Abstract
Cereal brans are by‐products of the milling of cereal grains, which are mainly used as low value ingredients in animal feed. Wheat and oat bran is a rich source of bioactives and phytochemicals, especially phenolic compounds. Within this study, the application of ultrasound (US) technology to assist the extraction of phenolics from oat and wheat bran was investigated (20–45 kHz). Peleg’s mathematical model was used to study the kinetics of ultrasound-assisted extraction (UAE) and subsequent stirring of total phenolic compounds (TPC). The surface morphology of cereal brans after extraction was studied using SEM analysis. The excellent agreement was determined between the values of TPC calculated from Peleg’s mathematical model and actual experimental results. The constant that represents a time required for the initial phenolic concentration to be extracted to one-half of its initial value has been introduced (K1/2). It was shown that the TPC extraction kinetics was dependent only on K1/2 enabling fast kinetics fitting and comparison between extraction rates. Moreover, different values of K1/2 constant could indicate the differences in brans composition and consequently different influence of US pretreatment on these samples.
Keywords: Cereal brans, Ultrasound-assisted extraction (UAE), Total phenolic content (TPC), Extraction kinetics, Peleg’s model
1. Introduction
Cereal brans are by‐products of the milling of cereal grains which are generated in enormous quantities during the production of refined cereal flours. It is estimated that 150 million tons of wheat bran are produced per year worldwide, which are mainly used as the low value ingredients in animal feeds [3]. Wheat bran is abundant in fibres (arabinoxylans, cellulose, β-glucans and lignin), oligosaccharides, polyphenols, carotenoids, phytic acid and other phytochemicals which can contribute to creation of added-value products [37]. Oat bran represents a good source of arabinoxylan, β-glucan, minerals, and potent antioxidant compounds – tocopherols, polyphenols, avenanthramides and phytic acid [4], [33]. Thus, the potential of cereal brans as a feedstock for the valorisation of bioactive compounds and phytochemicals is high, provided that the applied extraction techniques are enough sustainable and effective [18]. The extraction of phenolic compounds is usually done by alkali hydrolysis [1], [10], [23], but handling bases is dangerous at large scales and does not represent an environmentally safe process, because neutralization of bases with acids would only lead to production of salts, which, when introduced into water streams, would increase the salinity of the surrounding soils leading to soil infertility issues and other ecological changes [17]. However, the complete elimination of chemical solvent in extraction with satisfactory yield needs to be achieved [24]. Alternatively, the extraction of phenolic compounds can be enzymatically assisted, but enzymes are highly specific and lack practical implementation due to the high production costs [1], [12].
Extraction of phytochemicals can be conducted by novel extraction methods, which are environmentally friendly, provide shorter extraction time, reduce energy costs, increase the yield of the target compounds and improve the quality of extracts [16], [20], [42]. In this regard, ultrasound-assisted extraction (UAE) has been utilized in a number of studies with the aim to enhance the yields of phenolic compounds obtained from cereals and amplifying extraction efficiency [13], [18], [19], [38], [40]. To facilitate the optimisation, simulation, design and control of processes and contribute to the utilization of energy, time and solvent, different mathematical modelling methods are employed [9]. Peleg's model is an empirical and classic hyperbolic model initially developed to describe moisture sorption curves [31]. Since there is a similarity between the shape of the extraction and the sorption curves, Peleg’s model has been adapted and used to describe the solid–liquid extractions of various plant metabolites in general, and phenolics, in particular: from oregano [28], coffee silverskin [41], jamun (Syzygium cumini L.) seeds [7], forestry lignocellulosic by-products [43], brown seaweed (Ascophyllum nodosum) [20] etc.
The objective of this work was to study the potential of UAE to improve the extraction yields of phenolics from cereal brans (wheat and oat) and to conduct the kinetics study of UAE.
2. Materials and methods
2.1. Materials
Commercially available wheat and oat brans obtained from BioUna Ltd. (Novi Sad, Serbia) were ground using a laboratory cross beater mill (Retsch SK1, Retsch GmbH, Haan, Germany) equipped with a 0.8 mm sieve.
2.2. Ultrasound-assisted extraction (UAE)
Different ultrasound frequency exposures were examined using a 20 kHz probe system (VC 750, Sonics and Materials Inc., USA) and multiple bath systems operating at frequencies of 25 and 45 kHz (Elma IT H5, Germany), and 35 kHz (Jencons-PLS S1000, UK). For the 20 kHz ultrasound probe system, twenty four grams of wheat/oat bran were mixed with 480 mL of solvent (ethanol:water, 70:30, v:v) in a beaker and treated with a 13 mm diameter probe for 10 min. For the multiple bath systems, two grams of wheat/oat bran were mixed with 40 mL of solvent (ethanol:water, 70:30, v:v) in extraction tubes and treated for 10 min. For all treatments, the temperature was maintained at 25.0 ± 1.0 °C.
