Abstract
Background
Lung immune prognostic index (LIPI) refers to a biomarker combining derived neutrophil-to-lymphocyte ratio (dNLR) and lactate dehydrogenase (LDH). Its prognostic effect on advanced small cell lung cancer (SCLC) patients receiving programmed cell death 1/programmed cell death ligand-1 (PD-1/PD-L1) inhibitors plus chemotherapy as first-line treatment remains unclear. Our research investigated the relationship between pretreatment LIPI and the prognosis of patients receiving first-line PD-1/PD-L1 inhibitors plus chemotherapy.
Methods
Advanced SCLC patients receiving PD-1/PD-L1 inhibitors plus chemotherapy as first-line treatment from Jan 2015 to Oct 2020 were included. Based on the values of dNLR and LDH, the study population was divided into two groups: LIPI good and LIPI intermediate/poor. The Kaplan-Meier method was used to compute the median survival time and the log-rank test was used to compare the two groups. Univariate and multivariate analyses were used to examine the correlation between the pretreatment LIPI and clinical outcomes.
Results
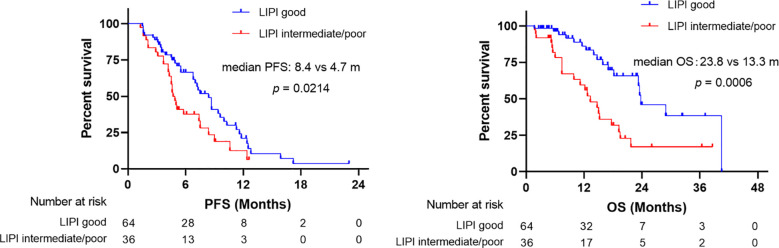
One hundred patients were included in this study, of which, 64% were LIPI good (dNLR < 4.0 and LDH < 283 U/L), 11% were LIPI poor (dNLR ≥ 4.0 and LDH ≥ 283 U/L), and the remaining 25% were LIPI intermediate. The LIPI good group had better progression-free survival (PFS) (median: 8.4 vs 4.7 months, p = 0.02) and overall survival (OS) (median: 23.8 vs 13.3 months, p = 0.0006) than the LIPI intermediate/poor group. Multivariate analysis showed that pretreatment LIPI intermediate/poor was an independent risk factor for OS (HR: 2.34; 95%CI, 1.13, 4.86; p = 0.02). Subgroup analysis showed that pretreatment LIPI good was associated with better PFS and OS in males, extensive disease (ED), PD-1 inhibitor treatment, smokers, and liver metastasis (p < 0.05).
Conclusions
Pretreatment LIPI could serve as a prognostic biomarker for advanced SCLC patients receiving first-line PD-1/PD-L1 inhibitors plus chemotherapy.
Keywords: small cell lung cancer, immune checkpoint inhibitor, first-line, lung immune prognostic index, prognosis
Introduction
Small-cell lung cancer (SCLC) constitutes 13 - 15% of total lung cancer cases, and is characterized by rapid progression and early distant metastasis (1, 2). Over 90% of SCLC patients are elders or past heavy smokers (3). One-third of SCLC patients are classified as having limited disease (LD), and the others as having extensive disease (ED) according to the Veteran’s Administration Lung Cancer Study Group Staging System (4, 5). Despite sensitivity to first-line chemotherapy, most SCLC cases recur in one year and are insensitive to second-line treatment (6). The median overall survival (OS) is 15−20 months for patients with LD, and 8−13 months for those with ED (7).
Immune checkpoint inhibitors (ICIs), especially programmed cell death 1/programmed cell death ligand-1 (PD-1/PD-L1) inhibitors, have revolutionized the treatment landscape of various cancers. Recently, the IMpower 133 and CASPIAN studies have demonstrated that a combination of atezolizumab or durvalumab and chemotherapy could improve clinical outcomes of SCLC patients as compared to those using chemotherapy alone (8, 9). The phase II EA5161 study has demonstrated the addition of nivolumab at first-line treatment significantly improved the progression-free survival (PFS) and OS of ES-SCLC patients (median PFS: 5.5 vs 4.6 months, p = 0.012; median OS: 11.3 vs 8.5 months, p = 0.038) (10). The phase III KEYNOTE-604 study showed that advanced SCLC patients receiving first-line pembrolizumab plus chemotherapy had better OS compared with those receiving chemotherapy alone, but the difference did not meet the predefined statistical threshold (11). A meta analysis study found that both PD-L1 inhibitors and PD-1 inhibitors plus chemotherapy as first-line treatment could provide a significant improvement of survival time compared with chemotherapy alone for advanced SCLC patients (12). FDA has approved PD-1 inhibitors as third-line treatment in 2018 and PD-L1 inhibitors as first-line treatment in 2020 for patients with ED or relapsed SCLC, which is an important advancement for SCLC patients.
