Abstract
Fluorescent staining of fungi in clinical specimens with the optical brightener Blankophor can be performed concomitantly with maceration of surrounding tissue and may be accelerated by heating. The procedure is suitable for disclosing fungi in gram-stained microscopical mounts and can be used for screening of tissue sections prior to immunofluorescence.
Fluorescent dyes of the diamino stilbene type are used as optical brighteners in washing agents and paper manufacture (1). Their high affinity for β-glycosidically linked polysaccharides (9) renders them useful for tracing glucan and chitin in the fungal cell wall. Optical brighteners have therefore been introduced for the histology of mycoses (2, 3), but they have also proved useful for the detection of fungal elements in all sorts of fresh clinical specimens (6).
Mostly, solutions of the compound Calcofluor white M2R in aqueous potassium hydroxide have been recommended for use at a concentration of 0.1% (wt/vol) (2, 4). A related compound is marketed as the ready-to-use Fungi-Fluor kit (Polysciences, Warrington, Pa.). Other compounds which have been recommended are Uvitex 2B (8) and Blankophor {4,4′-bis[(4-anilino-subst.1,3,5-triazin-2-yl)amino]stilben-2,2′-disulfonic acid} (3, 5). While Calcofluor white tends to form hair-like crystals at strongly alkaline pHs, Blankophor does not (5) and can be maintained as a working solution (a 1,000-fold diluted stock solution in 15 to 20% [wt/vol] aqueous potassium hydroxide) for more than 1 year if stored protected from light in the refrigerator. Fresh clinical specimens (e.g., sediment of bronchoalveolar lavage fluid) were treated with approximately one volume of the working solution, while solid tissues (e.g., from a biopsy specimen) were treated with two volumes. This allowed maceration of cellular debris or tissue by the base with simultaneous staining of fungal elements which are resistant to the base.
Depending on the size and consistency of the sample, maceration required 1 to 30 min for a pea-sized specimen. Alkaline maceration can be accelerated considerably by heating the specimen in the working solution at 56°C in a closed polyethylene vial. Larger specimens, such as whole murine liver or brain, took several hours to liquefy under such conditions. If fungal elements were scarce, gentle centrifugation of the digest and microscopy of the sediment were performed. Even under these conditions, fungal elements stained rapidly and remained physically stable when tested by overnight exposure to the alkaline working solution at 56°C or by storage in a tightly closed vial of liquefied and stained specimens containing Candida or Aspergillus elements for more than one year at room temperature. After it cooled to room temperature, the digest of a specimen was prepared for fluorescence microscopy as a wet mount, using standard slides and coverslips. Drying of the specimen caused artifactual fluorescence. Counterstaining with Evans blue was omitted. Fluorescence microscopic analysis was performed immediately (without rinsing) after preparation of the microscopical mount, using an excitation wavelength below 400 nm and a barrier filter at 420-nm wavelength (Carl Zeiss fluorescence filter kit 2). With an almost-black background, UV excitation yielded a stable bright blue fluorescent image of the fungal elements which changed slightly to a whitish yellow at extremely alkaline pH. In contrast, unbound dye proved to be highly unstable to exposure to UV light, which caused a rapid fading of the background fluorescence. This was thought to be due to a trans-cis isomerization of the unbound fluorescent dye (7).
Blankophor diluted in saline was used as a fast pilot stain for the localization in tissue of fungal elements which were subsequently treated for immunofluorescence by using polyclonal antibodies on sections of solid tissue collected from a patient with culture-proven candidiasis (Fig. 1) and monoclonal antibodies for zygomycosis (Fig. 2). Likewise, Blankophor staining was used after specific immunofluorescence on tissue sections collected from a patient with concomitant aspergillosis and zygomycosis (Fig. 3).
