Abstract
We describe four cases of disseminated infection caused by endemic Penicillium marneffei in human immunodeficiency virus (HIV)-infected patients from the Manipur state of India. The most common clinical features observed were fever, anorexia, weight loss, hepatosplenomegaly, and, more importantly, skin lesions resembling molluscum contagiosum. The diagnosis in each of the four cases was achieved by direct examination of smears, observance of intracellular yeast-like cells multiplying by fission in biopsied tissue from skin lesions, and isolation of the dimorphic P. marneffei in pure culture in each case. In one case, fluorescent antibody studies allowed specific diagnosis. This report documents a new area in which P. marneffei is endemic, located in eastern India, and describes the first occurrence in India of P. marneffei in HIV-infected patients as well as the extension of the areas of P. marneffei endemicity westward to the northeastern state of Manipur.
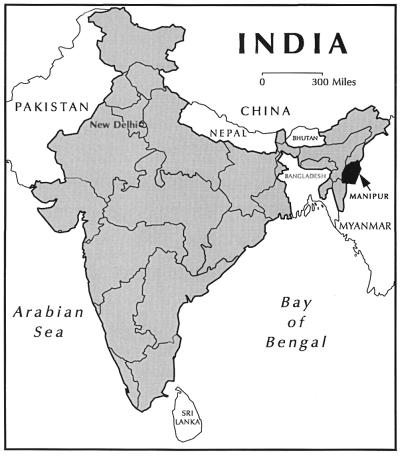
Penicilliosis marneffei is a disseminated and progressive fungal infection caused by Penicillium marneffei, a facultative intracellular pathogen and the only dimorphic species of the genus Penicillium (4, 15). In Southeast Asia, it is the third most common opportunistic infection in human immunodeficiency virus (HIV)-infected patients, following tuberculosis and cryptococcosis, in its prevalence and incidence (9, 17). It is included in the list of the indicator diseases for the diagnosis of AIDS in HIV-infected patients (9, 16). Infections by P. marneffei in HIV-infected individuals are life threatening, with fever, weight loss, anemia, and characteristic skin lesions (9, 17). Infections without timely diagnosis and appropriate treatment have a high rate of mortality (5, 6, 10, 17). Various studies of human infections and of bamboo rats naturally infected or colonized by P. marneffei have indicated that the fungus is endemic in China’s autonomous region of Zhuangzu and the special administrative region of Hong Kong (5, 6, 17), as well as Indonesia (2), Laos (8), Thailand (3, 10, 14, 16), and Vietnam (5, 7, 15). It has been speculated that this fungus would eventually be found in other Asian countries, such as Cambodia, Malaysia, and Myanmar (Burma) (17). In this report, we describe the occurrence of the first four cases of disseminated P. marneffei infection in HIV-infected patients from an eastern state of India, Manipur, which shares borders with Myanmar (Fig. 1), a country that has a high rate of HIV infection.
FIG. 1.
Map of India showing the location of the state of Manipur, which shares a border with Myanmar.
Case 1.
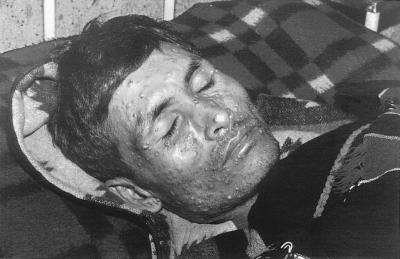
A 27-year-old male presented with recently developed multiple nodules on his face, fever, weight loss, anorexia, and general weakness. General examination revealed multiple molluscum contagiosum-like papules of various sizes with central umbilications mainly on his face, trunk, and upper and lower limbs (Fig. 2). He was diagnosed seropositive for HIV infection. Laboratory investigations revealed mild anemia, leukopenia, and a raised erythrocyte sedimentation rate (ESR). A chest X ray revealed bilateral patchy pneumonitis, and an ultrasonogram (USG) of the abdomen revealed mild hepatomegaly. Fine needle aspiration was carried out on skin lesions on patient’s face and trunk. A portion of the fine needle aspirate (FNA) when cultured yielded a Penicillium sp. producing a red diffusible pigment. Giemsa-stained smears of the aspirate showed numerous intracellular and extracellular oval, elongated or sausage-shaped yeast-like cells, many dividing by fission rather than by a budding process. Three days after hospitalization and before initiation of any form of treatment, the patient died.