SCLC patients have a relatively high tumor mutation burden (13), but it has not been proven to serve as a clear predictor in patients receiving ICI treatment (8, 14). PD-L1 expression is low or absent in SCLC patients, but it is still not used as a predictive biomarker in SCLC patients receiving ICI treatment (15). Currently, no prognostic biomarkers can definitely guide the application of ICIs in patients with SCLC. Therefore, identifying biomarkers to select patients who are likely to respond to immunotherapy is crucial. Systemic inflammation plays a critical role in the occurrence and development of cancer (16). Previous studies have reported the prognostic role of systemic inflammation indicators in non-small cell lung cancer (NSCLC) patients receiving immunotherapy, including neutrophil-to-lymphocyte ratio (NLR) and lactate dehydrogenase (LDH) (17–22). Several studies showed that the lung immune prognostic index (LIPI), combining derived NLR (dNLR, absolute neutrophil count/[white blood cell concentration−absolute neutrophil count]) and LDH, could predict survival in advanced NSCLC patients receiving immunotherapy (23, 24). However, there is a lack of studies describing the prognostic value of pretreatment LIPI in advanced SCLC patients receiving PD-1/PD-L1 inhibitor treatment. Therefore, we aim to investigate whether pretreatment LIPI was related to the prognosis of advanced SCLC patients treated with first-line PD-1/PD-L1 inhibitors plus chemotherapy.
Methods
Study Design and Patients
The study was carried out at the Chinese PLA general hospital (Beijing, China). Advanced SCLC patients receiving PD-1/PD-L1 inhibitors plus chemotherapy as first-line treatment from Jan 2015 to Oct 2020 were included. The inclusion criteria were as follows (1): patients who were diagnosed with SCLC (2); patients receiving first-line PD-1/PD-L1 inhibitors plus chemotherapy; and (3) patients who were treated with at least two cycles of PD-1/PD-L1 inhibitors. The exclusion criteria were (1): absence of efficacy assessment; and (2) absence of pretreatment blood test results. Clinical characteristics as well as pretreatment blood laboratory test results were recorded. Clinical characteristics included age, sex, stage, smoking history, ICI drugs, Eastern Cooperative Oncology Group Performance Status (ECOG PS), sites of metastasis and efficacy, and pretreatment blood test results included total white blood cell count, absolute neutrophil count, absolute lymphocyte count, and LDH levels. This research was authorized by the Ethics Committee of Chinese PLA General Hospital and performed according to the principles of the Declaration of Helsinki.
LD is defined as a disease limited to one hemithorax, local mediastinal lymph nodes, and ipsilateral supraclavicular lymph nodes, which can be included in a tolerable radiation field; ED includes the cases not classified as LD (25). Blood tests were conducted within 5 days before the first cycle of immunotherapy. LIPI was calculated by dNLR (absolute neutrophil count/[white blood cell concentration−absolute neutrophil count]) and LDH, and cutoff values of dNLR and LDH were calculated using X-tile software based on data (26), which were 4.0 U/L and 283 U/L, respectively. Patients were stratified into LIPI good (dNLR < 4.0 and LDH < 283 U/L) and LIPI intermediate/poor groups (intermediate: dNLR < 4.0 and LDH ≥ 283 U/L, or dNLR ≥ 4.0 and LDH < 283 U/L; poor: dNLR ≥ 4.0 and LDH ≥ 283 U/L) groups.
Treatment responses were assessed every two cycles of ICI treatment by two independent investigators (ZZ and LL) according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). PFS was defined as the period from the first ICI treatment to disease progression or death (whichever occurred first). OS was defined as the period from the first ICI treatment to death. All patients were followed up through telephone counseling and searching electronic medical records with a cutoff date of March 16, 2021.
Statistical Analysis
Statistical analyses were conducted using IBM SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 8 (La Jolla, CA, USA). X-tile 3.6.1 software (Yale University, New Haven, CT, USA) was used to identify the optimal cut-off values for dNLR and LDH. Kaplan-Meier curves were used to analyze OS and PFS, and the differences were evaluated by log-rank test. Chi-square or Fisher’s exact test was used to compare categorical variables. Hazard ratio (HR) with its 95% confidence interval (CI) was estimated by Cox proportional hazards models. Univariate and multivariate analyses were conducted to determine the independent prognostic value of pretreatment LIPI. The variables with p < 0.05 in the univariate analysis were eligible to be included in the multivariate analysis. Phi correlation coefficients were calculated to determine the association between each pair of the dichotomous variables. All statistical tests were two-sided with a statistical significance of p < 0.05.
