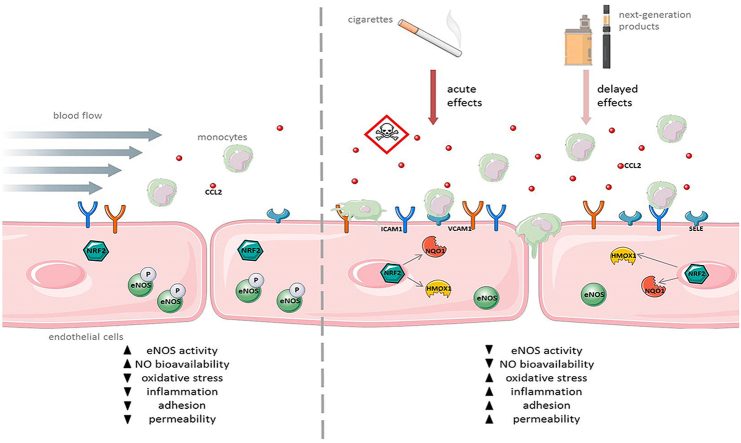
Abstract
Tobacco smoking and hemodynamic forces are key stimuli for the development of endothelial dysfunction. As an alternative to smoking, next generation tobacco and nicotine products (NGP) are now widely used. However, little is known about their potential pro-inflammatory and atherogenic effects on the endothelium. In this study, we analyzed key parameters of endothelial function after exposure to aqueous smoke extracts (AqE) of a heated tobacco product (HTP), an electronic cigarette (e-cig), a conventional cigarette (3R4F) and pure nicotine. All experiments were performed under atheroprotective high laminar or atherogenic low flow with primary human endothelial cells.
Treatment with 3R4F, but not alternative smoking products, reduced endothelial cell viability and wound healing capability via the PI3K/AKT/eNOS(NOS3) pathway. Laminar flow delayed detrimental effects on cell viability by 3R4F treatment. 3R4F stimulation led to activation of NRF2 antioxidant defense system at nicotine concentrations ≥0.56 μg/ml and increased expression of its target genes HMOX1 and NQO1. Treatment with HTP revealed an induction of HMOX1 and NQO1 at dosages with ≥1.68 μg/ml nicotine, whereas e-cig and nicotine exposure had no impact. Analyses of pro-inflammatory genes revealed an increased ICAM1 expression under 3R4F treatment. 3R4F reduced VCAM1 expression in a dose-dependent manner; HTP treatment had similar but milder effects; e-cig and nicotine treatment had no impact. SELE expression was induced by 3R4F under static conditions. High laminar flow prevented this upregulation. Stimulation with laminar flow led to downregulation of CCL2 (MCP-1). From this downregulated level, only 3R4F increased CCL2 expression at higher concentrations. Finally, under static conditions, all components increased adhesion of monocytes to endothelial cells. Interestingly, only stimulation with 3R4F revealed increased monocyte adhesion under atherosclerosis-prone low flow.
In conclusion, all product categories activated anti-oxidative or pro-inflammatory patterns. NGP responses were typically lower than in 3R4F exposed cells. Also, 3R4F stimulation led to an impaired endothelial wound healing and induced a pro-inflammatory phenotype compared to NGP treatment.
Keywords: 3R4F aqueous cigarette smoke extract (3R4F AqE), Next generation tobacco and nicotine products (NGP), Heated tobacco product (HTP), Electronic cigarette (e-cig), Endothelial dysfunction, Laminar flow, Monocyte adhesion, Wound healing
Graphical abstract
Highlights
-
•
3R4F, but not NGP and nicotine, impair endothelial cell viability and wound healing.
-
•
All tested product categories induce anti-oxidative enzymes and adhesion molecules in HUVEC.
-
•
Only 3R4F increases monocyte adhesion to endothelial cells under low laminar flow.
-
•
Protective effects of high laminar flow counteract with deleterious effects of smoking.
1. Introduction
Cardiovascular diseases are the leading cause of death globally. Major cardiovascular diseases are coronary heart disease affecting the blood vessels supplying the heart, cerebrovascular diseases affecting the blood vessels supplying the brain and peripheral arterial disease mainly affecting blood vessels supplying the arms and legs [1]. Most of these diseases are the consequence of atherosclerosis. The first step in the initiation of atherosclerosis is the development of endothelial dysfunction. Endothelial cells play a critical role in the control of vascular function and lacking of regulatory capacity leads to dysregulation of vascular homeostasis, inflammation and finally to the development of endothelial dysfunction [2]. This state is characterized by an imbalance between vasodilation and vasoconstriction, a pro-inflammatory phenotype of the endothelial cells, increased adhesion of monocytes and reduced bioavailability of nitric oxide (NO) and leads to plaque formation in the blood vessels [[3], [4], [5]].
Local hemodynamic forces are a key factor in the development of atherosclerosis. Low or disturbed blood flow has a pro-atherogenic effect on the endothelium at medium and large arteries, promoting differentiation into a pro-inflammatory phenotype [[6], [7], [8], [9]]. This type of flow typically occurs at the near side of arterial vessel branching [10]. High laminar flow has an atheroprotective effect on the endothelium resulting in a reduced response of endothelial cells to cardiovascular risk factors [11]. Alterations of flow will be immediately recognized by endothelial cells via transducing mechanical forces into biochemical signals [12]. This leads to changes in gene expression of different cellular systems, the increased release of vasoactive substances like nitric oxide (NO) and decreases permeability to plasma lipoproteins as well as adhesion of leukocytes [13,14].
Several risk factors for cardiovascular diseases are known. Importantly, behavioral risk factors are responsible for about 80% of coronary heart disease and cerebrovascular diseases. By life style changes, most of these diseases could be prevented. One of the most important behavioral risk factor of atherosclerosis as the underlying process of heart attack and stroke is tobacco smoking [[15], [16], [17]]. Stimulation with 3R4F aqueous smoke extract (AqE) leads to development of endothelial dysfunction and induces a multiplicity of changes in gene expression [[18], [19], [20]]. Increased expression of adhesion molecules promotes adhesion of monocytes to the endothelium which plays a key role within the further development of atherosclerotic plaques [20]. In addition, we have previously shown that improved endothelial wound healing under high laminar flow was inhibited by 3R4F AqE in a dose-dependent manner through the AKT/eNOS(NOS3) pathway [21].
One strategy for tobacco harm reduction is the development of next generation tobacco and nicotine products [22]. Various products for inhalation of nicotine (e‐cigarettes) are available on the market [23,24]. Recently, other potential less risky tobacco products based on heating rather than burning tobacco have been launched [[25], [26], [27]]. Both product types are developed closely to consumer preferences and are supposed to have a less potential to provoke diseases from smoking compared to conventional cigarette smoking [[28], [29], [30], [31]].
Own previous work showed the activation of major atherosclerotic key parameters by 3R4F AqE [21]. Within this process, high laminar flow could reduce the harmful effects of 3R4F AqE to a certain degree. In this follow-up study, we analyzed the impact of next generation tobacco and nicotine products on key parameters of endothelial function like monocyte adhesion, wound healing and the underlying protective molecular mechanisms.
2. Materials and methods
2.1. Cell culture
The collection of primary human umbilical vein endothelial cells (HUVEC) was approved by the ethical review board of the Medical Faculty Carl Gustav Carus of the TU Dresden (EK124082003). Primary cultures of HUVEC were isolated using 0.5% collagenase II solution (Worthington Biochemical Corp., Lakewood NJ, USA) [32,33]. Isolated HUVEC were cultured on 2% gelatine-coated plates in Medium 199 (Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% fetal calf serum (Biochrom, Berlin, Germany), 0.5% self-isolated retina calf eye growth supplement [34], 100 000 U/l Penicillin (Thermo Fisher Scientific, Waltham, MA, USA), 100 mg/l Streptomycin (Thermo Fisher Scientific, Waltham, MA, USA) and 500 μg/l Amphotericin B (Thermo Fisher Scientific, Waltham, MA, USA). Monocytic cell line THP-1 (ATCC# TIB-202) was provided by the Department of Cardiology, TU Dresden. Cultivation occurred in RPMI-1640 (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal calf serum. Cultivation and all experiments were done in a humidified environment with 5% CO2 at 37 °C. All experiments, unless otherwise indicated, were conducted 24 h after reaching confluence.
2.2. Aqueous smoke extract (AqE) production and stimulation
3R4F scientific conventional reference cigarettes (3R4F; University of Kentucky, Lexington, KY, USA) were used as reference cigarettes in this study. In addition, a heated tobacco product (HTP; iQOS, Philip Morris International, Lausanne, Switzerland) and a nicotine product (e-cig; VYPE ePen2, British American Tobacco, London, UK) were used in this study (for detailed information please see Table 1). 3R4F cigarettes were conditioned at 22 °C and 60% relative humidity for 48 h prior to use. iQOS consumables were stored at room temperature in original packing. Prior to smoking, iQOS was conditioned in closed pack with outer cellophane wrapper removed at 22 °C and 60% relative humidity for 48 h. Both alternative devices were fully charged before use and fresh consumables were used. All tested products were machine-puffed on a Borgwaldt-KC RM20H smoking engine (Borgwaldt KC, Hamburg, Germany), following the corresponding puffing regime (Table 1). All aqueous smoke extracts (AqE) were generated by bubbling a total of 10 puffs, with a puff volume of 55 ml each, through a glass impinger containing 20 ml Phenol Red-free M199 medium (without supplements; Thermo Fisher Scientific, Waltham, MA, USA). This procedure provided a stock aqueous smoke extract (100%) which was stored at -70 °C until further usage. AqE nicotine concentrations were determined as previously described and are shown in Table 2 [35].