FIG. 2.
Photograph of a patient (case 1) showing umbilicated molluscum contagiosum-like lesions caused by disseminated infection by P. marneffei. Printed with permission of the patient’s family.
Case 2.
A male 26-year-old intravenous drug user came in with complaints of fever, weight loss, anorexia, and cough with chest pain of a 3-month duration. He developed multiple molluscum contagiosum-like lesions mainly on his face and trunk. Blood examination revealed moderate anemia, mild leukopenia and an elevated ESR. A chest X ray showed pleural effusion in the left lung, and USG of the abdomen showed hepatosplenomegaly with ascites. The patient was HIV seropositive. Fine needle aspiration of the skin lesions of the face and trunk revealed numerous yeast-like cells multiplying by fission. A portion of the FNA when cultured yielded a Penicillium sp. producing red diffusible pigment, which was presumptively identified as P. marneffei. A presumptive diagnosis of penicilliosis marneffei was made, and oral administration of 400 mg of itraconazole per day was started. After 3 weeks of treatment, the cutaneous lesions were completely healed and the patient was considered clinically cured. His maintenance therapy with itraconazole (200 mg/day) continues.
Case 3.
A 26-year-old male habitual intravenous drug user was seen with complaints of skin lesions on his face after diagnosis of HIV infection. He had already received treatment for cervical lymphadenitis and Pneumocystis carinii pneumonia 8 months earlier. He later developed fever, oral candidiasis, and recurrent episodes of diarrhea. On examination, he had skin lesions resembling those of molluscum contagiosum and hepatosplenomegaly. Biopsy and FNA from his facial skin lesions showed numerous intracellular yeast-like cells multiplying by fission. A portion of the biopsy tissue was used to culture the causal agent. After 10 days of incubation of cultures at 25°C, several colonies of P. marneffei were evident. Treatment with itraconazole (400 mg/day) was instituted, and after 1 month of treatment, the patient was pronounced clinically cured. Maintenance therapy with itraconazole (200 mg/day) is being continued, and he has shown no signs of relapse to date.
Case 4.
A 30-year-old female HIV-positive patient, whose husband had died of HIV infection 5 years earlier, presented with weight loss, weakness, high-grade fever, anorexia, and pallor. She had papular lesions on her face, limbs, and trunk. A biopsy and fine needle aspiration of her skin papules, as well as an upper abdominal lump and cervical lymph nodes, were performed. Biopsy and FNA examination showed typical yeast-like cells dividing by fission, from which a tentative diagnosis of penicilliosis marneffei was made. After hospitalization, she was treated with 400 mg of itraconazole per day for 21 days, followed by 200 mg/day for 2 months along with other supportive therapy. The patient showed clinical improvement after a total dosage of 20.4 g of itraconazole and was clinically cured of her P. marneffei infection.
Histopathologic examination.
Biopsied tissue sections and aspirated material from skin lesions were stained by hematoxylin and eosin, periodic acid-Schiff stain, and Giemsa stain procedures. Microscopic examination of the tissue slides showed granuloma formation, necrosis, and neutrophilic infiltration. Numerous yeast-like cells were present that were round, ovate, elongated, multiplied by fission, and were located intracellularly and extracellularly. They often resembled the yeast cells of Histoplasma capsulatum var. capsulatum in size, shape, and invasion of the reticuloendothelial system. However, in tissue, the yeast-like cells of P. marneffei differ from those of the capsulatum variety of H. capsulatum by their schizogonous mode of reproduction, in contrast to those of H. capsulatum, which reproduce by a budding process.
Mycologic examination.