Results
Patient Clinical Characteristics
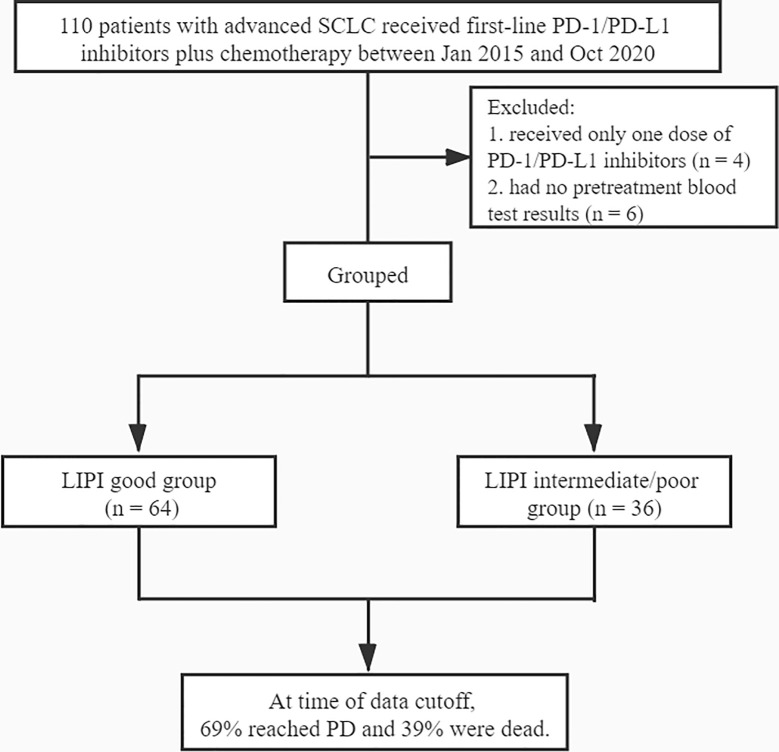
A total of 110 SCLC patients receiving first-line PD-1/PD-L1 inhibitors were identified, of which, four patients received only one dose of PD-1/PD-L1 inhibitors, and six patients had no pretreatment blood test results ( Figure 1 ). Finally, 100 SCLC patients were included for data analysis. Most of those patients (87%) received platinum-etoposide chemotherapy (45% carboplatin and 42% cis-platinum), and the other patients (13%) received nab-paclitaxel and etoposide. Moreover, 65% of the patients received PD-1 inhibitors (nivolumab, pembrolizumab or sintilimab), and 35% received PD-L1 inhibitors (atezolizumab or durvalumab). Patients had a maximum of 4-6 cycles of chemotherapy as first-line treatment. The median follow-up time was 19.2 months. Detailed clinical data of the patients are summarized in Table 1 . The median age was 60 years (range: 32−82). Among the 100 patients, 88% were males, 74% had an ED, 94% had an ECOG PS of 0−1, 79% had a smoking history, 22% had brain metastasis, 24% had liver metastasis, and 29% had bone metastasis. Of the patients, 60%, 31%, and 9% had PR, SD, and PD, respectively; 78% had dNLR < 4.0, and 75% had LDH < 283 U/L. Patients in the LIPI good, LIPI intermediate, and LIPI poor groups were 64%, 25%, and 11%, respectively.
Figure 1.
Diagram of the study.
Table 1.
Characteristics of patients with advanced SCLC.
| Characteristics | No. of patients (n = 100) | Percentage (%) |
|---|---|---|
| Age (year), median (range) | 60 (32−82) | |
| <60 | 48 | 48 |
| ≥60 | 52 | 52 |
| Sex | ||
| Male | 88 | 88 |
| Female | 12 | 12 |
| Stage | ||
| LD | 26 | 26 |
| ED | 74 | 74 |
| Smoking history | ||
| Never smoke | 21 | 21 |
| Smoke | 79 | 79 |
| ICI Drugs | ||
| PD-1 inhibitor | 65 | 65 |
| PD-L1 inhibitor | 35 | 35 |
| Chemotherapy | ||
| Platinum plus etoposide | 87 | 87 |
| Nab-paclitaxel plus etoposide | 13 | 13 |
| ECOG PS | ||
| 0−1 | 94 | 94 |
| ≥2 | 6 | 6 |
| Brain metastasis | ||
| Yes | 22 | 22 |
| No | 78 | 78 |
| Liver metastasis | ||
| Yes | 24 | 24 |
| No | 76 | 76 |
| Bone metastasis | ||
| Yes | 29 | 29 |
| No | 71 | 71 |
| Treatment efficacy | ||
| PR | 60 | 60 |
| SD | 31 | 31 |
| PD | 9 | 9 |
| dNLR | ||
| <4.0 | 78 | 78 |
| ≥4.0 | 22 | 22 |
| LDH (U/L) | ||
| <283 | 75 | 75 |
| ≥283 | 25 | 25 |
| Pretreatment LIPI | ||
| Good | 64 | 64 |
| Intermediate | 25 | 25 |
| Poor | 11 | 11 |
LD, limited disease; ED, extensive disease; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICI, immune checkpoint inhibitor; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1; dNLR, derived neutrophil-to-lymphocyte ratio; LDH, lactate dehydrogenase; LIPI, Lung immune prognostic index; PR, partial response; SD, steady disease; PD, progressive disease.
Univariate and Multivariate Analysis for PFS and OS
At time of data cutoff, 69% of the patients reached PD and 39% died. LIPI good was associated with better PFS than LIPI intermediate/poor (median: 8.4 vs 4.7 months, p = 0.02) ( Figure 2 ). Univariate analysis demonstrated that ECOG PS 0−1, no bone metastasis, and pretreatment LIPI good were related to better PFS in SCLC patients receiving first-line ICI treatment (p < 0.05). Before multivariate analysis, the pairwise correlation coefficients of ECOG PS, bone metastasis, and pretreatment LIPI were calculated to determine the potential correlation between each pair of these variables. All the correlation coefficients were below 0.5, indicating that there was a low correlation between each pair of these variables ( Table 2 ). After multivariate analysis, the results indicated that ECOG PS ≥ 2 (HR: 2.58; 95%CI, 1.10, 6.04; p = 0.03) and bone metastasis (HR: 2.53; 95%CI, 1.47, 4.37; p = 0.001) were independent risk factors for PFS. In contrast, pretreatment LIPI intermediate/poor (HR: 1.42; 95%CI, 0.84, 2.39; p = 0.19) was not an independent risk factor for PFS in multivariate analysis ( Table 3 ).
Figure 2.
Association between pretreatment LIPI with PFS and OS.
Table 2.