After the US treatments samples were transferred into an orbital shaker (S01, Stuart Scientific, UK), stirred at 250 rpm and withdrawn after 0 h, 1 h, 2 h, 3 h, 5 h, 21 h and 24 h and centrifuged at 5000 rpm for 10 min (Sigma 2-16PK, Osterode am Harz, Germany). Samples prepared by macerating the wheat/oat bran in solvent for 10 min without US treatment and stirring were marked as the Control, while the samples prepared by macerating the brans in solvent and stirring for 24 h without US treatment were marked as the Control (24 h) samples. Afterwards, the samples were centrifuged at 5000 rpm for 10 min (Sigma 2-16PK, UK). An aliquot of the supernatant (5 mL) was dried in a vacuum-evaporator (TurboVap LV, Caliper Life Science Inc., USA) below 40 °C. The dried extract was redissolved in methanol to 1 mL volume. The obtained extracts were used for further investigation of TPC.
2.3. Analysis of total phenolic compounds
Total phenolic content of wheat/oat bran extracts was determined spectrophotometrically using Folin-Ciocalteu's reagent [36]. Gallic acid was used as the standard and results were expressed as gallic acid equivalents (GAE) (μg GAE/g of sample on dry mass basis). The extracts (0.1 mL) of wheat and oat bran were diluted with pure water (7.9 mL). Folin-Ciocalteu's reagent (0.5 mL) and sodium carbonate solution (1.5 mL; concentration 20 g/100 mL) were added into the extracts, and the reaction mixture was mixed thoroughly. The mixture was allowed to stand for 120 min with intermittent shaking, and the absorbance at 750 nm was measured (Jenway, 6405 UV/Vis).
2.3.1. Extraction of phenolic compounds with sodium hydroxide
Alkali extraction of phenolic compounds was performed following the modified method of Gopalan and Nampoothiri [17], by treating 1 g of bran with 50 mL of 2 N NaOH at 25 °C for 12 h. The resulting slurry was then acidified using 5 N HCl till the pH dropped to 3.5. The acidified slurry was then extracted with three volumes of ethyl acetate. The extracted ethyl acetate volume was reduced by a rotary evaporation (R210, Buchi, Switzerland), and the yellowish liquid residue was then dissolved in ethanol/water (80:20, v/v) to 10 mL volume and used for further analyses using Folin-Ciocalteu's reagent following the same procedure as described in the previous section.
2.4. Extraction kinetics
Experimental data obtained for the extraction of total phenolic content from wheat and oat bran were fitted to Peleg’s kinetic model. Experimental data were normalised prior to nonlinear regression fitting. Empirical two parameter model proposed by Peleg [31] for sorption kinetics was employed:
| (1) |
where t (h) is the extraction time, C (t) represents the extraction yield at time t (mg/kg), C0 is initial extraction yield at time t = 0 (mg/kg GAE/kg), K1 (h kg/mg GAE) and K2 (kg/mg GAE) are Peleg’s constants (rate constant and capacity constant, respectively).
According to this model, reciprocal value of K1 is the initial rate constant B0 (mg GAE/kg h) at t = 0.
| (2) |
Similarly, reciprocal value of K2 allowing determining the equilibrium yield concentration or maximum capacity Ce (mg GAE/kg). Namely, when t→∞, Peleg’s equation represents equilibrium concentration.
| (3) |
Nonlinear regression constants (K1 and K2) were found by Mathcad software.
Having in mind that in experiments were used ultrasound bath and probe, the experimental data were normalised in order to enable better comparison between them. The following normalised model was developed
| (4) |
where K1/K2 (h) ratio represents the constant introduced for the first time in this study (K1/2) as a time required for the initial phenolic concentration to be extracted to one-half its initial value. In this way, kinetics was dependent only on one parameter that allowed fast comparison between experimental data.
2.5. Characterization of bran surface morphology by scanning electron microscope (SEM)
Bran surface morphology was characterized by obtaining the SEM images as described by Wen et al. [41]. Wheat/oat bran particles were mounted on stubs using double-sided carbon tape, firstly sputtered, and then coated with Gold by Emitech K575X Sputter Coating Unit. Coated bran samples were examined by a FEI Quanta 3D FEG DaulBeam (FEI Ltd, Hillsboro, OR), and the micrographs were recorded and analysed.
2.6. Statistical analysis
Results were expressed as mean ± standard deviation of triplicate analyses for all measurements. Statistical differences between samples were evaluated using one way analysis of variance (ANOVA) followed by Tukey‘s minimum square difference test. Difference between groups was considered significant at P ≤ 0.05. All data were analysed using the software package STATISTICA 10.0 (StatSoft Inc., Tulsa, OK, USA).
3. Results and discussion
Ultrasound has already proven its capability to assist extraction of phenolic compounds from plant material in simpler and more efficient manner, requiring the shorter extraction time and lower solvent consumption, which resulted in increased extraction yields and improved quality of extracts. Given that the extraction solvents significantly alter the total phenolics and the antioxidant activity of the obtained extracts [2], the selection of ethanol as the extraction solvent was based on the previous study of Wang et al. [40] who observed the highest efficacy of ethanol in the extraction of the total phenolic compounds from wheat bran, over methanol and acetone. Although there is some evidence that the use of 80% methanol results in the highest amount of phenolics and the best antioxidant capacity, the use of ethanol is more appropriate due to its non-toxicity and the fact that it can be easily recovered by reduced pressure distillation [6], [14], [35].