Samples collected by biopsy or aspiration were cultured on duplicate petri plates containing Sabouraud dextrose agar with chloramphenicol (Sab+C) and Sab+C containing cycloheximide (Sab+C+C). One set of cultures (Sab+C and Sab+C+C) was incubated in the dark at 25°C, and the other set was incubated at 37°C. The cultures were observed every 2 days. The colonies at 25°C were velvety to moist, glabrous, and pink to red in surface color. Some colonies were flat, umbonate, and exhibited radial striations. The reverse of the colonies produced a characteristic intense wine-red pigment that diffused into the medium within 7 days of incubation at 25°C. Colonies at 37°C were moist, yeast-like, white to brownish white, and with much-less-reddish diffusible pigment. Microscopically, mycelial-form cultures produced characteristic conidiophores bearing chains of ovate conidia characteristic of the Penicillium spp. Microscopically, the cultures incubated at 37°C showed a mixture of septate, branching hyphae and many ovate-to-cylindrical yeast-like cells dividing by septation. The fungus was tentatively identified as P. marneffei. Stained and unstained tissue slides prepared from biopsied and aspirated material from the case 4 patient and a subculture of P. marneffei isolated from the same patient were sent to the Fungus Reference Laboratory, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Ga., for specific identification of the etiologic agent.
The Indian isolate was subcultured on Sab+C and the cultures were incubated at 25°C in the dark for 2 weeks. After 2 weeks, the colonies were downy to finely powdery, moist, grayish pink to red, and 34 to 38 mm in diameter. The colonies produced a red pigment which diffused into the medium. Microscopically, the hyphae were hyaline, septate, and branched and bore lateral and terminal conidiophores. The conidiophores consisted of basal stripes, bearing terminal verticils of three to five metulae, bearing four to seven phialides in a verticillate manner. The phialides produced basipetal chains of smooth, spherical-to-ellipsoidal conidia often showing prominent disjunctors (7).
When grown on Pine’s medium and Kelley’s agar, the colonies at 37°C were yeast-like and white to tan with a cerebriform surface. A red diffusible pigment was not observed. Microscopically, growth consisted of yeast-like, ovate, oblong, tubular cells that had divided by a fission rather than budding process. Based on its macroscopic and microscopic morphology, the presence of a characteristic red diffusible pigment, and the dimorphic nature of the isolate, it was identified as P. marneffei. It has been deposited in the Centers for Disease Control and Prevention’s Mycotic Diseases Branch culture collection as B-5830; in the University of Alberta Microfungus Collection and Herbarium, Edmonton, Alberta, Canada (UAMH 9280); and at the Centraalbureau voor Schimmelcultures, Baarn, The Netherlands (CBS 101038).
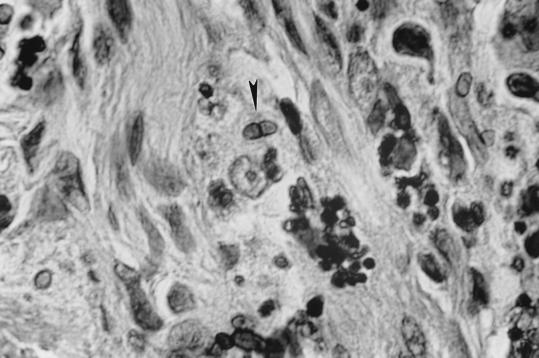
One of the unstained slides was stained by a combination of the Gomori methenamine silver and hematoxylin and eosin stains. Numerous ovate, round, elongated yeast-like cells measuring 2 to 3 by 2 to 6.5 μm were seen intra- and extracellularly. A careful examination revealed a few cells that had divided by fission (Fig. 3).
FIG. 3.
Biopsied skin tissue (case 4) showing yeast-like cells of P. marneffei. Note a cell dividing by fission (arrowhead). Staining was done with Gomori’s methenamine silver and hematoxylin and eosin combination stains. Magnification, ×875.
Immunofluorescence staining.