Correlation coefficient between each pair of the variables selected by univariate analysis.
| Correlation coefficients | ECOG PS | Bone metastasis | Pretreatment LIPI | ICI drugs | Stage | Liver metastasis |
|---|---|---|---|---|---|---|
| ECOG PS | – | 0.117 | 0.161 | -0.185 | 0.150 | 0.351 |
| Bone metastasis | 0.117 | – | 0.347 | 0.085 | 0.379 | 0.415 |
| Pretreatment LIPI | 0.161 | 0.347 | – | -0.026 | 0.160 | 0.310 |
| ICI drugs | -0.185 | 0.085 | -0.026 | – | -0.043 | 0.128 |
| Stage | 0.150 | 0.379 | 0.160 | -0.043 | – | 0.333 |
| Liver metastasis | 0.351 | 0.415 | 0.310 | 0.128 | 0.333 | – |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; LIPI, lung immune prognostic index; ICI, immune checkpoint inhibitor.
Table 3.
Univariate and multivariate analysis for PFS in SCLC patients treated with ICIs.
| Variable | Category | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95%CI) | p-value | ||
| Age (year) | ≥60 vs <60 | 1.14 (0.71, 1.84) | 0.59 | — | — |
| Sex | Female vs Male | 1.28 (0.63, 2.58) | 0.50 | — | — |
| Smoking history | Yes vs No | 0.58 (0.33, 1.02) | 0.06 | — | — |
| ICI drugs | PD-L1 inhibitors vs PD-1 inhibitors |
1.35 (0.82, 2.22) | 0.24 | — | — |
| Stage | ED vs LD | 0.99 (0.57, 1.72) | 0.98 | — | — |
| ECOG PS | ≥2 vs 0−1 | 2.71 (1.16, 6.35) | 0.02 | 2.58 (1.10, 6.04) | 0.03 |
| Brain metastasis | Yes vs No | 1.13 (0.64, 1.98) | 0.68 | — | — |
| Liver metastasis | Yes vs No | 1.57 (0.91, 2.69) | 0.10 | — | — |
| Bone metastasis | Yes vs No | 2.81 (1.67, 4.73) | <0.001 | 2.53 (1.47, 4.37) | 0.001 |
| Pretreatment LIPI | Intermediate/Poor vs Good |
1.76 (1.08, 2.89) | 0.03 | 1.42 (0.84, 2.39) | 0.19 |
ICI, immune checkpoint inhibitor; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1; LD, limited disease; ED, extensive disease; ECOG PS, Eastern Cooperative Oncology Group Performance Status; LIPI, lung immune prognostic index; HR, hazard ratio; CI, confidence interval.
As shown in Figure 2 , patients with LIPI good had better OS than those with LIPI intermediate/poor (median: 23.8 vs 13.3 months, p = 0.0006). Univariate analysis showed that PD-1 inhibitor treatment, LD, ECOG PS 0−1, no liver metastasis, no bone metastasis, and pretreatment LIPI good were related to better OS (p < 0.05). All the pairwise correlation coefficients of ICIs drugs, stage, ECOG PS, liver metastasis, bone metastasis, and pretreatment LIPI were below 0.5 ( Table 2 ). After multivariate analysis, the results showed that PD-L1 inhibitors (HR: 2.37; 95%CI, 1.10, 5.11; p = 0.03), ECOG PS ≥ 2 (HR: 6.96; 95%CI, 2.25, 21.55; p = 0.001), liver metastasis (HR: 2.66; 95%CI, 1.19, 5.93; p = 0.02), bone metastasis (HR: 4.61; 95%CI, 2.01, 10.59; p < 0.001), and LIPI intermediate/poor (HR: 2.34; 95%CI, 1.13, 4.86; p = 0.02) were independent risk factors for OS ( Table 4 ).
Table 4.
Univariate and multivariate analysis for OS in SCLC patients treated with ICIs.
| Variable | Category | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95%CI) | p-value | ||
| Age (year) | ≥60 vs <60 | 1.22 (0.65, 2.32) | 0.54 | — | — |
| Sex | Female vs Male | 0.34 (0.08, 1.40) | 0.13 | — | — |
| Smoking history | Yes vs No | 1.74 (0.68, 4.46) | 0.25 | — | — |
| ICI drugs | PD-L1 inhibitors vs PD-1 inhibitors |
2.20 (1.11, 4.35) | 0.02 | 2.37 (1.10, 5.11) | 0.03 |
| Stage | ED vs LD | 3.20 (1.13, 9.03) | 0.03 | 0.98 (0.29, 3.28) | 0.97 |
| ECOG PS | ≥2 vs 0−1 | 6.30 (2.58, 15.36) | <0.001 | 6.96 (2.25, 21.55) | 0.001 |
| Brain metastasis | Yes vs No | 1.83 (0.90, 3.71) | 0.09 | — | — |
| Liver metastasis | Yes vs No | 4.58 (2.39, 8.78) | <0.001 | 2.66 (1.19, 5.93) | 0.02 |
| Bone metastasis | Yes vs No | 5.61 (2.86, 10.97) | <0.001 | 4.61 (2.01, 10.59) | <0.001 |
| Pretreatment LIPI | Intermediate/Poor vs Good |
2.93 (1.54, 5.60) | 0.001 | 2.34 (1.13, 4.86) | 0.02 |
ICI, immune checkpoint inhibitor; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1; LD, limited disease; ED, extensive disease; ECOG PS, Eastern Cooperative Oncology Group Performance Status; LIPI, lung immune prognostic index; HR, hazard ratio; CI, confidence interval.
