatment with 100 mg/kg of mesalamine recovered the animals’ body weight at the end of the experiment (p < 0.001) compared to the CC group. Mesalamine is commonly utilized for IBDs, as it can block cyclooxygenases, interleukins, and TNF-α, therefore controlling the inflammatory process and contributing to preventing common intestinal symptoms and body weight loss (Lacucci et al., 2010). HT could not significantly reverse the body weight loss (p = 0.0560) in rats with colitis (group HC), but animals tended to return to their initial body weight before colitis induction (Fig. 2A).
Fig. 2.
Body weight, colon weight/length ratio, and appearance of necrotic lesions C: control, CC: control colitis, H: hawthorn, HC: hawthorn colitis, MC: mesalamine colitis. Results are presented as mean ± SEM. One-way or Two-way ANOVA followed by Tukey. Different letters indicate statistical significance (p < 0,05). A. Body weight alteration (%) after colitis induction (days 15–21). The calculation was determined in relation to day 14. B. Colon weight/colon length (g/cm) ratio as an inflammatory index. C. Photographic representation of the necrotic lesions on the colonic mucosa of rats with colitis.
Fig. 3.
Photographic representation of the score of stool consistency A. Score 0 - Normal stools. B. Score 1 – Soft pellets not adhering to the anus. C. Score 2 – Very soft pellets adhering to the anus. D. Score 3 – Liquid stool; wet anus. Score based on Gommeaux et al. (2007) and photographic scheme based on Nascimento, Lima et al. (2020).
Fig. 4.
Photographic representation of the score of bleeding A. Score 0 – No bleeding. B. Score 1 – Small spots of blood in stool; dry anal region. C. Score 2 – Large spots of blood in stool; blood appears through the anal orifice. D. Score 3 – Deep red stool; blood spreads largely around the anus. Score based on Gommeaux et al. (2007) and photographic scheme based on Nascimento, Lima et al. (2020). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Only the rats in the CC group presented a high colon weight/length ratio (p < 0.05) compared to the C group (Fig. 2C), potentially indicating the anti-inflammatory effects of the interventions with mesalamine and HT. The occurrence of necrotic lesions was evident in all rats that received TNBS (Fig. 2D). Both mesalamine and HT significantly decreased the length (p < 0.001) and the area (p < 0.01) of the most extensive brownish necrotic lesion, as well as the proportion of the colon affected by TNBS (p < 0,05) compared to the CC group. No differences were found between the MC and HC groups regarding such parameters (Table 3).
Table 3.
Macroscopic and microscopic parameters.
| Largest necrotic lesion | |||||
|---|---|---|---|---|---|
| Parameter | C | CC | H | HC | MC |
| Width (cm) | 0a | 2.04 ± 0.31b | 0a | 1.05 ± 0.28b | 1.13b ± 0.27b |
| Length (cm) | 0a | 2.38 ± 0.17b | 0a | 0.96 ± 0.22c | 0.92 ± 0.27c |
| Area (width × length, cm2) | 0a | 5.02 ± 1.13b | 0a | 1.31 ± 0.53a | 0.72 ± 0.27a |
| Lesion/colon length (%) | 0a | 11.51 ± 1.40b | 0a | 4.67 ± 1.20a | 4.83 ± 1.75a |
| Histology* | |||||
| Leukocyte infiltration | 0a | 8.20 ± 1.80b | 0a | 7.33 ± 0.98b | 4.85 ± 0.73b |
| Inflammation extent | 0a | 7.40 ± 1.69b | 0a | 7.00 ± 1.12b | 5.14 ± 0.59b |
| Crypt damage | 0a | 8.00 ± 2.21b | 0a | 6.00 ± 2.11b | 4.74 ± 0.60b |
| TOTAL (sum) | 0a | 23.60 ± 5.41b | 0a | 16.40 ± 1.03b** | 14.71 ± 1.79b |
C: control, CC: control colitis, H: hawthorn, HC: hawthorn colitis, MC: mesalamine colitis. Results are presented as mean ± SEM. One-way ANOVA followed by Tukey. Different letters indicate statistical significance (p < 0.05). *Score calculation: each parameter (crypt damage, inflammation extent, and leukocyte infiltration) was multiplied by the factor of involvement of the mucosa, and the total is the sum of the three results (Dieleman et al., 1998). **The sum of this group was not 20.33 ± 4.21, as the sum of the three parameters suggests (sum of the scores of leukocyte infiltration, inflammation extent, and crypt damage); this happened due to an outlier only found in the statistical analysis of the TOTAL.
The anti-necrotic potential of extracts from hawthorn is still poorly documented (Fujisawa et al., 2005, Zapatero, 1999), but evidence indicates that flavonoids and phenolic acids (e.g., chlorogenic acid), which were identified in the HT of our study (Fig. 1, Table 2), possess such properties. For example, chlorogenic acid, a polyphenol found in coffee beans, apple, and hawthorn species (Alirezalu et al., 2018), and the vitexin-rich plant Tragopogon graminifolius, are effective in reducing the typical macroscopic and necrotic lesions of TNBS-induced colitis (Farzaei et al., 2015, Zatorski et al., 2015, Zhou et al., 2016). Although necrosis is not a typical documented finding of patients with IBDs, it can work as a parameter to measure the possible anti-inflammatory and pro-homeostatic effects of natural products.
Although alterations in the liver, spleen, and kidney are described in few studies that use the model of TNBS-induced colitis (Jang et al., 2018, Patel and Trivedi, 2017, Zhi et al., 2017), in the present study, no differences were found between the experimental groups regarding the macroscopic aspect and weight of such organs (results not shown).