3.1. Extraction yield of total phenolics from wheat/oat bran
Wheat/oat phenolic extracts were obtained using ethanol–water solution without ultrasonic pretreatment (Control, Control (24 h)) and with the applied ultrasonic pretreatment at four different frequencies (20–45 kHz) associated with the expected physical and biochemical effects which are dominant at lower frequencies (20–100 kHz) [39]. The selection of applied frequency range was due to the high extraction yields reported in low frequency range (20–40 kHz) [39]. The obtained TPC yields are shown in Fig. 1.
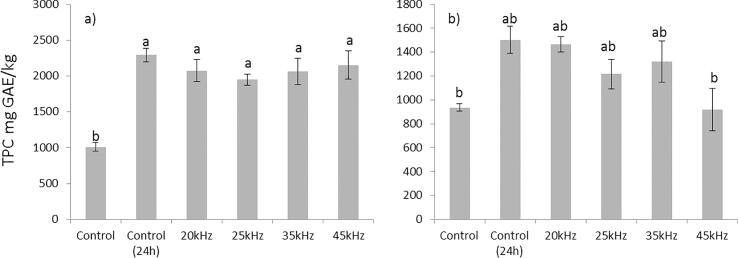
Fig. 1.
Effect of US pretreatment on extraction yield of total phenolics from wheat (a) and oat (b) bran (value is presented as mean (n = 3) ± standard deviation; Means marked with different letter are statistically different (P ≤ 0.05)).
The total phenolic content of US treated wheat bran without shaking ranged from 1949.5 ± 75.7 (25 kHz) to 2152.5 ± 195.9 (45 kHz) mg/kg. No significant differences (P < 0.05) in total phenolic content were detected between ultrasound treated samples and the Control (24 h) (Fig. 1A). The obtained results are lower than the results of Wang et al. [40] who used the same ethanol concentration, but applied higher tempertature regime (40–60 °C) and longer duration of sonication (15–25 min). The obtained results are in line with the results of Chen et al. [11] who applied US frequency and temperature in the range of 20–50 kHz and 30–70 °C, respectively, to treat black wheat breeding line with intrinsicly higher level of phenolic compounds. Untreated wheat bran after 24 h of shaking (Control 24 h) yielded 2293.5 ± 95.5 mg/kg of TPCs, which is higher than the results obtained by Abozed et al. [2] and Zhou & Yu [44] who applied 70% ethanol extraction. The comparison with the results of other authors is difficult due to the variability in the expression of results, variability in the utilization of the extraction solvents, but also variability in the cereal variety and bran particle size [2], [8].
Significantly lower TPC in the Control is due to the absence of mechanical stirring and/or shaking, which is common in conventional extraction procedures to assist the extraction of total phenolics [21]. Mechanical stirring/shaking provides the effective moistening of plant material and helps the interaction between the solid sample and the liquid medium which results in increased amount of extractive substances. The facilitation of extraction by mechanical stirring/shaking is achieved due to the increased molecular diffusion, and removal of concentrated solution from the sample surface, so that unsaturated solvent is in contact with the sample to achieve higher extraction yield.
The total phenolic content of US treated oat bran extract obtained without shaking ranged from 918.7 ± 177.1 (45 kHz) to 1467.5 ± 64.3 mg/kg (20 kHz). No significant changes (P < 0.05) in total phenolic content were observed with respect to ultrasound frequency and the Control (24 h), with an exception of 45 kHz and the Control being somewhat lower (Fig. 1B). Untreated oat bran extract after 24 h of shaking (Control 24 h) yielded 1503.5 ± 113.8 mg/kg of TPCs, being higher than TPC obtained by Hitayezu et al. (2015) who examined six commercial oat milling fractions.
The obtained results indicated a similar extraction efficiency of applyed ultrasonic pretreatment for 10 min and conventional solvent extraction for 24 h (Fig. 1A and 1B). Therefore, the possibility to obtain phenolic extracts from wheat and oat brans using shorter time of extraction (10 min instead of 24 h) could be beneficial regarding energy efficiency, which is especially important in the industrial production of extracts with potential application in the food and pharmaceutical industries.
Ultrasound technology is considered safe and clean as it is based on using non-ionizing radiation. However, referring to Rehman et al. [34], an ultrasound can induce chemical reactions through the occurrence of acoustic cavitation, formation and collapse of small gas bubbles during the treatment. The same authors state that the sonochemical reactions can take place in three different regions: 1) in the high temperature region of a collapsing gas bubble, 2) in the interfacial region between the surrounding liquid and a hot gas phase, 3) in the bulk of solution where the free radicals, formed in the cavitation bubbles and not scavenged in the interfacial region react with organic solutes. These radicals could cause breaking of covalent bonds in a way to cause hydrolysis of ester bonds, which is desired reaction in our case, but could also damage the extracted molecules. However, the applied ultrasound-assisted extraction procedures did not provide the breakage of ester bonds allowing the release of phenolic acids from the structural components of cell walls [18], [27], [29], so that the total phenolic content extractable from wheat/oat bran when alkaline hydrolysis is applied is much higher (10634 ± 268 mg/kg for oat and 14420 ± 523 mg/kg for wheat).