Subgroup Analysis of Relationship Between LIPI and Survival Outcomes
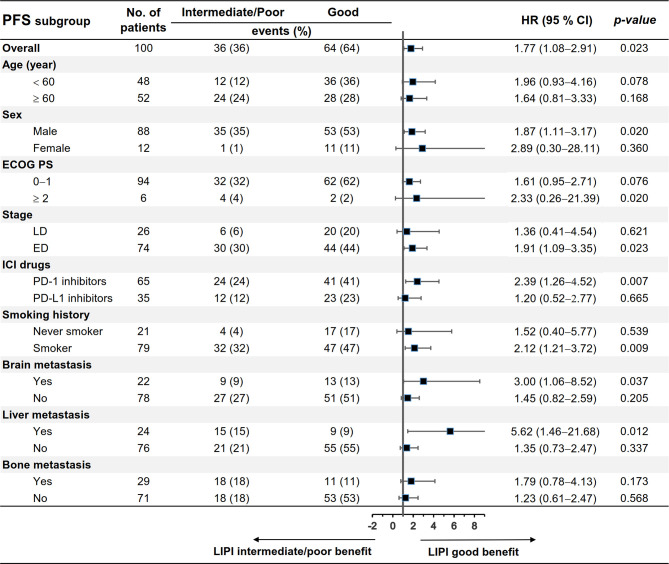
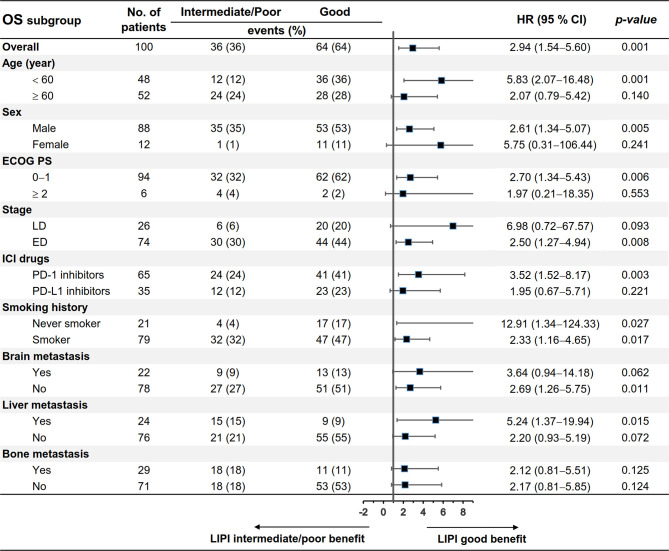
We evaluated the differences in patients’ characteristics between the LIPI good and LIPI intermediate/poor groups. The results indicated that age, liver metastasis, and bone metastasis were not balanced between the two groups (p < 0.05) ( Table 5 ). Subgroup analysis stratified by these characteristics was further conducted. As shown in Figures 3 , 4, LIPI good was associated with better PFS and OS compared with LIPI intermediate/poor in males, smokers, those with ED, those receiving PD-1 inhibitors, and those with liver metastasis (p < 0.05).
Table 5.
Differences of patients’ characteristics between the two groups.
| Characteristics | Pretreatment LIPI | p-value | |
|---|---|---|---|
| Good | Intermediate/poor | ||
| Age (year) | |||
| <60 | 36 | 12 | 0.037 |
| ≥60 | 28 | 24 | |
| Sex | |||
| Male | 53 | 35 | 0.051 |
| Female | 11 | 1 | |
| Stage | |||
| LD | 20 | 6 | 0.154 |
| ED | 44 | 30 | |
| Smoking history | |||
| Never smoke | 17 | 4 | 0.079 |
| Smoke | 47 | 32 | |
| ICI drugs | |||
| PD-1 inhibitors | 41 | 24 | 0.83 |
| PD-L1 inhibitors | 23 | 12 | |
| ECOG PS | |||
| 0−1 | 62 | 32 | 0.184 |
| ≥2 | 2 | 4 | |
| Brain metastasis | |||
| Yes | 13 | 9 | 0.621 |
| No | 51 | 27 | |
| Liver metastasis | |||
| Yes | 9 | 15 | 0.003 |
| No | 55 | 21 | |
| Bone metastasis | |||
| Yes | 11 | 18 | 0.001 |
| No | 53 | 18 | |
ICI, immune checkpoint inhibitor; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1; LD, limited disease; ED, extensive disease; ECOG PS, Eastern Cooperative Oncology Group Performance Status; LIPI, lung immune prognostic index; HR, hazard ratio; CI, confidence interval.
Figure 3.
Subgroup analysis of the association between pretreatment LIPI and PFS.
Figure 4.
Subgroup analysis of the association between pretreatment LIPI and OS.