Deparaffinized tissue sections were digested with 1% trypsin and treated with a rabbit P. marneffei antiglobulin adsorbed with yeast-form cells of H. capsulatum. Binding of the specific primary antiglobulin was determined by further treating the tissue sections with fluorescein isothiocyanate-conjugated goat anti-rabbit globulin. Tissue sections treated with the labeled antiglobulin were examined with a Leitz Ortholux II incident-light fluorescence microscope for yeast-like cells staining with a 1+ or greater intensity (11). P. marneffei was identified in the skin biopsy tissue sections from the case 4 patient treated with the specific P. marneffei antiglobulin in indirect fluorescent antibody tests. Yeast-like cells with and without cross-walls stained with a 2 to 3+ intensity.
The four HIV-positive Indian patients infected by P. marneffei described here were natives of India’s northeastern state of Manipur. None had a history of travel or residence in any of the countries of Southeast Asia in which P. marneffei is known to be endemic. The state of Manipur, one of the six eastern states of India, shares its eastern and southeastern border with Myanmar (Fig. 1). This report describes for the first time the occurrence of penicilliosis marneffei in HIV-positive patients residing in India and thus extends the known areas of P. marneffei endemicity westward into India. The state of Manipur shares common geographic, ethnic, ecologic, and climatic conditions with the Southeastern Asian regions of P. marneffei endemicity.
The most common clinical features observed in this series of four patients were fever, anorexia, weight loss, anemia, hepatosplenomegaly, and skin lesions resembling molluscum contagiosum. Similar observations have also been reported previously by Supparatpinyo et al. (17). At the J. N. Hospital of Manipur, we have presumptively diagnosed 20 additional cases of penicilliosis marneffei in the last few months. In many of these additional AIDS patients infected with P. marneffei in Manipur, molluscum contagiosum-like skin lesions are the prominent feature. Penicilliosis marneffei is being recognized as an important fungal infection following candidiasis, cryptococcosis, and P. carinii pneumonia and could become one of the AIDS-defining diseases in Manipur, as is the case in Thailand and China (17).
Early diagnosis and treatment of three of the four patients with itraconazole enabled clinical cure. One of the four patients (case 1), manifested a rapid advance of his infection and died before any form of antifungal therapy could be initiated. Itraconazole is an effective therapeutic agent. It provides a satisfactory clinical response with minimal side effects, is easy to administer, and is reasonably priced.
The first isolate of P. marneffei was recovered in 1956 from a captive bamboo rat (Rhizomys sinensis) in Vietnam (15). The isolation of P. marneffei from four species of bamboo rats (Cannomys badius, Rhizomys pruinosus, R. sinensis, and Rhizomys sumatrensis) in China, Thailand, and Vietnam suggests that these animals may have value in epidemiologic studies. The geographic distribution of these four species of bamboo rats appears to correlate with the distribution of P. marneffei. The monotypic genus Cannomys, as represented by C. badius, is described as occurring in northern Bangladesh, India (state of Assam, pre-1963), Laos, Myanmar, Nepal, Thailand, and northern Vietnam. During the period of 1963 to 1986, six new states, viz. Arunachal Pradesh, Meghalaya, Mizorum, Nagaland, Manipur, and Tripura, were derived from the original state of Assam (12). The three species of Rhizomys are geographically distributed as follows. R. pruinosus is from northeast India (state of Assam, pre-1963) to southeastern China and down through the Malay peninsula, which comprises Cambodia, Laos, Malaysia, Myanmar, Thailand, and Vietnam. R. sinensis occurs in central and southern China, northern Myanmar, and Vietnam. Finally, R. sumatrensis (Sumatran bamboo rat) is found in Cambodia, Indonesia, Laos, Malaysia, Myanmar, Thailand, and Vietnam (1, 13).
A skin test antigen for P. marneffei is not presently available. Accordingly, studies of the species of rats mentioned above and other related burrowing mammals in Manipur, other areas of northeastern India, and Bangladesh for the presence of P. marneffei could play an important role in providing valuable information regarding the ecologic niche and distribution of P. marneffei in the northeastern parts of India.