3.2. Extraction kinetics of total phenolics from wheat/oat bran
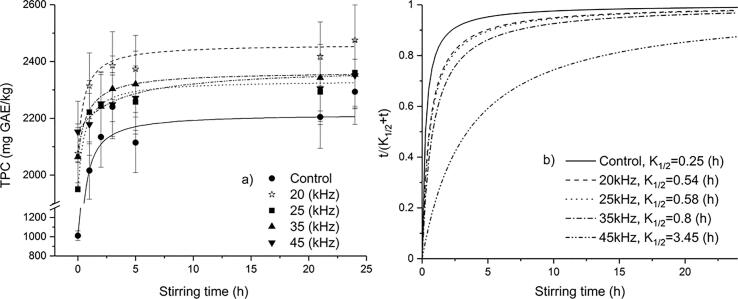
Fig. 2, Fig. 3 show the kinetic profile of phenolics extraction from wheat/oat bran with UAE treatment fitted by Peleg's model. The trend of extraction curves indicates similarity to the sorption process kinetics of Peleg's model [31]. The profiles of the total phenolics extraction curves for wheat bran indicate a high extraction rate in the initial stage of extraction up to 5 h followed by a reduced extraction rate presented by curves that asymptotically approaches an equilibrium concentration (Fig. 2a and 2b).
Fig. 2.
The extraction kinetics of total phenolics content from wheat bran subjected to ultrasound frequencies fitted to Peleg’s model (a) extraction yield, (b) extraction rate.
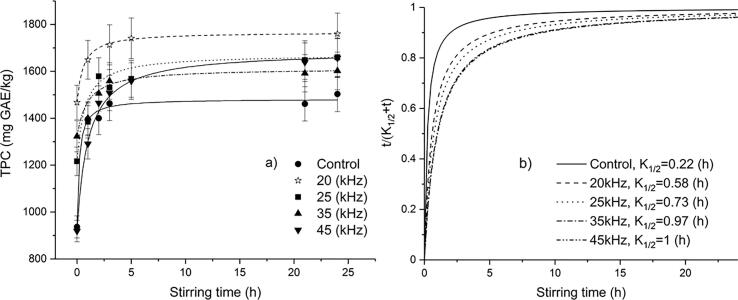
Fig. 3.
The extraction kinetics of total phenolics content from oat bran subjected to ultrasound frequencies fitted to Peleg’s model (a) extraction yield, (b) extraction rate.
Namely, extraction rate is controlled by mass transfer resistance in the liquid film and intra-particle diffusion [25]. Therefore, stirring up to 5 h reduced the resistance in the liquid film by creating the turbulence. A prolonged stirring time had no effect on the extraction rate of total phenolics from wheat bran due to the prevalence of the intra-particle diffusion.
In the case of oat bran, the initial extraction rate was high in the first 3 h and then the extraction rate gradually reached an equilibrium concentration (Fig. 3a and 3b).
Further prolongation of stirring (5 h, 21 h and 24 h) did not significantly improve the phenolics yield due to the large amounts of phenolics initially extracted from bran. This findings are in agreement with finding of Durling et al. [15] who suggested shorter shaking time during extraction of polyphenols, since the increased shaking time potentially may lead to the evaporation of solvent, which resulted in the decreased yield of TPCs.
The highest level of total phenolics content for both brans was obtained with the ultrasound probe system (20 kHz) (Fig. 2a and 3a) due to direct ultrasonic energy transfer to the sample and minimal energy losses [39].
The summary of kinetics parameters is presented in the Table 1.
Table 1.
Peleg’s model parameters for extraction kinetics of total phenolics (mg/kg) from wheat/oat bran.
| Peleg’s model | Control | 20 kHz | 25 kHz | 35 kHz | 45 kHz |
|---|---|---|---|---|---|
| Wheat bran | |||||
| K1 | 0.0002 | 0.0014 | 0.0015 | 0.0027 | 0.0152 |
| K2 | 0.0008 | 0.0026 | 0.0026 | 0.0033 | 0.0044 |
| K1/2 | 0.250 | 0.538 | 0.577 | 0.818 | 3.455 |
| B0 | 5000 | 714.286 | 666.667 | 370.37 | 65.789 |
| Ce | 2261 | 2461.12 | 2334.12 | 2367.03 | 1163.77 |
| R2 | 0.986 | 0.969 | 0.957 | 0.982 | 0.933 |
| Oat bran | |||||
| K1 | 0.0004 | 0.0019 | 0.0016 | 0.0033 | 0.0013 |
| K2 | 0.0018 | 0.0033 | 0.0022 | 0.0034 | 0.0013 |
| K1/2 | 0.222 | 0.576 | 0.727 | 0.971 | 1 |
| B0 | 2500 | 526.316 | 625 | 303.03 | 769.231 |
| Ce | 1492.06 | 1770.53 | 1671.17 | 1617.12 | 1687.98 |
| R2 | 0.99 | 0.998 | 0.931 | 0.93 | 0.996 |
K1 (h kg/mg GAE) – Peleg rate constant; K2 (kg/mg GAE) – Peleg capacity constant; K1/2 (h) – half-life constant; B0 (mg GAE/kg h) – initial extraction rate; Ce (mg GAE/kg) – equilibrium concentration; R2 –coefficient of determination.