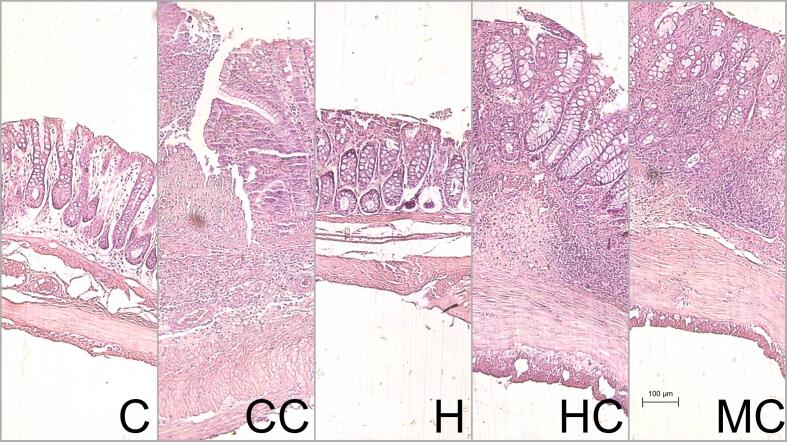
As far as the histological analysis, TNBS promoted extensive and severe ulceration in the epithelium, with loss of crypts, and a high inflammatory income in the mucosa and submucosa (Fig. 5). Results from the histological grading significantly shown this for all colitis groups (p < 0.05). Mesalamine (p = 0.103) and HT (p = 0.318) did not statistically decrease the histological score (Table 3), which could probably be related to two possibilities: 1. as expected for the TNBS model (Antoniou et al., 2016), the microscopic severity was extremely high, especially in the area selected for histology (distal colon), making it difficult for a clear observation of the benefits of the treatments; 2. the HT only had a mild preventive effect that could not be translated in the microscopic analysis. The next step would be to perform a study with other colitis model to understand if HT could exert preventive effects in the histology of the damaged intestinal mucosa. Cazarin et al. (2014), for example, by using the Passiflora edulis peel (rich in polyphenols and dietary fibers) could not find statistical significance in the microscopic score of rats with TNBS-induced colitis, but when they utilized the dextran sodium sulfate (DSS) model, a significant reduction in the histological damage in the colon was observed (Cinthia Baú Betim Cazarin et al., 2016). The DSS model results in less severe inflammation and more superficial ulcers in rats when compared to the TNBS acute model (Catana et al., 2018), which typically triggers extensive transmural inflammation (Antoniou et al., 2016). According to a recent review (Nascimento, Machado, et al., 2020), the DSS model best mimics the inflammation of UC, while the TNBS model, the inflammation of CD. In studies with colitis using products from hawthorn berries, the histological damage is improved, however, the models associated with the results are acetic acid and DSS, and not TNBS (Fujisawa et al., 2005, Guo et al., 2021, Malekinejad et al., 2013).
Fig. 5.
Photographic representation of the distal colon histology Hematoxylin and eosin stain. Settings: 10x/0.25 magnification, 6.31x/0.25 AxioCam ICc5 (Zeiss, Germany). Scale: 100 μm. C: control, CC: control colitis, H: hawthorn, HC: hawthorn colitis, MC: mesalamine colitis.
3.3. Serum antioxidant capacity
No statistical differences were found between all groups regarding the results from the FRAP and ORAC methods, meaning that the TNBS model did not worse the systemic antioxidant capacity; neither did the intervention with HT increased it (Table 4). In a study by Luana H. Maurer et al. (2020), similarly, ORAC and FRAP data were not changed by the TNBS-colitis model or by the intervention with a rich source of polyphenols (Luana H. Maurer et al., 2020). Since our study utilized an acute colitis model, we understand that the serum was not deeply affected. Maybe, a different dosage of HT may be necessary to achieve significance for such analyses.
Table 4.
Antioxidant capacity of the serum, inflammatory profile, and activity or concentration of oxidative metabolism enzymes.
| Antioxidant analyses (serum) | |||||
|---|---|---|---|---|---|
| Parameter | C | CC | H | HC | MC |
| FRAP (µmol Trolox/mL) | 0.62 ± 0.06a | 0.55 ± 0.04a | 0.60 ± 0.05a | 0.56 ± 0.01a | 0.59 ± 0.06a |
| ORAC (µmol Trolox/mL) | 4.31 ± 0.71a | 3.82 ± 0.81a | 7.26 ± 2.08a | 5.63 ± 0.47a | 4.04 ± 0.65a |
| Inflammatory profile (colon and serum) | |||||
| MPO (colon) (U/g protein) | 9.40 ± 0.50a | 39.80 ± 11.27b | 9.80 ± 1.49a | 11.67 ± 2.74a | 8.66 ± 0.61a |
| IL-1β (colon) (ng/g protein) | 111.20 ± 3.25a | 529.90 ± 127.30b | 81.34 ± 14.20a | 271.30 ± 39.32a | 208.00 ± 39.78a |
| IL-1β (serum) (ng/mL) | 0.40 ± 0.04a | 0.44 ± 0.08a | 0.44 ± 0.06a | 0.47 ± 0.09a | 0.34 ± 0.03a |
| TNF-α (colon) (ng/g protein) | 86.27 ± 2.37a | 77.64 ± 16.17a | 85.73 ± 4.25a | 106.50 ± 15.38a | 105.80 ± 19.70a |
| TNF-α (serum) (ng/mL) | 0.07 ± 0.01a | 0.17 ± 0.06a | 0.06 ± 0.01a | 0.13 ± 0.06a | 0.08 ± 0.01a |
| Oxidative metabolism enzymes (colon) | |||||
| CAT (U/g protein) | 87.60 ± 10.49a | 211.00 ± 33.48b | 56.00 ± 13.33a | 86.50 ± 20.63a | 209.90 ± 21.85b |
| GR (consumed NADPH/min/g protein) | 106.20 ± 19.7ab | 41.60 ± 5.11a | 198.00 ± 76.51bc | 324.50 ± 19.47c | 58.71 ± 9.38a |
| SOD (SOD/g protein) | 144.40 ± 45.57a | 56.00 ± 16.83a | 142.80 ± 43.34a | 105.00 ± 32.83a | 54.67 ± 4.81a |
| TBARS (nmol MDA/g protein) | 66.78 ± 12.42a | 77.58 ± 24.24a | 37.66 ± 10.84a | 68.30 ± 10.49a | 56.41 ± 8.02a |
| GSH (nmol GSH/mg protein) | 14.25 ± 2.85a | 9.39 ± 4.06a | 16.70 ± 2.08a | 11.22 ± 2.21a | 13.74 ± 1.94a |
C: control, CC: control colitis, H: hawthorn, HC: hawthorn colitis, MC: mesalamine colitis. FRAP: ferric reducing antioxidant power, ORAC: oxygen-radical absorbing capacity. CAT: catalase, GR: glutathione reductase, MPO: myeloperoxidase, SOD: superoxide dismutase, TBARS: thiobarbituric acid reactive substances, MDA: malondialdehyde, GSH: glutathione. IL-1β: interleukin-1beta, TNF-α: tumor necrosis factor-alpha. Results are presented as mean ± SEM. One-way ANOVA followed by Tukey. Different letters indicate statistical significance (p < 0,05).