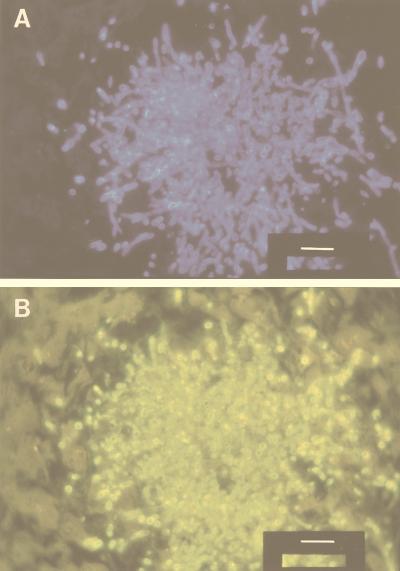
FIG. 1.
Blankophor staining and subsequent immunofluorescence for candidiasis. (A) A section of the liver of a leukemic patient who had succumbed to culture-proven C. albicans mycosis during neutropenia was deparaffinized and stained with Blankophor (1,000-fold diluted stock solution in saline) which proved the mycotic origin of the fatal disease. (B) Subsequently, indirect immunofluorescence for C. albicans was carried out on the same site in the same tissue section, using polyclonal rabbit antibodies against C. albicans (code B143; DAKO, Copenhagen, Denmark) and a suitable fluorescein-labeled second antibody. The bar corresponds to 25 μm.
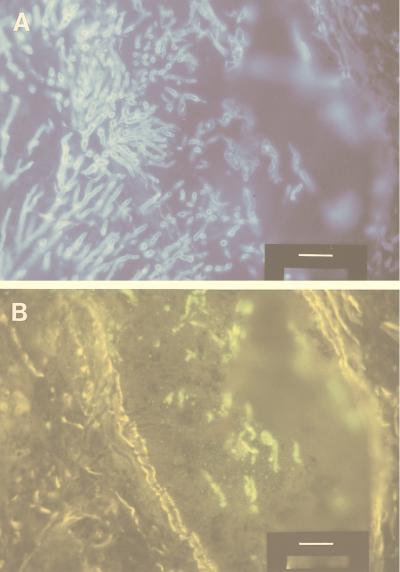
FIG. 2.
Blankophor staining and subsequent immunofluorescence for zygomycosis on a section of the lung of a neutropenic patient who had succumbed to double mycosis caused by culture-proven A. fumigatus and a noncultured zygomycete. (A) After deparaffinization, Blankophor in saline was applied as described in the legend for Fig. 1. (B) Subsequently, indirect immunofluorescence for zygomycosis was performed essentially as described in the legend for Fig. 1, using murine monoclonal antibodies against zygomycetes (code M3565; DAKO) and a suitable fluorescein-labeled second antibody. Comparison of panels A and B reveals the presence of elements of a second fungus at the same site. The bar corresponds to 25 μm.
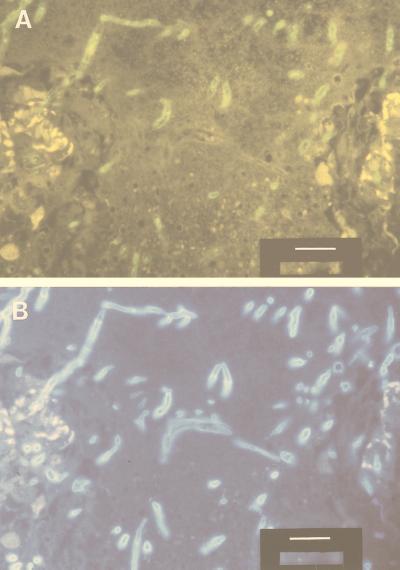
FIG. 3.
Immunofluorescence and subsequent staining with Blankophor of pulmonary tissue from the patient for whom sections are shown in Fig. 2. (A) First, murine monoclonal antibodies against A. fumigatus antigens (code M3564; DAKO) and an appropriate fluorescein-labeled second antibody were used. This yielded evidence of aspergillosis. (B) After subsequent removal of the coverslip, Blankophor working solution (in saline) was applied to the mount which, under appropriate conditions of fluorescence, confirmed the presence of a second fungus at the same site, which probably was a zygomycete (Fig. 2). The bar corresponds to 20 μm.