The extraction rate constants K1 and K2 increased with increasing ultrasound frequency for wheat bran, whilst for oat bran K1 and K2 increased with increasing US frequency up to 35 kHz, while further increase of US frequency affected decrease of K1 and K2. According to Equation (2), K1 is related to B0, which represents the initial extraction rate of the extraction curve. B0 was higher for the control samples (without UAE) than for sonicated samples, being in accordance with the results of Wen et al. [41]. This can be explained by two-stage extraction of phenolics whereby the first stage encompass the dissolution of the phenolics around the matrix surface (washing) which proceeds very rapidly, while the second stage encompass the slow diffusion of phenolics from the matrix to the solvent [28], [30], [41]. For the UAE treated samples, the first stage started from the beginning of the ultrasonic pretreatment during which most phenols were extracted out from brans. On contrary, the first stage of extraction for the control samples started with macerating. When the orbital shaker extraction started, the extraction of the UAE treated samples already started or got close to the second stage at which the rate was much slowed down. Therefore, B0 for the UAE treated samples was lower than the control groups. This means that UAE could shorten the first stage of extraction.
Maximal extraction capacity (Ce) was higher for sonicated samples than for the control samples. Higher values of Ce imply more extractable compounds from the samples. The present results revealed that the application of US probe system (20 kHz) corresponded to the highest Ce, implying that the cavitation caused by UAE probe system could disrupt structure of samples and enable more target compounds exposed to solvent, as well as break the bonds between target compounds and other molecules, which is in accordance with the results of extraction yield (Fig. 2a and 3a) and with the results of surface morphology of brans obtained by SEM (Fig. 4b and 5b). The coefficient of determinations for predicted total phenolics extraction yields showed a good correlation with the experimental data and indicated that the non-exponential Peleg's model could be employed to predict the extraction phenolics yields after ultrasonic treatment (R2 values was greater than 0.93).
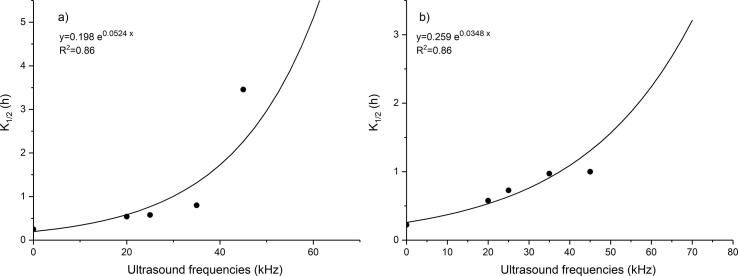
Fig. 4.
Effect of the ultrasound frequencies on the K1/2 for wheat bran (a), and oat bran (b).
In addition, by introducing the constant K1/2 defined as a time required for the initial phenolic concentration to be extracted to one-half of its initial value, it was shown that extraction kinetics was dependent only on one parameter that allowed fast comparison between extraction rates at different ultrasound frequencies. Furthermore, through the process of obtaining the constant K1/2 all experimental data were normalised and organised in order to show a more realistic comparison between ultrasound probe and bath. This is the first study in which the constant K1/2 was introduced. This value can be used to predict and evaluate the extraction kinetics as well as efficiency of different US pretreatment used for total phenolics extraction from wheat and oat brans. It was shown that with K1/2 increasing, the extraction rate is decreasing. The highest extraction rate (the smallest K1/2 value) for wheat bran could be noticed for the control sample (Fig. 2b) due to absence of US pretreatment which shortened the first stage of extraction during which the yield increased rapidly. Due to minor K1/2 differences between oat bran samples (Fig. 3b) extraction rate i.e. kinetics curves are more similar than wheat kinetics curves (Fig. 2b). However, differences in extraction rate between cereal brans could be influenced by the differences in their composition. According to our previously results, wheat bran had higher fibre and protein content in comparison to oat bran, while oat bran was characterized by almost two times higher starch content compared to wheat bran (Nedeljković et al., 2015). So, it is possible that ultrasonic treatment could be more effective in extracting phenolics from the wheat bran due to its microstructural characteristics. The dependency of model constant K1/2 on the ultrasound frequency is shown in Fig. 4. If the ultrasound frequency asymptotically approaches zero, the extraction is very fast, because there is no pre-treatment with ultrasound when much of initial phenols is extracted. This is in an agreement with Wen et al. [41]. On the other hand, when ultrasound frequency reaches infinity, the extraction of phenolics must be also infinitely long (or even impossible), because of the high ultrasound intensity that destructs samples and prevents the extraction. This is in agreement with the statement of Altemimi et al. [5] and Liu et al. [26] who pointed out that the higher frequencies (80 kHz) caused the collapse of cavitation bubbles in the samples not allowing them sufficient time to extract the target compounds which as a consequence have the prevention of the extraction of target compounds. Furthermore, Fig. 4 helps to predict unknown K1/2 values and consequently K1 and K2 values for wheat and oat brans with a certain accuracy, for a specific ultrasound frequency.
3.3. Microstructural changes of wheat/oat bran induced by ultrasonication
The enhancement in the extraction rate achieved by UAE is attributed to the cavitation phenomenon produced in the solvent by sonication [40]. Due to cavitation the cell membrane disruption occurs which alters microstructural properties and the porosity of matrix – micro fissures and channels are formed that improve the permeation of solvent into the matrix [22], [39], [41]. Microstructural changes of the control samples and samples treated by ultrasound were analysed with a scanning electron microscope (SEM) and presented in Fig. 5, Fig. 6.