3.4. Concentration or activity of inflammatory proteins and oxidative metabolism enzymes
As expected, MPO and IL-1β levels were significantly increased by the inflammatory process in the colon (p < 0.01). Both mesalamine and HT decreased the concentrations of MPO and IL-1β (p < 0.05) (Table 4). Similarly, Liu et al., 2020, Malekinejad et al., 2013, by using flavonoid-rich extracts from hawthorn berries, also found reduced levels of IL-1β and MPO, respectively, in their pre-clinical studies with colitis. Concentrations of IL-1β in serum and TNF-α in colon and serum were not changed in our study (Table 4).
Besides the common pathway of release of the IL-1β by macrophages (caspase-1 activation by inflammasome), neutrophil proteases can also cleave the precursor of this cytokine into its biologically active forms (Guma et al., 2009), following tissue injury and cell necrosis. Also, to help create an IL-1β-dependent inflammatory response (Lopez-Castejon & Brough, 2011), neutrophils can express MPO, a local mediator of tissue damage and inflammation ignition (Aratani, 2018). Patients with IBDs possess high serum and fecal levels of IL-1β and MPO (Peterson et al., 2007, Vasilyeva et al., 2016), and the typical inflammation of IBDs is largely attributed to the accumulation (UC) or impaired function (CD) of neutrophils (Wéra et al., 2016). In the present study, HT was able to decrease the levels of IL-1β and MPO in the colon, indicating its potential to block or regulate intestinal inflammations associated with the accumulation and activation of intestinal mucosal neutrophils (Therrien et al., 2019).
Regarding the results of oxidative metabolism enzymes, CAT activity was increased by the colitis model (p < 0.01). Experimental studies with mice supplemented with strains of genetically modified bacteria found that elevated CAT activity in a TNBS-induced CD murine model is linked to a decreased likelihood of worsening the disease and developing colon cancer (LeBlanc et al., 2011). Observing Table 4, only the colitis group treated with the HT (group HC) showed a normalization in CAT levels (p < 0.01), demonstrating a superior effect of a natural alternative intervention in comparison with a drug. Additionally, the colitis group receiving HT highly increased the levels of GR in comparison to CC and MC (p < 0.001) (Table 4). This increase possibly happened in response to the production of glutathione peroxidase (GPx), an antioxidant enzyme that works in conjunction with CAT by transforming hydrogen peroxide (H2O2) into water (H2O), thus decreasing oxidative stress caused by inflammation (Ighodaro & Akinloye, 2018). Briefly, in order for the peroxisome organelle to obtain GPx, it is necessary to convert reduced glutathione (GSH) into its oxidized form (GSSH). For the regeneration of GSH (glutathione cycle), the presence of GR is necessary (Moura et al., 2015).
Both CAT and GR are also known for their role in lipid peroxidation. CAT acts as a first-line defense against the formation of free radicals, while GR is responsible for the regeneration of GHS, the body master’s antioxidant (Fagan and Palfey, 2010, Ighodaro and Akinloye, 2018, Pérez et al., 2017). According to recent studies, GR can be increased in rats with improved TNBS-induced colitis, and that received rich sources of flavonoids (jaboticaba peel and grape peel residues) (da Silva-Maia et al., 2019, Maurer et al., 2019). This scenario suggests the importance of GR restoration to ameliorate intestinal conditions. On the other side, despite the well-known regulating role of CAT, in the present study, its activity was increased after colitis induction. This modification is probably due to adaptive or defective mechanisms, as this enzyme has also been found highly expressed in neutrophils from the intestinal mucosa of patients with IBDs (Kruidenier et al., 2003). Therefore, besides acting in the MPO-IL-1β axis, HT could also positively interfere in the mechanics of CAT and GR, first by avoiding the excessive or defective action of CAT, then by highly increasing the levels of GR to control the propagation of reactive oxygen species and restore redox balance.
The levels of SOD, TBARS, and GSH in the colon were not changed either by the colitis model or the interventions (Table 4).