Table 1.
Specifications of test products and parameters for aqueous smoke extracts (AqE) generation.
| 3R4F | e-cig | HTP | nic | |
|---|---|---|---|---|
| Product | 3R4F reference cigarette | VYPE ePen 2.0 | iQOS | nicotine solution |
| Manufacturer | University of Kentucky, Lexington, KY, USA | British American Tobacco, London, UK | Philip Morris International, Lausanne, Switzerland | Sigma-Aldrich, Munich, Germany |
| Consumables | n/a | Blended Tobacco e-liquid | Marlboro Essence HeatSticks | n/a |
| Nicotine content | 0.73 mg/cig | 18.00 mg/ml | 0.37 mg/stick | 1.00 g/ml |
| Puff Regime | HCI | CRM No. 81 | HCIm | n/a |
| Puff Volume | 55 ml | 55 ml | 55 ml | n/a |
| Puff Duration | 2 s | 3 s | 2 s | n/a |
| Puff Frequency | 30 s | 30 s | 30 s | n/a |
| Puff Profile | Bell | Square | Bell | n/a |
| Vent Blocking | Yes | n/a | No | n/a |
| Number of Puffs | 10 puffs | 10 puffs | 10 puffs | n/a |
| Capture Solvent | M199 w/o Phenol red | M199 w/o Phenol red | M199 w/o Phenol red | n/a |
| Volume of Capture Solvent | 20 ml | 20 ml | 20 ml | n/a |
| Settings | 90° vaping angle | High power (4.4 W); 4 s Button activated (1 s pre-activation followed by 3 s activation during puffing); 45° vaping angle |
4 s Button activated followed by 20 s pre-heating (no button pressed) prior 1st puff; 90° vaping angle |
n/a |
Table 2.
Amount of nicotine in different concentrations of aqueous extracts of tobacco and nicotine products.
| nicotine amount [μg/ml] |
concentration [%] |
||
|---|---|---|---|
| 3R4F | e-cig | HTP | |
| 0.56 | 10.0 | 11.5 | 18.5 |
| 1.12 | 20.0 | 23.0 | 37.0 |
| 1.68 | 30.0 | 34.4 | 55.5 |
| 2.68 | 47.8 | 54.8 | 88.3 |
| 4.32 | 76.9 | 88.3 | 142.2 |
| 4.96 | 88.3 | 101.4 | 163.3 |
| 5.613 | 100.0 | 114.8 | 184.9 |
Statistics: n ≥ 9.
HUVEC were exposed to increasing AqE dosages of the described scientific conventional reference cigarette (3R4F), heated tobacco product (HTP) and nicotine product (e-cig). Pure nicotine (nic) in different concentrations has been used as additional control. Due to the presence of media supplements (11.7 vol %), the maximum possible nicotine concentrations of used AqE within the cell culture media are: 3R4F = 4.96 μg/ml; HTP = 2.68 μg/ml; e-cig = 4.32 μg/ml; nic = 4.96 μg/ml (88.3 vol %; Table 2). Prior to treatment with AqE and nicotine, HUVEC were cultivated in Phenol Red-free Medium 199, supplemented with 10% fetal calf serum and 0.5% self-isolated retina calf eye growth supplement for 90 min. Stimulation with test compounds was performed in a nicotine concentration range from 0.56 to 4.96 μg/ml for the indicated time period with or without application of different types of flow. Each sample was accompanied by a control from the same cell preparation, incubated for the same period of time, without stimulation of AqE and nicotine or application of flow (static time-matched controls).
2.3. Application of different types of flow
HUVEC were subjected to flow using the ibidi pump system (ibidi, Martinsried, Germany) [36] or a cone-and-plate viscometer [[37], [38], [39], [40]]. High laminar flow was defined as 30 dyn/cm2 and low laminar flow as 1 dyn/cm2. 90 min prior to the application of flow, cells were cultivated in Phenol Red-free Medium 199, supplemented with 10% fetal calf serum and 0.5% growth supplement.
2.4. Analysis of cell viability of endothelial cells
The amount of ATP is an indicator of metabolically active cells and directly proportional to the number of cells present in culture [41]. By quantification of the amount of ATP present using CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Mannheim, Germany), the number of viable cells in culture can be determined. HUVEC were stimulated with the different test substances in a nicotine concentration range from 0 to 4.96 μg/ml for up to 24 h under static or flow conditions. Afterwards, the cell viability assay was performed and the emitted luminescence signal was measured using a luminometer.
2.5. Monocyte-endothelial cell adhesion assay under flow
HUVEC were seeded in μ-Slide I Luer (ibidi, Martinsried, Germany) and allowed to adhere for 3 h under static conditions. After adherence, HUVEC were perfused with Phenol Red-free Medium 199, supplemented with 10% fetal calf serum and 0.5% growth supplement with a flow rate of 15 to 30 dyn/cm2 until reaching confluence (24 h), followed by 24 h pre-incubation with the indicated type of flow. Subsequently, cells were stimulated for 24 h with AqE or nicotine in combination with or without flow.
Human monocytes were cultured in advance in RPMI-1640, supplemented with 10% fetal calf serum. Directly prior to the adhesion assay, monocytes were stained using a 10 μM Cell Tracker Orange CMRA-PBS solution according to manufacturer's instructions (Life Technologies, Darmstadt, Germany). Fluorescently labelled monocytes were resuspended in Phenol Red-free Medium 199, supplemented with 0.5% growth supplement. 175 000 monocytes/ml were added to the prestimulated HUVEC. For equal distribution of monocytes within the media laminar flow of 1 dyn/cm2 for 2 min was applied. After an incubation period of 20 min under static conditions, non-adherent monocytes were removed by application of 2 dyn/cm2 for 2 min. Cells were fixed using 4% formaldehyde/PBS solution (Merck, Darmstadt, Germany) for 20 min. Monocyte-endothelial interactions were visualized by 10 × 10 mosaic fluorescence/phase contrast images, taken using a Zeiss Axio Observer.Z1 ApoTome microscope (Carl Zeiss, Jena, Germany).
For evaluation of the experiments, each sample was accompanied by a control from the same cell preparation, incubated for the same time period, without stimulation of AqE and nicotine or application of flow (static time-matched controls).
2.6. Wound healing assay
A wound in the endothelial cell layer is considered as an initial stimulus for the development of atherosclerotic plaques (response-to injury model) [5]. To mimic this situation in an in vitro setup, HUVEC were cultivated in cell culture dishes containing cell culture inserts (ibidi, Martinsried, Germany), to create a defined wound area. Subsequently, cells were exposed to AqE and nicotine under high laminar flow conditions (30 dyn/cm2) using a plate-and-cone viscometer. Direction of flow was perpendicular to the wound in the cell layer. Each sample was accompanied by a static control without further treatment (static time-matched controls). The increasing closure of the wound was documented for a time period of 8 h and quantified using ImageJ [42].
2.7. Detection of nitric oxide
Nitric oxide (NO) was measured by Griess reaction as described previously [43]. In brief, 100 μl of supernatant from treated HUVEC was transferred to a 96-well plate. 50 μl of 2% aminobenzenesulphoamide in 2.5% phosphoric acid was added and incubated for 5 min, protected from light. Afterwards, 0.2% NED-reagent (N-(1-naphthyl) ethylenediamine dihydrochloride) was added to each well and incubated for 10 min, protected from light. Nitrite release was characterized by an increase in absorbance at 540 m and compared with a nitrite standard (0–100 μM) using linear regression analysis.
2.8. Real-time PCR
Total RNA from treated HUVEC was isolated using High Pure RNA Isolation Kit (Roche Diagnostics, Mannheim, Germany). Reverse transcription of mRNA into cDNA was performed with SuperScript II Reverse Transcriptase according to manufacturer's instructions (Thermo Fisher Scientific, Waltham, MA, USA) using 500 ng of total RNA and random hexamer primers. Quantification was performed by real-time PCR (7500 Fast Real-Time PCR System, Thermo Fisher Scientific, Waltham, MA, USA) using GoTaq qPCR Master Mix (Promega, Mannheim, Germany) with specific primers (Sigma-Aldrich, Munich, Germany) (for primer sequences see Table 3). POLR2A or TBP was used as reference gene for cDNA content normalization. Amplification started with an initial denaturation step at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s, specific annealing and extension for each gene for 60 s. Melt curve analysis was performed following every run to ensure a single amplified product in each reaction. Analysis of the raw data was performed with the 7500 Software Version 2.3 (Applied Biosystems by Life Technologies, Darmstadt, Germany). Data were evaluated using a mathematical model of relative expression ratio in real-time PCR under constant reference gene expression [44].
Table 3.