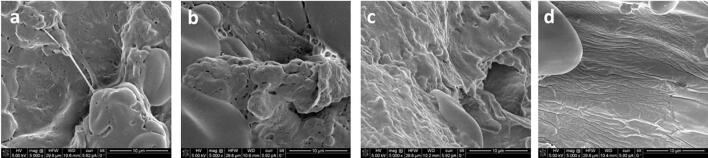
Fig. 5.
Scanning electron microscopy images of control sample of wheat bran (a) and US treated wheat bran at 20 kHz (b), 35 kHz (c) and 45 kHz (d).
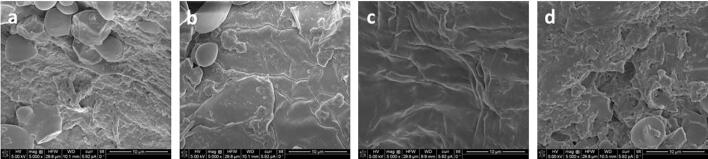
Fig. 6.
Scanning electron microscopy images of control sample of oat bran (a) and US treated oat bran at 20 kHz (b), 35 kHz (c) and 45 kHz (d).
Wheat and oat bran subjected to the conventional solvent extraction (control samples) underwent slight damage without pronounced fissures within the fibre matrix, mainly associated with the dissolution of the plant cell walls and membranes, due to which the phenolic compounds were released into the solvent (Fig. 5a and 6a).
The control samples exhibited different microstructural characteristics: wheat bran fibre matrix was covered with proteins (Fig. 5a), while oat bran was with visible residual starch particles (Fig. 6a), which may be the reason of the lower extraction rate of total phenolics obtained for oat bran in relation to wheat bran (K1/2 = 0.250 for wheat bran; K1/2 = 0.222 for oat bran).
The samples treated with UAE exhibited cell disruption, the emergence of micro fissures on the surface, indicating more intensive disruption of matrix surface than that obtained by conventional solvent extraction. Ultrasonic treatment weakened the protein network, “cleaned” the surfaces and exposed the fibre matrix. The surface of bran samples treated with the probe focussed sonication (at 20 kHz) (Fig. 5b and 6b) exhibited more intense matrix rupture compared to the bath focused sonication (Fig. 5c and 5d, Fig. 6c and 6d), with visible cracks caused not only by the higher ultrasound intensity, but a larger number of cavitation nucleus formed [32].
4. Conclusion
The results showed that the ultrasound application enhanced the solvent extraction of free phenolics from wheat and oat bran. Stirring times of 1 h to 5 h for wheat bran, and from 1 h to 3 h for oat bran improved the free phenolics extraction, but only 15% of total phenolic compounds present were extractable. Although UAE enhanced the extraction of free phenolic compounds by altering the microscopic structure of the brans tissue as shown by SEM images, the applied ultrasonic treatments did not provide sufficient energy to break ester and ether bonds, so that the appropriate selection of ultrasound intensities is crucial to enhance the isolation of total phenolics from cereal brans if the use of strong bases is to be avoided. Peleg’s model fits adequately extraction of the free phenolics from wheat/oat bran. For the first time, in this study the constant that represents a time required for the initial phenolic concentration to be extracted to one-half of its initial value has been introduced (K1/2). It was shown that the TPC extraction kinetics was dependent only on K1/2 enabling fast comparison between extraction rates at different ultrasound frequencies. It was shown that K1/2 exponentially increased with ultrasound frequencies which consequently prolonged the extraction of phenolics from cereal brans.
Funding
This work was financially supported by the Science Fund of Republic of Serbia (project number 6060592) and by the Ministry of Education, Science and Technological Development of the Republic of Serbia (contract No. 451-03-9/2021-14/ 200222).
CRediT authorship contribution statement
Nataša Milićević: Conceptualization, Methodology, Formal analysis. Predrag Kojić: Data curation. Marijana Sakač: Supervision. Aleksandra Mišan: Funding acquisition, Writing - review & editing. Jovana Kojić: Formal analysis. Camila Perussello: Methodology, Formal analysis. Vojislav Banjac: Formal analysis. Milica Pojić: Funding acquisition, Writing - review & editing. Brijesh Tiwari: Conceptualization, Supervision, Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The part of the experimental work on this paper is supported by project that has received funding from the European Union's Horizon 2020 Spreading Excellence and Widening Participation programme (under grant agreement number 692276).