3.5. Concentration of short-chain fatty acids
The extract of the Crataegus oxyacantha leaves is reported to increase the growth of Bifidobacterium and Lactobacillus species and the concentration of acetic acid, according to an in vitro study using skim milk as a fermentable matrix (Khaleel & Haddadin, 2013). Another study showed that flavonoids, including chlorogenic acid, which is abundant in Crataegus oxyacantha, slightly increased the counts of Bifidobacterium spp. and the concentration of SCFAs in vitro (Parkar et al., 2013). However, in the present study, the concentration of SCFAs (total, acetic, butyric, propionic) in feces from both cecum and colon of rats was not altered by the HT. Additionally, these compounds were not modulated by the TNBS-colitis induction model (data not shown). Perhaps, the polyphenols dosage in this study may not be sufficient to increase the levels of SCFAs, or the treatment period was short. Since our study used oral gavage to inoculate the proposed treatment, we decided to not extend its duration. Gavage, especially if done for a prolonged period and repeatedly, may cause reflux, irritation, and inflammation of the esophagus (Damsch et al., 2011, Kinder et al., 2014). Longer treatment periods using diets enriched with hawthorn products may encounter better results as far as SCFAs levels.
A recent investigation using a treatment with hawthorn for chemically-induced colitis found microbiota modulation and increased levels of SCFAs in vivo. However, a diversity of aspects were different from our study, which include: the animals used (C57BL/6J mice), the colitis model (DSS), the fraction and species of the hawthorn plant (fruit of Crataegus pinnatifida), and especially, the treatment applied and its period (an extracted polysaccharide for 42 days) (Guo et al., 2021). Such promising evidence indicates that the microbiome-related aspects of the therapy with the hawthorn plant are worthy of investigation and need further experiments to confirm its potential in vivo.
3.6. Correlation matrix
Colon weight/length ratio, the area of the necrotic lesions, and IL-1β levels presented the most numerous and diverse correlations. On the contrary, the DAI at its peak day had overall weak correlations with all other parameters (Fig. 6).
Fig. 6.
Heatmap correlation matrix Pearson’s correlation analysis. Correlations considered high (values between 0.7 and 1 or −0.7 and −1) are marked with an asterisk (*). Parameters: WEIGHT %: body weight alteration on euthanasia; DAI DAY 1: Disease Activity Index after one day of the administration of 2,4,6-trinitrobenzene sulfonic (peak day of symptoms); W/L COLON: colon weight/length ratio; LESION Wi: width of the largest necrotic lesion; LESION L: length of the largest necrotic lesion; LESION A: area of the largest necrotic lesion; LESION %: the proportion of the colon affected by the largest necrotic lesion; HISTOLOGY T: total score of the histology; HISTOLOGY LI: score of the leukocyte infiltration; HISTOLOGY IE: score of the inflammation extent; HISTOLOGY CD: score of the crypt damage; CAT: catalase levels in the colon; GR: glutathione reductase levels in the colon; MPO: myeloperoxidase levels in the colon; SOD: superoxide dismutase levels in the colon; IL-1β: interleukin-1beta levels in the colon.
The colon weight/length ratio, which is regarded as an inflammatory index (Sánchez-Fidalgo et al., 2010), was highly correlated with almost all the lesion parameters and the levels of IL-1β. On the other side, this cytokine was positively correlated with MPO levels (r = 0.846), probably due to mechanisms previously mentioned, associated with neutrophil infiltration and the release of inflammatory markers in the colonic mucosa. MPO also showed a high negative correlation with body weight alteration (r = −0.708). In a trial with patients with UC, MPO and IL-1β had good correlations with the histological, endoscopic, and clinical severity of the disease (Peterson et al., 2007). This scenario suggests the possibility of measuring such markers to improve prognostic in IBDs, in addition to targeting compounds capable of specifically blocking them, such as the ones found in the HT.
There was also a negative correlation (r = −0.732) between the levels of CAT and GR. As previously mentioned, this data shows that HT is mechanistically acting through an oxidative stress pathway, mainly by affecting in opposite ways GR and CAT in order to prevent the spread of free radicals and consequently oxidative stress.
Although the interventions did not reduce the histological score, there was a high positive correlation of such parameter with the necrotic lesion area (r = 0.708), which was diminished by the HT.
4. Conclusions
Animal studies directed towards IBDs lack analysis or do not present an adequate experimental model (e.g., acetic acid-induced colitis). This study is, to the best of our knowledge, the first successful investigation of the effects of Crataegus oxyacantha in IBDs. A HT prepared from the flowering tops (leaves and flowers), a source of polyphenols, effectively decreases inflammatory mediators (IL-1β, MPO) and regulates oxidative stress in TNBS-induced colitis. Additionally, administration of HT via oral gavage does not cause any harm in healthy rats. Such findings indicate that the hawthorn plant may serve as an alternative or complementary natural remedy that extends beyond its well-known cardiovascular protective and anti-hypertensive roles.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financed in part by the 1. Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Finance Code 001; 2. Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) - processes 140812/2019-9, 403328/2016-0, and 301496/2019-6; 3. Fundação de Amparo à Pesquisa do Estado de São Paulo – processes 2018/11069-5, 2017/23657-6, 2015/50333-1, and 2015/13320-9; 4. Ministry of Education and Training of Vietnam; and 5. Campus France. Special thanks to Red Iberomericana de Alimentos Autoctonos Subutilizados (ALSUB-CYTED, 118RT0543).
Contributor Information
Roberto de Paula do Nascimento, Email: roberto_beto1@hotmail.com.
Ana Paula da Fonseca Machado, Email: anapfonsecam@gmail.com.
Verena Silva Lima, Email: verena@ufam.edu.br.
Amanda Maria Tomazini Munhoz Moya, Email: amandamunhoz.moya@gmail.com.
Lívia Mateus Reguengo, Email: liviareguengo@gmail.com.
Stanislau Bogusz Junior, Email: stanislau@iqsc.usp.br.
Raquel Franco Leal, Email: raquelfl@fcm.unicamp.br.
Phu Cao-Ngoc, Email: tech.ngocphu@gmail.com.
Jean Christophe Rossi, Email: jean-christophe.rossi@umontpellier.fr.
Laurent Leclercq, Email: laurent.leclercq@umontpellier.fr.
Hervé Cottet, Email: herve.cottet@umontpellier.fr.