Primers used for analysis of human gene expression by real-time PCR.
|
target gene |
primer | sequence (5'→ 3′) |
|---|---|---|
| POLR2A | sense | ACCTGCGGTCCACGTTGTGT |
| antisense | CCACCATTTCCCCGGGATGCG | |
| TBP | sense | CGCCGGCTGTTTAACTTCG |
| antisense | AGAGCATCTCCAGCACACTC | |
| eNOS (NOS3) | sense | GAACCTGTGTGACCCTCACC |
| antisense | TGGCTAGCTGGTAACTGTGC | |
| NRF2 | sense | CCCAATTCAGCCAGCCCAGC |
| antisense | AACGGGAATGTCTGCGCCAA | |
| HMOX1 | sense | CGGATGGAGCGTCCGCAACC |
| antisense | TCACCAGCTTGAAGCCGTCTCG | |
| NQO1 | sense | CCCCGGACTGCACCAGAGC |
| antisense | CTGCAGCAGCCTCCTTCATGGC | |
| ICAM1 | sense | ACCATGGAGCCAATTTCTCG |
| antisense | GCGCCGGAAAGCTGTAGATG | |
| CCL2 | sense | CTCTCGCCTCCAGCATGAAA |
| antisense | AGGTGACTGGGGCATTGATT | |
| VCAM1 | sense | TGTGCCCACAGTAAGGCAGGC |
| antisense | AGCTGGTAGACCCTCGCTGGA | |
| SELE | sense | AGCCCAGAGCCTTCAGTGTA |
| antisense | CTCCAATAGGGGAATGAGCA |
Abbreviations: POLR2A: RNA polymerase II subunit A; TBP: TATA-box binding protein; eNOS (NOS3): endothelial NO synthase; NRF2: nuclear factor erythroid 2-related factor 2; HMOX1: heme oxygenase (decycling) 1; NQO1: NAD(P)H quinone dehydrogenase 1; ICAM-1: intercellular adhesion molecule-1; CCL2: chemokine (C-C motif) ligand 2/MCP-1: monocyte-chemoattractant protein-1; VCAM-1: vascular cell adhesion molecule-1; SELE: selectin E.
2.9. Western blot
Whole cell extracts were prepared using RIPA Buffer (Cell Signaling, Leiden, Netherlands) including Protease Inhibitor Cocktail (Sigma-Aldrich, Munich, Germany). Before lysis, cells were washed twice with ice-cold PBS containing phosphatase inhibitors (Active Motif, Carlsbad, CA, USA). Protein concentration was determined by BCA Protein Assay Reagent (Perbio Science, Bonn, Germany/Thermo Fisher Scientific, Waltham, MA, USA) to ensure equal amounts of protein used for subsequent analysis. Proteins were denaturated for 10 min at 95 °C, separated by 8% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated with the following antibodies for 1 h: P-eNOS(NOS3, Ser1177, Cell Signaling, Leiden, The Netherlands), eNOS (BD Transduction Laboratories, Heidelberg, Germany) and ACTB as loading control. Afterwards, membranes were incubated for 1 h with anti-rabbit (Acris, Herford, Germany) or anti-mouse (Life Technologies, Darmstadt, Germany) IgG HRP-conjugated secondary antibodies. Protein expression was detected with Western Lightning Chemiluminescence Reagent Plus (PerkinElmer, Rodgau, Germany) and quantified using AIDA Image Analyzer software (Raytest, Berlin, Germany).
2.10. Evaluation of data and statistical analysis
If not otherwise stated, time-matched controls were used for normalization of the data. All experiments, unless otherwise indicated, were started 24 h after reaching confluence.
Data are shown as mean ± standard deviation (SD). Statistical analysis was performed by One-Way ANOVA (SigmaPlot 13.0, Systat Software, Inc., San Jose, CA, USA). A value of p < 0.05 was considered as statistically significant.
3. Results
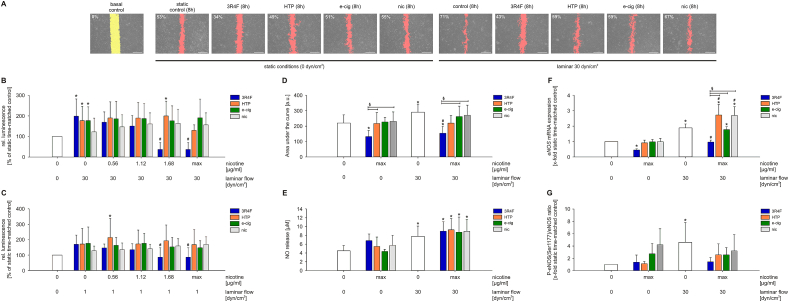
3.1. 3R4F stimulation, but not alternative smoking products, reduced endothelial cell viability and wound healing capability via PI3K/AKT/eNOS pathway
First, putative cytotoxic effects of AqE produced from the different tobacco/nicotine products and pure nicotine were studied. HUVEC were exposed to increasing dosages of 3R4F, HTP, e-cig AqE and pure nicotine (nic) under laminar flow conditions (Fig. 1B and C). High and low laminar flow increased cell viability compared to static conditions. This increased viability was reduced by stimulation with 3R4F containing 1.68 μg/ml nicotine. 3R4F doses higher than 1.68 μg/ml nicotine were leading to detachment of the cells under flow and could therefore not be examined.
Fig. 1.
Deleterious effects of next generation tobacco and nicotine products on primary human endothelial cells. (A) Representative pictures of wound healing after 8 h including degree of wound healing in % are shown. Direction of flow was perpendicular to the wound in the cell layer, n ≥ 8. Evaluation of endothelial cell viability under (B) high laminar flow conditions (30 dyn/cm2) or (C) low laminar flow conditions (1 dyn/cm2) using CellTiter-Glo Luminescent Cell Viability Assay. HUVEC monolayers were stimulated with aqueous extracts of the indicated tobacco products in a nicotine concentration range from 0.56 to 4.96 μg/ml for 24 h, n ≥ 3. (D) Evaluation of area under the curve data of endothelial wound healing capability under combined stimulation with aqueous extracts of different tobacco products (3R4F, HTP, e-cig and nic) and high laminar flow, n ≥ 8. (E) Regulation of endothelial nitric oxide (NO) release in response to flow and AqE stimulation. NO release was determined by Griess assay, n ≥ 4. (F) mRNA expression of eNOS in HUVEC after exposure to high laminar flow in combination with aqueous extracts for 8 h, n ≥ 4. (G) eNOS phosphorylation status after 8 h of AqE treatment under static and high laminar flow conditions, n ≥ 4. max = maximum nicotine concentration (Table 2). Data are shown as mean ± SD. Statistics: One-Way ANOVA, *p < 0.05 vs. static control, #p < 0.05 vs. time-matched control (0 μg/ml nicotine, 1 respectively 30 dyn/cm2), §p < 0.05.
Stimulation with HTP, e-cig and pure nicotine solution did not reduce the flow-dependent increase of cell viability, even if used maximum possible concentrations (HTP = 2.68 μg/ml; e-cig = 4.32 μl/ml; nic = 4.96 μg/ml). Therefore, stimulations with 3R4F in combination with laminar flow could only be carried out up to dosages of 1.12 μg/ml nicotine for the following experiments (Fig. 1A, D-G and 2–4). Stimulation with HTP, e-cig and pure nicotine solution could be performed up to the maximum possible concentrations (Table 2).
A wound in the endothelial cell layer is considered as an initial stimulus for the development of atherosclerotic plaques (response-to-injury model). Wound healing of HUVEC monolayers was analyzed in response to AqE of 3R4F, HTP and e-cig and pure nicotine. In addition, high laminar flow was applied to the endothelial cells.
Representative pictures of the endothelial cell layer after stimulation with the indicated AqE and and laminar flow are shown in Fig. 1A. Stimulation with atheroprotective high laminar flow for 8 h led to significantly accelerated wound closure (Fig. 1D). Additional treatment with 3R4F inhibited the positive effects of high laminar flow on wound healing even below the wound healing capability of the static time-matched control. A treatment with HTP or e-cig also inhibited the protective flow effect but not in the same degree as conventional cigarette smoke extract. Pure nicotine stimulation had no effect on the endothelial wound healing capability compared to the control.
As described in a previous study [21], the PI3K/AKT/eNOS pathway seems to be involved in the impaired endothelial wound healing under treatment with smoking products. As shown in Fig. 1E, the stimulation with AqE of smoking products and nicotine solution had no impact on the increased NO release of endothelial cells after high laminar flow stimulation. Interestingly, treatment with 3R4F completely abolished the protective effect of high laminar flow and showed decreased eNOS mRNA levels under static and flow conditions. Treatment with alternative products and nicotine had no impact on eNOS mRNA expression compared to the controls (Fig. 1F). The activation of eNOS is mediated by phosphorylation. Therefore, the phosphorylation of Ser1177 was analyzed. In Fig. 1G, an induced eNOS phosphorylation under atheroprotective flow is shown which is prohibited by 3R4F stimulation.