References
- 1.Aarabi A., Mizani M., Honarvar M., Faghihian H., Gerami A. Extraction of ferulic acid from sugar beet pulp by alkaline hydrolysis and organic solvent methods. J. Food Meas. Charact. 2016;10(1):42–47. doi: 10.1007/s11694-015-9274-z. [DOI] [Google Scholar]
- 2.Abozed S.S., El-kalyoubi M., Abdelrashid A., Salama M.F. Total phenolic contents and antioxidant activities of various solvent extracts from whole wheat and bran. Ann. Agric. Sci. 2014;59(1):63–67. doi: 10.1016/j.aoas.2014.06.009. [DOI] [Google Scholar]
- 3.Alonso E. The role of supercritical fluids in the fractionation pretreatments of a wheat bran-based biorefinery. J. Supercritical Fluids. 2018;133:603–614. doi: 10.1016/j.supflu.2017.09.010. [DOI] [Google Scholar]
- 4.Alrahmany R., Tsopmo A. Role of carbohydrases on the release of reducing sugar, total phenolics and on antioxidant properties of oat bran. Food Chem. 2012;132(1):413–418. doi: 10.1016/j.foodchem.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Altemimi A., Watson D.G., Choudhary R., Dasari M.R., Lightfoot D.A., Lin W.-X. Ultrasound assisted extraction of phenolic compounds from peaches and pumpkins. PLoS ONE. 2016;11(2):e0148758. doi: 10.1371/journal.pone.0148758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azwanida N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromatic Plants. 2015;4(196):412–2167. doi: 10.4172/2167-0412.1000196. [DOI] [Google Scholar]
- 7.Balyan U., Sarkar B. Aqueous extraction kinetics of phenolic compounds from jamun (Syzygium cumini L.) seeds. Int. J. Food Prop. 2017;20(2):372–389. doi: 10.1080/10942912.2016.1163266. [DOI] [Google Scholar]
- 8.Brewer L.R., Kubola J., Siriamornpun S., Herald T.J., Shi Y.-C. Wheat bran particle size influence on phytochemical extractability and antioxidant properties. Food Chem. 2014;152:483–490. doi: 10.1016/j.foodchem.2013.11.128. [DOI] [PubMed] [Google Scholar]
- 9.Bucić-Kojić A., Planinić M., Tomas S., Bilić M., Velić D. Study of solid-liquid extraction kinetics of total polyphenols from grape seeds. J. Food Eng. 2007;81(1):236–242. doi: 10.1016/j.jfoodeng.2006.10.027. [DOI] [Google Scholar]
- 10.Buranov A.U., Mazza G. Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents. Food Chem. 2009;115(4):1542–1548. doi: 10.1016/j.foodchem.2009.01.059. [DOI] [Google Scholar]
- 11.Chen X., Li X., Zhu X., Wang G., Zhuang K., Wang Y., Ding W. Optimization of extrusion and ultrasound-assisted extraction of phenolic compounds from Jizi439 Black wheat bran. Processes. 2020;8(9):1153. doi: 10.3390/pr8091153. [DOI] [Google Scholar]
- 12.Couteau D., Mathaly P. Purification of ferulic acid by adsorption after enzymic release from a sugar-beet pulp extract. Ind. Crops Prod. 1997;6(3):237–252. doi: 10.1016/S0926-6690(97)00014-9. [DOI] [Google Scholar]
- 13.Đorđević T., Antov M. Ultrasound assisted extraction in aqueous two-phase system for the integrated extraction and separation of antioxidants from wheat chaff. Sep. Purif. Technol. 2017;182:52–58. doi: 10.1016/j.seppur.2017.03.025. [DOI] [Google Scholar]
- 14.Drużyńska B., Stępniewska A., Wołosiak R. The influence of time and type of solvent on efficiency of the extraction of polyphenols from green tea and antioxidant properties obtained extracts. Acta Scientiarum Polonorum Technologia Alimentaria. 2007;6(1):27–36. http://www.food.actapol.net/issue1/volume/3_1_2007.pdf [Google Scholar]
- 15.Durling N.E., Catchpole O.J., Grey J.B., Webby R.F., Mitchell K.A., Foo L.Y., Perry N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007;101(4):1417–1424. doi: 10.1016/j.foodchem.2006.03.050. [DOI] [Google Scholar]
- 16.Galanakis C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: a viewpoint of opportunities and challenges. Food Bioprod. Process. 2013;91(4):575–579. doi: 10.1016/j.fbp.2013.01.004. [DOI] [Google Scholar]
- 17.Gopalan N., Nampoothiri K.M. Biorefining of wheat bran for the purification of ferulic acid. Biocatalysis Agric. Biotechnol. 2018;15:304–310. doi: 10.1016/j.bcab.2018.07.004. [DOI] [Google Scholar]
- 18.Guerrini A., Burlini I., Huerta Lorenzo B., Grandini A., Vertuani S., Tacchini M., Sacchetti G. Antioxidant and antimicrobial extracts obtained from agricultural by-products: Strategies for a sustainable recovery and future perspectives. Food Bioprod. Process. 2020;124:397–407. doi: 10.1016/j.fbp.2020.10.003. [DOI] [Google Scholar]
- 19.Hromádková Z., Košt’álová Z., Ebringerová A. Comparison of conventional and ultrasound-assisted extraction of phenolics-rich heteroxylans from wheat bran. Ultrason. Sonochem. 2008;15(6):1062–1068. doi: 10.1016/j.ultsonch.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Kadam S.U., Tiwari B.K., O’Donnell C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food. Chem. 2013;61(20):4667–4675. doi: 10.1021/jf400819p. [DOI] [PubMed] [Google Scholar]
- 21.Kasparavičienė G., Ramanauskienė K., Savickas A., Velžienė S., Kalvėnienė Z., Kazlauskienė D., Ivanauskas K. Evaluation of total phenolic content and antioxidant activity of different Rosmarinus officinalis L. ethanolic extracts. Biologija. 2013;59(1) doi: 10.6001/biologija.v59i1.2650. [DOI] [Google Scholar]
- 22.Khandare R.D., Tomke P.D., Rathod V.K. Kinetic modeling and process intensification of ultrasound-assisted extraction of d-limonene using citrus industry waste. Chem. Eng. Processing – Process Intensification. 2020;108181 doi: 10.1016/j.cep.2020.108181. [DOI] [Google Scholar]
- 23.Kim K.-H., Tsao R., Yang R., Cui S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006;95(3):466–473. doi: 10.1016/j.foodchem.2005.01.032. [DOI] [Google Scholar]
- 24.Kumar K., Srivastav S., Sharanagat V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O. Levenspiel, 2013. Chemical Reactor Omnibook-soft cover. Lulu. com.