Cinthia Baú Betim Cazarin, Email: cbetim@unicamp.br.
Mario Roberto Marostica Junior, Email: mmarosti@unicamp.br.
References
- Alirezalu A., Salehi P., Ahmadi N., Sonboli A., Aceto S., Maleki H.H., Ayyari M. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of Iran. International Journal of Food Properties. 2018 doi: 10.1080/10942912.2018.1446146. [DOI] [Google Scholar]
- Antoniou E., Margonis G.A., Angelou A., Pikouli A., Argiri P., Karavokyros I.…Pikoulis E. The TNBS-induced colitis animal model: An overview. Annals of Medicine and Surgery. 2016 doi: 10.1016/j.amsu.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aratani Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Archives of Biochemistry and Biophysics. 2018 doi: 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 1996 doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Billing E., Bech-Andersen J., Lelliott R.A. Fireblight in Hawthorn in England and Denmark. Plant Pathology. 1974 doi: 10.1111/j.1365-3059.1974.tb01837.x. [DOI] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976 doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campos M.R.S. Elsevier; 2019. Bioactive compounds: Health benefits and potential applications. [Google Scholar]
- Cardona F., Andrés-Lacueva C., Tulipani S., Tinahones F.J., Queipo-Ortuño M.I. Benefits of polyphenols on gut microbiota and implications in human health. Journal of Nutritional Biochemistry. 2013 doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. [59] Glutathione reductase. Methods in Enzymology. 1985 doi: 10.1016/S0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Catana C.S., Magdas C., Tabaran F.A., Craciun E.C., Deak G., Magdas V.A.…Dumitrascu D.L. Comparison of two models of inflammatory bowel disease in rats. Advances in Clinical and Experimental Medicine. 2018 doi: 10.17219/acem/69134. [DOI] [PubMed] [Google Scholar]
- Cazarin C.B.B., da Silva J.K., Colomeu T.C., Batista Â.G., Vilella C.A., Ferreira A.L.…Junior M.R.M. Passiflora edulis peel intake and ulcerative colitis: Approaches for prevention and treatment. Experimental Biology and Medicine. 2014 doi: 10.1177/1535370214525306. [DOI] [PubMed] [Google Scholar]
- Cazarin C.B., Betim R.-N.A., Algieri F., Utrilla M.P., Rodríguez-Cabezas M.E., Garrido-Mesa J., Guerra-Hernández E., de Braga P.A.C., Reyes F.G.R., Maróstica M.R., Gálvez J. Intestinal anti-inflammatory effects of Passiflora edulis peel in the dextran sodium sulphate model of mouse colitis. Journal of Functional Foods. 2016 doi: 10.1016/j.jff.2016.08.020. [DOI] [Google Scholar]
- Chang Q., Zuo Z., Harrison F., Chow M.S.S. Hawthorn. Journal of Clinical Pharmacology. 2002 doi: 10.1177/00970002042006003. [DOI] [PubMed] [Google Scholar]
- da Silva-Maia J.K., Batista Â.G., Cazarin C.B.B., Soares E.S., Junior S.B., Leal R.F.…Maróstica Junior M.R. Aqueous extract of Brazilian berry (Myrciaria jaboticaba) peel improves inflammatory parameters and modulates Lactobacillus and Bifidobacterium in rats with induced-colitis. Nutrients. 2019 doi: 10.3390/nu11112776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmer S., Scott E. Health effects of hawthorn. American Family Physician. 2010 [PubMed] [Google Scholar]
- Dalli E., Colomer E., Tormos M.C., Cosín-Sales J., Milara J., Esteban E., Sáez G. Crataegus laevigata decreases neutrophil elastase and has hypolipidemic effect: A randomized, double-blind, placebo-controlled trial. Phytomedicine. 2011 doi: 10.1016/j.phymed.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Damsch S., Eichenbaum G., Tonelli A., Lammens L., Van den Bulck K., Feyen B.…Kelley M. Gavage-related reflux in rats: Identification, pathogenesis, and toxicological implications (review) Toxicologic Pathology. 2011;39(2):348–360. doi: 10.1177/0192623310388431. [DOI] [PubMed] [Google Scholar]
- De Almeida C.S., Andrade-Oliveira V., Câmara N.O.S., Jacysyn J.F., Faquim-Mauro E.L. Crotoxin from Crotalus durissus terrificus is able to down-modulate the acute intestinal inflammation in mice. PLoS ONE. 2015 doi: 10.1371/journal.pone.0121427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman L.A., Palmen M.J.H.J., Akol H., Bloemena E., Peña A.S., Meuwissen S.G.M., Van Rees E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clinical and Experimental Immunology. 1998 doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J.E., Brown P.N., Talent N., Dickinson T.A., Shipley P.R. A review of the chemistry of the genus Crataegus. Phytochemistry. 2012 doi: 10.1016/j.phytochem.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Elango C., Devaraj S.N. Immunomodulatory effect of Hawthorn extract in an experimental stroke model. Journal of Neuroinflammation. 2010 doi: 10.1186/1742-2094-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango C., Jayachandaran K.S., Niranjali Devaraj S. Hawthorn extract reduces infarct volume and improves neurological score by reducing oxidative stress in rat brain following middle cerebral artery occlusion. International Journal of Developmental Neuroscience. 2009 doi: 10.1016/j.ijdevneu.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Fagan, R. L., & Palfey, B. A. (2010). Flavin-dependent enzymes. In Comprehensive Natural Products II: Chemistry and Biology. https://doi.org/10.1016/b978-008045382-8.00135-0.