REFERENCES
- 1.Ajello L, Padhye A A, Sukroongreung S, Nilakul C H, Tantimavanic S. Occurrence of Penicillium marneffei infections among wild bamboo rats in Thailand. Mycopathologia. 1995;131:1–8. doi: 10.1007/BF01103897. [DOI] [PubMed] [Google Scholar]
- 2.Ancelle T, Dupouy-Camet J, Pujol F, Nassif X, Ferradini L, Lapierre J. Un cas de penicilliose disseminee a Penicillium marneffei chez un malade atteint de SIDA. Bull Soc Fr Mycol Med. 1988;17:73–76. [PubMed] [Google Scholar]
- 3.Chiewchanvit S, Mahanupab P, Hirunsri P, Vanittanakom N. Cutaneous manifestations of disseminated Penicillium marneffei mycosis in five HIV-infected patients. Mycoses. 1991;34:245–249. doi: 10.1111/j.1439-0507.1991.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 4.Cooper C R, McGinnis M R. Pathology of Penicillium marneffei. An emerging acquired immunodeficiency syndrome-related pathogen. Arch Pathol Lab Med. 1997;121:798–804. [PubMed] [Google Scholar]
- 5.Deng Z, Connor D H. Progressive disseminated penicilliosis caused by Penicillium marneffei: report of eight cases and differentiation of the causative organism from Histoplasma capsulatum. Am J Clin Pathol. 1985;84:323–327. doi: 10.1093/ajcp/84.3.323. [DOI] [PubMed] [Google Scholar]
- 6.Deng Z, Ribas J L, Gibson D W, Connor D H. Infections caused by Penicillium marneffei in China and Southeast Asia: review of eighteen published cases and report of four more Chinese cases. Rev Infect Dis. 1988;10:640–652. doi: 10.1093/clinids/10.3.640. [DOI] [PubMed] [Google Scholar]
- 7.Drouhet E. Penicilliosis due to Penicillium marneffei: a new emerging systemic mycosis in AIDS patients travelling or living in Southeast Asia. Review of 44 cases reported in HIV infected patients during the last 5 years compared to 44 cases of non AIDS patients reported over 20 years. J Mycol Med. 1993;4:195–224. [Google Scholar]
- 8.Drouhet, E. 1998. Personal communication.
- 9.Duong T A. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. 1996;23:125–130. doi: 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 10.Jayanetra P, Nityanant P, Ajello L, Padhye A A, Lolekha S, Atichartakarn V, Vathesatogit P, Sathaphatayavongs B, Prajaktam R. Penicilliosis marneffei in Thailand: report of five human cases. Am J Trop Med Hyg. 1984;33:637–644. doi: 10.4269/ajtmh.1984.33.637. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman L, Standard P G, Anderson S A, Jalbert M, Swisher B L. Development of specific fluorescent-antibody test for tissue form of Penicillium marneffei. J Clin Microbiol. 1995;33:2136–2138. doi: 10.1128/jcm.33.8.2136-2138.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merriam Webster’s geographical dictionary, 3rd ed. 1997. Merriam-Webster, Inc., Springfield, Mass.
- 13.Nowak R M. Walker’s mammals of the world. 5th ed. Baltimore, Md: The John Hopkins University Press; 1991. [Google Scholar]
- 14.Sathapatayavongs B, Damrongkitchaiporn S, Saengditha P, Kiatboonsri S, Jayanetra P. Disseminated penicilliosis associated with HIV infection. J Infect. 1989;19:84–85. doi: 10.1016/s0163-4453(89)95136-0. [DOI] [PubMed] [Google Scholar]
- 15.Segretain G. Description d’une nouvelle espece de Penicillium marneffei n. sp. Bull Soc Mycol Fr. 1959;75:412–416. [Google Scholar]
- 16.Supparatpinyo K, Chiewchanvit S, Hirunsri P, Uthammchai C, Nelson K E, Sirisanthana T. Penicillium marneffei infection in patients infected with human immunodeficiency virus. Clin Infect Dis. 1992;14:871–874. doi: 10.1093/clinids/14.4.871. [DOI] [PubMed] [Google Scholar]
- 17.Supparatpinyo K, Khamwan C, Baosoung V, Nelson K E, Sirisanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]