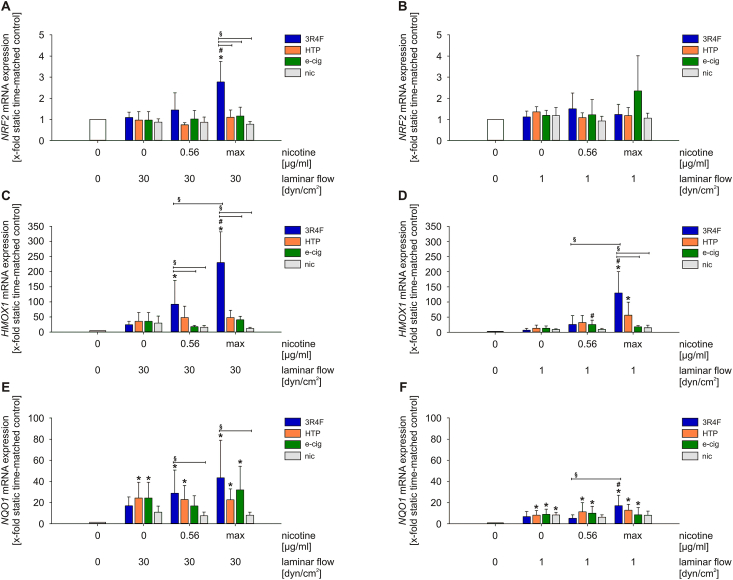
3.2. Aqueous extracts of different smoking products activates dose-dependent NRF2 antioxidant defense system in primary human endothelial cells
The nuclear factor, erythroid 2‐like 2 (NRF2) system plays an important role in the cellular response to oxidative stress. Upon activation, the transcriptional activator NRF2 binds to AREs (Antioxidant Response Elements) in the regulatory regions of a variety of detoxifying and antioxidant genes.
Stimulation with high laminar flow in combination with 3R4F increased NRF2 mRNA expression significantly. The combined stimulation with low laminar flow, however, could not trigger this effect. AqE exposure of next generation tobacco and nicotine products (NGP) as well as pure nicotine did not induce changes in NRF2 expression after 24 h under both flow conditions (Fig. 2A and B). NRF2 target genes heme oxygenase 1 (HMOX1) and NAD(P)H dehydrogenase (quinone 1) (NQO1) also showed increased mRNA expression in a dose‐dependent manner only under the treatment with 3R4F (Fig. 2C–F).
Fig. 2.
Treatment with aqueous extracts of different tobacco products leads to activation of NRF2 antioxidant defense system in a dose-dependent manner. mRNA expression of NRF2(A–B), HMOX1(C–D) and NQO1(E–F) under high and low laminar flow conditions were determined. max = maximum nicotine concentration (Table 2). Data are shown as mean (x-fold static time-matched controls) ± SD. Statistics: One-Way ANOVA *p < 0.05 vs. static time-matched controls, #p < 0.05 vs. time-matched control (0 μg/ml nicotine, 1 respectively 30 dyn/cm2), §p < 0.05, n ≥ 3.
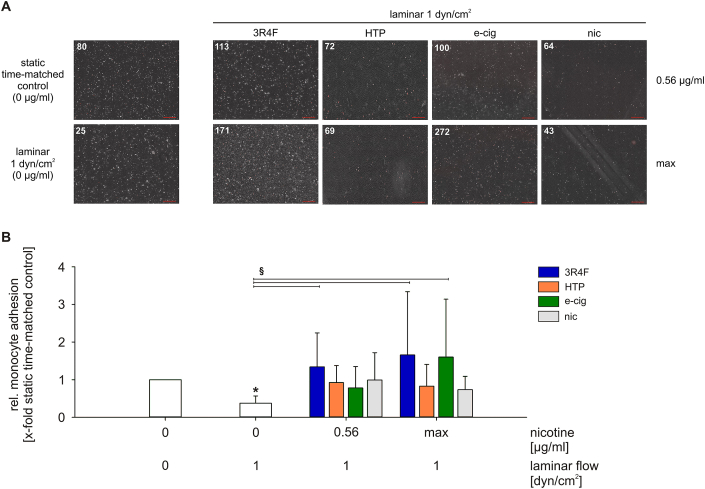
3.3. Smoking leads to increased adhesion of monocytes to endothelial cells in vitro
Adhesion of monocytes to the endothelium reflects a pro-inflammatory and thus atherosclerosis-prone phenotype. Stimulation with 3R4F can activate inflammatory pathways in endothelial cells. Monocyte-endothelial cell adhesion under atherosclerosis-prone static and low laminar flow conditions was investigated in response to the tested tobacco/nicotine products and pure nicotine. Application of low laminar flow without any further stimulation showed reduced monocyte-endothelial cell adhesion compared to static conditions (Fig. 3). All tested products, including nicotine, inhibited this effect. The comparative analysis under laminar flow revealed an increase of monocyte adhesion after stimulation with 3R4F and in addition increased monocyte adhesion under maximal e-cig dosage. Stimulation with HTP and pure nicotine did not show an additional effect on the monocyte-endothelial cell adhesion.
Fig. 3.
Increased adhesion of monocytes to endothelial cells after exposure to aqueous extracts of tobacco and nicotine products under atherosclerosis-prone flow conditions using the ibidi pump system. (A) Representative pictures showing monocyte-endothelial interactions. Fluorescently labelled THP-1 monocytes (red, numbers of monocytes are indicated) adhere to a pre-treated confluent endothelial cell layer. Scale bar represents 500 μm. (B) Evaluation of monocyte-endothelial cell adhesion after exposure to aqueous extracts in combination with low laminar flow conditions. max = maximum nicotine concentration (Table 2). Data are shown as mean (x-fold static time-matched controls) ± SD. Statistics: One-Way ANOVA, *p < 0.05 vs. static time-matched controls, §p < 0.05, n ≥ 6. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Stimulation with tobacco and nicotine products induces pro‐inflammatory/atherosclerosis‐prone endothelial phenotype
A pro-inflammatory phenotype of endothelial cells is characterized by an increased expression of adhesion molecules and cytokines. As we could show a functionally impaired endothelium under the stimulation with different smoking extracts, the mRNA expression of pro-inflammatory genes as markers for endothelial dysfunction and atherosclerosis was analyzed.
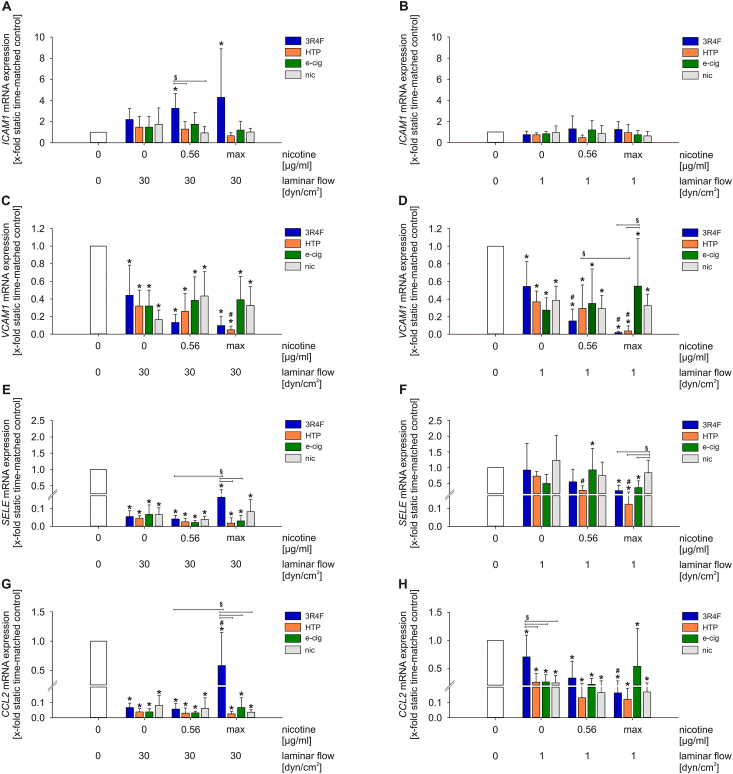
Gene expression of intercellular adhesion molecule 1 (ICAM1) was increased in response to stimulation with 3R4F under high flow conditions, whereas alternative tobacco products had no effect on ICAM1 expression. Combined stimulation of 3R4F with low laminar flow did lead to significant ICAM1 induction (Fig. 4A and B). The mRNA expression of Vascular cell adhesion molecule 1 (VCAM1) showed a regression under flow conditions with further dose-dependent downregulation due to 3R4F and HTP stimulation (Fig. 4C and D). Selectin E (SELE) plays an important role during the initial part of the interaction between endothelial cells and monocytes. In a previous study, stimulation with 3R4F showed increased SELE mRNA expression levels under static conditions [21]. Both flow conditions prevented this upregulation, while only high laminar flow was leading to a significant downregulation of SELE mRNA expression. From this level, 3R4F at maximum concentration could increase SELE expression. Interestingly, 3R4F and NGP stimulation, but not nicotine, resulted in a dose-dependent downregulation of SELE under low laminar flow conditions (Fig. 4E and F). Another marker for the progression of atherosclerosis is the pro‐inflammatory cytokine CCL2. Flow stimulation downregulated CCL2 significantly. From this downregulated level, high concentrations of 3R4F increased also CCL2 expression under high laminar flow (Fig. 4G and H). NGP extracts and pure nicotine had no further effect on CCL2 expression.
Fig. 4.
Treatment with aqueous extracts of different tobacco products leads to regulation of pro-inflammatory pathways in a dose-dependent manner. mRNA expression of ICAM1(A–B), VCAM1(C–D), SELE(E–F) and CCL2(G–H) under high and low laminar flow conditions were determined. max = maximum nicotine concentration (Table 2). Data are shown as mean (x-fold static time-matched controls) ± SD. Statistics: One-Way ANOVA, *p < 0.05 vs. static time-matched controls, #p < 0.05 vs. time-matched control (0 μg/ml nicotine, 1 respectively 30 dyn/cm2), §p < 0.05, n ≥ 3.