- Farzaei M.H., Ghasemi-Niri S.F., Abdolghafari A.H., Baeeri M., Khanavi M., Navaei-Nigjeh M.…Rahimi R. Biochemical and histopathological evidence on the beneficial effects of Tragopogon graminifolius in TNBS-induced colitis. Pharmaceutical Biology. 2015 doi: 10.3109/13880209.2014.923004. [DOI] [PubMed] [Google Scholar]
- Fujisawa M., Oguchi K., Yamaura T., Suzuki M., Cyong J.C. Protective effect of hawthorn fruit on murine experimental colitis. American Journal of Chinese Medicine. 2005 doi: 10.1142/S0192415X05002849. [DOI] [PubMed] [Google Scholar]
- Gao Y., Du Y., Ying Z., Leng A., Zhang W., Meng Y.…Kang T. Hepatic, gastric and intestinal first-pass effects of vitexin-2’’-O-rhamnoside in rats by ultra-high-performance liquid chromatography. Biomedical Chromatography. 2016 doi: 10.1002/bmc.3522. [DOI] [PubMed] [Google Scholar]
- Gommeaux J., Cano C., Garcia S., Gironella M., Pietri S., Culcasi M.…Carrier A. Colitis and colitis-associated cancer are exacerbated in mice deficient for tumor protein 53-induced nuclear protein 1. Molecular and Cellular Biology. 2007;27(6):2215–2228. doi: 10.1128/MCB.01454-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guma M., Ronacher L., Liu-Bryan R., Takai S., Karin M., Corr M. Caspase 1-independent activation of interleukin-1β in neutrophil-predominant inflammation. Arthritis and Rheumatism. 2009 doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Wang Y., Zhang S., Zhang X., Du Z., Li M., Ding K. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. International Journal of Biological Macromolecules. 2021;181:357–368. doi: 10.1016/J.IJBIOMAC.2021.03.137. [DOI] [PubMed] [Google Scholar]
- Hadwan M.H., Ali Kadhum S. New spectrophotometric assay for assessments of catalase activity in biological samples. Analytical Biochemistry. 2018 doi: 10.1016/j.ab.2017.11.013. [DOI] [PubMed] [Google Scholar]
- Hatipoʇlu M., Saʇlam M., Köseoʇlu S., Köksal E., Keleş A., Esen H.H. The effectiveness of Crataegus orientalis M Bieber. (Hawthorn) extract administration in preventing alveolar bone loss in rats with experimental periodontitis. PLoS ONE. 2015 doi: 10.1371/journal.pone.0128134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle, D. E., Wiersma, W., & Jurs, S. G. (2003). Applied statistics for the behavioral sciences (Mass: Houghton Mifflin (ed.); 3rd ed.).
- Horne R., Parham R., Driscoll R., Robinson A. Patient’s attitudes to medicines and adherence to maintenance treatment in inflammatory bowel disease. Inflammatory Bowel Diseases. 2009 doi: 10.1002/ibd.20846. [DOI] [PubMed] [Google Scholar]
- Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine. 2018 doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- Issaadi O., Fibiani M., Picchi V., Scalzo R.L., Madani K. Phenolic composition and antioxidant capacity of hawthorn (Crataegus oxyacantha L.) flowers and fruits grown in Algeria. Journal of Complementary and Integrative Medicine. 2020 doi: 10.1515/jcim-2018-0125. [DOI] [PubMed] [Google Scholar]
- Jang S.E., Jeong J.J., Kim J.K., Han M.J., Kim D.H. Simultaneous Amelioratation of Colitis and Liver Injury in Mice by Bifidobacterium longum LC67 and Lactobacillus plantarum LC27. Scientific Reports. 2018 doi: 10.1038/s41598-018-25775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata K., Yoshioka Y., Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules. 2019 doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keser S., Celik S., Turkoglu S., Yilmaz O., Turkoglu I. The Investigation of Some Bioactive Compounds and Antioxidant Properties of Hawthorn (Crataegus monogyna subsp. monogyna jacq.) Journal of Intercultural Ethnopharmacology. 2014 doi: 10.5455/jice.20140120103320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaleel S.M.J., Haddadin M.S.Y. The enhancement of hawthorn leaf extracts on the growth and production of short chain fatty acids of two probiotic bacteria. Pakistan Journal of Nutrition. 2013 doi: 10.3923/pjn.2013.144.149. [DOI] [Google Scholar]
- Kinder J.M., Then J.E., Hansel P.M., Molinero L.L., Bruns H.A. Long-term repeated daily use of intragastric gavage hinders induction of oral tolerance to ovalbumin in mice. Comparative Medicine. 2014;64(5):369–376. [PMC free article] [PubMed] [Google Scholar]
- Kruidenier L., Kuiper I., van Duijn W., Mieremet-Ooms M.A.C., van Hogezand R.A., Lamers C.B.H.W., Verspaget H.W. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. Journal of Pathology. 2003 doi: 10.1002/path.1408. [DOI] [PubMed] [Google Scholar]
- Kumar D., Arya V., Bhat Z.A., Khan N.A., Prasad D.N. The genus Crataegus: Chemical and pharmacological perspectives. Brazilian Journal of Pharmacognosy. 2012 doi: 10.1590/S0102-695X2012005000094. [DOI] [Google Scholar]
- Lacucci M., De Silva S., Ghosh S. Mesalazine in inflammatory bowel disease: A trendy topic once again? Canadian Journal of Gastroenterology. 2010 doi: 10.1155/2010/586092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaison, J. L., & Carnat, A. (1990). Teneur en principaux flavonoides des fleurs et des feuilles de Crataegus monogyna jacq. et de Crataegus laevigata (poiret) dc. (Rosaceae). Pharmaceutica Acta Helvetiae.