Discussion
Although ICIs have been established as an important option for treating patients with SCLC, these drugs are not beneficial for all patients. The method of selecting SCLC patients who could respond to immunotherapy remains unclear. Inflammatory markers have been found to be correlated with the survival of patients with lung cancer (27–34). The LIPI, calculated by dNLR and LDH, has been investigated as a prognostic factor for lung cancer. Mezquita et al. (23) reported that pretreatment LIPI was related to clinical outcomes of advanced NSCLC with ICI treatment, but not chemotherapy. However, Kazandjian et al. (35) demonstrated that LIPI was an important prognostic biomarker irrespective of treatment modality in NSCLC. Sonehara et al. (36) first revealed that LIPI could be used as a prognostic biomarker for SCLC patients, but the sample size was small, and the study involved patients without ICIs as first-line treatment. Other previous studies showed that pretreatment LIPI was a prognostic biomarker in ED-SCLC patients receiving chemotherapy or LD-SCLC patients (37, 38). In a recent retrospective study with data from a randomized clinical trial, inflammatory markers, including LIPI, were evaluated in ED-SCLC patients receiving atezolizumab and chemotherapy, and the results showed that LIPI was not an independent prognostic factor (39). However, their study had a small sample size and the patient population in the prospective clinical trial could not represent the entire SCLC population receiving first-line PD-1/PD-L1 inhibitor treatment.
To the best of our knowledge, this is the first study to demonstrate the relationship between pretreatment LIPI and the survival outcomes of SCLC patients receiving first-line ICI treatment. In previous studies, the included cohorts were divided into three groups (LIPI good, LIPI intermediate, and LIPI poor) (37–39). However, no obvious differences were reported between the LIPI intermediate and LIPI poor groups in terms of OS (37). In addition, few untreated patients had a poor LIPI score (11% patients in our study). Therefore, it might be more appropriate if the cohort was separated into two groups (LIPI good and LIPI intermediate/poor). In a previous study on the association of pretreatment LIPI with survival time in advanced hepatocellular carcinoma patients, the population was also divided into two groups (LIPI good and LIPI intermediate/poor) (40). Our findings showed that pretreatment LIPI was associated with PFS and OS in SCLC patients with first-line ICI treatment in univariate analysis. Multivariate analysis showed that pretreatment LIPI was an independent prognostic factor for OS, but not for PFS. However, the negative results of PFS should be interpreted with caution owing to the retrospective nature of this study. PFS was influenced by multiple factors, such as the frequency of evaluation of tumors. Conversely, the difference in OS between the LIPI good group and LIPI intermediate/poor group is more convincing. In addition, although multivariate analysis took many factors into consideration, other factors not included in the analysis, such as PD-L1, TMB and antibiotic therapy (41), may also affect the final results. We further conducted a subgroup analysis by patients’ characteristics, and the results indicated that the LIPI good group had better PFS and OS than the LIPI intermediate/poor group, especially in subgroups of males, smokers, those with ED, those receiving PD-1 inhibitor treatment, and those with liver metastasis, which revealed that the pretreatment LIPI might be prognostic only for specific subgroups of SCLC patients. However, these results need further investigation.
There were several limitations to this study. Firstly, it was a single-center retrospective study with a small sample size; therefore, some confounding factors and selective bias could not be avoided. Because the sample size of the LIPI poor group was too small, we divided the cohort into two groups (LIPI good and LIPI intermediate/poor) rather than three groups (LIPI good, LIPI intermediate, and LIPI poor) to conduct analyses. Secondly, considering the promising results of nivolumab plus chemotherapy as first-line treatment in SCLC patients in the EA5161 study and the accessibility and affordability of PD-L1 inhibitors in Chinese patients, 65% of the patients in this study were treated with PD-1 inhibitors, though only PD-L1 inhibitors have been approved as first-line treatment in SCLC patients by FDA. Thus, the interpretation of our results should be cautious due to drug selecting bias. Lastly, the cutoff values of dNLR and LDH were data-based and calculated using X-tile software, which may not have been optimal. Nevertheless, our study offered a simple and non-invasive method to help identify advanced SCLC patients who could benefit from first-line ICI plus chemotherapy treatment in clinical practice.
Our findings showed the prognostic value of pretreatment LIPI in advanced SCLC patients receiving first-line ICI treatment combined with chemotherapy, especially in males, those with ED, those receiving PD-1 inhibitor treatment, smokers, and those with liver metastasis. Pretreatment LIPI might serve as a useful tool to identify patients who may benefit from this treatment regimen.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study protocol was approved by the Ethics Committee of Chinese PLA general hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YH conceived the idea of this article. LL completed the work of acquisition of data. ZZ and LL shared the task of analysis, interpretation of data, and manuscript writing. All authors participated in discussing and revising the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Military Health Special Research Project Under Grant 20BJZ37.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. N. C. Institute . SEER Cancer Statistics Review. National Cancer Institute; (2009). pp. 1975–2006. Available at: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/755390. [Google Scholar]