4. Discussion
Tobacco smoking is the single most preventable risk factor related to the development of cardiovascular disease. Therefore, it is not surprising that innovative and potential less risky tobacco and nicotine alternative products were developed in order to reduce the harmful effects of conventional cigarette consumption. To get detailed information about the actual deleterious impact of these next generation tobacco and nicotine products on the vascular endothelium, this study investigated the effect of conventional cigarettes versus NGP on parameters of endothelial dysfunction, oxidative stress and inflammation. In addition, high laminar flow of 30 dyn/cm2 (to mimic atheroprotective flow conditions) or low laminar flow of 1 dyn/cm2 (representing pro-atherosclerotic flow conditions) were applied to primary human endothelial cells.
For toxicological evaluations, it is essential to provoke known pathophysiological parameters such as cytotoxicity and inflammatory cellular responses under defined conditions to make comparisons between different treatments subsequently. The stimulation with test compounds was performed in a nicotine concentration range from 0.56 to 4.96 μg/ml. Although depending on various factors (puff volume, depth of inhalation, inhalation frequency, amount and type of cigarettes, age, gender), this is an approximately 10 to 250-fold higher nicotine concentration compared to the physiological nicotine concentration in plasma of a chronic smoker which is around 20 to 50 ng/ml over a 24 h period [[45], [46], [47]]. Under normal conditions, the endothelium may not be exposed to such high concentrations in vivo. Nevertheless, these concentrations are commonly used to investigate the effect of test compounds on pathomechanisms in the vascular system in vitro (0.09–4.76 μg/ml nicotine) [48,49] and in vivo in mouse models of atherosclerosis (0.10–0.24 μg/ml nicotine in plasma) [50,51].
Stimulation with laminar flow itself increased cell viability. The combined treatment of shear stress and AqE revealed a major cytotoxic capacity of 3R4F, but not for the other product categories. The data demonstrate the reduced cytotoxic potential of NGP relative to a conventional cigarette. We were able to demonstrate beneficial effects of high laminar flow on endothelial viability in a previous study. The effects shown counteract the cytotoxicity of 3R4F treatment and can delay the progression of cell death [21]. Other studies showed similar conclusions by comparing traditional cigarettes and NGP regarding cytotoxicity [35]. The dose-dependent reduction of cell viability by conventional cigarettes was shown in human bronchial epithelial cells (HBEC), whereas stimulation with e-cigarettes had no impact on cell viability under the tested conditions [52]. Jaunky et al. demonstrated, in HBEC, a reduced cytotoxic potential of HTP relative to conventional cigarettes [53]. Further in vitro toxicological chemical analyses of HTP aerosol revealed significant reductions in cytotoxicity relative to cigarette smoke, which adds greater confidence to the observations in our study [54,55].
To determine the potential deleterious impact of the test agents on endothelial function, we performed functional in vitro assays and molecular analyses giving insights into the physiological status and transition of the endothelial phenotype. Endothelial damage is a main characteristic for endothelial dysfunction. Therefore, a wound in the endothelial cell layer and an impairment of vascular repair is considered as an initial stimulus for the development of atherosclerotic lesions [56]. By creating an artificial wound in an endothelial cell layer, we investigated the migration behavior of human endothelial cells under various treatments. Application of high laminar flow enhanced wound closure compared to static conditions. Additional treatment with 3R4F inhibited these protective effects of high laminar flow even below the level of the static non-treatment control. A similar reduction by 3R4F could be shown under static conditions. Our observations are in agreement with previous studies showing impaired wound healing in vivo and in vitro [57,58] and likewise enhanced endothelial repair under physiological flow levels [59]. The less potent HTP and e-cig exposure had less deleterious impact regarding endothelial wound healing compared to 3R4F. Taylor et al. recently published a comparative study of e-cigarette aerosols and cigarette smoke supporting our data [49]. Moreover, treatment with nicotine seems not to be responsible for the inhibition of endothelial wound healing capability that was induced by 3R4F [60].
A possible mechanism playing a substantial role in mediating protective effects of high laminar flow is the PI3K/AKT/eNOS pathway [61,62]. There is evidence that reduced NO formation due to impairment of eNOS phosphorylation is one of the main factors contributing to a declined wound healing capability [21,63]. NO release in response to high laminar flow was still increased under all test compounds but a decreased eNOS mRNA expression and an impaired eNOS phosphorylation at Ser1117 under 3R4F treatment was observed. NGP and nicotine treatment revealed rather contrary results on eNOS mRNA expression but seem to have the same deleterious impact on pathway activation via eNOS phosphorylation at Ser1117. To our knowledge, this study is the first demonstrating the impact of alternative tobacco products on the PI3K/AKT/eNOS pathway and will hopefully stimulate further investigations of the effect of NGP on this essential mechanism mediating endothelial homeostasis and vascular health.
NRF2 is known as a mechanosensitive transcription factor in endothelial cells [64] and there is growing evidence that supports NRF2-mediated atheroprotective and anti-oxidative effects in response to laminar flow [[65], [66], [67]]. Our data suggest a stronger activation of the NRF2 antioxidant defense system by combined exposure to laminar flow and 3R4F. NRF2 mRNA expression itself remained unaffected by all NGP stimulations. Whereas, 3R4F at maximum concentration increased NRF2 under high laminar flow. As NRF2 is a transcription factor, mainly regulated by translocation into the nucleus upon stimulation, the mRNA expression of HMOX1 and NQO1 as NRF2 target genes display changes of the cellular antioxidant defense very accurately. Only 3R4F showed clear induction of HMOX1 expression and significantly higher expression in comparison to HTP and nicotine. The expression of NQO1 reacts similar. The data is strongly supported by previous research showing induction of this system by traditional cigarettes in several cellular systems [[68], [69], [70], [71], [72]]. Recently, studies investigated the effect of HTP and e-cig on cellular oxidative stress and the activation of the NRF2 system. Teasdale et al. stimulated human coronary artery endothelial cells and revealed that conventional smoking but not e-cig or nicotine increase NRF2-regulated gene expression [73] which is supported by a comparative study in HBEC showing transcriptional activation of the antioxidant response element (ARE) after exposure to 3R4F, but not under e-cig treatment in vitro [52]. A comparative study from Taylor and colleagues between 3R4F and HTP in HBEC showed comparable results regarding smoke-induced oxidative stress [74]. All these findings reveal a reduced response of the NRF2 system regarding NGP versus traditional smoking. Often a delayed onset of the antioxidative cell response was demonstrated which goes in line with our own findings in this study.
Monocyte adhesion to the vascular wall is a marker for endothelial dysfunction. In a previous study, we showed an increased monocyte adhesion to endothelial cells treated with 3R4F, which goes in line with other studies showing similar effects of 3R4F stimulation [21,75,76]. Furthermore, Poussin et al. investigated MM6 monocyte-HCAEC (human coronary artery endothelial cell) adhesion after HTP stimulation under static conditions and revealed a reduced monocyte-endothelial cell adhesion compared to 3R4F [77]. These functional data could been shown to be related to an enhanced mRNA expression of several inflammatory marker genes as adhesion molecules and cytokines. In the present study, application of low laminar flow reduces monocyte endothelial cell adhesion compared to static conditions. Exposure to NGP and nicotine also inhibited the protective effects of laminar flow to a certain extent, but only e-cig stimulation under maximal dosage led to an additional increase in adhesion like the 3R4F treatment. Our gene expression analyses showed modulation of adhesion molecules and cytokines in response to 3R4F and HTP under flow. Nicotine and e-cig showed lesser responses. Typically, responses to NGP were lower and shifted to higher concentrations as for 3R4F. It can be assumed that also the use of HTP or e-cig leads to an inflammatory state in the vasculature but a higher concentration is needed to get changes on the functional level. Previous studies have shown an increased monocyte adhesion to endothelial cells in response to cigarette smoke in vitro and in vivo [[78], [79], [80]]. This stands in line with many reports revealing significantly higher levels of pro-inflammatory plasma markers in smokers than in non-smokers [[81], [82], [83]]. Nevertheless, there are studies showing increased expression of inflammatory markers in response to alternative tobacco products under static conditions, but so far, no study analyzing functional parameters of endothelial cells under NGP treatment in combination with flow application has been published [[84], [85], [86]].
Oxidative stress and inflammation are two closely related conditions in the pathogenesis of endothelial dysfunction and development of atherosclerosis [[87], [88], [89]]. Cigarette smoke-induced oxidative stress is responsible for the endothelial activation through promoting the expression of inflammatory genes and consequently the production of pro-atherogenic adhesion molecules and cytokines inducing a pro-inflammatory and thus atherosclerosis-prone phenotype [17,90].
The biological impact of next generation tobacco and nicotine products (NGP) are currently of substantial interest. Recently, animal studies stated reduced effects of NGP application compared to conventional cigarettes on the cardiovascular system [28,51,91,92]. These in vivo observations are consistent with our findings showing reduced deleterious effects of NGP relative to 3R4F on functional endothelial parameters, biomarkers for cellular stress response and inflammatory processes of the endothelium. A 6-month exposure response study in healthy smokers switching from conventional smoking to HTP usage showed comparable results and changes in endothelial dysfunction, mechanisms including oxidative stress and inflammation, oxygen transport, platelet activation and lung function [93]. Another study in healthy volunteers also showed unfavourable effects on parameters of endothelial function and flow-mediated dilation after e-cig usage compared to conventional smoking, although e-cig have a lesser impact [94].