- Lasa J., Correa G., Fuxman C., Garbi L., Linares M.E., Lubrano P.…Olivera P. Treatment adherence in inflammatory bowel disease patients from Argentina: A multicenter study. Gastroenterology Research and Practice. 2020;2020:4060648. doi: 10.1155/2020/4060648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc, J. G., del Carmen, S., Miyoshi, A., Azevedo, V., Sesma, F., Langella, P., Bermúdez-Humarán, L. G., Watterlot, L., Perdigon, G., & de Moreno de LeBlanc, A. (2011). Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. Journal of Biotechnology, 151(3), 287–293. https://doi.org/https://doi.org/10.1016/j.jbiotec.2010.11.008. [DOI] [PubMed]
- Leite A.V., Malta L.G., Riccio M.F., Eberlin M.N., Pastore G.M., Maróstica Júnior M.R. Antioxidant potential of rat plasma by administration of freeze-dried jaboticaba peel (Myrciaria jaboticaba Vell Berg) Journal of Agricultural and Food Chemistry. 2011;59(6):2277–2283. doi: 10.1021/jf103181x. [DOI] [PubMed] [Google Scholar]
- Liu, F., Zhang, X., & Ji, Y. (2020). Total Flavonoid Extract from Hawthorn (Crataegus pinnatifida) Improves Inflammatory Cytokines-Evoked Epithelial Barrier Deficit. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research. https://doi.org/10.12659/MSM.920170. [DOI] [PMC free article] [PubMed]
- Lopez-Castejon G., Brough D. Understanding the mechanism of IL-1β secretion. Cytokine and Growth Factor Reviews. 2011 doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekinejad H., Shafie-Irannejad V., Hobbenaghi R., Tabatabaie S.H., Moshtaghion S.M. Comparative protective effect of hawthorn berry hydroalcoholic extract, atorvastatin, and mesalamine on experimentally induced colitis in rats. Journal of Medicinal Food. 2013 doi: 10.1089/jmf.2012.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino E., Collina S., Rossi D., Bazzoni D., Gaggeri R., Bracco F., Azzolina O. Influence of the extraction mode on the yield of hyperoside, vitexin and vitexin-2-O-rhamnoside from Crataegus monogyna Jacq. (Hawthorn) Phytochemical Analysis. 2008 doi: 10.1002/pca.1081. [DOI] [PubMed] [Google Scholar]
- Maurer L.H., Cazarin C.B.B., Quatrin A., Minuzzi N.M., Nichelle S.M., de Lamas C.A., Cagnon V.H.A., Morari J., Velloso L.A., Maróstica Júnior M.R., Emanuelli T. Grape peel powder attenuates the inflammatory and oxidative response of experimental colitis in rats by modulating the NF-κB pathway and activity of antioxidant enzymes. Nutrition Research. 2020 doi: 10.1016/j.nutres.2020.01.006. [DOI] [PubMed] [Google Scholar]
- Maurer L.H., Cazarin C.B.B., Quatrin A., Nichelle S.M., Minuzzi N.M., Teixeira C.F.…Emanuelli T. Dietary fiber and fiber-bound polyphenols of grape peel powder promote GSH recycling and prevent apoptosis in the colon of rats with TNBS-induced colitis. Journal of Functional Foods. 2019 doi: 10.1016/j.jff.2019.103644. [DOI] [Google Scholar]
- Moura F.A., de Andrade K.Q., dos Santos J.C.F., Araújo O.R.P., Goulart M.O.F. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biology. 2015 doi: 10.1016/j.redox.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nascimento R.P., Lima A.V., Oyama L.M., Paiotti A.P.R., Cardili L., Martinez C.A.R., Pereira J.A., Silva M.F., Garofolo I.C., Silveira V.L.F., Caperuto L.C. Extra-virgin olive oil and flaxseed oil have no preventive effects on dss-induced acute ulcerative colitis. Nutrition. 2020;110731 doi: 10.1016/j.nut.2020.110731. [DOI] [PubMed] [Google Scholar]
- Nascimento, R. de P. do, Machado, A. P. da F., Galvez, J., Cazarin, C. B. B., & Maróstica Junior, M. R. (2020). Ulcerative colitis: Gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sciences, 118129. https://doi.org/10.1016/j.lfs.2020.118129. [DOI] [PubMed]
- Nathan M. The complete German Commission E monographs: Therapeutic guide to herbal medicines. Annals of Internal Medicine. 1999 doi: 10.7326/0003-4819-130-5-199903020-00024. [DOI] [Google Scholar]
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I.…Kaplan G.G. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. The Lancet. 2017;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- Ngoc P.C., Leclercq L., Rossi J.C., Desvignes I., Hertzog J., Fabiano-Tixier A.S.…Cottet H. Optimizing water-based extraction of bioactive principles of hawthorn: From experimental laboratory research to homemade preparations. Molecules. 2019 doi: 10.3390/molecules24234420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979 doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ou B., Chang T., Huang D., Prior R.L. Determination of total antioxidant capacity by oxygen radical absorbance capacity (ORAC) using fluorescein as the fluorescence probe: First action 2012.23. Journal of AOAC International. 2013 doi: 10.5740/jaoacint.13-175. [DOI] [PubMed] [Google Scholar]
- Paiotti A.P.R., Neto R.A., Marchi P., Silva R.M., Pazine V.L., Noguti J.…Ribeiro D.A. The anti-inflammatory potential of phenolic compounds in grape juice concentrate (G8000TM) on 2,4,6-trinitrobenzene sulphonic acid-induced colitis. British Journal of Nutrition. 2013 doi: 10.1017/S000711451300007X. [DOI] [PubMed] [Google Scholar]
- Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: An overview. Journal of Nutritional Science. 2016;5 doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkar S.G., Trower T.M., Stevenson D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe. 2013 doi: 10.1016/j.anaerobe.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Patel P.P., Trivedi N.D. Effect of karanjin on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in Balb/c mice. Indian Journal of Pharmacology. 2017 doi: 10.4103/ijp.IJP_234_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez S., Taléns-Visconti R., Rius-Pérez S., Finamor I., Sastre J. Redox signaling in the gastrointestinal tract. Free Radical Biology and Medicine. 2017 doi: 10.1016/j.freeradbiomed.2016.12.048. [DOI] [PubMed] [Google Scholar]