- 2. Bernhardt EB, Jalal SI. Small Cell Lung Cancer. Cancer Treat Res (2016) 170:301–22. doi: 10.1007/978-3-319-40389-2_14 [DOI] [PubMed] [Google Scholar]
- 3. Devesa SS, Bray F, Vizcaino AP, Parkin DM. International Lung Cancer Trends by Histologic Type: Male: Female Differences Diminishing and Adenocarcinoma Rates Rising. Int J Cancer (2005) 117:294–9. doi: 10.1002/ijc.21183 [DOI] [PubMed] [Google Scholar]
- 4. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin (2015) 65:87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 5. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing Epidemiology of Small-Cell Lung Cancer in the United States Over the Last 30 Years: Analysis of the Surveillance, Epidemiologic, and End Results Database. J Clin Oncol (2006) 24:4539–44. doi: 10.1200/jco.2005.04.4859 [DOI] [PubMed] [Google Scholar]
- 6. Rossi A, Tay R, Chiramel J, Prelaj A, Califano R. Current and Future Therapeutic Approaches for the Treatment of Small Cell Lung Cancer. Expert Rev Anticancer Ther (2018) 18:473–86. doi: 10.1080/14737140.2018.1453361 [DOI] [PubMed] [Google Scholar]
- 7. Lally BE, Urbanic JJ, Blackstock AW, Miller AA, Perry MC. Small Cell Lung Cancer: Have We Made Any Progress Over the Last 25 Years? Oncol (2007) 12:1096–104. doi: 10.1634/theoncologist.12-9-1096 [DOI] [PubMed] [Google Scholar]
- 8. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab Plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med (2018) 379:2220–29. doi: 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 9. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab Plus Platinum-Etoposide Versus Platinum-Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet (2019) 394:1929–39. doi: 10.1016/s0140-6736(19)32222-6 [DOI] [PubMed] [Google Scholar]
- 10. Randomized Phase II Clinical Trial of Cisplatin/Carboplatin and Etoposide (CE) Alone or in Combination With Nivolumab as Frontline Therapy for Extensive-Stage Small Cell Lung Cancer (ES-SCLC): ECOG-ACRIN Ea5161. Available at: https://ascopubs.org/doi/abs/ 10.1200/JCO.2020.38.15_suppl.9000. [DOI]
- 11. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol (2020) 38(21):2369–79. doi: 10.1200/JCO.20.00793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu H, Chen P, Cai X, Chen C, Zhang X, He L, et al. Efficacy and Safety of PD-L1 Inhibitors Versus PD-1 Inhibitors in First-Line Treatment With Chemotherapy for Extensive-Stage Small-Cell Lung Cancer. Cancer Immunol Immunother (2021). doi: 10.1007/s00262-021-03017-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of Mutational Processes in Human Cancer. Nat (2013) 500:415–21. doi: 10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination With Ipilimumab in Small-Cell Lung Cancer. Cancer Cell (2018) 33:853–61.e4. doi: 10.1016/j.ccell.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol (2021) 39(6):619–30. doi: 10.1200/JCO.20.01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Visser KE, Eichten A, Coussens LM. Paradoxical Roles of the Immune System During Cancer Development. Nat Rev Cancer (2006) 6:24–37. doi: 10.1038/nrc1782 [DOI] [PubMed] [Google Scholar]
- 17. Zhang Z, Li Y, Yan X, Song Q, Wang G, Hu Y, et al. Pretreatment Lactate Dehydrogenase may Predict Outcome of Advanced Non-Small-Cell Lung Cancer Patients Treated With Immune Checkpoint Inhibitors: A Meta-Analysis. Cancer Med (2019) 8:1467–73. doi: 10.1002/cam4.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y, Zhang Z, Hu Y, Yan X, Song Q, Wang G, et al. Pretreatment Neutrophil-to-Lymphocyte Ratio (NLR) may Predict the Outcomes of Advanced Non-Small-Cell Lung Cancer (NSCLC) Patients Treated With Immune Checkpoint Inhibitors (ICIs). Front Oncol (2020) 10:654. doi: 10.3389/fonc.2020.00654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment Neutrophil-to-Lymphocyte Ratio as a Marker of Outcomes in Nivolumab-Treated Patients With Advanced Non-Small-Cell Lung Cancer. Lung Cancer (2017) 106:1–7. doi: 10.1016/j.lungcan.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 20. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-To-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as Prognostic Markers in Patients With non-Small Cell Lung Cancer (NSCLC) Treated With Nivolumab. Lung Cancer (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024 [DOI] [PubMed] [Google Scholar]
- 21. Taniguchi Y, Tamiya A, Isa SI, Nakahama K, Okishio K, Shiroyama T, et al. Predictive Factors for Poor Progression-Free Survival in Patients With non-Small Cell Lung Cancer Treated With Nivolumab. Anticancer Res (2017) 37:5857–62. doi: 10.21873/anticanres.12030 [DOI] [PubMed] [Google Scholar]
- 22. De Castro Am, Navarro A, Perez SC, Martinez A, Pardo N, Hernando A, et al. P3.02c-063 Lactate Dehydrogenase (LDH) as a Surrogate Biomarker to Checkpoint-Inhibitors for Patient With Advanced Non-Small-Cell Lung Cancer (NSCLC). JTO (2017) 12:S1313–S14. doi: 10.1016/j.jtho.2016.11.1858 [DOI] [Google Scholar]