In summary, various data from in vitro, animal in vivo and human clinical studies show comparable results of the biological impact of NGP relative to conventional cigarettes. This corroborates the relevance of in vitro approaches in research to characterize the molecular and functional effects of test compounds under simplified and defined in vitro conditions that mimic in vivo conditions.
5. Conclusion
This study suggests that stimulation of endothelial cells with conventional cigarette smoke leads to an earlier onset of antioxidative and pro-inflammatory mechanisms compared to next generation tobacco and nicotine products. Atheroprotective high laminar flow did not completely abrogate the detrimental effects of 3R4F stimulation but the effects were clearly delayed. Exposition to NGP typically shifted the response rightward to higher doses compared to 3R4F.
Compliance with ethical standards
This study is approved by the ethical review board of the Medical Faculty Carl Gustav Carus of the Technische Universität Dresden, Dresden, Germany (EK124082003).
Declaration of competing interest
The work was funded by British American Tobacco (BAT), London, UK. All authors were employees of BAT or Technische Universität Dresden at the time of study conduct. Frazer Lowe has since left BAT. Vype is a registered trademark and is manufactured by BAT. All test articles were generated and supplied by BAT.
Acknowledgements
We kindly would thank Katherine Hewitt and Stela Bozhilova for the production of aqueous extracts used in this study.
References
- 1.Organization W.H. WHO Press; 2019. World Health Statistics 2019: Monitoring Health for the SDGs; pp. 1–132. [Google Scholar]
- 2.Cybularz M., Langbein H., Zatschler B., Brunssen C., Deussen A., Matschke K., Morawietz H. Endothelial function and gene expression in perivascular adipose tissue from internal mammary arteries of obese patients with coronary artery disease. Atherosclerosis Suppl. 2017;30:149–158. doi: 10.1016/j.atherosclerosissup.2017.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 4.Langbein H., Brunssen C., Hofmann A., Cimalla P., Brux M., Bornstein S.R., Deussen A., Koch E., Morawietz H. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Eur. Heart J. 2016;37:1753–1761. doi: 10.1093/eurheartj/ehv564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis is an inflammatory disease. Am. Heart J. 1999;138:S419–S420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 6.Chatzizisis Y.S., Coskun A.U., Jonas M., Edelman E.R., Feldman C.L., Stone P.H. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 7.Coppola G., Caro C. Arterial geometry, flow pattern, wall shear and mass transport: potential physiological significance. J. R. Soc. Interface/Roy. Soc. 2009;6:519–528. doi: 10.1098/rsif.2008.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies P.F., Spaan J.A., Krams R. Shear stress biology of the endothelium. Ann. Biomed. Eng. 2005;33:1714–1718. doi: 10.1007/s10439-005-8774-0. [DOI] [PubMed] [Google Scholar]
- 9.Hastings N.E., Simmers M.B., McDonald O.G., Wamhoff B.R., Blackman B.R. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am. J. Physiol. Cell Physiol. 2007;293:C1824–C1833. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- 10.Cheng C., Tempel D., van Haperen R., van der Baan A., Grosveld F., Daemen M.J., Krams R., de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 11.Chiu J.J., Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn C., Schwartz M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks A.R., Lelkes P.I., Rubanyi G.M. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol. Genom. 2002;9:27–41. doi: 10.1152/physiolgenomics.00075.2001. [DOI] [PubMed] [Google Scholar]
- 14.Pan S. Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxidants Redox Signal. 2009;11:1669–1682. doi: 10.1089/ars.2009.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunssen C., Giebe S., Hofmann A., Brux M., Morawietz H. Evaluation of cytotoxic, oxidative and pro-inflammatory effects of aqueous cigarette smoke extract on human monocytes: a potential model system for assessment of next generation tobacco and nicotine products. Appl. In Vitro Toxicol. 2017;3:121–130. [Google Scholar]
- 16.Csordas A., Kreutmayer S., Ploner C., Braun P.R., Karlas A., Backovic A., Wick G., Bernhard D. Cigarette smoke extract induces prolonged endoplasmic reticulum stress and autophagic cell death in human umbilical vein endothelial cells. Cardiovasc. Res. 2011;92:141–148. doi: 10.1093/cvr/cvr165. [DOI] [PubMed] [Google Scholar]
- 17.Messner B., Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014;34:509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 18.Jorge P.A., Ozaki M.R., Almeida E.A. Endothelial dysfunction in coronary vessels and thoracic aorta of rats exposed to cigarette smoke. Clin. Exp. Pharmacol. Physiol. 1995;22:410–413. doi: 10.1111/j.1440-1681.1995.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 19.Maresh J.G., Xu H., Jiang N., Gairola C.G., Shohet R.V. Tobacco smoke dysregulates endothelial vasoregulatory transcripts in vivo. Physiol. Genom. 2005;21:308–313. doi: 10.1152/physiolgenomics.00310.2004. [DOI] [PubMed] [Google Scholar]
- 20.Vita J.A. Endothelial function. Circulation. 2011;124:e906–912. doi: 10.1161/CIRCULATIONAHA.111.078824. [DOI] [PubMed] [Google Scholar]
- 21.Giebe S., Cockcroft N., Hewitt K., Brux M., Hofmann A., Morawietz H., Brunssen C. Cigarette smoke extract counteracts atheroprotective effects of high laminar flow on endothelial function. Redox Biol. 2017;12:776–786. doi: 10.1016/j.redox.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stratton K., Shetty P., Wallace R., Bondurant S. Clearing the smoke: the science base for tobacco harm reduction--executive summary. Tobac. Contr. 2001;10:189–195. doi: 10.1136/tc.10.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farsalinos K.E., Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther. Adv. Drug Saf. 2014;5:67–86. doi: 10.1177/2042098614524430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grana R., Benowitz N., Glantz S.A. E-cigarettes: a scientific review. Circulation. 2014;129:1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogden M.W., Marano K.M., Jones B.A., Morgan W.T., Stiles M.F. vol. 20. 2015. Switching from usual brand cigarettes to a tobacco-heating cigarette or snus: Part 2. Biomarkers of exposure; pp. 391–403. (Biomarkers : Biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogden M.W., Marano K.M., Jones B.A., Morgan W.T., Stiles M.F. vol. 20. 2015. Switching from usual brand cigarettes to a tobacco-heating cigarette or snus: Part 3. Biomarkers of biological effect; pp. 404–410. (Biomarkers : Biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogden M.W., Marano K.M., Jones B.A., Stiles M.F. vol. 20. 2015. Switching from usual brand cigarettes to a tobacco-heating cigarette or snus: Part 1. Study design and methodology; pp. 382–390. (Biomarkers : Biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kogel U., Schlage W.K., Martin F., Xiang Y., Ansari S., Leroy P., Vanscheeuwijck P., Gebel S., Buettner A., Wyss C. A 28-day rat inhalation study with an integrated molecular toxicology endpoint demonstrates reduced exposure effects for a prototypic modified risk tobacco product compared with conventional cigarettes. Food Chem. Toxicol. : Int. J. Publ. Br. Industr. Biol. Res. Assoc. 2014;68:204–217. doi: 10.1016/j.fct.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Patskan G., Reininghaus W. Toxicological evaluation of an electrically heated cigarette. Part 1: overview of technical concepts and summary of findings. J. Appl. Toxicol. : JAT. 2003;23:323–328. doi: 10.1002/jat.923. [DOI] [PubMed] [Google Scholar]
- 30.Stabbert R., Voncken P., Rustemeier K., Haussmann H.J., Roemer E., Schaffernicht H., Patskan G. Toxicological evaluation of an electrically heated cigarette. Part 2: chemical composition of mainstream smoke. J. Appl. Toxicol. : JAT. 2003;23:329–339. doi: 10.1002/jat.924. [DOI] [PubMed] [Google Scholar]
- 31.van der Toorn M., Frentzel S., Goedertier D., Peitsch M., Hoeng J., De Leon H. A prototypic modified risk tobacco product exhibits reduced effects on chemotaxis and transendothelial migration of monocytes compared with a reference cigarette. Food Chem. Toxicol. 2015;80:277–286. doi: 10.1016/j.fct.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Korten S., Brunssen C., Poitz D.M., Grossklaus S., Brux M., Schnittler H.J., Strasser R.H., Bornstein S.R., Morawietz H., Goettsch W. Impact of Hey2 and COUP-TFII on genes involved in arteriovenous differentiation in primary human arterial and venous endothelial cells. Basic Res. Cardiol. 2013;108:362. doi: 10.1007/s00395-013-0362-0. [DOI] [PubMed] [Google Scholar]