- Peterson C.G.B., Sangfelt P., Wagner M., Hansson T., Lettesjö H., Carlson M. Fecal levels of leukocyte markers reflect disease activity in patients with ulcerative colitis. Scandinavian Journal of Clinical and Laboratory Investigation. 2007 doi: 10.1080/00365510701452838. [DOI] [PubMed] [Google Scholar]
- Pieper C., Haag S., Gesenhues S., Holtmann G., Gerken G., Jöckel K.-H. Guideline adherence and patient satisfaction in the treatment of inflammatory bowel disorders–an evaluation study. BMC Health Services Research. 2009;9:17. doi: 10.1186/1472-6963-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter L.J., Hrstich L.N., Chan B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry. 1985 doi: 10.1016/S0031-9422(00)94533-3. [DOI] [Google Scholar]
- Rauf, A., Imran, M., Abu-Izneid, T., Iahtisham-Ul-Haq, Patel, S., Pan, X., Naz, S., Silva], A. [Sanches, Saeed, F., & Suleria], H. A. [Rasul. (2019). Proanthocyanidins: A comprehensive review. Biomedicine & Pharmacotherapy, 116, 108999. https://doi.org/10.1016/j.biopha.2019.108999. [DOI] [PubMed]
- Sánchez-Fidalgo S., Villegas I., Cárdeno A., Talero E., Sánchez-Hidalgo M., Motilva V., Alarcón de la Lastra C. Extra-virgin olive oil-enriched diet modulates DSS-colitis-associated colon carcinogenesis in mice. Clinical Nutrition. 2010;29(5):663–673. doi: 10.1016/j.clnu.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Simpson B., Aryee A.N., Toldrá F. Wiley; 2019. Byproducts from agriculture and fisheries: Adding value for food, feed, pharma and fuels. [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965;16(3):144–158. https://www.ajevonline.org/content/16/3/144 [Google Scholar]
- Therrien A., Chapuy L., Bsat M., Rubio M., Bernard G., Arslanian E.…Sarfati M. Recruitment of activated neutrophils correlates with disease severity in adult Crohn’s disease. Clinical and Experimental Immunology. 2019;195(2):251–264. doi: 10.1111/cei.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungaro R., Mehandru S., Allen P.B., Peyrin-biroulet L., Colombel J. Ulcerative colitis. Lancet. 2017;389(10080) doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilyeva E., Abdulkhakov S., Cherepnev G., Martynova E., Mayanskaya I., Valeeva A.…Rizvanov A. Serum cytokine profiles in children with Crohn’s disease. Mediators of Inflammation. 2016 doi: 10.1155/2016/7420127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A.F., Marakis G., Simpson E., Hope J.L., Robinson P.A., Hassanein M., Simpson H.C.R. Hypotensive effects of hawthorn for patients with diabetes taking prescription drugs: A randomised controlled trial. British Journal of General Practice. 2006 [PMC free article] [PubMed] [Google Scholar]
- Weber R.W. On the cover – Hawthorn. Annals of Allergy, Asthma and Immunology. 2010 doi: 10.1016/j.anai.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Wei W., Ying X., Zhang W., Chen Y., Leng A., Jiang C., Liu J. Effects of vitexin-2“-O-rhamnoside and vitexin-4”-O-glucoside on growth and oxidative stress-induced cell apoptosis of human adipose-derived stem cells. The Journal of Pharmacy and Pharmacology. 2014;66(7):988–997. doi: 10.1111/jphp.12225. [DOI] [PubMed] [Google Scholar]
- Wéra O., Lancellotti P., Oury C. The dual role of neutrophils in inflammatory bowel diseases. Journal of Clinical Medicine. 2016 doi: 10.3390/jcm5120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C.C., Hawkins R.E., Brian M., Carrell R.W. The estimation of red cell superoxide dismutase activity. The Journal of Laboratory and Clinical Medicine. 1975 doi: 10.5555/uri:pii:0022214375904394. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Naito Y., Kishi A., Tomii T., Kaneko T., Iinuma S.…Kondo M. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut. 1993 doi: 10.1136/gut.34.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapatero J.M. Selections from current literature: Effects of Hawthorn on the cardiovascular system. Family Practice. 1999 doi: 10.1093/fampra/16.5.534. [DOI] [PubMed] [Google Scholar]
- Zatorski H., Sałaga M., Zielińska M., Piechota-Polańczyk A., Owczarek K., Kordek R.…Fichna J. Experimental colitis in mice is attenuated by topical administration of chlorogenic acid. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2015 doi: 10.1007/s00210-015-1110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Nyman M., Jonsson J. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomedical Chromatography : BMC. 2006;20:674–682. doi: 10.1002/bmc.580. [DOI] [PubMed] [Google Scholar]
- Zhi C.Y., Lv Z., Kong N., Chen Y., Wang Z.H., Gao H.C. Effect on various organs in TNBS-induced ulcerative colitis. Biomedical Research (India) 2017 [Google Scholar]
- Zhou Y., Zhou L., Ruan Z., Mi S., Jiang M., Li X.…Yin Y. Chlorogenic acid ameliorates intestinal mitochondrial injury by increasing antioxidant effects and activity of respiratory complexes. BioScience, Biotechnology and Biochemistry. 2016 doi: 10.1080/09168451.2015.1127130. [DOI] [PubMed] [Google Scholar]
- Zhu X.X., Li L.D., Liu J.X., Liu Z.Y., Ma X.Y. Effect of vitexia-rhamnoside (V-R) on vasomotor factors expression of endothelial cell. Zhongguo Zhongyao Zazhi. 2006 [PubMed] [Google Scholar]