- 26.Liu B., Ma Y., Liu Y., Yang Z., Zhang L. Ultrasonic-assisted extraction and antioxidant activity of flavonoids from Adinandra nitida leaves. Trop. J. Pharm. Res. 2013;12(6):1045–1051. doi: 10.4314/tjpr.v12i6.27. [DOI] [Google Scholar]
- 27.Liyana-Pathirana C.M., Shahidi F. Importance of insoluble-bound phenolics to antioxidant properties of wheat. J. Agric. Food. Chem. 2006;54(4):1256–1264. doi: 10.1021/jf052556h. [DOI] [PubMed] [Google Scholar]
- 28.Oreopoulou A., Goussias G., Tsimogiannis D., Oreopoulou V. Hydro-alcoholic extraction kinetics of phenolics from oregano: Optimization of the extraction parameters. Food Bioprod. Process. 2020;123:378–389. doi: 10.1016/j.fbp.2020.07.017. [DOI] [Google Scholar]
- 29.Parenti O., Guerrini L., Zanoni B. Techniques and technologies for the breadmaking process with unrefined wheat flours. Trends Food Sci. Technol. 2020;99:152–166. doi: 10.1016/j.tifs.2020.02.034. [DOI] [Google Scholar]
- 30.Paunović D.Đ., Mitić S.S., Kostić D.A., Mitić M.N., Stojanović B.T., Pavlović J.L. Kinetics and thermodynamics of the solid-liquid extraction process of total polyphenols from barley. Adv. Technol. 2014;3(2):58–63. [Google Scholar]
- 31.PELEG M. An empirical model for the description of moisture sorption curves. J. Food Sci. 1988;53(4):1216–1217. doi: 10.1111/jfds.1988.53.issue-410.1111/j.1365-2621.1988.tb13565.x. [DOI] [Google Scholar]
- 32.Pereira S., Fonseca L.P., Capelo J.L., Armas T., Vilhena F., Pinto A.P. Comparative study between probe focussed sonication and conventional stirring in the evaluation of cadmium and copper in plants. Anal. Bioanal. Chem. 2010;398(5):2315–2324. doi: 10.1007/s00216-010-4178-6. [DOI] [PubMed] [Google Scholar]
- 33.Peterson D.M. Oat antioxidants. J. Cereal Sci. 2001;33(2):115–129. doi: 10.1006/jcrs.2000.0349. [DOI] [Google Scholar]
- 34.Rehman M.U., Jawaid P., Uchiyama H., Kondo T. Comparison of free radicals formation induced by cold atmospheric plasma, ultrasound, and ionizing radiation. Arch. Biochem. Biophys. 2016;605:19–25. doi: 10.1016/j.abb.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Qasim M., Aziz I., Rasheed M., Gul B., Khan M.A. Effect of extraction solvents on polyphenols and antioxidant activity of medicinal halophytes. Pak. J. Bot. 2016;48(2):621–627. [Google Scholar]
- 36.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 37.Stevenson L., Phillips F., O’Sullivan K., Walton J. Wheat bran: its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012;63(8):1001–1013. doi: 10.3109/09637486.2012.687366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabaraki R., Nateghi A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason. Sonochem. 2011;18(6):1279–1286. doi: 10.1016/j.ultsonch.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Tiwari B.K. Ultrasound: A clean, green extraction technology. TrAC, Trends Anal. Chem. 2015;71:100–109. doi: 10.1016/j.trac.2015.04.013. [DOI] [Google Scholar]
- 40.Wang J., Sun B., Cao Y., Tian Y., Li X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106(2):804–810. doi: 10.1016/j.foodchem.2007.06.062. [DOI] [Google Scholar]
- 41.Wen L., Zhang Z., Rai D., Sun D.-W., Tiwari B.K. Ultrasound-assisted extraction (UAE) of bioactive compounds from coffee silverskin: Impact on phenolic content, antioxidant activity, and morphological characteristics. J. Food Process Eng. 2019;42(6) doi: 10.1111/jfpe.13191. [DOI] [Google Scholar]
- 42.Wijngaard H.H., Trifunovic O., Bongers P. Novel extraction techniques for phytochemicals. Handbook of Plant Food Phytochemicals. 2013:412–433. doi: 10.1002/9781118464717.ch18. [DOI] [Google Scholar]
- 43.Xavier L., Freire M.S., González-Álvarez J. Modeling and optimizing the solid–liquid extraction of phenolic compounds from lignocellulosic subproducts. Biomass Convers. Biorefin. 2019;9(4):737–747. doi: 10.1007/s13399-019-00401-9. [DOI] [Google Scholar]
- 44.Zhou K., Yu L. Effects of extraction solvent on wheat bran antioxidant activity estimation. LWT – Food Sci. Technol. 2004;37(7):717–721. doi: 10.1016/j.lwt.2004.02.008. [DOI] [Google Scholar]