- 33.Brock T., Boudriot E., Klawitter A., Grosser M., Nguyen T.T.P., Giebe S., Klapproth E., Temme A., El-Armouche A., Breier G. The influence of VE-cadherin on adhesion and incorporation of breast cancer cells into vascular endothelium. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22116049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunssen C., Korten S., Brux M., Seifert S., Roesler J., Bornstein S.R., Morawietz H., Goettsch W. COUP-TFII is regulated by high glucose in endothelial cells. Horm. Metab. Res. 2010;42:81–87. doi: 10.1055/s-0029-1241862. [DOI] [PubMed] [Google Scholar]
- 35.Bozhilova S., Baxter A., Bishop E., Breheny D., Thorne D., Hodges P., Gaca M. Optimization of aqueous aerosol extract (AqE) generation from e-cigarettes and tobacco heating products for in vitro cytotoxicity testing. Toxicol. Lett. 2020;335:51–63. doi: 10.1016/j.toxlet.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Cockcroft N.Y., Oke O., Cunningham F., Bishop E., Fearon I.M., Zantl R., Gaca M.D. An in vitro perfusion system to examine the responses of endothelial cells to simulated flow and inflammatory stimulation. Alternat. Laboratory Anim. : ATLA. 2009;37:657–669. doi: 10.1177/026119290903700610. [DOI] [PubMed] [Google Scholar]
- 37.Duerrschmidt N., Stielow C., Muller G., Pagano P.J., Morawietz H. NO-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells. J. Physiol. 2006;576:557–567. doi: 10.1113/jphysiol.2006.111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goettsch C., Goettsch W., Brux M., Haschke C., Brunssen C., Muller G., Bornstein S.R., Duerrschmidt N., Wagner A.H., Morawietz H. Arterial flow reduces oxidative stress via an antioxidant response element and Oct-1 binding site within the NADPH oxidase 4 promoter in endothelial cells. Basic Res. Cardiol. 2011;106:551–561. doi: 10.1007/s00395-011-0170-3. [DOI] [PubMed] [Google Scholar]
- 39.Morawietz H., Talanow R., Szibor M., Rueckschloss U., Schubert A., Bartling B., Darmer D., Holtz J. Regulation of the endothelin system by shear stress in human endothelial cells. J. Physiol. (Lond.) 2000;525:761–770. doi: 10.1111/j.1469-7793.2000.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schubert A., Cattaruzza M., Hecker M., Darmer D., Holtz J., Morawietz H. Shear stress-dependent regulation of the human b-tubulin folding cofactor D gene. Circ. Res. 2000;87:1188–1194. doi: 10.1161/01.res.87.12.1188. [DOI] [PubMed] [Google Scholar]
- 41.Crouch S.P., Kozlowski R., Slater K.J., Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods. 1993;160:81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- 42.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goettsch C., Goettsch W., Arsov A., Hofbauer L.C., Bornstein S.R., Morawietz H. Long-term cyclic strain downregulates endothelial Nox4. Antioxidants Redox Signal. 2009;11:2385–2397. doi: 10.1089/ars.2009.2561. [DOI] [PubMed] [Google Scholar]
- 44.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hukkanen J., Jacob P., 3rd, Benowitz N.L. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 46.Benowitz N.L., Kuyt F., Jacob P., 3rd, Jones R.T., Osman A.L. Cotinine disposition and effects. Clin. Pharmacol. Ther. 1983;34:604–611. doi: 10.1038/clpt.1983.222. [DOI] [PubMed] [Google Scholar]
- 47.Gourlay S.G., Benowitz N.L. Arteriovenous differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray, and intravenous nicotine. Clin. Pharmacol. Ther. 1997;62:453–463. doi: 10.1016/S0009-9236(97)90124-7. [DOI] [PubMed] [Google Scholar]
- 48.Bishop E., Breheny D., Hewitt K., Taylor M., Jaunky T., Camacho O.M., Thorne D., Gaca M. Evaluation of a high-throughput in vitro endothelial cell migration assay for the assessment of nicotine and tobacco delivery products. Toxicol. Lett. 2020;334:110–116. doi: 10.1016/j.toxlet.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Taylor M., Jaunky T., Hewitt K., Breheny D., Lowe F., Fearon I.M., Gaca M. A comparative assessment of e-cigarette aerosols and cigarette smoke on in vitro endothelial cell migration. Toxicol. Lett. 2017;277:123–128. doi: 10.1016/j.toxlet.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Wong E.T., Szostak J., Titz B., Lee T., Wong S.K., Lavrynenko O., Merg C., Corciulo M., Simicevic J., Auberson M. A 6-month inhalation toxicology study in Apoe(-/-) mice demonstrates substantially lower effects of e-vapor aerosol compared with cigarette smoke in the respiratory tract. Arch. Toxicol. 2021;95:1805–1829. doi: 10.1007/s00204-021-03020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips B., Szostak J., Titz B., Schlage W.K., Guedj E., Leroy P., Vuillaume G., Martin F., Buettner A., Elamin A. A six-month systems toxicology inhalation/cessation study in ApoE(-/-) mice to investigate cardiovascular and respiratory exposure effects of modified risk tobacco products, CHTP 1.2 and THS 2.2, compared with conventional cigarettes. Food Chem. Toxicol. : Int. J. Publ. Br. Industr. Biol. Res. Assoc. 2019;126:113–141. doi: 10.1016/j.fct.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Taylor M., Carr T., Oke O., Jaunky T., Breheny D., Lowe F., Gaca M. E-cigarette aerosols induce lower oxidative stress in vitro when compared to tobacco smoke. Toxicol. Mech. Methods. 2016;26:465–476. doi: 10.1080/15376516.2016.1222473. [DOI] [PubMed] [Google Scholar]
- 53.Jaunky T., Adamson J., Santopietro S., Terry A., Thorne D., Breheny D., Proctor C., Gaca M. Assessment of tobacco heating product THP1.0. Part 5: in vitro dosimetric and cytotoxic assessment. Regul. Toxicol. Pharmacol. 2018;93:52–61. doi: 10.1016/j.yrtph.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 54.Schaller J.P., Pijnenburg J.P.M., Ajithkumar A., Tricker A.R. Evaluation of the Tobacco Heating System 2.2. Part 3: influence of the tobacco blend on the formation of harmful and potentially harmful constituents of the Tobacco Heating System 2.2 aerosol. Regul. Toxicol. Pharmacol. 2016;81(Suppl 2):S48–S58. doi: 10.1016/j.yrtph.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Smith M.R., Clark B., Ludicke F., Schaller J.P., Vanscheeuwijck P., Hoeng J., Peitsch M.C. Evaluation of the tobacco heating system 2.2. Part 1: description of the system and the scientific assessment program. Regul. Toxicol. Pharmacol. 2016;81(Suppl 2):S17–S26. doi: 10.1016/j.yrtph.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Ross R., Glomset J., Harker L. Response to injury and atherogenesis. Am. J. Pathol. 1977;86:675–684. [PMC free article] [PubMed] [Google Scholar]
- 57.Martin J.W., Mousa S.S., Shaker O., Mousa S.A. The multiple faces of nicotine and its implications in tissue and wound repair. Exp. Dermatol. 2009;18:497–505. doi: 10.1111/j.1600-0625.2009.00854.x. [DOI] [PubMed] [Google Scholar]
- 58.Su Y., Cao W., Han Z., Block E.R. Cigarette smoke extract inhibits angiogenesis of pulmonary artery endothelial cells: the role of calpain. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L794–L800. doi: 10.1152/ajplung.00079.2004. [DOI] [PubMed] [Google Scholar]
- 59.Albuquerque M.L., Waters C.M., Savla U., Schnaper H.W., Flozak A.S. Shear stress enhances human endothelial cell wound closure in vitro. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H293–H302. doi: 10.1152/ajpheart.2000.279.1.H293. [DOI] [PubMed] [Google Scholar]
- 60.Snajdar R.M., Busuttil S.J., Averbook A., Graham D.J. Inhibition of endothelial cell migration by cigarette smoke condensate. J. Surg. Res. 2001;96:10–16. doi: 10.1006/jsre.2000.6055. [DOI] [PubMed] [Google Scholar]
- 61.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflügers Archiv. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- 62.Soneja A., Drews M., Malinski T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol. Rep. 2005;57(Suppl):108–119. [PubMed] [Google Scholar]
- 63.Kolluru G.K., Bir S.C., Kevil C.G. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int. J. Vasc. Med. 2012;2012:918267. doi: 10.1155/2012/918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McSweeney S.R., Warabi E., Siow R.C. Nrf2 as an endothelial mechanosensitive transcription factor: going with the flow. Hypertension. 2016;67:20–29. doi: 10.1161/HYPERTENSIONAHA.115.06146. [DOI] [PubMed] [Google Scholar]
- 65.Fledderus J.O., Boon R.A., Volger O.L., Hurttila H., Yla-Herttuala S., Pannekoek H., Levonen A.L., Horrevoets A.J. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:1339–1346. doi: 10.1161/ATVBAHA.108.165811. [DOI] [PubMed] [Google Scholar]