There was no need to remove the optical brightener before exposure to antibodies, as Blankophor binds exclusively to polysaccharides whereas antibodies usually bind to proteinaceous antigens. Conditions should be tested individually if monoclonal antibodies are to be used. If the tissue is suspected to be infected with a second fungus and specific immunofluorescence for one fungus has already been performed, Blankophor can be applied again to disclose elements of this second fungus (Fig. 3). When appropriate fluorescence filters were used, interference of the fluorescence of Blankophor with that of fluorescein was not encountered (Fig. 1 to 3). This allows the inspection of identical sites of the specimen for both fluorescent markers by change of the fluorescence filters. In fact, photographic documentation for both modes of fluorescence can be obtained concomitantly at the end of the experiment.
Filamentous fungi like aspergilli, which stain poorly by the Gram procedure, may be unveiled on gram-stained microscopic mounts after removal of immersion oil by subsequent Blankophor staining (6). In such mounts, fungi do not stain as rapidly as in fresh specimens. In this case, mounts can be examined for fungi approximately 15 min after application of alkaline Blankophor, and the image of the fungal elements improves over the course of 24 h if the mounts are kept in a moist chamber at room temperature.
Alkaline digests of clinical specimens containing fungal elements can be stored for future microscopical inspection at room temperature in closed polyethylene vials for more than 1 year if protected from light, as has been confirmed in tests with Candida albicans and Aspergillus fumigatus. Complete microscopic mounts may be stored in polyethylene containers in the freezer. Even repeated examination of these stained mounts after warming to room temperature in the closed container did not cause any substantial loss of fluorescence intensity.
Acknowledgments
Blankophor-P (aqueous stock solution 20% [wt/vol] or dry compound) was kindly provided by Bayer AG, Dept. Spezialprodukte, Leverkusen, Germany. We are indebted to U. Vogt, Bayer AG, for helpful comments. The generous donation by H. E. Jensen (The Royal Veterinary University, Copenhagen, Denmark) of monoclonal antibodies for initial trials with zygomycetous tissue is gratefully acknowledged.
REFERENCES
- 1.Anliker R. History of whitening. In: Anliker R, Müller G, editors. Fluorescent whitening agents. Stuttgart, Germany: Georg Thieme; 1975. pp. 12–18. [Google Scholar]
- 2.Hageage G J, Harrington B J. Use of Calcofluor white in clinical mycology. Lab Med. 1984;15:109–112. [Google Scholar]
- 3.Holländer H, Keilig W, Bauer J, Rothemund E. A reliable stain for fungi in tissue sections and clinical specimens. Mycopathologia. 1984;88:131–134. doi: 10.1007/BF00436443. [DOI] [PubMed] [Google Scholar]
- 4.Monheit J E, Cowan D F, Moore D G. Rapid detection of fungi in tissues using calcofluor white and fluorescence microscopy. Arch Pathol Lab Med. 1984;108:616–618. [PubMed] [Google Scholar]
- 5.Monod M, Baudraz-Rosselet F, Ramelet A A, Frenk E. Direct mycological examination in dermatology: a comparison of different methods. Dermatologica. 1989;179:183–186. doi: 10.1159/000248356. [DOI] [PubMed] [Google Scholar]
- 6.Rüchel R, Margraf S. Rapid microscopical diagnosis of deep-seated mycoses following maceration of fresh specimens and staining with optical brighteners. Mycoses. 1993;36:239–242. doi: 10.1111/j.1439-0507.1993.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 7.Vogt, U. (Bayer AG). 1997. Personal communication.
- 8.Wachsmuth E D. Visualization of fungi in histological sections. Virchows Arch B Cell Pathol. 1988;56:1–4. doi: 10.1007/BF02889994. [DOI] [PubMed] [Google Scholar]
- 9.Wood P J, Fulcher R G. A basis for specific detection and histochemistry of polysaccharides. J Histochem Cytochem. 1983;31:823–826. doi: 10.1177/31.6.6841974. [DOI] [PubMed] [Google Scholar]