- 23. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol (2018) 4:351–57. doi: 10.1001/jamaoncol.2017.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meyers DE, Stukalin I, Vallerand IA, Lewinson RT, Suo A, Dean M, et al. The Lung Immune Prognostic Index Discriminates Survival Outcomes in Patients With Solid Tumors Treated With Immune Checkpoint Inhibitors. Cancers (Basel) (2019) 11(11):1713. doi: 10.3390/cancers11111713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Socinski MA, Bogart JA. Limited-Stage Small-Cell Lung Cancer: The Current Status of Combined-Modality Therapy. J Clin Oncol (2007) 25:4137–45. doi: 10.1200/jco.2007.11.5303 [DOI] [PubMed] [Google Scholar]
- 26. Camp RL, Dolled-Filhart M, Rimm DL, X-Tile. A New Bio-Informatics Tool for Biomarker Assessment and Outcome-Based Cut-Point Optimization. Clin Cancer Res (2004) 10:7252–59. doi: 10.1158/1078-0432.CCR-04-0713 [DOI] [PubMed] [Google Scholar]
- 27. Paramanathan A, Saxena A, Morris DL. A Systematic Review and Meta-Analysis on the Impact of Preoperative Neutrophil-Lymphocyte Ratio on Long Term Outcomes After Curative-Intent Resection of Solid Tumors. Surg Oncol (2014) 23:31–9. doi: 10.1016/j.suronc.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 28. Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment Neutrophil-to-Lymphocyte Ratio as a Marker of Outcomes in Nivolumab-Treated Patients With Advanced non-Small-Cell Lung Cancer. Lung Cancer (2017) 106:1–7. doi: 10.1016/j.lungcan.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 29. Lu Y, Jiang J, Ren C. The Clinicopathological and Prognostic Value of the Pretreatment Neutrophil-to-Lymphocyte Ratio in Small Cell Lung Cancer: A Meta-Analysis. PloS One (2020) 15:e0230979. doi: 10.1371/journal.pone.0230979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shao N, Cai Q. High Pretreatment Neutrophil-Lymphocyte Ratio Predicts Recurrence and Poor Prognosis for Combined Small Cell Lung Cancer. Clin Transl Oncol (2015) 17:772–8. doi: 10.1007/s12094-015-1289-8 [DOI] [PubMed] [Google Scholar]
- 31. Zhang X, Guo M, Fan J, Lv Z, Huang Q, Han J, et al. Prognostic Significance of Serum LDH in Small Cell Lung Cancer: A Systematic Review With Meta-Analysis. Cancer Biomark (2016) 16:415–23. doi: 10.3233/cbm-160580 [DOI] [PubMed] [Google Scholar]
- 32. Lenci E, Cantini L, Pecci F, Cognigni V, Agostinelli V, Mentrasti G, et al. The Gustave Roussy Immune (GRIm)-Score Variation Is an Early-On-Treatment Biomarker of Outcome in Advanced Non-Small Cell Lung Cancer (NSCLC) Patients Treated With First-Line Pembrolizumab. J Clin Med (2021) 10(5):1005. doi: 10.3390/jcm10051005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Banna GL, Signorelli D, Metro G, Galetta D, De Toma A, Cantale O, et al. Neutrophil-To-Lymphocyte Ratio in Combination With PD-L1 or Lactate Dehydrogenase as Biomarkers for High PD-L1 Non-Small Cell Lung Cancer Treated With First-Line Pembrolizumab. Trans Lung Cancer Res (2020) 9(4):1533–42. doi: 10.21037/tlcr-19-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Banna GL, Cortellini A. The Lung Immuno-Oncology Prognostic Score (LIPS-3): A Prognostic Classification of Patients Receiving First-Line Pembrolizumab for PD-L1 ≥ 50% Advanced Non-Small-Cell Lung Cancer. ESMO Open (2021) 6(2):100078. doi: 10.1016/j.esmoop.2021.100078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kazandjian D, Gong Y, Keegan P, Pazdur R, Blumenthal GM. Prognostic Value of the Lung Immune Prognostic Index for Patients Treated for Metastatic Non-Small Cell Lung Cancer. JAMA Oncol (2019) 5:1481–5. doi: 10.1001/jamaoncol.2019.1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sonehara K, Tateishi K, Komatsu M, Yamamoto H, Hanaoka M. Lung Immune Prognostic Index as a Prognostic Factor in Patients With Small Cell Lung Cancer. Thorac Cancer (2020) 11:1578–86. doi: 10.1111/1759-7714.13432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qi W, Zhao S, Chen J. Prognostic Role of Pretreatment Lung Immune Prognostic Index in Extensive-Stage Small-Cell Lung Cancer Treated With Platinum Plus Etoposide Chemotherapy. Cancer Biomarkers Sect A Dis Markers (2021) 31(2):177–85. doi: 10.3233/CBM-201502 [DOI] [PubMed] [Google Scholar]
- 38. SchnÖller L, KÄsmann L, Taugner J, Abdo R, Eze C, Manapov F. Prognostic Role of Lung Immune Scores for Prediction of Survival in Limited-Stage Small Cell Lung Cancer. In Vivo (Athens Greece) (2021) 35(2):929–35. doi: 10.21873/invivo.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qi W-X, Xiang Y, Zhao S, Chen J. Assessment of Systematic Inflammatory and Nutritional Indexes in Extensive-Stage Small-Cell Lung Cancer Treated With First-Line Chemotherapy and Atezolizumab. Cancer Immunol Immunother CII (2021). doi: 10.1007/s00262-021-02926-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen S, Huang Z, Jia W, Tao H, Zhang S, Ma J, et al. Association of the Pretreatment Lung Immune Prognostic Index With Survival Outcomes in Advanced Hepatocellular Carcinoma Patients Treated With PD-1 Inhibitors. J Hepatocell Carcinoma (2020) 7:289–99. doi: 10.2147/JHC.S277453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cortellini A, Di Maio M, Nigro O, Leonetti A, Cortinovis DL, Aerts JG, et al. Differential Influence of Antibiotic Therapy and Other Medications on Oncological Outcomes of Patients With Non-Small Cell Lung Cancer Treated With First-Line Pembrolizumab Versus Cytotoxic Chemotherapy. J Immunother Cancer (2021) 9(4):e002421. doi: 10.1136/jitc-2021-002421 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.