- 66.Hsieh C.Y., Hsiao H.Y., Wu W.Y., Liu C.A., Tsai Y.C., Chao Y.J., Wang D.L., Hsieh H.J. Regulation of shear-induced nuclear translocation of the Nrf2 transcription factor in endothelial cells. J. Biomed. Sci. 2009;16:12. doi: 10.1186/1423-0127-16-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones C.I., 3rd, Zhu H., Martin S.F., Han Z., Li Y., Alevriadou B.R. Regulation of antioxidants and phase 2 enzymes by shear-induced reactive oxygen species in endothelial cells. Ann. Biomed. Eng. 2007;35:683–693. doi: 10.1007/s10439-007-9279-9. [DOI] [PubMed] [Google Scholar]
- 68.Cheng S.E., Lee I.T., Lin C.C., Kou Y.R., Yang C.M. Cigarette smoke particle-phase extract induces HO-1 expression in human tracheal smooth muscle cells: role of the c-Src/NADPH oxidase/MAPK/Nrf2 signaling pathway. Free Radical Biol. Med. 2010;48:1410–1422. doi: 10.1016/j.freeradbiomed.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 69.Kode A., Rajendrasozhan S., Caito S., Yang S.R., Megson I.L., Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 70.Pickett G., Seagrave J., Boggs S., Polzin G., Richter P., Tesfaigzi Y. Effects of 10 cigarette smoke condensates on primary human airway epithelial cells by comparative gene and cytokine expression studies. Toxicol. Sci. : Off. J. Soc. Toxicol. 2010;114:79–89. doi: 10.1093/toxsci/kfp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shih R.H., Lee I.T., Hsieh H.L., Kou Y.R., Yang C.M. Cigarette smoke extract induces HO-1 expression in mouse cerebral vascular endothelial cells: involvement of c-Src/NADPH oxidase/PDGFR/JAK2/STAT3 pathway. J. Cell. Physiol. 2010;225:741–750. doi: 10.1002/jcp.22270. [DOI] [PubMed] [Google Scholar]
- 72.Wang L., Kondo N., Cano M., Ebrahimi K., Yoshida T., Barnett B.P., Biswal S., Handa J.T. Nrf2 signaling modulates cigarette smoke-induced complement activation in retinal pigmented epithelial cells. Free Radic. Biol. Med. 2014;70:155–166. doi: 10.1016/j.freeradbiomed.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teasdale J.E., Newby A.C., Timpson N.J., Munafo M.R., White S.J. Cigarette smoke but not electronic cigarette aerosol activates a stress response in human coronary artery endothelial cells in culture. Drug Alcohol Depend. 2016;163:256–260. doi: 10.1016/j.drugalcdep.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor M., Thorne D., Carr T., Breheny D., Walker P., Proctor C., Gaca M. Assessment of novel tobacco heating product THP1.0. Part 6: a comparative in vitro study using contemporary screening approaches. Regul. Toxicol. Pharmacol. 2018;93:62–70. doi: 10.1016/j.yrtph.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 75.Poussin C., Gallitz I., Schlage W.K., Steffen Y., Stolle K., Lebrun S., Hoeng J., Peitsch M.C., Lietz M. Mechanism of an indirect effect of aqueous cigarette smoke extract on the adhesion of monocytic cells to endothelial cells in an in vitro assay revealed by transcriptomics analysis. Toxicol. Vitro : Int. J. Publ. Assoc. BIBRA. 2014;28:896–908. doi: 10.1016/j.tiv.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 76.Poussin C., Laurent A., Peitsch M.C., Hoeng J., De Leon H. Systems biology reveals cigarette smoke-induced concentration-dependent direct and indirect mechanisms that promote monocyte-endothelial cell adhesion. Toxicol. Sci. : Off. J. Soc. Toxicol. 2015;147:370–385. doi: 10.1093/toxsci/kfv137. [DOI] [PubMed] [Google Scholar]
- 77.Poussin C., Laurent A., Peitsch M.C., Hoeng J., De Leon H. Systems toxicology-based assessment of the candidate modified risk tobacco product THS2.2 for the adhesion of monocytic cells to human coronary arterial endothelial cells. Toxicology. 2016;339:73–86. doi: 10.1016/j.tox.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 78.Dovgan P.S., Edwards J.D., Zhan X., Wilde M., Agrawal D.K. Cigarette smoking increases monocyte adherence to cultured endothelial cell monolayer. Biochem. Biophys. Res. Commun. 1994;203:929–934. doi: 10.1006/bbrc.1994.2271. [DOI] [PubMed] [Google Scholar]
- 79.Duplaa C., Couffinhal T., Labat L., Fawaz J., Moreau C., Bietz I., Bonnet J. Monocyte adherence to endothelial cells in patients with atherosclerosis: relationships with risk factors. Eur. J. Clin. Invest. 1993;23:474–479. doi: 10.1111/j.1365-2362.1993.tb00793.x. [DOI] [PubMed] [Google Scholar]
- 80.Kalra V.K., Ying Y., Deemer K., Natarajan R., Nadler J.L., Coates T.D. Mechanism of cigarette smoke condensate induced adhesion of human monocytes to cultured endothelial cells. J. Cell. Physiol. 1994;160:154–162. doi: 10.1002/jcp.1041600118. [DOI] [PubMed] [Google Scholar]
- 81.Bermudez E.A., Rifai N., Buring J.E., Manson J.E., Ridker P.M. Relation between markers of systemic vascular inflammation and smoking in women. Am. J. Cardiol. 2002;89:1117–1119. doi: 10.1016/s0002-9149(02)02284-1. [DOI] [PubMed] [Google Scholar]
- 82.Mazzone A., Cusa C., Mazzucchelli I., Vezzoli M., Ottini E., Ghio S., Tossini G., Pacifici R., Zuccaro P. Cigarette smoking and hypertension influence nitric oxide release and plasma levels of adhesion molecules. Clin. Chem. Lab. Med. 2001;39:822–826. doi: 10.1515/CCLM.2001.136. [DOI] [PubMed] [Google Scholar]
- 83.Winkelmann B.R., Boehm B.O., Nauck M., Kleist P., Marz W., Verho N.K., Ranjith N., Kneissl G. Cigarette smoking is independently associated with markers of endothelial dysfunction and hyperinsulinaemia in nondiabetic individuals with coronary artery disease. Curr. Med. Res. Opin. 2001;17:132–141. [PubMed] [Google Scholar]
- 84.Muthumalage T., Prinz M., Ansah K.O., Gerloff J., Sundar I.K., Rahman I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front. Physiol. 2017;8:1130. doi: 10.3389/fphys.2017.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scott A., Lugg S.T., Aldridge K., Lewis K.E., Bowden A., Mahida R.Y., Grudzinska F.S., Dosanjh D., Parekh D., Foronjy R. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax. 2018;73:1161–1169. doi: 10.1136/thoraxjnl-2018-211663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ween M.P., Whittall J.J., Hamon R., Reynolds P.N., Hodge S.J. Phagocytosis and Inflammation: exploring the effects of the components of E-cigarette vapor on macrophages. Physiol. Rep. 2017;5 doi: 10.14814/phy2.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borgerding M., Klus H. Analysis of complex mixtures--cigarette smoke. Exp. Toxicol. Pathol. 2005;57(Suppl 1):43–73. doi: 10.1016/j.etp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 88.Das A., Dey N., Ghosh A., Das S., Chattopadhyay D.J., Chatterjee I.B. Molecular and cellular mechanisms of cigarette smoke-induced myocardial injury: prevention by vitamin C. PloS One. 2012;7 doi: 10.1371/journal.pone.0044151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karademirci M., Kutlu R., Kilinc I. Relationship between smoking and total antioxidant status, total oxidant status, oxidative stress index, vit C, vit E. Clin. Res. J. 2018;12:2006–2012. doi: 10.1111/crj.12757. [DOI] [PubMed] [Google Scholar]
- 90.Colarusso C., Terlizzi M., Molino A., Pinto A., Sorrentino R. Role of the inflammasome in chronic obstructive pulmonary disease (COPD) Oncotarget. 2017;8:81813–81824. doi: 10.18632/oncotarget.17850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Phillips B., Veljkovic E., Boue S., Schlage W.K., Vuillaume G., Martin F., Titz B., Leroy P., Buettner A., Elamin A. An 8-month systems toxicology inhalation/cessation study in Apoe-/- mice to investigate cardiovascular and respiratory exposure effects of a candidate modified risk tobacco product, THS 2.2, compared with conventional cigarettes. Toxicol. Sci. : Off. J. Soc. Toxicol. 2016;149:411–432. doi: 10.1093/toxsci/kfv243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Szostak J., Wong E.T., Titz B., Lee T., Wong S.K., Low T., Lee K.M., Zhang J., Kumar A., Schlage W.K. A 6-month systems toxicology inhalation study in ApoE(-/-) mice demonstrates reduced cardiovascular effects of E-vapor aerosols compared with cigarette smoke. Am. J. Physiol. Heart and circulatory physiology. 2020;318:H604–H631. doi: 10.1152/ajpheart.00613.2019. [DOI] [PubMed] [Google Scholar]
- 93.Ludicke F., Ansari S.M., Lama N., Blanc N., Bosilkovska M., Donelli A., Picavet P., Baker G., Haziza C., Peitsch M. Effects of switching to a heat-not-burn tobacco product on biologically relevant biomarkers to assess a candidate modified risk tobacco product: a randomized trial. Cancer Epidemiol. Biomark. Prev. 2019;28:1934–1943. doi: 10.1158/1055-9965.EPI-18-0915. [DOI] [PubMed] [Google Scholar]
- 94.Carnevale R., Sciarretta S., Violi F., Nocella C., Loffredo L., Perri L., Peruzzi M., Marullo A.G., De Falco E., Chimenti I. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest. 2016;150:606–612. doi: 10.1016/j.chest.2016.04.012. [DOI] [PubMed] [Google Scholar]